Summary
Background
The workplace offers a unique opportunity for effective health promotion. We aimed to comprehensively study the effectiveness of multicomponent worksite wellness programmes for improving diet and cardiometabolic risk factors.
Methods
We did a systematic literature review and meta-analysis, following PRISMA guidelines. We searched PubMed-MEDLINE, Embase, the Cochrane Library, Web of Science, and Education Resources Information Center, from Jan 1, 1990, to June 30, 2020, for studies with controlled evaluation designs that assessed multicomponent workplace wellness programmes. Investigators independently appraised the evidence and extracted the data. Outcomes were dietary factors, anthropometric measures, and cardiometabolic risk factors. Pooled effects were calculated by inverse-variance random-effects meta-analysis. Potential sources of heterogeneity and study biases were evaluated.
Findings
From 10 169 abstracts reviewed, 121 studies (82 [68%] randomised controlled trials and 39 [32%] quasi-experimental interventions) met the eligibility criteria. Most studies were done in North America (57 [47%]), and Europe, Australia, or New Zealand (36 [30%]). The median number of participants was 413·0 (IQR 124·0–904·0), and median duration of intervention was 9·0 months (4·5–18·0). Workplace wellness programmes improved fruit and vegetable consumption (0·27 servings per day [95% CI 0·16 to 0·37]), fruit consumption (0·20 servings per day [0·11 to 0·28]), body-mass index (−0·22 kg/m2 [−0·28 to −0·17]), waist circumference (−1·47 cm [−1·96 to −0·98]), systolic blood pressure (−2·03 mm Hg [−3·16 to −0·89]), and LDL cholesterol (−5·18 mg/dL [−7·83 to −2·53]), and to a lesser extent improved total fat intake (−1·18% of daily energy intake [−1·78 to −0·58]), saturated fat intake (−0·70% of daily energy [−1·22 to −0·18]), bodyweight (−0·92 kg [−1·11 to −0·72]), diastolic blood pressure (−1·11 mm Hg [−1·78 to −0·44]), fasting blood glucose (−1·81 mg/dL [−3·33 to −0·28]), HDL cholesterol (1·11 mg/dL [0·48 to 1·74]), and triglycerides (−5·38 mg/dL [−9·18 to −1·59]). No significant benefits were observed for intake of vegetables (0·03 servings per day [95% CI −0·04 to 0·10]), fibre (0·26 g per day [−0·15 to 0·67]), polyunsaturated fat (−0·23% of daily energy [−0·59 to 0·13]), or for body fat (−0·80% [−1·80 to 0·21]), waist-to-hip ratio (−0·00 ratio [−0·01 to 0·00]), or lean mass (1·01 kg [−0·82 to 2·83]). Heterogeneity values ranged from 46·9% to 91·5%. Between-study differences in outcomes were not significantly explained by study design, location, population, or similar factors in heterogeneity analyses.
Interpretation
Workplace wellness programmes are associated with improvements in specific dietary, anthropometric, and cardiometabolic risk indicators. The heterogeneity identified in study designs and results should be considered when using these programmes as strategies to improve cardiometabolic health.
Introduction
Adults spend most of their weekday waking hours at work, and thus the workplace offers a unique opportunity to promote health. Both the World Health Assembly in 20041 and the UN high-level meeting on the prevention and control of non-communicable diseases in 2011 called on the private sector to promote enabling environments and worksite wellness programmes for healthy behaviours among workers.2 In addition to improved employee health, workplace wellness programmes might benefit companies through higher employee satisfaction, increased loyalty, improved productivity, and lower health-care costs.3,4 In 2017, WHO identified workplace wellness programmes as a best-buy option for the prevention and control of non-communicable diseases including mental health.5
Although narrative reviews have suggested benefits of workplace wellness programmes for lifestyle behaviours and cardiometabolic health,6–10 few quantitative meta-analyses have been done to identify magnitudes of benefits for specific outcomes or employee-related or intervention-related factors that may influence effectiveness.11–13 Also, most of these analyses were limited to a specific population, intervention targets (eg, diet, physical activity), or outcomes (eg, fruit intake, bodyweight, glycaemia), with few included studies (eg, ten total studies11) in each. To our knowledge, no previous systematic review and meta-analysis has comprehensively assessed the effect of workplace wellness programmes on a broad set of dietary and diet-related health indicators. Additionally, few studies have quantitatively explored potential heterogeneity in outcomes, for instance based on employee characteristics or intervention components.14 To address these gaps in knowledge, we did a systematic review and meta-analysis of the effects of multicomponent workplace wellness programmes on dietary behaviours and major diet-related cardiometabolic risk factors.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15 The protocol is presented in the appendix (pp 5–6).
See Online for appendix
Reports from health agencies related to workplace wellness programmes were first reviewed by the investigators for development of the protocol, contextualisation of the research, and identification of key scientific literature. This preliminary review included policy statements and guidance from the Institute of Medicine, WHO, Centers for Disease Control and Prevention, US Department of Health and Human Services, and other similar international, national, and local agencies.
The systematic search was done in PubMed-MEDLINE, Embase, the Cochrane Library, Web of Science, and the Education Resources Information Center, for studies published in English from Jan 1, 1990, to June 30, 2020. The search strategy was developed and implemented under the guidance of experts of library services from Tufts University (Boston, MA, USA). The search terms included different synonyms and combinations of words for workplaces, health promotion, weight loss, diet, and cardiometabolic factors (appendix pp 7–8). Online searches were supplemented by hand searches of reference lists of the first 20 related articles suggested in PubMed for each of the final included articles. Titles and abstracts were screened in duplicate and, for all potentially relevant articles, full-text manuscripts were retrieved for further review and eligibility check.
Studies were selected if they were interventional controlled trials, either randomised or quasi-experimental with external control or comparison group. Commentaries, protocols, or review articles were also assessed as sources of potential references. Studies were excluded if they were quasi-experimental without an external control or comparison group, observational, ecological, theoretical (laboratory experiments), or simulation (modelling) study designs; or duplicate publications from the same study (after full-text review). Studies were eligible if they reported any worksite-based intervention that targeted the overall workplace. Studies of non-employed individuals, children, or an intervention aimed solely at disease management (eg, control of existing type 2 diabetes) were excluded. The study setting could be any type of workplace, apart from schools targeting students and non-workplace organisations (eg, community centres or religious organisations).
Eligible studies assessed multicomponent workplace wellness programmes based on two or more intervention components that targeted improved health, such as the use of educational messages, cafeteria or vending machine interventions, promotion of stair use, financial incentives, changes to health insurance policies, or improved accessibility or discounts for gym memberships. Only multicomponent programmes were included because there is evidence of this being the most effective approach, particularly when targeting diet and physical activity,16,17 and multicomponent workplace wellness programmes are recommended by WHO for the prevention of non-communicable diseases.18,19 Studies were excluded if they assessed single-component programmes or tailored individual-level interventions not part of a multicomponent intervention, routine workplace health screening programmes without additional multicomponent intervention, work–life balance programmes that did not target improvements in health (eg, stress management), or smoking cessation-only programmes.
We included studies if they reported any of the following outcomes: change in dietary habits (measured by food frequency questionnaires, 24-hour recall, or dietary records), markers of adiposity (eg, bodyweight, body-mass index [BMI], waist circumference, skinfolds, body fat percentage), cardiometabolic risk factors (eg, blood pressure, lipids, glucose, insulin), cardiovascular risk scores (eg, Framingham risk score), or disease outcomes (eg, diabetes) if available. Studies had to report the differential change (intervention vs control) in the outcome of interest plus a measure of uncertainty for the reported difference, or information to compute these numbers. We did not include studies if they only reported changes in knowledge or attitudes regarding health, diet, or physical activity; changes in health-care costs to the company; absenteeism; or changes in mental health, general wellbeing, or quality of life.
Data extraction
All of the following steps were done in duplicate by two investigators. Studies selected for full-text review were evaluated independently for inclusion, with any differences resolved by consensus. Data were extracted individually using standardised electronic templates. The extracted information is detailed in the appendix (p 8). Study quality was assessed using a previously established and utilised scoring system based on five criteria: study design, assessment of exposure, assessment of outcome, control for confounding, and evidence of selection bias (appendix p 9).20–22 Each criterion received a score of 0 or 1 (with 1 being better) and an overall score was calculated as the sum of individual scores; with 0–3 considered as lower quality and 4–5 considered higher quality. Balance between intervention and control groups, differential attrition rate, and fit of the statistical model chosen to the study design, in terms of unit of analysis, covariate adjustment, and uncertainty of the estimate were also used to assess study quality. To address and assess the variability in the intervention components and targets used across the studies and facilitate identification of potentially more influential intervention design characteristics of workplace wellness programmes, individual components and targets of the intervention (eg, focus on physical activity or dietary habits) were classified into different domains. These categories (appendix p 9) were assessed in exploration of heterogeneity. Missing data or unclear information were attempted to be resolved by direct contact with authors; when feedback was not received, assumptions were discussed and agreed upon by the investigators (appendix pp 10–11). Differences in data extraction and bias assessment between investigators were infrequent and resolved by the duplicate abstractors and a third investigator.
For randomised and quasi-experimental interventional studies, the differential (between-group, intervention vs comparator) change at follow-up adjusted for baseline values and relevant covariates was extracted when available or calculated based on the information reported. Statistical uncertainty (standard error, SE) of effect sizes was extracted or calculated based on other statistics, or in the absence of other information, assumed based on information provided in the article (appendix pp 12–15). For paired observations without reported covariance, we used a correlation coefficient of 0·9 if no loss to follow-up, 0·5 if loss to follow-up, and 0 if independent samples. Effects were extracted as continuous effect sizes when available, and as other effect sizes otherwise (eg, percentage meeting a cutpoint, odds ratio), together with their statistical uncertainty. Multiple trial groups were handled as separate intervention groups from the same study and were included as separate estimates in the meta-analyses; subgroup findings from the same intervention group (eg, by sex, age) were first combined using study-specific meta-analysis. Effect sizes were standardised to servings per day for fruits and vegetables, g per day for dietary fibre, percentage daily energy for fats and fatty acids, kg for bodyweight and lean mass, kg/m2 for BMI, cm for waist circumference, percentage for body mass, mm Hg for blood pressure, and mg/dL for plasma glucose and lipid fractions. Information on outcomes related to intake of sodium, monounsaturated fatty acids, red meat, and whole grains; insulin and apolipoprotein concentrations; disease risk scores (eg, Framingham risk score); and disease outcomes (eg, cardiovascular disease) was scarce, and not enough for meta-analysis; thus, these outcomes were only included in the qualitative assessment of the evidence.
Data analysis
Study-specific effect sizes and corresponding SE were pooled using inverse-variance random-effects meta-analysis to estimate an overall summary effect size (95% CI) and to obtain the corresponding forest plots for each of the outcomes of interest. All analyses were done with Stata (release 16/SE).
Cochran’s Q and I2 statistics were used to assess between-study heterogeneity.23 For outcomes with ten or more study estimates, univariate and multivariate meta-regressions explored prespecified sources of potential heterogeneity including study publication, location, study design, bias score, type of workplace, duration of the intervention, number of intervention components, mean employee age, and sex. Because analyses of heterogeneity were exploratory across multiple comparisons, a Bonferroni-adjusted p<0·001 was considered to be significant in multivariate meta-regressions. Potential small-study effects or public bias were assessed by visual inspection of funnel plots, and computation of Egger’s test and Begg’s test.24 The trim-and-fill method25 was used to adjust for potential small-study effects or publication bias and compare with unadjusted findings. To further evaluate the consistency of the results depending on study design, a sensitivity analysis was done excluding quasi-experimental studies (ie, including randomised controlled trials only) for both meta-analyses of effects, and trim-and-fill for small-study effects.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of 10 169 identified abstracts and 958 articles identified for full-text review, 121 interventional studies met the inclusion and exclusion criteria, including 82 (68%) randomised controlled trials and 39 (32%) quasi-experimental trials (figure 1). Study characteristics are summarised in table 1, with details of each individual study reported in the appendix (pp 16–31). Most studies (72 [60%] of 121) were published from 2010 onwards. Studies were from North America (57 [47%]), western Europe, Australia, and New Zealand (36 [30%]), Asia (22 [18%]), and other countries (Brazil, South Africa, and Tunisia; 6 [5%]). Workplace settings included factories, offices, hospitals, and schools (employees), and mixed settings were predominant (56 [48%] of 117). Numbers of participating employees varied (median 413·0 employees [IQR 124·0–904·0]), as did duration of the intervention (median 9·0 months [4·5–18·0]). Behavioural intervention targets included dietary habits (94 [78%] of 121), physical activity (81 [67%]), and weight loss (53 [44%]), with most studies (101 [84%]) having more than one target. More than 50 individual intervention components were identified across the retrieved studies, which were classified into nine intervention domains (appendix p 9): screening, individual education, group education, food environment, labelling, financial incentives, physical activity, self-awareness, and other (eg, employees’ advisory committees). Identified trials compared workplace wellness programme interventions with a comparison group that was either a base case (usual care) control or a less intensive intervention (eg, basic education about healthy diet only). Assessment of bias scores were higher (more favourable) for randomised controlled interventions (mean 3·8 [SD 0·7]) versus non-randomised designs (2·4 [0·9]).
Figure 1:
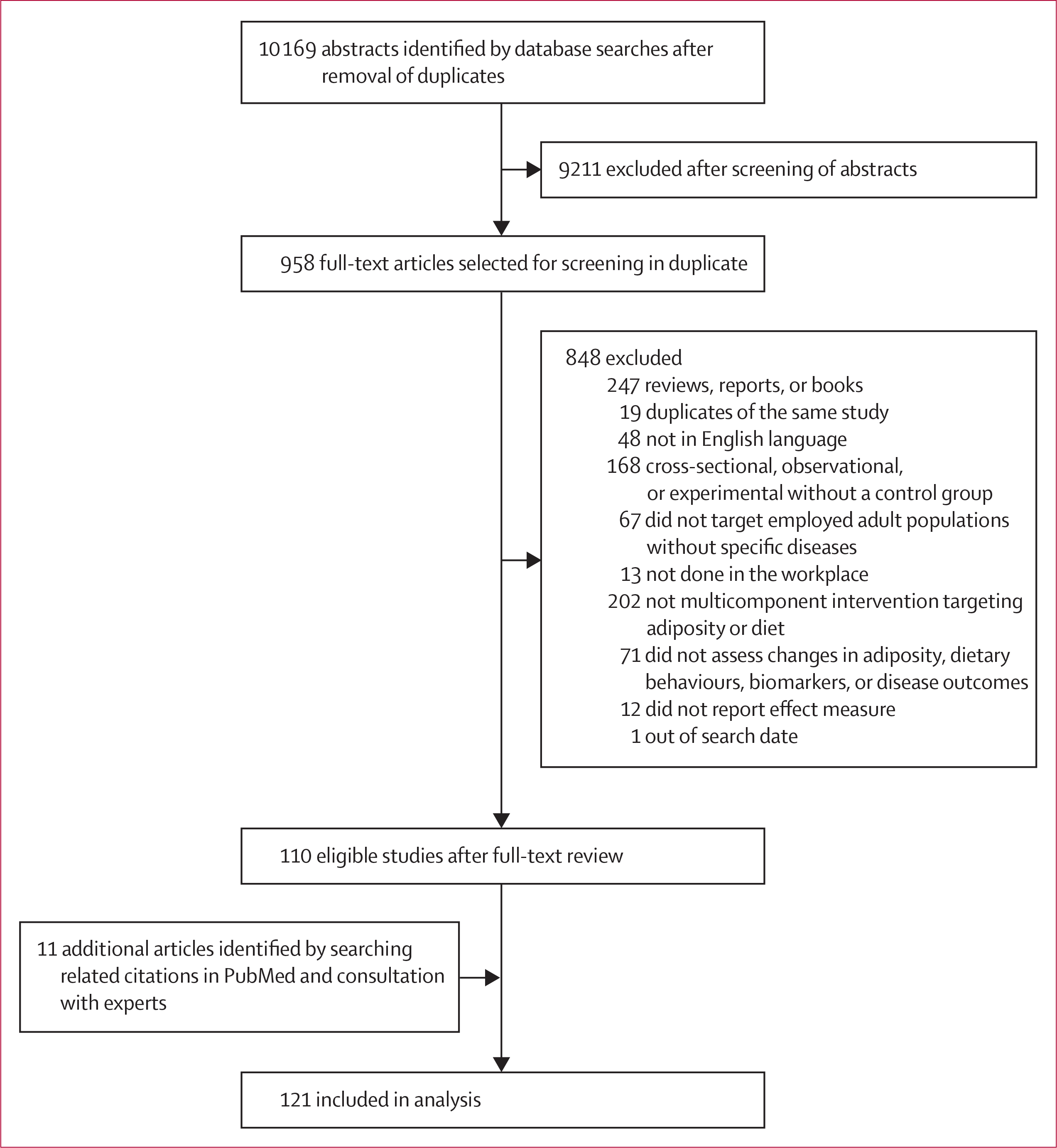
Study selection
Table 1:
Main characteristics of the 121 studies reporting assessment of the effect of workplace wellness programmes
| All studies (n=121) | Randomised controlled trials (n=82) | Quasi-experimental studies (n=39) | |
|---|---|---|---|
|
| |||
| Study details | |||
| Publication date | |||
| Before 2000 | 18 (15%) | 13 (16%) | 5 (13%) |
| 2000–09 | 31 (26%) | 14 (17%) | 17 (44%) |
| 2010 or later | 72 (60%) | 55 (67%) | 17 (44%) |
| Country | |||
| North America (US and Canada) | 57 (47%) | 46 (56%) | 11 (28%) |
| Europe*, Australia, New Zealand | 36 (30%) | 22 (27%) | 14 (36%) |
| Asia† | 22 (18%) | 9 (11%) | 13 (33%) |
| Other‡ | 6 (5%) | 5 (6%) | 1 (3%) |
| Unit of randomisation | |||
| Individual | ·· | 24 (29%) | ·· |
| Cluster | ·· | 58 (71%) | ·· |
| Lost to follow-up (%) | 26·0% (20·9) | 24·4% (20·2) | 29·6% (22·2) |
| Median (IQR) | 20·9% (12·0–37·4) | 19·8% (12·0–34·6) | 27·9% (12·2–43·3) |
| Bias score§ | 3·4 (1·0) | 3·8 (0·7) | 2·4 (0·9) |
| Median (IQR) | 4·0 (3·0–4·0) | 4·0 (3·0–4·0) | 2·0 (2·0–3·0) |
| Workplace settings | |||
| Type¶ | |||
| Office | 19 (16%) | 13 (16%) | 6 (16%) |
| Hospital | 15 (13%) | 14 (18%) | 1 (3%) |
| School | 5 (4%) | 5 (6%) | 0 |
| Factory | 22 (19%) | 11 (14%) | 11 (30%) |
| Mixed or other | 56 (48%) | 37 (46%) | 19 (51%) |
| Number of sites | 9·4 (14·2) | 10·5 (15·3) | 7·2 (11·2) |
| Median (IQR) | 4·0 (2·0–13·0) | 4·0 (2·0–16·0) | 3·0 (2·0–7·5) |
| Employee characteristics | |||
| Number of participating employees | 1231 (3147) | 886 (1532||) | 1964 (4870) |
| Median (IQR) | 413·0 (124·0–904·0) | 314·0 (98·0–782·0) | 547·0 (253·0–1749·0) |
| Age, years | 42·7 (5·7) | 43·0 (5·8) | 41·8 (5·4) |
| Median (IQR) | 43·0 (38·9–46·0) | 43·6 (39·9–46·5) | 42·0 (37·3–44·6) |
| Sex, percentage male** | 51·4% (30·8) | 48·4% (31·4) | 58·0% (29·1) |
| Median (IQR) | 49·2% (24·6–76·1) | 46·8% (22·6–74·4) | 60·1% (37·7–77·1) |
| Race or ethnicity, percentage white†† | 56·0% (34·2) | 62·9% (30·8) | 36·3% (36·7) |
| Median (IQR) | 61·3% (32·4–89·8) | 73·1% (42·4–90·8) | 38·1% (0·0–68·3) |
| BMI (kg/m2) | 28·0 (3·3) | 28·7 (3·5) | 26·5 (2·6) |
| Median (IQR) | 27·5 (25·5–29·7) | 28·2 (25·7–31·6) | 26·4 (25·2–28·1) |
| Intervention characteristics | |||
| Duration, months | 13·3 (13·8) | 10·1 (8·6) | 20·0 (19·3) |
| Median (IQR) | 9·0 (4·5–18·0) | 6·0 (3·0–15·0) | 12·0 (8·0–24·0) |
| Primary specified target‡‡ | |||
| Diet | 94 (78%) | 60 (73%) | 34 (87%) |
| Physical activity | 81 (67%) | 51 (62%) | 30 (77%) |
| Weight management | 53 (44%) | 36 (44%) | 17 (44%) |
| Other | 76 (63%) | 52 (63%) | 24 (62%) |
| Number of components | |||
| 2 | 12 (10%) | 6 (7%) | 6 (15%) |
| 3 | 31 (26%) | 16 (20%) | 15 (38%) |
| 4 | 22 (18%) | 15 (18%) | 7 (18%) |
| 5 | 14 (12%) | 11 (13%) | 3 (8%) |
| 6 | 10 (8%) | 9 (11%) | 1 (3%) |
| 7 | 5 (4%) | 5 (6%) | 0 |
| 8 | 8 (7%) | 6 (7%) | 2 (5%) |
| 9 | 9 (7%) | 6 (7%) | 3 (8%) |
| 10 | 10 (8%) | 8 (10%) | 2 (5%) |
Data are n (%) or mean (SD), unless otherwise specified.
=not applicable. BMI=body-mass index.
Europe includes the Netherlands, Norway, Denmark, Belgium, the UK, Finland, Germany, Iceland, Ireland, and Switzerland.
Asia includes Japan, Taiwan, South Korea, Malaysia, Singapore, India, China, Jordan, and Bangladesh.
Other countries are Brazil, Tunisia, and South Africa.
The bias score assigned to each study ranged from 1 to 5. This value represents the mean (SD) of the score and the corresponding median (IQR).
Denominator is 117; four studies did not report the type of workplace. Workplace settings were classified into the following types: office, hospital, school, factory, mixed, or others. Mixed is defined as a combination of the prespecified workplaces, whereas others differ from the prespecified categories.
A mean of 1188 participants (SD 1739) were cluster randomised and 171 (183) participants were individually randomised.
Data on number of women were not extracted and therefore are not available.
When available, data on race or ethnicity usually referred to white people, and so this group was chosen to represent this variable in the meta-analyses.
Primary specified target indicates the most frequent, but not limited to, intervention focus per outcome identified subjectively by the investigators (diet quality, weight loss, physical activity, and other such as reduction in cardiovascular disease risk factors, smoking cessation, stress reduction, diabetes, or cancer prevention); for instance the primary specified target for the interventions reporting fruit intake was diet quality (93%), meaning diet quality was the most frequent target, but not exclusively because the intervention could also target physical activity or weight loss less frequently.
Pooled quantitative estimates were derived for seven dietary factors, six anthropometric measures, and seven clinical risk factors (table 2). The most assessed outcome among anthropometric factors was BMI (67 intervention groups); for dietary habits, total fruit and vegetable consumption as well as total fat were the most assessed outcomes (both with 18 intervention groups); and for clinical risk factors, blood pressure was the most assessed outcome (41 intervention groups).
Table 2:
Pooled estimates of the effect (change) of workplace wellness programmes on dietary habits, anthropometric measurements, and clinical parameters
| Number of studies (number of intervention groups)* | Number of intervention groups from RCT (%) | Number of participants, median (IQR) | Primary specified targets, n (%)† | Duration, months (SD) | Pooled effect size (95% CI)‡ | I2, % | p asymmetry (Egger’s test) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Dietary habits | ||||||||
| Fruits and vegetables, servings per day | 16 (18) | 15 (83%) | 550 (397–1359) | 18 (100%)§ | 15·6 (8·9) | 0·27 (0·16 to 0·37) | 88·7 | 0·0004 |
| Fruits, servings per day | 13 (15) | 13 (87%) | 430 (257–730) | 14 (93%)§ | 6·5 (5·4) | 0·20 (0·11 to 0·28) | 59·9 | 0·0092 |
| Vegetables, servings per day | 12 (14) | 10 (71%) | 419 (314–515) | 13 (93%)§ | 6·8 (5·4) | 0·03 (−0·04 to 0·10) | 75·6 | 0·010 |
| Fibre, g per day | 8 (15) | 11 (73%) | 433 (174–850) | 15 (100%)§ | 11·8 (6·6) | 0·26 (−0·15 to 0·67) | 61·3 | 0·63 |
| Total fat, % daily energy | 14 (18) | 12 (67%) | 478 (362–850) | 18 (100%)§ | 11·7 (7·4) | –1·18 (−1·78 to −0·58) | 80·1 | 0·44 |
| Saturated fat, % energy | 4 (6) | 2 (33%) | 850 (770–850) | 6 (100%)§ | 11·0 (7·4) | –0·70 (−1·22 to −0·18) | 46·9 | 0·046 |
| Polyunsaturated fat, % energy | 3 (3) | 2 (67%) | 770 (186–3076) | 3 (100%)§ | 14·0 (10·5) | –0·23 (−0·59 to 0·13) | 62·6 | 0·23 |
| Anthropometric measures | ||||||||
| BMI, kg/m2 | 57 (67) | 41 (61%) | 447 (110–904) | 53 (79%)¶|| | 14·0 (12·1) | –0·22 (−0·28 to −0·17) | 86·9 | 0·0013 |
| Bodyweight, kg | 47 (59) | 35 (59%) | 269 (77–850) | 45 (76%)|| | 10·0 (8·7) | –0·92 (−1·11 to −0·72) | 86·8 | 0·0068 |
| Waist circumference, cm | 31 (37) | 18 (49%) | 265 (95–553) | 30 (81%)|| | 10·3 (12·6) | –1·47 (−1·96 to −0·98) | 81·9 | 0·061 |
| Body fat, % | 11 (13) | 10 (77%) | 60 (58–77) | 13 (100%)|| | 4·8 (2·9) | –0·80 (−1·80 to 0·21) | 87·0 | 0·84 |
| Waist-to-hip, ratio | 6 (8) | 8 (100%) | 169 (45–592) | 7 (88%)** | 10·4 (12·6) | –0·00 (−0·01 to 0·00) | 79·8 | 0·17 |
| Lean mass, kg | 4 (4) | 4 (100%) | 50 (30–189) | 4 (100%)|| | 3·3 (0·5) | 1·01 (−0·82 to 2·83) | 89·8 | 0·45 |
| Cardiometabolic risk factors | ||||||||
| Systolic blood pressure, mm Hg | 34 (41) | 22 (54%) | 228 (102–817) | 33 (80%)¶ | 13·4 (18·0) | –2·03 (−3·16 to −0·89) | 89·5 | 0·019 |
| Diastolic blood pressure, mm Hg | 34 (41) | 22 (54%) | 228 (102–817) | 33 (80)¶ | 13·4 (18·0) | –1·11 (−1·78 to −0·44) | 74·7 | 0·51 |
| Fasting glucose, mg/dL | 21 (26) | 12 (46%) | 190 (70–1371) | 23 (88%)|| | 12·7 (11·6) | –1·81 (−3·33 to −0·28) | 91·5 | 0·22 |
| HDL cholesterol, mg/dL | 29 (32) | 15 (47%) | 190 (74–532) | 28 (88%)|| | 14·2 (16·0) | 1·11 (0·48 to 1·74) | 89·6 | 0·077 |
| LDL cholesterol, mg/dL | 20 (22) | 11 (50%) | 162 (70–447) | 21 (95%)|| | 11·9 (13·2) | –5·18 (−7·83 to −2·53) | 87·5 | 0·0022 |
| Triglycerides, mg/dL | 23 (26) | 12 (46%) | 142 (61–490) | 23 (88%)|| | 12·3 (14·6) | –5·38 (−9·18 to −1·59) | 67·8 | 0·013 |
| Total cholesterol, mg/dL | 32 (36) | 17 (47%) | 292 (104–1138) | 31 (86%)¶|| | 15·7 (15·2) | –1·75 (−2·59 to −0·91) | 83·6 | 0·0056 |
References per outcome: fruits and vegetables,26–41 fruits,30,36,39,42–51 vegetables,30,36,39,42–44,46,48,50–53 fibre,27,35,36,40,41,54–56 total fat,26,31,36,37,40,41,43,55–61 saturated fat,55,58,60,62 polyunsaturated fat,58,60,62 BMI,26,29,34,35,37,40,43,46,48,51,55,57,59–61,63–104 bodyweight,26,29,35,40,43,46,48,55,57,59,61,64,66–75,79–81,87–92,96,98,100,102,103,105–115 waist circumference,40,46,48,55,57,61,66,68,71–73,75,79,81,84,85,87,88,90,93,96,100,103,106,112,114–119 body fat,61,67,71,80,90,100,103,105,109,115,116 waist-to-hip ratio,34,60,67,79,89,94 lean mass,80,105,109,116 systolic blood pressure,46,48,53,55,57,61,63,65,67,69,70,73,74,76,77,79,81,82,85,87–89,91,92,100,103,104,112,114,115,117–120 diastolic blood pressure,46,48,53,55,57,61,63,65,67,69,70,73,74,76,77,79,81,82,85,87–89,91,92,100,103,104,112,114,115,117–120 fasting glucose,57,61,68,69,71,72,76,77,79,81,87,90–92,103,109,112,114,118,119,121 HDL cholesterol,57,60,61,63,65,68,70–73,76,77,79,81,85,87,90–93,103,104,109,112,114,117–119,121 LDL cholesterol,57,61,68,71–74,77,79,81,85,87,90–93,103,104,114,121 triglycerides,57,61,68,70–73,77,79,81,87,90–93,103,104,109,112,114,118,119,121 and total cholesterol.26,27,36,37,40,41,43,55–61 BMI=body-mass index. RCT=randomised control trial.
Some studies included more than two intervention groups, which were analysed separately.
Primary specified target indicates the main target (but not the only target) of the intervention per outcome identified subjectively by the investigators (diet quality, weight loss, physical activity, and others such as reduction in cardiovascular disease risk factors, smoking cessation, stress reduction, diabetes, or cancer prevention); for instance the primary specified target for the interventions reporting fruit intake was diet quality (93%), meaning diet quality was the most frequent target, but not exclusively because the intervention could, and frequently did, also target physical activity or bodyweight less intensively.
Pooled effect sizes were calculated using inverse-variance random-effects meta-analysis.
Diet quality.
Weight loss.
Physical activity.
Other.
Among dietary factors, workplace wellness programmes increased intake of total fruits and vegetables (0·27 servings per day [95% CI 0·16 to 0·37]) and intake of fruits (0·20 servings per day [0·11 to 0·28]; figure 2), and decreased intake of total fat (−1·18% of daily energy intake [−1·78 to −0·58]) and saturated fat (−0·70% of daily energy [−1·22 to −0·18]). No significant changes were identified for intake of vegetables (0·03 servings per day [95% CI −0·04 to 0·10]), dietary fibre (0·26 g per day [−0·15 to 0·67]), or polyunsaturated fat (−0·23% of daily energy intake [−0·59 to 0·13]). Workplace wellness programmes significantly reduced BMI (−0·22 kg/m2 [95% CI −0·28 to −0·17]), bodyweight (−0·92 kg [−1·11 to −0·72]), and waist circumference (−1·47 cm [−1·96 to −0·98]; figure 3). A numerical decrease was seen for body fat percentage (−0·80% [95% CI −1·80 to 0·21]) and an increase for lean mass (1·01 kg [−0·82 to 2·83]), but these changes were not significant. No significant changes were seen in waist-to-hip ratio (−0·00 ratio [95% CI −0·01 to 0·00]); eight intervention groups).
Figure 2: Forest plot of intake of fruits.
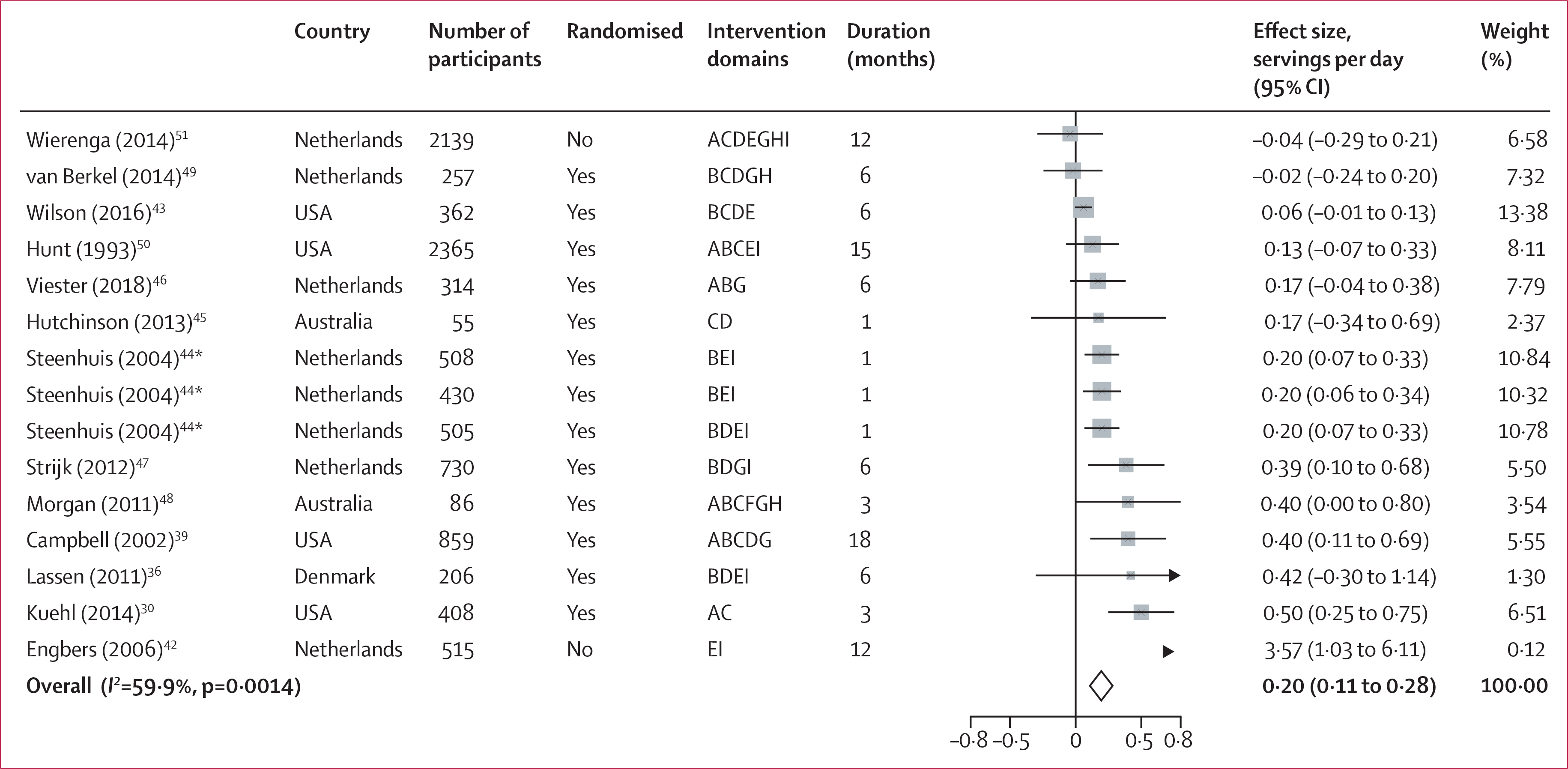
Intervention domains correspond to screening (A), individual education (B), group education (C), food environment (D), labelling (E), financial incentives (F), physical activity (G), self-awareness (H), and others (I). Weights are from random-effects analysis. *Different intervention groups from the same study (Steenhuis 2004).
Figure 3: Forest plot of waist circumference.
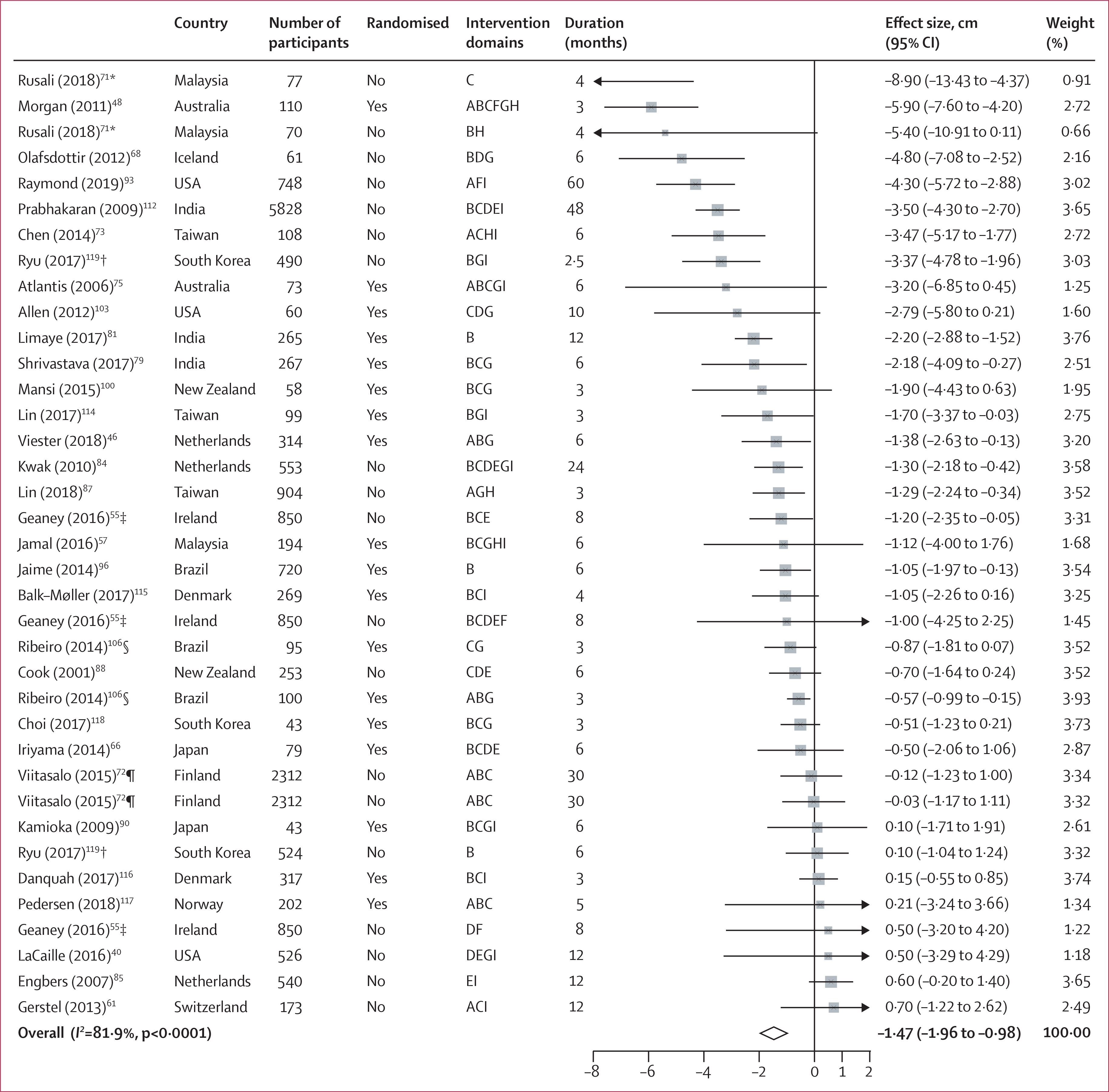
Intervention domains correspond to screening (A), individual education (B), group education (C), food environment (D), labelling (E), financial incentives (F), physical activity (G), self-awareness (H), and others (I). Weights are from random-effects analysis. *Different intervention groups from the same study (Rusali 2018). †Different intervention groups from the same study (Ryu 2017). ‡Different intervention groups from the same study (Geaney 2016). §Different intervention groups from the same study (Ribeiro 2014). ¶Different intervention groups from the same study (Viitasalo 2015).
Significant improvements were identified in all cardiometabolic risk factors (table 2). Systolic blood pressure declined by 2·03 mm Hg (95% CI −3·16 to −0·89) and diastolic blood pressure by 1·11 mm Hg (−1·78 to −0·44). Fasting glucose decreased by 1·81 mg/dL (95% CI −3·33 to −0·28), LDL cholesterol by 5·18 mg/dL (−7·83 to −2·53; figure 4), triglycerides by 5·38 mg/dL (− 9·18 to −1·59), and HDL cholesterol increased by 1·11 mg/dL (0·48 to 1·74). Forest plots for all outcomes are presented in the appendix (pp 32–41).
Figure 4: Forest plot of LDL cholesterol.
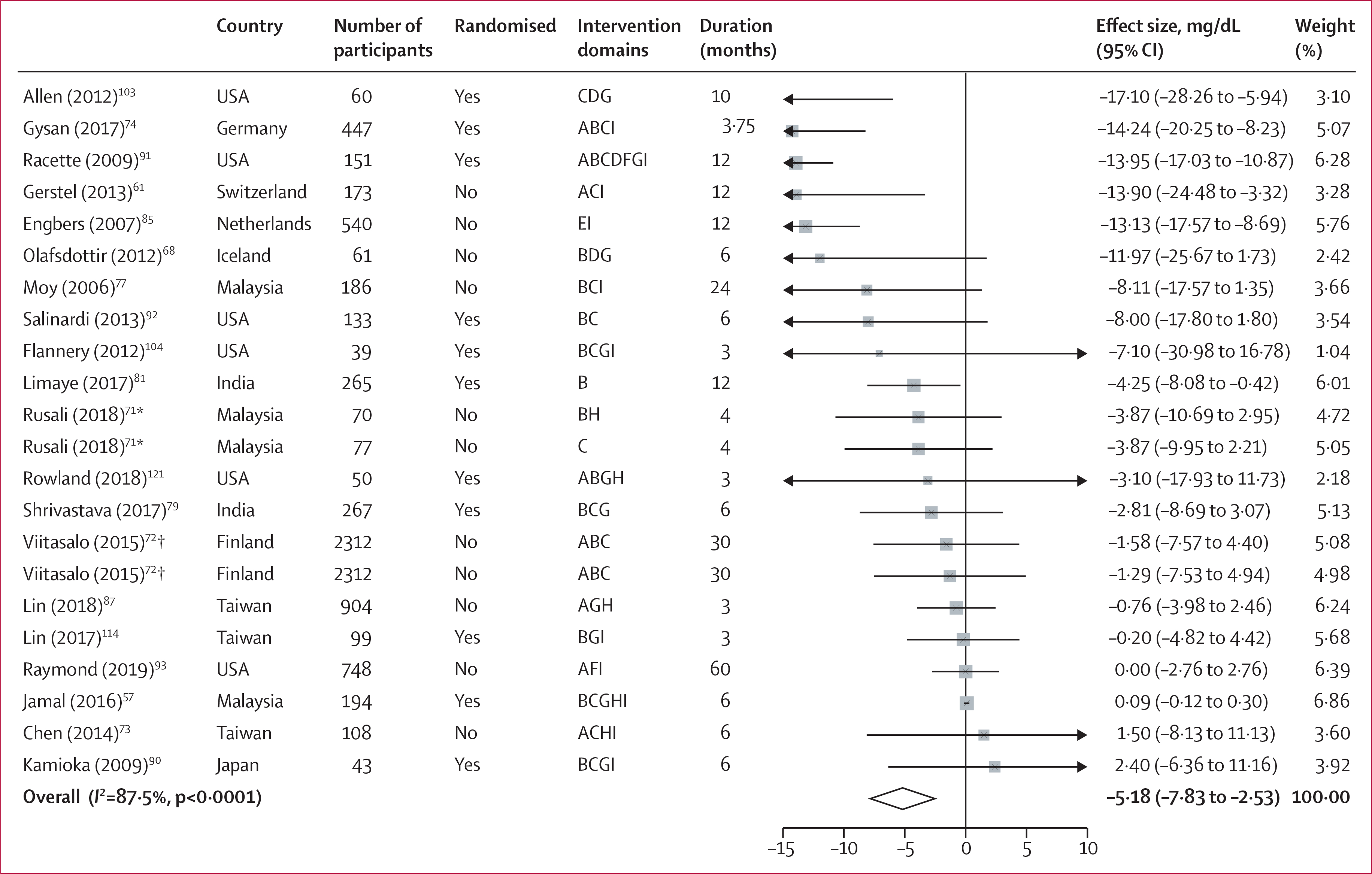
Intervention domains correspond to screening (A), individual education (B), group education (C), food environment (D), labelling (E), financial incentives (F), physical activity (G), self-awareness (H), and others (I). Weights are from random-effects analysis. *Different intervention groups from the same study (Rusali 2018).
†Different intervention groups from the same study (Viitasalo 2015).
Substantial levels of heterogeneity (I2>60%) were observed for most of the outcomes, except for fruit intake and saturated fat intake, which showed moderate heterogeneity (I2<60%). Results for prespecified sources of heterogeneity are presented in the appendix (pp 42–57). The multivariate meta-regressions corrected for multiple comparisons suggested that changes towards healthier food environments seem to be a relevant intervention component for improvements in blood lipids, particularly in LDL cholesterol (pinteraction<0·001). Stratified analysis for this finding could not be reliably done because of the simultaneity of several intervention components (eg, an intervention includes, concurrently, a food environment component such as providing free fruit at work, and a physical activity component such as using a pedometer). No other statistically significant effect modifier was identified by the exploratory analyses, indicating that the heterogeneity in several outcomes could not be explained by the potential moderating factors that we investigated here. Scores for assessment of bias were not observed to be a significant source of differential effects (appendix pp 42–44), nor a source of heterogeneity.
Visual inspection of funnel plots and Egger’s regression tests was done to assess small-study effects or publication bias (appendix pp 58–67). Neither plot asymmetry nor significant Egger’s tests were observed for several outcomes, including intakes of dietary fibre, total fat, or polyunsaturated fat, waist circumference, body fat percentage, waist-to-hip ratio, lean mass, diastolic blood pressure, fasting plasma glucose, or HDL cholesterol. Egger’s test suggested potential small-study effects or publication bias for intakes of fruits and vegetables (p=0·0004), fruits (p=0·0092), or vegetables (p=0·010), and saturated fat (p=0·046), BMI (p=0·0013), bodyweight (p=0·0068), systolic blood pressure (p=0·019), LDL cholesterol (p=0·0022), triglycerides (p=0·013), and total cholesterol (p=0·0056); further supported by visual asymmetry in the funnel plots. The trim-and-fill method was used to adjust for these potential effects and compared with the unadjusted pooled effect sizes (appendix p 68). After adjustment, the estimates of the effect of workplace wellness programmes on dietary intake of total fruits and vegetables (0·05 servings per day [95% CI −0·05 to 0·16]) and saturated fat (−0·31% of daily energy intake [−0·87 to 0·25]) were no longer statistically significant; intake of vegetables remained not significant (−0·06 servings per day [−0·13 to 0·02]) and the effect on intake of fruits (0·12 servings per day [0·11 to 0·28]) was smaller but still significant. Similarly, effect sizes were smaller but still significant for BMI (−0·12 kg/m2 [95% CI −0·18 to −0·06]) and bodyweight (−0·52 kg [−0·72 to −0·31]). Effects of workplace wellness programmes were no longer significant for systolic blood pressure (−0·06 mm Hg [95% CI −1·31 to 1·20]), LDL cholesterol (−0·41 mg/dL [−3·00 to 2·18]), triglycerides (−2·14 mg/dL [−6·39 to 2·11]), and total cholesterol (−0·30 mg/dL [−1·20 to 0·59]) after correction.
In sensitivity analysis considering randomised controlled trials only (appendix p 69), overall similar or slightly higher pooled effects were observed for dietary and anthropometric outcomes (eg, intake of fruits and vegetables 0·34 servings per day [95% CI 0·21 to 0·47]). For cardiometabolic risk factors, however, estimates were slightly smaller or became non-significant for fasting glucose, HDL cholesterol, and triglycerides. Small-study effect or publication bias was suggested for the estimates of fruits and vegetables intake combined or separately, also for anthropometric outcomes (BMI and bodyweight) and some cardiometabolic risk factors (systolic blood pressure, LDL cholesterol, and total cholesterol). After adjusting for small-study effects or publication bias by use of the trim-and-fill method, only the estimated change in intake of fruits, BMI, and bodyweight (eg, BMI −0·16 kg/m2 [95% CI −0·29 to −0·02]) remained significant (appendix p 70).
Discussion
This systematic review and meta-analysis summarised and quantified the effect of 121 multicomponent workplace wellness programmes, based on scientific evidence accumulated in interventional trials during the past 30 years, on dietary habits, anthropometrics, and cardiometabolic risk markers. In overall pooled results, our investigation found that workplace wellness programmes influence specific dietary habits, anthropometric parameters, and cardiometabolic risk factors. We identified significant unexplained heterogeneity between studies, as well as potential for small-study effects or potential publication bias, that influenced the statistical significance of changes in cardiometabolic risk markers. Although magnitudes of effects were often modest, such effects are crucial and provide meaningful risk reduction when shown across populations, as opposed to through individual-focused clinical treatment.
One of the most salient findings of our comprehensive review is the wide variation in worksite settings, employee populations, intervention components, intervention durations, and outcomes assessed in studies of workplace wellness programmes. Despite comprehensive analyses to explore these factors as potential sources of heterogeneity, no definitive drivers were identified for the outcomes. One exception was a potentially larger effect on blood LDL cholesterol when interventions included changes in the food environment. A previous narrative review of 22 studies122 identified the food environment (eg, availability of healthy foods, cost of healthy option) as one of the intervention components most often included in workplace wellness programmes, but neither quantitative pooling of studies nor quantitative evaluation of heterogeneity was done. We identified a stronger effect of food environment interventions on LDL cholesterol, and this observation should be viewed cautiously and might be a chance finding. Overall, our detailed review and pooled analyses support potential health benefits of workplace wellness programmes and highlight the need for greater standardisation of approaches and methods for assessing which intervention characteristics are most relevant for different populations and outcomes.
Previous meta-analyses of workplace wellness programmes have included far fewer studies. For example, Hwang and colleagues11 summarised ten studies and found significant effects on systolic blood pressure (0·66 mm Hg [95% CI 0·27 to 1·60]), diastolic blood pressure (0·63 mm Hg [0·21 to 1·06]), and BMI (0·71 kg/m2 [0·15 to 1·11]), but not bodyweight or LDL cholesterol. Another systematic review and meta-analysis of workplace wellness programmes focused on obesity and included only seven studies,13 finding significant reductions in BMI with short-term interventions (<6 months: −1·26 kg/m2 [95% CI −1·98 to −0·55]), but not longer-term interventions (>6 months: −1·68 kg/m2 [−4·12 to 0·76]). A third meta-analysis including 24 studies12 identified reductions in bodyweight (−2·61 kg [95% CI −3·89 to −1·33]), BMI (−0·42 kg/m2 [−0·69 to −0·15]), and waist circumference (−1·92 cm [−3·25 to −0·60]), but not blood pressure, lipids, or plasma glucose; effects on dietary habits were not evaluated. By comparison with these previous studies, our investigation of 121 workplace wellness programmes builds upon and extends the previous body of evidence by retrieving and analysing a far larger number of interventional trials, providing substantially greater statistical power, generalisability of findings, and ability to assess small-study effects.
Although several significant benefits were identified in the overall pooled findings, our analysis suggested the potential for small-study effects that influence several of these outcomes. Visual inspection of funnel plots and corresponding statistical tests cannot distinguish between true differences in efficacy of smaller versus larger studies (ie, caused by other, unidentified characteristics) versus publication bias.123 Trim-and-fill methods suggested robustness of efficacy of workplace wellness programmes for fruit intake, BMI, and bodyweight, but not necessarily for other dietary factors or clinical biometrics. A sensitivity analysis restricted to randomised controlled trials showed generally similar or stronger effects for dietary and anthropometric outcomes and slightly smaller effects for cardiometabolic outcomes, although none of these differences were significantly different from the overall pooled findings. Our novel findings emphasise the need to further understand the relevance of these potential differences in additional, large, well powered intervention studies of workplace wellness programmes.
The majority of the identified studies were from high-income countries. However, non-communicable diseases are increasing rapidly in low-income and middle-income countries.124 In line with this, we encourage the prioritisation of the development of workplace wellness programmes and their evaluation in other geographical and socioeconomic contexts. Our results highlight the generally moderate duration (about 1 year) of most workplace wellness programmes and the limited assessment of the sustainability of behaviour change after the programme ends, raising important unanswered questions about long-term effectiveness. Thus, further research with extended assessments of workplace wellness programmes should be prioritised, including assessment of the costs and cost-effectiveness of different approaches, which were often not reported in the identified studies.
This research has several strengths. This study represents the largest and most comprehensive systematic review and meta-analysis of workplace wellness programmes, allowing evaluation of various important health outcomes. We focused on multicomponent interventions, identified and recommended as most likely to be successful.16–18 We explored multiple potentially important sources of heterogeneity. Trimand-fill sensitivity analyses were done to quantify the potential influence of small-study effects of publication bias. The majority of studies were randomised, and findings were similar in sensitivity analyses restricted to randomised trials.
Limitations should be considered. Although study quality was assessed with a published tool used in previous meta-analyses, our quality assessment might differ from standardised methods such as GRADE or the Cochrane risk of bias tool. Study design was not identified as a source of heterogeneity in prespecified analysis, and studies were combined for the meta-analysis. This limitation should be considered when interpreting our pooled estimates. Most studies did not clearly report which outcomes were primary versus secondary in the analysis, raising the potential for biased reporting in the studies of outcomes with observed benefits. On the basis of the extensive nature of the review and number of identified studies, this research was limited to scientific literature published in English; the results might be less applicable to non-English socioeconomic settings, and potentially relevant evidence in other languages might not have been captured. As is common in meta-analyses, unexplained heterogeneity was present for most outcomes.
This study represents a characterisation of workplace wellness programmes across many settings and countries. In the past year, job dynamics have been disrupted globally by the COVID-19 pandemic, with unclear long-term repercussions. Our new findings serve as a benchmark and could be helpful in the context of considering and developing novel, more virtual workplace wellness programmes that address the shifting nature of work, especially remote work, after the pandemic.
In conclusion, this systematic review and meta-analysis suggests that workplace wellness programmes can improve specific dietary, anthropometric, and cardiometabolic risk indicators, supporting their use and further investigation as effective strategies to improve cardiometabolic health.
Supplementary Material
Research in context.
Evidence before this study
We did a preliminary review of scientific and policy statements and guidance from organisations such as WHO, the Institute of Medicine, US Department of Health and Human Services, and other similar international and national health agencies using synonyms and combinations of keywords for workplaces, health promotion, and health outcomes. We found extensive but dispersed evidence suggesting potential health benefits of workplace wellness programmes in improving several lifestyle and cardiometabolic risk factors among staff members. Mostly narrative reviews, and only a few quantitative meta-analyses, have been done to identify magnitudes of benefits, but these were limited to specific populations or targets and outcomes of workplace wellness programmes. Therefore, we searched PubMed-MEDLINE, Embase, Cochrane Library, Web of Science, and Education Resources Information Center, from Jan 1, 1990, to June 30, 2020, for studies with controlled evaluation designs published in English that assessed multicomponent workplace wellness programmes. We aimed to provide the most comprehensive review of workplace wellness programme designs, quantify their effectiveness in improving several risk factors and outcomes, and identify the major drivers of impact.
Added value of this study
We systematically extracted, reviewed, and catalogued information on the design and characteristics of available workplace wellness programmes that have been assessed for effectiveness in studies with controlled designs. From the 121 studies reviewed, we identified enough information to calculate pooled estimates for 20 different outcomes, of which 13 were found to be positively affected by workplace wellness programmes, especially fruit and vegetable consumption and markers of adiposity. This research compiles and provides novel findings that integrate the scientific evidence accumulated during the past 30 years on the effects of workplace wellness programmes on dietary factors, body anthropometrics, and cardiometabolic risk.
Implications of all the available evidence
International health organisations recommend workplace programmes as one of the key strategies for improving population health. Our work supports this recommendation by demonstrating that workplace programmes are associated with improvements in fruit and vegetable consumption, bodyweight, and body-mass index, and potentially improvements in cardiometabolic risk factors. Promoting a healthy diet and increasing physical activity among workers, by means of education strategies and accompanying improvements in the food environment of the workplace, were the most common elements in these interventions. These results support the implementation of workplace programmes, and highlight the need for further research to identify the most cost-effective approaches, and how to tailor these programmes in different socioeconomic contexts.
Acknowledgments
We thank Colin Rehm and Ashkan Afshin for their input and earlier contributions. Funding was obtained from the National Heart, Lung, and Blood Institute.
Declaration of interests
JS is a current employee of General Mills, a food manufacturer; her contribution to this work took place during her affiliation with Tufts University. Her salary at the time of this work was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research. RM reports grants to her institution from the Bill & Melinda Gates Foundation, Nestle, and Danone, outside the submitted work; and consulting fees from Development Initiatives, outside the submitted work. All other authors declare no competing interests.
Footnotes
Data sharing
The data used in this study may be accessible under request by contacting the corresponding author.
Contributor Information
José L Peñalvo, Non-Communicable Diseases Unit, Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium; Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Diana Sagastume, Non-Communicable Diseases Unit, Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium.
Elly Mertens, Non-Communicable Diseases Unit, Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium.
Irina Uzhova, Department of Health and Nutritional Sciences, Institute of Technology Sligo, Sligo, Ireland.
Jessica Smith, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA; Bell Institute of Health and Nutrition, General Mills, Minneapolis, MN, USA.
Jason H Y Wu, George Institute for Global Health, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia.
Eve Bishop, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Jennifer Onopa, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Peilin Shi, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Renata Micha, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA; Department of Food Science and Human Nutrition, University of Thessaly, Thessaly, Greece.
Dariush Mozaffarian, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
References
- 1.WHO. WHA57.17 Global strategy on diet, physical activity and health. The Fifty-seventh World Health Assembly. 2004. https://apps.who.int/gb/ebwha/pdf_files/WHA57/A57_R17-en.pdf (accessed Jun 22, 2021). [Google Scholar]
- 2.Quintiliani L, Sattelmair J, Activity P, Sorensen G. The workplace as a setting for interventions to improve diet and promote physical activity. Geneva, 2008. https://www.who.int/dietphysicalactivity/Quintiliani-workplace-as-setting.pdf?ua=1 (accessed Jun 22, 2021). [Google Scholar]
- 3.Cancelliere C, Cassidy JD, Ammendolia C, Côté P. Are workplace health promotion programs effective at improving presenteeism in workers? A systematic review and best evidence synthesis of the literature. BMC Public Health 2011; 11: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng L, Wolff MB, Mattick KA, DeJoy DM, Wilson MG, Smith ML. Strategies for worksite health interventions to employees with elevated risk of chronic diseases. Saf Health Work 2017; 8: 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Tackling NCDs: ‘best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases. 2017. https://apps.who.int/iris/handle/10665/259232 (accessed Jun 22, 2021).
- 6.Schliemann D, Woodside JV. The effectiveness of dietary workplace interventions: a systematic review of systematic reviews. Public Health Nutr 2019; 22: 942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandercock V, Andrade J. Evaluation of worksite wellness nutrition and physical activity programs and their subsequent impact on participants’ body composition. J Obes 2018; 2018: 1035871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassen AD, Fagt S, Lennernäs M, et al. The impact of worksite interventions promoting healthier food and/or physical activity habits among employees working ‘around the clock’ hours: a systematic review. Food Nutr Res 2018; published online Aug 2. 10.29219/fnr.v62.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weerasekara YK, Roberts SB, Kahn MA, LaVertu AE, Hoffman B, Das SK. Effectiveness of workplace weight management interventions: a systematic review. Curr Obes Rep 2016; 5: 298–306. [DOI] [PubMed] [Google Scholar]
- 10.Brown SA, García AA, Zuñiga JA, Lewis KA. Effectiveness of workplace diabetes prevention programs: a systematic review of the evidence. Patient Educ Couns 2018; 101: 1036–50. [DOI] [PubMed] [Google Scholar]
- 11.Hwang WJ, Kang SJ. Interventions to reduce the risk of cardiovascular disease among workers: a systematic review and meta-analysis. Int J Environ Res Public Health 2020; 17: 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulchandani R, Chandrasekaran AM, Shivashankar R, et al. Effect of workplace physical activity interventions on the cardio-metabolic health of working adults: systematic review and meta-analysis. Int J Behav Nutr Phys Act 2019; 16: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SH, Kim SY. Effectiveness of worksite-based dietary interventions on employees’ obesity: a systematic review and meta-analysis. Nutr Res Pract 2019; 13: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robroek SJ, Oude Hengel KM, van der Beek AJ, et al. Socio-economic inequalities in the effectiveness of workplace health promotion programmes on body mass index: an individual participant data meta-analysis. Obes Rev 2020; 21: e13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshin A, Penalvo J, Del Gobbo L, et al. CVD prevention through policy: a review of mass media, food/menu labeling, taxation/subsidies, built environment, school procurement, worksite wellness, and marketing standards to improve diet. Curr Cardiol Rep 2015; 17: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Afshin A, Benowitz NL, et al. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation 2012; 126: 1514–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Interventions on diet and physical activity. What works? Summary report. Geneva: World Health Organization, 2009. https://apps.who.int/iris/handle/10665/44140 (accessed June 22, 2021). [PubMed] [Google Scholar]
- 19.Burton J WHO healthy workplace framework and model: background and supporting literature and practices. 2010. https://apps.who.int/iris/handle/10665/113144 (accessed June 22, 2021).
- 20.Afshin A, Peñalvo JL, Del Gobbo L, et al. The prospective impact of food pricing on improving dietary consumption: a systematic review and meta-analysis. PLoS One 2017; 12: e0172277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010; 121: 2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shangguan S, Afshin A, Shulkin M, et al. A meta-analysis of food labeling effects on consumer diet behaviors and industry practices. Am J Prev Med 2019; 56: 300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–101. [PubMed] [Google Scholar]
- 25.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000; 95: 89–98. [Google Scholar]
- 26.Elliot DL, Goldberg L, Kuehl KS, Moe EL, Breger RK, Pickering MA. The PHLAME (Promoting Healthy Lifestyles: Alternative Models’ Effects) firefighter study: outcomes of two models of behavior change. J Occup Env Med 2007; 49: 204–13. [DOI] [PubMed] [Google Scholar]
- 27.Emmons KM, Linnan LA, Shadel WG, Marcus B, Abrams DB. The Working Healthy Project: a worksite health-promotion trial targeting physical activity, diet, and smoking. J Occup Env Med 1999; 41: 545–55. [DOI] [PubMed] [Google Scholar]
- 28.Bandoni DH, Sarno F, Jaime PC. Impact of an intervention on the availability and consumption of fruits and vegetables in the workplace. Public Health Nutr 2011; 14: 975–81. [DOI] [PubMed] [Google Scholar]
- 29.French SA, Harnack LJ, Hannan PJ, Mitchell NR, Gerlach AF, Toomey TL. Worksite environment intervention to prevent obesity among metropolitan transit workers. Prev Med 2010; 50: 180–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuehl KS, Elliot DL, Goldberg L, et al. The safety and health improvement: enhancing law enforcement departments study: feasibility and findings. Front Public Health 2014; 2: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosliner WA, James P, Yancey AK, Ritchie L, Studer N, Crawford PB. Impact of a worksite wellness program on the nutrition and physical activity environment of child care centers. Am J Health Promot 2010; 24: 186–89. [DOI] [PubMed] [Google Scholar]
- 32.Beresford SA, Thompson B, Feng Z, Christianson A, McLerran D, Patrick DL. Seattle 5 a Day worksite program to increase fruit and vegetable consumption. Prev Med 2001; 32: 230–38. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds KD, Gillum JL, Hyman DJ, et al. Comparing two strategies to modify dietary behavior and serum cholesterol. J Cardiovasc Risk 1997; 4: 1–5. [DOI] [PubMed] [Google Scholar]
- 34.Siegel JM, Prelip ML, Erausquin JT, Kim SA. A worksite obesity intervention: results from a group-randomized trial. Am J Public Health 2010; 100: 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almeida FA, You W, Harden SM, et al. Effectiveness of a worksite-based weight loss randomized controlled trial: the worksite study. Obesity 2015; 23: 737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassen AD, Thorsen AV, Sommer HM, et al. Improving the diet of employees at blue-collar worksites: results from the ‘Food at Work’ intervention study. Public Health Nutr 2011; 14: 965–74. [DOI] [PubMed] [Google Scholar]
- 37.Ostbye T, Stroo M, Brouwer RJN, et al. Steps to Health employee weight management randomized control trial: short-term follow-up results. J Occup Environ Med 2015; 57: 188–95. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen G, Stoddard A, Peterson K, et al. Increasing fruit and vegetable consumption through worksites and families in the treatwell 5-a-day study. Am J Public Health 1999; 89: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell MK, Tessaro I, DeVellis B, et al. Effects of a tailored health promotion program for female blue-collar workers: health works for women. Prev Med 2002; 34: 313–23. [DOI] [PubMed] [Google Scholar]
- 40.LaCaille LJ, Schultz JF, Goei R, et al. Go!: results from a quasi-experimental obesity prevention trial with hospital employees. BMC Public Health 2016; 16: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen G, Thompson B, Glanz K, et al. Work site-based cancer prevention: primary results from the Working Well Trial. Am J Public Health 1996; 86: 939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engbers LH, van Poppel MN, Chin APM, van Mechelen W. The effects of a controlled worksite environmental intervention on determinants of dietary behavior and self-reported fruit, vegetable and fat intake. BMC Public Health 2006; 6: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson MG, Dejoy DM, Vandenberg R, Padilla H, Davis M. FUEL Your Life: a translation of the diabetes prevention program to worksites. Am J Health Promot 2016; 30: 188–97. [DOI] [PubMed] [Google Scholar]
- 44.Steenhuis I, Van Assema P, Van Breukelen G, Glanz K, Kok G, De Vries H. The impact of educational and environmental interventions in Dutch worksite cafeterias. Health Promot Int 2004; 19: 335–43. [DOI] [PubMed] [Google Scholar]
- 45.Hutchinson AD, Howlett G, Wilson C. Increasing employees’ fruit consumption through access and peer support at work. Food Nutr Sci 2013; 4: 88–95. [Google Scholar]
- 46.Viester L, Verhagen E, Bongers PM, van der Beek AJ. Effectiveness of a worksite intervention for male construction workers on dietary and physical activity behaviors, body mass index, and health outcomes: results of a randomized controlled trial. Am J Health Promot 2018; 32: 795–805. [DOI] [PubMed] [Google Scholar]
- 47.Strijk JE, Proper KI, van der Beek AJ, van Mechelen W. A worksite vitality intervention to improve older workers’ lifestyle and vitality-related outcomes: results of a randomised controlled trial. J Epidemiol Community Health 2012; 66: 1071–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan PJ, Collins CE, Plotnikoff RC, et al. Efficacy of a workplace-based weight loss program for overweight male shift workers: the Workplace POWER (Preventing Obesity Without Eating like a Rabbit) randomized controlled trial. Prev Med (Baltim) 2011; 52: 317–25. [DOI] [PubMed] [Google Scholar]
- 49.van Berkel J, Boot CR, Proper KI, Bongers PM, van der Beek AJ. Effectiveness of a worksite mindfulness-based multi-component intervention on lifestyle behaviors. Int J Behav Nutr Phys Act 2014; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt MK, Hebert JR, Sorensen G, et al. Impact of a worksite cancer prevention program on eating patterns of workers. J Nutr Educ 1993; 25: 236–44. [Google Scholar]
- 51.Wierenga D, Engbers LH, Van Empelen P, et al. The implementation of multiple lifestyle interventions in two organizations: a process evaluation. J Occup Env Med 2014; 56: 1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kushida O, Murayama N. Effects of environmental intervention in workplace cafeterias on vegetable consumption by male workers. J Nutr Educ Behav 2014; 46: 350–58. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Wu X, Gu D. Hypertension and cardiovascular diseases intervention in the capital steel and iron company and Beijing Fangshan community. Obes Rev 2008; 9 (suppl 1): 142–45. [DOI] [PubMed] [Google Scholar]
- 54.Fitzgerald S, Buckley L, Perry IJ, et al. The impact of a complex workplace dietary intervention on Irish employees’ off-duty dietary intakes. Health Promot Int 2020; 35: 544–54. [DOI] [PubMed] [Google Scholar]
- 55.Geaney F, Kelly C, Di Marrazzo JS, et al. The effect of complex workplace dietary interventions on employees’ dietary intakes, nutrition knowledge and health status: a cluster controlled trial. Prev Med 2016; 89: 76–83. [DOI] [PubMed] [Google Scholar]
- 56.Sorensen G, Morris DM, Hunt MK, et al. Work-site nutrition intervention and employees’ dietary habits: the Treatwell program. Am J Public Health 1992; 82: 877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamal SN, Moy FM, Mohamed MNA, Mukhtar F. Effectiveness of a Group Support Lifestyle Modification (GSLiM) programme among obese adults in workplace: a randomised controlled trial. PLoS One 2016; 11: e0160343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moy FM, Ab Sallam A, Wong ML. Dietary modification in a workplace health promotion program in Kuala Lumpur, Malaysia. Asia Pac J Public Health 2008; 20 (suppl): 166–72. [PubMed] [Google Scholar]
- 59.Wilson MG, DeJoy DM, Vandenberg RJ, Corso P, Padilla H, Zuercher H. Effect of intensity and program delivery on the translation of diabetes prevention program to worksites: a randomized controlled trial of Fuel Your Life. J Occup Environ Med 2016; 58: 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braeckman L, De Bacquer D, Maes L, De Backer G. Effects of a low-intensity worksite-based nutrition intervention. Occup Med (Lond) 1999; 49: 549–55. [DOI] [PubMed] [Google Scholar]
- 61.Gerstel E, Pataky Z, Busnel C, et al. Impact of lifestyle intervention on body weight and the metabolic syndrome in home-care providers. Diabetes Metab 2013; 39: 78–84. [DOI] [PubMed] [Google Scholar]
- 62.Hebert JR, Harris DR, Sorensen G, Stoddard AM, Hunt MK, Morris DH. A work-site nutrition intervention: its effects on the consumption of cancer-related nutrients. Am J Public Health 1993; 83: 391–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu T, Horiguchi I, Kato T, Nagata S. Relationship between an interview-based health promotion program and cardiovascular risk factors at Japanese companies. J Occup Health 2004; 46: 205–12. [DOI] [PubMed] [Google Scholar]
- 64.Lemon SC, Zapka J, Li W, et al. Step ahead a worksite obesity prevention trial among hospital employees. Am J Prev Med 2010; 38: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furuki K, Honda S, Jahng D, Ikeda M, Okubo T. The effects of a health promotion program on body mass index. J Occup Health 1999; 41: 19–26. [Google Scholar]
- 66.Iriyama Y, Murayama N. Effects of a worksite weight-control programme in obese male workers: a randomized controlled crossover trial. Health Educ J 2014; 73: 247–61. [Google Scholar]
- 67.Sforzo GA, Kaye MP, Calleri D, Ngai N. Free choice access to multipoint wellness education and related services positively impacts employee wellness: a randomized and controlled trial. J Occup Env Med 2012; 54: 471–77. [DOI] [PubMed] [Google Scholar]
- 68.Olafsdottir AS, Johannsdottir SS, Arngrimsson SA, Johannsson E. Lifestyle intervention at sea changes body composition, metabolic profile and fitness. Public Health 2012; 126: 888–90. [DOI] [PubMed] [Google Scholar]
- 69.Goetzel RZ, Roemer EC, Pei X, et al. Second-year results of an obesity prevention program at the Dow Chemical Company. J Occup Environ Med 2010; 52: 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johanning E, Landsbergis P, Geissler H, Karazmann R. Cardiovascular risk and back-disorder intervention study of mass transit operators. Int J Occup Environ Health 1996; 2: 79–87. [DOI] [PubMed] [Google Scholar]
- 71.Rusali R Comparison of the effectiveness of online and face-to-face weight-loss interventations in the workplace: evidence from Malaysia. Sains Malaysiana 2018; 47: 2437–45. [Google Scholar]
- 72.Viitasalo K, Hemio K, Puttonen S, et al. Prevention of diabetes and cardiovascular diseases in occupational health care: feasibility and effectiveness. Prim Care Diabetes 2015; 9: 96–104. [DOI] [PubMed] [Google Scholar]
- 73.Chen MM, Tsai AC, Wang JY. The effectiveness and barriers of implementing a workplace health promotion program to improve metabolic disorders in older workers in Taiwan. Glob Health Promot 2014; 23: 6–14. [DOI] [PubMed] [Google Scholar]
- 74.Gysan DB, Millentrup S, Albus C, et al. Substantial improvement of primary cardiovascular prevention by a systematic score-based multimodal approach: a randomized trial: the PreFord-Study. Eur J Prev Cardiol 2017; 24: 1544–54. [DOI] [PubMed] [Google Scholar]
- 75.Atlantis E, Chow CM, Kirby A, Fiatarone Singh MA. Worksite intervention effects on physical health: a randomized controlled trial. Health Promot Int 2006; 21: 191–200. [DOI] [PubMed] [Google Scholar]
- 76.Song Z, Baicker K. Effect of a workplace wellness program on employee health and economic outcomes: a randomized clinical trial. JAMA 2019; 321: 1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moy F, Sallam AA, Wong M. The results of a worksite health promotion programme in Kuala Lumpur, Malaysia. Health Promot Int 2006; 21: 301–10. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez ID, Chin NP, Devine CM, et al. Images of a healthy worksite: a group-randomized trial for worksite weight gain prevention with employee participation in intervention design. Am J Public Health 2015; 105: 2167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shrivastava U, Fatma M, Mohan S, Singh P, Misra A. Randomized control trial for reduction of body weight, body fat patterning, and cardiometabolic risk factors in overweight worksite employees in Delhi, India. J Diabetes Res 2017; 2017: 7254174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tucker S, Farrington M, Lanningham-Foster LM, et al. Worksite physical activity intervention for ambulatory clinic nursing staff. Workplace Health Saf 2016; 64: 313–25. [DOI] [PubMed] [Google Scholar]
- 81.Limaye T, Kumaran K, Joglekar C, et al. Efficacy of a virtual assistance-based lifestyle intervention in reducing risk factors for type 2 diabetes in young employees in the information technology industry in India: LIMIT, a randomized controlled trial. Diabet Med 2017; 34: 563–68. [DOI] [PubMed] [Google Scholar]
- 82.Doran K, Resnick B, Zhu S. Testing the impact of the Worksite Heart Health Improvement Project on cardiovascular disease risk factors over time. J Occup Environ Med 2018; 60: 717–23. [DOI] [PubMed] [Google Scholar]
- 83.Lemon SC, Wang ML, Wedick NM, et al. Weight gain prevention in the school worksite setting: results of a multi-level cluster randomized trial. Prev Med 2014; 60: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwak L, Kremers SP, Candel MJ, Visscher TL, Brug J, van Baak MA. Changes in skinfold thickness and waist circumference after 12 and 24 months resulting from the NHF-NRG In Balance-project. Int J Behav Nutr Phys Act 2010; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engbers LH, van Poppel MN, van Mechelen W. Modest effects of a controlled worksite environmental intervention on cardiovascular risk in office workers. Prev Med 2007; 44: 356–62. [DOI] [PubMed] [Google Scholar]
- 86.Meenan RT, Vogt TM, Williams AE, Stevens VJ, Albright CL, Nigg C. Economic evaluation of a worksite obesity prevention and intervention trial among hotel workers in Hawaii. J Occup Environ Med 2010; 52 (suppl 1): S8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin TY, Liao PJ, Ting MK, Hsu KH. Lifestyle characteristics as moderators of the effectiveness of weight control interventions among semiconductor workers. Biomed J 2018; 41: 376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cook C, Simmons G, Swinburn B, Stewart J. Changing risk behaviours for non-communicable disease in New Zealand working men--is workplace intervention effective? N Z Med J 2001; 114: 175–78. [PubMed] [Google Scholar]
- 89.Faghri PD, Li R. Effectiveness of financial incentives in a worksite diabetes prevention program. Open Obes J 2014; 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamioka H, Nakamura Y, Okada S, et al. Effectiveness of comprehensive health education combining lifestyle education and hot spa bathing for male white-collar employees: a randomized controlled trial with 1-year follow-up. J Epidemiol 2009; 19: 219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Racette SB, Deusinger SS, Inman CL, et al. Worksite Opportunities for Wellness (WOW): effects on cardiovascular disease risk factors after 1 year. Prev Med 2009; 49: 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salinardi TC, Batra P, Roberts SB, et al. Lifestyle intervention reduces body weight and improves cardiometabolic risk factors in worksites. Am J Clin Nutr 2013; 97: 667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raymond LW, Roy DM, Mullinax SL, Yanni A, Pentek KC, Isaacs SE. Preventing diabetes in the workplace: effects of coaching and monetary incentives. J Occup Environ Med 2019; 61: e308–11. [DOI] [PubMed] [Google Scholar]
- 94.Williams AE, Stevens VJ, Albright CL, Nigg CR, Meenan RT, Vogt TM. The results of a 2-year randomized trial of a worksite weight management intervention. Am J Health Promot 2014; 28: 336–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scoggins JF, Sakumoto KN, Schaefer KS, Bascom B, Robbins DJ, Whalen CL. Short-term and long-term weight management results of a large employer-sponsored wellness program. J Occup Env Med 2011; 53: 1215–20. [DOI] [PubMed] [Google Scholar]
- 96.Jaime PC, Bandoni DH, Sarno F. Impact of an education intervention using email for the prevention of weight gain among adult workers. Public Health Nutr 2014; 17: 1620–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linde JA, Nygaard KE, MacLehose RF, et al. HealthWorks: results of a multi-component group-randomized worksite environmental intervention trial for weight gain prevention. Int J Behav Nutr Phys Act 2012; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mache S, Jensen S, Jahn R, Steudtner M, Ochsmann E, Preuss G. Worksite health program promoting changes in eating behavior and health attitudes. Health Promot Pract 2015; 16: 826–36. [DOI] [PubMed] [Google Scholar]
- 99.Addley K, Boyd S, Kerr R, McQuillan P, Houdmont J, McCrory M. The impact of two workplace-based health risk appraisal interventions on employee lifestyle parameters, mental health and work ability: results of a randomized controlled trial. Health Educ Res 2014; 29: 247–58. [DOI] [PubMed] [Google Scholar]
- 100.Mansi S, Milosavljevic S, Tumilty S, Hendrick P, Higgs C, Baxter DG. Investigating the effect of a 3-month workplace-based pedometer-driven walking programme on health-related quality of life in meat processing workers: a feasibility study within a randomized controlled trial. BMC Public Health 2015; 15: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeffery RW, Forster JL, French SA, et al. The Healthy Worker Project: a work-site intervention for weight control and smoking cessation. Am J Public Health 1993; 83: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kouwenhoven-Pasmooij TA, Robroek SJW, Kraaijenhagen RA, et al. Effectiveness of the blended-care lifestyle intervention ‘PerfectFit’: a cluster randomised trial in employees at risk for cardiovascular diseases. BMC Public Health 2018; 18: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen JC, Lewis JB, Tagliaferro AR. Cost-effectiveness of health risk reduction after lifestyle education in the small workplace. Prev Chronic Dis 2012; 9: E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flannery K, Resnick B, Galik E, Lipscomb J, McPhaul K, Shaughnessy M. The Worksite Heart Health Improvement Project (WHHIP): feasibility and efficacy. Public Health Nurs 2012; 29: 455–66. [DOI] [PubMed] [Google Scholar]
- 105.Vilela BL, Silva AAB, de Lira CAB, Andrade MD. Workplace exercise and educational program for improving fitness outcomes related to health in workers: a randomized controlled trial. J Occup Environ Med 2015; 57: 235–40. [DOI] [PubMed] [Google Scholar]
- 106.Ribeiro MA, Martins MA, Carvalho CRF. Interventions to increase physical activity in middle-age women at the workplace: a randomized controlled trial. Med Sci Sports Exerc 2014; 46: 1008–15. [DOI] [PubMed] [Google Scholar]
- 107.Cawley J, Price JA. Outcomes in a program that offers financial rewards for weight loss. Cambridge, MA, USA: National Bureau of Economic Research, 2009. [Google Scholar]
- 108.Kwak L, Kremers SP, Visscher TL, van Baak MA, Brug J. Behavioral and cognitive effects of a worksite-based weight gain prevention program: the NHF-NRG in balance-project. J Occup Env Med 2009; 51: 1437–46. [DOI] [PubMed] [Google Scholar]
- 109.Thompson WG, Koepp GA, Levine JA. Increasing physician activity with treadmill desks. Work 2014; 48: 47–51. [DOI] [PubMed] [Google Scholar]
- 110.Hossain M, Islam Z, Sultana S, et al. Effectiveness of workplace nutrition programs on anemia status among female readymade garment workers in Bangladesh: a program evaluation. Nutrients 2019; 11: 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robbins AS, Chao SY, Baumgartner N, Runyan CN, Oordt MS, Fonseca VP. A low-intensity intervention to prevent annual weight gain in active duty Air Force members. Mil Med 2006; 171: 556–61. [DOI] [PubMed] [Google Scholar]
- 112.Prabhakaran D, Jeemon P, Goenka S, et al. Impact of a worksite intervention program on cardiovascular risk factors: a demonstration project in an Indian industrial population. J Am Coll Cardiol 2009; 53: 1718–28. [DOI] [PubMed] [Google Scholar]
- 113.Stites SD, Singletary SB, Menasha A, et al. Pre-ordering lunch at work. Results of the what to eat for lunch study. Appetite 2015; 84: 88–97. [DOI] [PubMed] [Google Scholar]
- 114.Lin YP, Lin CC, Chen MM, Lee KC. Short-term efficacy of a “sit less, walk more” workplace intervention on improving cardiometabolic health and work productivity in office workers. J Occup Environ Med 2017; 59: 327–34. [DOI] [PubMed] [Google Scholar]
- 115.Balk-Møller NC, Poulsen SK, Larsen TM. Effect of a nine-month web- and app-based workplace intervention to promote healthy lifestyle and weight loss for employees in the social welfare and health care sector: a randomized controlled trial. J Med Internet Res 2017; 19: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danquah IH, Kloster S, Holtermann A, et al. Take a Stand! A multicomponent intervention aimed at reducing sitting time among office workers-a cluster randomized trial. Int J Epidemiol 2017; 46: 128–40. [DOI] [PubMed] [Google Scholar]
- 117.Pedersen C, Halvari H, Williams GC. Worksite intervention effects on motivation, physical activity, and health: a cluster randomized controlled trial. Psychol Sport Exerc 2018; 35: 171–80. [Google Scholar]
- 118.Choi YS, Song R, Ku BJ. Effects of a t’ai chi-based health promotion program on metabolic syndrome markers, health behaviors, and quality of life in middle-aged male office workers: a randomized trial. J Altern Complement Med 2017; 23: 949–56. [DOI] [PubMed] [Google Scholar]
- 119.Ryu H, Jung J, Cho J, Chin DL. Program development and effectiveness of workplace health promotion program for preventing metabolic syndrome among office workers. Int J Environ Res Public Health 2017; 14: 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindquist T, Cooper C. Using lifestyle and coping to reduce job stress and improve health in “at risk” office workers. Stress Med 1999; 15: 143–52. [Google Scholar]
- 121.Rowland SA, Berg KE, Kupzyk KA, et al. Feasibility and effect of a peer modeling workplace physical activity intervention for women. Workplace Health Saf 2018; 66: 428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clohessy S, Walasek L, Meyer C. Factors influencing employees ‘ eating behaviours in the office-based workplace: a systematic review. Obes Rev 2019; 20: 1771–80. [DOI] [PubMed] [Google Scholar]
- 123.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 124.WHO. Regional high-level consultation in the Eastern Mediterranean region on the prevention and control of non-communicable diseases in low-and middle-income countries. 2010. https://www.who.int/nmh/events/2010/Tehran_Background_Paper.pdf (accessed June 22, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


