SUMMARY / ABSTRACT
Background:
In an aging population with cardiovascular comorbidities, anticoagulant (AC), anti-platelet (AP), and non-steroidal anti-inflammatory (NSAID) use is increasing. It remains unclear whether these agents pose increased bleeding risk in cirrhosis. This study aimed to assess the association between these medications and bleeding and portal hypertension complications in cirrhosis.
Methods:
The IMS PharMetrics database was used to identify privately insured adults diagnosed with cirrhosis from 2007 to 2015, stratified as compensated or decompensated based on the presence of portal hypertensive complications 1 year prior to cirrhosis diagnosis. Bleeding or decompensation outcomes were assessed 6–18 months after cirrhosis diagnosis using a landmark analysis design. Multivariable Cox proportional hazards regression modeling assessed associations between AC, AP, and NSAID drug exposures and outcomes adjusting for covariates.
Results:
18,070 cirrhosis patients were analyzed; 57% male; 74% ages 50–64 years; 34% with a prior decompensation. Overall, 377 (2%) had claims for ACs; 385 (2%) APs; and 1231 (7%) NSAIDS. APs were associated with increased bleeding (aHR 1.31; 95% 1.00,1.72) and decompensation events (aHR 1.44; 95% CI 1.06, 1.95) in a 9-month landmark analysis. NSAIDS were significantly associated with bleeding events (aHR 1.29; 95% CI 1.06, 1.57) on 3-month landmark analysis. No statistically significant associations were seen between ACs and bleeding or decompensation outcomes in adjusted analyses.
Conclusion:
AP use was associated with increased bleeding and decompensation events among privately insured patients with cirrhosis. NSAID use was associated with significant early bleeding, but not decompensations. Lastly, ACs were not associated with bleeding or decompensation outcomes.
Keywords: Anticoagulation, Anti-Platelet, NSAIDS, Bleeding outcomes, Portal hypertension, Cirrhosis
INTRODUCTION
Amidst an aging population with advanced cardiac, vascular, and musculoskeletal comorbidities, the use of anticoagulation (AC), antiplatelet (AP), and non-steroidal anti-inflammatory (NSAID) prescription medications is on the rise.1–3 While there has been great interest in AC and direct oral anticoagulants (DOACs) in cirrhosis, little attention has been paid to whether APs and NSAIDs are safe in cirrhosis, particularly among patients with a history of portal hypertension.
The contention surrounding AC in cirrhosis relates to an increased risk of bleeding, especially among those with more advanced portal hypertension and history of varices. However, there has been limited evaluation of these risks in AP and NSAID users. Some studies suggest that AC, particularly in the setting of portal vein thrombus (PVT), may improve outcomes including survival by reducing portal hypertension,4–6 or reducing risk of repeat cardiovascular event.7–9 This benefit may come at the cost of increased gastrointestinal bleeding.7,8 Furthermore, there is limited data investigating DOACs among patients with cirrhosis.10 Given the delicate coagulopathic state associated with end stage liver disease, AC may be essential to preventing further complications of venous thromboembolism (VTE).11,12 Still, the role of AC among more advanced liver disease including Child-Turcotte Pugh classes B/C remains controversial, with unclear morbidity and survival benefit in this population.
While PVT has been the most investigated thrombotic condition in cirrhosis, it is not the only thromboembolic event that affects this population. The risk of myocardial infarction, ischemic cerebrovascular accidents, and other vascular events amidst a growing population of non-alcoholic fatty liver disease (NAFLD) patients, necessitates an examination of whether APs, in addition to ACs, are safe to use in cirrhosis. Additionally, the use of NSAIDS to manage pain and inflammatory conditions also needs to be explored, especially among our aging cirrhosis population. This study aimed to investigate the association between all cause bleeding outcomes (including gastrointestinal bleeding) and exposure to anticoagulant, antiplatelet, and NSAID prescriptions among patients with cirrhosis. Lastly, we aimed to investigate associations between these drugs and non-bleeding portal hypertensive complications.
METHODS
Data Source & Study Population
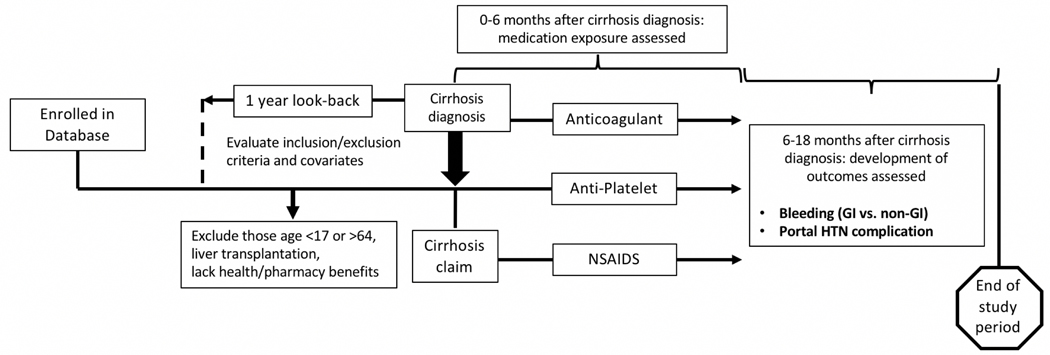
The IQVIA PharMetrics database was used to identify privately insured patients aged 18–64 years from January 1st 2007 to September 30th 2015. This database includes claims data on pharmacy and medical coverage for millions of Americans with private health insurance, and has been shown to be representative of commercially insured US patients.13 The study population included those with a new diagnosis of cirrhosis (at least two International Classification of Diseases, Ninth Revision (ICD-9) codes for cirrhosis: 571.2 or 571.5) using previously validated methods.14–16 These methods are described elsewhere.17 The “diagnosis date” of cirrhosis was based on the first ICD-9 code present in the database (Figure 1).
FIGURE 1.
A landmark analysis design featuring the IMS Pharmetrics database was performed. This methods overview includes the assessment of: (1) exposure to prescription anticoagulants, antiplatelets, NSAIDs, and (2) outcomes including any bleeding or portal hypertension decompensation events. GI indicates gastrointestinal; HTN, hypertension; NSAID, nonsteroidal anti-inflammatory drug.
Patients with cirrhosis were further stratified as “compensated” or “decompensated” based on the presence of a decompensation event identified by ICD-9 code or Current Procedural Terminology (CPT) code (Supplemental Table 1). Patients with decompensated cirrhosis were identified as having any administrative codes from 1 year prior to cirrhosis diagnosis to the index date of cirrhosis diagnosis. Decompensations included evidence of ascites, hepatic encephalopathy, hepatocellular carcinoma, hepatorenal syndrome/acute kidney failure/end stage renal disease, jaundice, spontaneous bacterial peritonitis, transjugular intrahepatic portosystemic shunt (TIPS) procedure, or variceal hemorrhage.
To ensure that exposures and outcomes were adequately assessed, participants were included in the analysis if they had 12 months of continuous health plan enrollment with pharmacy benefits before the diagnosis of cirrhosis, as well as 6 months of coverage after the diagnosis of cirrhosis. Individuals >64 years old with dual Medicare enrollment were excluded given their claims may not be accurately captured in the PharMetrics database. Individuals with prior liver transplantation were excluded. This study was approved by the University of North Carolina Institutional Review Board.
Exposures & Outcomes
The drug exposures of interest included prescription claims for an anticoagulant (AC), antiplatelet (AP), or NSAID medication 0–6 months after index cirrhosis date (Supplemental Table 2). Individuals could have been exposed to the drug prior to cirrhosis diagnosis, but had to have a pharmacy claim for the drug up to 6 months after their cirrhosis diagnosis. Pharmacy claims included new claims and refills both for the inpatient and outpatient setting. If patients were only exposed to a drug after the end of the assessment window, they were counted as not having received the drug. The main drug exposures of interest were AC, AP, and prescription NSAIDS, which were compared to no drug exposure, as well as statins and levothyroxine as a negative control group (i.e. medications unlikely to cause the outcome of interest) to assess for healthy user bias. If individuals were exposed to multiple drugs, they were assigned to one drug category based on a hierarchy designated a priori (AC > AP > NSAIDS).
The primary outcome of interest was any bleeding event (i.e. gastrointestinal and non-gastrointestinal) assessed 6–18 months after index cirrhosis diagnosis (Supplemental Table 1). Bleeding outcomes were identified using ICD-9 codes cited in previously validated methods.18 Secondary outcomes included claims for any portal hypertensive complication. Finally, we performed sensitivity analyses to examine whether levothyroxine and statins were associated with bleeding or decomposition outcomes.
Statistical Analysis
Our descriptive statistics included medians and first and third quartiles for continuous variables, and frequencies for categorical variables stratified by drug exposure type. We used a landmark analysis study design to estimate the time-to-event probabilities in each exposure category, conditional on the exposure status of patients at the landmark time.19 In a landmark analysis, a fixed exposure window is defined from the index cirrhosis diagnosis; the analysis is limited to patients who have not yet experienced the outcome by the landmark time. Hence, individuals had to be free of bleeding or decompensation outcomes during the drug exposure window to be included in the respective analyses. This strategy aims to eliminate error from an immortal time bias. In this study, we chose the landmark windows a priori, and investigated other windows as sensitivity analyses.
For our main analysis, the drug exposure assessment window for the landmark analysis was from cirrhosis index date to six months afterward; development of the outcome was assessed between 6 and 18 months after cirrhosis index date. Multivariable Cox proportional hazards regression was used to assess associations between drug exposures and hazard of bleeding outcomes adjusting for age, sex, region, cirrhosis etiology, Charlson comorbidity index (CCI),20 prior decompensation, and presence of cardiac/vascular comorbidities. Unadjusted and adjusted analyses were performed and sensitivity analyses were conducted examining different drug exposure windows (i.e. receiving drug within 3, 9, and 12 months of index cirrhosis diagnosis). The data were artificially censored at one year after the exposure assessment window ended in order to ensure proximity to drug exposure. Significant findings were defined using a prespecified alpha of 0.05. Analyses were performed using R version 3.6.1 (R Core Team, Vienna, Austria, 2019).
RESULTS
Population: Sociodemographic & Clinical Characteristics
For the six-month landmark analysis, a total of 18,070 patients with cirrhosis were identified from 2007 to 2015. A majority of the population was between the ages of 50 and 64 (74%) with just over half including males (57%). The most common etiologies of cirrhosis were non-alcoholic fatty liver disease or cryptogenic cirrhosis (41%) followed by viral hepatitis (34%) as identified by ICD-9 code. Among the 18,070 patients, 6,215 (34%) had a prior portal hypertensive decompensation, with ascites being the most common complication (4041/6215; 65%), followed by hepatic encephalopathy (17%) and varices (13%). About 48% of the population had a CCI score >= 2. Regarding specific comorbidities, 26% of the population had diabetes, 6% congestive heart failure (CHF), and 3% atrial fibrillation (Table 1). The median duration of total healthcare coverage including prescription benefits was 69 months (1Q-3Q: 45–100).
Table 1.
Sociodemographic and Clinical Characteristics of Cirrhosis Patients in Pharmetrics Database from 2007 to 2015 (N= 18,070)
| VARIABLE | FREQUENCY+ n (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| TOTAL n=18,070 | AC n=377 | AP n=385 | NSAIDS n=1,231 | NONE n=16,077 | |
| Age | |||||
| 18 – 49 | 4686 (25.9%) | 73 (19.4%) | 36 (9.4%) | 339 (27.5%) | 4238 (26.4%) |
| 50 – 64 | 13384 (74.1%) | 304 (80.6%) | 349 (90.6%) | 892 (72.5%) | 11839 (73.6%) |
|
| |||||
| Sex & | |||||
| Female | 7691 (42.6%) | 155 (41.1%) | 132 (34.3%) | 569 (46.2%) | 6835 (42.5%) |
| Male | 10376 (57.4%) | 222 (58.9%) | 253 (65.7%) | 662 (53.8%) | 9239 (57.5%) |
|
| |||||
| US Region | |||||
| East | 3201 (17.7%) | 57 (15.1%) | 86 (22.3%) | 156 (12.7%) | 2902 (18.1%) |
| Midwest | 5224 (28.9%) | 132 (35.0%) | 95 (24.7%) | 287 (23.3%) | 4710 (29.3%) |
| South | 5259 (29.1%) | 105 (27.9%) | 140 (36.4%) | 442 (35.9%) | 4572 (28.4%) |
| West | 4386 (24.3%) | 83 (22.2%) | 64 (16.6%) | 346 (28.1%) | 3893 (24.2%) |
|
| |||||
| Cirrhosis Etiology | |||||
| Alcohol Only | 3294 (18.2%) | 55 (14.6%) | 66 (17.1%) | 183 (14.9%) | 2990 (18.6%) |
| Viral Hepatitis Only | 6143 (34.0%) | 79 (21.0%) | 108 (28.1%) | 426 (34.6%) | 5530 (34.4%) |
| Both Alcohol/Viral | 532 (2.9%) | 6 (1.6%) | 9 (2.3%) | 42 (3.4%) | 475 (3.0%) |
| NAFLD/Cryptogenic | 7475 (41.4%) | 222 (58.9%) | 189 (49.1%) | 546 (44.4%) | 6518 (40.5%) |
| Other (PBC, PSC, AIH) | 626 (3.5%) | 15 (4.0%) | 13 (3.4%) | 34 (2.8%) | 564 (3.5%) |
|
| |||||
| Charlson Comorbidity Index (CCI) ^ | |||||
| CCI 0 | 662 (3.7%) | 8 (2.1%) | 7 (1.8%) | 40 (3.2%) | 607(3.8%) |
| CCI 1 | 8745 (48.4%) | 112 (29.7%) | 95 (24.7%) | 522 (42.4%) | 8016 (49.9%) |
| CCI 2 | 4031 (22.3%) | 88 (23.3%) | 99 (25.7%) | 359 (29.2%) | 3485 (21.7%) |
| CCI > 2 | 4632 (25.6%) | 169 (44.8%) | 184 (47.8%) | 310 (25.2%) | 3969 (24.7%) |
|
| |||||
| Prior Portal HTN Complications by ICD-9 or CPT ^ | |||||
| Any Portal HTN Complication | 6215 (34.4%) | 210 (55.7%) | 168 (43.6%) | 372 (30.2%) | 5465 (34.0%) |
| Ascites | 4041 (22.4%) | 178 (47.2%) | 117 (30.4%) | 232 (18.8%) | 3514 (21.9%) |
| Hepatic Encephalopathy | 1085 (6.0%) | 22 (5.8%) | 33 (8.6%) | 69 (5.6%) | 961 (6.0%) |
| HCC | 267 (1.5%) | 3 (0.8%) | 9 (2.3%) | 17 (1.4%) | 238 (1.5%) |
| Hepatorenal Syndrome / Acute Kidney Failure / CKD | 663 (3.7%) | 39 (10.3%) | 35 (9.1%) | 31 (2.5%) | 558 (3.5%) |
| Jaundice | 928 (5.1%) | 15 (4.0%) | 7 (1.8%) | 42 (3.4%) | 864 (5.4%) |
| SBP | 59 (0.3%) | 3 (0.8%) | 1 (0.3%) | 3 (0.2%) | 52 (0.3%) |
| TIPS | 3 (0.0%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 2 (0.0%) |
| Varices | 839 (4.6%) | 12 (3.2%) | 14 (3.6%) | 45 (3.7%) | 768 (4.8%) |
|
| |||||
| Cardiac / Vascular Comorbidities ^ | |||||
| Any Comorbidity | 6339 (35.1%) | 293 (77.7%) | 269 (69.9%) | 500 (40.6%) | 5277 (32.8%) |
| Atrial Fibrillation | 530 (2.9%) | 154 (40.8%) | 25 (6.5%) | 21 (1.7%) | 330 (2.1%) |
| CHF | 1032 (5.7%) | 116 (30.8%) | 69 (17.9%) | 59 (4.8%) | 788 (4.9%) |
| CVA/TIA | 773 (4.3%) | 35 (9.3%) | 71 (18.4%) | 57 (4.6%) | 610 (3.8%) |
| Diabetes | 4774 (26.4%) | 136 (36.1%) | 189 (49.1%) | 394 (32.0%) | 4055 (25.2%) |
| MI | 261 (1.4%) | 20 (5.3%) | 51 (13.2%) | 11 (0.9%) | 179 (1.1%) |
| PE / DVT | 279 (1.5%) | 66 (17.5%) | 6 (1.6%) | 18 (1.5%) | 189 (1.2%) |
| PVD | 398 (2.2%) | 24 (6.4%) | 40 (10.4%) | 35 (2.8%) | 299 (1.9%) |
| PVT | 87 (0.5%) | 15 (4.0%) | 1 (0.3%) | 6 (0.5%) | 65 (0.4%) |
|
| |||||
| Prior Medication ^ | |||||
| Levothyroxine | 421 (2.3%) | 13 (3.4%) | 14 (3.6%) | 35 (2.8%) | 359 (2.2%) |
| Statins | 2106 (11.7%) | 99 (26.3%) | 184 (47.8%) | 156 (12.7%) | 1667 (10.4%) |
Anticoagulant, antiplatelet, and prescription NSAIDs evaluated from cirrhosis index to six months after index; demographics provided for six-month landmark analysis of all bleeding events.
3 participants had missing values for sex: 2 in the statin group and 1 in the none group.
Conditions, comorbidities, CCI, and prior medications evaluated in the year prior to cirrhosis index.
Abbreviations: Anticoagulant (AC); Anti-platelet (AP); Charlson Comorbidity Index (CCI); Congestive heart failure (CHF); Chronic kidney disease (CKD); Current Procedural Terminology (CPT); Deep vein thrombosis (DVT); Hypertension (HTN); International Classification of Diseases (ICD); Non-steroidal anti-inflammatory drugs (NSAIDS); Pulmonary embolus (PE); Peripheral vascular disease (PVD); Portal vein thrombosis (PVT); Spontaneous bacterial peritonitis (SBP); Transjugular intrahepatic portosystemic shunt (TIPS)
In total, 377 patients (2%) were classified as AC, 385 (2%) as AP, and 1,231 (7.0%) as prescription NSAIDS. Among those on ACs, 51 (14%) received DOACs. Regarding likely indication for AC, 41% had atrial fibrillation, 18% pulmonary embolus (PE) or deep vein thrombosis (DVT), 6% peripheral vascular disease (PVD) and 4% portal vein thrombosis (PVT). For AP use, 18% had congestive heart failure, 18% cerebrovascular accident (CVA) or transient ischemic attack (TIA), 13% myocardial infarction, and 10% PVD. Just under a third of NSAID users had diabetes (32%) or a history of prior portal hypertensive complication (30%), including 19% with ascites and 3% with pre-existing renal disease. Of note, 12% of the total population were on statins, including 26% of AC and 48% of AP users (Table 1).
Bleeding Outcomes Stratified by Drug Exposure and Decompensation History
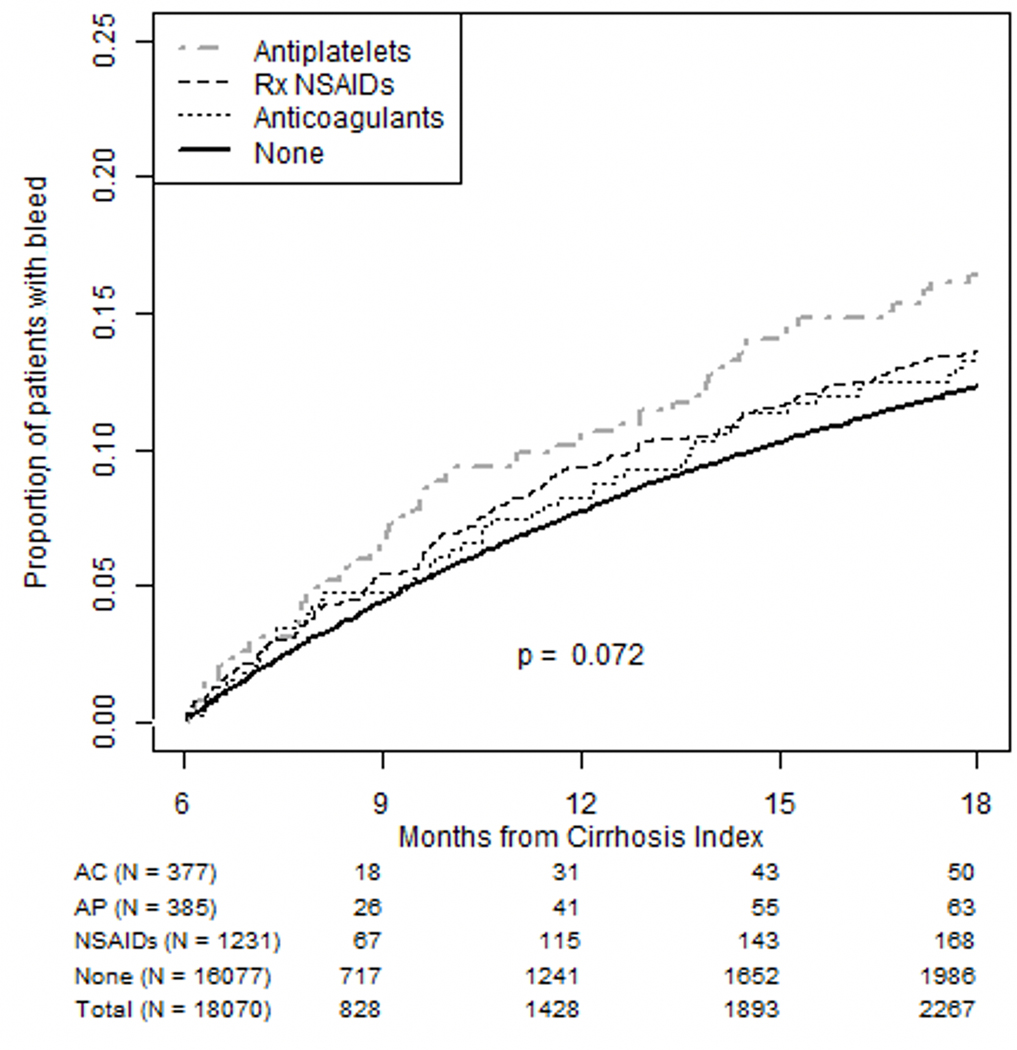
18,070 patients were included in our main 6-month landmark analysis regarding bleeding outcomes. In total, 13% of the population had a bleeding event during the outcome assessment period including 3% with a variceal gastrointestinal (GI) bleed and 7% with a non-variceal GI bleed (Supplemental Table 3). Among those on ACs, 13% had any type of bleeding event including 5% with a non-variceal GI bleed and 0.5% with a variceal GI bleed. Fifty-one patients received DOACS (14% of all AC users); 3 of these patients experienced any bleeding outcome (6%), none of which were variceal. For individuals on APs, 16% had a bleeding event, 1% of which were variceal. Among NSAID users, 14% had any bleeding, 2% of which were variceal. The cumulative incidence of bleeding events among AP users was higher than non-AP users (Figure 2), and did not vary based on decompensation history (Supplemental Figure 1a and 1b). Overall, cumulative incidence of bleeding was significantly higher among individuals with a prior decompensation (Supplemental Figure 2A).
FIGURE 2.
The cumulative incidence of any bleeding event (gastrointestinal and nongastrointestinal) is featured here for all 3 drug exposures: anticoagulant (AC), antiplatelet (AP), and nonsteroidal anti-inflammatory drug (NSAID) medications. The cumulative incidence of bleeding events among AP users was higher than non-AP users. Rx indicates prescription.
ACs were not significantly associated with increased bleeding during the 6-month period after cirrhosis diagnosis on unadjusted (HR 1.14; 95% CI (0.86, 1.51)) or adjusted analyses (aHR 1.00; 95% CI (0.75, 1.33)) (Table 2). On the other hand, APs were significantly associated with bleeding on unadjusted (HR 1.31; 95% CI (1.02, 1.69)) but not adjusted (aHR 1.21; 95% CI (0.93, 1.56)) analyses. We additionally conducted 3-, 9- and 12-month landmark analyses as sensitivities, comprising 20,994, 15,868, and 13,819 eligible patients, respectively. In examining these pre-specified sensitivity analyses, APs were significantly associated with bleeding on both unadjusted (HR 1.36; 95% CI (1.05, 1.78)) and adjusted 9-month landmark analyses (aHR 1.31; 95% CI (1.00, 1.72)) and NSAIDs were significantly associated with bleeding on unadjusted (HR 1.31; 95% CI (1.07, 1.59)) and adjusted (aHR 1.29; 95% (1.06, 1.57)) 3-month landmark analyses.
Table 2.
Associations Between Medication Exposure and Bleeding Events Up to 12 Months After Landmark Time*
| MEDICATION EXPOSURE | UNADJUSTED HR (95% CI) | ADJUSTED HR^ (95% CI) |
|---|---|---|
| Landmark: 3 months after cirrhosis index (n=20,994) | ||
|
| ||
| Anticoagulants (n=300) | 1.16 (0.82, 1.62); p=0.405 | 1.01 (0.72, 1.43); p=0.946 |
| Antiplatelets (n=314) | 1.39 (1.04, 1.85); p=0.026 | 1.26 (0.94, 1.70); p=0.119 |
| NSAIDs (n=824) | 1.31 (1.07, 1.59); p=0.007 | 1.29 (1.06, 1.57); p=0.010 |
| None of the above (n=16451) | 1.00 (ref.) | 1.00 (ref.) |
|
| ||
| Landmark: 6 months after cirrhosis index (n=18,070) | ||
|
| ||
| Anticoagulants (n=377) | 1.14 (0.86, 1.51); p=0.354 | 1.00 (0.75, 1.33); p=0.987 |
| Antiplatelets (n=385) | 1.31 (1.02, 1.69); p=0.033 | 1.21 (0.93, 1.56); p=0.157 |
| NSAIDs (n=1231) | 1.16 (1.00, 1.57); p=0.052 | 1.16 (0.99, 1.36); p=0.061 |
| None of the above (n=16077) | 1.00 (ref.) | 1.00 (ref.) |
|
| ||
| Landmark: 9 months after cirrhosis index (n=15,868) | ||
|
| ||
| Anticoagulants (n=373) | 1.22 (0.91, 1.63); p=0.176 | 1.09 (0.81, 1.46); p=0.566 |
| Antiplatelets (n=375) | 1.36 (1.05, 1.78); p=0.021 | 1.31 (1.00, 1.72); p=0.054 |
| NSAIDs (n=1372) | 1.16 (0.99, 1.36); p=0.069 | 1.17 (0.99, 1.37); p=0.058 |
| None of the above (n=13748) | 1.00 (ref.) | 1.00 (ref.) |
|
| ||
| Landmark: 12 months after cirrhosis index (n=13,819) | ||
|
| ||
| Anticoagulants (n=356) | 1.19 (0.87, 1.62); p=0.274 | 1.12 (0.82, 1.54); p=0.467 |
| Antiplatelets (n=347) | 1.29 (0.96, 1.73); p=0.087 | 1.31 (0.97, 1.78); p=0.075 |
| NSAIDs (n=1404) | 1.06 (0.90, 1.26); p=0.480 | 1.08 (0.91, 1.29); p=0.361 |
| None of the above (n=11712) | 1.00 (ref.) | 1.00 (ref.) |
Significant findings presented in bold.
Drug exposures evaluated from cirrhosis index through assessment window ending at landmark time of interest; bleeds are assessed from landmark time to one year after landmark time.
Adjusted for sex, region, age, etiology, Charlson comorbidity index (CCI), prior decompensation, use of levothyroxine and/or statins during drug assessment window, and presence of cardiac / vascular comorbidities in the year prior to cirrhosis index
Abbreviations: Hazard ratio (HR); Non-steroidal anti-inflammatory drugs (NSAIDs)
Portal Hypertension Complication Stratified by Drug Exposure and Decompensation History
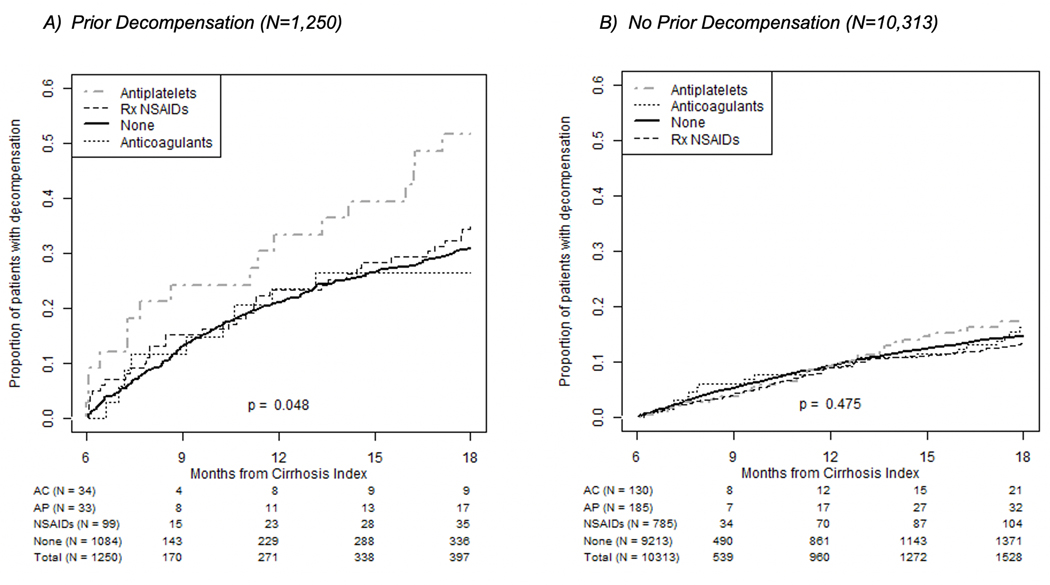
11,563 patients were included in our main 6-month landmark analysis regarding bleeding outcomes. Among them, 17% had a decompensation event during the outcome assessment period (Supplemental Table 3). The highest frequency of portal hypertensive complications was among AP users (23%), followed by AC users (18%). Varices were evident in 6% of the population and renal injury in 2%. The cumulative incidence of portal hypertensive decompensation was higher among AP users as compared to non-AP users, but this was not statistically significant. Cumulative incidence of portal hypertension complications was significantly higher among AP users who had a prior history of decompensation (Figure 3A); whereas this relationship was not seen among individuals with no prior history of decompensation (Figure 3B). Overall, cumulative incidence of portal hypertensive complication was significantly higher among individuals with a prior decompensation (Supplemental Figure 2B).
FIGURE 3.
Portal hypertension complications were significantly higher among patients taking APs who had a decompensation event (A). They were not significantly higher among those with no history of decompensation event before cirrhosis index (B). AC indicates anticoagulant; AP, antiplatelet; NSAID, nonsteroidal anti-inflammatory drug; Rx, prescription.
In unadjusted analyses, AP drug exposures were associated with increased decompensations (HR 1.37; 95% CI 1.03, 1.82) for the 11,563 patients included in the 6-month landmark analysis; however, this relationship was no longer significant when adjusting for potential confounders including a history of decompensation event (aHR 1.28; 95% CI 0.95, 1.73)). In 9- and 12-month landmark analyses of 10,859 and 10,341 patients, respectively, APs were significantly associated with portal hypertensive complications in adjusted analyses (9-month: aHR 1.44; 95% CI 1.06, 1.95 and 12-month: aHR 1.43; 95% CI 1.01, 2.01). ACs and NSAIDS were not significantly associated with decompensation events in adjusted analyses (Table 3). As final sensitivity analyses, levothyroxine or statins were not associated with either bleeding or decompensation outcomes (chi-square tests at alpha = 0.05). Additionally, all analyses were conducted excluding cases of hepatocellular carcinoma (HCC) as a type of decompensation outcome, with no significant differences found in the results reported above.
Table 3.
Associations Between Drug Exposure and Decompensation Events Up to 12 Months After Landmark Time*
| MEDICATION EXPOSURE | UNADJUSTED HR (95% CI) | ADJUSTED HR^ (95% CI) |
|---|---|---|
| Landmark: 3 months after cirrhosis index (n=12,653) | ||
|
| ||
| Anticoagulants (n=154) | 0.90 (0.54, 1.50); p=0.694 | 0.75 (0.45, 1.25); p=0.268 |
| Antiplatelets (n=210) | 1.39 (1.00, 1.94); p=0.049 | 1.30 (0.93, 1.83); p=0.130 |
| NSAIDs (n=679) | 0.95 (0.76, 1.19); p=0.656 | 0.95 (0.76, 1.20); p=0.687 |
| None of the above (ref.) (n=11610) | 1.00 | 1.00 |
|
| ||
| Landmark: 6 months after cirrhosis index (n=11,563) | ||
|
| ||
| Anticoagulants (n=164) | 1.21 (0.85, 1.74); p=0.293 | 1.03 (0.71, 1.48); p=0.889 |
| Antiplatelets (n=218) | 1.37 (1.03, 1.82); p=0.033 | 1.28 (0.95, 1.73); p=0.100 |
| NSAIDs (n=884) | 0.99 (0.83, 1.18); p=0.895 | 0.99 (0.83, 1.18); p=0.950 |
| None of the above (ref.) (n=10297) | 1.00 | 1.00 |
|
| ||
| Landmark: 9 months after cirrhosis index (n=10,859) | ||
|
| ||
| Anticoagulants (n=172) | 1.44 (1.00, 2.07); p=0.049 | 1.24 (0.86, 1.79); p=0.254 |
| Antiplatelets (n=234) | 1.50 (1.12, 2.01); p=0.007 | 1.44 (1.06, 1.95); p=0.020 |
| NSAIDs (n=1060) | 0.98 (0.82, 1.18); p=0.851 | 0.99 (0.82, 1.18); p=0.877 |
| None of the above (ref.) (n=9393) | 1.00 | 1.00 |
|
| ||
| Landmark: 12 months after cirrhosis index (n=10,341) | ||
|
| ||
| Anticoagulants (n=176) | 1.24 (0.81, 1.89); p=0.317 | 1.11 (0.73, 1.71); p=0.626 |
| Antiplatelets (n=233) | 1.47 (1.06, 2.05); p=0.020 | 1.43 (1.01, 2.01); p=0.042 |
| NSAIDs (n=1145) | 0.97 (0.80, 1.18); p=0.795 | 0.99 (0.82, 1.20); p=0.921 |
| None of the above (ref.) (n=8787) | 1.00 | 1.00 |
Significant findings presented in bold.
Drug exposures evaluated from cirrhosis index through assessment window ending at landmark time of interest; decompensation events are assessed from landmark time to one year after landmark time.
Adjusted for sex, region, age, etiology, Charlson comorbidity index (CCI), prior decompensation, use of levothyroxine and/or statins during drug assessment window, and presence of cardiac / vascular comorbidities in the year prior to cirrhosis index
Abbreviations: Hazard ratio (HR); Non-steroidal anti-inflammatory drugs (NSAIDs)
DISCUSSION
In a large cohort of >18,000 privately-insured patients with compensated and decompensated cirrhosis, antiplatelet (AP) use was associated with increased risk of bleeding and decompensation events. AP users with a prior history of decompensation were at significantly increased risk of developing a portal hypertensive complication as compared to non-AP users. Of interest, 7% of the population were prescribed NSAIDS despite this not being recommended in cirrhosis; 30% of NSAID users had a history of prior portal hypertensive complication. NSAID use was associated with significant bleeding during a shorter 3-month landmark analysis adjusting for potential confounders. Although we did not find anticoagulant (AC) or NSAID use to be significantly associated with increased bleeding or other hepatic decompensation events, point estimates were in the direction of increased bleeding. Our overall findings suggest that APs may incur a higher risk of bleeding and decompensation outcomes as compared to AC or NSAIDS among individuals with cirrhosis, and that this risk is further amplified by a history of prior decompensation.
The risks of ACs in cirrhosis has dominated the literature, with limited inquiry into the role of APs and NSAIDs in propagating bleeding or portal hypertensive complications. The extant literature on APs in cirrhosis is limited by small, retrospectively designed studies focused on aspirin and clopidogrel use with minimal consideration of other AP agents. Since most of the cytochrome P2Y12 inhibitors are hepatically metabolized, these drugs have not been thoroughly investigated in individuals with severe liver disease or decompensated cirrhosis.21 Russo et al. examined bleeding events in a retrospective study of patients with cirrhosis receiving coronary artery stents followed by clopidogrel and aspirin use. Patients with cirrhosis were compared to age and sex-matched controls. No significant differences in bleeding events and transfusion requirements were found between the two groups; only 16 patients received a stent plus antiplatelet agent, with a majority of individuals receiving aspirin.22 Another larger retrospective study using the National Health Insurance Research Database found no significant difference in GI bleeding events among 170 aspirin and 70 clopidogrel users with cirrhosis as compared to non-AP users, adjusting for age, sex, and type of cirrhosis.23 It did not account for pre-existing portal hypertensive complications or severity of liver disease. A large Taiwanese study including cirrhosis patients with acute myocardial infarction on dual AP therapy found significantly increased GI bleeding (HR = 1.49, 95% CI 1.31,1.70) as compared to those without cirrhosis, but did not account for history of portal hypertensive complication.7 Additionally, a case control study among 200 cirrhosis patients with esophageal varices found that aspirin use with or without NSAIDs was associated with first time variceal bleeds (OR 4.9; p=0.007). NSAID use was independently associated with bleeding (OR 2.9; 95% CI 1.8, 4.7) adjusting for age, Child-Turcotte Pugh class, ascites, variceal size and recent beta blocker use.24 None of the above studies investigated the association between APs and portal hypertensive complications apart from variceal bleeding.
In this study, we found that APs were associated with increased bleeding and decompensation events, and this relationship was likely amplified by a prior history of decompensation. In fact, the cumulative incidence of portal hypertensive complications was significantly higher among AP users with a history of decompensated cirrhosis. The current study focused on prescription APs and did not examine the role of aspirin use given its availability over-the-counter. However, several AP agents in addition to clopidogrel were examined, allowing for a more in-depth investigation of this medication class than previously reported.
The literature has focused on AC use in liver disease and the controversy surrounding bleeding and portal hypertensive complications related to AC exposure. In this study, we found that ACs were not significantly associated with increased bleeding during a 6-month period after cirrhosis diagnosis, especially when adjusting for potential confounders such as prior portal hypertensive decompensation. Among patients with atrial fibrillation and liver disease, some studies have suggested increased GI bleeding events associated with warfarin,25 unfractionated heparin,26 and DOACs.9,27 However, other studies, mostly focused on AC in portal vein thrombosis found no increased risk of bleeding.6,11,28–30 Given the small number of DOAC users (51 out of 377 AC users), we could not adequately assess the question of whether DOACS were associated with significant bleeding events or decompensations. Other studies have alluded to the safety of DOACS in cirrhosis. More specifically, a recent meta-analysis of DOACs and vitamin K antagonists (VKAs) among patients with advanced fibrosis/cirrhosis and atrial fibrillation found that DOACs were associated with significantly less major bleeding events including GI bleeds compared to VKAs.31 A total of 4 studies were included in the analysis and the results may not be applicable to individuals with decompensated cirrhosis.
NSAID use is discouraged among patients with cirrhosis. Yet, this study found about 7% of patients with cirrhosis were taking prescribed NSAIDS; just under a third of NSAID users had a history of prior portal hypertensive complication including 19% with ascites and 3% with pre-existing renal disease. NSAID use was associated with significant early bleeding events (3-month sensitivity landmark analysis), but was not associated with increased decompensations including renal injury. These findings do not account for potential over-the-counter use of NSAIDs, and likely underestimate complications associated with this drug class.
This study has limitations, which are related to using a database and identifying a cohort based on ICD-9 and CPT codes. While markers of disease severity, such as Charlson Comorbidity Index (CCI) and clinical features of decompensated cirrhosis were investigated, specific data such as laboratory values were not available. This limited our ability to more fully account for liver disease severity including not being able to assess Model for End Stage Liver Disease (MELD) or Child-Turcotte Pugh scores. Of note, a recently published single center study reported no association between these scores and DOAC-related bleeding risk.27 Additionally, the CCI is a comprehensive scale accounting for 17 comorbidities including diabetes, which has been associated with portal hypertension decompensation events.32 Lastly, the use of claims databases such as these do not allow us to examine mortality. Many of the limitations of this study were mitigated by the large number of patients in this cohort and the fact that we were able to identify those with portal hypertensive complications and decompensated cirrhosis based on ICD-9 and CPT codes. Moreover, the investigation of anticoagulation, antiplatelet, and prescriptions NSAIDs was novel compared to prior studies that focused only on AC use.
Despite the attention anticoagulants have received in the extant literature, they were not associated with statistically significant bleeding and decompensation events among individuals with cirrhosis. Antiplatelet use, however, was associated with increased risk of bleeding and decompensation events. Although NSAIDS are discouraged in cirrhosis, they continue to be prescribed, even among those with a history of decompensation. This study found that NSAID use was significantly associated with early bleeding events, adding further evidence to support the clinical recommendation of NSAID avoidance in cirrhosis. In conclusion, attention to anticoagulants should be shifted to antiplatelet and NSAID medications, which should be used with caution, especially among patients with decompensated cirrhosis.
Supplementary Material
Supplemental Figure 1: Bleeding complications among patients who had a decompensation event (A) or did not have a decompensation event (B) prior to cirrhosis index. All individuals did not have a bleeding event during the 6-month drug assessment window, and then were followed from 6-months through up to 18 months after cirrhosis index for whether they experienced a bleeding outcome.
Supplemental Figure 2: The cumulative incidences are depicted here for: A) any bleeding event (gastrointestinal and non-gastrointestinal) and B) portal hypertension complications stratified by history of prior decompensation. Overall, cumulative incidence of any bleeding and portal hypertension complication was significantly higher among individuals with a prior decompensation.
Grant Support:
This research was supported, in part, by the National Institutes of Health (T32DK007634).
Abbreviations:
- AC
Anticoagulant
- AP
Anti-platelet
- CVA
Cerebrovascular accident
- CCI
Charlson comorbidity index
- CPT
Current Procedural Terminology
- DVT
Deep vein thrombosis
- DOACs
Direct oral anticoagulants
- GI
Gastrointestinal
- ICD-9
International Classification of Diseases, Ninth Revision
- MI
Myocardial infarction
- NAFLD
Non-alcoholic fatty liver disease
- NSAID
Non-steroidal anti-inflammatory
- PVD
Peripheral vascular disease
- PVT
Portal vein thrombosis
- PE
Pulmonary embolus
- TIA
Transient ischemic attack
- VTE
Venous thromboembolism
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Esteve-Pastor MA, Ruíz-Nodar JM, Orenes-Piñero E, et al. Temporal Trends in the Use of Antiplatelet Therapy in Patients With Acute Coronary Syndromes. J Cardiovasc Pharmacol Ther. 2018;23(1):57–65. [DOI] [PubMed] [Google Scholar]
- 2.Rowan SB, Bailey DN, Bublitz CE, Anderson RJ. Trends in Anticoagulation for Atrial Fibrillation in the U.S. An Analysis of the National Ambulatory Medical Care Survey Database. J Am Coll Cardiol. 2007;49(14):1561–1565. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23(1):43–50. [DOI] [PubMed] [Google Scholar]
- 4.Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of Anticoagulants in Patients With Cirrhosis and Portal Vein Thrombosis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;153(2):480–487.e1. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28(1):82–89. [DOI] [PubMed] [Google Scholar]
- 6.Noronha Ferreira C, Reis D, Cortez-Pinto H, et al. Anticoagulation in Cirrhosis and Portal Vein Thrombosis Is Safe and Improves Prognosis in Advanced Cirrhosis. Dig Dis Sci. March 2019. [DOI] [PubMed] [Google Scholar]
- 7.Wu VC-C, Chen S-W, Chou A-H, et al. Dual antiplatelet therapy in patients with cirrhosis and acute myocardial infarction - A 13-year nationwide cohort study. PLoS One. 2019;14(10):e0223380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qamar A, Antman EM, Ruff CT, et al. Edoxaban Versus Warfarin in Patients With Atrial Fibrillation and History of Liver Disease. J Am Coll Cardiol. 2019;74(2):179–189. [DOI] [PubMed] [Google Scholar]
- 9.Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral Anticoagulation in Patients With Liver Disease. J Am Coll Cardiol. 2018;71(19):2162–2175. [DOI] [PubMed] [Google Scholar]
- 10.Intagliata NM, Henry ZH, Maitland H, et al. Direct Oral Anticoagulants in Cirrhosis Patients Pose Similar Risks of Bleeding When Compared to Traditional Anticoagulation. Dig Dis Sci. 2016;61(6):1721–1727. [DOI] [PubMed] [Google Scholar]
- 11.Cerini F, Gonzalez JM, Torres F, et al. Impact of anticoagulation on upper-gastrointestinal bleeding in cirrhosis. A retrospective multicenter study. Hepatology. 2015;62(2):575–583. [DOI] [PubMed] [Google Scholar]
- 12.Pettinari I, Vukotic R, Stefanescu H, et al. Clinical Impact and Safety of Anticoagulants for Portal Vein Thrombosis in Cirrhosis. Am J Gastroenterol. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Stempel DA, Mauskopf J, Mclaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med. 2001;95(3):227–234. [DOI] [PubMed] [Google Scholar]
- 14.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47(5):e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clin Gastroenterol Hepatol. 2018;16(10):1677–1678. [DOI] [PubMed] [Google Scholar]
- 16.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2007;27(3):274–282. [DOI] [PubMed] [Google Scholar]
- 17.Moon AM, Jiang Y, Rogal SS, Tapper EB, Lieber SR, Barritt AS. Opioid prescriptions are associated with hepatic encephalopathy in a national cohort of patients with compensated cirrhosis. Aliment Pharmacol Ther. 2020;51(6):652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–77. [DOI] [PubMed] [Google Scholar]
- 19.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 21.Lisman T, Kamphuisen PW, Northup PG, Porte RJ. Established and new-generation antithrombotic drugs in patients with cirrhosis - Possibilities and caveats. J Hepatol. 2013;59(2):358–366. [DOI] [PubMed] [Google Scholar]
- 22.Russo MW, Pierson J, Narang T, Montegudo A, Eskind L, Gulati S. Coronary Artery Stents and Antiplatelet Therapy in Patients With Cirrhosis. J Clin Gastroenterol. 2012;46(4):339–344. [DOI] [PubMed] [Google Scholar]
- 23.Chen C-Y, Lee K-T, Lee CT-C, Lai W-T, Huang Y-B. Effectiveness and safety of antiplatelet therapy in stroke recurrence prevention in patients with liver cirrhosis: a 2-year follow-up study. Pharmacoepidemiol Drug Saf. 2012;21(12):1334–1343. [DOI] [PubMed] [Google Scholar]
- 24.De Lédinghen V, Heresbach D, Fourdan O, et al. Anti-inflammatory drugs and variceal bleeding: A case-control study. Gut. 1999;44(2):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi J, Kim J, Shim JH, Kim M, Nam G-B. Risks Versus Benefits of Anticoagulation for Atrial Fibrillation in Cirrhotic Patients. J Cardiovasc Pharmacol. 2017;70(4):255–262. [DOI] [PubMed] [Google Scholar]
- 26.Kwon J, Koh Y, jong Yu S, Yoon J-H. Low-molecular-weight heparin treatment for portal vein thrombosis in liver cirrhosis: Efficacy and the risk of hemorrhagic complications. Thromb Res. 2018;163:71–76. [DOI] [PubMed] [Google Scholar]
- 27.Mort JF, Davis JP, Giselle M, Stotts MJ, Intagliata NM, Northup PG. Rates of Bleeding and Discontinuation of Direct Oral Anticoagulants in Patients with Decompensated Cirrhosis. Clin Gastroenterol Hepatol. August 2020. [DOI] [PubMed] [Google Scholar]
- 28.Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Cardiovasc Qual Outcomes. 2011;4(3):363–371. [DOI] [PubMed] [Google Scholar]
- 29.Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488–493. [DOI] [PubMed] [Google Scholar]
- 30.La Mura V, Braham S, Tosetti G, et al. Harmful and Beneficial Effects of Anti-coagulants in Patients With Cirrhosis and Portal Vein Thrombosis. Clin Gastroenterol Hepatol. 2018;16(7):1146–1152. [DOI] [PubMed] [Google Scholar]
- 31.Violi F, Vestri A, Menichelli D, Di Rocco A, Pastori D, Pignatelli P. Direct Oral Anticoagulants in Patients With Atrial Fibrillation and Advanced Liver Disease: An Exploratory Meta-Analysis. Hepatol Commun. May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu TL, Trogdon J, Weinberger M, Fried B, Barritt AS. Diabetes Is Associated with Clinical Decompensation Events in Patients with Cirrhosis. Dig Dis Sci. 2016;61(11):3335–3345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Bleeding complications among patients who had a decompensation event (A) or did not have a decompensation event (B) prior to cirrhosis index. All individuals did not have a bleeding event during the 6-month drug assessment window, and then were followed from 6-months through up to 18 months after cirrhosis index for whether they experienced a bleeding outcome.
Supplemental Figure 2: The cumulative incidences are depicted here for: A) any bleeding event (gastrointestinal and non-gastrointestinal) and B) portal hypertension complications stratified by history of prior decompensation. Overall, cumulative incidence of any bleeding and portal hypertension complication was significantly higher among individuals with a prior decompensation.