Abstract
Purpose:
Functional magnetic resonance imaging (fMRI) has promise for understanding neural mechanisms of neurogenic speech and voice disorders. However, performing vocal tasks within the fMRI environment may not always be analogous to performance outside of the scanner. Using a mock MRI scanner, this study examines the effects of a simulated scanning environment on vowel intensity in individuals with Parkinson’s disease (PD) and hypophonia and older healthy control (OHC) participants.
Method:
Thirty participants (15 PD, 15 OHC) performed a sustained /ɑ/ vowel production task in three conditions: 1) Upright, 2) Mock Scanner + No Noise, and 3) Mock Scanner + MRI noise. We used a linear mixed-effects (multi-level) model to evaluate the contributions of group and recording environment to vowel intensity. A second linear mixed-effects model was also used to evaluate the contributions of PD medication state (On vs. Off) to voice intensity.
Results:
Vowel intensity was significantly lower for PD compared to the OHC group. The intensity of vowels produced in the Upright condition was significantly lower compared to the Mock Scanner + No Noise condition, while vowel intensity in the Mock Scanner + MRI Noise condition was significantly higher compared to the Mock Scanner + No Noise condition. A group by condition interaction also indicated that the addition of scanner noise had a greater impact on the PD group. A second analysis conducted within the PD group showed no effects of medication state on vowel intensity.
Conclusion:
Our findings demonstrate that performance on voice production tasks is altered for PD and OHC groups when translated into the fMRI environment, even in the absence of acoustic scanner noise. For fMRI studies of voice in PD hypophonia, careful thought should be given to how the presence of acoustic noise may differentially affect PD and OHC, for both group and task comparisons.
Keywords: Parkinson’s disease, Hypophonia, Voice, fMRI, Lombard effect, Body position, Voice intensity
1. INTRODUCTION
Functional magnetic resonance imaging (fMRI) can be a powerful tool for examining brain activity during voice production. However, performing a task during fMRI may not always be analogous to performance outside of the scanner. Differences between the fMRI and out-of-scanner testing environments typically include a change in body position (laying supine vs. sitting upright) and the presence of loud background noise. While these differences may have little consequence to tasks such as visual discrimination, performance during voice production tasks may be critically impacted by this change in environment. Furthermore, the effects of scanning environment on voice production may differentially impact those with communication disorders compared to healthy individuals. As fMRI studies of voice production are important for understanding the neural mechanisms of neurogenic speech disorders, it is important to not only consider the effects of scanning environment on healthy individuals, but also the effect that scanning environment may have on the clinical populations under investigation.
In this study, we focus on the effects of the scanning environment on voice intensity among individuals with hypophonia resulting from Parkinson’s disease (PD) and older healthy adults. Individuals with hypophonia secondary to PD (herein referred to as PD hypophonia) speak with a lower voice intensity than healthy adults of the same age and may present with other dysarthric speech characteristics (Duffy, 2013). The neural mechanisms of speech and voice changes in PD are not well characterized and fMRI provides a promising approach for understanding the neural underpinnings of PD hypophonia (Arnold et al., 2014; Maillet et al., 2012; Narayana et al., 2020; Pinto et al., 2011; Rektorova et al., 2007). However, in order to accurately interpret the results of fMRI studies using voice production tasks in this population, it is important to consider whether or not the effects of scanning environment differ for individuals with PD hypophonia compared to older healthy controls. Further, as fMRI studies of voice in PD may also seek to examine participants both on and off of their antiparkinsonian medication, it is worth considering whether any effects of scanning environment on this population might be dependent on medication status.
1.1. Acoustic noise during fMRI scanning
The acoustic noise generated during fMRI is a major limiting factor when conducting overt speech and voice production studies in the scanner. The sound exposure during echo-planar imaging (EPI; the standard approach for fMRI studies) has been estimated to range from 122–138 dB sound pressure level (SPL) inside the head coil (Foster et al., 2000; Ravicz et al., 2000). This is attenuated by the use of hearing protection during fMRI scanning. Combined, the use of earplugs and earmuffs can reduce the sound exposure by 39–41 dB SPL (Ravicz & Melcher, 2001). Thus, sound exposure during echo planar imaging should range between 81–99 dB SPL when this combined hearing protection is used. During a typical task fMRI scan, the data is collected in a continuous manner, with the scanner actively collecting data and producing acoustic noise while the participant simultaneously performs the task of interest. Other neuroimaging techniques, such as sparsely-sampled fMRI (Perrachione & Ghosh, 2013) or positron emission tomography (PET), may be used as alternative approaches that do not involve concurrent scanner noise during task performance. Using the sparsely sampled fMRI approach, the task is executed by the participant during an acoustically silent period (when the gradient is off) and followed immediately after by the collection of fMRI data (Gracco et al., 2005; Hall et al., 1999). The slow nature of the blood-oxygen level dependent (BOLD) response measured by fMRI allows researchers to capture the peak of the BOLD response 4–5 seconds after the task begins, thereby giving the participant a short window to perform the task without the interference of loud background noise. However, the sparse sampling approach can also come at the cost of longer scan times or reduced statistical power (Nebel et al., 2005). PET scans, by contrast, do not generate loud background acoustic noise, and have been used in a number of studies examining speech and voice production in PD (Liotti et al., 2003; Narayana et al., 2010; Narayana et al., 2020; Pinto et al., 2004). However, the use of PET scans is not always feasible. There are a limited number of research and clinical institutions with access to PET scanners, and such studies involve additional risks associated with radiation exposure.
When continuous fMRI scanning protocols are used, it is almost certain that the acoustic background noise will impact the participant’s voice intensity. It is well established that speaking in the presence of background noise prompts healthy individuals to systematically increase voice intensity (Lane & Tranel, 1971; Lombard, 1911; Zollinger & Brumm, 2011a, 2011b). This phenomenon, known as the “Lombard effect”, has been demonstrated in the presence of several different noise types and intensities (Egan, 1971; Garnier et al., 2010). Studies have shown that individuals with PD hypophonia also experience the Lombard effect, elevating their vocal intensity when speaking under conditions of 50–70 dB background noise (Adams, Dykstra, et al., 2006; Adams et al., 2005; Adams, Moon, et al., 2006; Dykstra et al., 2012), as well as conditions of 90 dB background noise (Adams & Lang, 1992). Interestingly, although individuals with PD hypophonia speak at a lower voice intensity compared to controls, the slope at which voice intensity increases as a function of background noise intensity appears to be comparable between the two groups at noise levels of 50–70 dB (Adams, Dykstra, et al., 2006; Adams et al., 2005; Adams,. Moon, et al., 2006; Dykstra et al., 2012). During a continuous fMRI experiment one could expect that individuals with hypophonia vocalize with lower mean and maximum intensity levels compared to controls, but that the magnitude of the Lombard response would be similar between the groups. However, testing this will be important for the interpretation of findings across imaging modalities in which scanner noise is or is not present during voice production.
1.2. Supine posture
In addition to scanner noise, participant position is another important consideration in fMRI speech task designs. In fMRI experiments, participants are performing speech and voice tasks while laying supine, which differs from the upright posture typically used in natural speech and voice production. The effect of supine body position on voice intensity has not been systematically studied in healthy adults or in individuals with PD hypophonia. However, the mechanics of speech breathing (Hoit, 1995) are known to differ between the upright and supine positions, which may affect the respiratory strategies used to achieve a desired vocal intensity. Unlike upright voice production, the gravitational force exerted on the body in the supine position works to move both the rib cage wall and abdominal wall in the expiratory direction (Duffy, 2013; Hixon et al., 2018). The resultant increase in relaxation pressure during supine vowel production means that less muscular effort is required to produce an utterance during expiration (Hixon et al., 2018). Individuals with PD may exhibit rigidity and weakness of the rib cage musculature (Hovestadt et al., 1989; Sabate et al., 1996; Solomon & Hixon, 1993), increased reliance on abdominal muscles for expiration (Huber et al., 2003; Solomon & Hixon, 1993), as well as patterns of lung volume initiations and terminations which differ from those seen in older healthy adults (Huber and Darling 2011, Bunton, 2005). Additional abdominal effort may be needed to overcome restrictions due to rigidity and weakness of the rib cage in PD. When translated into the supine position, individuals with PD may benefit from the gravitational pull on the breathing apparatus when supine. Collectively, the literature on supine respiration and respiratory changes in PD highlight the need to understand positional influences on voice intensity when performing voice tasks during fMRI.
1.3. PD medication
Another factor for consideration is whether PD medication state has any effect on speech performance in the scanning environment. In the fMRI literature, studies examining the speech of individuals with PD can be found both in the on medication and off medication states (Maillet et al., 2012; Pinto et al., 2011; Rektorova et al., 2007). Testing individuals with PD on their typical antiparkinsonian medication can help to reduce tremor and head movement artifacts during fMRI; however, some research questions may require testing after medication withdrawal or a comparison of on versus off medication states. In general, dopaminergic therapy does not appear to have a robust or consistent effect on voice intensity (Fabbri et al., 2017; Ho et al., 2008; Jiang et al., 1999; Kompoliti et al., 2000; Skodda et al., 2010), thus it seems unlikely to have a meaningful impact on voice intensity during fMRI. Still, there are a few reasons to test this empirically. First, medication can improve some aspects of respiratory function in PD, including vital capacity (De Letter et al., 2007; De Letter et al., 2010; Monteiro et al., 2012) and peak expiratory flow (de Bruin et al., 1993; Monteiro et al., 2012). If speech breathing in PD becomes more effortful in the supine position, it is possible that medication related improvements in respiratory function could enable target voice intensities to be more easily reached when the individual is lying down. Second, the tasks previously used to evaluate the effects of dopaminergic medication on voice intensity include only a small number of trials (Fabbri et al., 2017; Ho et al., 2008; Jiang et al., 1999; Kompoliti et al., 2000). By contrast, fMRI typically requires a minimum of 25 trials in order to accurately estimate the hemodynamic response of a given brain region (Huettel & McCarthy, 2001). The greater number of trials required for fMRI studies makes it more likely that the participants with PD will experience vocal fatigue. As there is some evidence to suggest that levodopa can reduce motor fatigue in individuals with PD (Lou et al., 2003), it is worth examining the effects of PD medication on voice intensity using a task more typical of an fMRI experiment. By doing so, we can instill greater confidence that fMRI experiments of speech and voice production in PD may be accurately interpreted across and between medication states.
1.4. Study objective
The objective of this study is to examine the effects of a simulated fMRI environment on the voice intensity of individuals with PD hypophonia and older healthy controls (OHC). To accomplish this, we utilize a mock MRI scanner and ask participants to perform a sustained vowel production task in three conditions: 1) seated upright outside of the mock scanner (“Upright”), 2) laying supine inside the mock scanner with no MRI sounds (“Mock Scanner + No Noise”), and 3) laying supine inside the mock scanner with MRI sounds played over headphones (“Mock Scanner + MRI Noise”). The Upright condition is used to represent a typical recording setup for acoustic speech analysis. The Mock Scanner + No Noise condition is used to approximate a scanning environment, in which the participant is supine and wearing headphones, but no MRI sounds are present. Finally, the Mock Scanner + MRI Noise condition is used to approximate the environment of a continuously sampled fMRI experiment. We then evaluate the data using two linear mixed effects models – with the first model examining group effects and the second examining medication effects in the PD group. We predict that PD will have a negative effect on vowel intensity. With respect to recording environment, we predict that the Mock Scanner + MRI Noise condition will result in higher voice intensity, in line with prior studies of Lombard effect in older healthy adults and those with PD hypophonia (Adams, Dykstra, et al., 2006; Adams et al., 2005; Adams & Lang, 1992; Adams, Moon, et al., 2006; Dykstra et al., 2012). Within the PD group, we further examine the effects of medication state (on medication vs. 12-hour withdrawal) on vowel SPL across each of the three recording conditions. As there is not strong evidence to suggest that PD medication leads to meaningful improvements in voice intensity (Daniels et al., 1996; Fabbri et al., 2017; Kompoliti et al., 2000; Skodda et al., 2010), we predict that PD participants will produce vowels at a comparable voice intensity when on versus off medication.
2. METHODS
2.1. Subjects
We recruited 15 participants who presented with PD hypophonia (10 male, 5 female) and 15 older healthy controls (OHC; 10 male, 5 female). The mean age of the PD group was 63.13 years and the mean age of the OHC group was 61.47 years. All participants were right-handed, native English speakers between 40–80 years old with a score of either ≥26 on the Montreal Cognitive Assessment (MoCA) or ≥18 on the MoCA-Blind (if screened over the phone). Additional cognitive testing using the Mattis Dementia Rating Scale (DRS-2) (Matteau et al., 2011; Matteau et al., 2012) showed comparable performance between OHC (M = 141.40, SD = 1.76) and PD groups (M = 140.47, SD = 2.03). Hearing threshold was calculated for each participant using a bilateral pure tone average threshold of 0.5, 1, and 2 kHz (Oscilla SM910-B). Pure-tone hearing thresholds for all participants were ≤ 35 dB HL and did not differ significantly between OHC (M = 17.33 dB HL, SD = 7.55) and PD (M = 21.39 dB HL, SD = 5.22) groups when tested using an independent samples t-test, t(25) = −1.71, p = 0.100.
PD participants with hypophonia were either referred by a movement disorders neurologist at Northwestern Memorial Hospital, recruited from a laboratory participant registry, or recruited through PD community events. PD participants were only referred from the Movement Disorders Clinic if the neurologist judged them to have hypophonia based on clinical experience. All participants with PD were judged to have hypophonia by one of two trained movement disorders researchers, each of which had 3+ years of experience conducting PD research and was certified to administer the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). The researchers’ judgements of hypophonia were made based on experience, paying specific attention to speech intensity. The presence of hypophonia was later confirmed by three experienced raters based on perceptual ratings of collected speech samples completed in the ‘on’ medication state (testing Day 1). Procedures used to obtain perceptual ratings are described briefly in section 2.1.1, with additional details provided as Supplementary Material.
2.1.1. Parkinson’s disease characteristics
All participants in the PD group were being treated with antiparkinsonian medication. To characterize the severity of disease, participants in the PD group were administered the MDS-UPDRS, with Part III motor testing completed after 12-hour medication withdrawal. The MDS-UPDRS was administered and video recorded by a certified rater and any ratings that were unclear were reviewed by a second trained rater until a consensus was reached. Parkinson’s disease characteristics, including MDS-UPDRS ratings, levodopa equivalent daily dose (Tomlinson et al., 2010), Hoehn and Yahr stage (Goetz et al., 2004; Hoehn & Yahr, 1967), and PD subtype (Stebbins et al., 2013) are reported in Table I. In order to describe the voice and communication symptoms of the PD group, we obtained perceptual measures of voice quality (Kempster et al., 2009), as well as measures of sentence intelligibility (Yorkston et al., 1996) and communicative participation (Baylor et al., 2013). Perceptual ratings were performed by three raters with experience evaluating speech of individuals with PD (2 speech-language pathologists and 1 research assistant). The raters included one of the study authors as well as two independent raters – all of whom were blinded to participant ID and were not involved in data collection, analysis of primary study variables, or recruitment of participants. Table II reports voice quality and speech intelligibility characteristics of the PD group while in their ‘on’ medication state, as well as communicative participation scores. A detailed description of the perceptual rating and transcription procedures can be found in Supplementary Materials.
Table I.
Parkinson’s disease characteristics
| MDS-UPDRS1 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Participant | Part I | Part II | Part III | Part IV | Total | LEDD | H&Y | PD Subtype |
|
| ||||||||
| PD03 | 19 | 16 | 35 | 1 | 71 | 600 | 2 | TD |
| PD04 | 9 | 7 | 48 | 0 | 64 | 1140 | 2 | PIGD |
| PD05 | 10 | 22 | 43 | 1 | 76 | 1020 | 3 | TD |
| PD06 | 4 | 10 | 41 | 5 | 60 | 920 | 2 | Intermediate |
| PD07 | 14 | 17 | 36 | 5 | 72 | 1563 | 2 | PIGD |
| PD08 | 7 | 11 | 41 | 0 | 59 | 600 | 2 | TD |
| PD09 | 4 | 3 | 6 | 0 | 13 | 300 | 2 | PIGD |
| PD11 | 19 | 17 | 26 | 1 | 63 | 1260 | 2 | PIGD |
| PD12 | 4 | 1 | 22 | 5 | 32 | 120 | 1 | TD |
| PD13 | 13 | 7 | 42 | 4 | 66 | 627 | 2 | TD |
| PD14 | 10 | 18 | 45 | 7 | 80 | 900 | 2 | TD |
| PD15 | 5 | 18 | 23 | 0 | 46 | 400 | 2 | PIGD |
| PD16 | 12 | 14 | 31 | 0 | 57 | 450 | 2 | PIGD |
| PD17 | 5 | 11 | 41 | 1 | 58 | 400 | 2 | PIGD |
| PD18 | 1 | 11 | 44 | 0 | 56 | 650 | 2 | Intermediate |
H&Y, Hoehn and Yahr Stage
LEDD, Levodopa Equivalent Daily Dose
MDS-UPDRS, Movement Disorders Society Parkinson’s Disease Rating Scale
PIGD, Postural Instability and Gait Disturbances
TD, Tremor Dominant
Performed after 12-hr medication withdrawal
Table II.
Descriptive measures of voice quality, speech intelligibility, and communication participation of the PD group
| CAPE-V |
||||||||
|---|---|---|---|---|---|---|---|---|
| Participant | Roughness | Breathiness | Strain | Pitch | Loudness | Intelligibility (Scaled) | Intelligibility (% correct words) | CPIB T (logit) |
|
| ||||||||
| PD03 | 55 | 25 | 22.50 | 12.50 | 15 | 95.00 | 100 | 52.7 (0.27) |
| PD04 | 37.50 | 10 | 40 | 15 | 15 | 95.00 | 96.90 | 52.7 (0.27) |
| PD05 | 17.50 | 22.50 | 20 | 22.50 | 15 | 96.00 | 99.03 | 45.5 (−0.45) |
| PD06 | 49.50 | 17.50 | 32.50 | 11.50 | 27.50 | 96.00 | 96.30 | 59.2 (0.92) |
| PD07 | 70 | 20 | 40 | 17.50 | 15 | 97.50 | 98.44 | 51.5 (0.15) |
| PD08 | 55 | 45 | 34 | 35 | 42.50 | 91.50 | 88.81 | 52.7 (0.27) |
| PD09 | 20 | 27.50 | 20 | 10 | 30 | 100 | 98.68 | 71 (2.1) |
| PD11 | 47.50 | 22.50 | 25 | 17.50 | 25 | 97.50 | 99.09 | 52.7 (0.27) |
| PD12 | 50 | 70 | 40 | 40 | 50 | 100 | 98.68 | 71 (2.1) |
| PD13 | 37.50 | 20 | 30 | 30 | 30 | 100 | 98.18 | 62.2 (1.22) |
| PD14 | 20 | 15 | 18.50 | 34 | 36.50 | 99.00 | 97.20 | 71 (2.1) |
| PD15 | 50 | 52.50 | 40 | 20 | 30 | 92.50 | 89.63 | 49 (−0.1) |
| PD16 | 32.50 | 10 | 30 | 15 | 10 | 100 | 93.87 | 52.7 (0.27) |
| PD17 | 31 | 21 | 25 | 10 | 15 | 100 | 97.27 | 62.2 (1.22) |
| PD18 | - | - | - | - | - | - | - | 51.5 (0.15) |
Note. Audio recordings from a standardized reading passage (either the Rainbow Passage (Fairbanks, 1960) or Grandfather Passage (Darley et al., 1975)) and a maximum sustained phonation task were used to generate perceptual ratings. Scores were averaged across the three experienced raters (2 speech-language pathologists and one research assistant) to derive a mean rating for each voice quality dimension. All intraclass correlation coefficients were >0.81 (Range 0.81 to 0.96). All ratings made in medication ‘on’ state. Roughness, breathiness, strain, pitch, loudness perceptual ratings are based on sustained phonation for the lax vowel /ɑ/ and a standardized reading passage using the 100-millimeter visual analog scale (VAS) from the Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V; (Kempster et al., 2009)). Intelligibility (perceptual) ratings are based on a standardized reading passage using the VAS from the CAPE-V. Intelligibility (% correct words) scores are based on 11 sentences from the Sentence Intelligibility Test (Yorkston et al., 1996). PD18 had missing audio data in medication ‘on’ state. Scores on the Communicative Participant Item Bank (CPIB) are provided as T and logit scores for interpretability across studies, where higher scores on the CPIB are more favorable and indicate greater communicative participation.
2.2. Data Collection
2.2.1. fMRI simulation
To create a simulated fMRI environment, we utilized a mock MRI scanner with the bore and internal dimensions identical to those of a Siemens 3T TIM-TRIO MRI scanner. Visual and auditory stimuli for the sustained vowel task were presented using E-Prime software (https://pstnet.com/products/e-prime/). The mock head coil was affixed with an angled mirror so that task instructions and stimuli could be viewed on a display monitor placed outside of the mock scanner. Pre-recorded MRI sounds were delivered via over-the-ear headphones. The volume of the MRI sounds was held constant across sessions and subjects at 90 dB(A) and specifically simulated the EPI noise generated during an fMRI experiment. The intensity of 90 dB was chosen to approximate the mid-range of noise exposure experienced during fMRI when both earplugs and earmuffs are used (Foster et al., 2000; Ravicz & Melcher, 2001; Ravicz et al., 2000). The 90 dB output volume of the headphones was confirmed using a sound level meter (SLM; Extech 407736).
2.2.2. Sustained vowel production task
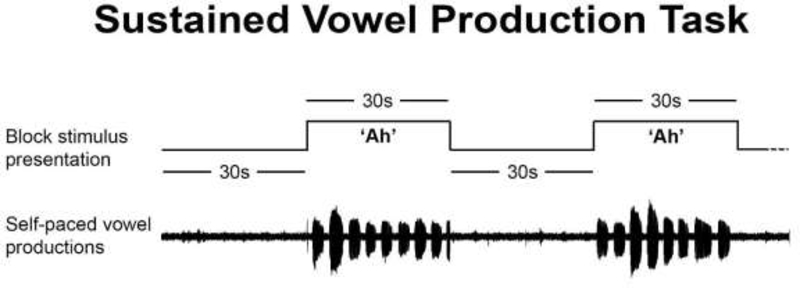
We collected voice recordings while participants performed a sustained vowel production task. This quasi speech task was chosen as it is one that requires minimal movement of the lips and jaw, thereby reducing the likelihood of excessive head movement in the scanner. The task consisted of alternating vowel production blocks (30s each) and rest blocks (30s each). During the task, participants were presented with either a “+” symbol (rest) or “Ah” (vowel production). During the “Ah” blocks, subjects were instructed to produce an /ɑ/ vowel for approximately 3–5 seconds at their normal conversational loudness and repeat for the duration of the block. This self-paced paradigm was designed so that participants would rely primarily on internal, rather than external cueing mechanisms for vowel initiation and production (Darling & Huber, 2011; Sadagopan & Huber, 2007; Tjaden et al., 2013; Tjaden & Wilding, 2004). Participants practiced the sustained vowel production task for 1–2 blocks before beginning the experiment to ensure that they understood the self-paced paradigm and did not try to hold the vowel for the full 30 second duration of the stimulus. We did not provide a model of the vowel productions for participants but gave them verbal feedback as needed until the task was performed correctly. Participants were not provided with any feedback regarding vocal intensity. Figure 1 illustrates the task design.
Figure 1.
Illustration of two alternating rest blocks and sustained vowel production blocks. Above: block stimulus presentation consisting of 30s rest blocks, alternated with 30s sustained vowel blocks (‘Ah’) in which participants were instructed to produce 3–5 second self-paced /ɑ/ vowels. Below: example head microphone recording of self-paced /ɑ/ vowels produced during the 30s sustained vowel blocks. Example recording taken from a healthy control participant.
2.2.3. Procedure
All testing was performed at Northwestern University’s Center for Translational Imaging. The study was approved by the Institutional Review Board at Northwestern University and informed consent was obtained in accordance with Northwestern University’s guidelines. Participants performed the sustained vowel production task in the three different recording environments: 1) Upright, 2) Mock Scanner + No Noise, and 3) Mock Scanner + MRI Noise. Recording during the Upright condition took place after the practice task, but before the mock scanner conditions. This was done in an effort to alleviate the discomfort of PD participants repeatedly going in and out of the mock scanner. Once inside the mock scanner, the order of the conditions (Mock Scanner + No Noise vs. Mock Scanner + MRI Noise) was counterbalanced. Participants completed 5 task blocks in the Upright condition (where one task block includes one 30s rest block and one 30s vowel production block; 5 minutes), 10 task blocks in the Mock Scanner + No Noise condition (10 minutes), and 10 task blocks in the Mock Scanner + MRI Noise condition (10 minutes). The 5–10 minute duration of each condition is typical of what one might see in a typical fMRI task run (Coutanche & Thompson-Schill, 2012).
Speech and voice samples were recorded using a head mounted, unidirectional microphone (Shure SM10A) positioned 3 cm from the lower lip. The distance of 3 cm was chosen in order to accommodate the head coil in the mock MRI scanner while maintaining a consistent recording setup when the participant was seated upright. The microphone was channeled through a pre-amplifier (ART Project Series USB Dual Pre) and then relayed onto a laptop computer for recording in Audacity. Speech samples were recorded at a sampling frequency of 44.1kHz.
To achieve accurate SPL measurements, calibration recordings were taken before each condition’s task run using a SLM (A-weighted; Extech 407736) positioned 30 cm from the lower lip and the head mounted microphone positioned 3 cm from the lower lip (Svec and Granqvist 2018). A 94 dB 1kHz pure tone sound level calibrator (Extech 407744) was applied to the SLM and the SLM reading was read out loud and recorded. Participants were then instructed to produce an /ɑ/ phonation and hold it at a constant sound level for several seconds, while head mic and SLM signal were recorded on two different channels. Once a stable sound level was achieved, the value on the SLM was read out loud and recorded. To calibrate the sound levels in the software, we first adjusted the SLM recording to match the known level of the 94 dB calibration sound. Second, we used the sustained /ɑ/ recording as a reference signal to equalize the sound levels of the head mic and the SLM. As the internal bore of the mock MRI scanner was too small to take SLM measurements at 30 cm, calibration recordings for the mock scanner conditions were taken outside of the bore while the participant was positioned supine on the table with the head microphone at its fixed 3 cm distance and SLM positioned at 30 cm. The table was then immediately rolled into the bore of the mock MRI scanner and the task was started.
Testing for PD participants took place on two consecutive days. On Day 1, testing was conducted in the afternoon. Participants took antiparkinsonian medication on their typical schedules, and data collection appointments were scheduled to capture the participant’s “on” medication state relative to their individualized medication schedules (typically within 1 hour of their most recent dose). On Day 2, testing was conducted the next morning following 12-hour medication withdrawal. Voice recordings for the three conditions were collected on both days to capture performance both on and off medication.
2.3. Data analysis
2.3.1. Voice intensity
We extracted voice SPL from the vowel production recordings collected in the three recording environments. Any vowel production that lasted less than 0.5 seconds was excluded from analysis. Voice intensity measures were extracted using Praat (Boersma, 2017). Intensity traces were extracted from each block and then automatically segmented into individual vowel segments. Segmentations were visually inspected to confirm accurate onset/offset boundaries for each vowel segment. After visual inspection, we extracted raw voice intensity values for each full vowel segment. The raw voice intensity values were then adjusted using the calibration procedures described above and averaged within each block.
2.3.2. Statistical analysis of voice SPL
Because each participant was sampled multiple times per condition (5 blocks in the Upright condition, and 10 blocks in both the Mock Scanner + No Noise and Mock Scanner + MRI Noise conditions), we fit a series of mixed effects (multi-level) models using the lmer command in the R package lme4 (version 1.1–21, Bates et al., 2015). One advantage of using a mixed effects model approach was that it allowed for occasional missing data and for the unbalanced number of blocks in the different test conditions (Cnaan et al., 1997; Spilke et al., 2005). In these mixed effects models, random effects characterized the degree of difference across individual intercepts and slopes. The fixed effects estimates were drawn from the average of these individual intercepts and slopes. Estimated p values were obtained via Kenward-Roger’s degrees of freedom method using the lmerTest package (version 3.1–0, Kuznetsova et al., 2015).
We first modeled the effect of group (OHC vs. PD off-medication) on vowel intensity, with fixed effects estimates of condition, group, and block and their interactions. Unlike ANOVAs, which can quantify main effects and all pair-wise contrasts for factors with more than two levels, mixed effects models output only the pairwise comparisons specified by the chosen contrast codes (Clopper, 2013). We coded factors using effects (simple) coding, thus making the fixed effects estimates roughly analogous to an ANOVA main effect. Group was coded as −0.5 for OHC and +0.5 for PD participants. For condition, Mock Scanner + No Noise was set as the reference level, enabling us to test for significant differences between the Upright and Mock Scanner + No Noise conditions; and between the Mock Scanner + No Noise and Mock Scanner + MRI Noise conditions. Finally, block was treated as numeric variable to account for possible vocal decay across blocks, with the first block of each condition coded as zero. This model included random intercepts and random linear slopes for each subject, to account for individual differences in average vowel intensity and individual differences in the effect of block on vowel intensity (vocal decay). Random effects were estimated with an unstructured covariance matrix and restricted maximum likelihood estimation, using the Nelder-Mead optimization function.
We then modeled the effect of medication status on vowel intensity for PD participants, with fixed effects of condition, medication status, and block and their interactions. This model used the same coding scheme as the previous model: effects coding was used for factor variables (with Mock Scanner + No Noise chosen as the reference condition), and block was treated as a numeric variable where the first block was coded as zero. Random slopes for subjects were not included in this model because block accounted for minimal variability in data and including random slopes would have caused a singular model fit (overfitting). Random effects were again estimated with an unstructured covariance matrix and restricted maximum likelihood estimation, using the Nelder-Mead optimization function.
3. RESULTS
3.1. Group effects model (OHC vs PD off-medication)
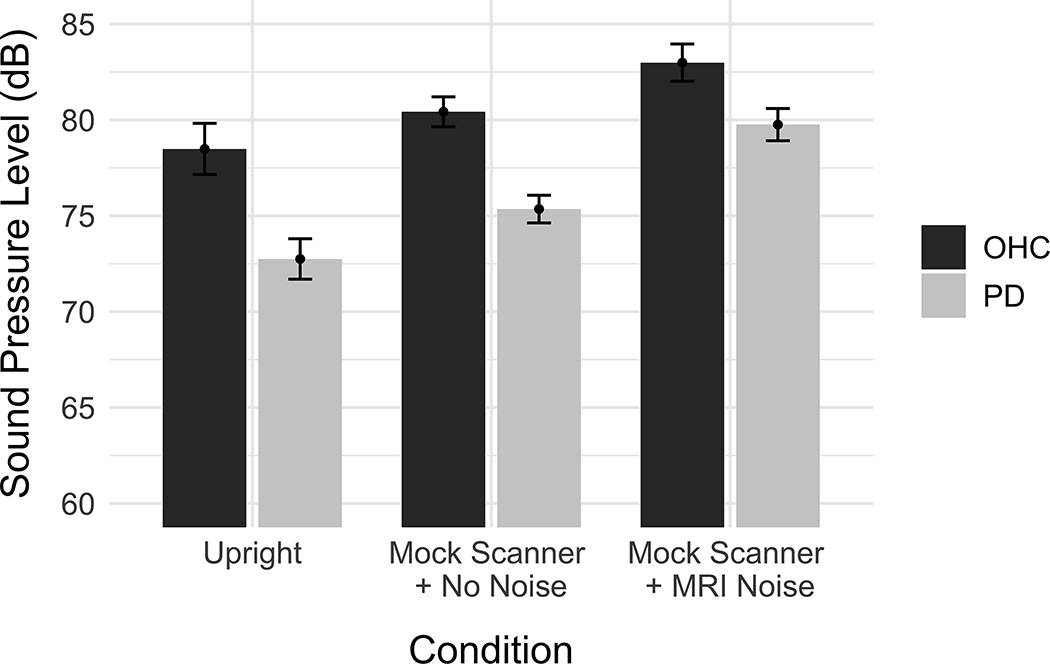
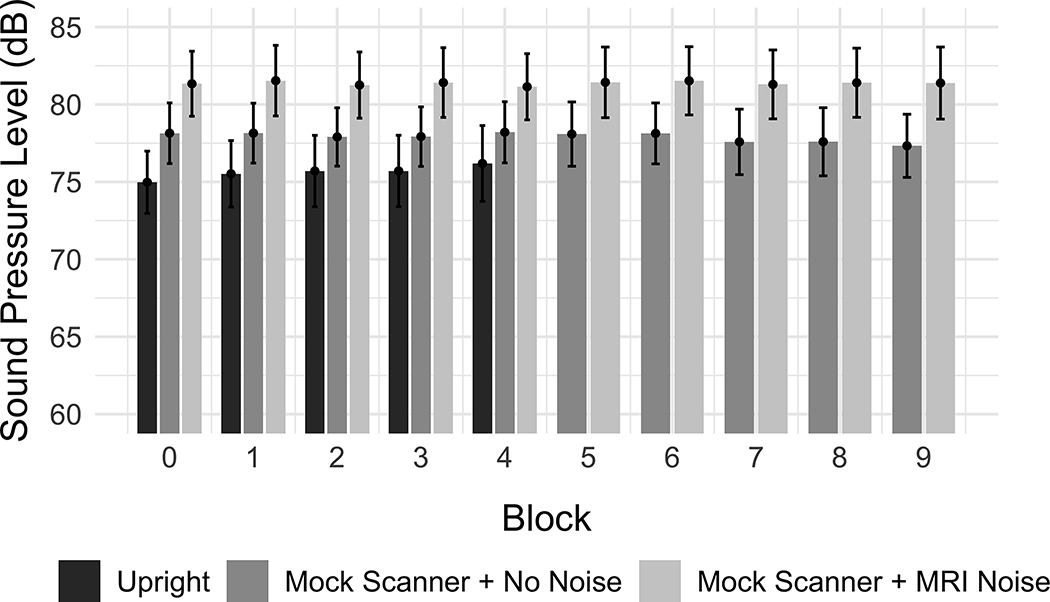
The output of the first linear mixed effect model is reported in Table III. Figure 2 depicts the mean SPL for OHC and PD groups across the three recording conditions. There was a significant effect of PD on voice SPL (β = −4.153, SE = 1.609, p = 0.015). Participants in the PD group produced vowels with a lower voice SPL compared to those in the OHC group. Using Mock Scanner + No Noise as the condition reference level, there was a significant effect of the Upright condition (β = −3.140, SE = 0.430, p < 0.001) as well as a significant effect of the Mock Scanner + MRI Noise condition (β = 3.127, SE = 0.368, p < 0.001). Vowels produced in the Upright recording condition had a lower voice SPL compared to the Mock Scanner + No Noise condition, while vowels produced in the Mock Scanner + MRI Noise condition had a higher SPL compared to the Mock Scanner + No Noise condition. There was also a significant interaction between the Mock Scanner + MRI Noise and the PD group (β =1.477, SE = 0.735, p = 0.045). The difference between PD and OHC voice SPL was smaller in the Mock Scanner + MRI Noise condition than in the Mock Scanner + No Noise condition, whereas the difference between OHC and PD participants was relatively consistent between the Mock Scanner + No Noise and Upright conditions. An interaction between the Upright condition and block was also observed (β = 0.330, SE = 0.148, p = 0.026). Figure 3 depicts the mean SPL for the recording conditions across each block, showing that voice SPL increased across the 5 blocks of the Upright condition, but stayed relatively stable across the Mock Scanner + No Noise and Mock Scanner + MRI Noise conditions.
Table III.
Output of Linear Mixed Effects Model for Group Effects on Voice SPL
| SPL | |||||
|---|---|---|---|---|---|
| Predictors | Estimates | SE | CI | df | p |
|
| |||||
| Intercept | 78.23 | 0.80 | 76.58 – 79.88 | 28.40 | <0.001 |
| Upright | −3.14 | 0.43 | −3.98 – −2.30 | 680.00 | <0.001 |
| Mock Scanner + MRI Noise | 3.13 | 0.37 | 2.41 – 3.85 | 680.00 | <0.001 |
| PD | −4.15 | 1.61 | −7.45 – −0.86 | 28.40 | 0.015 |
| Block | 0.07 | 0.06 | −0.06 – 0.19 | 85.11 | 0.305 |
| Upright * PD | −0.70 | 0.86 | −2.39 – 0.99 | 680.00 | 0.415 |
| Mock Scanner + MRI Noise * PD | 1.48 | 0.74 | 0.03 – 2.92 | 680.00 | 0.045 |
| Upright * Block | 0.33 | 0.15 | 0.04 – 0.62 | 680.01 | 0.026 |
| Mock Scanner + MRI Noise * Block | 0.07 | 0.07 | −0.07 – 0.21 | 680.04 | 0.309 |
| PD * Block | −0.17 | 0.13 | −0.42 – 0.08 | 85.11 | 0.182 |
| (Upright * PD) * Block | −0.18 | 0.30 | −0.77 – 0.40 | 680.01 | 0.535 |
| (Mock Scanner + MRI Noise * PD) * Block | 0.06 | 0.14 | −0.21 – 0.33 | 680.04 | 0.650 |
| Random Effects | |||||
| σ2 | 5.87 | ||||
| τ00 Sub_ID | 18.57 | ||||
| τ11 Sub ID.Block | 0.04 | ||||
| ρ01 Sub_ID | 0.41 | ||||
| ICC | 0.79 | ||||
| N Sub_ID | 30 | ||||
|
| |||||
| Observations | 748 | ||||
| Marginal R2 / Conditional R2 | 0.267 / 0.847 | ||||
Figure 2.
Effect of group and recording condition on voice SPL. The vertical axis indicates the mean sound pressure level (dB). Error bars represent the 95% confidence intervals.
Figure 3.
Effect of condition and block on voice SPL, collapsed across PD and OHC groups. The vertical axis indicates the mean sound pressure level (dB). Error bars represent the 95% confidence intervals.
3.2. Medication effects model (PD on- vs off-medication)
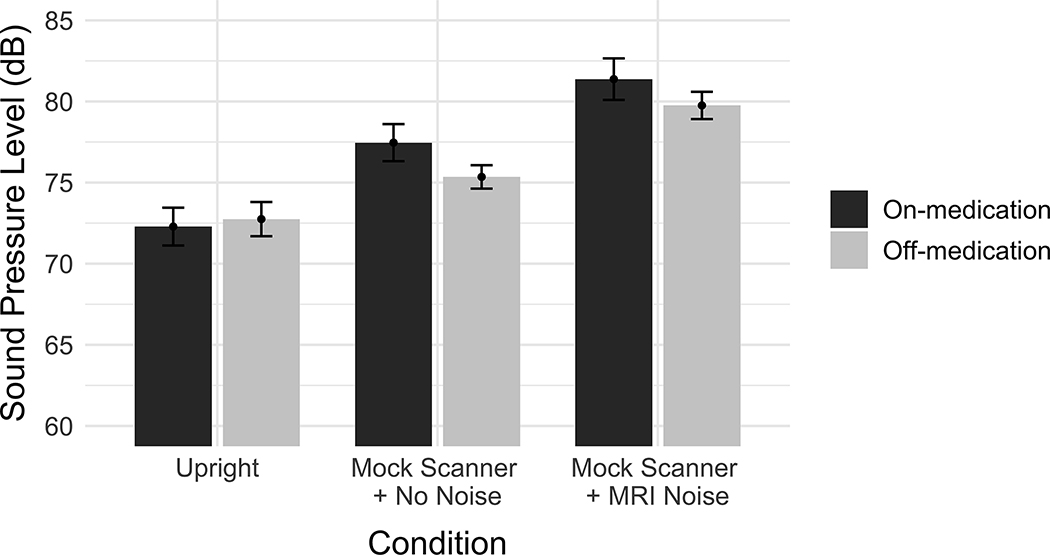
The output of the second linear mixed effects model is reported in Table IV. Figure 4 depicts the mean vowel SPL across conditions and medication state. Using Mock Scanner + No Noise as the condition reference level, there was once again significant effect of the Upright condition (β = −4.175, SE = 0.529, p < 0.001) as well as a significant effect of the Mock Scanner + MRI Noise condition (β = 4.230, SE = 0.453, p < 0.001) on voice SPL. No significant effect of medication state was observed, nor were any interactions between medication state and any of the other fixed factors.
Table IV.
Output of Linear Mixed Effects Model for Medication Effects on Voice SPL
| SPL | |||||
|---|---|---|---|---|---|
| Predictors | Estimates | SE | CI | df | p |
|
| |||||
| Intercept | 76.15 | 1.27 | 73.43 – 78.86 | 14.55 | <0.001 |
| Upright | −4.18 | 0.53 | −5.22 – −3.14 | 647.00 | <0.001 |
| Mock Scanner + MRI Noise | 4.23 | 0.45 | 3.34 – 5.12 | 647.00 | <0.001 |
| Off-med | 0.03 | 0.42 | −0.80 – 0.85 | 647.51 | 0.950 |
| Block | 0.03 | 0.06 | −0.09 – 0.16 | 647.00 | 0.606 |
| Upright * Off-med | 1.35 | 1.06 | −0.73 – 3.43 | 647.00 | 0.203 |
| Mock Scanner + MRI Noise * Off-med | −0.75 | 0.91 | −2.53 – 1.02 | 647.00 | 0.405 |
| Upright * Block | 0.12 | 0.18 | −0.23 – 0.48 | 647.00 | 0.495 |
| Mock Scanner + MRI Noise * Block | −0.02 | 0.08 | −0.19 – 0.14 | 647.00 | 0.769 |
| Off-med * Block | −0.11 | 0.13 | −0.36 – 0.14 | 647.00 | 0.402 |
| (Upright * Off-med) * Block | 0.23 | 0.36 | −0.48 – 0.95 | 647.00 | 0.525 |
| (Mock Scanner + MRI Noise * Off-med) * Block | 0.26 | 0.17 | −0.08 – 0.59 | 647.00 | 0.129 |
| Random Effects | |||||
| σ2 | 7.90 | ||||
| τ00 Sub_ID | 23.60 | ||||
| ICC | 0.75 | ||||
| N Sub_ID | 15 | ||||
|
| |||||
| Observations | 673 | ||||
| Marginal R2 / Conditional R2 | 0.229 / 0.807 | ||||
Figure 4.
Effect of recording condition and medication state on voice SPL. The vertical axis indicates the mean sound pressure level (dB). Error bars represent the 95% confidence intervals.
4. DISCUSSION
There were six main observations in this study. First, our initial mixed effects model indicated a significant effect of group, in which the sustained vowel intensity produced by the PD group was significantly lower than that of the OHC group. Second, both models indicated a significant effect of recording condition, with pairwise contrasts demonstrating that sustained vowel intensity was lower in the Upright condition compared to the Mock Scanner + No Noise condition. Third, pairwise contrasts from both models also indicated that vowel intensity was higher in the Mock Scanner + MRI Noise condition compared to the Mock Scanner + No Noise condition. Fourth, our mixed effects model indicated a significant interaction between PD and the Mock Scanner + MRI Noise condition, showing that the difference in voice intensity between PD and OHC was smaller when scanner noise was present in the mock scanner. Fifth, we observed a significant interaction between the Upright condition and experimental block. Sixth, we did not observe any significant effects of medication state on vowel intensity when PD participants were tested on- versus off-medication.
4.1. Lower voice intensity in PD
With respect to our first finding, we observed lower voice intensity in the PD group, as expected. Prior research has reported that individuals with PD hypophonia present with an approximate 2–5 dB reduction in voice SPL across different production tasks (Dykstra et al., 2012; Dykstra et al., 2015; Fox, 1997). Our β-coefficient estimate indicated that the effect of PD reduced vocal intensity by approximately 4.1 dB SPL during sustained vowel production. This is in line with the findings reported by Fox and Ramig (1997), who demonstrated that individuals with PD hypophonia had an approximate 4 dB reduction in voice SPL during sustained vowel production compared to healthy controls.
4.2. Lower voice intensity in the Upright recording condition
Our second finding demonstrated that sustained vowel intensity was lower in the Upright condition relative to the Mock Scanner + Nos Noise condition. The observed increase in SPL may be attributable in part to assistance of gravitational forces acting in the expiratory direction on the breathing apparatus (Hixon et al., 2018). By reducing the demand on active muscular forces for expiration, participants may have been able to achieve greater vocal intensity in the Mock Scanner + No Noise condition using the same effort required in the Upright condition. Another possible contribution to this is the dampening of auditory feedback caused by the over-the-ear headphones. Although the headphones used in this study were not designed for noise attenuation, the passive occlusion of auditory feedback was likely sufficient to prompt some increase in vocal intensity, even when MRI noise was not being administered. It is important to note that the Mock Scanner + No Noise condition did not include brief periods of scanner noise between trials, as would be the case in a true sparse-sampled fMRI experiment. While the current study design provided us with a simplified framework for examining the effects of supine posture and background noise, it is possible that exposure to 90 dB scanner noise between trials could lead to additional adaptation effects not seen in the present study. More sophisticated simulations could be used in the future to examine whether the presentation of fMRI noise between trials impacts behavioral performance.
4.3. Higher voice intensity when presented with fMRI background noise
Regarding our third finding, this study is the first to explicitly examine how the Lombard effect impacts voice intensity when translated into the fMRI environment. As expected, the addition of 90 dB scanner noise led to increased voice intensity during sustained vowel production (Adams, Dykstra, et al., 2006; Adams et al., 2005; Adams, Moon, et al., 2006; Dykstra et al., 2012; Sadagopan & Huber, 2007). The effects observed during our fMRI simulation suggest that performing vowel production tasks during continuous fMRI scanning will cause individuals to vocalize at a significantly greater intensity compared to performance during PET or sparsely sampled fMRI scans. The effects of background noise during continuous scanning could have implications for the interpretation of fMRI results, as one may observe neural processes related to this adaptation in addition to any effects of interest. For example, a small scaled fMRI study by Meekings et al., (2016) showed changes in the activity of the superior temporal gyrus (STG) related to speaking under different noise masking conditions. If discrepancies in patterns of STG activation are found between continuous fMRI paradigms and PET or sparsely sampled fMRI paradigms, it will be worth considering whether the differences are related to the engagement of the Lombard effect. It is also important to note that, because the Lombard effect is an involuntary response, it is likely that the Lombard effect will result in changes in brain activity that differ from activity observed during high intensity speech (Baumann et al., 2018) or following intensity-driven voice therapy for PD (Baumann et al., 2018; Narayana et al., 2010). By eliminating concurrent scanner noise during experimental trials, the use of PET or sparsely sampled fMRI paradigms may help alleviate these effects. However, as mentioned above, these should not be taken as analogous to typical upright voice experiments. It is also worth noting that additional levels of background noise over 90 dB raise the potential of masking the ability to hear one’s own voice. Given that fMRI noise exposure can range from 81–99 dB with hearing protection (Foster et al., 2000; Ravicz & Melcher, 2001; Ravicz et al., 2000), future research could investigate the degree to which EPI noise is able to mask the perception of one’s voice at various sound levels within the 80–100 dB range.
4.4. Interaction between PD group and MRI noise
With respect to our fourth finding, the significant interaction between PD and the Mock Scanner + MRI Noise condition reduced the differences in voice intensity between PD and OHC groups. This was unexpected given the number of studies which have documented the slope of Lombard responses to be roughly parallel in PD compared to OHC (Adams, Dykstra, et al., 2006; Adams et al., 2005; Adams, Moon, et al., 2006; Dykstra et al., 2012), as well as research showing that both PD and OHC groups utilize more efficient respiratory strategies when speaking in noise (Sadagopan and Huber 2007). One possible reason for this discrepancy is the intensity of the background noise used. Studies reporting parallel Lombard responses in PD and OHC had only examined background noise at the levels of 50–70 dB (Adams, Dykstra, et al., 2006; Adams et al., 2005; S. Adams, Moon, et al., 2006; Dykstra et al., 2012). The present study specifically examined the effects of background noise presented at 90 dB in order to more accurately represent the sound profile of the fMRI environment (Foster et al., 2000). Although the Lombard effect has been previously described in PD in the presence of 90 dB white noise (Adams & Lang, 1992), this analysis was performed within group and was not directly compared to healthy controls. It is therefore possible that the parallel Lombard responses seen between 50–70 dB begin to deviate once background noise levels reach 90 dB. Another possibility is that we reached a ceiling effect with the OHC group. While there are fewer studies reporting Lombard responses at 90 dB or above, decreases in the slope of the Lombard response have been reported once participants approach the peak of their performance range (Hanley & Steer, 1949; Lane & Tranel, 1971; Pickett, 1958). It is possible that the OHC group began to approach this peak when presented with 90 dB scanner noise, while PD participants were able to continue increasing voice SPL at the same rate. A further comparison of OHC and PD Lombard response functions at higher noise levels will be useful in determining a) whether these functions begin to deviate under conditions of very loud noise and b) whether noise levels of 90 dB are able to induce a ceiling effect in OHC participants.
Despite the known respiratory deficits in PD (Hovestadt et al., 1989; Huber & Darling-White, 2017; Huber et al., 2003; Sabate et al., 1996; Solomon & Hixon, 1993), the absence of a significant PD * Upright interaction in our first mixed effects model shows that body position did not have a differential impact on SPL production between OHC and PD groups in the present study. However, it should be cautioned that the PD participants in this study were largely in the early-mid stages of the disease and may have experienced less rigidity and weakness within the respiratory apparatus. In a longitudinal study of speech breathing, Huber and Darling-White (2017) found that individuals with PD had comparable lung volume initiations and terminations to controls at baseline, but later deviated as the disease progressed. It is therefore possible that interactions between group and body position would be found when examining individuals with PD at later stages of the disease. Alternatively, individuals with PD may have been able to successfully adapt to the change in body position by employing additional respiratory or laryngeal strategies (Stathopoulos et al., 2014) or by benefitting from gravitational forces exerted in the expiratory direction on the breathing apparatus (Duffy, 2013; Hixon et al., 2018). Furthermore, this study employed vowel productions that were relatively short (3–5 s). Research using dynamic MRI of the lungs in professional singers found rib cage lowering only after 50% of maximum phonation time (i.e., lower lung volumes; Traser et al., 2017). Due to the shorter length of phonations used in the present study, participants may not have exerted sufficient lung volume to require more active engagement of the rib cage and abdomen. Further research is needed to extend these findings to individuals who are in the later stages of PD, to directly examine respiratory as well as laryngeal strategies for supine voice production, and to investigate the effects of supine position across a variety of speech and voice tasks.
4.5. Voice intensity across task blocks
Our fifth finding showed that voice SPL increased slightly as a function of experimental block in the Upright recording condition. However, this was not the case for either the Mock Scanner + No Noise or the Mock Scanner + MRI Noise recording conditions. The estimates for this effect were quite small (β = 0.330) and could be explained in part due to the order in which the conditions were tested as well as the lower number of blocks in the Upright condition, both of which are limitations of the study design. Notably, we did not detect any significant decay in vocal intensity across our experiment blocks for either the OHC or PD groups.
4.6. No effects of PD medication
Regarding our sixth finding, we did not find an effect of PD medication on vowel intensity in the PD group. This is consistent with prior studies showing that voice intensity is unresponsive to dopaminergic stimulation (Daniels et al., 1996; Fabbri et al., 2017; Kompoliti et al., 2000; Skodda et al., 2010). There were also no interactions between medication status and recording condition or block. Thus, the effects of the fMRI recording environment on vowel intensity appear to be comparable regardless of medication status. It should be noted that the results of our medication analysis come with two important limitations. First, the Day 1 (on medication) speech and vowel production measures were collected during the afternoon, while Day 2 (off medication) measures were collected in the morning after overnight medication withdrawal. Thus, there is a possibility of diurnal differences between the two testing sessions, including afternoon decline in levodopa response during Day 1 testing (Nutt & Holford, 1996). Second, we did not evaluate MDS-UPDRS Part III scores during both on and off medication states, so we could not confirm whether motor function did in fact decrease after medication withdrawal. While the data in the present study are in line with our hypothesized outcomes, the results of the medication analysis should still be interpreted with caution until replicated using peak morning levodopa responses and with the comparison of motor function in the on and off medication states.
4.7. Limitations and future research
This study provides an intriguing look into changes in voice production within an analogous fMRI environment. Still, it is important to note the degree to which these findings may or may not generalize to other studies. First, the group differences in this study were only examined using measures collected after 12-hour medication withdrawal in the PD group. Although our second mixed-effects model indicated no significant effect of medication state on vowel intensity, caution should be taken when generalizing these findings to PD participants who are in the ‘on’ medication state. Second, this study specifically focused on measures of voice intensity during sustained vowel production. It is possible that other acoustic measures might respond differently to changes in background noise or body position. Furthermore, adding respiratory outcome measures could provide more detailed information on the respiratory mechanics underlying voice SPL changes in the fMRI environment. Finally, the study focused exclusively on vowel production and did not examine the effects of fMRI on voice intensity during connected speech tasks (e.g., reading or conversational tasks). Although the use of connected speech tasks comes with the risk of additional head motion artefact, some studies have been able to successfully utilize connected speech tasks during continuous fMRI by applying advanced motion correction techniques during image pre-processing (e.g., Liu et al., 2012; Xu et al., 2014). Recently, it has also been shown that the use of continuous speech tasks with minimal movement artefact is feasible during continuous fMRI, when participants are instructed to maintain a closed jaw and limit the movement of the lips (Narayana et al., 2020). Of course, speaking with a closed jaw position and limited lip movement is not representative of articulatory patterns outside of the scanner. Exploration of the effects of scanning environment on more ecologically valid, continuous speech tasks will be critical moving forward. As studies observing the Lombard effect have been conducted using overt word production (Junqua, 1993; Van Summers et al., 1988), sentence reading (Arciuli et al., 2014; Castellanos et al., 1996; Darling & Huber, 2011), passage reading (Sadagopan & Huber, 2007; Vogel et al., 2014), and conversational interactions (Garnier et al., 2010; Patel & Schell, 2008; Stathopoulos et al., 2014; Vogel et al., 2014) it is likely that the presence of MRI noise would lead to increased voice intensity during these tasks as well. Investigating the influence of fMRI scanning environment across different acoustic measures, respiratory measures, and tasks would be a useful extension of the present research.
In sum, the present study suggests that the effects of scanning environment should be taken into consideration when conducting voice production tasks during fMRI experiments, particularly when investigating clinical populations such as PD. Collecting voice recordings in a simulated fMRI environment could provide a more ecologically valid estimate of behavior during fMRI experiments. Given our observed interactions between scanner noise and PD, quantifying a participant’s Lombard response at fMRI noise levels (~90 dB) may be useful when comparing OHC and PD groups using continuous scanning protocols. Techniques for reducing noise exposure during neuroimaging experiments could help to mitigate the effects of concurrent scanner noise on vocal intensity. Still, our findings suggest that elevated voice intensity may also be present while laying supine in the absence of scanner noise. Taken together, our results stress the importance of understanding how behavior during out-of-scanner tasks translates to behavior within the fMRI scanning environment, and whether the fMRI environment itself may lead to different neuro-behavioral responses in different populations of interest.
Supplementary Material
Highlights.
PD and OHC groups produce louder vowels lying supine compared to sitting upright
Background fMRI noise leads to greater voice intensity compared to no fMRI noise
Effects of fMRI noise on voice intensity differentially affect PD and OHC groups
ACKNOWLEDGMENTS
We would like to extend a special thanks to Alyssa Penn and Michelle Murashev for assisting with clinical perceptual ratings of our PD group. Funding for this project was provided by the National Institute on Deafness and Other Communication Disorders (F31 DC015717 and T32 DC013017).
ABBREVIATIONS
- fMRI
functional magnetic resonance imaging
- PD
Parkinson’s disease
- BOLD
blood oxygen level dependent
- SPL
sound pressure level
- OHC
older healthy controls
- MoCA
Montreal Cognitive Assessment
- DRS-2
Mattis Dementia Rating Scale 2
- CPIB
Communicative Participation Item Bank
- MDS-UPDRS
Movement Disorders Society Unified Parkinson’s Disease Rating Scale
- SLM
sound level meter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams MR, & Hutchinson J (1974). The effects of three levels of auditory masking on selected vocal characteristics and the frequency of disfluency of adult stutterers. J Speech Hear Res, 17(4), 682–688. 10.1044/jshr.1704.682 [DOI] [PubMed] [Google Scholar]
- Adams MR, & Moore WH Jr. (1972). The effects of auditory masking on the anxiety level, frequency of dysfluency, and selected vocal characteristics of stutterers. J Speech Hear Res, 15(3), 572–578. 10.1044/jshr.1503.572 [DOI] [PubMed] [Google Scholar]
- Adams S, Dykstra A, Abrams K, Winnell J, Jenkins M, & Jog M (2006). Conversational speech intensity under different noise conditions in hypophonia and Parkinson’s disease. Canadian Acoustics, 34(3), 96–97. [Google Scholar]
- Adams S, Haralabous O, Dykstra A, Abrams K, & Jog M (2005). Effects of multi-talker background noise on the intensity of spoken sentences in Parkinson’s disease. Canadian Acoustics, 33(3), 94–95. [Google Scholar]
- Adams S, Moon B-H, Dykstra A, Abrams K, Jenkins M, & Jog M (2006). Effects of multitalker noise on conversational speech intensity in Parkinson’s disease. Journal of Medical Speech-Language Pathology, 14(4), 221–229. [Google Scholar]
- Adams SG, Dykstra A, Abrams K, Winnell J, Jenkins M, & Jog M (2006). Conversational speech intensity under different noise conditions in hypophonia and Parkinson’s disease. Canadian Acoustics, 34(3), 96–97. [Google Scholar]
- Adams SG, Haralabous O, Dykstra A, Abrams K, & Jog M (2005). Effects of multi-talker background noise on the intensity of spoken sentences in Parkinson’s disease. Canadian Acoustics, 33(3), 94–95. [Google Scholar]
- Adams SG, & Lang AE (1992). Can the Lombard Effect Be Used to Improve Low Voice Intensity in Parkinsons-Disease. European Journal of Disorders of Communication, 27(2), 121–127. [DOI] [PubMed] [Google Scholar]
- Adams SG, Moon B-H, Dykstra A, Abrams K, Jenkins M, & Jog M (2006). Effects of multitalker noise on conversational speech intensity in Parkinson’s disease. Journal of Medical Speech-Language Pathology, 14(4), 221–229. [Google Scholar]
- Arciuli J, Simpson BS, Vogel AP, & Ballard KJ (2014). Acoustic changes in the production of lexical stress during Lombard speech. Lang Speech, 57(Pt 2), 149–162. 10.1177/0023830913495652 [DOI] [PubMed] [Google Scholar]
- Arnold C, Gehrig J, Gispert S, Seifried C, & Kell CA (2014). Pathomechanisms and compensatory efforts related to Parkinsonian speech. Neuroimage Clin, 4, 82–97. 10.1016/j.nicl.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Baumann A, Nebel A, Granert O, Giehl K, Wolff S, Schmidt W, Baasch C, Schmidt G, Witt K, Deuschl G, Hartwigsen G, Zeuner KE, & van Eimeren T (2018). Neural Correlates of Hypokinetic Dysarthria and Mechanisms of Effective Voice Treatment in Parkinson Disease. Neurorehabil Neural Repair, 32(12), 1055–1066. 10.1177/1545968318812726 [DOI] [PubMed] [Google Scholar]
- Baylor C, Yorkston K, Eadie T, Kim J, Chung H, & Amtmann D (2013). The Communicative Participation Item Bank (CPIB): item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res, 56(4), 1190–1208. 10.1044/1092-4388(2012/12-0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, Weenink D (2017). Praat: doing phonetics by computer [Computer Program]. Retrieved May 24 2019 from http://www.praat.org
- Bunton K (2005). Patterns of lung volume use during an extemporaneous speech task in persons with Parkinson disease. Journal of communication disorders, 38(5), 331–348. [DOI] [PubMed] [Google Scholar]
- Castellanos A, Benedí J-M, & Casacuberta F (1996). An analysis of general acoustic-phonetic features for Spanish speech produced with the Lombard effect. Speech Communication, 20(1–2), 23–35. [Google Scholar]
- Clopper CG (2013). Modeling multi-level factors using linear mixed effects
- Cnaan A, Laird NM, & Slasor P (1997). Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in Medicine, 16, 2349–2380. [DOI] [PubMed] [Google Scholar]
- Coutanche MN, & Thompson-Schill SL (2012). The advantage of brief fMRI acquisition runs for multi-voxel pattern detection across runs. Neuroimage, 61(4), 1113–1119. 10.1016/j.neuroimage.2012.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels N, Oates J, Phyland D, Feiglin A, & Hughes A (1996). Vocal characteristics and response to levodopa in Parkinson’s disease. Movement Disorders, 11(Suppl. 1), 117. [Google Scholar]
- Darley FL, Aronson AE, & Brown JR (1975). Motor speech disorders. WB Saunders Company. [Google Scholar]
- Darling M, & Huber JE (2011). Changes to articulatory kinematics in response to loudness cues in individuals with Parkinson’s disease. J Speech Lang Hear Res, 54(5), 1247–1259. 10.1044/1092-4388(2011/10-0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin PF, de Bruin VM, Lees AJ, & Pride NB (1993). Effects of treatment on airway dynamics and respiratory muscle strength in Parkinson’s disease. Am Rev Respir Dis, 148(6 Pt 1), 1576–1580. 10.1164/ajrccm/148.6_Pt_1.1576 [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Van Maele G, Van Borsel J, & Boon P (2007). The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson’s disease. Clin Neurol Neurosurg, 109(6), 495–500. 10.1016/j.clineuro.2007.04.003 [DOI] [PubMed] [Google Scholar]
- De Letter M, Van Borsel J, Boon P, De Bodt M, Dhooge I, & Santens P (2010). Sequential changes in motor speech across a levodopa cycle in advanced Parkinson’s disease. Int J Speech Lang Pathol, 12(5), 405–413. 10.3109/17549507.2010.491556 [DOI] [PubMed] [Google Scholar]
- Duffy JR (2013). Motor speech disorders: Substrates, differential diagnosis, and management. Elsevier Health Sciences. [Google Scholar]
- Dykstra AD, Adams SG, & Jog M (2012). The effect of background noise on the speech intensity of individuals with hypophonia associated with Parkinson’s disease. Journal of Medical Speech-Language Pathology, 20(3), 19–31.26157329 [Google Scholar]
- Dykstra AD, Adams SG, & Jog M (2015). Examining the relationship between speech intensity and self-rated communicative effectiveness in individuals with Parkinson’s disease and hypophonia. J Commun Disord, 56, 103–112. 10.1016/j.jcomdis.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Egan JJ (1971). The Lombard reflex. Historical perspective. Arch Otolaryngol, 94(4), 310–312. 10.1001/archotol.1971.00770070502004 [DOI] [PubMed] [Google Scholar]
- Fabbri M, Guimaraes I, Cardoso R, Coelho M, Guedes LC, Rosa MM, Godinho C, Abreu D, Goncalves N, Antonini A, & Ferreira JJ (2017). Speech and Voice Response to a Levodopa Challenge in Late-Stage Parkinson’s Disease. Front Neurol, 8, 432. 10.3389/fneur.2017.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G (1960). The rainbow passage. Voice and articulation drillbook, 2. [Google Scholar]
- Foster JR, Hall DA, Summerfield AQ, Palmer AR, & Bowtell RW (2000). Sound-level measurements and calculations of safe noise dosage during EPI at 3 T. J Magn Reson Imaging, 12(1), 157–163. [DOI] [PubMed] [Google Scholar]
- Foster JR, Hall DA, Summerfield AQ, Palmer AR, & Bowtell RW (2000). Sound-level measurements and calculations of safe noise dosage during EPI at 3 T. Journal of Magnetic Resonance Imaging, 12(1), 157–163. [DOI] [PubMed] [Google Scholar]
- Fox CR, L. O. (1997). Vocal Sound Pressure Level and Self-Perception of Speech and Voice in Men and Women With Idiopathic Parkinson Disease. Am J Speech Lang Pathol, 6(2), 85–94. [Google Scholar]
- Garnier M, Henrich N, & Dubois D (2010). Influence of sound immersion and communicative interaction on the Lombard effect. J Speech Lang Hear Res, 53(3), 588–608. 10.1044/1092-4388(2009/08-0138) [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, & Movement Disorder Society Task Force on Rating Scales for Parkinson’s, D. (2004). Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord, 19(9), 1020–1028. 10.1002/mds.20213 [DOI] [PubMed] [Google Scholar]
- Gracco VL, Tremblay P, & Pike B (2005). Ismaging speech production using fMRI. Neuroimage, 26(1), 294–301. 10.1016/j.neuroimage.2005.01.033 [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, & Bowtell RW (1999). “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp, 7(3), 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley TD, & Steer MD (1949). Effect of level of distracting noise upon speaking rate, duration and intensity. J Speech Disord, 14(4), 363–368. 10.1044/jshd.1404.363 [DOI] [PubMed] [Google Scholar]
- Hixon TJ, Weismer G, & Hoit JD (2018). Preclinical speech science: Anatomy, physiology, acoustics, and perception. Plural Publishing. [Google Scholar]
- Ho AK, Bradshaw JL, & Iansek R (2008). For better or worse: The effect of levodopa on speech in Parkinson’s disease [Research Support, Non-U.S. Gov’t]. Mov Disord, 23(4), 574–580. 10.1002/mds.21899 [DOI] [PubMed] [Google Scholar]
- Hoehn MM, & Yahr MD (1967). Parkinsonism: onset, progression and mortality. Neurology, 17(5), 427–442. 10.1212/wnl.17.5.427 [DOI] [PubMed] [Google Scholar]
- Hoit JD (1995). Influence of body position on breathing and its implications for the evaluation and treatment of speech and voice disorders. J Voice, 9(4), 341–347. [DOI] [PubMed] [Google Scholar]
- Hovestadt A, Bogaard JM, Meerwaldt JD, van der Meche FG, & Stigt J (1989). Pulmonary function in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 52(3), 329–333. 10.1136/jnnp.52.3.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, & Darling M (2011). Effect of Parkinson’s disease on the production of structured and unstructured speaking tasks: Respiratory physiologic and linguistic considerations. Journal of Speech and Hearing Research, 54(1), 33–46. 10.1044/1092-4388(2010/09-0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, & Darling-White M (2017). Longitudinal Changes in Speech Breathing in Older Adults with and without Parkinson’s Disease. Semin Speech Lang, 38(3), 200–209. 10.1055/s-0037-1602839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Stathopoulos ET, Ramig LO, & Lancaster SL (2003). Respiratory function and variability in individuals with Parkinson disease: Pre-and post-Lee Silverman Voice Treatment. Journal of Medical Speech-Language Pathology, 11(4), 185–202. [Google Scholar]
- Huettel SA, & McCarthy G (2001). The effects of single-trial averaging upon the spatial extent of fMRI activation. Neuroreport, 12(11), 2411–2416. 10.1097/00001756-200108080-00025 [DOI] [PubMed] [Google Scholar]
- Jiang J, Lin E, Wang J, & Hanson DG (1999). Glottographic measures before and after levodopa treatment in Parkinson’s disease. Laryngoscope, 109(8), 1287–1294. 10.1097/00005537-199908000-00019 [DOI] [PubMed] [Google Scholar]
- Junqua JC (1993). The Lombard reflex and its role on human listeners and automatic speech recognizers. J Acoust Soc Am, 93(1), 510–524. 10.1121/1.405631 [DOI] [PubMed] [Google Scholar]
- Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, & Hillman RE (2009). Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech Lang Pathol, 18(2), 124–132. 10.1044/1058-0360(2008/08-0017) [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Wang QE, Goetz CG, Leurgans S, & Raman R (2000). Effects of central dopaminergic stimulation by apomorphine on speech in Parkinson’s disease. Neurology, 54(2), 458–462. 10.1212/wnl.54.2.458 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2015). Package ‘lmertest’. R package version, 2(0). [Google Scholar]
- Lane H, & Tranel B (1971). The Lombard sign and the role of hearing in speech. Journal of Speech and Hearing Research, 14(4), 677–709. [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, Ingham JC, & Fox PT (2003). Hypophonia in Parkinson’s disease: neural correlates of voice treatment revealed by PET. Neurology, 60(3), 432–440. [DOI] [PubMed] [Google Scholar]
- Liu S, Chow HM, Xu Y, Erkkinen MG, Swett KE, Eagle MW, Rizik-Baer DA, & Braun AR (2012). Neural correlates of lyrical improvisation: an fMRI study of freestyle rap. Scientific reports, 2(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard E (1911). Le signe de l’elevation de la voix. Ann. Mal. de L’Oreille et du Larynx, 101–119. [Google Scholar]
- Lou JS, Kearns G, Benice T, Oken B, Sexton G, & Nutt J (2003). Levodopa improves physical fatigue in Parkinson’s disease: a double-blind, placebo-controlled, crossover study. Mov Disord, 18(10), 1108–1114. 10.1002/mds.10505 [DOI] [PubMed] [Google Scholar]
- Maillet A, Krainik A, Debu B, Tropres I, Lagrange C, Thobois S, Pollak P, & Pinto S (2012). Levodopa effects on hand and speech movements in patients with Parkinson’s disease: a FMRI study [Research Support, Non-U.S. Gov’t]. PLoS One, 7(10), e46541. 10.1371/journal.pone.0046541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteau E, Dupre N, Langlois M, Jean L, Thivierge S, Provencher P, & Simard M (2011). Mattis Dementia Rating Scale 2: screening for MCI and dementia. Am J Alzheimers Dis Other Demen, 26(5), 389–398. 10.1177/1533317511412046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteau E, Dupre N, Langlois M, Provencher P, & Simard M (2012). Clinical validity of the Mattis Dementia Rating Scale-2 in Parkinson disease with MCI and dementia. J Geriatr Psychiatry Neurol, 25(2), 100–106. 10.1177/0891988712445086 [DOI] [PubMed] [Google Scholar]
- Meekings S, Evans S, Lavan N, Boebinger D, Krieger-Redwood K, Cooke M, & Scott SK (2016). Distinct neural systems recruited when speech production is modulated by different masking sounds. J Acoust Soc Am, 140(1), 8. 10.1121/1.4948587 [DOI] [PubMed] [Google Scholar]
- Monteiro L, Souza-Machado A, Valderramas S, & Melo A (2012). The effect of levodopa on pulmonary function in Parkinson’s disease: a systematic review and meta-analysis. Clin Ther, 34(5), 1049–1055. 10.1016/j.clinthera.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Narayana S, Fox PT, Zhang W, Franklin C, Robin DA, Vogel D, & Ramig LO (2010). Neural correlates of efficacy of voice therapy in Parkinson’s disease identified by performance-correlation analysis [Research Support, N.I.H., Extramural]. Hum Brain Mapp, 31(2), 222–236. 10.1002/hbm.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana S, Parsons MB, Zhang W, Franklin C, Schiller K, Choudhri AF, Fox PT, LeDoux MS, & Cannito M (2020). Mapping typical and hypokinetic dysarthric speech production network using a connected speech paradigm in functional MRI. Neuroimage Clin, 27, 102285. 10.1016/j.nicl.2020.102285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel K, Stude P, Wiese H, Muller B, de Greiff A, Forsting M, Diener HC, & Keidel M (2005). Sparse imaging and continuous event-related fMRI in the visual domain: a systematic comparison. Hum Brain Mapp, 24(2), 130–143. 10.1002/hbm.20075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, & Holford NH (1996). The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol, 39(5), 561–573. 10.1002/ana.410390504 [DOI] [PubMed] [Google Scholar]
- Patel R, & Schell KW (2008). The influence of linguistic content on the Lombard effect. J Speech Lang Hear Res, 51(1), 209–220. 10.1044/1092-4388(2008/016) [DOI] [PubMed] [Google Scholar]
- Perrachione TK, & Ghosh SS (2013). Optimized design and analysis of sparse-sampling FMRI experiments. Front Neurosci, 7, 55. 10.3389/fnins.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett J (1958). Limits of direct speech communication in noise. The Journal of the Acoustical Society of America, 30(4), 278–281. [Google Scholar]
- Pinto S, Mancini L, Jahanshahi M, Thornton JS, Tripoliti E, Yousry TA, & Limousin P (2011). Functional magnetic resonance imaging exploration of combined hand and speech movements in Parkinson’s disease. Mov Disord, 26(12), 2212–2219. 10.1002/mds.23799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Thobois S, Costes N, Le Bars D, Benabid AL, Broussolle E, Pollak P, & Gentil M (2004). Subthalamic nucleus stimulation and dysarthria in Parkinson’s disease: a PET study [Research Support, Non-U.S. Gov’t]. Brain, 127(Pt 3), 602–615. 10.1093/brain/awh074 [DOI] [PubMed] [Google Scholar]
- Ravicz ME, & Melcher JR (2001). Isolating the auditory system from acoustic noise during functional magnetic resonance imaging: examination of noise conduction through the ear canal, head, and body. J Acoust Soc Am, 109(1), 216–231. 10.1121/1.1326083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz ME, Melcher JR, & Kiang NY (2000). Acoustic noise during functional magnetic resonance imaging. J Acoust Soc Am, 108(4), 1683–1696. 10.1121/1.1310190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rektorova I, Barrett J, Mikl M, Rektor I, & Paus T (2007). Functional abnormalities in the primary orofacial sensorimotor cortex during speech in Parkinson’s disease [Research Support, Non-U.S. Gov’t]. Mov Disord, 22(14), 2043–2051. 10.1002/mds.21548 [DOI] [PubMed] [Google Scholar]
- Sabate M, Rodriguez M, Mendez E, Enriquez E, & Gonzalez I (1996). Obstructive and restrictive pulmonary dysfunction increases disability in Parkinson disease. Arch Phys Med Rehabil, 77(1), 29–34. 10.1016/s0003-9993(96)90216-6 [DOI] [PubMed] [Google Scholar]
- Sadagopan N, & Huber JE (2007). Effects of loudness cues on respiration in individuals with Parkinson’s disease. Mov Disord, 22(5), 651–659. 10.1002/mds.21375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S, Visser W, & Schlegel U (2010). Short- and long-term dopaminergic effects on dysarthria in early Parkinson’s disease. J Neural Transm (Vienna), 117(2), 197–205. 10.1007/s00702-009-0351-5 [DOI] [PubMed] [Google Scholar]
- Solomon NP, & Hixon TJ (1993). Speech breathing in Parkinson’s disease. J Speech Hear Res, 36(2), 294–310. 10.1044/jshr.3602.294 [DOI] [PubMed] [Google Scholar]
- Spilke J, Piepho HP, & Hu X (2005). Analysis of Unbalanced Data by Mixed Linear Models Using the mixed Procedure of the SAS System. J. Agronomy & Crop Science, 191, 47–54. [Google Scholar]
- Stathopoulos ET, Huber JE, Richardson K, Kamphaus J, DeCicco D, Darling M, Fulcher K, & Sussman JE (2014). Increased vocal intensity due to the Lombard effect in speakers with Parkinson’s disease: simultaneous laryngeal and respiratory strategies. J Commun Disord, 48, 1–17. 10.1016/j.jcomdis.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, & Tilley BC (2013). How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord, 28(5), 668–670. 10.1002/mds.25383 [DOI] [PubMed] [Google Scholar]
- Svec JG, & Granqvist S (2018). Tutorial and Guidelines on Measurement of Sound Pressure Level in Voice and Speech. J Speech Lang Hear Res, 61(3), 441–461. 10.1044/2017_JSLHR-S-17-0095 [DOI] [PubMed] [Google Scholar]
- Thijs Z, & Watts CR (2020). Perceptual Characterization of Voice Quality in Nonadvanced Stages of Parkinson’s Disease. J Voice. 10.1016/j.jvoice.2020.05.007 [DOI] [PubMed] [Google Scholar]
- Tjaden K, Lam J, & Wilding G (2013). Vowel acoustics in Parkinson’s disease and multiple sclerosis: comparison of clear, loud, and slow speaking conditions. J Speech Lang Hear Res, 56(5), 1485–1502. 10.1044/1092-4388(2013/12-0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K, & Wilding GE (2004). Rate and loudness manipulations in dysarthria: acoustic and perceptual findings. J Speech Lang Hear Res, 47(4), 766–783. 10.1044/1092-4388(2004/058) [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, & Clarke CE (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord, 25(15), 2649–2653. 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- Traser L, Burk F, Ozen AC, Burdumy M, Bock M, Blaser D, Richter B, & Echternach M (2020). Respiratory kinematics and the regulation of subglottic pressure for phonation of pitch jumps - a dynamic MRI study. PLoS One, 15(12), e0244539. 10.1371/journal.pone.0244539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traser L, Ozen AC, Burk F, Burdumy M, Bock M, Richter B, & Echternach M (2017). Respiratory dynamics in phonation and breathing-A real-time MRI study. Respir Physiol Neurobiol, 236, 69–77. 10.1016/j.resp.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Van Summers W, Pisoni DB, Bernacki RH, Pedlow RI, & Stokes MA (1988). Effects of noise on speech production: Acoustic and perceptual analyses. The Journal of the Acoustical Society of America, 84(3), 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AP, Fletcher J, & Maruff P (2014). The impact of task automaticity on speech in noise. Speech Communication, 65, 1–8. [Google Scholar]
- Wingate ME (1970). Effect on stuttering of changes in audition. J Speech Hear Res, 13(4), 861–873. 10.1044/jshr.1304.861 [DOI] [PubMed] [Google Scholar]
- Xu Y, Tong Y, Liu S, Chow HM, AbdulSabur NY, Mattay GS, & Braun AR (2014). Denoising the speaking brain: toward a robust technique for correcting artifact-contaminated fMRI data under severe motion. Neuroimage, 103, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston K, Beukelman D, & Tice R (1996). Sentence intelligibility test. Lincoln, NE: Tice Technologies. [Google Scholar]
- Zollinger SA, & Brumm H (2011a). The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour, 148(11–13), 1173–1198. [Google Scholar]
- Zollinger SA., & Brumm H. (2011b). The Lombard effect. Curr Biol, 21(16), R614–615. 10.1016/j.cub.2011.06.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.