Abstract
Proteolysis by the ubiquitin-proteasome system is highly selective. Specificity is achieved by the cooperation of diverse ubiquitin-conjugating enzymes (Ubcs or E2s) with a variety of ubiquitin ligases (E3s) and other ancillary factors. These recognize degradation signals characteristic of their target proteins. In a previous investigation, we identified signals directing the degradation of β-galactosidase and Ura3p fusion proteins via a subsidiary pathway of the ubiquitin-proteasome system involving Ubc6p and Ubc7p. This pathway has recently been shown to be essential for the degradation of misfolded and regulated proteins in the endoplasmic reticulum (ER) lumen and membrane, which are transported to the cytoplasm via the Sec61p translocon. Mutant backgrounds which prevent retrograde transport of ER proteins (hrd1/der3Δ and sec61-2) did not inhibit the degradation of the β-galactosidase and Ura3p fusions carrying Ubc6p/Ubc7p pathway signals. We therefore conclude that the ubiquitination of these fusion proteins takes place on the cytosolic face of the ER without prior transfer to the ER lumen. The contributions of different sequence elements to a 16-amino-acid-residue Ubc6p-Ubc7p-specific signal were analyzed by mutation. A patch of bulky hydrophobic residues was an essential element. In addition, positively charged residues were found to be essential. Unexpectedly, certain substitutions of bulky hydrophobic or positively charged residues with alanine created novel degradation signals, channeling the degradation of fusion proteins to an unidentified proteasomal pathway not involving Ubc6p and Ubc7p.
As intracellular proteolysis by the ubiquitin-proteasome system plays a crucial role in complex processes such as the cell cycle, development, signal transduction, and transcriptional regulation, it is not surprising that it is exquisitely controlled. This means that degradation must be not only highly specific for particular proteins, but also must occur at appropriate times and places dictated by the state of the cell or the organism. Selection of specific proteins for destruction is usually achieved at the level of ligation of ubiquitin to the target. Diverse ubiquitin-conjugating enzymes (E2s or Ubcs) cooperate with numerous ubiquitin ligases (E3s) and other factors to attach ubiquitin to the appropriate target protein (reviewed in references 8 and 15). Each combination of E2s, E3s, and other factors is thought to recognize a specific degradation signal on a target protein. Often the signal is conditional and must be covalently modified to be activated or, alternatively, a cryptic signal must be exposed to ensure that ubiquitination and degradation occur at the appropriate time or physiological condition of the cell. Although a large number of combinations of E2s and E3s and other ancillary factors have been found to participate in the ubiquitination of different targets, relatively few protein degradation signals are known (reviewed in references 8, 15, 16, 19, 20, 22, 27, and 32).
In an earlier investigation, we made lacZ and URA3 libraries with random yeast genomic fragments inserted at the C terminus of the genes. These libraries were screened for clones producing β-galactosidase or Ura3p fusion proteins with C-terminal extensions making them unstable. By determining which ubiquitin system mutants stabilize such fusion proteins, the subsidiary pathways involved in their degradation were identified (11). The clones most frequently isolated in this way produced unstable fusion proteins which were degraded via the Ubc6p-Ubc7p pathway. The C-terminal extensions of these fusion proteins consisted of short, nonphysiological reading frames (16 to 60 amino acid residues) and contained strongly hydrophobic stretches (Table 1). In a parallel investigation, Johnson et al. (21) provided evidence that the Deg1 signal of the Matα2 repressor, which is recognized by the Ubc6p-Ubc7p pair, has the characteristics of an amphipathic α-helix. Only some of the signals found in our laboratory had predicted amphipathic α-helix sequences. Taken together, these results suggest that a feature of the signal can be a cluster of large hydrophobic residues or an amphipathic α-helix. Interestingly, the Deg1 signal of Matα2p is cryptic in diploid yeast cells because of its association with Mata1p, revealing oligomerization as a novel mechanism regulating degradation (21).
TABLE 1.
C-terminal signal sequences of unstable Ura3p fusion proteins stabilized in a ubc6 ubc7 background
| Clone | Deduced C-terminal sequencea |
|---|---|
| CL1 | ACKNWFSSLSHFVIHL |
| CL2 | SLISLPLPTRVKFSSLLLIRIMKIITMTFPKKLRS |
| CL6 | FYYPIWFARVLLVHYQ |
| CL9 | SNPFSSLFGASLLIDSVSLKSNWDTSSSSCLISFFSSVMFSSTTRRS |
| CL10 | CRQRFSCHLTASYPQSTVTPFLAFLRRDFFFLRHNSSAD |
| CL11 | GAPHVVLFDFELRITNPLSHIQSVSLQITLIFCSLPSLILSKFLQV |
| CL12 | NTPLFSKSFSTTCGVAKKTLLLAQISSLFFLLLSSNIAV |
| CL15 | PTVKNSPKIFCLSSSSPYLAFNLEYLSLRIFSTLSKCSNTLLTSLS |
| CL16 | SNQLKRLWVLWLLEVRSFDRTLRRPWIHLPS |
| SL17 | SISFVIRSHASIRMGASNDFFHKLYFTKCLTSVILSKFLIHLLLRSTPRV |
Hydrophobic regions in a Kyte-Doolittle hydropathy plot (window size = 7) are underlined, and positively charged residues are boldface.
Ubc6p and Ubc7p have recently been shown to be implicated in the degradation of misfolded or regulated proteins localized in the endoplasmic reticulum (ER) lumen or membrane. The degradation of these ER proteins occurs via the ubiquitin-proteasome system, following retrograde transport to the cytoplasm via the Sec61p translocon (reviewed in references 5, 26, and 31). The ubiquitinating enzymes Ubc6p and Ubc7p have been implicated in this process (18). Ubc6p is attached to the cytoplasmic face of the ER by a C-terminal membrane anchor, and Ubc7p is moored to the same face of the membrane by a recently discovered protein, Cue1p (3). Ubiquitination of the unstable ER proteins by Ubc6p, Ubc7p, and Cue1p is essential for their translocation to the cytoplasm. Signals required for the degradation of aberrant proteins in the ER lumen have been described and have been found to consist of a C-terminal hydrophobic region with embedded charged residues (4, 23).
In light of these recent developments, a number of questions arise regarding the fusion proteins with Ubc6p-Ubc7p-specific signals which we described previously (11). One question is if the unstable fusion proteins interact directly with Ubc6p and Ubc7p, which are located at the cytosolic face of the ER, or if transport to the ER lumen followed by export via the Sec61p translocon, leading to ubiquitination, is a prerequisite for degradation. Another question is if Cue1p is involved in ubiquitination of the Ubc6p-Ubc7p target proteins identified in our laboratory, as in the case of the ER proteins studied in other laboratories (3). An additional question is whether a strongly hydrophobic region is sufficient to channel protein degradation to the Ubc6p-Ubc7p pathway or whether other sequence elements are required. The present investigation indicates that ubiquitination of the fusion proteins studied in our laboratory occurs on the cytosolic face of the ER without prior transport to the lumen. Mutational analysis of a 16-amino-acid-residue signal indicates that positively charged residues and a strongly hydrophobic region are essential elements of this signal.
MATERIALS AND METHODS
Media.
Cells carrying plasmids derived from pOC9 (11) were grown on minimal (SD) medium without tryptophan (30). To distinguish between cells with high and low levels of Ura3p, uracil was also omitted from the medium. Cells carrying plasmids derived from pBRR88 (11) were grown on SD without uracil.
Yeast strains.
The following strains of Saccharomyces cerevisiae were used: SUB62/DF5a [MATa ura3-52 leu2-3 his3Δ200 lys2-801 trp1-1(am)] (10); SS414 (ubc6::HIS3 ubc7::LEU2 in SUB62) (7); rpt2RF (in SUB62) (28); SUB61/DF5α [MATα ura3-52 leu2-3 his3Δ200 lys2-801 trp1-1(am)] (10); YTX115 (cue1::LEU2) in SUB61 (3); W303-IC (prc1-1 ade2-loc ura3-1 his3-11,15 leu2-3,112 trp1-1 can1-100); W303-ICΔ3 (der3::HIS3 in W303-IC) (6); YS181 (MATa ura3 leu2-3,112 his3-11,5 trp1Δ); YS182 (pre1-1 pre2-1 in YS181) (14); MHY501 (MATα ura3-52 lys2-80 his3-Δ200 trp1-1 leu2-3,112); MHY623 (doa4::LEU2 in MHY501) (24); and RSY333 (MATα sec61-2 leu2-3,112 ade2 ura3-52 pep4-3) (9).
Plasmids.
pBRR88 is a 2 μm lacZ expression vector with a GAL1 promoter (11); pBRR88-SL17 is derived from the same vector but expresses β-galactosidase with a 50-amino-acid-residue C-terminal degradation signal (11). K-β-gal is a PLGSD5-derived expression vector expressing β-galactosidase with an N-terminal lysine (2, 12). pOC9 is a centromeric URA3 expression vector under the CUP1 promoter (11). CL1, CL2, CL6, CL9, CL10, CL11, CL12, CL15, and CL16 are derived from the pOC9 vector and express Ura3p with various C-terminal degradation signals (Table 1) (11).
Mutant plasmid construction.
Two PCR approaches were used to mutate the CL1 signal. Mutations close to the C terminus of the signal were introduced by PCR with an SK sense primer and a mutagenic antisense primer. The product, which included the URA3 gene plus the mutated C-terminal extension, was inserted into the pOC9 vector from which URA3 had been excised with EcoRI and SalI. Mutations proximal to the URA3 gene were introduced by PCR with a sense mutagenic primer and an antisense primer complementary to a sequence in the CL1 insert downstream of the translational stop codon of the signal sequence. The resulting fragment was inserted into the BamHI site of the pOC9 vector. All mutations were verified by sequencing.
Pulse-chase analysis.
Pulse-chase analyses were done as described in detail previously (11). Briefly, mid-exponential-phase cells grown in selective medium without methionine in the presence of 200 μM CuSO4 were labeled for 5 min with l-[35S]-methionine. After washing, they were incubated in the same medium supplemented with l-methionine (1 mg/ml) plus cycloheximide (0.5 mg/ml). Samples were suspended in disruption buffer containing 100 μM N-ethylmaleimide and protease inhibitor cocktail (4 μl/ml; cocktail 1 for yeast and fungi; Sigma) and broken with glass beads. After centrifugation, trichloroacetic acid (TCA)-insoluble counts in the supernatant were measured. Samples of the supernatant containing equal amounts of TCA-soluble counts were immunoprecipitated with anti-HA epitope antibody plus protein A beads and after elution were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The radioactivity patterns of the gels were monitored with a phosphoimager.
β-Galactosidase assay.
The enzymatic activity of β-galactosidase of mid-exponential-phase cultures growing on minimal medium without glucose plus 2% galactose and 2% raffinose was measured as described by Guarente (12).
RESULTS
Role of the ER in Ura3 fusion protein degradation.
In a previous paper, we described nine Ura3p fusion proteins (CL1, CL2, CL6, CL9, CL10, CL11, CL12, CL15, and CL16) and one β-galactosidase fusion protein (SL17), each with a different C-terminal degradation signal channeling the proteins for degradation via Ubc6p-Ubc7p (Table 1). As outlined in the Introduction, Ubc6p and Ubc7p have been implicated in ER protein degradation involving their retrograde transport to the cytoplasm. Thus, the question arose whether the fusion proteins studied in our laboratory must first be imported into the ER and subsequently undergo retrograde transport to the cytoplasm, followed by degradation by the proteasome, similarly to proteins normally resident in the ER (5, 26, 31). In order to test this we examined the effect of mutating genes essential for retrograde transport from the ER on the stability of our Ura3p fusion proteins. Cells of a cue1 null mutant (3) carrying clones of the fusion proteins, with signals shown in Table 1, grew in the absence of uracil, indicating stabilization of the fusion protein in this background. Thus, Cue1p is involved in the degradation of all the fusion proteins. In contrast, no growth was observed with the same clones in an hrd1/der3 null mutant (6, 13) background, indicating that this gene is unnecessary for degradation of their products. Similarly, the instability of the β-gal-SL17 fusion protein, as indicated by β-galactosidase activity, was unaffected in a sec61-2 mutant (9) at the nonpermissive temperature and in an hrd1 null mutant (Fig. 1B). Our observations do not indicate whether in the hrd1 or sec61-2 mutant degradation of the fusion proteins still occurs via Ubc6p and Ubc7p, but a change of degradative pathway as a result of the mutations seems unlikely. Taken together, the results indicate that the components of the retrograde transport machinery examined are not required for the degradation of any of the presumably cytosolic fusion proteins studied here. Recent reports indicate that ER membrane proteins can be degraded with various degrees of dependence of on UBC6, UBC7, or HRD genes and even complete independence of these genes (17, 33). Cue1p, which anchors Ubc7p to the cytoplasmic face of the ER membrane (3), was essential for the degradation of the fusion proteins tested. This indicates that the Ubc7p-Cue1p complex is required for the proteolysis of proteins resident in the cytosol as well as for that of proteins located in the ER. Our findings are consistent with an earlier report (25) that the Matα2-derived Deg1–β-galactosidase fusion protein is degraded in the cytosol independently of the ER translocation system.
FIG. 1.
Effect of cue1Δ, hrd1/der3Δ, and sec61-2 mutations on Ura3 fusion protein stability. (A) Growth without uracil of wild-type (WT), cue1Δ (3), and hrd1 der3Δ (6) cells carrying plasmids expressing fusion proteins with Ubc6p-Ubc7p-specific signals (11). pOC9 is the parent plasmid, which expresses stable Ura3p. Patches of mutant and isogenic wild-type cells were grown on SD plus uracil without tryptophan and replica plated onto SD without uracil and tryptophan. Growth on plates without uracil was taken as an indicator of Ura3p stability. (B) β-Galactosidase activity of sec61-2 mutant cells carrying plasmids expressing β-galactosidase (β-gal), the β-galactosidase-SL17 fusion protein (β-gal–SL17) (11), or β-galactosidase with an N-terminal lysine residue (K-β-gal) (2) at permissive and nonpermissive temperatures. (C) β-Galactosidase activity of wild-type and hrd1/der3Δ mutant cells carrying plasmids expressing β-galactosidase (β-gal), β-galactosidase-SL17 fusion protein (β-gal–SL17) (11), or β-galactosidase with an N-terminal lysine residue (K-β-gal) (2).
Importance of bulky hydrophobic amino acid residues.
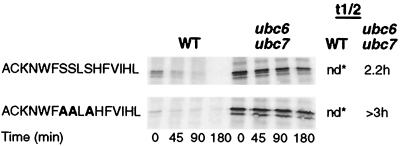
To further analyze the essential elements of signals channeling proteins for degradation via the Ubc6p-Ubc7p-Cue1p pathway, we performed a mutational analysis of the CL1 signal. This signal consists of 16 amino acid residues whose sequence corresponds to an almost perfect predicted amphipathic α-helix (Fig. 2). Substitution of W5 and F6 with A led to complete stabilization of the CL1 protein (Fig. 3). Single substitutions of either W5 or F6 similarly led to marked stabilization. In contrast, replacement of four C-terminal bulky hydrophobic residues (F, V, I, and L) with A did not stabilize the fusion protein. Interestingly, the fusion protein mutated in this way was not stabilized in a ubc6 ubc7 mutant background (Fig. 3). This indicates that the altered signal channeled degradation of the protein to an unknown pathway other than the Ubc6p-Ubc7p pathway. A mutant in which both W5 and F6 and the C-terminal bulky hydrophobic residues were replaced with A was stable. Thus, W5 and F6 appear to be important for the newly created signal as well as for the Ubc6p-Ubc7p pathway signal. These experiments confirmed the conclusions of the previous comparative study that hydrophobic regions are an important element of the Ubc6p-Ubc7p pathway signal (11). They show that even small changes in hydrophobicity can greatly alter the effectiveness of the signal.
FIG. 2.
Sequence and helical wheel representation of the CL1 degradation signal. Boxes identify hydrophobic residues.
FIG. 3.
Effects of replacing bulky hydrophobic residues of the CL1 degradation signal with alanine or serine. Mutated residues are shown in boldface. Fusion protein stability was determined by pulse-chase analysis. nd, not determined because of rapid degradation rate; estimated t1/2, <5 min.
In most of the pulse-chase experiments, two or more immunoprecipitated bands were visible. These were not the result of ubquitination, as molecular weight differences were too small. The possibility that some of the bands were phosphorylated species of the C terminus seems unlikely, as a fusion protein in which all the serines of the C-terminal extension were replaced with alanine still formed multiple bands. Although there is no verification for this, the most likely possibility is that the bands were products of limited proteolysis which did not affect the hemagglutinin (HA) epitope.
Importance of positively charged amino acid residues.
All the Ubc6p-Ubc7p degradation signals described previously have positively charged amino acid residues adjacent to or embedded in a hydrophobic region (Table 1) (11). We therefore mutated these residues in the CL1 signal in order to determine their importance. Figure 4 shows that a K3R substitution did not alter the instability of the CL1 protein. This showed that K3 is not the ubiquitination site of the CL1 fusion protein. Substitution of K3 with A or T slowed degradation but did not abolish it. Similarly, even a reversal of charge by substitution of K3 with E slowed but did not abolish degradation. Taken together, these substitutions show that a positively charged residue at position 3 is not an essential element of the signal, although it is important for maximum degradation rate.
FIG. 4.
Effects of replacing lysine 3 of the CL1 degradation signal with other residues. Residue replacements are shown in boldface. Fusion protein stability was determined by pulse-chase analysis. nd, not determined because of rapid degradation rate; estimated t1/2, <5 min.
In contrast, positively charged residues at positions 11 and 15 were found to be essential for degradation via the Ubc6p-Ubc7p pathway (Fig. 5). Mutation of residues H11 and H15 to R slowed but did not abolish degradation via the Ubc6p-Ubc7p pathway. On the other hand, charge reversal at positions 11 and 15 by substitution with D abolished the degradation of the fusion protein. Interestingly, mutation of H11 and H15, either singly or together, to small uncharged residues (A or T) channeled degradation to an unknown pathway other than that involving Ubc6p and Ubc7p. A mutant in which all of the positively charged amino acid residues (K3, H11, and H15) were replaced with A was unstable and not stabilized in a ubc6 ubc7 mutant background.
FIG. 5.
Effects of replacing histidines 11 and/or 15 of the CL1 degradation signal with other residues. Mutated residues are shown in boldface. Fusion protein stability was determined by pulse-chase analysis. nd, not determined because of rapid degradation rate; estimated t1/2, <5 min.
Role of serine residues.
All three serines of the CL1 signal are located on the hydrophilic face of a predicted amphipathic α-helix. Mutation of all of these S residues to A did not significantly alter the degradation rate via the Ubc6p-Ubc7p pathway (Fig. 6). This mutation would significantly increase the hydrophobicity of the hypothetical hydrophilic face of the α-helix. Thus, it is doubtful if amphipathic character plays a role in the CL1 signal. The lack of effect of the serine-to-alanine mutation also indicated that phosphorylation of the signal was not a prerequisite for fusion protein degradation.
FIG. 6.
Effect of replacing serine with alanine in the CL1 degradation signal. Residue replacements are shown in boldface. Fusion protein stability was determined by pulse-chase analysis. nd, not determined because of rapid degradation rate; estimated t1/2, <5 min.
Degradation of fusion proteins with novel signals created by mutation is proteasomal.
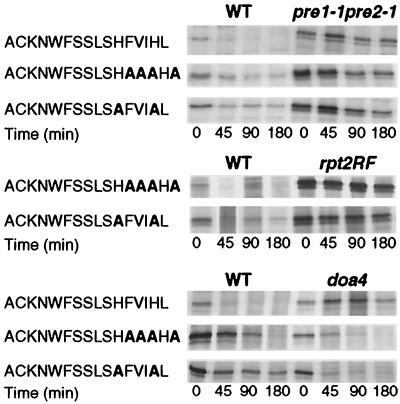
The above experiments showed that both the quadruple mutant F12A V13A I14A L16A (Fig. 3) and the double mutant H11A H15A (Fig. 5) were unstable and were not stabilized in the ubc6 ubc7 double mutant. This indicated that the mutations had created novel signals which, instead of channeling degradation via the Ubc6p-Ubc7p pathway, directed it to an unidentified degradation pathway(s). The first question arising was whether this redirection of pathway terminated at the proteasome. Figure 7 shows that both mutant fusion proteins were stabilized both in a proteasome core particle subunit mutant, pre1 pre2 (14), and in a regulatory particle ATPase mutant, rpt2RF (28). Interestingly, unlike the parent CL1 fusion protein, neither of the newly created fusion proteins was stabilized in the doa4 (ubiquitin hydrolase) mutant, which indirectly inhibits proteasome function in some instances (24).
FIG. 7.
Stability of fusion proteins with newly created degradation signals in proteasome subunit and ubiquitin hydrolase mutant backgrounds. Fusion protein stability was determined by pulse-chase analysis. The proteasome mutants tested were pre1-1 pre2-1 (14) and rpt2RF (28). The ubiquitin hydrolase mutant was doa4 (24).
DISCUSSION
The degradation of the Ura3p and β-galactosidase fusion proteins bearing Ubc6p-Ubc7p-specific degradation signals is not affected by the hrd1/der3 null mutation, which abolishes the transport and degradation of misfolded ER proteins (6, 13) (Fig. 1A and C). In addition, the degradation of the β-galactosidase fusion protein is not affected by the sec61-2 mutation at the nonpermissive temperature, which inactivates translocon function (Fig. 1B). In contrast, the cue1 null mutation stabilizes all the fusion proteins tested (Fig. 1A). We therefore conclude that the fusion proteins studied in this investigation reach the ER-anchored Ubc6p-Ubc7p-Cue1p complex directly from the cytosol. It was found previously (25) that the proteolysis of the Matα2 Deg1–β-galactosidase fusion protein is similarly unaffected by sec61-2 and sec63-1 mutations, indicating that its degradation does not involve prior transport to the ER. It may be significant that the cytosolic face of the ER is continuous with the inner face of the nuclear envelope, which could facilitate intranuclear degradation of Matα2. Thus, it seems that the Ubc6p-Ubc7p-Cue1p pathway is involved in the degradation of cytosolic proteins carrying the appropriate signal as well as that of ER proteins. It is possible that the Ubc6p-Ubc7p-Cue1p system is involved in a regulatory or scavenging function which disposes of cytosolic proteins displaying signals which are cryptic in native proteins or cryptic under specific physiological conditions, as in the case of Matα2p (21). As Hrd1p is homologous to components of known E3 ubiquitin ligases with an H2-RING finger motif, it has been suggested that it might be a component of an E3 complex which targets a subset of ER substrates for degradation (33). The failure of the hrd1 mutation to inhibit the degradation of the fusion proteins studied in this work could suggest two alternatives. One possibility is that targeting of these substrates for degradation requires an as yet unknown E3 cooperating with Ubc6p and Ubc7p. Another even more intriguing possibility is that Ubc6p and Ubc7p are sufficient by themselves for the ubiquitination and degradation of these substrates, although there are no precedents for such a mechanism in vivo.
Analysis of a 16-amino-acid-residue signal (CL1) which targets proteins to Ubc6p plus Ubc7p-Cue1p shows that replacing bulky hydrophobic residues with A or other small residues destroys the effectiveness of the signal (Fig. 3). This is consistent with our previous conclusion that large hydrophobic residues constitute an important element of the Ubc6p-Ubc7p pathway signal (11) (Table 1). Other laboratories have found that signals channeling degradation to the Ubc6p-Ubc7p pathway have the characteristics of predicted amphipathic α-helices. One such signal is the Deg1 sequence of the Matα2 transcriptional regulator (21). Another is a synthetic signal generated by fusing random polypeptides to the N terminus of β-galactosidase (29). The 16-residue sequence studied here conforms to an almost perfect predicted amphipathic α-helix with a hydrophobic face made up of six bulky hydrophobic residues (Fig. 2). Our results are consistent with the hypothesis that eliminating a single large hydrophobic residue from this face is sufficient to greatly reduce the strength of the signal. Mutagenesis studies on the Matα2 Deg1 signal and the synthetic signal are also consistent with this view (21, 29). In our study, mutagenesis of all three serine residues of the 16-residue signal to alanine did not reduce the instability of the fusion protein, suggesting that the hydrophilic character of the putative helix is less crucial than its hydrophobicity.
A striking finding of this study is the importance of positively charged residues (H or K) interspersed with the hydrophobic residues. It seems significant that all the Ubc6p-Ubc7p signals studied have positively charged residues embedded in or flanking a highly hydrophobic region (Table 1). The K at position 3 of the 16-residue signal is not essential for instability, although its substitution with residues other than R slows the degradation of the fusion protein (Fig. 4). The lack of effect of a K-to-R substitution at position 3 shows that K3 of the signal is not the ubiquitination site. In contrast, histidine residues at positions 11 and 15 are an essential component of the signal (Fig. 5). Only another positively charged residue (R) can substitute for histidine and preserve the degradation involving Ubc6p and Ubc7p. Substitution with acidic residues abolishes degradation. Previous analyses by Bonifacino and his associates (4, 23) of ER lumen protein sequences promoting proteolysis showed that the degradation signal consists of a C-terminal cluster of large hydrophobic residues with charged residues embedded in it. In contrast to the signal analyzed in the present investigation, negatively charged residues as well as positively charged residues could confer instability (4, 23). At the time of these earlier studies, it was not realized that degradation of ER proteins involved retrograde transport to the cytosol. The question arises whether proteins reaching the Ubc6p-Ubc7p-Cue1p complex via the Sec-61p translocon use the same signals as those reaching it from the cytoplasm. A difference seems to be that a positive charge seems to be mandatory for the latter. It is possible that this is required for interaction with the cytosolic face of the ER membrane, which is negatively charged (1).
An unexpected result of the present study (Fig. 3 and 5) is that certain mutations of positively charged residues to A (H11A and H15A) as well as mutations of C-terminal bulky hydrophobic residues (F, V, I, and L) to A do not abolish the degradation of the fusion protein but channel it into an unidentified pathway other than that involving Ubc6p and Ubc7p. We have thus generated new degradation signals for an unidentified pathway(s). Both types of novel signal channel degradation of the fusion protein to the proteasome (Fig. 7). The possible involvement of Ubcs and other ubiquitin system components in the degradation of the novel fusion proteins is being investigated.
ACKNOWLEDGMENTS
This work was supported by a grant from the Israel Science Foundation.
We thank the following persons for generous gifts of yeast strains and plasmids: Daniel Finley, Mark Hochstrasser, Stefan Jentsch, Randy Scheckman, Thomas Sommer, and Dieter Wolf.
REFERENCES
- 1.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular biology of the cell. 2nd ed. New York, N.Y: Garland Publishing; 1989. [Google Scholar]
- 2.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 3.Biederer T, Volkwein C, Sommer T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 1997;278:1806–1809. doi: 10.1126/science.278.5344.1806. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino J S, Cosson P, Shah N, Klausner R D. Role of potentially charged transmembrane residues in targetting proteins for retention and degradation within the endoplasmic reticulum. EMBO J. 1991;10:2783–2793. doi: 10.1002/j.1460-2075.1991.tb07827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifacino J S, Weissman A M. Ubiquitin and the control of protein fate in the secretory and endocytic pathway. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordallo J, Plemper R K, Finger A, Wolf D H. Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded luminal and integral membrane proteins. Mol Biol Cell. 1998;9:209–222. doi: 10.1091/mbc.9.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Johnson P, Sommer T, Jentsch S, Hochstrasser M. Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell. 1993;74:357–369. doi: 10.1016/0092-8674(93)90426-q. [DOI] [PubMed] [Google Scholar]
- 8.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshaies R J, Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987;105:633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation and other stresses. Cell. 1987;48:1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- 11.Gilon T, Chomsky O, Kulka R G. Degradation signals for ubiquitin system proteolysis in Saccharomyces cerevisiae. EMBO J. 1998;17:2759–2766. doi: 10.1093/emboj/17.10.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarente L. Yeast promoters and LacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 13.Hampton R Y, Gardner R G, Rine J. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum protein. Mol Biol Cell. 1996;7:2029–2044. doi: 10.1091/mbc.7.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinemeyer W, Gruhler A, Mohrle V, Mahe Y, Wolf D H. PRE2, highly homologous to the human major histocompatibility complex RING10 gene, codes for a yeast proteasome subunit necessary for chymotryptic activity and degradation of ubiquitinated proteins. J Biol Chem. 1993;268:5115–5120. [PubMed] [Google Scholar]
- 15.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 16.Hicke L. Gettin' down with ubiquitin: turning off cell surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 17.Hill K B, Cooper A A. Degradation of unassembled Vph1p reveals novel aspects of the yeast ER quality control system. EMBO J. 2000;19:550–561. doi: 10.1093/emboj/19.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiller M M, Finger A, Schweiger M, Wolf D H. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- 19.Hilt W, Wolf D H. Proteasomes: destruction as a programme. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 20.Johnson E S, Ma P C, Ota I M, Varshavsky A. A proteolytic pathway that recognizes ubiquitin as a degradation signal. J Biol Chem. 1995;270:17442–17456. doi: 10.1074/jbc.270.29.17442. [DOI] [PubMed] [Google Scholar]
- 21.Johnson P R, Swanson R, Rakhilina L, Hochstrasser M. Degradation signal masking by heterodimerization of Matα2 and Mata1 blocks their mutual destruction by the ubiquitin-proteasome pathway. Cell. 1998;94:217–227. doi: 10.1016/s0092-8674(00)81421-x. [DOI] [PubMed] [Google Scholar]
- 22.Laney J D, Hochstrasser M. Substrate targetting in the ubiquitin system. Cell. 1999;97:427–430. doi: 10.1016/s0092-8674(00)80752-7. [DOI] [PubMed] [Google Scholar]
- 23.Lankford S P, Cosson P, Bonifacino J S, Klausner R D. Transmembrane domain length affects charge-mediated retention and degradation of proteins within the endoplasmic reticulum. J Biol Chem. 1993;268:4814–4820. [PubMed] [Google Scholar]
- 24.Papa F R, Hochstrasser M. The yeast DOA4 gene encodes a deubiquitinating enzyme related to a product of the human tre-2 oncogene. Nature. 1993;366:313–319. doi: 10.1038/366313a0. [DOI] [PubMed] [Google Scholar]
- 25.Plemper R K, Bomler S, Bordallo J, Sommer T, Wolf D H. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 26.Plemper R K, Wolf D H. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24:266–270. doi: 10.1016/s0968-0004(99)01420-6. [DOI] [PubMed] [Google Scholar]
- 27.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 28.Rubin D M, Glickman M H, Larsen C N, Dhruvakumar S, Finley D. Active site mutants in the six regulatory particle ATPases reveal multiple roles for ATP in the proteasome. EMBO J. 1998;17:4909–4919. doi: 10.1093/emboj/17.17.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadis S, Atienza C, Jr, Finley D. Synthetic signals for ubiquitin-dependent proteolysis. Mol Cell Biol. 1995;15:4086–4094. doi: 10.1128/mcb.15.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman F, Fink G R, Hicks J. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1981. [Google Scholar]
- 31.Sommer T, Wolf D H. Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J. 1997;11:1227–1233. doi: 10.1096/fasebj.11.14.9409541. [DOI] [PubMed] [Google Scholar]
- 32.Varshavsky A. The N-end rule: functions, mysteries, uses. Proc Natl Acad Sci USA. 1996;93:12142–12149. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilhovsky S, Gardner R, Hampton R. HRD gene dependence of endoplasmic reticulum-associated degradation. Mol Biol Cell. 2000;11:1697–1708. doi: 10.1091/mbc.11.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]