Abstract
Biomolecular condensates are cellular compartments that can form by phase separation in the absence of limiting membranes. Studying the P granules of Caenorhabditis elegans, we find that condensate dynamics are regulated by protein clusters that adsorb to the condensate interface. Using in vitro reconstitution, live observations, and theory, we demonstrate that localized assembly of P granules is controlled by MEG-3, an intrinsically disordered protein that forms low dynamic assemblies on P granules. Following classic Pickering emulsion theory, MEG-3 clusters lower surface tension and slow down coarsening. During zygote polarization, MEG-3 recruits the DYRK family kinase MBK-2 to accelerate spatially regulated growth of the P granule emulsion. By tuning condensate-cytoplasm exchange, interfacial clusters regulate the structural integrity of biomolecular condensates, reminiscent of the role of lipid bilayers in membrane-bound organelles.
Liquid-liquid phase separation has emerged as a new principle for cellular organization (1). Phase separation of proteins, frequently with RNA, can create dense condensates that are visible by microscopy as micron-scale assemblies containing dozens or more protein and RNA species. Condensates have been reconstituted in vitro using purified proteins that interact by using multivalent, low-affinity binding sites to generate large, interconnected networks. Some proteins form condensates that exhibit fast internal dynamics and rapid exchange with the dilute phase on a time scale of seconds or minutes (1). Other proteins form more viscous condensates with slower internal and exchange dynamics, in some cases becoming glass-like with time (2, 3). All condensate systems (emulsions) are predicted to evolve (coarsen) toward a single-condensate equilibrium at a rate that correlates positively with their internal and exchange dynamics (4–8) (see supplementary text). Like condensates assembled in vitro, condensates in cells exhibit a range of dynamic behaviors, but these do not always fit theoretical predictions (9, 10). For example, some condensates exhibit little coarsening over hour time scales, yet maintain low viscosity, maintain fast exchange dynamics, and dissolve within minutes in response to changes in the cellular environment (11). Several hypotheses have been put forward to explain the lack of coarsening of cellular condensates with fast internal dynamics, including physical barriers that keep condensates away from each other (5, 12, 13), active mechanisms that continuously regenerate small condensates (14), and chemical reactions and protein gradients that suppress Ostwald ripening (15–18) (see supplementary text). In this study, we investigate the mechanisms that control the coarsening and dynamics of P granules, condensates in the Caenorhabditis elegans germline. We find that P granule coarsening is controlled by nanoscale protein clusters that adsorb to the condensate interface, a phenomenon first described by Ramsden (in 1904) and Pickering (in 1907) for inorganic emulsions (19, 20).
P granules were the first cellular condensates proposed to form by liquid-liquid phase separation (21). At the core of P granules is a liquid-like phase assembled by PGL proteins, paralogs PGL-1 and PGL-3 (21–23). During most of the C. elegans life cycle, PGL condensates associate stably with the cytoplasmic face of nuclei (24). During the oocyte-to-zygote transition, PGL condensates redistribute to the cytoplasm and undergo two rapid cycles of dissolution and condensation. The first cycle occurs during oocyte maturation when most PGL condensates dissolve before reassembling after fertilization in the zygote. The second cycle occurs during zygote polarization when PGL condensates dissolve in anterior cytoplasm and assemble in posterior cytoplasm. Both cycles are completed within minutes without substantial coarsening. Factors that regulate P granule dynamics during oocyte maturation have not yet been identified. Factors that regulate dynamics during polarization include MEX-5, an RNA-binding protein; MEG proteins MEG-3 and MEG-4, two paralogous intrinsically disordered proteins; and MBK-2, a DYRK family kinase that interacts physically and genetically with MEG-3 (22, 25–28). During polarization, MEX-5 becomes enriched in the anterior cytoplasm, where it promotes P granule dissolution, possibly by competing with P granule proteins for RNA (22, 27). In zygotes lacking mbk-2 or meg-3 and meg-4 activity, P granules do not dissolve in the anterior cytoplasm, despite a normal MEX-5 gradient (28). In this study, we investigate how the MEGs and MBK-2 collaborate with MEX-5 to regulate P granule dynamics.
MEG-3 forms low-dynamic clusters that adsorb to the PGL-3 interface
MEG-3 has been reported to form assemblies on the surface of PGL-3 condensates in newly fertilized zygotes (28, 29) and in PGL-3 and MEG-3 co-condensates reconstituted in vitro (29). Superresolution three-dimensional confocal microscopy (see methods) confirmed that MEG-3 forms diffraction-limited clusters (<160 nm) at the PGL-3 interface in vivo and in vitro (Fig. 1A and fig. S1). Consistent with MEG-3 clusters adsorbing to the interface, MEG-3 modifies the wetting behavior of PGL-3 condensates assembled in vitro, reducing the extent to which PGL-3 droplets wet the surface of untreated glass slides (Fig. 1, B and C).
Fig. 1. MEG-3 forms low-dynamic clusters that adsorb to the surface of PGL-3 condensates.
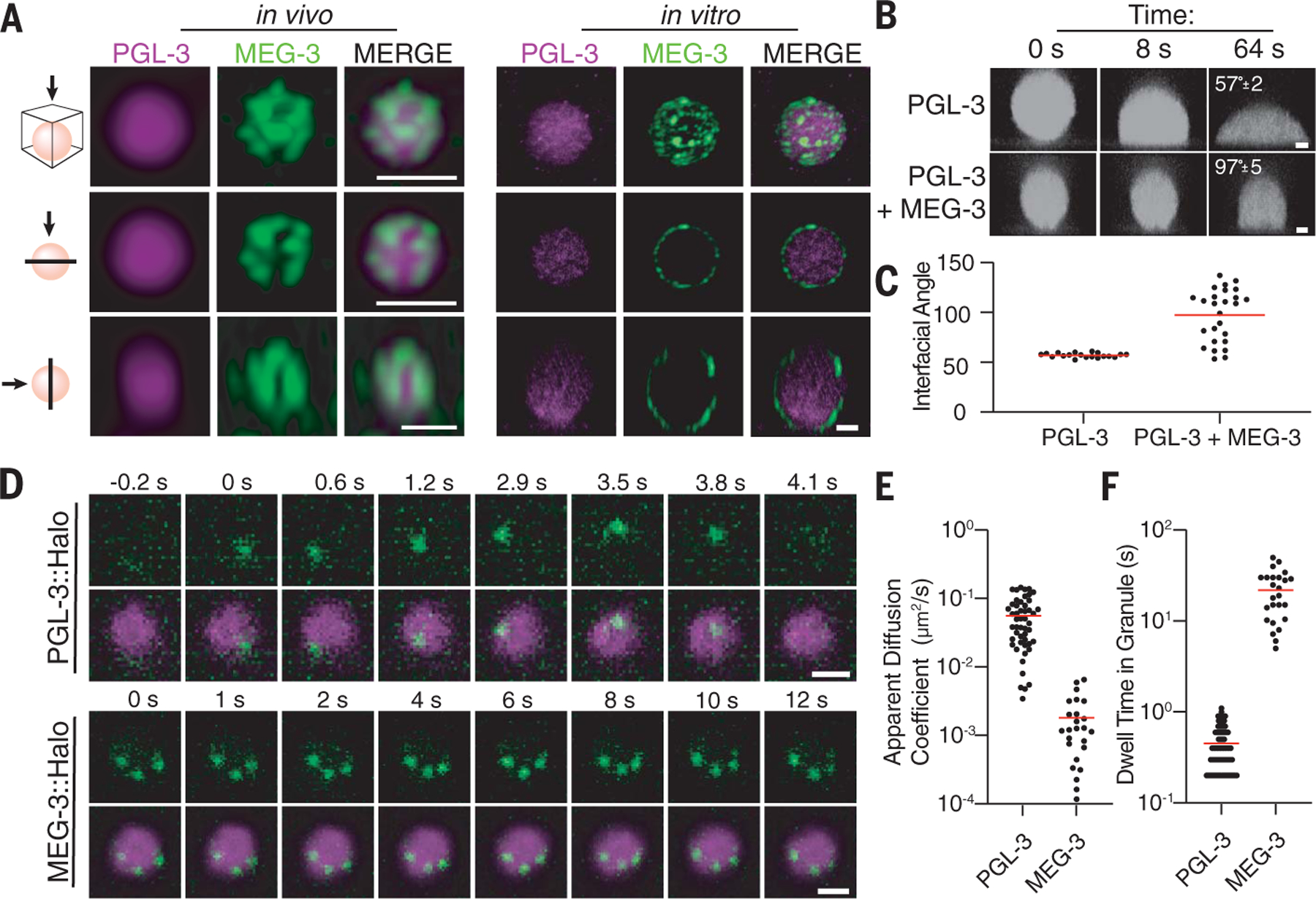
(A) Photomicrographs of a P granule in vivo labeled with PGL-3::mCherry and MEG-3::meGFP (GFP, green fluorescent protein) and of a P granule reconstituted in vitro with purified PGL-3 and MEG-3 trace-labeled with Dylight 488 and Alexa 647, respectively. Scale bars are 500 nm (in vivo, each scale bar applies to all images in the same row) and 3 µm (in vitro, scale bar applies to all images in the set). The top panels are a maximum projection of a z-stack through the granule. The middle panels are a single x-y plane through the middle of the same granule. The lower panels are a single z-x plane through the middle of the same granule. See fig. S1A for additional examples of P granules captured in vivo. (B) In vitro time-lapse series showing PGL-3 droplets trace-labeled with Dylight 488 wetting the surface of a glass slide with 3 µM PGL-3, with 80 ng/µl nos-2 RNA, and with or without 0.5 µM MEG-3. Average contact angles at 64 s are indicated. Scale bars are 1 µm, and each scale bar applies to all images in the same row. (C) Contact angles measured as in (B). Each dot represents a droplet, and red lines represent the mean. (D) In vivo time-lapse series showing single molecules (green) of PGL-3::Halo and MEG-3::Halo in P granules (magenta). Scale bars are 1 µm, and each scale bar applies to all images in the respective set. (E) Graph depicting the apparent diffusion coefficients of PGL-3::Halo and MEG-3::Halo molecules in P granules. Each dot represents one trajectory, and the red line represents the mean. (F) Graph depicting the dwell time of PGL-3::Halo and MEG-3::Halo molecules in P granules. Each dot represents one trajectory, and the red line represents the mean.
MEG-3 clusters are resistant to dilution, high temperature, and salt treatment; by contrast, PGL-3 condensates readily dissolve in dilute conditions and at increased temperatures (29). MEG-3 condensates exchange more slowly than PGL-3 condensates, as measured by fluorescence recovery after photobleaching (FRAP) in vitro and in vivo (29). Using a single-molecule method adapted from Wu et al. (30), we measured the dynamics of MEG-3 and PGL-3 molecules in P granules in vivo (Fig. 1, D to F, and movies S1 and S2). Most PGL-3 molecules exhibited short-lived trajectories in P granules with an average apparent diffusion coefficient of D = 0.056 µm2/s (Fig. 1,E and F). By contrast, all MEG-3 molecules exhibited restricted long-lived trajectories with an average apparent diffusion coefficient of D = 0.0018 µm2/s (Fig. 1, E and F). In three of three cases where we captured the trajectories of three labeled MEG-3 molecules in the same P granule, their relative position remained fixed over time (Fig. 1D and movie S2). Together these observations confirm that PGL-3 molecules exist primarily in a dynamic liquid-like phase (albeit highly viscous), whereas MEG-3 molecules experience much slower dynamics, resembling solid clusters within our experimental time scales.
MEG-3 reduces the surface tension of PGL-3 condensates without changing viscosity
Solid particulates that adsorb to liquid surfaces reduce surface tension without affecting internal dynamics (viscosity) (31). Previous studies have shown that PGL-3 condensates are “aging Maxwell fluids” whose viscosity increases over days, eventually adopting glass-like properties [see supplementary text and (2, 32)]. To focus on the time scales experienced by PGL-3 condensates during the oocyte-to-embryo transition, we examined PGL-3 condensates within the first 3 hours of assembly for all in vitro studies. To probe internal dynamics, we measured the movement of fluorescent microspheres embedded in PGL-3 condensates with and without MEG-3 (fig. S2, A and B, and movie S3). Fitting the mean squared displacement (MSD) of microspheres to the equation MSD = 4Dtα, we calculated a diffusion coefficient (D) and anomalous diffusion exponent (α). We observed an α ≈ 1 for PGL-3, consistent with newly formed PGL-3 condensates behaving as viscous liquids (fig. S2C). Using the Stokes-Einstein relation, we calculated a viscosity of η = 5.4 Pa⋅s in the range estimated previously for PGL-3 condensates in vivo and in vitro (fig. S2D) (2, 21). Addition of MEG-3 did not have a notable effect on P L-3 viscosity (η = 5.6 Pa⋅s) (fig. S2, B to D, and vie S3), as expected for a surface agent that, on its own, does not affect internal dynamics.
To measure PGL-3 surface tension, we examined the coalescence behavior of PGL-3 droplets (Fig. 2A). Relaxation time of coalescing condensates can be expressed by τ ≈ ℓ(η/γ), where ℓ is the geometric mean of the diameters of the droplets at the onset of fusion, τ is the viscosity of the droplets, γ is the surface tension, and η/γ is the inverse capillary velocity. PGL-3 condensates coalesce with a linear relationship over a range of condensate sizes, yielding an inverse capillary velocity of 0.25 s/µm and surface tension of 19.4 µN/m (Fig. 2B and movie S4), comparable to previous estimates for P granules in vivo (21). Addition of MEG-3 slowed coalescence of PGL-3 droplets, yielding an inverse capillary velocity of 1.2 s/µm and a surface tension of 4.7 µN/m (500 nM MEG-3; Fig. 2B and movie S5).
Fig. 2. MEG-3 reduces the surface tension of PGL-3 condensates and prevents coarsening.
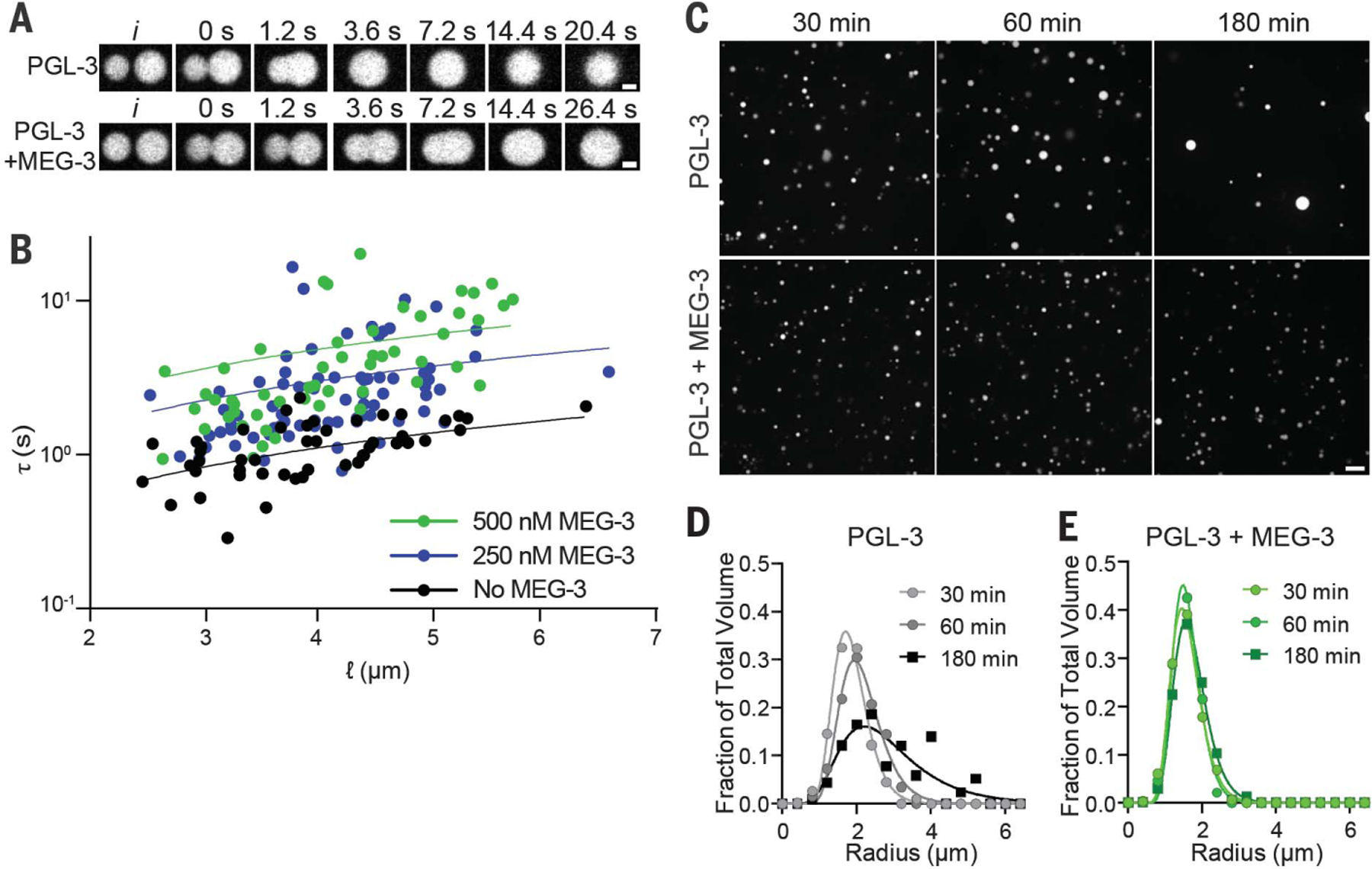
(A) Photomicrographs of PGL-3 droplets (3 µM PGL-3 and 80 ng/µl nos-2 RNA) coalescing with or without 0.5 µM MEG-3. (B) Relaxation time (τ) of fusing PGL-3 droplets (as above) is plotted versus length scale (ℓ) with varying concentrations of MEG-3 as indicated. Each dot represents a single fusion event. The linear slope represents the inverse capillary velocity (η/γ). (C) Photomicrographs of a PGL-3 emulsion (max projections) at the indicated time points after assembly. Three micromolar PGL-3 and 80 ng/µl nos-2 RNA were incubated in condensation buffer in the presence or absence of 0.5 µM MEG-3. Scale bar is 5 µm and applies to all images in the set. (D and E) Histograms plotting the size distribution of PGL condensates assembled as in (C). Each data point indicates the fraction of total PGL-3 condensate volume represented by condensates binned by radius from 80 images [as in (C)] collected in four replicates. Lines were fit to a log normal distribution.
In addition to modulating surface tension, Pickering agents can also inhibit coalescence by steric hindrance and slow rearrangement at the condensate interface (33). We observed examples of MEG-3–coated PGL-3 droplets that did not relax to a sphere (movie S6) or remained in contact without fusing during imaging (movie S7). Higher concentrations of MEG-3 (1 µm) increased surface coverage and caused PGL-3 droplets to flocculate without fusing (fig. S2E). We conclude that, as expected for a Pickering agent, MEG-3 clusters lower the surface tension of PGL-3 condensates and also form a physical barrier to coalescence.
MEG-3 prevents coarsening of the PGL-3 emulsion without eliminating surface exchange
To determine whether MEG-3 stabilizes the PGL-3 emulsion against coarsening, we examined the evolution of a newly assembled PGL-3 emulsion over time. Over the course of 180 min, the PGL-3 emulsion coarsened substantially: Droplets increased in size on average and decreased in number without a change in the total volume of PGL-3 in droplets (Fig. 2, C and D, and fig. S2, F to H). Addition of MEG-3 reduced coarsening, stabilizing droplet size and number over the 180 min of the experiment (Fig. 2, C and E, and fig. S2, F to H). MEG-3 concentrations constrained the size of PGL-3 droplets in a dose-dependent manner (fig. S2, I to M).
MEG-3 does not affect the internal dynamics of PGL-3 condensates as measured by FRAP (29). To examine whether MEG-3 prevents exchange of PGL-3 molecules at the interface, we used PGL-3 preparations trace-labeled with different fluorophores and examined mixing kinetics. Unlike MEG-3 clusters, which do not mix after assembly, PGL-3 condensates continue to exchange after assembly (fig. S3, A to C). Addition of soluble PGL-3 to a preformed emulsion leads to the formation of new condensates that, over the course of an hour, completely mix with the old condensates (fig. S3, D to F). Addition of MEG-3 prevented coarsening but did not affect the rate of new and old PGL-3 mixing (fig. S3, D to F). We conclude that MEG-3 clusters stabilize the PGL-3 emulsion by lowering surface tension without completely blocking surface exchange, as described for other Pickering agents (34).
MEG-3 stabilizes PGL-3 condensates against DYRK3-accelerated coarsening
The relative high viscosity of the PGL-3 emulsion is consistent with the apparent stability of P granules during most of germline development and suggests that active processes must operate to dissolve P granules during oocyte maturation and polarization. MBK-2 kinase is required for P granule dissolution during polarization, and its mammalian homolog DYRK3 has been implicated in the dissolution of condensates in mammalian cells (28, 35, 36). We found that recombinant DYRK3 phosphorylates PGL-3 efficiently in vitro (fig. S4A). Addition of DYRK3 and adenosine triphosphate (ATP) to preassembled PGL-3 condensates (2.5 µM) led to their complete dissolution over 60 min (Fig. 3, A and B). The effect did not depend on the hydrotrope properties of ATP (37) because dissolution was not observed in the presence of guanosine triphosphate (GTP) (Fig. 3, A and B). Sedimentation experiments confirmed that phosphorylation by DYRK3 increases the fraction of PGL-3 in the soluble pool (fig. S4B). At a higher concentration of PGL-3 (5 µM), condensates could be maintained in the presence of DYRK3 (Fig. 3C). We used these supersaturated conditions to conduct microrheology experiments on PGL-3 droplets treated with DYRK3 (Fig. 3D and movie S8). We found that DYRK3 decreases the viscosity of PGL-3 droplets (Fig. 3D). Consistent with accelerated internal dynamics, DYRK3-treated PGL-3 condensates also coarsened rapidly (Fig. 3, C and E). Addition of MEG-3 did not interfere with PGL-3 phosphorylation but led to a ~3-fold increase in the frequency of small PGL-3 droplets (Fig. 3, C and E, and fig. S4D). The small droplets were covered with MEG-3 and remained stable for 120 min, even in the presence of much larger condensates (fig. S4, E to G). These observations indicate that MEG-3 stabilizes the PGL-3 emulsion against coarsening, even under conditions where PGL-3 dynamics have been accelerated by phosphorylation.
Fig. 3. MEG-3 stabilizes PGL-3 condensates against kinase accelerated coarsening.
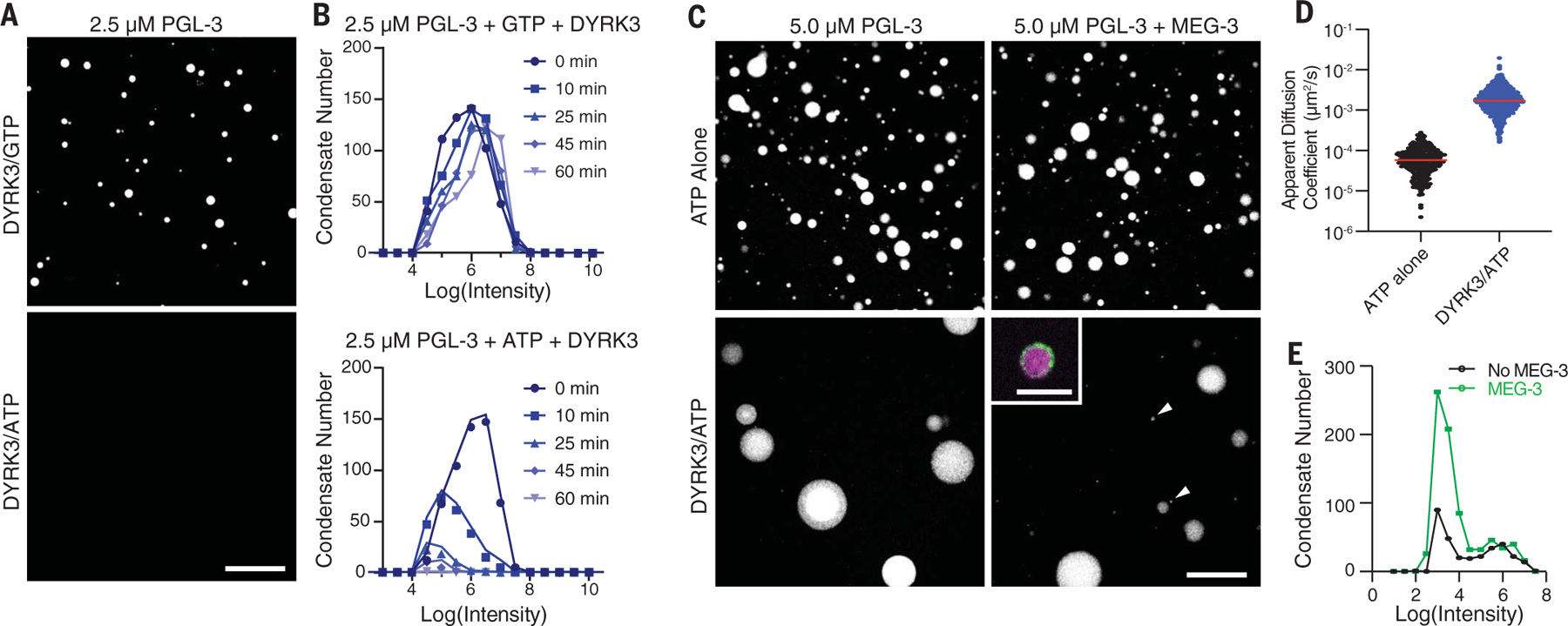
(A) Photomicrographs of a 2.5 µM PGL-3488 emulsion after a 60-min treatment with 100 nM DYRK3 kinase in the presence of GTP (top) or ATP (bottom). Scale bar is 50 µm and applies to both images. (B) Histograms of PGL condensates assembled as in (A) with GTP and DYRK3 or ATP and DYRK3 at indicated time points. Circles indicate the number of PGL-3 condensates binned by the log(intensity) of each condensate. Colors indicate the time after addition of DYRK3. (C) Photomicrographs of PGL-3 condensates (max projections) assembled with and without 70 nM MEG-3 and captured 60 min after addition of 100 µM ATP with and without 100 nM DYRK3. Arrows point to small PGL-3 and MEG-3 co-condensates. The inset is a high-resolution image with PGL-3 in magenta and MEG-3 in green (see fig. S4, F and G, for additional examples). Scale bars are 50 µm (applies to all images in the set) and 5 µm (inset). (D) Diffusion coefficients of 200-nm microspheres in PGL-3 condensates (5 µM PGL-3 and 100 µM ATP) with and without 100 nM DYRK3. Each dot represents a single microsphere trajectory. The red line represents the mean. (E) Histograms of PGL-3 condensates assembled with and without 70 nM MEG-3 and incubated for 60 min in 100 µM ATP with 100 nM DYRK3. Circles indicate the number of PGL-3 condensates binned by the log (intensity) of each condensate captured from 17 images. See fig. S4, D and E, for additional MEG-3 concentrations and an additional time point.
MEG-3 and MEG-4 stabilize P granules against coarsening during the oocyte-to-zygote transition
To examine the impact of MEG-3 on P granule dynamics in vivo, we used quantitative livecell imaging to measure the number and size of PGL-3 condensates in wild type and meg-3 meg-4 mutants (where meg-3 and meg-4 are deleted). We began by imaging eggs in utero as they progress through oocyte maturation and fertilization (Fig. 4, A and B). We found that the volume of PGL-3 in condensates decreased 10-fold during oocyte maturation and remained low in newly fertilized zygotes as they completed the meiotic divisions (Fig. 4, C to F, and fig. S5A). Total PGL-3 levels did not change during this period (fig. S5B), consistent with a transient increase in PGL-3 solubility. We observed the same decrease in PGL-3 condensate volume in wild-type and meg-3 meg-4 oocytes, indicating that the increase in PGL-3 solubility during the oocyte-to-zygote transition is not dependent on meg-3 and meg-4 (Fig. 4, C to F, and fig. S5, A and B). The size distribution of PGL-3 condensates after dissolution, however, was different in the two genotypes. The PGL-3 emulsion coarsened rapidly in meg-3 meg-4 zygotes with fewer larger condensates dominating the emulsion (Fig. 4, B and E). By contrast, wild-type zygotes maintained many small PGL-3 condensates, consistent with MEG-3 stabilizing the PGL-3 emulsion against coarsening (Fig. 4, B and E). Zygotes in late meiosis can survive outside of the uterus, allowing for the acquisition of high-resolution images ex utero. These images confirmed that wild-type zygotes contain dozens of <1-mm condensates not observed in meg-3 meg-4 zygotes (Fig. 4, B and F).
Fig. 4. MEG-3 and MEG-4 stabilize P granules against coarsening during the oocyte-to-zygote transition.
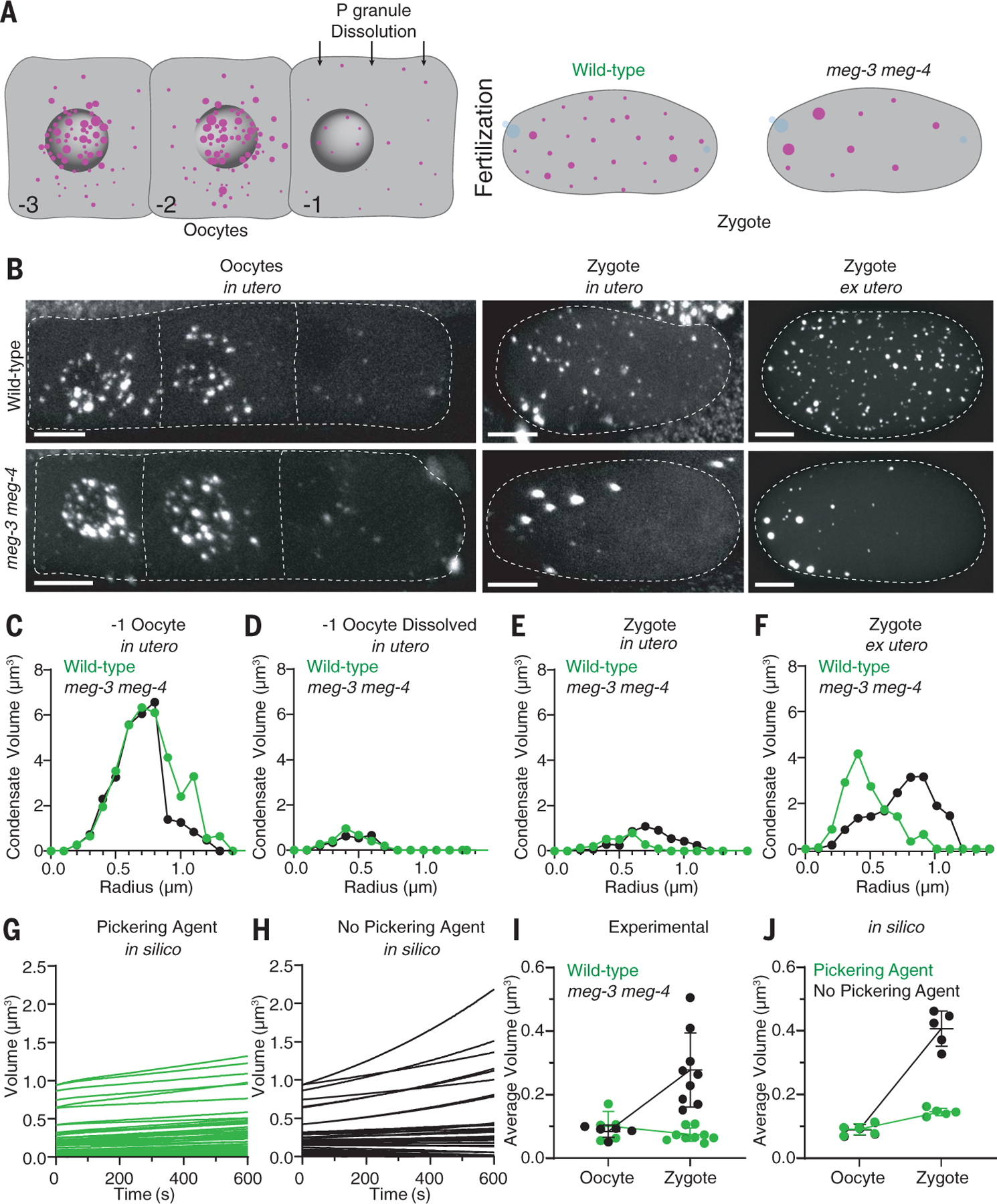
(A) Schematics depicting the dissolution of P granules (magenta) during the transition from oocyte to fertilized zygote. The numbers indicate the relative position of each oocyte in the germline, and blue represents DNA. (B) Photomicrographs of wild-type and meg-3 meg-4 oocytes and zygotes expressing PGL-3::mCherry (white). Photomicrographs were captured in live adult hermaphrodites (in utero) or after dissection out of the uterus (ex utero). Representative photomicrographs are max projections corresponding to ~20% of oocyte volume and ~80% of zygote volume. The anterior (left) bias for PGL condensates in meg-3 meg-4 zygotes correlates with anterior displacement of the oocyte nucleus (and associated P granules) that occurs immediately before fertilization. The white dashed lines indicate the boundary of each oocyte or zygote. Scale bars are 10 µm. (C to F) Histograms of PGL condensate volumes measured from images captured as in (B) representing 100% of oocyte and zygote volumes. Circles indicate the volume of individual PGL-3 in condensates binned by condensate radius in wild-type (green) and meg-3 meg-4 (black) oocytes and zygotes. Volumes are higher in (F) than in (E) owing to higher detection sensitivity ex utero. (G and H) Graphs showing the evolution of individual PGL condensates in a 10-min period starting after dissolution in wild-type and meg-3 meg-4 oocytes and zygotes under simulated conditions. Each line represents the evolution of a single condensate over time. (I and J) Graphs showing the average volume of individual PGL condensates in oocytes and zygotes under experimental and simulated conditions. For (I), each dot corresponds to an oocyte [same dataset as shown in (D)] or zygote [same data set as shown in (F)] of the indicated genotypes. For (J), each dot corresponds to one simulation. Simulations were run in the presence or absence of the Pickering agent. Horizontal and vertical lines represent the mean SD.
These observations suggest that MEG-3 functions as a Pickering agent for the PGL-3 emulsion. To examine the physical plausibility of this hypothesis, we modeled in silico the kinetics of an idealized PGL-3 emulsion in the presence or absence of a Pickering agent that lowers surface tension. To account for the intrinsically slow dynamics of PGL-3 condensates, we modeled PGL-3 dynamics under a “conversion-limited” scheme, where the soluble-to-condensate conversion rate of PGL-3 molecules is much slower than their diffusion-limited adsorption-desorption rate and is therefore rate limiting for condensate growth and degrowth (see supplementary text). To model dissolution of PGL-3 condensates during oocyte maturation, we assigned a relatively high conversion rate to PGL-3 condensates and a high critical concentration for PGL-3 phase separation because most PGL-3 condensates dissolve during this period. To model MEG-3 as a Pickering agent, we reduced the Gibbs-Thomson length (proportional to surface tension) of MEG-3–coated PGL-3 condensates by 100-fold compared with PGL-3–only condensates. Parameter sweeps revealed that reductions in the Gibbs-Thomson length as low as twofold, within the range observed in vitro (Fig. 2B), were sufficient to show the same quantitative behavior (see supplementary text). We used the model to run simulations tracking the dynamics of condensates with starting sizes matching the distribution of PGL-3 condensates in oocytes after dissolution (Fig. 4, G and H). Coarsening was notably different in the presence or absence of the Pickering agent, with the stabilization of smaller condensates requiring the Pickering agent. The simulations reproduced the increase in average condensate volume observed in meg-3 meg-4 zygotes in comparison to wild-type zygotes (Fig. 4, I and J). These results support the hypothesis that MEG-3 stabilizes the PGL-3 emulsion against an increase in PGL-3 dynamics in newly fertilized zygotes by lowering the surface tension of PGL-3 condensates.
MEG-3 and MEG-4 drive asymmetric growth of the P granule emulsion during polarization
Next, we examined PGL-3 dynamics as zygotes transition from unpolarized to polarized. During this period, zygotes exit meiosis, assemble pronuclei that migrate to the center of the zygote, and fuse and initiate the first mitotic division, and MEX-5 redistributes in an anterior-rich gradient (Fig. 5A). During pronuclear migration, we observed new PGL-3 condensates appearing throughout the cytoplasm in both wild-type and meg-3 meg-4 zygotes (Fig. 5, B and C, and fig. S6A). The total volume of PGL-3 in condensates also increased (fig. S6B), without a change in total PGL-3 (fig. S6C), consistent with a return to low PGL-3 solubility in both genotypes. During this period, total condensate volume in the anterior half of the zygote decreased steadily, eventually reaching zero, whereas total condensate volume in the posterior increased (Fig. 5D). Notably, in meg-3 meg-4 zygotes, we also observed a decrease and increase in total condensate volume in the anterior and posterior, respectively, but the amplitude of the change was greatly reduced (Fig. 5E). By mitosis, in wild-type zygotes, all PGL-3 condensates were restricted to the posterior cytoplasm; by contrast, in meg-3 meg-4 zygotes, PGL-3 condensates remained stable throughout the cytoplasm through the first cell division (Fig. 5, A to E), suggesting that in meg-3 meg-4 mutants, the PGL-3 emulsion does not respond efficiently to the MEX-5–driven solubility gradient.
Fig. 5. MEG-3 and MEG-4 drive asymmetric growth of the P granule emulsion during polarization.
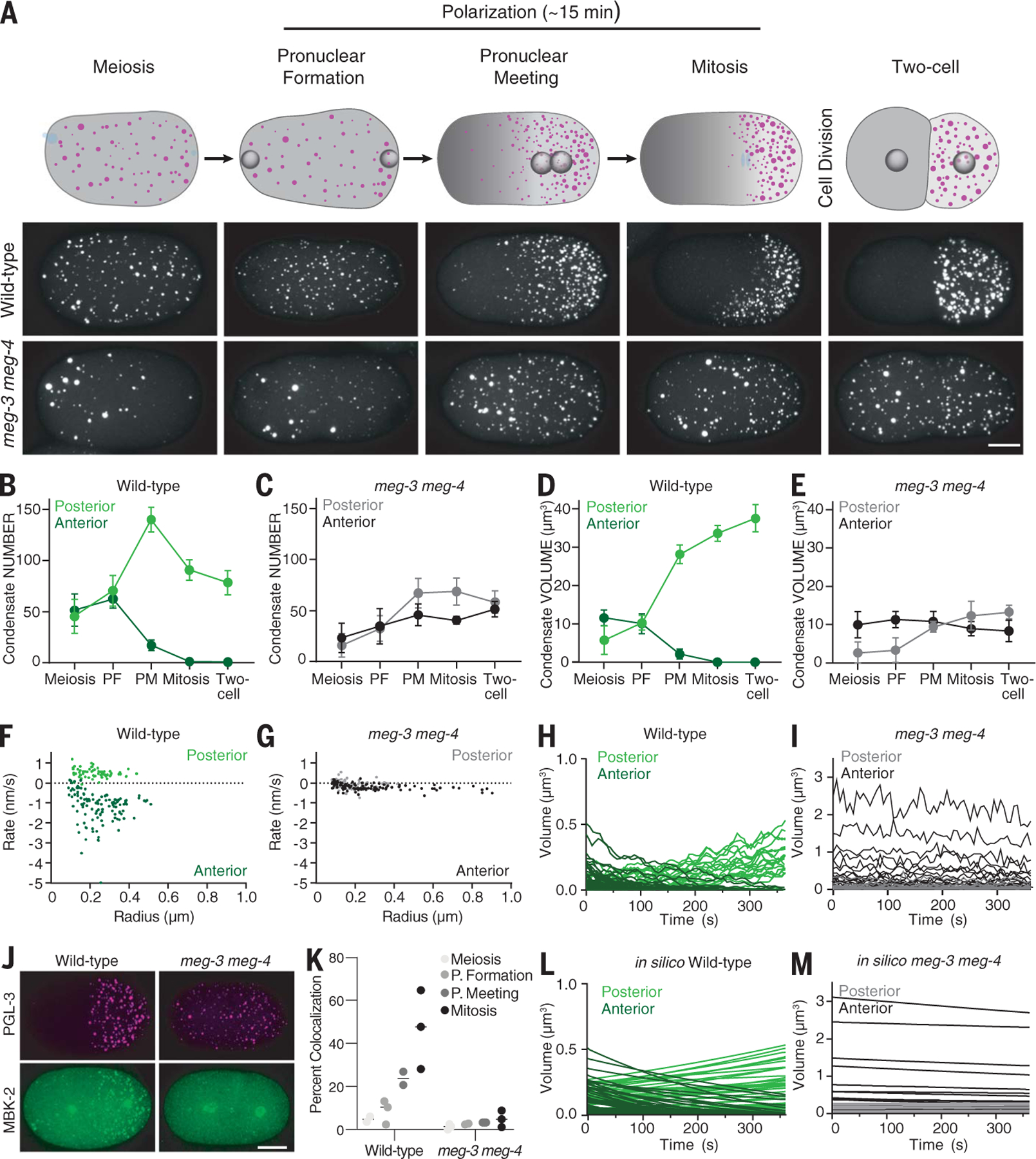
(A) Top row: Cartoons depicting PGL-3 condensates (magenta) and MEX-5 (gray) at different stages during the transition from unpolarized to polarized zygote. Blue represents DNA. Bottom two rows: Photomicrographs of wild-type and meg-3 meg-4 zygotes (ex utero) expressing PGL-3::mCherry (white) and matching the stages shown in the cartoons above. Scale bar is 10 µm and applies to all images. (B to E) Graphs showing the total number [(B) and (C)] and total volume [(D) and (E)] of PGL-3::mCherry condensates in the posterior (light color) or anterior (dark color) half of wild-type and meg-3 meg-4 zygotes calculated from the photomicrographs shown in (A). Circles represent the average from five zygotes, and error bars represent the SD. PF, pronuclear formation; PM, pronuclear meeting. (F and G) Graphs showing the rate of change in radius of PGL-3::mCherry condensates in wild-type and meg-3 meg-4 zygotes calculated from traces shown in (H) and (I). (H and I) Graphs showing the evolution of individual PGL-3::mCherry condensates in anterior and posterior regions during polarization in wild-type and meg-3 meg-4 zygotes. Traces begin at pronuclear formation and end just before pronuclear meeting. (J) Photomicrographs of fixed zygotes (mitosis) of indicated genotypes showing the distribution of MBK-2::OLLAS (green) and PGL-3::mCherry (magenta). Note that, in addition to P granules, MBK-2 localizes to centrosomes. Scale bar is 10 µm and applies to all images in the set. (K) Graph showing the percentage of PGL-3:mCherry condensates colocalized with MBK-2::OLLAS puncta in wild-type or meg-3 meg-4 zygotes at the indicated developmental stages. Each circle represents one zygote (>50 puncta), and each line represents the mean. (L and M) Graphs showing the evolution of individual anterior (dark color) and posterior (light color) PGL-3 condensates under conditions simulating “wild-type” (starting condensate sizes as in wild-type zygotes, high conversion rates, and Pickering agent) and “meg-3 meg-4” (starting condensate sizes as in meg-3 meg-4 zygotes, low conversion rates, and no Pickering agent). Compare with experimental data in (H) and (I).
To examine condensate dynamics directly, we tracked individual PGL-3 condensates in live zygotes from pronuclear formation to pronuclear meeting (Fig. 5, F to I, and movie S9). These observations confirmed that in meg-3 meg-4 zygotes, condensates experience very slow growth and dissolution rates during polarization, with a slight bias for decay in the anterior [anterior rate (kA,avg) = −0.19 nm/s; posterior rate (kP,avg) = 0.06 nm/s; Fig. 5, G and I]. Growth and decay rates were more than fivefold faster in wild-type zygotes, with a clear bias for dissolution in the anterior and condensation in the posterior (kA,avg = −1.18 nm/s; kP,avg = 0.50 nm/s; Fig. 5, F and H). These findings suggest that unlike in oocytes where PGL-3 dissolution occurs independently of meg-3 and meg-4, during polarization, meg-3 and meg-4 are required to accelerate PGL-3 condensate dynamics.
In addition to meg-3 and meg-4, P granule dissolution during polarization requires the DYRK family kinase MBK-2 (38, 39). Genetic epistasis experiments have shown that dissolution activity of MBK-2 is dependent on meg-3 and meg-4 (28) (see supplementary text). Consistent with these observations, using an epitope-tagged allele of endogenous MBK-2, we found that MBK-2 is recruited to PGL-3 condensates during polarization and that this recruitment is diminished in meg-3 meg-4 mutant embryos (Fig. 5, J and K). MEG-3 and MBK-2 levels varied more than fivefold among PGL-3 condensates (fig. S6, D to G). Condensate growth rates during polarization were also heterogeneous in a manner that did not correlate with initial condensate size (Fig. 5, F and G). Together, these observations suggest that heterogeneous recruitment of MEG-3 and MBK-2 variably accelerates PGL-3 condensate dynamics during polarization.
Based on these findings, we built on our theoretical model of the PGL-3 emulsion, adding three new features specific to polarization: (i) higher variable conversion rates for wild-type versus meg-3 meg-4 PGL-3 condensates to reflect heterogeneous fluidization by MEG proteins and MBK-2, (ii) higher solubility for PGL-3 in anterior cytoplasm to reflect the influence of the MEX-5 gradient, and (iii) lower Gibbs-Thomson lengths for posterior condensates to reflect sustained coverage of posterior condensates by MEG proteins (“asymmetric Pickering agent”). Parameters were benchmarked to allow for complete dissolution of anterior granules in ~6 min, as observed in vivo. We used the model to run simulations tracking the growth and decay of condensates matching the size distribution of PGL-3 condensates in vivo before polarization. The simulations faithfully recapitulated the coarsening-free dissolution of anterior condensates and growth of posterior condensates that were observed in wild-type zygotes (Fig. 5L and movie S10). Simulations mimicking conditions in meg-3 meg-4 mutants reproduced the slow dynamics observed in those mutants (Fig. 5M and movie S10). The simulations also reproduced the rapid increase in PGL-3 condensate volume (fig. S6H) that is observed during mitosis in wild-type but not meg-3 meg-4 zygotes (figs. S5A and S6B). The rapid rise is a consequence of the faster approach to equilibrium in the supersaturated environment of the posterior cytoplasm by PGL-3 condensates with accelerated dynamics driven by MEG proteins and MBK-2.
To test the importance of each feature in the model, we reran the wild-type simulations, changing one model feature at a time. Simulations where condensates were assigned uniformly low conversion rates yielded decay rates that were too slow to clear anterior granules under the in vivo time constraints (fig. S6I and movie S10). Simulations with low PGL-3 solubility across the cytoplasm led to the coarsening of anterior condensates (fig. S6J and movie S10). Simulations lacking the Pickering agent led to the coarsening of posterior condensates (fig. S6K and movie S10). Together with the in vitro and in vivo findings, the theory supports a model where MEG proteins facilitate P granule polarization in response to the MEX-5–induced saturation gradient by accelerating PGL-3 conversion dynamics (through recruitment of MBK-2) and by functioning as Pickering agents to lower surface tension on posterior condensates, thus preventing coarsening during periods of fast PGL dynamics (Fig. 6; see supplementary text for a full discussion of theoretical considerations).
Fig. 6. Pickering agents stabilize dynamic emulsions against kinase-accelerated coarsening.
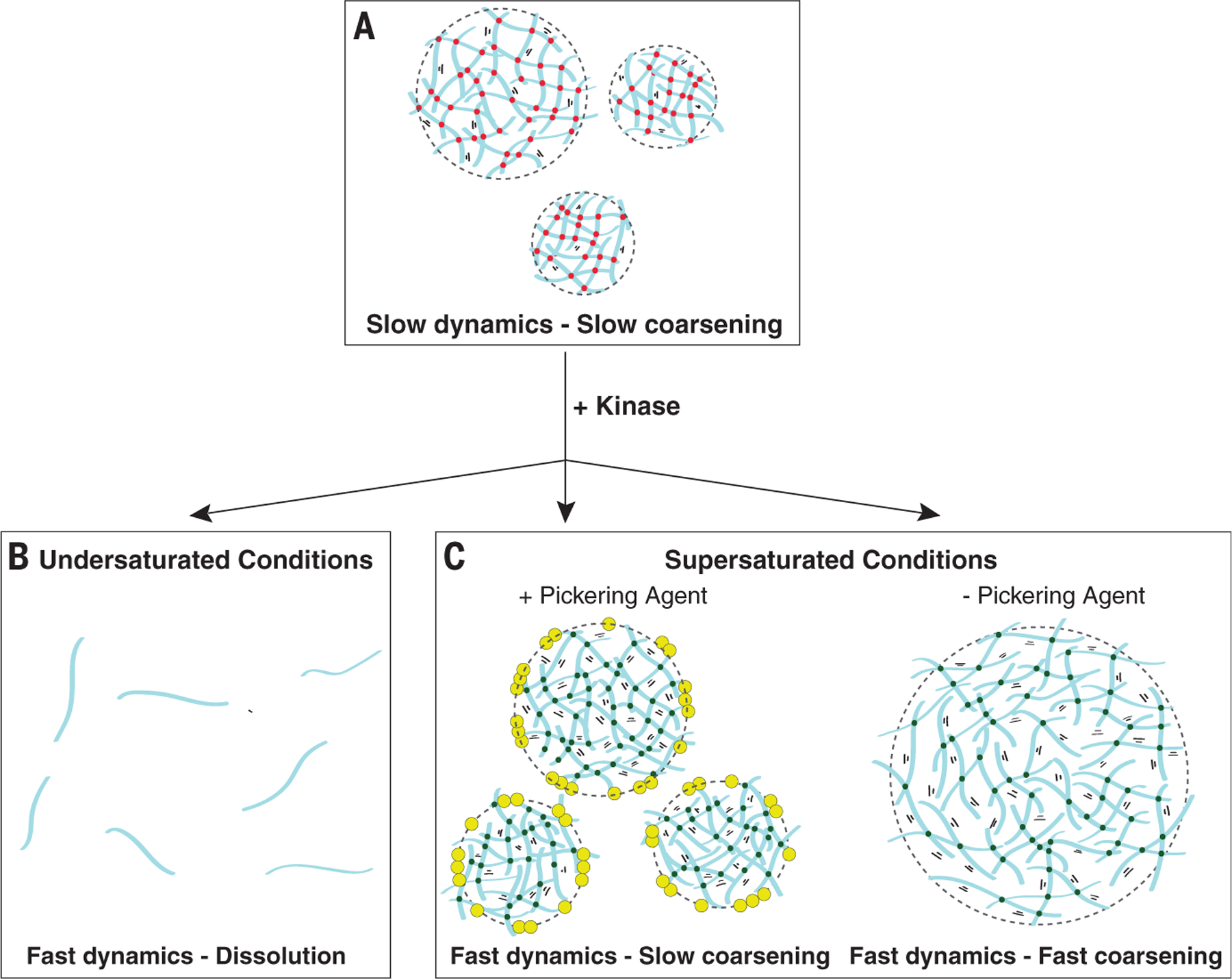
Schematics showing an idealized emulsion of a self-interacting polymer (blue). (A) In untreated condensates, polymer-polymer binding and unbinding events are slow (red dots), allowing the emulsion to persist with minimal coarsening over short time scales. (B and C) Phosphorylation by kinase increases solubility and accelerates internal dynamics [green dots in (C)]. In undersaturated conditions (B), kinase-accelerated dynamics cause the condensates to dissolve, as is observed in the anterior of wild-type polarized zygotes. In saturated conditions (C), kinase-accelerated dynamics allow the condensates to rapidly grow by allowing new molecules from the dilute phase to enter the condensates. In the presence of the Pickering agent (yellow), the condensates are stabilized against coarsening, as observed in the posterior of wild-type polarized zygotes. In the absence of the Pickering agent, the condensates coarsen, as observed in meg-3 meg-4 mutants during the oocyte-to-embryo transition. During polarization, MEG-3 and MEG-4 function both as Pickering agents and recruiters of the kinase MBK-2. In meg-3 meg-4 zygotes undergoing polarization, PGL condensates are maintained throughout the cytoplasm with minimal coarsening, because MBK-2 kinase is not recruited to the condensates and PGL dynamics remain slow, as in (A).
Discussion
Since their description by Ramsden and Pickering in the early 1900s (19, 20), Pickering agents have been used widely to stabilize emulsions in the pharmaceutical, energy, and food industries (40–42). Unlike surfactants (amphiphilic molecules that insert at interfaces), Pickering agents are nanoscale solid particulates that adsorb to interfaces upon partial wetting by both phases. Adsorption is energetically favored and balances the drive to reduce interfacial area, stabilizing the emulsion against coarsening (43). Many types of solid particulates have been shown to function as Pickering agents, from silica to denatured proteins (44). The first described intrinsically disordered protein, casein, functions as a natural Pickering agent in homogenized milk (45). We characterized intrinsically disordered protein MEG-3 as an intracellular Pickering agent, and we speculate that other self-assembling biopolymers will exhibit similar properties. In somatic cells, PGL droplets are covered by EPG-2 clusters that may function like MEG-3 to regulate the size and dynamics of PGL droplets in preparation for autophagy (46). Artificial protein-RNA assemblies that adsorb to the surface of stress granules have been reported to influence their size and coalescence (47). mRNAs that accumulate on the surface of protein condensates could also serve as stabilizing agents (48, 49). Given the rich diversity of biopolymers in cells, it is tempting to speculate that biopolymers acting as Pickering agents will prove a general organizing principle for biomolecular condensates. By interacting with enzymes like the DYRK family kinase MBK-2, biological Pickering agents also regulate interfacial exchange to control the flux of molecules in and out of condensates in response to environmental changes. In this respect, surface-adsorbed biopolymers fulfill a boundary function for biomolecular condensates, reminiscent of the role of lipid bilayers and associated machineries (e.g., channels) in membrane-bound organelles. Indeed, it has been speculated that lipid membranes arose from liposomes that functioned as Pickering stabilizers for aqueous emulsions (50).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Johns Hopkins Integrated Imaging Center (S10OD023548) for microscopy support. We thank the Lavis lab for HaloTag ligands JF549 and JF646, the Griffin lab for the MEG-3:: Halo strain, the Waugh lab for TEV protease (pRK793, Addgene), T. Hyman, the Baltimore Worm Club, and the Seydoux lab for many helpful discussions.
Funding:
This work was supported by the National Institutes of Health (grant numbers R37HD037047 to G.S. and F32GM134630 to A.P.). G.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Competing interests: G.S. serves on the scientific advisory board of Dewpoint Therapeutics, Inc. A.W.F., A.P., and G.S. are inventors on provisional application #63/094,987 filed on 10/22/2020 held by Johns Hopkins University that covers the use of intrinsically disordered proteins as Pickering agents.
Data and materials availability: All data are available in the manuscript or the supplementary materials. Code for simulations is deposited and accessible at Zenodo (51).
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Hyman AA, Weber CA, Jülicher F., Annu. Rev. Cell Dev. Biol 30, 39–58 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Jawerth L et al. , Science 370, 1317–1323 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Boeynaems S et al. , Trends Cell Biol 28, 420–435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman Rosenzweig ES et al. , Cell 171, 148–162.e19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feric M, Brangwynne CP, Nat. Cell Biol 15, 1253–1259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP, Proc. Natl. Acad. Sci. U.S.A 112, E5237–E5245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbaum-Garfinkle S et al. , Proc. Natl. Acad. Sci. U.S.A 112, 7189–7194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranganathan S, Shakhnovich EI, eLife 9, e56159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry J, Brangwynne CP, Haataja M., Rep. Prog. Phys 81, 046601 (2018). [DOI] [PubMed] [Google Scholar]
- 10.McSwiggen DT, Mir M, Darzacq X, Tjian R., Genes Dev 33, 1619–1634 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R., eLife 5, e18413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JE, Cathey PI, Wu H, Parker R, Voeltz GK, Science 367, eaay7108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DSW, Wingreen NS, Brangwynne CP, Nat. Phys 17, 531–538 (2021). [Google Scholar]
- 14.Lee CF, Brangwynne CP, Gharakhani J, Hyman AA, Jülicher F., Phys. Rev. Lett 111, 088101 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Weber CA, Lee CF, Jülicher F., New J. Phys 19, 053021 (2017). [Google Scholar]
- 16.Zwicker D, Hyman AA, Jülicher F., Phys. Rev. E 92, 012317 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Bressloff PC, Phys. Rev. E 101, 042804 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Wurtz JD, Lee CF, Phys. Rev. Lett 120, 078102 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Ramsden W, Gotch F., Proc. R. Soc. London 72, 156–164 (1904). [Google Scholar]
- 20.Pickering SU, J. Chem. Soc. Trans 91, 2001–2021 (1907). [Google Scholar]
- 21.Brangwynne CP et al. , Science 324, 1729–1732 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Saha S et al. , Cell 166, 1572–1584.e16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanazawa M, Yonetani M, Sugimoto A., J. Cell Biol 192, 929–937 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheth U, Pitt J, Dennis S, Priess JR, Development 137, 1305–1314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert CM, Lin R, de Vries CJ, Plasterk RH, Priess JR, Mol. Cell 5, 671–682 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Chen J-X et al. , Mol. Cell. Proteomics 15, 1642–1657 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J et al. , eLife 5, e21337 (2016).27914198 [Google Scholar]
- 28.Wang JT et al. , eLife 3, e04591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putnam A, Cassani M, Smith J, Seydoux G., Nat. Struct. Mol. Biol 26, 220–226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y et al. , Mol. Biol. Cell 30, 333–345 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngai T, Bon S, Eds., Particle-Stabilized Emulsions and Colloids (Royal Society of Chemistry, 2014); https://pubs.rsc.org/en/content/ebook/978-1-84973-881-1. [Google Scholar]
- 32.Jawerth LM et al. , Phys. Rev. Lett 121, 258101 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Pawar AB, Caggioni M, Ergun R, Hartel RW, Spicer PT, Soft Matter 7, 7710–7716 (2011). [Google Scholar]
- 34.Crowe CD, Keating CD, Interface Focus 8, 20180032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang KM et al. , Dev. Biol 265, 127–139 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Rai AK, Chen J-X, Selbach M, Pelkmans L., Nature 559, 211–216 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Patel A et al. , Science 356, 753–756 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Quintin S, Mains PE, Zinke A, Hyman AA, EMBO Rep 4, 1175–1181 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellettieri J, Reinke V, Kim SK, Seydoux G., Dev. Cell 5, 451–462 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Berton-Carabin CC, Schroën K., Annu. Rev. Food Sci. Technol 6, 263–297 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Goodarzi F, Zendehboudi S., Can. J. Chem. Eng 97, 281–309 (2019). [Google Scholar]
- 42.Albert C et al. , J. Control. Release 309, 302–332 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez Ortiz D, Pochat-Bohatier C, Cambedouzou J, Bechelany M, Miele P., Engineering 6, 468–482 (2020). [Google Scholar]
- 44.Yang Y et al. , Front. Pharmacol 8, 287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redwan EM, Xue B, Almehdar HA, Uversky VN, Curr. Protein Pept. Sci 16, 228–242 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, Wang Z, Du Z, Zhang H., Cell 174, 1492–1506.e22 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Jove Navarro M et al. , Nat. Commun 10, 3230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampoelz B et al. , Cell 179, 671–686.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tauber D et al. , Cell 180, 411–426.e16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewey DC, Strulson CA, Cacace DN, Bevilacqua PC, Keating CD, Nat. Commun 5, 4670 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Lee CF, chiufanlee/Pickering-stabilization, Version v1.0, Zenodo (2021); 10.5281/zenodo.5083065. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


