ABSTRACT
Hepatic lipid homeostasis depends on intracellular pathways that respire fatty acid in peroxisomes and mitochondria, and on systemic pathways that secrete fatty acid into the bloodstream, either free or condensed in very-low-density lipoprotein (VLDL) triglycerides. These systemic and intracellular pathways are interdependent, but it is unclear whether and how they integrate into a single cellular circuit. Here, we report that mouse liver wrappER, a distinct endoplasmic reticulum (ER) compartment with apparent fatty acid- and VLDL-secretion functions, connects peroxisomes and mitochondria. Correlative light electron microscopy, quantitative serial section electron tomography and three-dimensional organelle reconstruction analysis show that the number of peroxisome-wrappER-mitochondria complexes changes throughout fasting-to-feeding transitions and doubles when VLDL synthesis stops following acute genetic ablation of Mttp in the liver. Quantitative proteomic analysis of peroxisome-wrappER-mitochondria complex-enriched fractions indicates that the loss of Mttp upregulates global fatty acid β-oxidation, thereby integrating the dynamics of this three-organelle association into hepatic fatty acid flux responses. Therefore, liver lipid homeostasis occurs through the convergence of systemic and intracellular fatty acid-elimination pathways in the peroxisome-wrappER-mitochondria complex.
KEY WORDS: Peroxisome, Mitochondria, Inter-organelle contacts, Fatty acid, Liver lipid homeostasis
Summary: In liver hepatocytes, the wrappER, a wrapping type of rough ER, simultaneously connects mitochondria and peroxisomes to form a three-organelle complex that responds to changes in hepatic lipid flux.
INTRODUCTION
The liver receives large amounts of fatty acid during the postprandial phase, from the diet and from the adipose tissue during fasting. When in excess, fatty acid elimination occurs through a combination of events, including respiration in mitochondria and peroxisomes (Lodish et al., 2000), secretion into the bloodstream (bound to lipocalins and albumin), and condensation as triglycerides, which are then accumulated as lipid droplet or secreted as very-low-density lipoprotein (VLDL). A single pool of hepatic fatty acid feeds these processes, but the cellular basis linking fatty acid respiration to fatty acid secretion remains poorly understood.
Critical intracellular and systemic cellular functions originate at sites where two organelles either adhere or juxtapose at a distance of 10 to 60 nm (Giacomello and Pellegrini, 2016; Scorrano et al., 2019; Vance, 2020). Studies on inter-organelle contacts revealed that most membrane-bound and membraneless organelles establish contacts with the endoplasmic reticulum (ER) (Csordás et al., 2018; Desai et al., 2020; Lee et al., 2020; Phillips and Voeltz, 2016), a heterogeneous collection of morphologically distinct tubular and sheet-like membrane compartments with distinctive proteomic and lipid signatures (Anastasia et al., 2021; Carter et al., 2020; Hoffman et al., 2020; Nixon-Abell et al., 2016). One of the various ER types is the wrappER, a curved wrapping type of rough ER widely present in hepatocytes and enterocytes, in which it plays a crucial role in systemic lipid homeostasis (Anastasia et al., 2021). The wrappER accumulates fatty acid and fatty acid-binding proteins of the lipocalin family; further, it produces VLDL and establishes extensive contacts with nearly every mitochondrion of the liver hepatocyte to regulate intracellular and systemic lipid flux (Anastasia et al., 2021). However, whether the function of the wrappER in hepatic lipid metabolism occurs through the contacts with other organelles remains unknown.
Here, we report that, in mouse livers, the wrappER contacts both mitochondria and peroxisomes to form a three-organelle complex that responds to physiological and pathological hepatic lipid flux changes. The physical and dynamic association between a VLDL-producing type of ER and the two fatty acid-respiring organelles of the cell indicates that hepatic lipid homeostasis occurs by integrating into a single multi-organelle complex the cellular platforms that decrease the fatty acid content via systemic and intracellular pathways.
RESULTS
WrappER-associated peroxisomes in mouse liver hepatocytes
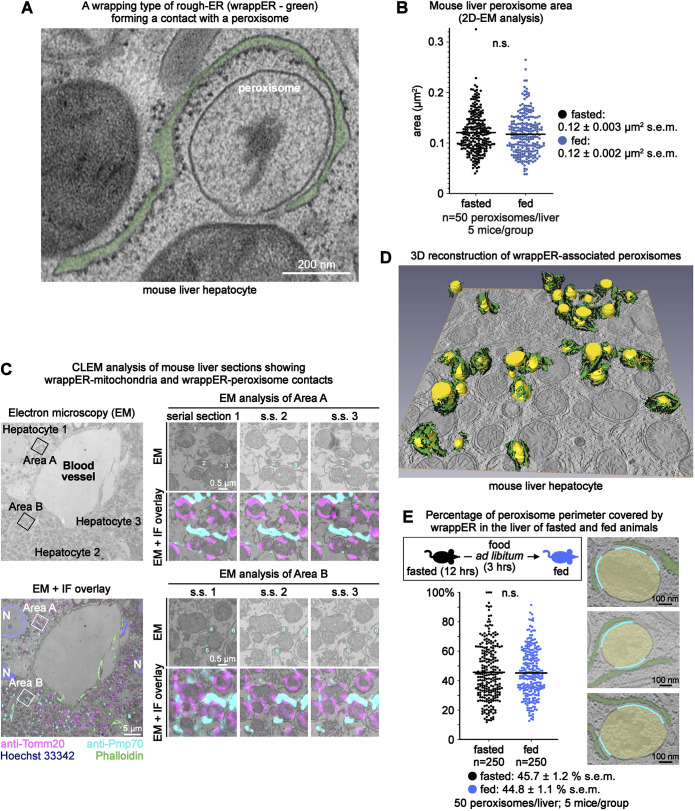
Electron microscopy analysis of mouse liver hepatocytes showed curved sheets of rough ER wrapped around organelles with the typical shape and size of peroxisomes (Fig. 1A,B). These organelles also contained a large electron-dense area of irregular shape (Fig. 1A), a signature electron microscope feature of peroxisomes. Correlative light-electron microscopy (CLEM) analysis of mouse liver sections immunolabelled with anti-Pmp70, a peroxisomal marker, confirmed that these organelles were peroxisomes (Fig. 1C). Further, it revealed that the wrapping type of rough ER surrounding peroxisomes established contacts with Tomm-20-labelled mitochondria (Fig. 1C), which qualified it to be the wrappER (Anastasia et al., 2021).
Fig. 1.
Extensive WrappER-peroxisome contacts in mouse liver hepatocytes. (A) Transmission electron microscope image showing a wrappER-peroxisome contact. (B) Dot plot of the data collected by the quantitative electron microscope (EM) morphometric analysis showing peroxisome area in the liver of fasted (14 h) and fed mice (3 h postprandial). (C) CLEM analysis of mouse liver hepatocytes showing wrappER-mitochondria and wrappER-peroxisome contacts. The lower left panel shows an overlay of electron microscope and immunofluorescence (IF) images. The right panels show high magnification images of peroxisomes and mitochondria from the indicated areas. (D) SSET plus three-dimensional reconstruction analysis of wrapping rough ER (wrappER; green, with black ribosomes) associated with peroxisomes (yellow). (E) Percentage of peroxisome perimeter covered by wrappER in hepatocytes of livers collected from fasted and fed mice. In the electron microscope images on the right, the cyan line labels the portion of the peroxisome perimeter that is in contact with the wrappER. The P values were calculated by a two-tailed, unpaired Student's t-test. Data were collected from mouse livers at 3 h postprandial, unless otherwise indicated.
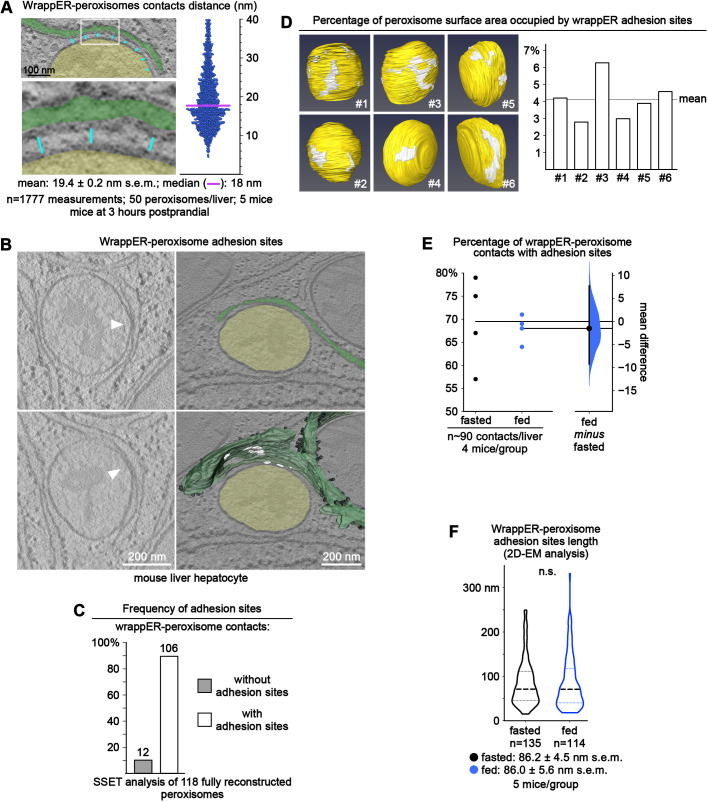
To confirm this finding, we studied the wrappER-peroxisome contact structure by analyzing a large portion of the cell volume of the hepatocytes by serial section electron tomography (SSET) coupled to three-dimensional reconstruction. We found that almost every peroxisome was extensively associated with the wrappER (Fig. 1D; Movie 1). Quantitative electron microscope morphometric analysis supported this observation by showing that, on average, ∼44% of the peroxisome perimeter was surrounded by wrappER, a value that remained unchanged irrespective of the nutritional status of the animal (n=250 peroxisomes; 5 mice/group; Fig. 1E). To further the ultrastructural characterization of the wrappER-peroxisome contact, we combined quantitative electron microscopy approaches with SSET imaging studies. These studies showed that the wrappER-peroxisome contact consists of three distinct domains. The first comprises short-range ribosome-free inter-organelle juxtapositions; the median distance separating the two organelles is ∼18 nm (MAM-like; n=1777 measurements from 250 peroxisomes; Fig. 1A, Fig. 2A) (Giacomello and Pellegrini, 2016; Sood et al., 2014; Vance, 1990). The second type of wrappER-peroxisome contact domain is characterized by medium-range (30-60 nm) inter-organelle juxtapositions and by the presence of ribosomes (Fig. 1A). The third domain consists of sites where the membranes of the two organelles physically adhere to each other (Fig. 2B). These previously unreported adhesion sites are similar to those occurring between wrappER and mitochondria (Anastasia et al., 2021), the function of which remains unknown.
Fig. 2.
The wrappER-peroxisome contact contains MAM-like domains and adhesion sites. (A) Dot plot of the wrappER-peroxisome contact distance; the line denotes the median distance separating these organelles (18 nm). This analysis was completed by measuring the distance between wrappER and peroxisome every 100 nm (cyan lines), as depicted in the left panels. Ribosome-containing long-range contacts (40-60 nm) were not included in this analysis. (B) SSET analysis showing that the wrappER-peroxisome contact contains adhesion sites. The images on the left are two consecutive virtual slices from a tomogram showing sites of physical contact (adhesion sites, white arrows) between wrappER (green) and peroxisome (yellow). Lower right: three-dimensional reconstruction of the wrappER with the adhesion sites (white areas). (C) Frequency of adhesion sites in wrappER-peroxisome contacts. The data were collected from SSET analysis of 118 fully reconstructed peroxisomes that have a contact with the wrappER (from ten tomograms). (D) Area occupied by wrappER peroxisome adhesion sites on the surface of six peroxisomes. The data were collected from SSET plus three-dimensional reconstruction analysis. Data are expressed as a percentage (4.1±1.3%; mean±s.d.). (E) Fasting-to-feeding transition does not change the percentage of wrappER-peroxisome contacts with ≥1 adhesion site (n=∼90 wrappER-peroxisome contacts/liver, five mice/group; each dot indicates the value per liver). Data were analyzed by estimation statistics and plotted in a Gardner–Altman graph; the mean difference is indicated by the dot on the right panel and is plotted as a bootstrap sampling distribution. The 95% c.i. is indicated by the vertical error bar (Bernard, 2019; Ho et al., 2019). (F) Violin plot of the data collected by quantitative electron microscope (EM) morphometric analysis investigating the average length of wrappER-peroxisome adhesion sites in the liver of fasted and fed mice (n=135 measurements, five mice/group). The P value was calculated by a two-tailed, unpaired Student's t-test. Data were collected from mouse livers at 3 h postprandial, unless otherwise indicated.
The SSET analysis of 118 fully reconstructed peroxisomes showed that ∼90% of wrappER-peroxisome contacts contained at least one adhesion site (Fig. 2C), which, on average, occupied 4.1±1.3% (mean±s.d.) of the peroxisome surface area (n=6 fully reconstructed peroxisomes; Fig. 2D). Multiple adhesion sites could populate a single wrappER-peroxisome contact (Fig. 2B; Movie 2). Still, neither their number nor their size appeared to change during fasting-to-feeding transitions (Fig. 2E,F). Collectively, these data show that, in mouse liver hepatocytes, wrappER and peroxisomes engage in stable and extensive contacts that harbour ribosome-free short-range (MAM-like) domains, ribosome-containing inter-organelle juxtapositions and adhesion sites where the membranes of the two organelles are in touch but not fused. Such structure is remarkably similar to that observed in wrappER-mitochondria contacts (Anastasia et al., 2021), and is unique among the various types of contacts that other types of ER establish with other cellular organelles.
The wrappER organizes peroxisomes and mitochondria into a three-organelle complex
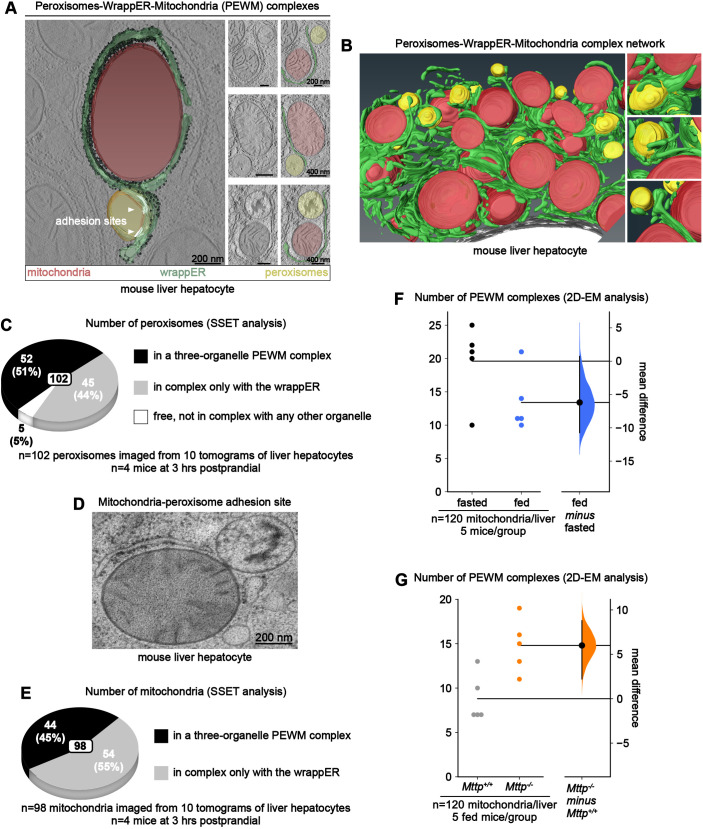
The association of mitochondria and peroxisomes to a single sheet of wrappER (Fig. 1C) suggests the possibility that this type of ER may organize these organelles into a single complex. To address this possibility, we returned to SSET analysis to visualize them simultaneously reconstructed in three dimensions. This approach allowed us to observe that these organelles can be organized into a single peroxisome-wrappER-mitochondria (PEWM) complex (Fig. 3A; Movie 2) that is seamlessly integrated within the ER network of the hepatocyte (Fig. 3B; Movie 3). However, although almost all peroxisomes were associated with the wrappER, only a fraction of them appeared to be recruited within PEWM complexes (Movie 3).
Fig. 3.
Peroxisome-wrappER-mitochondria complex dynamics are regulated by hepatic lipid flux. (A) Left: SSET plus three-dimensional analysis of a PEWM complex. Right: electron microscope images of PEWM complexes. (B) SSET plus three-dimensional analysis of PEWM complexes integrated in the ER network of a mouse liver hepatocyte. (C,E) Percentage of fully reconstructed peroxisomes (C) and mitochondria (E) that are engaged in PEWM complexes. These data were obtained by visually inspecting tomograms obtained through SSET analysis. (D) Example of a peroxisome-mitochondria adhesion site; this type of contact is rarely observed in both fasting and 3 h postprandial mouse livers. (F,G) Fasting-to-feeding transition (F) and loss of Mttp expression in the liver (G) changes the number of PEWM complexes (each dot indicates the number of PEWM complexes observed per liver). This data was obtained by counting, on liver hepatocyte electron microscope (EM) images, the number of wrappER-associated mitochondria that are in complex also with a peroxisome, as shown in A. Data were then analyzed by estimation statistics and plotted in a Gardner–Altman graph; the mean difference is indicated by the dot on the right panel and is plotted as a bootstrap sampling distribution. The 95% c.i. is indicated by the vertical error bar. Data were collected from mouse livers at 3 h postprandial, unless otherwise indicated.
This observation prompted us to estimate the proportion of peroxisomes in a three-organelle PEWM complex. To this end, we conducted extensive SSET analyses to visualize and inspect 102 peroxisomes. This study revealed that, at 3 h postprandial, these organelles were distributed in three distinct pools. In the first pool, 50% of the peroxisomal population was organized into a three-organelle complex with wrappER and mitochondria. In the second pool, 45% of the peroxisomes formed a two-organelle complex with the wrappER. The third pool of peroxisomes consisted of organelles that were free from any type of inter-organelle contact; they amounted to ∼5% of the peroxisomal population. Of note, none of these 102 peroxisomes made direct physical contact with mitochondria; in fact, this type of contact is extremely rare to observe even in traditional electron microscopy analysis, in which a larger cohort of peroxisomes was investigated (Fig. 3D).
We also used SSET analyses to estimate the share of mitochondrial population organized in PEWM complexes by visually inspecting 98 mitochondria. This study showed that ∼50% of the mitochondria were part of a PEWM complex (Fig. 3E). Collectively, these observations show that, at 3 h postprandial, the wrappER organizes nearly 50% of the liver mitochondrial and peroxisomal populations into three-organelle PEWM complexes.
Changes in hepatic fatty acid flux regulate peroxisome-wrappER-mitochondria complex dynamics
It is well established that although during fasting fatty acid absorption and VLDL synthesis and secretion reach a maximum level, these hepatic activities are substantially reduced upon feeding (Wei et al., 2010). We recently showed that the wrappER-mitochondria contacts are dynamic because they are regulated by changes in the hepatic lipid flux, such as those associated with fasting-to-feeding transitions (Anastasia et al., 2021). However, the wrappER-peroxisome contact does not appear to be subject to the same type of control. Indeed, unlike what was observed for the wrappER-mitochondria contact (Anastasia et al., 2021), neither the extent of the contact between wrappER and peroxisome (Fig. 1E) nor the number of adhesion sites between these organelles changes during the transition from the fasting to the fed state (Fig. 2E,F). However, because both mitochondria and peroxisomes are capable of β-oxidative respiration, we hypothesized that changes in fatty acid flux might rather change the pool of peroxisomes recruited within the PEWM complex.
To address this possibility, we first compared the PEWM complex in the livers of mice fasted for 14 h and at 3 h postprandial. Estimation statistics analysis from data generated by quantitative electron microscopy analysis showed that fasting was associated with ∼47% more PEWM complexes (Fig. 3F; n=600, 120/mouse, 5 mice/group). This observation implies that a substantial pool of peroxisomes is released from PEWM complexes within 3 h from feeding. To confirm that changes in hepatic lipid flux caused this phenotype, we next calculated the frequency of PEWM complexes in mouse livers upon acute liver-specific genetic ablation of Mttp, the most upstream regulator of VLDL biogenesis (Raabe et al., 1998, 1999). To this goal, we used Mttp-floxed mice (Chang et al., 1999) injected with an AAV8 vector that expresses the Cre recombinase under the control of the liver-specific thyroxine-binding globulin (TBG) promoter; in these mice, 3 weeks after virus injection, hepatic expression of Mttp is almost eliminated (Fig. 4B), and lipid droplet accumulation is quite pronounced due to the interruption of fatty acid flux into VLDL (Anastasia et al., 2021; Raabe et al., 1999). This study showed that compared to control mice, loss of Mttp expression nearly doubles the number of PEWM complexes (Fig. 3G; n=600; 120/mouse; 5 mice/group) without, however, affecting peroxisome content (Fig. 4B) and wrappER-peroxisome contact structure (Fig. S1A-E). Therefore, we infer that the PWEM complex is dynamic and that changes in fatty acid flux change the number of peroxisomes recruited to PEWM complexes.
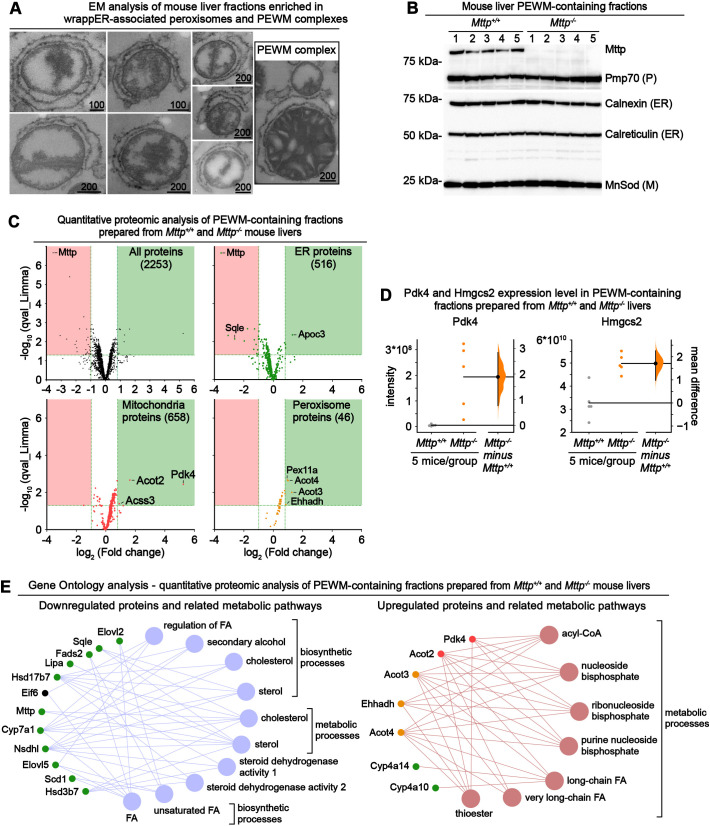
Fig. 4.
Comparative quantitative proteomic analysis of PEWM complexes containing fractions isolated from control and Mttp−/− mouse livers. (A,B) Electron microscopy (EM) (A) and immunoblot (B) analysis of mouse liver fractions enriched in wrappER-associated peroxisomes and PEWM complexes. Scale bars indicate values expressed in nm. ER, endoplasmic reticulum; M, mitochondria; P, peroxisome. (C) Volcano plot comparing the quantitative proteomic analysis of fractions enriched in PEWM complexes isolated from control Mttp+/+ and Mttp−/− mice (n=5). The upper left panel plots all the proteins identified by label-free LC-MS/MS analysis; the other panels display only the proteins assigned to the indicated organelle. Proteins that are upregulated and downregulated in a statistically significant manner are plotted in the green and red boxes, respectively (q-value <0.05; fold change <0.5 and >1.76). (D) Estimation statistics analysis (Bernard, 2019; Ho et al., 2019) of the expression level of Pdk4 and Hmgcs2 proteins in PEWM complex-enriched fractions analyzed by quantitative proteomic analysis. Pdk4 is a negative regulator of pyruvate respiration (Grassian et al., 2011), and Hmgcs2 is a positive regulator of mitochondrial fatty acid β-oxidation (Puchalska and Crawford, 2017). Each dot indicates the expression level of the indicated protein in one sample. In the y-axis, the intensity refers to the quantity of peptides that make the analyzed protein. Data were plotted using the Gardner–Altman graph; the mean difference is indicated by the dot on the right panel and is plotted as a bootstrap sampling distribution. The 95% c.i. is indicated by the vertical error bar. (E) Gene ontology analysis of the data plotted in C showing the metabolic pathways downregulated (left) and upregulated (right) by the loss of Mttp expression. Data were collected from mouse livers at 3 h postprandial.
Peroxisome recruitment to PEWM complexes is associated with global β-oxidation activation
Next, we addressed whether a higher number of PEWM complexes in Mttp−/− livers (Fig. 3G) correlates with the activation of FA β-oxidation in mitochondria and peroxisomes. Our previous study developed a protocol (Ilacqua et al., 2021) that yields mouse liver fractions enriched in wrappER-associated mitochondria that are structurally and functionally competent to synthesize and transfer phospholipids between wrappER and mitochondria (Anastasia et al., 2021). Electron microscopy and immunoblot analysis indicated that this fraction contains wrappER-associated peroxisome and PEWM complexes (Fig. 4A,B). We leveraged this observation to find evidence indicating activation of mitochondrial and peroxisomal β-oxidation in these fractions. To this end, we prepared PEWM-containing fractions from control and Mttp−/− livers and subjected them to label-free liquid chromatography with tandem mass spectrometry (LC-MS/MS) proteomic analysis. This study showed that loss of Mttp upregulates ∼40-fold the expression of the mitochondrial pyruvate kinase Pdk4, the inhibitor of pyruvate respiration (Grassian et al., 2011), and doubles the expression of key enzymes of mitochondrial β-oxidation, namely Hmgcs2, Acss3 and Acot2 (Fig. 4C,D; Table S1). Simultaneously, ablation of Mttp led to a significant increase in the expression of key enzymes of peroxisomal β-oxidation, namely Acot3, Acot4 and Ehhadh (Fig. 4C) (Houten et al., 2012). Interrogation of the Gene Ontology Resource database with this proteomic dataset (Table S1) confirmed that loss of Mttp expression upregulates fatty acid catabolism (Fig. 4E; Fig. S2A) and, as expected, downregulates cholesterol synthesis (Fig. 4E; Fig. S2B). The latter was evidenced by a 6-fold reduction in squalene epoxidase expression (Sqle; Fig. 4C), a rate-limiting enzyme in cholesterol biosynthesis and a target for the treatment of hypercholesteremia (Padyana et al., 2019), and by a significant reduction in Scap expression (Fig. S2B,D), a critical positive regulator of cholesterol biosynthesis (Brown et al., 2018). These findings collectively suggest that shifts in hepatic lipid flux might regulate PEWM dynamics, which could be part of the adaptive response governing hepatic lipid homeostasis.
DISCUSSION
This study shows that loss of VLDL synthesis activates fatty acid β-oxidation in mitochondria and peroxisomes (Fig. 4C-E; Table S1), and that this metabolic state is associated with a 2-fold increase in the number of PEWM complexes, thus recruiting nearly all peroxisomes into this subcellular structure (Fig. 3G). These observations suggest that proper elimination of fatty acid from the liver cell requires both types of fatty acid-respiring organelles, mitochondria and peroxisomes, to be physically close and connected through the same wrappER sheet. This could be because such a three-organelle complex allows for the parallel catabolism of small-, medium-, long-, and very-long-chain fatty acids, which are known to be differentially respired in mitochondria and peroxisomes (Lodish et al., 2000). Indeed, peroxisomes do not completely degrade fatty acid; they import long- and very-long-chain fatty acids and degrade them to short-chain fatty acids (∼C8) that are then respired by mitochondria (Lodish et al., 2000). The reason for respiring fatty acid in such manner is unclear (Speijer, 2017). However, within this context, the discovery of the PEWM complex is significant because, via the wrappER, the various fatty acid species can be confined and differentially distributed to the two organelles that respire them. Therefore, the PEWM complex emerges as a candidate subcellular complex that, in the liver, allows the compartmentalization of long- and very-long-chain fatty acid respiration.
Sites of physical contact between peroxisomes and the ER were first identified in yeast (David et al., 2013; Knoblach et al., 2013). Subsequent studies confirmed this observation in mammalian cells and demonstrated that the peroxisome-ER contact occurs through the tethering of ACBD5 on the peroxisome to VAPB on the ER (Costello et al., 2017; Hua et al., 2017). In mouse livers, genetic ablation of ACBD5 eliminates ∼90% of the peroxisome-ER contacts and causes very-long-chain fatty acid accumulation (Darwisch et al., 2020), a finding that is consistent with the role of wrappER as a fatty acid-distributing circuit to β-oxidative organelles. By integrating mitochondria and peroxisomes into a single dynamically regulated three-organelle complex, the wrappER appears to regulate lipid homeostasis by governing lipid balance and flux. This process occurs through the apparent competence of the PEWM complex to lower hepatic fatty acid content through systemic and intracellular pathways. Here, the systemic elimination of fatty acid is provided by the wrappER, which can secrete them into the bloodstream, either directly, bound to albumin and lipocalins, or indirectly, condensed as triglycerides in VLDLs (Anastasia et al., 2021); the intracellular elimination of fatty acid is instead provided by mitochondria and peroxisomes, which eliminate them through β-oxidative respiration. An additional intracellular route is provided by storing fatty acid as triglycerides in lipid droplets (Walther et al., 2017). It is widely assumed that the systemic and intracellular pathways run in parallel. However, the discovery of the PEWM complex challenge this view by showing that intracellular and systemic fatty acid-lowering pathways converge in a single subcellular compartment capable of storing, respiring and secreting fatty acid and VLDL (Fig. 3A,B; Anastasia et al., 2021).
The findings reported in this study echo a discovery made in yeast, in which the lysosome, lipid droplets and ER interact to regulate sterol distribution and utilization, and drive adaptive responses that protect against fatty acid toxicity (Henne, 2019). The wrappER is enriched in medium- and long-chain fatty acids, both saturated and unsaturated, and contacts nearly the entire population of β-oxidative organelles of the hepatocyte (Fig. 3C; Anastasia et al., 2021). How fatty acids flux within the PEWM complex remains unknown. Nonetheless, the use of multi-organelle complexes to govern lipid flux in yeast and mammals suggests that the multi-organelle compartmentalization observed in such distant organisms is at the heart of lipid biology in all eukaryotes, and most likely evolved already at the onset of the evolution of eukaryotes when the intracellular membrane system was being established.
In this study, we showed that the number of PEWM complexes in the liver is highest when the hepatic cell switches from pyruvate to fatty acid respiration. Thus, other factors could come into play in regulating the assembly and disassembly of this cellular structure, such as changes in insulin levels, the amount of dietary cholesterol, a ketogenic diet and, in general, any other factors known to regulate liver energy metabolism (Puchalska and Crawford, 2017; Yki-Järvinen et al., 2021). Therefore, we anticipate that studies addressing the role of PEWM complex dynamics will help understand the etiology of liver metabolic syndromes and diseases.
MATERIALS AND METHODS
Mice
Adult male C57BL/6N mice (8 weeks old) were purchased from Charles River. Mttp-floxed mice were a generous gift from Dr Mahmood M. Hussain and Dr Chan Lawrence (Chang et al., 1999). The mice were housed in a pathogen-free animal facility under a 12 h light/dark cycle at constant temperature and humidity, and fed standard rodent chow and water ad libitum. All experiments were conducted with male mice 9-12 weeks old. For fasting/refeeding studies, animals were either fasted for 14 h overnight with water ad libitum and sacrificed in the morning, or fasted for 12 h overnight with water ad libitum and, in the morning, they were provided standard rodent chow and sugary water (30% sucrose) ad libitum for 3 hours. Livers were removed immediately after anesthetization of the animals with 2% isoflurane, which were sacrificed through cervical dislocation. For liver samples cryofixation, animals were anesthetized by intraperitoneal injection with ketamine; 3-5 tissue biopsies were collected using the Rapid Transfer System (Leica) and vitrified by high-pressure freezing (Leica EM PACT2). Mice were then sacrificed by cervical dislocation. All experiments involving animals were approved by the Animal Protection Committee of Laval University (CPAUL) and performed in accordance with its guidelines for animal welfare.
Immunoblot analysis
Total protein concentration was determined using bicinchoninic acid assay protein assay reagent (Thermo Fisher Scientific). Protein samples were analyzed by SDS-PAGE using the precast Bolt 8% Bis-Tris Plus gels or Bolt 4-12% Bis-Tris Plus gels (Thermo Fisher Scientific) according to the manufacturer's instructions. Western blotting was performed by transferring proteins for 60 min at 100 V to a PVDF membrane (Immobilon, Millipore; 0.45 μm pore size) in transfer buffer [20% methanol; 320 mM glycine, 20 mM Tris-base (pH 8.4)]. Membranes were blocked for 60 min at room temperature with 7.5% nonfat milk in Tris-buffered saline with 0.1% Tween 20. Primary antibodies were incubated overnight at 4°C in 5% nonfat milk. The primary antibodies used in this study were as follows: Calnexin (StressMarq Biosciences, SPC-127; 1:30,000); Calreticulin (Cell Signaling Technology, 12238; 1:100,000); Mn SOD (Enzo Life Sciences, ADI-SOD-110-D; 1:10,000); Mttp (Atlas Antibodies, HPA054862; 1:750); and PMP70 (Sigma-Aldrich, SAB4200181; 1:20,000). The horseradish peroxidase-conjugated secondary antibodies used in this study were as follows: anti-mouse IgG (Jackson ImmunoResearch, 115-035-062; 1:5000) and anti-rabbit IgG (GE Healthcare, NA934; 1:5000). Protein bands were detected by chemiluminescence using the SuperSignal ELISA Femto substrate (Thermo Fisher Scientific) and the VersaDoc 3000 charged-coupled device (CCD) imaging system (Bio-Rad). The specificity of the anti-Mttp antibody in immunoblot analysis was validated by pre-absorbing it with a 10-fold excess of the recombinant protein (Atlas Antibodies, APrEST85548, lot PRL02977), and by the knockout experiments shown in Fig. 4B.
Preparation of wrappER-associated mitochondria-enriched fractions containing peroxisome-WrappER-mitochondria complexes from mouse liver
The wrappER-associated mitochondria-enriched fractions protocol has been described previously (Ilacqua et al., 2021). Briefly, a mouse liver was quickly excided, rinsed three times with Elution Buffer (EB; 10 mM Tris-HCl, 1 mM MgCl2 and 0.1 mM EGTA (pH 7.4)] supplemented with 250 mM sucrose (EB10), chopped in small pieces and resuspended in 5 ml EB10 supplemented with protease inhibitor cocktail (PIC; Thermo Fisher Scientific, 78429). The sample was mechanically homogenized with 16 strokes in a glass-Teflon dounce homogenizer, brought to a 12 ml volume with EB10 supplemented with PIC, and poured in two 30 ml glass centrifuge tubes. The homogenate was spun three times at 400 g for 10 min at 4°C, and, at the end of the centrifugation, the supernatants were collected and mixed together to obtain the mouse liver whole lysate. The whole lysate (7 ml) was then carefully layered on top of 7 ml of a 27% (w/w) sucrose solution (27% sucrose in EB) inside a 50 ml Falcon tube and spun at 2000 g for 20 min at 4°C; the pellet thus obtained represented the wrapper-associated mitochondria-enriched fraction containing PEWM complexes.
PEWM-enriched fraction fixation and embedding for electron microscopy
PEWM-enriched fractions were gently resuspended in 27% w/w sucrose solution (27% sucrose in EB), pelleted at 3000 g for 10 min at 4°C, and incubated overnight in 500 µl of 27% (w/w) sucrose solution, containing 2% glutaraldehyde, at 4°C. The pellet was rinsed three times with 27% (w/w) sucrose solution and incubated in 1% (w/v) osmium tetroxide in double-distilled water for 40 min at room temperature, in the dark. After three washes with water, the pellet was dehydrated in 50%, 70% (supplemented with 1% uranyl acetate, for 1 h in the dark), 90% and 100% ethanol, followed by two incubations in 100% propylene oxide for 20 min at room temperature, and embedding in epoxy resin.
Tissue sample preparation for electron microscopy analysis, electron tomography imaging, three-dimensional reconstruction and correlative light-electron microscopy analysis
Mouse livers were quickly biopsied using the Rapid Transfer System (Leica). High-pressure freezing (Leica EM PACT2) was used for cryofixation of the samples. Freeze substitution was performed with the Leica automatic freeze substitution chamber. The substitution fluid was acetone containing 1% osmium tetroxide and 0.1% uranyl acetate. The procedure started at −90°C for 8 h and warmed up to −60°C at the speed of 5°C/h. The substitution medium was replaced with pure acetone after the temperature reached 0°C. Samples were embedded in Araldite/epon/dodecenylsuccinic anhydride (DDSA) and 2,4,6-tris (dimethylaminomethyl) phenol (DMP30) mixture [araldite/epon stock, 41% epoxy (w/w), 54% durcupan Araldite casting resin M (w/w), 5% dibutylphthalate (w/w); Araldite/epon complete formulation, 49% Araldite/epon stock (w/w), 49% hardener DDSA (w/w) and 2% accelerator DMP-30 (w/w)]. The procedure was performed stepwise: 33% resin in water-free acetone for 4 h, 66% resin in water-free acetone for hours, 100% resin overnight, and one 100% resin change before polymerization. All samples were polymerized at 58°C for at least 48 h. Samples were cut (50 nm) and put on single-slot copper grids using a Leica Ultramicrotome. After counterstaining with lead citrate, samples were viewed using a Tecnai-12 (Philips) with a Megaview camera using the Analysis software.
For SSET, serial thick sections were collected on formvar-coated copper slot grids and gold fiducials (10 nm) were applied on both surfaces of the grids. The samples were imaged using a 200 kV Tecnai G2 20 electron microscope (FEI) or a 120 kV Talos L120C (Thermo Fisher Scientific). Tilted images (+65/−65 according to a Saxton scheme) were acquired using Xplorer 3D (FEI) with an Eagle 2k×2k CCD camera (FEI) or transmission electron microscopy (TEM) Tomography 4.0 acquisition software using a 4kx4k Ceta16M camera (Thermo Fisher Scientific). Tilted series alignment and tomography reconstruction was performed using the IMOD software package (Mastronarde, 1997).
Three-dimensional reconstruction analysis
For each electron tomography analysis, serial tomograms were compiled as a single TIFF file and analyzed using Amira Software (v. 2020.3; Thermo Fisher Scientific). The z scale was stretched using a 1.6 factor to correct for resin shrinkage. The structure of interest (e.g. peroxisome, mitochondria, wrappER, ribosome, etc.) was carefully manually traced on each virtual slice using a graphic tablet and then reconstructed in three dimensions, with rendering generated using unconstrained smoothing parameters (for smaller structures like ribosomes and adhesion sites, existing weights parameters were used). The surfaces of adhesion sites and peroxisomes were measured using the Surface Area Volume module. Movies were generated using Amira Software (v. 2020.3, Thermo Fisher Scientific) and then edited with iMovie (v. 10.1.10; Apple).
Two-dimensional electron microscopy morphometric analysis
Quantitative morphometric analyses were performed on high-quality TEM images of hepatocytes using a graphics tablet as a hand-drawing tool. For this analysis, hepatocytes were identified as cells when they had the following attributes: (1) a polarized cellular architecture; (2) polygonal in shape; (3) sides in contact either with sinusoids (sinusoidal face) or neighboring hepatocytes (lateral faces); (4) microvilli abundantly present on the sinusoidal face and projecting sparsely into bile canaliculi; and (5) containing glycogen granules as chrysanthemum-like clusters of electron-dense particles (Burdon and van Knippenberg, 1991; Hooser et al., 1990; Sorenson and Brelje, 2014).
Peroxisome-wrappER distance, coverage, peroxisome areas and adhesion site analysis were measured using ImageJ (NIH). In the analysis of the number of PEWMs, mitochondria were used as a reference, and we counted how many were in contact with a wrapper that was also simultaneously in contact with a peroxisome. Data were analyzed and plotted using Excel (v.16.25; Microsoft), Prism (v.9; GraphPad) or https://www.estimationstats.com/#/.
Correlative light electron microscopy analysis
CLEM was performed on 500-700 nm Tokuyasu cryosections as described by Mateos et al., (2016), with some modifications. Mouse livers were fixed in situ by portal vein injection (4% formaldehyde and 4% sucrose in 0.1 M HEPES buffer at pH 7.4). After perfusion, livers were removed and left in the same fixative solutions overnight at 4°C. The liver tissues were cut into 1 mm3 pieces and infiltrated in 2.3 M sucrose overnight at 4°C. Samples were mounted on cryo pins (Electron Microscopy Sciences), frozen in liquid nitrogen, and mounted onto the ultramicrotome (FC7 Leica Microsystem). Frozen blocks were trimmed and cut at a thickness of 500-700 nm at −60 to −70°C using a glass knife. Each thick Tokuyasu liver section was retrieved using a perfect loop with a drop of methylcellulose solution (1% methylcellulose and 1.15% sucrose) and transferred to a 24 mm round glass coverslip. Coverslips were stored at 4°C until processed for immunolabeling.
For immunolabeling, Tokuyasu liver sections were washed with PBS, treated with 50 mM glycine for 10 min, and incubated with blocking buffer (1% bovine serum albumin in PBS) for 30 min. Primary antibodies anti-Pmp70 (Novas Bio, NBP2-36770; 1:500) and anti-Tomm20 (Novas Bio, Nbp1-81556; 1:200) were diluted in blocking buffer and incubated overnight at 4°C. After several washes in PBS (5×2 min), samples were incubated with secondary antibodies (goat anti-mouse-IgG conjugated to Alexa Fluor 647 and goat anti-rabbit-IgG conjugated to Alexa Fluor 546, Invitrogen, 1:1000) and Alexa Fluor 488–phallodin (Invitrogen, 1:200) in blocking buffer for 1 h at room temperature. After several washes in PBS, sections were counterstained with Hoechst 33342, washed and fixed with 0.2% glutaraldehyde for 5 min, and left in PBS at 4°C until imaged.
Sections were imaged in PBS with a Deltavision wide-field microscope (GE Healthcare). To acquire a large field of view, tiled z-stacked images were acquired with an Olympus 100x UPLSAPO oil objective with a pixel size of 0.0646, 0.0646 and 0.300 m. After acquisition, images were stitched and deconvolved using softWoRx and Huyens software (Scientific Volume Imaging).
After imaging, sections were fixed with 2.5% glutaraldehyde, postfixed using the reduced osmium-thiocarbohydrazide-osmium (ROTO) technique, dehydrated in ethanol and embedded in epon, as described previously (Guidotti et al., 2015). Resin blocks were detached from glass coverslips using liquid nitrogen. Thin sections (70-90 nm) were collected using an ultramicrotome (UC7 Leica microsystem) and deposited on copper carbon-coated slot grids. Grids were contrasted with uranyl acetate and Sato's lead solution, and imaged using a TALOS L120C transmission electron microscope. Fluorescent images were imported using MAPS software. Hoechst and Phalloidin staining were used as a reference in order to relocate the same region in the electron microscope ultrathin sections. Large view fields were acquired by tile image acquisition and stitched using MAPS software.
Fluorescent signals were aligned to electron microscope images using IMOD programs, as described previously (Sun et al., 2019). Briefly, using 3dmod model points, pairs were placed over corresponding nuclei in immunofluorescence and electron microscope images, and used to calculate linear transformation in order to roughly align immunofluorescence to electron microscope images. Following this step, mitochondrial-stained immunofluorescence images were carefully manually aligned over electron microscope images using MIDAS, and the resulting transformation was applied to all channels. Finally, aligned electron microscope and immunofluorescence stacks images were overlaid using ImageJ.
Protein mass spectrometry analysis
Sodium deoxycholate (SDC) was added to PEWM-enriched fractions containing PEWM complexes to obtain a final concentration of 1% SDC. The samples were then sonicated 20 times (1 s on/1 s off) and centrifuged. The resulting supernatant was precipitated with five volumes of acetone. After precipitation, protein pellets were resuspended in Buffer 1 [50 mM ammonium bicarbonate and 1% sodium deoxycholate (pH 8)], and quantified using the micro-Bradford assay (Bio-Rad). A 10 µg volume of proteins was reduced at 37°C with dithiothreitol, alkylated with iodoacetamide, and digested overnight with trypsin. Proteolysis was stopped by acidification [3% acetonitrile, 1% trifluoroacetic acid (TFA) and 0.5% acetic acid], and peptides were purified on stage tip (C18), vacuum dried and resuspended in 50 ml 0.1% formic acid. One-tenth of this volume (1 µg) was separated by online reversed-phase nanoscale capillary liquid chromatography and analyzed by electrospray mass spectrometry (LC MS/MS) using a Dionex UltiMate 3000 nanoRSLC chromatography system (Thermo Fisher Scientific) connected to an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) equipped with a nanoelectrospray ion source.
Peptides were trapped at 20 μl/min in loading solvent (2% acetonitrile and 0.05% TFA) on a 5 mm×300 μm C18 pepmap cartridge pre-column (Thermo Fisher Scientific) for 5 min. Then, the pre-column was switched online with a Pepmap Acclaim (Thermo Fisher Scientific) 50 cm×75 µm internal diameter separation column and the peptides were eluted with a linear gradient from 5-40% solvent B (A, 0.1% formic acid; B, 80% acetonitrile and 0.1% formic acid) in 90 min for a total run of 120 min, at 300 nl/min. Mass spectra were acquired using a data-dependent acquisition mode using Thermo XCalibur software (v. 4.1.50). Full scan mass spectra (350 to 1800 m/z) were acquired in the Orbitrap using an automatic gain control (AGC) target of 4e5, a maximum injection time of 50 ms and a resolution of 120,000. Internal calibration using lock mass on the m/z 445.12003 siloxane ion was used. Each MS scan was followed by the acquisition of fragmentation MS/MS spectra of the most intense ions for a total cycle time of 3 s (top speed mode). The selected ions were isolated using the quadrupole analyzer in a window of 1.6 m/z and fragmented by higher energy collision-induced dissociation with 35% of collision energy. The resulting fragments were detected by the linear ion trap at rapid scan rate with an AGC target of 1e4 and a maximum injection time of 50 ms. Dynamic exclusion of previously fragmented peptides was set for a period of 30 s and a tolerance of 10 ppm.
Database searching and label-free quantification
Spectra were searched against the Uniprot Ref Mus musculus database (July 2020 release/63807entries) using the Andromeda module of MaxQuant software (v. 1.6.10.43) (Cox and Mann, 2008). Trypsin/P enzyme parameter was selected with two possible missed cleavages. Carbamidomethylation of cysteines was set as fixed modification, and methionine oxidation and protein N-terminal acetylation were set as variable modifications. Mass search tolerances were 5 ppm and 0.5 Da for MS and MS/MS, respectively. For protein validation, a maximum false discovery rate of 1% at the peptide and protein level was used based on a target/decoy search. MaxQuant was also used for label-free quantification (LFQ). The ‘match between runs’ option was used with a 20 min value as the alignment time window and 3 min as the match time window. Only unique and razor peptides were used for quantification. Normalization (LFQ intensities) was performed by MaxQuant.
RStudio (v. 1.2.5019) was used for data processing. Missing peptide intensity values were replaced by a noise value corresponding to the 1% percentile of the normalized value for each condition. A peptide was considered to be quantifiable only if at least five intensity values were present in the five replicates of each condition. The ratio between conditions, Limma q-value (with Benjamini-Hochberg correction) and z-score were calculated. At least two quantified peptides were needed to obtain quantification for the protein. To be significantly differentially expressed, a protein needed to have a q-value (adjusted Benjamini-Hochberg corrected P value) less than 0.05 and a z-score lower than −1.96 (underexpressed) or higher than 1.96 (overexpressed). To graphically represent these data, the volcano plot [−log10(Limma q value) versus log2(fold change)] was constructed using Prism (v. 9, GraphPad Software).
Gene ontology analysis of the biological processes associated with downregulated and upregulated proteins (Fig. 4E) was conducted by uploading the protein lists in the Cytoscape (v. 3.7.2) (Shannon et al., 2003) plug-in ClueGO (Bindea et al., 2009). Heatmaps in Fig. S2 were generated with Excel using the conditional formatting color-scales feature row by row (v.16.25; Microsoft). The data are organized into a matrix in which each column contains the spectral count from a single sample and each row corresponds to a single protein. The color palette is set to gradually change from green, representing high spectral count, to red, representing low spectral count.
Protein digestion, mass spectrometry analyses, protein identification and proteomic analysis were performed by the Proteomics Platform of the Centre Hospitalier Universitaire de Québec-Université Laval Research Center, Québec City, Canada.
Inactivation of Mttp with Cre adenovirus in the liver
Liver-specific Mttp-knockout mice were generated from Mttp-floxed mice (Chang et al., 1999) by injecting male animals (9-12 weeks old) with either control vector (pENN.AAV.TBG.PI.ffLuciferase.RBG, Addgene, 105538) or AAV.TBG.PI.Cre.rBG (Addgene, 107787) via tail vein injection (1.8×1010 genome copies per mouse) with BD Ultra-Fine Insulin Syringes. Experiments were commenced 21 days after injection.
Quantification and statistical analysis
An unpaired t-test was used for comparisons involving two groups. P values were calculated using Prism (v. 9, GraphPad Software) as specified in the figure legends. P<0.05 were considered statistically significant [not significant (n.s.) P≥0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001]. Sample sizes for each experiment are displayed on the figures. For estimation statistics based on confidence intervals (Bernard, 2019; Ho et al., 2019), we directly introduced the raw data in estimationstats.com and downloaded the results and graphs. In these graphs, the mean difference between the groups is depicted as the dot within the vertical error bar that represents the 95% c.i.
Supplementary Material
Acknowledgements
We thank Sylvie Bourassa (Centre Hospitalier Universitaire de Québec) for support with proteomic analysis; Rana Ghandehari-Alavijeh and Leonardo Pellegrini for ET-3D reconstruction; and Lawrence C. B. Chan (Baylor College of Medicine) and Mahmood M. Hussein (New York University) for the Mttp-floxed mice and valuable discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: N.I., I.A., T.Q.d.A.V., K.T., E.V.K., L.P.; Methodology: N.I., I.A., A.R., P.L.; Validation: N.I., I.A., L.P.; Formal analysis: N.I., I.A., K.T., E.V.K., L.P.; Investigation: N.I., I.A., A.R., L.P.; Resources: A.R., P.L., T.Q.d.A.V.; Data curation: L.P.; Writing - original draft: N.I., I.A., E.V.K., L.P.; Supervision: L.P.; Project administration: L.P.; Funding acquisition: L.P.
Funding
T.Q.d.A.V. is supported by the National Institutes of Health (NIH, HL122677 and DK112119); K.T. is supported by the Canadian Institutes of Health Research (CIHR, MOP-81142); and E.V.K. is supported by intramural funds from the National Center for Biotechnology Information of the NIH. I.A. and N.I. are both recipients of PhD scholarships from the Centre Thématique de Recherche en Neurosciences and the Fonds de recherche du Québec – Santé. This study was funded by grants from the CIHR (201603PJT-365052) and the Natural Sciences and Engineering Research Council of Canada (RGPIN-2017-06130) to L.P. Deposited in PMC for release after 12 months.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the MassIve partner repository with the dataset identifier PXD025598.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259091.
References
- Anastasia, I., Ilacqua, N., Raimondi, A., Lemieux, P., Ghandehari-Alavijeh, R., Faure, G., Mekhedov, S. L., Williams, K. J., Caicci, F., Valle, G.et al. (2021). Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Rep. 34, 108873. 10.1016/j.celrep.2021.108873 [DOI] [PubMed] [Google Scholar]
- Bernard, C. (2019). Changing the way we report, interpret, and discuss our results to rebuild trust in our research. eNeuro 6, ENEURO.0259-19.2019. 10.1523/ENEURO.0259-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea, G., Mlecnik, B., Hackl, H., Charoentong, P., Tosolini, M., Kirilovsky, A., Fridman, W.-H., Pagès, F., Trajanoski, Z. and Galon, J. (2009). ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091-1093. 10.1093/bioinformatics/btp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M. S., Radhakrishnan, A. and Goldstein, J. L. (2018). Retrospective on cholesterol homeostasis: the central role of scap. Annu. Rev. Biochem. 87, 783-807. 10.1146/annurev-biochem-062917-011852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon, R. H. and van Knippenberg, P. H. (1991). Microscopy of isolated hepatocytes. In Laboratory Techniques in Biochemistry and Molecular Biology (eds Burdon R. H. and van Knippenberg P. H.), pp. 99-120. Elsevier. [Google Scholar]
- Carter, S. D., Hampton, C. M., Langlois, R., Melero, R., Farino, Z. J., Calderon, M. J., Li, W., Wallace, C. T., Tran, N. H., Grassucci, R. A.et al. (2020). Ribosome-associated vesicles: a dynamic subcompartment of the endoplasmic reticulum in secretory cells. Sci. Adv. 6, eaay9572. 10.1126/sciadv.aay9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, B. H.-J., Liao, W., Li, L., Nakamuta, M., Mack, D. and Chan, L. (1999). Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274, 6051-6055. 10.1074/jbc.274.10.6051 [DOI] [PubMed] [Google Scholar]
- Costello, J. L., Castro, I. G., Hacker, C., Schrader, T. A., Metz, J., Zeuschner, D., Azadi, A. S., Godinho, L. F., Costina, V., Findeisen, P.et al. (2017). ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J. Cell Biol. 216, 331-342. 10.1083/jcb.201607055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, J. and Mann, M. (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367-1372. 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- Csordás, G., Weaver, D. and Hajnóczky, G. (2018). Endoplasmic reticulum–mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 28, 523-540. 10.1016/j.tcb.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwisch, W., von Spangenberg, M., Lehmann, J., Singin, Ö., Deubert, G., Kühl, S., Roos, J., Horstmann, H., Körber, C., Hoppe, S.et al. (2020). Cerebellar and hepatic alterations in ACBD5-deficient mice are associated with unexpected, distinct alterations in cellular lipid homeostasis. Commun. Biol. 3, 713. 10.1038/s42003-020-01442-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, C., Koch, J., Oeljeklaus, S., Laernsack, A., Melchior, S., Wiese, S., Schummer, A., Erdmann, R., Warscheid, B. and Brocard, C. (2013). A combined approach of quantitative interaction proteomics and live-cell imaging reveals a regulatory role for endoplasmic reticulum (ER) reticulon homology proteins in peroxisome biogenesis. Mol. Cell. Proteomics 12, 2408-2425. 10.1074/mcp.M112.017830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, R., East, D. A., Hardy, L., Faccenda, D., Rigon, M., Crosby, J., Alvarez, M. S., Singh, A., Mainenti, M., Hussey, L. K.et al. (2020). Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci. Adv. 6, eabc9955. 10.1126/sciadv.abc9955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello, M. and Pellegrini, L. (2016). The coming of age of the mitochondria–ER contact: a matter of thickness. Cell Death Differ. 23, 1417-1427. 10.1038/cdd.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian, A. R., Metallo, C. M., Coloff, J. L., Stephanopoulos, G. and Brugge, J. S. (2011). Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 25, 1716-1733. 10.1101/gad.16771811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti, L. G., Inverso, D., Sironi, L., Di Lucia, P., Fioravanti, J., Ganzer, L., Fiocchi, A., Vacca, M., Aiolfi, R., Sammicheli, S.et al. (2015). Immunosurveillance of the liver by intravascular effector CD8+ T cells. Cell 161, 486-500. 10.1016/j.cell.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne, M. (2019). And three's a party: lysosomes, lipid droplets, and the ER in lipid trafficking and cell homeostasis. Curr. Opin. Cell Biol. 59, 40-49. 10.1016/j.ceb.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Ho, J., Tumkaya, T., Aryal, S., Choi, H. and Claridge-Chang, A. (2019). Moving beyond P values: data analysis with estimation graphics. Nat. Methods 16, 565-566. 10.1038/s41592-019-0470-3 [DOI] [PubMed] [Google Scholar]
- Hoffman, D. P., Shtengel, G., Xu, C. S., Campbell, K. R., Freeman, M., Wang, L., Milkie, D. E., Pasolli, H. A., Iyer, N., Bogovic, J. A.et al. (2020). Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science 367, eaaz5357. 10.1126/science.aaz5357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooser, S. B., Beasley, V. R., Basgall, E. J., Carmichael, W. W. and Haschek, W. M. (1990). Microcystin-LR-induced ultrastructural changes in rats. Vet. Pathol. 27, 9-15. 10.1177/030098589002700102 [DOI] [PubMed] [Google Scholar]
- Houten, S. M., Denis, S., Argmann, C. A., Jia, Y., Ferdinandusse, S., Reddy, J. K. and Wanders, R. J. A. (2012). Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J. Lipid Res. 53, 1296-1303. 10.1194/jlr.M024463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, R., Cheng, D., Coyaud, É., Freeman, S., Di Pietro, E., Wang, Y., Vissa, A., Yip, C. M., Fairn, G. D., Braverman, N.et al. (2017). VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J. Cell Biol. 216, 367-377. 10.1083/jcb.201608128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilacqua, N., Anastasia, I. and Pellegrini, L. (2021). Isolation and analysis of fractions enriched in WrappER-associated mitochondria from mouse liver. STAR Protoc. 2, 100752. 10.1016/j.xpro.2021.100752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach, B., Sun, X., Coquelle, N., Fagarasanu, A., Poirier, R. L. and Rachubinski, R. A. (2013). An ER-peroxisome tether exerts peroxisome population control in yeast. EMBO J. 32, 2439-2453. 10.1038/emboj.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. E., Cathey, P. I., Wu, H., Parker, R. and Voeltz, G. K. (2020). Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367, eaay7108. 10.1126/science.aay7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish, H., Berk, A., Zipursky, S. L., Matsudaira, P., Baltimore, D. and Darnell, J. (2000). Oxidation of Glucose and Fatty Acids to CO2, 4th edn. New York: W. H. Freeman. [Google Scholar]
- Mastronarde, D. N. (1997). Dual-axis tomography: an approach with alignment methods that preserve resolution. J. Struct. Biol. 120, 343-352. 10.1006/jsbi.1997.3919 [DOI] [PubMed] [Google Scholar]
- Mateos, J. M., Guhl, B., Doehner, J., Barmettler, G., Kaech, A. and Ziegler, U. (2016). Topographic contrast of ultrathin cryo-sections for correlative super-resolution light and electron microscopy. Sci. Rep. 6, 34062. 10.1038/srep34062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon-Abell, J., Obara, C. J., Weigel, A. V., Li, D., Legant, W. R., Xu, C. S., Pasolli, H. A., Harvey, K., Hess, H. F., Betzig, E.et al. (2016). Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 354, aaf3928. 10.1126/science.aaf3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padyana, A. K., Gross, S., Jin, L., Cianchetta, G., Narayanaswamy, R., Wang, F., Wang, R., Fang, C., Lv, X., Biller, S. A.et al. (2019). Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat. Commun. 10, 97. 10.1038/s41467-018-07928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. J. and Voeltz, G. K. (2016). Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69-82. 10.1038/nrm.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalska, P. and Crawford, P. A. (2017). Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262-284. 10.1016/j.cmet.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe, M., Flynn, L. M., Zlot, C. H., Wong, J. S., Veniant, M. M., Hamilton, R. L. and Young, S. G. (1998). Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA 95, 8686-8691. 10.1073/pnas.95.15.8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe, M., Véniant, M. M., Sullivan, M. A., Zlot, C. H., Björkegren, J., Nielsen, L. B., Wong, J. S., Hamilton, R. L. and Young, S. G. (1999). Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103, 1287-1298. 10.1172/JCI6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano, L., De Matteis, M. A., Emr, S., Giordano, F., Hajnóczky, G., Kornmann, B., Lackner, L. L., Levine, T. P., Pellegrini, L., Reinisch, K.et al. (2019). Coming together to define membrane contact sites. Nat. Commun. 10, 1287. 10.1038/s41467-019-09253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., Amin, N., Schwikowski, B. and Ideker, T. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498-2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood, A., Jeyaraju, D. V., Prudent, J., Caron, A., Lemieux, P., McBride, H. M., Laplante, M., Tóth, K. and Pellegrini, L. (2014). A Mitofusin-2–dependent inactivating cleavage of Opa1 links changes in mitochondria cristae and ER contacts in the postprandial liver. Proc. Natl. Acad. Sci. USA 111, 16017-16022. 10.1073/pnas.1408061111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson, R. L. and Brelje, T. C. (2014). The Atlas of Human Histology: A Guide to Microscopic Structure of Cells, Tissues and Organs, 3rd edn. (ed. Sorenson R. L. and Brelje T. C.); https://histologyguide.com/. [Google Scholar]
- Speijer, D. (2017). Evolution of peroxisomes illustrates symbiogenesis. BioEssays 39, 1700050. 10.1002/bies.201700050 [DOI] [PubMed] [Google Scholar]
- Sun, R., Liu, Y.-T., Tao, C.-L., Qi, L., Lau, P.-M., Zhou, Z. H. and Bi, G.-Q. (2019). An efficient protocol of cryo-correlative light and electron microscopy for the study of neuronal synapses. Biophys. Rep. 5, 111-122. 10.1007/s41048-019-0092-4 [DOI] [Google Scholar]
- Vance, J. E. (1990). Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265, 7248-7256. 10.1016/S0021-9258(19)39106-9 [DOI] [PubMed] [Google Scholar]
- Vance, J. E. (2020). Inter-organelle membrane contact sites: implications for lipid metabolism. Biol. Direct 15, 24. 10.1186/s13062-020-00279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, T. C., Chung, J. and Farese, R. V. (2017). Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 33, 491-510. 10.1146/annurev-cellbio-100616-060608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, E., Ben Ali, Y., Lyon, J., Wang, H., Nelson, R., Dolinsky, V. W., Dyck, J. R. B., Mitchell, G., Korbutt, G. S. and Lehner, R. (2010). Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 11, 183-193. 10.1016/j.cmet.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen, H., Luukkonen, P. K., Hodson, L. and Moore, J. B. (2021). Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 10.1038/s41575-021-00472-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.