ABSTRACT
Copper (Cu) homeostasis is essential for the development and function of many organisms. In humans, Cu misbalance causes serious pathologies and has been observed in a growing number of diseases. This Review focuses on mammalian Cu(I) transporters and highlights recent studies on regulation of intracellular Cu fluxes. Cu is used by essential metabolic enzymes for their activity. These enzymes are located in various intracellular compartments and outside cells. When cells differentiate, or their metabolic state is otherwise altered, the need for Cu in different cell compartments change, and Cu has to be redistributed to accommodate these changes. The Cu transporters SLC31A1 (CTR1), SLC31A2 (CTR2), ATP7A and ATP7B regulate Cu content in cellular compartments and maintain Cu homeostasis. Increasing numbers of regulatory proteins have been shown to contribute to multifaceted regulation of these Cu transporters. It is becoming abundantly clear that the Cu transport networks are dynamic and cell specific. The comparison of the Cu transport machinery in the liver and intestine illustrates the distinct composition and dissimilar regulatory response of their Cu transporters to changing Cu levels.
KEY WORDS: Copper, Transport, SLC31A1, SLC31A2, ATP7A, ATP7B
Summary: This Review describes accumulating evidence for diverse and cell-specific regulation of copper ion transporters that cells employ to adjust copper fluxes to their changing metabolic needs.
Introduction
The essential role of copper (Cu) homeostasis in human physiology is often illustrated by existence of severe pathologies associated with either inborn Cu deficiency (Menkes disease) or Cu overload (Wilson disease) (Kaler, 2013). The discovery of genes mutated in Menkes disease (ATP7A), and Wilson disease (ATP7B) led to the identification of the first Cu transporters and subsequent characterization of mechanisms regulating intracellular Cu distribution and signaling (La Fontaine et al., 2010). Both reduced and oxidized forms of Cu [generally referred to as Cu(I) and Cu(II)] are used in cells. As a rule, copper is transported as Cu(I) in mammalian cells. These mechanisms are necessary for normal cell growth, differentiation, and many specialized cell functions.
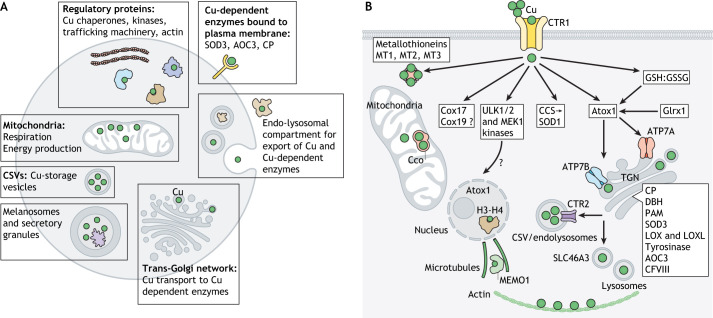
Cells utilize Cu in several compartments (Fig. 1). Following Cu entry into a cell, the group of proteins, named Cu chaperones, distribute Cu to specific protein targets in the cytosol, mitochondria, and the secretory pathway (Box 1). In addition, within the cytosol, the Cu-regulated kinases ULK1 and UKL2, and MEK1 (also known as MAP2K1) (Brady et al., 2014; Tsang et al., 2020), and the cyclic AMP (cAMP)-degrading phosphodiesterase PDE3B mediate Cu-dependent signaling (Krishnamoorthy et al., 2016), which impacts autophagosome formation, cell proliferation and metabolism (Brady et al., 2014; He et al., 2019; Tsang et al., 2020). Close spatial proximity of Cu to F-actin, especially at the basis of dendritic protrusions, has led to the suggestion that Cu modulates cytoskeleton morphology in dendrites and spines (Domart et al., 2020). Furthermore, it has been shown that the redox activity of mediator of ERBB2 driven cell motility 1 (MEMO1), a protein that regulates microtubule growth into lamellipodia (Schotanus and Van Otterloo, 2020), is Cu dependent (MacDonald et al., 2014). The cytoskeleton remodeling in response to Cu elevation has been directly demonstrated in the bone marrow mesenchymal stem cells, although in this case, Cu appears to act indirectly by stimulating the expression of Rho family GTPase Rnd3 (Chen et al., 2020). Also in the cytosol, the metallothioneins MT1, MT2 and MT3, metal-binding proteins with high Cu-binding capacity, sequester excess Cu when the Cu efflux mechanisms are saturated or fail to work (Shishido et al., 2001; Koh and Lee, 2020).
Fig. 1.
Sites of Cu utilization in a generalized cell and major Cu-handling proteins. (A) Cellular compartments in which Cu is used and respective cell functions. (B) Protein machinery involved in the Cu transport and regulation network. Cu enters the cells primarily through the high-affinity Cu transporter CTR1 but additional Cu-uptake mechanisms exist. In the cytosol, the Cu chaperones CCS, Atox1, and possibly Cox17 and Cox19 serve as compartment-specific Cu shuttles. The cytosolic redox environment, which is maintained by the glutathione pair (GSH:GSSG), influences the rate of Cu uptake via CTR1, as well as the oxidation state of Atox1. Cytosolic Cu-dependent SOD1 detoxify reactive oxygen species. Also in the cytosol, the Cu-sensing kinases ULK1, ULK2 and MEK1 regulate cell proliferation and autophagy, whereas the metallothioneins MT1, MT2, and MT3 bind to and store excess Cu. In the trans-Golgi network (TGN), the ATP-driven Cu transporters ATP7A and/or ATP7B receive Cu from Atox1 and activate the listed Cu-dependent enzymes. Glutaredoxin Grx1 regulates glutathionylation and Cu transfer to ATP7A. In mitochondria, Cu enables respiration and ATP production as a cofactor of cytochrome c oxidase (Cco). Secretory granules and specialized compartments such as melanosomes contain Cu-dependent enzymes, as well as Cu. Lysosomal Cu content is affected by the transporter SLC46A3. Cu-storage vesicles (CSVs) are cellular compartments with a very high Cu content. In the nucleus, histones H3-H4 act as Cu-dependent reductases, and Atox1 can be transiently found in the nucleus. CP, ceruloplasmin.
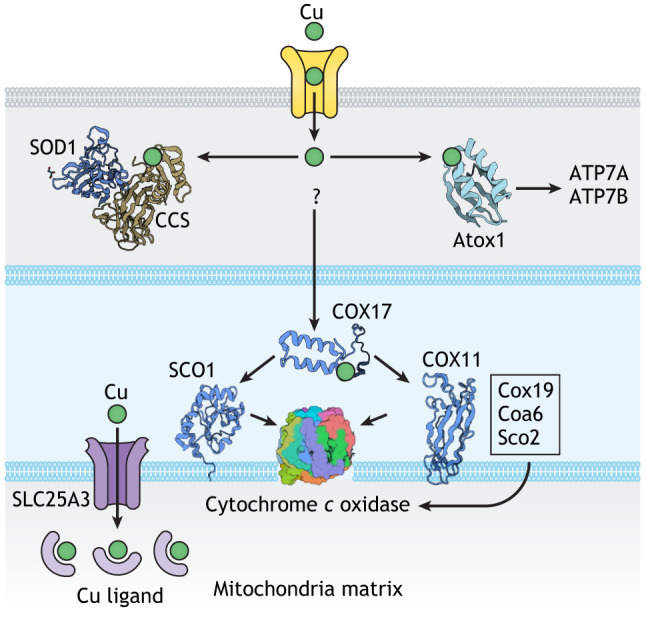
Box 1. Cu chaperones and their known protein targets.
Cu chaperones are small cytosolic proteins that bind Cu(I) and transfer it to the metal-binding site(s) of specific protein targets (Palumaa, 2013). Two classic Cu chaperones, copper chaperone for SOD1 (CCS) and antioxidant protein 1 (Atox1), operate in the cytosol and have similar Cu-binding sites, which are based on the CxxC motif (see figure). CCS transfers Cu to SOD1 by forming a highly specific protein complex (Banci et al., 2012). Atox1 delivers Cu to two ATP-driven Cu transporters, ATP7A and ATP7B, which are located in the membranes of secretory pathway, and regulates their activity (Yu et al., 2017). Unlike CCS, Atox1 interacts weakly with the transporters and may have additional protein targets in the cytosol and nucleus. The Cu transfer machinery for mitochondria cytochome c oxidase (Cco) is complex – both in its protein composition and the mechanism of Cu transfer (Baker et al., 2017a,b) (illustrated in simplified form in the figure). The soluble inner membrane Cu chaperone Cox17 has three pairs of cysteine residues, one of which is reduced and binds Cu. Cox17 transfers Cu along with two electrons to an oxidized Sco1, a membrane-bound Cco-assembly factor, thus both reducing Sco1 and loading it with Cu (Banci et al., 2008). Sco2 is a membrane-bound thiol reductase, which makes the Cu-binding site in Cco available for Cu insertion. Coa6 is a thiol-reductase, which reduces Sco2 (Maghool et al., 2019; Soma et al., 2019). Also in the inner-membrane space, another soluble chaperone, Cox19, facilitates Cu insertion into Cco by interacting with another assembly factor, Cox11. Both Cox17 and Cox19 can also be found in the cytosol; however, it remains unclear whether they represent the major Cu shuttles on the route from CTR1 to mitochondria.
Mitochondria and the secretory pathway are two other major sites of Cu utilization. The delivery of Cu to mitochondria is obligatory for cell survival, because Cu is essential for activity of cytochrome c oxidase, the major component of respiratory chain (Cobine et al., 2021). Cu transport to the secretory pathway enables differentiated cells to perform their specialized functions by activating specific Cu-dependent enzymes (Fig. 1). The Cu-dependent enzymes that mature and function in the secretory pathways make it possible for cells to produce neuromodulators and pigments (Hatori et al., 2016), form and maintain extracellular matrix (Mäki et al., 2005; Shanbhag et al., 2019), regulate iron efflux (Jiang et al., 2016), mediate wound healing (Das et al., 2016), and regulate the uptake of metabolic fuels (Yang et al., 2018). The compartments of the secretory pathway, which are heavily involved in Cu processing and utilization, include the trans-Golgi network (TGN), endo-lysosomes, secretory granules and Cu-storage vesicles (Fig. 1A). Little is known about the role of Cu in the nucleus. However, the inhibitory effect of elevated Cu on histone acetylation (Sarode et al., 2021) and the recently discovered ability of histones H3-H4 to act as Cu reductases (Attar et al., 2020) illustrate that nucleus is an important component of the Cu distribution network, which deserves more studies.
Accumulating data indicate that the distribution of Cu between cellular compartments is non-static and is determined by cellular needs, such as differentiation, mitochondria biogenesis, changes in cellular redox status, hormonal signaling, hypoxia and others. The Cu transporters SLC31A1, SLC31A2, ATP7A and ATP7B, together with their regulators (see below) act as critical determinants of intracellular Cu distribution, when metabolic needs are changed. This Review provides an update on the roles of these essential Cu transporters in mammalian cells and the mechanisms of their regulation. The main objective is to illustrate that, depending on specific metabolic needs, cells restructure their Cu transport networks to route Cu to destinations where it is used to satisfy these needs.
CTR1 is the primary regulator of Cu uptake
The high-affinity Cu transporter (CTR1, also known as SLC31A1) is responsible for the majority of Cu uptake into cells. This homo-trimeric N- and O-glycosylated protein is located at the plasma membrane; its large extracellular domain is enriched in the Cu-binding amino acids histidine and methionine, and is thought to guide Cu towards the transmembrane channel located in the center of the trimer (Pope et al., 2012; Ren et al., 2019). This domain also plays a regulatory role. Loss of O-glycosylation within this domain is coupled to a proteolytic cleavage at residues Ala29–His33, which decreases the rate of Cu transport (Maryon et al., 2007). It has been suggested that the cleavage is facilitated by interactions between CTR1 and CTR2 (also known as SLC31A2), a structural homolog of CTR1, which lacks the Cu-binding domain (Ohrvik et al., 2013).
CTR1 and CTR2 have distinct cellular locations and functions (Fig. 2). CTR1 is ubiquitously expressed and has the same Cu-uptake function in most cells (Öhrvik and Thiele, 2014), whereas CTR2 expression is cell specific. For example, in the brain, CTR2 is thought to be expressed in specialized astrocytes that harbor Cu-storage vesicles and are enriched in lateral ventricles (Leary and Ralle, 2020). CTR1 works at the plasma membrane, and genetic inactivation of CTR1 causes Cu deficiency in cells (Lee et al., 2002). In contrast, CTR2 is located in endo-lysosomes, where it facilitates Cu release from the lumen of vesicles into cytosol (Öhrvik et al., 2015). Inactivation of CTR2 is associated with Cu accumulation in cells, presumably due to retention of Cu in endo-lysosomes (Ohrvik et al., 2013). The essential role of CTR1 in copper homeostasis has been demonstrated (Lee et al., 2001, 2002). By contrast, little is known about the contribution of CTR2 to mammalian cell physiology, although an intriguing role in the maturation of secretory granules has been shown in mast cells (Öhrvik et al., 2015; Hu Frisk et al., 2017).
Fig. 2.
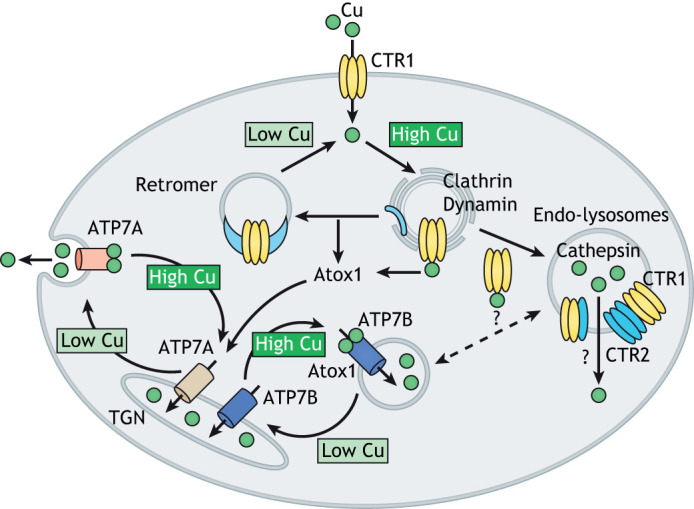
Cu-mediated regulation of the distribution of Cu transporters between cellular compartments. The amount of Cu taken into cells depends on the abundance of CTR1 at the plasma membrane. Excess Cu stimulates the clathrin- and dynamin-dependent endocytosis of CTR1. Atox1 removes Cu from the cytosolic-binding sites on CTR1 and also transfers Cu to the regulatory metal-binding sites on the ATP-driven Cu transporters ATP7A and ATP7B. From the recycling endosomes, CTR1 either returns to the plasma membrane with the assistance of retromer or moves to endo-lysosomes, where it interacts with the low-affinity Cu transporter CTR2 and regulates Cu release to the cytosol. Cu levels in the cytosol are maintained by the ATP-driven Cu transporters ATP7A and ATP7B. These transporters are typically targeted to sub-compartments of TGN but can also be found in endosomes and specialized compartments such as melanosomes. They transfer Cu from the cytosol to the TGN lumen to activate the Cu-dependent enzymes within the secretory pathway. When cytosolic Cu is high, ATP7A and ATP7B leave the TGN to facilitate Cu export (see Box 2 for details). Whether ATP7B and CTR2 move Cu in opposite direction in the same vesicular compartment is unclear.
The relationship between CTR1- and CTR2-mediated Cu transport in the cytosol is complicated by the fact that CTR2 by itself does not have a significant Cu-transport activity and is degraded in the absence of CTR1, at least in some cells (Tsai et al., 2015). Unlike CTR1, which was convincingly shown to form a stable homotrimer (Pope et al., 2012), the oligomerization status of functional CTR2 is unclear. CTR2 and CTR1 may interact as pre-assembled homotrimers; alternatively, it seems possible that CTR2 could heterotrimerize with CTR1 to form a new complex with distinct transport characteristics (Fig. 2). How these two proteins work together to mediate Cu release into a cytosol is a fascinating mechanistic question, which awaits further studies.
Cellular Cu uptake depends on the Cu status of cytosol and mitochondria
The amount of Cu uptake into cells is determined largely by the abundance of CTR1 at the plasma membrane, which is regulated by Cu. In in vitro experiments, addition of as little as 0.5–1 μM of Cu to HEK293 cells triggers the retrieval of CTR1 from the plasma membrane to recycling endosomes via clathrin- and dynamin-dependent endocytosis (Clifford et al., 2016). CTR1 traffics back to the plasma membrane when the amount of cytosolic Cu is decreased (Fig. 2). The return is facilitated by retromer, as evident by colocalization of CTR1 with the retromer component VPS35 in endosomes and the disruption of CTR1 trafficking upon VPS35 knockout (Curnock and Cullen, 2020). Relocation of CTR1 to endosomes is also required for its proteolytic cleavage, which is mediated by cathepsins L and B (Öhrvik et al., 2016). Whether this proteolytic cleavage enhances the interactions between CTR1 and CTR2 is an intriguing question for future studies.
CTR1 abundance at the plasma membrane can also be modulated by regulation of its expression at the mRNA level. In the liver, chronic Cu overload decreases CTR1 mRNA abundance (Gray et al., 2012); this effect is associated with an upregulation of several RNA-binding proteins (snRNP-F, KHDRBS1, DDX21 and others), including PTBP1 (Burkhead et al., 2011). In osteosarcoma cells, PTBP1 has been shown to directly bind to CTR1 mRNA, and knockdown of PTBP1 increased the CTR1 transcript levels (Cheng et al., 2020). CTR1 abundance and localization also show a remarkable dependence on the Cu status of mitochondria, specifically on the presence of active SCO1, a protein necessary for Cu delivery to mitochondria cytochrome c oxidase (Cco) (Leary et al., 2004) (discussed below). In the liver, genetic deletion of SCO1 is associated with a significant decrease of CTR1 due to its degradation (Hlynialuk et al., 2015), whereas in SCO1-mutated cardiomyoblasts, a lower abundance of CTR1 at the plasma membrane is caused by its re-localization to cytosolic vesicles (Baker et al., 2017a,b). Interestingly, in colorectal cancer, a coordinated upregulation of SLC31A1, SCO1 and COX11 transcripts was observed (Barresi et al., 2016), further highlighting a relationship between the machinery involved in Cu transfer to Cco and Cu uptake at the plasma membrane. How SCO1 communicates its status to CTR1 is another fascinating question for future studies.
ATP7A and ATP7B are responsible for intracellular Cu distribution, efflux and storage
The ATP-driven Cu transporters ATP7A and ATP7B contribute to cellular Cu homeostasis in several critical ways (Linz and Lutsenko, 2007; La Fontaine et al., 2010). Their primary function is to transport Cu from the cytosol across the membranes of several compartments, including trans-Golgi network, various endosomes and specialized organelles, such as melanosomes (Fig. 1). Cu transport into these compartments is required for activation of Cu-dependent enzymes that mature within the secretory pathway (biosynthetic function), for the export of excess Cu from cells and, in some cells, for Cu storage (homeostatic function).
ATP7A and ATP7B are structurally similar membrane proteins. They belong to the family of P1B-type ATPases and use the energy of ATP hydrolysis to transfer Cu from the cytosol across the membranes (Linz and Lutsenko, 2007; Lutsenko et al., 2008). Despite their structural and biochemical similarities, the physiological roles of ATP7A and ATP7B are different and determined by their tissue-specific expression, and differences in intracellular localization and trafficking, as well as distinct regulation (Polishchuk and Lutsenko, 2013). Available single-cell RNA sequencing data (see Single Cell Portal https://singlecell.broadinstitute.org/single_cell) indicate that many cells and tissues express both ATP7A and ATP7B at various ratios; however, how these transporters work together to balance cellular Cu remains to be established. In cells and tissues characterized so far, ATP7A is usually more abundant than ATP7B and plays a primary role in Cu delivery to Cu-dependent enzymes as well as in Cu efflux (Telianidis et al., 2013), whereas ATP7B has a regulatory function – it buffers Cu in the cytosol and sequesters Cu in vesicles for storage (see Pierson et al., 2018 and below). In hepatocytes of adult liver, however, ATP7B is the primary Cu transporter, and expression of ATP7A is low (Lenartowicz et al., 2010).
In cells with only one ATP-driven Cu transporter [for example, ATP7A in adipocytes (Yang et al., 2018) or ATP7B in hepatocytes (Lenartowicz et al., 2010)], either ATP7A or ATP7B are targeted to the TGN, where they activate Cu-dependent enzymes. ATP7A has been shown to transfer Cu to peptidyl-α-monooxygenase (PAM) (El Meskini et al., 2003), tyrosinase (Petris et al., 2000), dopamine-β-hydroxylase (DBH) (Xiao et al., 2018), amino oxidase 3 (AOC3; also known as SSAO) (Yang et al., 2018), extracellular superoxide dismutase 3 (Qin et al., 2006) and the lysyl-oxidase (LOX) family of proteins (Shanbhag et al., 2019), whereas ATP7B transfers Cu to ceruloplasmin (Hellman et al., 2002). In tissues, it is not always clear which of these two Cu transporters supplies Cu to specific enzymes. For example, liver is the major source of the Cu-containing blood clotting factor VIII (CFVIII), and ATP7B is the primary Cu transporter in hepatocytes. However, CFVIII is produced by liver sinusoidal endothelial cells (Pradhan-Sundd et al., 2021), and whether ATP7B functions in these cells is currently unknown. It was also thought that Cu-dependent enzymes all obtain their Cu cofactor in the same manner – by directly capturing Cu released from the transporter, without assistance of other proteins (El Meskini et al., 2003). However, the recent discovery of the involvement of fibulin-4 in Cu incorporation into LOX (Noda et al., 2020) suggests that transporter-mediated Cu transfer to an acceptor enzyme is more complex than previously anticipated. Therefore, the full elucidation of the mechanisms of Cu transfer to acceptor proteins require further study.
In cells in which both ATP7A and ATP7B are expressed, they localize to different sub-compartments of secretory pathway and have non-redundant functions. In SH-SY5Y cells, a cell model of noradrenergic neurons, ATP7A transports Cu to Cu-dependent enzymes in the TGN and exports Cu from cells by trafficking towards the plasma membrane, whereas ATP7B pumps Cu into vesicles and thus regulates the availability of Cu to ATP7A (Schmidt et al., 2018). The activity of both ATP7A and ATP7B is regulated by the Cu chaperone Atox1, which transfers Cu to the N-terminal metal-binding domains of these transporters (Banci et al., 2009; Yu et al., 2017). Early biochemical studies did not find marked differences between ATP7A and ATP7B in their interactions with Atox1 (Lutsenko et al., 2007). Therefore, Cu is likely to be distributed between ATP7A and ATP7B based on their relative abundance in cells. In addition, the occupancy of metal-binding sites of Cu(I) transporters can be altered by the glutaredoxin Grx1, which regulates glutathionylation of ATP7A and ATP7B and Cu levels in cells (Singleton et al., 2010). Further studies are needed to determine how Atox1 and Glrx1, which have similar CxxC-based metal-binding sites and a similar sensitivity to changes in the cellular redox status (Hatori et al., 2012), work together to regulate ATP7A and ATP7B.
Multilayered regulation of ATP7A and ATP7B determines the intracellular Cu fluxes
Regulation of ATP7A and ATP7B localization and function is central to intracellular Cu distribution and is multilayered. Changes in cellular Cu levels affect both ATP7A and ATP7B. In polarized cells, intracellular Cu elevation triggers kinase-mediated phosphorylation and trafficking of ATP7A from the TGN towards the basolateral membrane, whereas ATP7B moves from the TGN towards the apical membrane via endolysosomes (Fig. 2; Box 2). Decrease in the intracellular Cu content is associated with dephosphorylation of ATP7A and/or ATP7B and return to the TGN. The Cu-dependent change in the intracellular localization represents the major mechanism that enables ATP7A and ATP7B to switch from their biosynthetic function (Cu delivery to Cu-dependent enzymes) to homeostatic function (sequestration of excess Cu in vesicles and Cu export). The biosynthetic function becomes particularly important when cells acquire their functional identity during differentiation (see below). The homeostatic function (Cu sequestration and export) is critical in tissues with high Cu fluxes, such as the small intestine, liver, kidneys, brain endothelial cells and choroid plexus. Whether and how Cu-dependent endocytosis of CTR1 and trafficking of ATP7A and ATP7B are coordinated remains to be characterized.
Box 2. Simplified overview of ATP7A and ATP7B trafficking.
Cu-dependent trafficking of ATP7A and ATP7B from the TGN is initiated when the luminal Cu acceptor proteins are saturated with Cu and further transport is stalled (see box figure). Excess Cu binds to the regulatory metal-binding domains; this causes structural changes in this domain and increases kinase-mediated phosphorylation (indicated by ‘P’ in the box figure). Phosphorylation weakens interactions between the cytosolic domains and accelerates interactions with the trafficking machinery (Hasan et al., 2012; Nyasae et al., 2014). ATP7A-containing vesicles move towards the basolateral membrane, where they fuse with the membrane and either recycle following clathrin- and AP-2-mediated endocytosis via endosomes or return back to the TGN with the assistance of retromer. The COMMD1–CCDC22–CCDC93 (CCC) complex together with the WASH complex control movement of ATP7A from endosomes (Phillips-Krawczak et al., 2015) ATP7B-containing vesicles first move towards the basolateral membrane and then, via transcytosis, relocate to the apical membrane. This latter step depends on the clathrin adaptor protein AP-2α, dynamin and microtubules (Lalioti et al., 2016). ATP7B trafficking involves interactions between the N-terminal domain of ATP7B and dynactin 4 (Lim et al., 2006). Myosin Vb, which is located near the F-actin-rich apical membrane, is required for ATP7B targeting to the apical region, where ATP7B colocalizes with Rab11 (Gupta et al., 2016). COMMD1 binds to endosomes via phosphatidylinositol (4,5)-bisphosphate and regulates ATP7B distribution along the endocytic pathway (Stewart et al., 2019). COG7, a component of the COG complex that regulates vesicle fusion with Golgi, interacts with ATP7A and regulates its abundance (Comstra et al., 2017). Cu depletion tightens the TGN localization for both ATP7A and ATP7B. Cytosolic metallothioneins participate in Cu ‘rationing’ for Cu transporters (Gudekar et al., 2020).
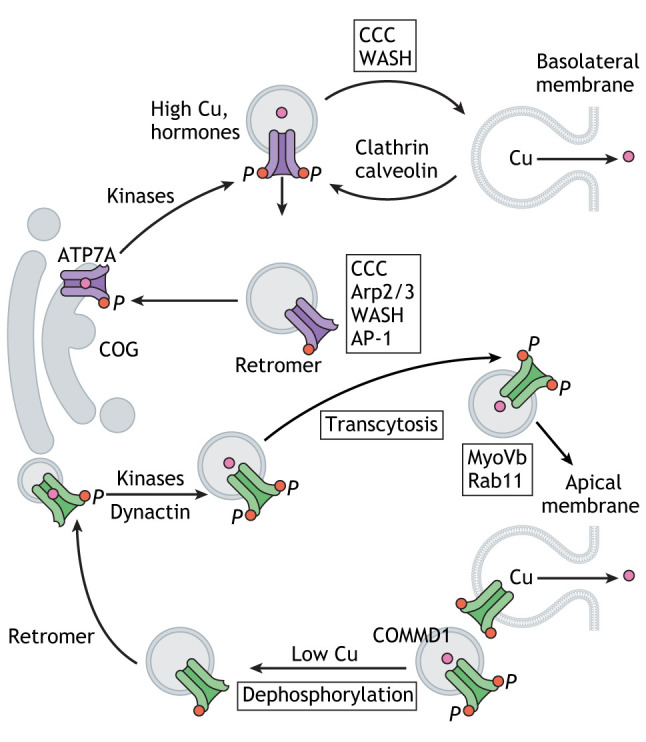
Studies of the ATP7A interactome have determined that a conserved oligomeric Golgi complex (COG) contributes to the trafficking of ATP7A to the plasma membrane (Comstra et al., 2017). The trafficking also involves the adaptor proteins AP1 and AP2, retromer, Arp2/3, the WASH complex and BLOC-1, as well as COMMD1-based protein complexes (Box 2) (for details, see a recent review by Hartwig et al., 2019). The conserved di-leucine motifs in the C-terminus of ATP7A and ATP7B are important for retrograde trafficking to the TGN as they interact with the σ1 subunit of the clathrin adaptor AP-1 (Holloway et al., 2013; Jain et al., 2015; Zhu et al., 2016). Less is known about kinases and phosphatases involved in phosphorylation and dephosphorylation of ATP7A and ATP7B. In vascular smooth muscle cells, Akt2 phosphorylates ATP7A at one of the C-terminal residues Ser1424, Ser1463 or Ser1466, and promotes its trafficking from the TGN to endosomes (Sudhahar et al., 2018). Protein kinase D has been implicated in the phosphorylation of ATP7B in response to Cu elevation in COS-7 and HepG2 cells (Pilankatta et al., 2011). It is currently unknown whether these kinases somehow ‘sense’ changes in the cellular Cu levels and/or detect Cu-induced structural changes within the Cu transporters. The presence of numerous phosphorylation sites in the N- and C-termini of both ATP7A and ATP7B suggests that more than one kinase may regulate Cu transporters in response to different stimuli (Braiterman et al., 2015). Indeed, insulin, estrogen and glucagon have been shown to regulate activity and trafficking of ATP7A and ATP7B in a Cu-independent manner (Hardman et al., 2007b; Hilário-Souza et al., 2016; Sudhahar et al., 2018), whereas PDGF stimulates relocation of ATP7A to the leading edge of migrating vascular muscle cells by stimulating cellular Cu uptake by CTR1 (Ashino et al., 2010). It is thus likely that many more metabolic regulators will emerge.
In addition to trafficking, the activity of ATP7A and ATP7B is regulated through changes in their abundance. In BE(2)-C and SH-SY5Y cells, ATP7A expression is regulated by retinoic acid receptor (Bohlken et al., 2009). In muscle cells, during differentiation, the length of the 3′UTR of ATP7A mRNA shortens owing to utilization of an alternative polyadenylation signal (Vest et al., 2018), and the presence of this shorter 3′UTR is associated with an increased stability of ATP7A mRNA (Vest et al., 2018). In C2C12 cells, PTBP1, which downregulates CTR1, also binds to Atp7a mRNA and negatively regulates its abundance (Whitlow et al., 2021). An upregulation of ATP7A and associated Cu export takes place in the liver in response to Cu deficit in the heart, demonstrating reprogramming of the Cu transport network driven by inter-organ communication (Kim et al., 2010).
For ATP7B, changes in mRNA and protein abundance have been reported in response to tissue-specific stimuli, such as circadian rhythms in pineal gland (Borjigin et al., 1999), changes in dietary Cu in intestine (Pierson et al., 2018) and hormonal signaling in placental cells (Hardman et al., 2007b). In squamous cell carcinoma of the head and neck (SCCHN), the levels of ATP7B mRNA are influenced by TMEM16A, a Ca2+-dependent chloride channel (Vyas et al., 2019), possibly reflecting a previously demonstrated chloride-dependence of Cu transfer to the ceruloplasmin orthologue FET3 (Davis-Kaplan et al., 1998). A strong positive correlation between TMEM16 and ATP7B expression was observed in SCCHN tumors (Vyas et al., 2019); however, the specific role of ATP7B in metabolic reprogramming of tumors remains unclear. The reader is referred to recent reviews that summarize the current view on Cu involvement in cancer biology (Chang et al., 2021; Tsang et al., 2021). Taken together, the available data appear to suggest that an increase of Cu transfer to enzymes within the secretory pathway is associated with the coordinated increase in abundance of ATP7A and CTR1, whereas the regulation of ATP7B is somewhat independent. Interestingly, in senescent cells, coordinated upregulation of CTR1 and ATP7A is not observed (ATP7A is downregulated) (Masaldan et al., 2018). It would be highly significant to determine how important regulatory signals modulate the entire Cu handling network in health and disease.
Cu storage
Intracellular Cu is efficiently buffered by thiol-containing molecules, such as metallothioneins and glutathione (Morgan et al., 2019), but can be released from these labile pools through oxidation and possibly other yet-to-be-identified mechanisms. Cu is also stored in specialized vesicles (Pushkar et al., 2013; Leary and Ralle, 2020) and in secretory granules (Bonnemaison et al., 2016). In the intestine, ATP7B sequesters Cu to large vesicles (Pierson et al., 2018). Although it has been convincingly shown that Cu accumulates in lysosomes (Myers et al., 1993), especially when Cu export from cell is disrupted, it is unclear whether these lysosomes represent a specialized subset for Cu storage or a general compartment where protein degradation occurs. The exact nature and the relationship between various Cu storage compartments is a fascinating topic for future studies. The available data suggest that CTR2 plays a role in releasing Cu from endolysosmal vesicles (Ohrvik et al., 2013), whereas the activity of lysosomal transporter SLC46A3 impacts the lysosomal Cu content (Kim et al., 2021). It has also been suggested that Niemann–Pick protein, an endosomal cholesterol transporter, may regulate Cu levels in tissues (Yanagimoto et al., 2011). The deletion of this protein in mice causes Cu accumulation in several tissues (Baguña Torres et al., 2019). Given that Cu overload markedly decreases hepatic cholesterol (Huster et al., 2007) and that ATP7B-dependent Cu storage in the intestine affects formation of chylomicrones (Pierson et al., 2018), it is tempting to speculate that cholesterol and Cu balance in cells could be interdependent, and further studies would be illuminating.
Mechanisms coordinating activity of Cu-containing proteins and Cu transport
Cu-dependent enzymes (cuproenzymes) are major users of cellular Cu, and a wealth of information is available about their catalytic properties, organization of Cu-binding sites, and roles in normal physiology and disease; for the full list of enzymes see Horn and Wittung-Stafshede (2021). By comparison, much less is known about how cellular Cu transport is adjusted to accommodate changes in the abundance and activity of specific cuproenzymes located in different cellular compartments, when metabolic conditions change. One obvious mechanism is an upregulation of Cu uptake and Cu-trafficking proteins in response to an increase in the abundance of a Cu-requiring enzyme. Several cuproenzymes rely on this mechanism; one example is LOX, which catalyzes the crosslinking of collagen and elastin and requires Cu for this activity. Upregulation of LOX in the breast cancer cells has been shown to coincide with an increased expression of several Cu-handling proteins, which together facilitate a Cu delivery to the secretory pathway (Ashino et al., 2010; Blockhuys and Wittung-Stafshede, 2017), where Cu binds to LOX and also facilitates the autocatalytic formation of a lysine tyrosylquinone cofactor necessary for LOX activity (Vallet and Ricard-Blum, 2019). Another example of a restructuring of the Cu transport network is the macrophage response to hypoxia, which stimulates Cu uptake by upregulating CTR1 and prioritizes Cu transfer to secretory pathway, while decreasing Cu delivery to cytosolic SOD1 and mitochondrial Cco (White et al., 2009).
Cco is an essential Cu-dependent component of the mitochondria respiratory chain. Cco requires a complex protein machinery for the delivery of Cu to its two Cu-binding sites (see Box 1) (Timón-Gómez et al., 2018). In mitochondria, small soluble Cu carriers work together with the membrane-bound Cu chaperones/oxidoreductases (Sco1, Sco2 and Coa6) in protein ‘conveyer belt’ (Box 2). Here, the redox reactions that maintain Cu-coordinating sites in a state that is necessary for binding of Cu are coupled to the Cu-transfer steps (for a recent review see Timón-Gómez et al., 2018). In addition, the Cu and phosphate transporter SLC25A3 brings Cu to mitochondria matrix (Boulet et al., 2018), where Cu binds to an as-yet-uncharacterized Cu ligand, which also influences Cu insertion into Cco.
It is thought that Cu delivery to mitochondria is prioritized over other intracellular destinations because of the critical importance of mitochondria for cell viability; however, the mechanism remains uncertain. It was suggested that Cu distribution to mitochondria and other organelles is governed by the differential affinities of cytosolic Cu chaperones for the metal (Banci et al., 2010), but the methodology for measuring affinity for Cu in this study was questioned (Xiao et al., 2011). It seems likely that not only Cu-binding affinity, but also increased abundance of Cu chaperones could contribute to an enhanced Cu flow to mitochondria, particularly during mitochondria biogenesis. Indeed, the mRNA levels of the cytosolic mitochondria-directed Cu chaperones Cox17 and Cox19 increase significantly upon initiation of mitochondria biogenesis (Couvillion et al., 2016).
Oxygen metabolism in mitochondria is associated with the generation of superoxide, which is harmful to the cell. (Cu+ and Zn2+)-dependent superoxide dismutase 1 (SOD1) is an abundant enzyme that detoxifies the superoxide radical by converting it into molecular oxygen and peroxide (Leitch et al., 2009). The cytosolic Cu chaperone for SOD1 (CCS) facilitates Cu delivery to SOD1 (Box 1) and also assists in its folding, disulfide bond formation and zinc binding (Boyd et al., 2020). SOD1 is also involved in the regulation of metabolism, metal buffering and peroxide signaling, as well as other cellular processes (Banks and Andersen, 2019). These functions require changes in SOD1 abundance and/or activity. A rapid increase in SOD1 activity in response to metabolic and environmental changes can be mediated through the CCS-dependent activation of the pre-existing pool of a fully-folded apo-SOD1 (Brown et al., 2004). The source of extra Cu could be either increased activity of the high-affinity copper transporter CTR1, which was shown to interact with CCS (Skopp et al., 2019) or, in the case of oxidative stress, the oxidation-dependent release of Cu from labile pools (Morgan et al., 2019).
Cell differentiation is associated with Cu transport network remodeling
Cell differentiation is associated with changes in expression of Cu transporters, acceptor proteins and changes in the cellular Cu content (Hatori et al., 2016; Vest et al., 2018; Yang et al., 2018). A proper Cu supply has been shown to be necessary for myoblast differentiation (Vest et al., 2018). In male gonads, CTR1, ATP7A and ATP7B regulate Cu homeostasis during spermatogenesis (Ogórek et al., 2017). An increase in expression of both CTR1 and ATP7A during spermatogenesis suggests that increased Cu flux through the secretory pathway contributes to functional maturation of male germ cells. Furthermore, changes in ATP7A expression parallel changes in testicular Cu content (Ogórek et al., 2017). Similarly, in differentiating adipocytes, ATP7A is upregulated during differentiation to accommodate the necessary increase in Cu delivery to AOC3, which becomes highly abundant in mature fat cells (Yang et al., 2018).
Over the course of cell differentiation, the ratio between ATP7A and ATP7B may change. In non-differentiated proliferating neuronal cells (Hatori et al., 2016) and immature adipocytes (Yang et al., 2018), both ATP7A and ATP7B are present in measurable quantities, whereas in myoblasts, only ATP7B is detected (Vest et al., 2018). When these cells complete differentiation, ATP7A is significantly upregulated, whereas ATP7B is downregulated or remains unchanged (Hatori et al., 2016; Vest et al., 2018; Yang et al., 2018). Increased abundance of ATP7A accelerates Cu transfer to the secretory pathway (Hatori et al., 2016). In addition, Cu distribution to the secretory pathway is regulated by the redox status of the cell cytosol, specifically the ratio of reduced and oxidized glutathione (Hatori et al., 2016). The Cu chaperone Atox1 transfers Cu to the N-terminal domains of ATP7A and ATP7B, which stimulates their activity and thus accelerates Cu transport to the secretory pathway (Yu et al., 2017). Atox1 is a redox-sensitive molecule. Its Cu-binding cysteines form a disulfide bond when the cytosolic environment becomes more oxidizing, which prevents Cu binding, and bind Cu when the environment becomes reducing (Hatori and Lutsenko, 2013). Changes in the redox state of glutathione occur during neuronal cell differentiation, increasing the amount of reduced Atox1 molecules and thus enhancing Cu binding and its transfer to ATP7A (Hatori et al., 2016). This process augments the increase in ATP7A protein abundance, which is necessary to accommodate a marked upregulation of peptidyl-α-monooxygenase and dopamine-β-hydroxylase within the secretory pathway (Hatori et al., 2016; Yang et al., 2018). These developmental changes are disrupted in many cancers, resulting in Cu dis-homeostasis, altered activity of Cu-dependent enzymes and dysregulated signaling mechanisms (Shanbhag et al., 2021). Whether or not the increased Cu flow to one compartment negatively affects the others remains to be determined.
Cell-specific Cu transport mechanisms vary significantly
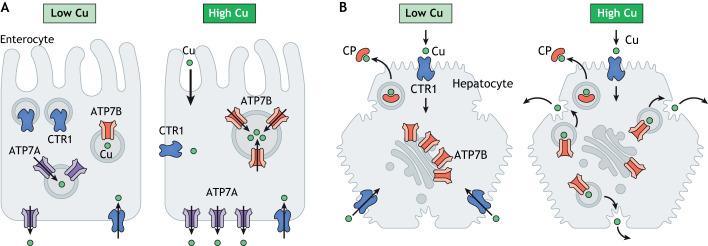
Cell-specific Cu transport networks remain greatly understudied. Only limited data exist on the role of ATP7A and ATP7B in balancing Cu content in tissues, such as placenta (Hardman et al., 2007a,b), mammary gland (Michalczyk et al., 2000; Llanos et al., 2008) and kidneys (Linz et al., 2008), which are all tissues where both transporters are relatively highly expressed, but the mechanistic details are lacking. Discussed below are the examples of enterocytes and hepatocytes, which exhibit marked differences in the regulation and function of Cu transport networks in the respective tissues (Fig. 3).
Fig. 3.
A comparison of the Cu transport machinery and its response to Cu in enterocytes and hepatocytes. In enterocytes when Cu is limited, CTR1 is upregulated and can be found at the basolateral membrane and in vesicles in the vicinity to apical membrane. In low Cu, ATP7B is downregulated and is targeted to the TGN and endocytic vesicles. ATP7A is predominantly found in vesicles near the basolateral membrane. When Cu increases, intestinal CTR1 is downregulated, ATP7A relocates to the plasma membrane to facilitate Cu export. In response to Cu elevation, ATP7B is upregulated and ATP7B-containing vesicles become more numerous and larger in size. In hepatocytes, under basal or low Cu, CTR1 is targeted to the basolateral membranes and ATP7B is targeted predominantly to the TGN, where it delivers Cu to ceruloplasmin (CP). Cu elevation (in response to increased dietary intake or in Wilson disease) decreases CTR1 abundance at the plasma membrane via endocytosis or changes in the mRNA abundance. In high Cu, ATP7B levels do not change, instead ATP7B relocates to the endolysosomal compartment to facilitate Cu export.
Regulation of Cu transport in enterocytes
The stomach and small intestine are the major sites for dietary Cu absorption (Van campen and Mitchell, 1965). How Cu enters the enterocytes from the lumen of the gut has been a subject of significant interest, but the mechanism is still not fully understood (Fig. 3). CTR1 has been proposed to play a central role in this process, as it can be detected at the apical membrane facing the gut lumen (Nose et al., 2010). However, the targeted deletion of CTR1 in enterocytes does not prevent Cu entry; instead, it causes significant Cu accumulation and the loss of Cu export from the gut (Nose et al., 2006). This phenotype is also associated with significant morphological changes in intestine, abnormal cell patterning, as well as overexpression of ATP7B, which appears to be responsible for sequestration of Cu into vesicles and making it unavailable for export (Pierson et al., 2018). Immunostaining of CTR1 in the intestinal tissue also yielded conflicting results, as both basolateral plasma membrane localization (Zimnicka et al., 2007; Pierson et al., 2019) and apical membrane targeting (Nose et al., 2010) were observed in different studies. These differences may reflect different ages of mice [2-week-old pups showed clear basolateral staining of CTR1 (Pierson et al., 2019), whereas apical surface biotinylation of CTR1 was seen in adult mice (Nose et al., 2010)], and the very different diet of suckling pups compared to adult animals along with other potential metabolic and developmental differences. The relationship between CTR1 and CTR2 in the intestine has not been investigated; therefore, it is not clear whether the different pools of CTR1 contain only CTR1 homotrimer or also the CTR1–CTR2 complex. Nevertheless, it is clear that the activity of intestinal CTR1 is required for normal intestinal morphology, proper cell differentiation and patterning, as well as the processing of dietary Cu for export into the blood.
Export of Cu from enterocytes into the bloodstream is mediated by the Cu(I) transporter ATP7A (Wang et al., 2012). Immunostaining of intestinal tissue shows ATP7A to be predominantly located at the basolateral membrane in agreement with its major role in Cu export (Nyasae et al., 2007). Studies in cells and animals suggest that, in enterocytes, ATP7A does not permanently reside at the basolateral membrane. Instead, ATP7A is targeted to vesicles in the vicinity of the membrane where it sequesters Cu from the cytosol (Nyasae et al., 2007; Pierson et al., 2018). Cu export is mediated, presumably, by the fusion of vesicles with the plasma membrane. This process is stimulated by elevated Cu and is essential for the dietary Cu export into the bloodstream (Nyasae et al., 2007). Genetic inactivation of Atp7a in mouse enterocytes is associated with Cu retention in the intestine, and a significant Cu deficiency in the rest of the organism, which in turn causes heart hypertrophy and neurological impairment (Wang et al., 2012).
The abundance of intestinal ATP7A is regulated by the Cu status of the whole organism (Chun et al., 2017) and represents a perfect example of the tissue-specific regulation of Cu transporters. Specifically, changes in the intestinal ATP7A levels are opposite to changes in ATP7A within peripheral tissues. Under conditions of systemic Cu deficiency, peripheral ATP7A is downregulated to preserve tissue Cu content, whereas intestinal ATP7A is upregulated to increase Cu delivery to the blood (Chun et al., 2017). Systemic Cu overload has an opposite effect; downregulation of intestinal ATP7A (to decrease dietary Cu absorption) is accompanied by ATP7A upregulation in peripheral tissues (to prevent toxic Cu overload) (Chun et al., 2017). By contrast, acute changes of Cu levels in the diet do not have a notable effect on ATP7A expression in enterocytes. Instead, short-term elevation of dietary Cu correlates with an increased frequency of fusion of ATP7A-carrying vesicles with the plasma membrane (Nyasae et al., 2007).
Although higher amounts of Cu in the diet increase Cu flux through enterocytes, within the enterocytes, the intracellular Cu concentration is tightly controlled by ATP7B (Pierson et al., 2018). Enteric ATP7B is targeted primarily to vesicles, presumably endosomes, although targeting to the TGN has also been observed (Weiss et al., 2008). Cu elevation is associated with an increased ATP7B abundance, whereas Cu depletion decreases ATP7B protein levels in enterocytes (Fig. 3 and Pierson et al., 2018). This Cu-dependent regulation of ATP7B along with its vesicular localization led to the suggestion that intestinal ATP7B functions to buffer cytosolic Cu (Pierson et al., 2018). The significance of such a buffering is apparent in Atp7b−/− knockout mice, which show intestinal fat accumulation, mislocalization of the triglyceride carrier protein ApoB and a diminished amount of lipoprotein particles called chylomicrones (Pierson et al., 2018). Further studies of Atp7b- knockout mice have also revealed that these mice show significant iron accumulation in enterocytes (Pierson et al., 2018).
It is worth noting that the total amount of Cu that enters the gastrointestinal tract is not limited to the dietary Cu. Saliva, pancreatic juices and gastric juices contain significant amount of Cu, most of which is being recycled by the body (Linder, 2020). The forms in which Cu is carried in the bloodstream and other biological fluids have been investigated, but more studies are needed to established the precise molecular nature of the carriers involved (reviewed in Linder, 2020).
Regulation of Cu homeostasis in the liver
The liver is a uniquely important organ for whole-body Cu homeostasis, as it receives and processes the majority of dietary Cu from the bloodstream. It also produces ceruloplasmin, the most abundant Cu-containing protein in serum, and removes excess Cu to the bile (Cartwright and Wintrobe, 1964; Owen and Orvis, 1970). The molecular details of how the liver performs these functions have been largely worked out, although several mysteries remain (Fig. 3). Cu enters hepatocytes predominantly through CTR1. Accordingly, targeted deletion of CTR1 in mice hepatocytes decreases the total Cu content of the liver by half and reduces incorporation of Cu into ceruloplasmin and other Cu-dependent enzymes (Kim et al., 2009). However, over time, the Cu deficit becomes less pronounced, suggesting the presence of yet-unidentified transporter(s) that compensate for the loss of CTR1 function (Kim et al., 2009).
ATP7B exerts a central role in the Cu homeostasis in the liver. In hepatocytes, ATP7B is targeted primarily to the TGN, where it transfers Cu to ceruloplasmin, which is subsequently secreted (Hellman et al., 2002). Unlike enteric ATP7B, the abundance of hepatic ATP7B is not regulated by Cu. When Cu levels exceed the amount needed for activation of cuproproteins, ATP7B leaves the TGN and traffics to a vesicular compartment in the vicinity of the canalicular (apical) membrane, which appears to be a part of the late endosome/lysosome network (Polishchuk and Polishchuk, 2019). Cu-dependent trafficking is a complex process that is only partially understood (Box 2).
Although experimental evidence is still absent, it is thought that ATP7B-positive vesicles fuse with the apical membranes of hepatocytes to release Cu into the bile (Bartee and Lutsenko, 2007; Hubbard and Braiterman, 2008). Additional mechanisms for Cu export, such as regulated lysosomal exocytosis, have also been proposed (Polishchuk et al., 2014). It is possible that more than one route of Cu export exists and that one of them becomes predominant depending on the extent of Cu elevation in the liver and on whether this increase is gradual or acute (Dijkstra et al., 1997). As long as Cu levels in the cell remain high, ATP7B remains in the apical (cathepsin-B)-positive endo-lysosomal (recycling) compartment where it sequesters excess Cu (Das et al., 2020). When cytosolic Cu decreases, ATP7B returns to the TGN (Box 2). Three members of COMMD family (COMMD1, COMMD6 and COMMD9) form a protein complex that facilitates ATP7B recycling and Cu efflux from cells (Singla et al., 2021). In particular, COMMD1 binds to PtdIns(4,5)P2-containing vesicles and facilitates retention of ATP7B in the recycling vesicular pool (Stewart et al., 2019). The decrease in the cytosolic Cu content is associated with the dephosphorylation of ATP7B, its retrieval from the recycling pool and a retromer-assisted return of ATP7B back to the TGN (Das et al., 2020).
Disruption of ATP7B function in hepatocytes causes accumulation of Cu in the liver, and dysregulation of lipid and nucleic acid metabolisms, as well as increased abundance of respiratory chain components and redox-balancing enzymes (Muchenditsi et al., 2021). Systemic inactivation of Atp7b (i.e. in all liver cells and elsewhere) in mice markedly augments the hepatocyte response, causing further metabolic changes (Wooton-Kee et al., 2020) and an increase in autophagy (Polishchuk et al., 2019), as well as eventual loss of normal liver morphology and function (Zischka et al., 2011; Polishchuk et al., 2019; Wooton-Kee et al., 2020; Muchenditsi et al., 2021). This response not only further illustrates the importance of Cu homeostasis in various cells, but also the significance of intercellular metabolic interactions that contribute to tissue response to Cu misbalance.
Conclusions
Several sophisticated mechanisms of Cu transport and distribution operate in mammalian cells and tissues to sustain their basic metabolism, as well as specialized functions. The multifaceted role of Cu in cell metabolism is facilitated by a highly regulated dynamic Cu transport network. Many regulators of this network have now been discovered, including copper chaperones, kinases (Brady et al., 2014; Tsang et al., 2020) and trafficking proteins (Hartwig et al., 2019; Curnock and Cullen, 2020). These findings begin to explain why Cu imbalance is increasingly being observed in human disorders in which the primary Cu transporters are not mutated, such as in the case of MEDNIK syndrome, which is caused by mutation in a subunit of AP-1, a regulator of ATP7A and ATP7B trafficking. It has also become clear that Cu transporters and their cellular levels change with aging, during immune response and in various diseases, especially in cancer, Alzheimer's disease and obesity, pointing to the possibility of modulating Cu for therapeutic purpose (Jain et al., 2013; Chan et al., 2017; Hunsaker and Franz, 2019). The important next frontier in Cu biology is to understand how Cu is employed by various highly specialized cells, how Cu fluxes between the cell compartments are coordinated, and how cells and tissues communicate their Cu status. Many exciting discoveries await those who takes the road to this new frontier.
Footnotes
Competing interests
I declare no competing or financial interests.
Funding
My work in this area is supported by the National Institutes of Health (grants R01GM101502, R01DK117396 and R01DK071865). Deposited in PMC for release after 12 months.
References
- Ashino, T., Sudhahar, V., Urao, N., Oshikawa, J., Chen, G.-F., Wang, H., Huo, Y., Finney, L., Vogt, S., McKinney, R. D.et al. (2010). Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ. Res. 107, 787-799. 10.1161/CIRCRESAHA.110.225334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attar, N., Campos, O. A., Vogelauer, M., Cheng, C., Xue, Y., Schmollinger, S., Salwinski, L., Mallipeddi, N. V., Boone, B. A., Yen, L.et al. (2020). The histone H3-H4 tetramer is a copper reductase enzyme. Science 369, 59-64. 10.1126/science.aba8740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguña Torres, J., Yu, Z., Bordoloi, J., Sunassee, K., Smith, D., Smith, C., Chen, O., Purchase, R., Tuschl, K., Spencer, J.et al. (2019). Imaging of changes in copper trafficking and redistribution in a mouse model of Niemann-Pick C disease using positron emission tomography. Biometals 32, 293-306. 10.1007/s10534-019-00185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, Z. N., Cobine, P. A. and Leary, S. C. (2017a). The mitochondrion: a central architect of copper homeostasis. Metallomics 9, 1501-1512. 10.1039/C7MT00221A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, Z. N., Jett, K., Boulet, A., Hossain, A., Cobine, P. A., Kim, B.-E., El Zawily, A. M., Lee, L., Tibbits, G. F., Petris, M. J.et al. (2017b). The mitochondrial metallochaperone SCO1 maintains CTR1 at the plasma membrane to preserve copper homeostasis in the murine heart. Hum. Mol. Genet. 26, 4617-4628. 10.1093/hmg/ddx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci, L., Bertini, I., Ciofi-Baffoni, S., Hadjiloi, T., Martinelli, M. and Palumaa, P. (2008). Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc. Natl. Acad. Sci. USA 105, 6803-6808. 10.1073/pnas.0800019105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci, L., Bertini, I., Calderone, V., Della-Malva, N., Felli, I. C., Neri, S., Pavelkova, A. and Rosato, A. (2009). Copper(I)-mediated protein-protein interactions result from suboptimal interaction surfaces. Biochem. J. 422, 37-42. 10.1042/BJ20090422 [DOI] [PubMed] [Google Scholar]
- Banci, L., Bertini, I., Ciofi-Baffoni, S., Kozyreva, T., Zovo, K. and Palumaa, P. (2010). Affinity gradients drive copper to cellular destinations. Nature 465, 645-648. 10.1038/nature09018 [DOI] [PubMed] [Google Scholar]
- Banci, L., Bertini, I., Cantini, F., Kozyreva, T., Massagni, C., Palumaa, P., Rubino, J. T. and Zovo, K. (2012). Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc. Natl. Acad. Sci. USA 109, 13555-13560. 10.1073/pnas.1207493109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, C. J. and Andersen, J. L. (2019). Mechanisms of SOD1 regulation by post-translational modifications. Redox Biol. 26, 101270. 10.1016/j.redox.2019.101270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi, V., Trovato-Salinaro, A., Spampinato, G., Musso, N., Castorina, S., Rizzarelli, E. and Condorelli, D. F. (2016). Transcriptome analysis of copper homeostasis genes reveals coordinated upregulation of SLC31A1,SCO1, and COX11 in colorectal cancer. FEBS Open Bio 6, 794-806. 10.1002/2211-5463.12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee, M. Y. and Lutsenko, S. (2007). Hepatic copper-transporting ATPase ATP7B: function and inactivation at the molecular and cellular level. Biometals 20, 627-637. 10.1007/s10534-006-9074-3 [DOI] [PubMed] [Google Scholar]
- Blockhuys, S. and Wittung-Stafshede, P. (2017). Roles of copper-binding proteins in breast cancer. Int. J. Mol. Sci. 18, 871. 10.3390/ijms18040871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlken, A., Cheung, B. B., Bell, J. L., Koach, J., Smith, S., Sekyere, E., Thomas, W., Norris, M., Haber, M., Lovejoy, D. B.et al. (2009). ATP7A is a novel target of retinoic acid receptor β2 in neuroblastoma cells. Br. J. Cancer 100, 96-105. 10.1038/sj.bjc.6604833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemaison, M. L., Duffy, M. E., Mains, R. E., Vogt, S., Eipper, B. A. and Ralle, M. (2016). Copper, zinc and calcium: imaging and quantification in anterior pituitary secretory granules. Metallomics 8, 1012-1022. 10.1039/C6MT00079G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjigin, J., Payne, A. S., Deng, J., Li, X., Wang, M. M., Ovodenko, B., Gitlin, J. D. and Snyder, S. H. (1999). A novel pineal night-specific ATPase encoded by the Wilson disease gene. J. Neurosci. 19, 1018-1026. 10.1523/JNEUROSCI.19-03-01018.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet, A., Vest, K. E., Maynard, M. K., Gammon, M. G., Russell, A. C., Mathews, A. T., Cole, S. E., Zhu, X., Phillips, C. B., Kwong, J. Q.et al. (2018). The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 293, 1887-1896. 10.1074/jbc.RA117.000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, S. D., Ullrich, M. S., Skopp, A. and Winkler, D. D. (2020). Copper sources for Sod1 activation. Antioxidants 9, 500. 10.3390/antiox9060500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, D. C., Crowe, M. S., Turski, M. L., Hobbs, G. A., Yao, X., Chaikuad, A., Knapp, S., Xiao, K., Campbell, S. L., Thiele, D. J.et al. (2014). Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509, 492-496. 10.1038/nature13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiterman, L. T., Gupta, A., Chaerkady, R., Cole, R. N. and Hubbard, A. L. (2015). Communication between the N and C termini is required for copper-stimulated Ser/Thr phosphorylation of Cu(I)-ATPase (ATP7B). J. Biol. Chem. 290, 8803-8819. 10.1074/jbc.M114.627414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N. M., Torres, A. S., Doan, P. E. and O'Halloran, T. V. (2004). Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu,Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 101, 5518-5523. 10.1073/pnas.0401175101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhead, J. L., Ralle, M., Wilmarth, P., David, L. and Lutsenko, S. (2011). Elevated copper remodels hepatic RNA processing machinery in the mouse model of Wilson's disease. J. Mol. Biol. 406, 44-58. 10.1016/j.jmb.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright, G. E. and Wintrobe, M. M. (1964). Copper metabolism in normal subjects. Am. J. Clin. Nutr. 14, 224-232. 10.1093/ajcn/14.4.224 [DOI] [PubMed] [Google Scholar]
- Chan, N., Willis, A., Kornhauser, N., Ward, M. M., Lee, S. B., Nackos, E., Seo, B. R., Chuang, E., Cigler, T., Moore, A.et al. (2017). Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res. 23, 666-676. 10.1158/1078-0432.CCR-16-1326 [DOI] [PubMed] [Google Scholar]
- Chang C, G. E., Bush, A., Casini, A., Cobine, P., Cross, J., DeNicola, G., Dou, Q. P., Franz, K., Gohil, V., Gupta, S.et al. (2021). Connecting copper and cancer from transition metal signaling to metalloplasia in the clinic. Nat. Rev. Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Hu, J.-G., Huang, Y.-Z., Li, S., Li, S.-F., Wang, M., Xia, H.-W., Li-Ling, J. and Xie, H.-Q. (2020). Copper promotes the migration of bone marrow mesenchymal stem cells via Rnd3-dependent cytoskeleton remodeling. J. Cell. Physiol. 235, 221-231. 10.1002/jcp.28961 [DOI] [PubMed] [Google Scholar]
- Cheng, C., Ding, Q., Zhang, Z., Wang, S., Zhong, B., Huang, X. and Shao, Z. (2020). PTBP1 modulates osteosarcoma chemoresistance to cisplatin by regulating the expression of the copper transporter SLC31A1. J. Cell. Mol. Med. 24, 5274-5289. 10.1111/jcmm.15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, H., Catterton, T., Kim, H., Lee, J. and Kim, B.-E. (2017). Organ-specific regulation of ATP7A abundance is coordinated with systemic copper homeostasis. Sci. Rep. 7, 12001. 10.1038/s41598-017-11961-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford, R. J., Maryon, E. B. and Kaplan, J. H. (2016). Dynamic internalization and recycling of a metal ion transporter: Cu homeostasis and CTR1, the human Cu+ uptake system. J. Cell Sci. 129, 1711-1721. 10.1242/jcs.173351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobine, P. A., Moore, S. A. and Leary, S. C. (2021). Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1868, 118867. 10.1016/j.bbamcr.2020.118867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstra, H. S., McArthy, J., Rudin-Rush, S., Hartwig, C., Gokhale, A., Zlatic, S. A., Blackburn, J. B., Werner, E., Petris, M., D'Souza, P.et al. (2017). The interactome of the copper transporter ATP7A belongs to a network of neurodevelopmental and neurodegeneration factors. eLife 6, e24722. 10.7554/eLife.24722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillion, M. T., Soto, I. C., Shipkovenska, G. and Churchman, L. S. (2016). Synchronized mitochondrial and cytosolic translation programs. Nature 533, 499-503. 10.1038/nature18015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnock, R. and Cullen, P. J. (2020). Mammalian copper homeostasis requires retromer-dependent recycling of the high-affinity copper transporter 1. J. Cell Sci. 133, jcs249201. 10.1242/jcs.249201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, A., Sudhahar, V., Chen, G.-F., Kim, H. W., Youn, S.-W., Finney, L., Vogt, S., Yang, J., Kweon, J., Surenkhuu, B.et al. (2016). Endothelial Antioxidant-1: a key mediator of copper-dependent wound healing in vivo. Sci. Rep. 6, 33783. 10.1038/srep33783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S., Maji, S., Ruturaj, Bhattacharya, I., Saha, T., Naskar, N. and Gupta, A. (2020). Retromer retrieves the Wilson disease protein ATP7B from endolysosomes in a copper-dependent manner. J. Cell Sci. 133, jcs246819. 10.1242/jcs.246819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Kaplan, S. R., Askwith, C. C., Bengtzen, A. C., Radisky, D. and Kaplan, J. (1998). Chloride is an allosteric effector of copper assembly for the yeast multicopper oxidase Fet3p: an unexpected role for intracellular chloride channels. Proc. Natl. Acad. Sci. USA 95, 13641-13645. 10.1073/pnas.95.23.13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra, M., Kuipers, F., van den Berg, G. J., Havinga, R. and Vonk, R. J. (1997). Differences in hepatic processing of dietary and intravenously administered copper in rats. Hepatology 26, 962-966. 10.1002/hep.510260424 [DOI] [PubMed] [Google Scholar]
- Domart, F., Cloetens, P., Roudeau, S., Carmona, A., Verdier, E., Choquet, D. and Ortega, R. (2020). Correlating STED and synchrotron XRF nano-imaging unveils cosegregation of metals and cytoskeleton proteins in dendrites. eLife 9, e62334. 10.7554/eLife.62334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Meskini, R., Culotta, V. C., Mains, R. E. and Eipper, B. A. (2003). Supplying copper to the cuproenzyme peptidylglycine α-amidating monooxygenase. J. Biol. Chem. 278, 12278-12284. 10.1074/jbc.M211413200 [DOI] [PubMed] [Google Scholar]
- Gray, L. W., Peng, F., Molloy, S. A., Pendyala, V. S., Muchenditsi, A., Muzik, O., Lee, J., Kaplan, J. H. and Lutsenko, S. (2012). Urinary copper elevation in a mouse model of Wilson's disease is a regulated process to specifically decrease the hepatic copper load. PLoS ONE 7, e38327. 10.1371/journal.pone.0038327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudekar, N., Shanbhag, V., Wang, Y., Ralle, M., Weisman, G. A. and Petris, M. J. (2020). Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess. Sci. Rep. 10, 7856. 10.1038/s41598-020-64521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A., Schell, M. J., Bhattacharjee, A., Lutsenko, S. and Hubbard, A. L. (2016). Myosin Vb mediates Cu+ export in polarized hepatocytes. J. Cell Sci. 129, 1179-1189. 10.1242/jcs.175307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman, B., Michalczyk, A., Greenough, M., Camakaris, J., Mercer, J. and Ackland, L. (2007a). Distinct functional roles for the Menkes and Wilson copper translocating P-type ATPases in human placental cells. Cell Physiol. Biochem. 20, 1073-1084. 10.1159/000110718 [DOI] [PubMed] [Google Scholar]
- Hardman, B., Michalczyk, A., Greenough, M., Camakaris, J., Mercer, J. F. B. and Ackland, M. L. (2007b). Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem. J. 402, 241-250. 10.1042/BJ20061099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, C., Zlatic, S. A., Wallin, M., Vrailas-Mortimer, A., Fahrni, C. J. and Faundez, V. (2019). Trafficking mechanisms of P-type ATPase copper transporters. Curr. Opin. Cell Biol. 59, 24-33. 10.1016/j.ceb.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, N. M., Gupta, A., Polishchuk, E., Yu, C. H., Polishchuk, R., Dmitriev, O. Y. and Lutsenko, S. (2012). Molecular events initiating exit of a copper-transporting ATPase ATP7B from the trans-Golgi network. J. Biol. Chem. 287, 36041-36050. 10.1074/jbc.M112.370403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori, Y. and Lutsenko, S. (2013). An expanding range of functions for the copper chaperone/antioxidant protein Atox1. Antioxid. Redox Signal. 19, 945-957. 10.1089/ars.2012.5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori, Y., Clasen, S., Hasan, N. M., Barry, A. N. and Lutsenko, S. (2012). Functional partnership of the copper export machinery and glutathione balance in human cells. J. Biol. Chem. 287, 26678-26687. 10.1074/jbc.M112.381178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori, Y., Yan, Y., Schmidt, K., Furukawa, E., Hasan, N. M., Yang, N., Liu, C.-N., Sockanathan, S. and Lutsenko, S. (2016). Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat. Commun. 7, 10640. 10.1038/ncomms10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, F., Chang, C., Liu, B., Li, Z., Li, H., Cai, N. and Wang, H.-H. (2019). Copper (II) ions activate ligand-independent Receptor Tyrosine Kinase (RTK) signaling pathway. Biomed. Res. Int. 2019, 4158415. 10.1155/2019/4158415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman, N. E., Kono, S., Mancini, G. M., Hoogeboom, A. J., De Jong, G. J. and Gitlin, J. D. (2002). Mechanisms of copper incorporation into human ceruloplasmin. J. Biol. Chem. 277, 46632-46638. 10.1074/jbc.M206246200 [DOI] [PubMed] [Google Scholar]
- Hilário-Souza, E., Cuillel, M., Mintz, E., Charbonnier, P., Vieyra, A., Cassio, D. and Lowe, J. (2016). Modulation of hepatic copper-ATPase activity by insulin and glucagon involves protein kinase A (PKA) signaling pathway. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1862, 2086-2097. 10.1016/j.bbadis.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Hlynialuk, C. J., Ling, B., Baker, Z. N., Cobine, P. A., Yu, L. D., Boulet, A., Wai, T., Hossain, A., El Zawily, A. M., McFie, P. J.et al. (2015). The mitochondrial metallochaperone SCO1 is required to sustain expression of the high-affinity copper transporter CTR1 and preserve copper homeostasis. Cell Rep. 10, 933-943. 10.1016/j.celrep.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Holloway, Z. G., Velayos-Baeza, A., Howell, G. J., Levecque, C., Ponnambalam, S., Sztul, E. and Monaco, A. P. (2013). Trafficking of the Menkes copper transporter ATP7A is regulated by clathrin-, AP-2-, AP-1-, and Rab22-dependent steps. Mol. Biol. Cell 24, 1735-1748. S1731-1738. 10.1091/mbc.e12-08-0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, N. and Wittung-Stafshede, P. (2021). ATP7A-regulated enzyme metalation and trafficking in the menkes disease puzzle. Biomedicines 9, 391. 10.3390/biomedicines9040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Frisk, J. M., Kjellén, L., Kaler, S. G., Pejler, G. and Öhrvik, H. (2017). Copper regulates maturation and expression of an MITF:tryptase axis in mast cells. J. Immunol. 199, 4132-4141. 10.4049/jimmunol.1700786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, A. L. and Braiterman, L. T. (2008). Could ATP7B export Cu(I) at the tight junctions and the apical membrane? Gastroenterology 134, 1255-1257. 10.1053/j.gastro.2008.02.073 [DOI] [PubMed] [Google Scholar]
- Hunsaker, E. W. and Franz, K. J. (2019). Emerging opportunities to manipulate metal trafficking for therapeutic benefit. Inorg. Chem. 58, 13528-13545. 10.1021/acs.inorgchem.9b01029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster, D., Purnat, T. D., Burkhead, J. L., Ralle, M., Fiehn, O., Stuckert, F., Olson, N. E., Teupser, D. and Lutsenko, S. (2007). High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J. Biol. Chem. 282, 8343-8355. 10.1074/jbc.M607496200 [DOI] [PubMed] [Google Scholar]
- Jain, S., Cohen, J., Ward, M. M., Kornhauser, N., Chuang, E., Cigler, T., Moore, A., Donovan, D., Lam, C., Cobham, M. V.et al. (2013). Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann. Oncol. 24, 1491-1498. 10.1093/annonc/mds654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, S., Farías, G. G. and Bonifacino, J. S. (2015). Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1. Mol. Biol. Cell 26, 218-228. 10.1091/mbc.E14-07-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, B., Liu, G., Zheng, J., Chen, M., Maimaitiming, Z., Chen, M., Liu, S., Jiang, R., Fuqua, B. K., Dunaief, J. L.et al. (2016). Hephaestin and ceruloplasmin facilitate iron metabolism in the mouse kidney. Sci. Rep. 6, 39470. 10.1038/srep39470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler, S. G. (2013). Inborn errors of copper metabolism. Handb. Clin. Neurol. 113, 1745-1754. 10.1016/B978-0-444-59565-2.00045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Son, H.-Y., Bailey, S. M. and Lee, J. (2009). Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G356-G364. 10.1152/ajpgi.90632.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. E., Turski, M. L., Nose, Y., Casad, M., Rockman, H. A. and Thiele, D. J. (2010). Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 11, 353-363. 10.1016/j.cmet.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.-H., Matsubara, T., Lee, J., Fenollar-Ferrer, C., Han, K., Kim, D., Jia, S., Chang, C. J., Yang, H., Nagano, T.et al. (2021). Lysosomal SLC46A3 modulates hepatic cytosolic copper homeostasis. Nat. Commun. 12, 290. 10.1038/s41467-020-20461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, J.-Y. and Lee, S.-J. (2020). Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain 13, 116. 10.1186/s13041-020-00654-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy, L., Cotruvo, J. A., Jr., Chan, J., Kaluarachchi, H., Muchenditsi, A., Pendyala, V. S., Jia, S., Aron, A. T., Ackerman, C. M., Wal, M. N.et al. (2016). Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 12, 586-592. 10.1038/nchembio.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fontaine, S., Ackland, M. L. and Mercer, J. F. B. (2010). Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B: emerging roles. Int. J. Biochem. Cell Biol. 42, 206-209. 10.1016/j.biocel.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalioti, V., Peiró, R., Pérez-Berlanga, M., Tsuchiya, Y., Muñoz, A., Villalba, T., Sanchez, C. and Sandoval, I. V. (2016). Basolateral sorting and transcytosis define the Cu+-regulated translocation of ATP7B to the bile canaliculus. J. Cell Sci. 129, 2190-2201. 10.1242/jcs.184663 [DOI] [PubMed] [Google Scholar]
- Leary, S. C. and Ralle, M. (2020). Advances in visualization of copper in mammalian systems using X-ray fluorescence microscopy. Curr. Opin. Chem. Biol. 55, 19-25. 10.1016/j.cbpa.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary, S. C., Kaufman, B. A., Pellecchia, G., Guercin, G.-H., Mattman, A., Jaksch, M. and Shoubridge, E. A. (2004). Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 13, 1839-1848. 10.1093/hmg/ddh197 [DOI] [PubMed] [Google Scholar]
- Lee, J., Prohaska, J. R. and Thiele, D. J. (2001). Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA 98, 6842-6847. 10.1073/pnas.111058698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., Petris, M. J. and Thiele, D. J. (2002). Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J. Biol. Chem. 277, 40253-40259. 10.1074/jbc.M208002200 [DOI] [PubMed] [Google Scholar]
- Leitch, J. M., Yick, P. J. and Culotta, V. C. (2009). The right to choose: multiple pathways for activating copper,zinc superoxide dismutase. J. Biol. Chem. 284, 24679-24683. 10.1074/jbc.R109.040410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz, M., Wieczerzak, K., Krzeptowski, W., Dobosz, P., Grzmil, P., Starzynski, R. and Lipinski, P. (2010). Developmental changes in the expression of the Atp7a gene in the liver of mice during the postnatal period. J. Exp. Zool. A Ecol. Genet. Physiol. 313A, 209-217. 10.1002/jez.586 [DOI] [PubMed] [Google Scholar]
- Lim, C. M., Cater, M. A., Mercer, J. F. B. and La Fontaine, S. (2006). Copper-dependent interaction of dynactin subunit p62 with the N terminus of ATP7B but not ATP7A. J. Biol. Chem. 281, 14006-14014. 10.1074/jbc.M512745200 [DOI] [PubMed] [Google Scholar]
- Linder, M. C. (2020). Copper homeostasis in mammals, with emphasis on secretion and excretion. a review. Int. J. Mol. Sci. 21, 4932. 10.3390/ijms21144932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz, R. and Lutsenko, S. (2007). Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. J. Bioenerg. Biomembr. 39, 403-407. 10.1007/s10863-007-9101-2 [DOI] [PubMed] [Google Scholar]
- Linz, R., Barnes, N. L., Zimnicka, A. M., Kaplan, J. H., Eipper, B. and Lutsenko, S. (2008). Intracellular targeting of copper-transporting ATPase ATP7A in a normal and Atp7b−/− kidney. Am. J. Physiol. Renal Physiol. 294, F53-F61. 10.1152/ajprenal.00314.2007 [DOI] [PubMed] [Google Scholar]
- Llanos, R. M., Michalczyk, A. A., Freestone, D. J., Currie, S., Linder, M. C., Ackland, M. L. and Mercer, J. F. B. (2008). Copper transport during lactation in transgenic mice expressing the human ATP7A protein. Biochem. Biophys. Res. Commun. 372, 613-617. 10.1016/j.bbrc.2008.05.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko, S., LeShane, E. S. and Shinde, U. (2007). Biochemical basis of regulation of human copper-transporting ATPases. Arch. Biochem. Biophys. 463, 134-148. 10.1016/j.abb.2007.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko, S., Gupta, A., Burkhead, J. L. and Zuzel, V. (2008). Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch. Biochem. Biophys. 476, 22-32. 10.1016/j.abb.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, G., Nalvarte, I., Smirnova, T., Vecchi, M., Aceto, N., Doelemeyer, A., Frei, A., Lienhard, S., Wyckoff, J., Hess, D.et al. (2014). Memo is a copper-dependent redox protein with an essential role in migration and metastasis. Sci. Signal. 7, ra56. 10.1126/scisignal.2004870 [DOI] [PubMed] [Google Scholar]
- Maghool, S., Cooray, N. D. G., Stroud, D. A., Aragão, D., Ryan, M. T. and Maher, M. J. (2019). Structural and functional characterization of the mitochondrial complex IV assembly factor Coa6. Life Sci. Alliance 2, e201900458. 10.26508/lsa.201900458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki, J. M., Sormunen, R., Lippo, S., Kaarteenaho-Wiik, R., Soininen, R. and Myllyharju, J. (2005). Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am. J. Pathol. 167, 927-936. 10.1016/S0002-9440(10)61183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon, E. B., Molloy, S. A. and Kaplan, J. H. (2007). O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J. Biol. Chem. 282, 20376-20387. 10.1074/jbc.M701806200 [DOI] [PubMed] [Google Scholar]
- Masaldan, S., Clatworthy, S. A. S., Gamell, C., Smith, Z. M., Francis, P. S., Denoyer, D., Meggyesy, P. M., Fontaine, S. and Cater, M. A. (2018). Copper accumulation in senescent cells: Interplay between copper transporters and impaired autophagy. Redox Biol. 16, 322-331. 10.1016/j.redox.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk, A. A., Rieger, J., Allen, K. J., Mercer, J. F. B. and Ackland, M. L. (2000). Defective localization of the Wilson disease protein (ATP7B) in the mammary gland of the toxic milk mouse and the effects of copper supplementation. Biochem. J. 352, 565-571. 10.1042/bj3520565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, M. T., Bourassa, D., Harankhedkar, S., McCallum, A. M., Zlatic, S. A., Calvo, J. S., Meloni, G., Faundez, V. and Fahrni, C. J. (2019). Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells. Proc. Natl. Acad. Sci. USA 116, 12167-12172. 10.1073/pnas.1900172116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchenditsi, A., Talbot, C. C., Jr., Gottlieb, A., Yang, H., Kang, B., Boronina, T., Cole, R., Wang, L., Dev, S., Hamilton, J. P.et al. (2021). Systemic deletion of Atp7b modifies the hepatocytes’ response to copper overload in the mouse models of Wilson disease. Sci .Rep. 11, 5659. 10.1038/s41598-021-84894-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, B. M., Prendergast, F. G., Holman, R., Kuntz, S. M. and Larusso, N. F. (1993). Alterations in hepatocyte lysosomes in experimental hepatic copper overload in rats. Gastroenterology 105, 1814-1823. 10.1016/0016-5085(93)91080-2 [DOI] [PubMed] [Google Scholar]
- Noda, K., Kitagawa, K., Miki, T., Horiguchi, M., Akama, T. O., Taniguchi, T., Taniguchi, H., Takahashi, K., Ogra, Y., Mecham, R. P.et al. (2020). A matricellular protein fibulin-4 is essential for the activation of lysyl oxidase. Sci. Adv. 6, eabc1404. 10.1126/sciadv.abc1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose, Y., Kim, B.-E. and Thiele, D. J. (2006). Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 4, 235-244. 10.1016/j.cmet.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Nose, Y., Wood, L. K., Kim, B.-E., Prohaska, J. R., Fry, R. S., Spears, J. W. and Thiele, D. J. (2010). Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem. 285, 32385-32392. 10.1074/jbc.M110.143826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasae, L., Bustos, R., Braiterman, L., Eipper, B. and Hubbard, A. (2007). Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1181-G1194. 10.1152/ajpgi.00472.2006 [DOI] [PubMed] [Google Scholar]
- Nyasae, L. K., Schell, M. J. and Hubbard, A. L. (2014). Copper directs ATP7B to the apical domain of hepatic cells via basolateral endosomes. Traffic 15, 1344-1365. 10.1111/tra.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogórek, M., Lenartowicz, M., Starzyński, R., Jończy, A., Staroń, R., Doniec, A., Krzeptowski, W., Bednarz, A., Pierzchała, O., Lipiński, P.et al. (2017). Atp7a and Atp7b regulate copper homeostasis in developing male germ cells in mice. Metallomics 9, 1288-1303. 10.1039/C7MT00134G [DOI] [PubMed] [Google Scholar]
- Öhrvik, H. and Thiele, D. J. (2014). How copper traverses cellular membranes through the mammalian copper transporter 1, Ctr1. Ann. N. Y. Acad. Sci. 1314, 32-41. 10.1111/nyas.12371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrvik, H., Nose, Y., Wood, L. K., Kim, B.-E., Gleber, S.-C., Ralle, M. and Thiele, D. J. (2013). Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. USA 110, E4279-E4288. 10.1073/pnas.1311749110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhrvik, H., Logeman, B., Noguchi, G., Eriksson, I., Kjellén, L., Thiele, D. J. and Pejler, G. (2015). Ctr2 regulates mast cell maturation by affecting the storage and expression of tryptase and proteoglycans. J. Immunol. 195, 3654-3664. 10.4049/jimmunol.1500283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhrvik, H., Logeman, B., Turk, B., Reinheckel, T. and Thiele, D. J. (2016). Cathepsin protease controls copper and cisplatin accumulation via cleavage of the Ctr1 metal-binding ectodomain. J. Biol. Chem. 291, 13905-13916. 10.1074/jbc.M116.731281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, C. A., Jr. and Orvis, A. L. (1970). Release of copper by rat liver. Am. J. Physiol. 218, 88-91. 10.1152/ajplegacy.1970.218.1.88 [DOI] [PubMed] [Google Scholar]
- Palumaa, P. (2013). Copper chaperones. The concept of conformational control in the metabolism of copper. FEBS Lett. 587, 1902-1910. 10.1016/j.febslet.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Petris, M. J., Strausak, D. and Mercer, J. F. B. (2000). The Menkes copper transporter is required for the activation of tyrosinase. Hum. Mol. Genet. 9, 2845-2851. 10.1093/hmg/9.19.2845 [DOI] [PubMed] [Google Scholar]
- Phillips-Krawczak, C. A., Singla, A., Starokadomskyy, P., Deng, Z., Osborne, D. G., Li, H., Dick, C. J., Gomez, T. S., Koenecke, M., Zhang, J. S.et al. (2015). COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol. Biol. Cell 26, 91-103. 10.1091/mbc.e14-06-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, H., Muchenditsi, A., Kim, B.-E., Ralle, M., Zachos, N., Huster, D. and Lutsenko, S. (2018). The function of ATPase copper transporter ATP7B in intestine. Gastroenterology 154, 168-180.e5. 10.1053/j.gastro.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, H., Yang, H. and Lutsenko, S. (2019). Copper transport and disease: what can we learn from organoids? Annu. Rev. Nutr. 39, 75-94. 10.1146/annurev-nutr-082018-124242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilankatta, R., Lewis, D. and Inesi, G. (2011). Involvement of protein kinase D in expression and trafficking of ATP7B (copper ATPase). J. Biol. Chem. 286, 7389-7396. 10.1074/jbc.M110.171454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk, R. and Lutsenko, S. (2013). Golgi in copper homeostasis: a view from the membrane trafficking field. Histochem. Cell Biol. 140, 285-295. 10.1007/s00418-013-1123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk, R. S. and Polishchuk, E. V. (2019). From and to the Golgi - defining the Wilson disease protein road map. FEBS Lett. 593, 2341-2350. 10.1002/1873-3468.13575 [DOI] [PubMed] [Google Scholar]
- Polishchuk, E. V., Concilli, M., Iacobacci, S., Chesi, G., Pastore, N., Piccolo, P., Paladino, S., Baldantoni, D., van IJzendoorn, S. C., Chan, J.et al. (2014). Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell 29, 686-700. 10.1016/j.devcel.2014.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk, E. V., Merolla, A., Lichtmannegger, J., Romano, A., Indrieri, A., Ilyechova, E. Y., Concilli, M., De Cegli, R., Crispino, R., Mariniello, M.et al. (2019). Activation of autophagy, observed in liver tissues from patients with wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 156, 1173-1189.e5. 10.1053/j.gastro.2018.11.032 [DOI] [PubMed] [Google Scholar]
- Pope, C. R., Flores, A. G., Kaplan, J. H. and Unger, V. M. (2012). Structure and function of copper uptake transporters. Curr. Top. Membr. 69, 97-112. 10.1016/B978-0-12-394390-3.00004-5 [DOI] [PubMed] [Google Scholar]
- Pradhan-Sundd, T., Gudapati, S., Kaminski, T. W. and Ragni, M. V. (2021). Exploring the complex role of coagulation factor VIII in chronic liver disease. Cell. Mol. Gastroenterol. Hepatol. 12, 1061-1072. 10.1016/j.jcmgh.2021.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkar, Y., Robison, G., Sullivan, B., Fu, S. X., Kohne, M., Jiang, W., Rohr, S., Lai, B., Marcus, M. A., Zakharova, T.et al. (2013). Aging results in copper accumulations in glial fibrillary acidic protein-positive cells in the subventricular zone. Aging Cell 12, 823-832. 10.1111/acel.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Z., Itoh, S., Jeney, V., Ushio-Fukai, M. and Fukai, T. (2006). Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J. 20, 334-336. 10.1096/fj.05-4564fje [DOI] [PubMed] [Google Scholar]
- Ren, F., Logeman, B. L., Zhang, X., Liu, Y., Thiele, D. J. and Yuan, P. (2019). X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 10, 1386. 10.1038/s41467-019-09376-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarode, G. V., Neier, K., Shibata, N. M., Shen, Y., Goncharov, D. A., Goncharova, E. A., Mazi, T. A., Joshi, N., Settles, M. L., LaSalle, J. M.et al. (2021). Wilson disease: intersecting DNA methylation and histone acetylation regulation of gene expression in a mouse model of hepatic copper accumulation. Cell Mol. Gastroenterol. Hepatol. 12, 1457-1477. 10.1016/j.jcmgh.2021.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, K., Ralle, M., Schaffer, T., Jayakanthan, S., Bari, B., Muchenditsi, A. and Lutsenko, S. (2018). ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-β-hydroxylase. J. Biol. Chem. 293, 20085-20098. 10.1074/jbc.RA118.004889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotanus, M. D. and Van Otterloo, E. (2020). Finding MEMO—emerging evidence for MEMO1's function in development and disease. Genes 11, 1316. 10.3390/genes11111316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag, V., Jasmer-McDonald, K., Zhu, S., Martin, A. L., Gudekar, N., Khan, A., Ladomersky, E., Singh, K., Weisman, G. A. and Petris, M. J. (2019). ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl. Acad. Sci. USA 116, 6836-6841. 10.1073/pnas.1817473116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag, V. C., Gudekar, N., Jasmer, K., Papageorgiou, C., Singh, K. and Petris, M. J. (2021). Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1868, 118893. 10.1016/j.bbamcr.2020.118893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido, N., Nakayama, K., Takazawa, A., Ohyama, T. and Nakamura, M. (2001). Cu-metallothioneins (Cu(I)8-MTs) in LEC rat livers 13 weeks after birth still act as antioxidants. Arch. Biochem. Biophys. 387, 216-222. 10.1006/abbi.2000.2233 [DOI] [PubMed] [Google Scholar]
- Singla, A., Chen, Q., Suzuki, K., Song, J., Fedoseienko, A., Wijers, M., Lopez, A., Billadeau, D. D., van de Sluis, B. and Burstein, E. (2021). Regulation of murine copper homeostasis by members of the COMMD protein family. Dis. Model Mech. 14, dmm045963. 10.1242/dmm.045963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, W. C. J., McInnes, K. T., Cater, M. A., Winnall, W. R., McKirdy, R., Yu, Y., Taylor, P. E., Ke, B.-X., Richardson, D. R., Mercer, J. F. B.et al. (2010). Role of glutaredoxin1 and glutathione in regulating the activity of the copper-transporting P-type ATPases, ATP7A and ATP7B. J. Biol. Chem. 285, 27111-27121. 10.1074/jbc.M110.154468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopp, A., Boyd, S. D., Ullrich, M. S., Liu, L. and Winkler, D. D. (2019). Copper-zinc superoxide dismutase (Sod1) activation terminates interaction between its copper chaperone (Ccs) and the cytosolic metal-binding domain of the copper importer Ctr1. Biometals 32, 695-705. 10.1007/s10534-019-00206-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma, S., Morgada, M. N., Naik, M. T., Boulet, A., Roesler, A. A., Dziuba, N., Ghosh, A., Yu, Q., Lindahl, P. A., Ames, J. B.et al. (2019). COA6 is structurally tuned to function as a thiol-disulfide oxidoreductase in copper delivery to mitochondrial cytochrome c oxidase. Cell Rep. 29, 4114-4126.e5. 10.1016/j.celrep.2019.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, D. J., Short, K. K., Maniaci, B. N. and Burkhead, J. L. (2019). COMMD1 and PtdIns(4,5)P2 interaction maintain ATP7B copper transporter trafficking fidelity in HepG2 cells. J. Cell Sci. 132, jcs231753. 10.1242/jcs.231753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhahar, V., Okur, M. N., Bagi, Z., O'Bryan, J. P., Hay, N., Makino, A., Patel, V. S., Phillips, S. A., Stepp, D., Ushio-Fukai, M.et al. (2018). Akt2 (Protein Kinase B Beta) stabilizes ATP7A, a copper transporter for extracellular superoxide dismutase, in vascular smooth muscle: novel mechanism to limit endothelial dysfunction in type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 38, 529-541. 10.1161/ATVBAHA.117.309819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telianidis, J., Hung, Y. H., Materia, S. and Fontaine, S. L. (2013). Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis. Front. Aging Neurosci. 5, 44. 10.3389/fnagi.2013.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timón-Gómez, A., Nývltová, E., Abriata, L. A., Vila, A. J., Hosler, J. and Barrientos, A. (2018). Mitochondrial cytochrome c oxidase biogenesis: recent developments. Semin. Cell Dev. Biol. 76, 163-178. 10.1016/j.semcdb.2017.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, C.-Y., Liebig, J. K., Tsigelny, I. F. and Howell, S. B. (2015). The copper transporter 1 (CTR1) is required to maintain the stability of copper transporter 2 (CTR2). Metallomics 7, 1477-1487. 10.1039/C5MT00131E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, T., Posimo, J. M., Gudiel, A. A., Cicchini, M., Feldser, D. M. and Brady, D. C. (2020). Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 22, 412-424. 10.1038/s41556-020-0481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, T., Davis, C. I. and Brady, D. C. (2021). Copper biology. Curr. Biol. 31, R421-R427. 10.1016/j.cub.2021.03.054 [DOI] [PubMed] [Google Scholar]
- Vallet, S. D. and Ricard-Blum, S. (2019). Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem. 63, 349-364. 10.1042/EBC20180050 [DOI] [PubMed] [Google Scholar]
- Van campen, D. R. and Mitchell, E. A. (1965). Absorption of Cu-64, Zn-65, Mo-99, and Fe-59 from ligated segments of the rat gastrointestinal tract. J. Nutr. 86, 120-124. 10.1093/jn/86.2.120 [DOI] [PubMed] [Google Scholar]
- Vest, K. E., Paskavitz, A. L., Lee, J. B. and Padilla-Benavides, T. (2018). Dynamic changes in copper homeostasis and post-transcriptional regulation of Atp7a during myogenic differentiation. Metallomics 10, 309-322. 10.1039/C7MT00324B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas, A., Duvvuri, U. and Kiselyov, K. (2019). Copper-dependent ATP7B up-regulation drives the resistance of TMEM16A-overexpressing head-and-neck cancer models to platinum toxicity. Biochem. J. 476, 3705-3719. 10.1042/BCJ20190591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Zhu, S., Hodgkinson, V., Prohaska, J. R., Weisman, G. A., Gitlin, J. D. and Petris, M. J. (2012). Maternofetal and neonatal copper requirements revealed by enterocyte-specific deletion of the Menkes disease protein. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1236-G1244. 10.1152/ajpgi.00339.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, K. H., Wurz, J., Gotthardt, D., Merle, U., Stremmel, W. and Füllekrug, J. (2008). Localization of the Wilson disease protein in murine intestine. J. Anat. 213, 232-240. 10.1111/j.1469-7580.2008.00954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, C., Kambe, T., Fulcher, Y. G., Sachdev, S. W., Bush, A. I., Fritsche, K., Lee, J., Quinn, T. P. and Petris, M. J. (2009). Copper transport into the secretory pathway is regulated by oxygen in macrophages. J. Cell Sci. 122, 1315-1321. 10.1242/jcs.043216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow, T., Zhang, Y. and Vest, K. (2021). Defining post-transcriptional regulation of the copper transporter ATP7A in skeletal muscle cell. FASEB J. 35, 10.1096/fasebj.2021.35.S1.02428 [DOI] [Google Scholar]
- Wooton-Kee, C. R., Robertson, M., Zhou, Y., Dong, B., Sun, Z., Kim, K. H., Liu, H., Xu, Y., Putluri, N., Saha, P.et al. (2020). Metabolic dysregulation in the Atp7b−/− Wilson's disease mouse model. Proc. Natl. Acad. Sci. USA 117, 2076-2083. 10.1073/pnas.1914267117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Z., Brose, J., Schimo, S., Ackland, S. M., La Fontaine, S. and Wedd, A. G. (2011). Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J. Biol. Chem. 286, 11047-11055. 10.1074/jbc.M110.213074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, T., Ackerman, C. M., Carroll, E. C., Jia, S., Hoagland, A., Chan, J., Thai, B., Liu, C. S., Isacoff, E. Y. and Chang, C. J. (2018). Copper regulates rest-activity cycles through the locus coeruleus-norepinephrine system. Nat. Chem. Biol. 14, 655-663. 10.1038/s41589-018-0062-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimoto, C., Harada, M., Kumemura, H., Abe, M., Koga, H., Sakata, M., Kawaguchi, T., Terada, K., Hanada, S., Taniguchi, E.et al. (2011). Copper incorporation into ceruloplasmin is regulated by Niemann-Pick C1 protein. Hepatol. Res. 41, 484-491. 10.1111/j.1872-034X.2011.00788.x [DOI] [PubMed] [Google Scholar]
- Yang, H., Ralle, M., Wolfgang, M. J., Dhawan, N., Burkhead, J. L., Rodriguez, S., Kaplan, J. H., Wong, G. W., Haughey, N. and Lutsenko, S. (2018). Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 16, e2006519. 10.1371/journal.pbio.2006519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. H., Yang, N., Bothe, J., Tonelli, M., Nokhrin, S., Dolgova, N. V., Braiterman, L., Lutsenko, S. and Dmitriev, O. Y. (2017). The metal chaperone Atox1 regulates the activity of the human copper transporter ATP7B by modulating domain dynamics. J. Biol. Chem. 292, 18169-18177. 10.1074/jbc.M117.811752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S., Shanbhag, V., Hodgkinson, V. L. and Petris, M. J. (2016). Multiple di-leucines in the ATP7A copper transporter are required for retrograde trafficking to the trans-Golgi network. Metallomics 8, 993-1001. 10.1039/C6MT00093B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimnicka, A. M., Maryon, E. B. and Kaplan, J. H. (2007). Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J. Biol. Chem. 282, 26471-26480. 10.1074/jbc.M702653200 [DOI] [PubMed] [Google Scholar]
- Zischka, H., Lichtmannegger, J., Schmitt, S., Jägemann, N., Schulz, S., Wartini, D., Jennen, L., Rust, C., Larochette, N., Galluzzi, L.et al. (2011). Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J. Clin. Invest. 121, 1508-1518. 10.1172/JCI45401 [DOI] [PMC free article] [PubMed] [Google Scholar]