Abstract
Mitochondria are both a primary source of reactive oxygen species (ROS) and a sensitive target of oxidative stress; damage to mitochondria can result in bioenergetic dysfunction and both necrotic and apoptotic cell death. These relationships between mitochondria and cell death are particularly strong in both acute and chronic neurodegenerative disorders. ROS levels are affected by both the production of superoxide and its toxic metabolites and by antioxidant defense mechanisms. Mitochondrial antioxidant activities include superoxide dismutase 2, glutathione peroxidase and reductase, and intramitochondrial glutathione. When intracellular conditions disrupt the homeostatic balance between ROS production and detoxification, a net increase in ROS and an oxidized shift in cellular redox state ensues. Cells respond to this imbalance by increasing the expression of genes that code for proteins that protect against oxidative stress and inhibit cytotoxic oxidation of proteins, DNA, and lipids. If, however, the genomic response to mitochondrial oxidative stress is insufficient to maintain homeostasis, mitochondrial bioenergetic dysfunction and release of pro-apoptotic mitochondrial proteins into the cytosol initiate a variety of cell death pathways, ultimately resulting in potentially lethal damage to vital organs, including the brain. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a translational activating protein that enters the nucleus in response to oxidative stress, resulting in increased expression of numerous cytoprotective genes, including genes coding for mitochondrial and non-mitochondrial antioxidant proteins. Many experimental and some FDA-approved drugs promote this process. Since mitochondria are targets of ROS, it follows that protection against mitochondrial oxidative stress by the Nrf2 pathway of gene expression contributes to neuroprotection by these drugs. This document reviews the evidence that Nrf2 activation increases mitochondrial antioxidants, thereby protecting mitochondria from dysfunction and protecting neural cells from damage and death. New experimental results are provided demonstrating that post-ischemic administration of the Nrf2 activator sulforaphane protects against hippocampal neuronal death and neurologic injury in a clinically-relevant animal model of cardiac arrest and resuscitation.
Keywords: Oxidative stress, Reactive oxygen species, Reactive nitrogen species, Inflammation, Calcium, Excitotoxicity, Sulforaphane
1. Introduction
Following acute brain injury, secondary neurodegeneration often increases the extent and severity of the insult (Schimmel et al., 2017; Visser et al., 2019). Several secondary processes have been implicated in chronic neurodegeneration including altered energy metabolism, oxidative stress, inflammation, and cell death (Hiebert et al., 2015; Narne et al., 2017). Despite their apparent heterogeneity, mitochondria are a common factor that links these processes together. As a result, pharmaceutical interventions that target mitochondria have the potential to impact multiple mechanisms that drive the ongoing injury (Bhatti et al., 2017). This review focuses on nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that interacts with mitochondria to inhibit neurodegenerative processes and provides neuroprotection in numerous animal models of neurologic disorders. New experimental results obtained with an animal model of cardiac arrest are included, demonstrating the neuroprotective efficacy of sulforaphane, a drug that activates the Nrf2 pathway of cytoprotective gene expression and that protects mitochondria from damage caused by elevated Ca2+ and oxidized redox state.
2. Acute brain injury
2.1. Traumatic brain injury
Traumatic brain injury (TBI) is a major cause of death and disability worldwide with an estimated 70 million people experiencing a TBI each year (Dewan et al., 2018). The primary injury stems from direct transfer of mechanical energy on the brain, acceleration/deceleration, or pressure waves associated with explosions. Depending on the severity and type of energy injury, the initial insult may cause primarily a focal injury, associated with contusions, lacerations, and/or bleeds, or a disseminated injury, characterized by diffuse axonal injury from stretching/tearing of tissue (Blennow et al., 2012). TBI in patients and lab animals is associated with inhibition of aerobic and stimulation of anaerobic brain energy metabolism within a few hours after the initial injury (DeVience et al., 2017; Robertson et al., 2006b; Xu et al., 2011). Severely damaged neurons and other brain cells may undergo necrotic cell death at this stage, thereby releasing a number of danger-associated molecular patterns (DAMPs), including HMGB1, mitochondrial DNA (mtDNA), ATP, and other molecules that stimulate neuroinflammation (Braun et al., 2017). The excitatory neurotransmitter glutamate is also released which, along with axonal damage, can increase intracellular Ca2+ and cause excitotoxicity (Hinzman et al., 2016; Siedler et al., 2014). Elevated intracellular Ca2+ can also increase mitochondrial Ca2+ levels, which promote reactive oxygen species (ROS) production and opening of the mitochondrial inner membrane permeability transition pore (mPTP), leading to mitochondrial osmotic swelling, energy failure, and cell death (Hurst et al., 2017).
2.2. Ischemic brain injury
According to the World Health Organization, cerebrovascular accidents, including cerebral ischemia, are the second leading cause of death and the third leading cause of disability worldwide (Johnson et al., 2016). Most commonly, ischemia is caused by diminished or interrupted blood supply to tissues from a blockage or cardiac arrest. Due to decreased blood flow, insufficient oxygen supply to the brain disrupts energy metabolism and leads to an ischemic injury, either to just a portion of the brain (focal cerebral ischemia, as occurs with an ischemic stroke), or to the whole brain (global cerebral ischemia, as occurs with cardiac arrest). This lack of sufficient oxygen impairs aerobic energy metabolism and increases glycolysis, resulting in a net decrease in intracellular ATP. Eventually, inadequate energy production promotes several conditions including ionic exchange pump failure, hyperosmolarity, and metabolic lactic acidosis, which can cause necrotic cell death (Sanganalmath et al., 2017; Wu et al., 2018). Necrosis releases glutamate, thereby promoting excitotoxicity and, like TBI, mitochondrial dysfunction through elevated intracellular Ca2+ (Andreyev et al., 2018). For a comprehensive review of calcium, ischemia, and excitotoxicity, see Szydlowska and Tymianski (2010). Cerebral ischemia also induces hypoxia inducible factor-1α (HIF-1α)-linked transcription which, in turn, activates NADPH oxidase (NOX) enzymes. NOX are membrane-bound complexes that are a major source of ROS through the generation of superoxide (O2−) (Ma et al., 2017).
Ischemic cells that survive the initial injury have a lower concentration of antioxidants (e.g., glutathione) than healthy cells, making them vulnerable to oxidative stress (Wu et al., 2018). As a result, in cases where blood supply is restored to ischemic tissues either through resuscitation or resolution of a blockage, reperfusion can often exacerbate the ischemic injury (Pan et al., 2007). While reperfusion injury occurs due to a variety of mechanisms, excess ROS production is considered a primary driver (Granger and Kvietys, 2015). In the ischemic brain, this increased ROS production comes from a variety of sources including NOX (Walder et al., 1997) and mitochondria (Piantadosi et al., 2008).
2.3. Brain injury treatment window
Given the overlapping mechanisms that drive oxidative stress, inflammation, and energy metabolism dysfunction (for a review, see Witte et al. (2010)), it is not surprising that acute brain injuries can worsen over time due to ongoing pathogenic processes (Balan et al., 2013; Frati et al., 2017; Hazelton et al., 2010; Lee et al., 2018; Proctor et al., 2014; Schimmel et al., 2017). As acute brain injuries generally occur outside of a hospital environment and access to immediate intervention is limited, the hours/days immediately following the injury is a potential target for translational studies aimed to find pharmacologic interventions that prevent functional deterioration and maximize post-injury outcomes. Compounds that activate the Nrf2 pathway of cytoprotective gene expression may be particularly effective due to their ability to modulate multiple mechanisms of cell injury and repair.
3. Nrf2 regulation
Nrf2, the basic leucine zipper transcription factor encoded by the NFE2L2 gene (Alam et al., 1999), is an approximately 100 kilodalton cap’n’collar transcription factor. Nrf2 is a member of a family of conserved proteins that serve important roles in homeostasis and adaptive response to cellular stress in metazoans (Sykiotis and Bohmann, 2010). Nrf2 protein is constitutively expressed and localized in the cytosol [Fig. 1A] (Stewart et al., 2003). Under unstressed conditions, a homodimer of Kelch-like ECH-associated protein (KEAP1) sequesters Nrf2 along the actin cytoskeleton in the cytosol. KEAP1, a Cullin-3 (Cul3)-based E3 ubiquitin ligase adapter, acts to facilitate binding of Nrf2 to the Cul3 ubiquitin ligase complex, thereby promoting the ubiquitination of Nrf2 and subsequent proteasomal degradation (Cullinan et al., 2004; Eggler et al., 2009) [Fig. 1B].
Fig. 1.
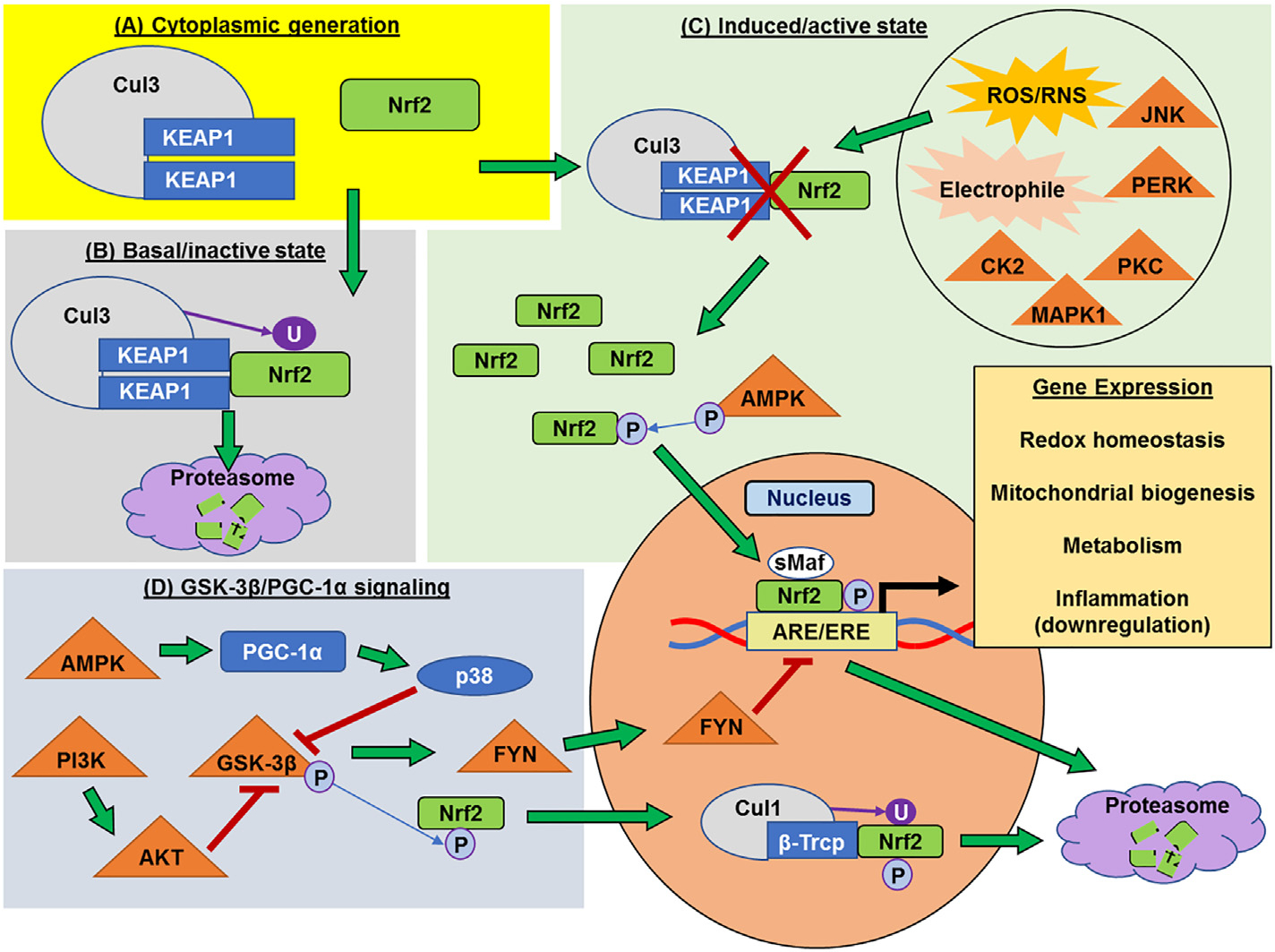
Regulation of Nrf2. (A) Nuclear factor erythroid 2-related factor 2 (Nrf2) protein is constitutively generated and, along with the Kelch-like ECH associated protein (KEAP1), is located in the cytoplasm. (B) In the basal state, Nrf2 associates with the Keap1/Cullin3 (KEAP1/Cul3) ubiquitin ligase complex in the cytosol, which ubiquitinates Nrf2 and targets it for proteasomal degradation. (C) Exposure to reactive oxygen/nitrogen species (ROS/RNS), electrophiles, or phosphorylation by certain kinases, such as protein kinase C (PKC), PRK-ER-related kinase (PERK), mitogen activated protein kinase 1 (MAPK1), c-Jun-NH2-kinase (JNK), or casein kinase 2 (CK2), inhibits the association of the Keap1/Cul3 complex with Nrf2, causing it to build up in the cytoplasm. Cytoplasmic AMP-activated protein kinase (AMPK) phosphorylation of Nrf2 allows it to translocate to the nucleus. Phosphorylated Nrf2 then binds to small Maf (sMaf) proteins and promotes gene expression via binding to antioxidant/electrophile response elements (ARE/EREs) in the promoter regions of genes that affect redox homeostasis, mitochondrial biogenesis, and metabolic up-regulators. Collectively this pattern of gene expression inhibits inflammation. (D) Glycogen synthase kinase-3β (GSK-3β) inhibits Nrf2 expression. This is carried out either through phosphorylation on a different site and recognition by the β-transducin repeats-containing protein/Cullin-1 (β-Trcp/Cul1) ubiquitin ligase complex that targets it for nuclear export and degradation, or by activation and translocation of the nuclear kinase FYN, which may cause dissociation of Nrf2 from ARE/EREs, thereby halting gene expression and leading to proteasomal degradation. GSK-3β can be inhibited by AMPK through activation of peroxisome proliferator-activated receptor-γ coactivator alpha (PGC-1α) and subsequent expression of protein p38, as well as through the phosphatidyl inositol 3-kinase/AKT (PI3K/AKT) pathway.
In the presence of increased ROS, reactive nitrogen species (RNS), electrophiles, or xenobiotics, cysteine thiols within KEAP1 become oxidized (Kansanen et al., 2013) [Fig. 1C], which causes a conformational change and the liberation of Nrf2, allowing it to translocate into the nucleus. Once in the nucleus, Nrf2 forms a heterodimer with small Maf (sMaf) proteins and binds to antioxidant response elements (AREs) or electrophile response elements (EREs). This promotes the expression of proteins involved in the response to oxidative stress and inflammation as well as metabolism and mitochondrial biogenesis (Dinkova-Kostova and Abramov, 2015; Friling et al., 1990; Motohashi and Yamamoto, 2004; Rushmore et al., 1991; Rushmore and Pickett, 1990). There is also evidence that Nrf2-KEAP1 is tethered to the outer mitochondrial membrane, perhaps to react most efficiently to increased mitochondrial ROS generation (Lo and Hannink, 2008; Strom et al., 2016).
While the above pathway is well-defined, the regulation of Nrf2 is complex, with post-translational phosphorylation playing a major role in its release, nuclear translocation, and degradation. Several sites on Nrf2 can be phosphorylated by different kinases to elicit different effects. For instance, it was thought that phosphorylation via protein kinase C (PKC) was necessary for dissociation from the KEAP1 dimer (Huang et al., 2000), but several other kinases have been implicated as potential dissociation agents, including the endoplasmic reticulum-localized PRK-ER-related kinase (PERK) (Cullinan et al., 2003), mitogen-activated protein kinase 1 (MAPK1/ERK), stress response signaler c-Jun-NH2-kinase (JNK) (Xu et al., 2006), and casein kinase 2 (CK2) (Pi et al., 2007) [Fig. 1C]. Nrf2 translocation into the nucleus may also be stimulated by phosphorylation of Nrf2 via AMP-activated protein kinase (AMPK) (Joo et al., 2016; Wang et al., 2016a). Phosphorylation also plays a critical role in inhibition of Nrf2 activity, especially via glycogen synthase kinase-3β (GSK-3β), a master regulator for several cellular processes including glycogen metabolism, insulin signaling, and apoptosis (Cohen and Frame, 2001). GSK-3β phosphorylation causes sequestration of Nrf2 in the cytosol thereby preventing its influence over gene expression (Salazar et al., 2006); however, phosphorylation of Nrf2 by GSK-3β can also stimulate nuclear translocation, recognition by the nuclear β-transducin repeats containing protein ligase (β-Trcp), a Cullin1 (Cul1)-based E3 ubiquitin ligase adaptor, and degradation (Chowdhry et al., 2013; Rada et al., 2011; Rada et al., 2012). Alternatively, GSK-3β activation of the Src-family tyrosine kinase FYN drives nuclear export of Nrf2 and possibly serves to control gene expression and maintain homeostatic balance (Rizvi et al., 2014; Tebay et al., 2015). GSK-3β can itself be inhibited by the pro-survival phosphatidyl inositol 3-kinase/AKT (PI3K-AKT) signaling pathway (Hayes et al., 2015; Nakaso et al., 2003; Tebay et al., 2015) as well as AMPK (Joo et al., 2016; Wang et al., 2016a), which serve to increase Nrf2 activity [Fig. 1D].
Other mechanisms of Nrf2 regulation have been reported. The protein p38 MAPK enhances the interaction between KEAP1 and Nrf2 (Keum et al., 2006), while the autophagy adapter protein p62 interferes with this interaction (Komatsu et al., 2010). The BTB and CNC homology 1 (BACH1) transcription factor competes with Nrf2 for DNA binding sites and is itself exported from the nucleus via antioxidant-induced phosphorylation (Wiel et al., 2019). In addition, the ER-associated E3 ubiquitin ligase synoviolin 1 (SYVN1/Hrd1) has an affinity for Nrf2 and may act independently of the KEAP1/Cul3 and GSK-3β/β-Trcp/Cul1 pathways (Wu et al., 2014).
4. Antioxidant and redox status
The human brain accounts for 20% of the body’s oxygen consumption, despite comprising just 2% of the body weight (Kety, 1957). As a result of its relatively high rate of oxygen consumption and low capacity for ROS detoxification, the brain is particularly vulnerable to oxidative injury (Salim, 2017). The mitochondrial electron transport chain (ETC), a series of complexes involved in the aerobic production of ATP through oxidative phosphorylation, is a primary endogenous source of ROS in the brain. Under basal conditions, low levels of O2− can be converted from oxygen due to electron leak from the ETC (Handy and Loscalzo, 2012). Despite the constant generation of ROS, cells also possess a robust antioxidant defense capacity that is responsible for ROS detoxification including both enzymatic (e.g. superoxide dismutase 1 (SOD1), catalase (CAT), glutathione peroxidase (GPX)) and non-enzymatic antioxidants (e.g. glutathione (GSH) and coenzyme Q10 (CoQ10)). These phase II antioxidants, a classification for detoxifying enzymes that reduce reactivity of intermediate metabolites, can maintain redox homeostasis under healthy conditions but, in the event of acute injury, the antioxidant defense system may not be sufficient to counteract increased ROS production in the brain.
The Nrf2 pathway is most well-established in its role as a cellular defense mechanism against oxidative and electrophilic stress. Along this pathway, AREs have been identified in the promoter regions of antioxidant genes such as heme oxygenase 1 (HO-1), SOD1, glutamate cysteine ligase catalytic subunit (GCLc), and NAD(P)H quinone dehydrogenase 1 (NQO1), among many others (Hayes et al., 2000; Itoh et al., 1999; Nguyen et al., 2003). In fact, Nrf2 plays a role in the regulation of hundreds of genes, many of which are cytoprotective in nature (Malhotra et al., 2010; Tebay et al., 2015). It follows that ROS levels are usually higher in Nrf2 knockout models (Holmström et al., 2013; Kovac et al., 2015).
One of the most important influences of Nrf2-mediated gene expression is its regulation of redox homeostasis via glutathione regulation. Nrf2 mediates the expression of necessary subunits of GCL, a key enzyme responsible for the biosynthesis of GSH (Wild et al., 1999). Furthermore, Nrf2-controlled expression of glutathione reductase 1 enables the maintenance of glutathione in its reduced state (Harvey et al., 2009; MacLeod et al., 2009). Several enzymes responsible for the generation of NADPH necessary for GSH reduction, including malic enzyme 1 (ME1), isocitrate dehydrogenase 1 (IDH1), 6-phosphogluconate dehydrogenase (PGD), and glucose-6-phosphate dehydrogenase (G6PD), are upregulated by Nrf2 (Lee et al., 2003; Mitsuishi et al., 2012; Thimmulappa et al., 2002; Wu et al., 2011). As shown with regard to antioxidant capacity, Nrf2 deficiency results in lower levels of reduced GSH (Benedict et al., 2012; Higgins and Hayes, 2011; Wakabayashi et al., 2004) as well as a decreased mitochondrial NAD(P) H pool (Holmström et al., 2013).
5. Nrf2 and inflammation
Oxidative stress plays a vital role in many facets of the inflammatory response, including the induction of inflammatory markers such as cytokines, proteins that relay pro-inflammatory signals to other cells, and chemokines, which attract immune cells to the injury site. Activation of these cells can lead to further ROS generation. Thus, ROS removal is the most direct way in which Nrf2 works to mediate inflammatory signaling. For example, the Nrf2 transcription target NQO1 affects the activity of the NLR family pyrin domain containing 3 (NLRP3) inflammasome by preventing the priming necessary for NLRP3 activation by ROS. It follows that NLRP3 is then unable to cleave caspase-1, thereby blocking the production of mature interleukin (IL)-1β, a major pro-inflammatory cytokine (Liu et al., 2017).
Nrf2 also inhibits inflammation by mechanisms other than detoxification of ROS. Carbon monoxide-induced Nrf2 results in an increase in the expression of anti-inflammatory cytokine IL-10 and a decrease in pro-inflammatory tumor necrosis factor-α (TNFα) (MacGarvey et al., 2012). Kobayashi et al. (2016) showed that in mouse macrophages, Nrf2 interferes with the upregulation of the pro-inflammatory genes IL-6 and IL-1β through binding in proximity to their respective gene loci and blocking recruitment of RNA polymerase II. Furthermore, Hu et al. (2018) showed that the mitochondrially-targeted, synthetic antioxidant MitoQ enhances nuclear translocation of Nrf2. The resulting transcriptional activity reduced circulating mtDNA, which acts as a DAMP. They also observed decreased amounts of the cytokines IL-6 and IL-1β. However, this effect may be tissue dependent, as activation of the ARE in proximity to IL-6 causes upregulation of that gene in hepatocytes (Wruck et al., 2011). There is also much evidence that Nrf2 both directly and indirectly suppresses the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), a major activator of the inflammatory response (for review, see Ahmed et al. (2017)). For example, Nrf2-induced HO-1 expression inhibits NFκB -linked pro-inflammatory signaling, including the production of cytokines, chemokines, and prostaglandins, in a variety of tissues (Bellezza et al., 2012; Chi et al., 2015; Lee and Jeong, 2014). In Nrf2 knockouts, NFκB activity and proinflammatory cytokine expression is elevated (Jin et al., 2008; Reddy et al., 2011). The protective effects of Nrf2 activation toward inflammation is further demonstrated by improvement in cardiac function and survival in mouse models of sepsis (Liang et al., 2018; MacGarvey et al., 2012).
There is also evidence that Nrf2 may interact with a class of receptors that mediate the immune response to both infectious and noninfectious systemic pathologies. Toll-like receptors (TLRs), the most well-studied being TLR4, are pattern recognition receptors that can recognize both pathogen-associated molecular patterns (PAMPs), such as a component of gram negative bacteria (lipopolysaccharide; LPS), as well as DAMPs, such as circulating mtDNA and proteins that are released by stress or injured cells. Ligand binding of these molecules to TLRs induces the production of cytokines, chemokines, and interferons. TLR agonists also activate the Nrf2 pathway (Nadeem et al., 2016) which attenuates TLR-induced inflammatory signaling (Chen et al., 2017; Townsend and Johnson, 2016). For a review, see Mohan and Gupta (2018).
6. Nrf2 and mitochondrial biogenesis
Mitochondrial bioenergetic dysregulation is a hallmark of most neuropathological disorders. Although controlled ROS generation is an important component of homeostatic cell signaling (Shadel and Horvath, 2015), uncontrolled ROS generation leads to oxidative damage, including DNA damage. The contribution of mitochondria to ROS production taken together with the proximity of mitochondrial DNA to these ROS may explain why mitochondrial DNA is relatively sensitive to oxidative damage (Mikhed et al., 2015). Moreover, a disruption in the regular turnover of mitochondria, through both mitochondrial biosynthesis and the removal of damaged mitochondria, a process known as mitophagy, contributes to neurodegeneration (Lemasters, 2005). As a result, interventions that aim to restore healthy mitochondrial stores may be beneficial for the treatment of neurodegeneration in both natural aging (Fivenson et al., 2017; Palikaras and Tavernarakis, 2012; Sun et al., 2016) as well as neurodegenerative disorders such as Alzheimer’s disease (Wilkins and Swerdlow, 2016), Parkinson’s disease (Narendra et al., 2008; Pickrell and Youle, 2015; Ryan et al., 2015), Huntington’s disease (Jodeiri Farshbaf and Ghaedi, 2017; Khalil et al., 2015), and acute brain injury (Hiebert et al., 2015; Niizuma et al., 2009).
The connection between Nrf2 and mitochondrial biogenesis is a more recent discovery (Gureev et al., 2019). Piantadosi et al. (2008) first showed that HO-1, a well-known transcriptional target of Nrf2, enhances the nuclear translocation of nuclear respiratory factor 1 (NRF1), which controls the expression of several ETC components encoded in the nucleus (Evans and Scarpulla, 1990; Li et al., 2017b) and is an ARE target (Picca and Lezza, 2015). NRF1 subsequently promotes the expression of mitochondrial transcription factor A (TFAM), a protein that enters the mitochondrial matrix and binds to the “D-Loop” promoter region of the mitochondrial genome, thereby stimulating mtRNA expression and mtDNA replication (Piantadosi and Suliman, 2006). Upregulation of TFAM and other mitochondrial markers such as citrate synthase and mtDNA copy number led MacGarvey et al. (2012) to conclude that Nrf2-induced mitochondrial biogenesis is a primary driver of increased survival in a rat sepsis model. Nrf2-mediated mitochondrial biogenesis also contributes to normal lipid metabolism. Fatty acid oxidation is decreased in mitochondria isolated from Nrf2 genetic knockout (Nrf2−/−) mice but is increased in KEAP1 knockout mice (Ludtmann et al., 2014). Furthermore, abnormal lipid metabolism is curbed by the administration of sulforaphane, which increases mitochondrial membrane potential, ETC activity, and utilization of lipids via the promotion of mitochondrial turnover (Lei et al., 2019). The p62-mediated mitophagy inducer (PMI), which acts to disrupt the Nrf2-KEAP1 association and promotes Nrf2-associated activity, induces mitophagy independent of the traditional PINK/Parkin pathway (Bertrand et al., 2015; East et al., 2014). This demonstrates that Nrf2 not only contributes to mitochondrial biogenesis but also to mitophagy, indicating that it is an important regulator of mitochondrial homeostasis.
There is significant crosstalk between mitochondrial biogenesis and the AMP-activated protein kinase (AMPK). AMPK activates peroxisome proliferator-activated receptor-γ (PPAR-γ) coactivator α (PGC-1α), known as the master regulator of mitochondrial biogenesis (Jäger et al., 2007), as a deficiency in AMPK results in decreased mitochondrial complex subunits and mtDNA copy number (Cao et al., 2014). Moreover, p38, which is positively regulated by PGC-1α, inhibits GSK-3β (Choi et al., 2017) [Fig. 1D]. Therefore, while Nrf2’s activity is regulated by PGC-1α activity (and therefore AMPK), they work in tandem to induce mitochondrial biogenesis (Bruni et al., 2010). To further illustrate this point, an Nrf2-sensitive ARE is located in the promoter region for PPAR-γ, the co-activator of PGC-1α (Pi et al., 2010).
Nrf2 may also play a more direct role in mitochondrial metabolic activity and ROS formation. Many transcriptional targets of Nrf2 positively affect mitochondrial membrane potential and the production of ATP (Dinkova-Kostova and Abramov, 2015). A byproduct of increased ETC activity is increased ROS generation, but this effect might be more than compensated by an increase in Nrf2-associated mitochondrial antioxidant gene expression (Holmström et al., 2013). The mitochondrial electron transport chain component NADH dehydrogenase [ubiquinone] 1 α subcomplex subunit 4 (NDUFA4) is upregulated in human breast epithelial cells by treatment with sulforaphane (Agyeman et al., 2012). Similarly, ARE/ARE-like sequences are present in the promoter region of ATP synthase F1 subunit α (atp5F1α) (Abdullah et al., 2012). Overexpression of uncoupling proteins (UCP) can inhibit superoxide formation through increased inner membrane proton conductance (Kim-Han and Dugan, 2005; Mattiasson et al., 2003) and provides neuroprotection in mouse models of Parkinson’s disease (Conti et al., 2005). One of the UCPs, UCP3, is a transcriptional target of Nrf2 (Anedda et al., 2013).
7. Sex differences in Nrf2
Sex is an important biological variable in the response to brain injury. For example, microglia, which are the resident macrophages in the brain, present a sexually dimorphic presence across anatomical regions, including the hippocampus and cortex, that are particularly vulnerable to acute brain injury (Schwarz et al., 2012). Sexually dichotomized responses to inflammatory stimuli have also been observed. In several models of acute brain injury, female animals exhibit milder inflammatory responses to injury than males (Acaz-Fonseca et al., 2015; Bodhankar et al., 2015; Caplan et al., 2017). While the mechanism that underlies these sex differences is unknown, clinical studies indicate that pre-menopausal adult females are more resistant to ischemic brain injury than age-matched males; however, post-menopausal females are more vulnerable (Rahimian et al., 2019) (but see Gupte et al. (2019)). As a result, female sex hormones estrogen and progesterone have been investigated as a potential mediator of the protective effect of sex on brain injury (Robertson et al., 2006a) although clinical trials using estrogen therapy have not yielded promising results (Hendrix et al., 2006). Therefore, alternative explanations to sex hormones for female resilience to injury still need to be investigated. There are several recent reviews of current research on sex differences after brain injury (Bushnell et al., 2018; Gupte et al., 2019; Mollayeva et al., 2018; Spychala et al., 2017).
To date, sex differences in the expression, activation, and signaling of Nrf2 in the brain have not been adequately explored. Indeed, most animal studies using Nrf2 activators rely on a single sex, making it impossible to determine if Nrf2 pathway activation is sexually dimorphic. For example, basal Nrf2 mRNA and/or protein are lower in females compared to males in brain (Zawada et al., 2015) and in liver (Rohrer et al., 2014). It is unclear if these basal differences translate into sex-specific responses to injury or drugs. A cerebral ischemia study showed that estrogen increases Nrf2 signaling in ovariectomized female mice and improves short-term outcomes, including inflammatory signaling, edema, and neurologic function (Li et al., 2017a); it is unclear how intact male or female mice would respond to similar treatment. A study examining sulforaphane induction of glutathione S-transferase activity in the liver failed to reveal sex differences in healthy Fischer and Sprague-Dawley rats, though it is unknown how injured animals may respond. Further research should examine the time course of Nrf2 pathway induction in the brain following trauma or ischemia in both sexes and compare responses to Nrf2 inducers. For recent reviews on sex differences and mitochondrial function in health and disease, see Ventura-Clapier et al. (2017) and Demarest and McCarthy (2015).
8. Nrf2 in animal models of acute brain injury
The Nrf2 pathway is activated in response to several forms of acute brain injury. Following TBI, Nrf2 immunoreactivity is increased in pericontusional neurons (but see Shih et al. (2003)), astrocytes, and microglia, with neuronal-selective localization peaking at 1 day post injury (dpi) and glial expression at 7 dpi (Dong et al., 2019). In both TBI and cerebral ischemia models, Nrf2 is decreased in the cytoplasm and/or increased in the nucleus at 1 dpi (Dai et al., 2018a; Ding et al., 2017b; Li et al., 2013; Li et al., 2016b), indicating release of Nrf2 from KEAP1 and nuclear translocation soon after injury. However, studies on the time course of Nrf2 and oxidative damage following acute brain injury suggest that while the insult activates the Nrf2 pathway, its induction is delayed, thus diminishing its potential to provide antioxidant protection during initial injury-induced increases in ROS production (Miller et al., 2014; Srivastava et al., 2013; Tanaka et al., 2011). The authors suggest that earlier activation with pharmacological Nrf2 inducers could help minimize oxidative damage and improve neurologic outcomes.
Several studies indicate that genetic knockout of Nrf2 results in increased injury severity. In a weight-drop TBI model, Nrf2−/− mice exhibit increased edema, oxidative stress, inflammation, and cell death. Nrf2−/− mice are also characterized by reduced glutathione and worse neurological severity scores (NSS) after TBI relative to wild-type mice (Ding et al., 2017b; Jin et al., 2008; Jin et al., 2009; Lu et al., 2015). Similar results were obtained using CCI-induced TBI, with Nrf2−/− mice showing increased oxidative stress and decreased activation of antioxidant pathways (Cheng et al., 2013; Hong et al., 2010). The same effects of Nrf2 knockout are observed in models of cerebral ischemia. Following focal ischemia (i.e., stroke), Nrf2−/− animals exhibit increased cerebral infarct, decreased antioxidant gene expression pathway induction, reduced angiogenesis, and worse neurological deficit scores relative to wild-type (Chen et al., 2019; Li et al., 2013; Shih et al., 2005). Importantly, differences in infarct volume between Nrf2−/− and wild-type are not evident at 24 h; it is only after a delay of several days that this difference becomes apparent, suggesting that persistent oxidative stress, inflammation, and/or mitochondrial dysfunction due to Nrf2 deficiency is to blame rather than a difference in the severity of the initial injury (Shih et al., 2005). Nrf2 knockouts also display increased expression of TLR4, NLRP3, and NFκB, key regulators of inflammatory signaling (Hou et al., 2018; Li et al., 2013; Molteni et al., 2016).
9. Pharmacologic activation of Nrf2 pathways in acute brain injury
The activity of many cytoprotective electrophilic compounds depend at least in part on Nrf2-dependent gene expression. These include sulforaphane, dimethyl fumarate (Tecfidera®), terpenes/terpenoids (including CDDO-Me, carnosic acid, oleanolic acid, and ursolic acid), and polyphenols (including curcumin, resveratrol, quercetin). Other compounds also directly or indirectly activate Nrf2, such as omega 3 fatty acids and valproic acid (Chen et al., 2018; Zhu et al., 2018), but for the sake of this review, we will focus on the major known activators. See Table 1 for a summary of Nrf2 activators in animal models of acute brain injury.
Table 1.
A summary of animal studies employing agents that target Nrf2 in traumatic and ischemic brain injury including: the injury models used, evidence of Nrf2 involvement, and outcome measures with a fraction of the cited studies reporting this outcome in parentheses.
| Drug | Injury model | Evidence of Nrf2 involvement | Outcome measures | References |
|---|---|---|---|---|
|
| ||||
| Sulforaphane | Cerebral Ischemia (middle cerebral artery occlusion; MCAO, carotid artery occlusion) | ↑Nrf2 downstream factors ↓Nuclear Nrf2 |
↑Neurobehavioral function (2/5) ↓Cell death factors (1/5) ↓Lesion volume (3/5) ↓Inflammatory markers (3/5) ↓Oxidative stress markers (1/5) |
(Ma et al., 2015; Mao et al., 2019; Ping et al., 2010; Yu et al., 2017; Zhao et al., 2006) |
| TBI (controlled cortical impact; CCI) | ↑Nrf2 downstream factors ↓Drug effect in Nrf2−/− ↓Nuclear Nrf2 |
↑Neurobehavioral function (2/3) ↓Cell death factors (1/3) ↓Oxidative stress markers (2/3) ↓Lesion volume (1/3) ↓Edema (1/3) |
(Dash et al., 2009; Hong et al., 2010; Zhao et al., 2005) | |
| Dimethyl or Monomethyl Fumarate | Cerebral Ischemia (middle cerebral artery occlusion; MCAO) | ↑Nrf2 downstream factors ↓Drug effect in Nrf2−/− ↑Antioxidant measures |
↑Neurobehavioral function (3/4) ↓Cell death factors (1/4) ↓Lesion volume (2/4) ↓Inflammatory markers (3/4) ↓Oxidative stress markers (3/4) |
(Clausen et al., 2017; Kunze et al., 2015; Singh et al., 2019a; Yao et al., 2016) |
| TBI (controlled cortical impact; CCI) | ↑Nrf2 downstream factors ↑Antioxidant measures |
↓Edema (3/4) ↑Neurobehavioral function (2/2) ↓Lesion volume (2/2) ↓Cell death factors (1/2) ↓Inflammatory markers (1/2) ↓Oxidative stress markers (2/2) |
(Casili et al., 2018; Kramer et al., 2017) | |
| Terpenes/Terpenoids (ursolic acid, oleanolic acid, carnosic acid, bardoxolone) | Cerebral Ischemia (carotid artery occlusion, middle cerebral artery occlusion; MCAO) | ↑Nrf2 downstream factors ↓Drug effect in Nrf2−/− ↓Nuclear Nrf2 |
↑Neurobehavioral function (4/5) ↓Cell death factors (3/5) ↓Lesion volume (5/5) ↓Inflammatory markers (3/5) ↓Oxidative stress markers (2/5) |
(Li et al., 2013; Wang et al., 2016b; Wang et al., 2018; Yamauchi et al., 2016; Zhang et al., 2012) |
| TBI (weight drop, controlled cortical impact; CCI) | ↑Nrf2 downstream factors ↓Nuclear Nrf2 ↓Drug effect in Nrf2−/− ↑Antioxidant measures |
↑Neurobehavioral function (2/3) ↓Cell death factors (1/3) ↓Oxidative stress markers (2/3) |
(Ding et al., 2017a; Maynard et al., 2019; Miller et al., 2015) | |
| Polyphenols (curcumin, resveratrol, quercetin, epicatechin, tBHQ) | Cerebral Ischemia (middle cerebral artery occlusion; MCAO) | ↑Nrf2 downstream factors ↓Drug effect in Nrf2−/− ↓Nuclear Nrf2 ↑Antioxidant measures |
↑Neurobehavioral function (6/7) ↓Cell death factors (3/7) ↓Lesion volume (6/7) ↓Inflammatory markers (3/7) ↓Oxidative stress markers (2/7) |
(Chen et al., 2019; Dai et al., 2018b; Leonardo et al., 2013; Li et al., 2016a; Narayanan et al., 2015; Wicha et al., 2017; Yang et al., 2009) |
| TBI (weight drop, controlled cortical impact; CCI) | ↑Nrf2 downstream factors ↓Drug effect in Nrf2−/− ↓Nuclear Nrf2 ↑ Antioxidant measures |
↑Neurobehavioral function (3/5) ↓Cell death factors (4/5) ↓Lesion volume (2/5) ↓Inflammatory markers (2/5) ↓Oxidative stress markers (5/5) ↓Edema (3/5) |
(Cheng et al., 2016; Dai et al., 2018a; Ding et al., 2017b; Dong et al., 2018; Li et al., 2016b) | |
9.1. Sulforaphane
Sulforaphane is an isothiocyanate derived from cruciferous vegetables, such as broccoli sprouts, and is among the most potent inducers of the Nrf2 expression pathway, including both upregulation of Nrf2-linked antioxidants as well as mitochondrial biogenesis (Lei et al., 2019; Posner et al., 1994; Prestera and Talalay, 1995; Zhu et al., 2008). Sulforaphane has been shown to decrease MPTP sensitivity to Ca2+, likely due to increased antioxidant levels in mitochondria following Nrf2-induced gene expression (Greco and Fiskum, 2010; Greco et al., 2011). A synthetic version, SFX-01, is currently in Phase II clinical trials for the prevention of vasospasm and delayed cerebral ischemia following subarachnoid hemorrhage (National Clinical Trial (NCT02614742).
9.2. Monomethyl and dimethyl fumarate
Of all the Nrf2 activators, only one compound is approved for the treatment of a neurodegenerative disease in humans in the USA and Europe. Tecfidera® (BG-12 or dimethyl fumarate; DMF) is prescribed for relapsing-remitting multiple sclerosis (MS) (Gold et al., 2012). DMF and its metabolite monomethyl fumarate (MMF) have also been investigated in animal models of acute neurological injury. As a pro-drug, DMF is converted into MMF by GSH (Schmidt et al., 2007). DMF and MMF activate the Nrf2 pathway. However, DMF also exhibits immunomodulatory properties, even in Nrf2−/− mice, confirming that it acts through multiple mechanisms, including mitochondrial biogenesis and NFκB inhibition (Gillard et al., 2015; Hayashi et al., 2017; Linker et al., 2011; Schulze-Topphoff et al., 2016). DMF has also been shown to be protective in an animal model of subarachnoid hemorrhage (Iniaghe et al., 2015), another form of acute brain injury associated with significant oxidative stress linked to neuronal death and mitochondrial dysfunction (Chen et al., 2015; Han et al., 2017).
9.3. Terpenes/terpenoids
Terpenes/Terpenoids are compounds most commonly derived from natural sources such as rosemary, olive oil, garlic, and apples. A related synthetic compound has also been developed, CDDO methyl (CDDO-Me; also called RTA 402 or bardoxolone methyl), that made it to phase 3 clinical trials (BEACON) for chronic kidney disease and type 2 diabetes. While the trial was terminated early due to an increased rate of heart failure, retrospective analyses suggest that, in the absence of certain predictors of heart failure, the risk of cardiac events in drug- and placebo-treated patients was the same (Chin et al., 2014; de Zeeuw et al., 2013), suggesting that the drug may be tested again for other conditions.
9.4. Polyphenols
Polyphenols are naturally-occurring chemicals found in a variety of consumables including tea, red wine, spices, fruits and vegetables, etc. Many polyphenol-rich foods form important parts of diets associated with longevity, such the Mediterranean diet, making them attractive compounds in studies focused on enhancing health (Martinez-Huelamo et al., 2017). In addition to Nrf2 activation, polyphenols have been investigated for their ability to control inflammatory signaling as well as diminish protein aggregation associated with neurodegenerative disease in animal models (Almeida et al., 2016; Caruana et al., 2016; Shimizu, 2017).
10. Nrf2 activators in other neurodegenerative disorders
In addition to testing Nrf2 activators for neuroprotection in acute CNS injury models, they are also being tested for treatment of chronic neurological disorders. As previously mentioned, Tecfidera® is FDA-approved to treat the relapsing-remitting form of MS, an autoimmune demyelinating disease; it is also being investigated in the primary-progressive form of MS (NCT02959658). A new form of the drug, diroximel fumarate (which is also converted into MMF) is currently in phase 3 trials and may have better gastrointestinal tolerability (Palte et al., 2019). A terpenoid compound (omaveloxolone or RTA 408; NCT02255435) and a polyphenol (micronized resveratrol; NCT03933163) are both in phase 2 trials as therapies for Friedrich’s ataxia, a genetic disorder characterized by progressive ataxia, neurodegeneration, mitochondrial dysfunction, and oxidative stress (Lynch et al., 2018; Rezende et al., 2019).
In animal epilepsy models, sulforaphane acts as an anticonvulsant (Carrasco-Pozo et al., 2015) whereas other drugs with Nrf2-activating properties, such as the type-2 diabetes medication sitagliptin and the vasodilator pentoxifylline, are neuroprotective, decreasing neuroinflammation, oxidative stress, and cell death (Ahmad, 2013; Kaur et al., 2014; Pauletti et al., 2019; Singh et al., 2019b). Perhaps most important for long-term health implications, acute treatment with the KEAP1 inhibitor RTA 408 after an epileptic event resulted in greatly reduced frequency of subsequent occurrences for as much as multiple months, as well as increased ATP production and decreased neuronal loss (Shekh-Ahmad et al., 2018). In age-related neurodegenerative diseases like Parkinson’s and Alzheimer’s disease, pharmacological modification of Nrf2 signaling is similarly beneficial (Ahuja et al., 2016; Campolo et al., 2017; Choi and Lim, 2010; Jazwa et al., 2011; Lastres-Becker et al., 2016; Majkutewicz et al., 2018; Morroni et al., 2013; Pu et al., 2018; Rojo et al., 2018; Siebert et al., 2009; Zhang et al., 2014; Zhou et al., 2016).
Natural aging may also be a target for Nrf2 activators since aging is associated with oxidative stress and mitochondrial dysfunction (Fivenson et al., 2017; Sykiotis and Bohmann, 2010). Impaired Nrf2 signaling has been observed in aged mouse cardiac and human skeletal muscle (Gounder et al., 2012; Safdar et al., 2010). Huang et al. (2019) concluded that decreased physical activity and mitochondrial mass in aged mice is directly caused by diminished Nrf2 activity, as evidenced by middle-aged Nrf2−/− displaying characteristics of frailty and sarcopenia. In the brain, Navarro et al. (2016) showed that Nrf2-mediated biogenesis can decelerate age-related microglial dysfunction and hinder the progression of neurodegenerative disorders.
11. Protection against cardiac arrest-induced neuronal death and neurologic injury by post-ischemic administration of sulforaphane
11.1. Background
As discussed earlier, a variety of drugs and nutraceuticals have been tested for neuroprotection in animal models of acute ischemic and traumatic brain injury. Sulforaphane is one of these agents that is therapeutically effective in many of these models but has not been tested for neuroprotection in a clinically-relevant model. A study was therefore conducted focusing on the ability of post-ischemic sulforaphane administration to improve neurohistologic and neurobehavioral outcomes in a highly clinically-relevant large animal model of cardiac arrest and resuscitation.
11.2. Methods
A detailed description of this model, the stereologic quantification of hippocampal neuronal death, and the neurologic deficit scoring system can be found in Balan et al. (2006). All animal procedures were performed as approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee and in accordance with ARRIVE guidelines. Ventricular fibrillation cardiac arrest was induced in chloralose-anesthetized and intubated adult female (9–12 kg) purebred beagles (Covance) by stopping the ventilator and applying electrical stimulation to the cardiac surface with a Grass stimulator. Ten min later, dogs were ventilated with 100% O2 and the heart resuscitated using 3 min open-chest CPR and electrical defibrillation. Following defibrillation, animals were maintained on 100% oxygen for one hr and then 28–35% oxygen for an additional 23 hr. All 11 animals received constant critical care with a morphine drip and additional boluses as needed for analgesia. A subgroup of animals (n=4) received an intravenous 5 min infusion of sulforaphane (LKT Laboratories Inc., catalog number S-8044) at 1 mg per kg in saline containing 0.6% dimethyl sulfoxide immediately after defibrillation. Pancuronium was used intermittently, only as needed, to facilitate ventilation and only after verification that the animal was adequately anesthetized. Ventilation was adjusted to maintain arterial carbon dioxide and oxygen in normal ranges.
At hr 20, anesthesia and analgesia were lightened, and animals weaned from controlled ventilation. At hr 23, the effects of morphine were reversed with naloxone and the neurological deficit score (NDS) was measured by two examiners blinded to treatment protocol. The NDS used has been validated in other laboratories (Bircher and Safar, 1985), as well as ours (Rosenthal et al., 1992) and is a multisystem test of 18 parameters in 5 categories (level of consciousness, respiration, cranial nerve, motor, sensory, behavior), yielding a score between 0 (normal) and 100 (brain death). Following the 5 min NDS procedure, the dogs were re-anesthetized with pentobarbital and α-chloralose, intubated, and mechanically ventilated. At 24 hr post-resuscitation, the deeply anesthetized animals were perfusion-fixed with paraformaldehyde. The fixed brains were removed and processed for histopathology.
11.3. Stereologic quantification
Six dorsal hippocampus sections from a 1-in-24 series representing the entire rostral caudal formation were stained with cresyl violet and quantified with unbiased stereology by a microscopist blinded to treatment. Boundaries of the hippocampal CA1 fields were outlined at low magnification (4x), after which StereoInvestigator software placed within each subfield boundary a set of optical counting frames (55×55 μm) in a systematic random fashion. Cresyl violet stained normal and dying neurons (ie, necrotic and apoptotic cells) were then counted in optical dissectors 7 μm in depth at high magnification (100x), according to stereological principles.
11.4. Statistical analysis
All data are expressed as mean ± SE. NDS scores and cell death counts were analyzed using a t-test where significance was set at p <0.05.
11.5. Results
As described by many labs using cardiac arrest with several species, the CA1 region of the hippocampus is one of the more vulnerable brain regions that undergo considerable neuronal death within just 24 hr following arrest and resuscitation (Back et al., 2004). Fig. 2 provides representative images of hippocampal brain slices at an anteroposterior distance of 40 mm from the rostral olfactory bulb tip that were stained with either cresyl violet or Fluoro Jade B. Cresyl violet–stained neurons in a representative non-ischemic surgical Sham animal appeared normal, as expected. At high resolution, dying neurons in a representative sample from a dog that underwent arrest/resuscitation appeared shrunken, with condensed nuclei lacking a nucleolus. Hippocampal neurons in dogs that received sulforaphane immediately after resuscitation were not entirely normal but displayed identifiable nuclei and were not as condensed as neurons in the brains of dogs that did not receive sulforaphane. Brain slices were also stained with Fluoro Jade B, which is selective for dead or dying neurons (Balan et al., 2006; Park et al., 2013). Sections stained with Fluoro Jade B exhibited little staining in Shams and in brains of dogs treated with sulforaphane; however, Fluoro Jade B staining was much greater in animals that underwent cardiac arrest.
Fig. 2.
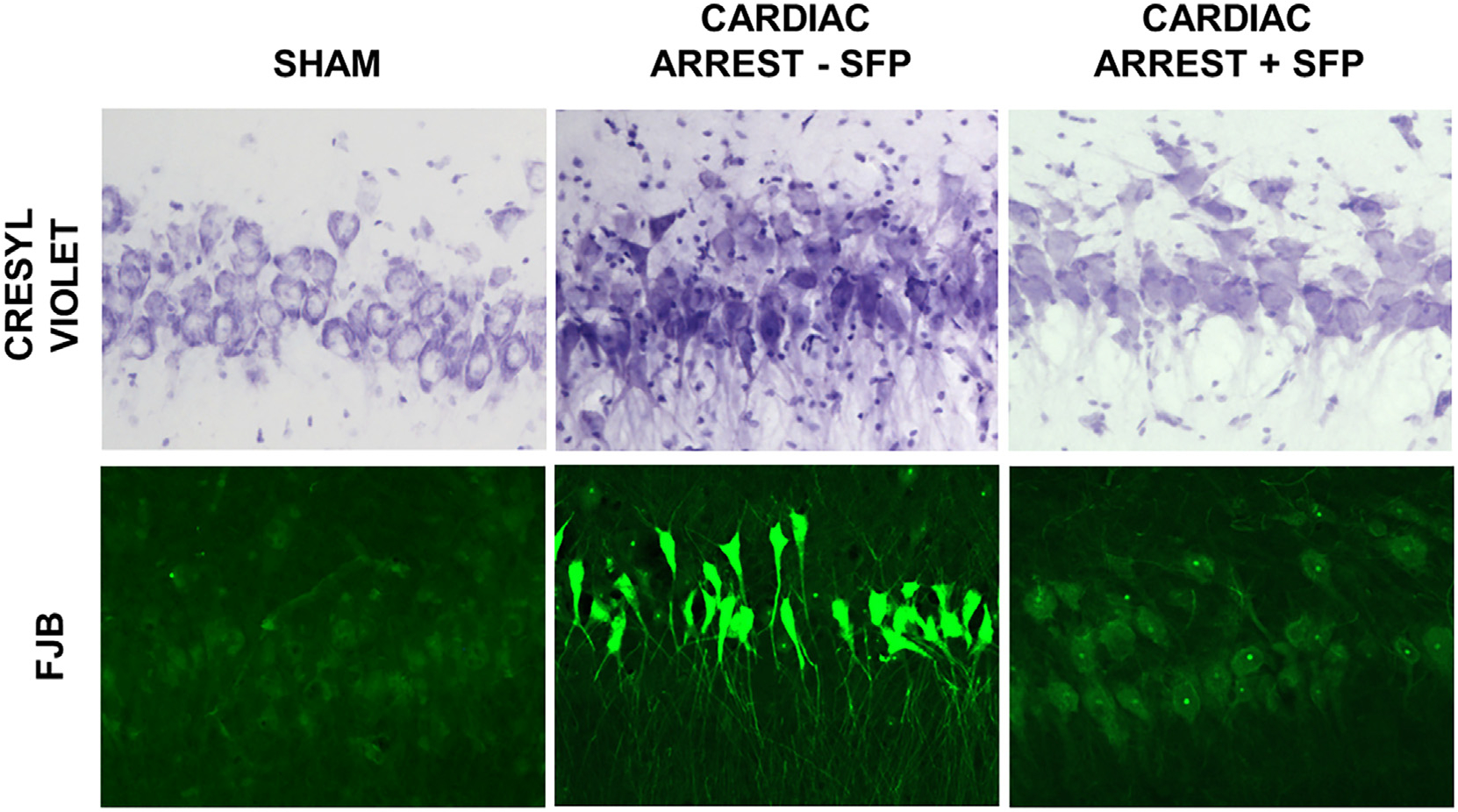
Hippocampal neuronal death at 24 hr after cardiac arrest and protection by sulforaphane. Canine brain sections were stained with cresyl violet for morphologic alterations and with Fluoro Jade B (FJB) for neuron-specific damage and death. Following Sham cardiac arrest, hippocampal CA1 neurons appeared healthy with homogeneous discrete nuclei and cell as shown with cresyl violet-stained sections. Following cardiac arrest and 24 hr resuscitation, most neurons displayed dark, pyknotic nuclei and were surrounded by cell debris. Sulforaphane administration reduced but did not eliminate neuronal abnormalities. Sections stained with Fluoro Jade B exhibited little staining in Shams and in dogs following cardiac arrest and treatment with sulforaphane. Fluoro Jade B was much greater in animals that underwent cardiac arrest with no sulforaphane treatment.
Fig. 3 provides the results of comparisons between neurologic deficit scores and cresyl violet-stained hippocampal CA1 neuronal death for dogs treated with sulforaphane and those used previously without sulforaphane administration. The NDS for sulforaphane-treated dogs was significantly lower than the NDS reported previously for animals that received hyperoxic resuscitation but no sulforaphane (24.1 ± 3.8% vs 61.2 ± 4.9%, p < 0.001, t-test, n=4 and 7 for sulforaphane treated and untreated, respectively). The fraction of neurons that were dead or dying was significantly lower in the hippocampus after administration of sulforaphane compared to the fraction previously reported for hyperoxic resuscitation without sulforaphane (6.8 ± 2.5% vs 63.7±3.1%, p< 0.001, t-test, n=4 and 7 for sulforaphane-treated and untreated, respectively).
Fig. 3.
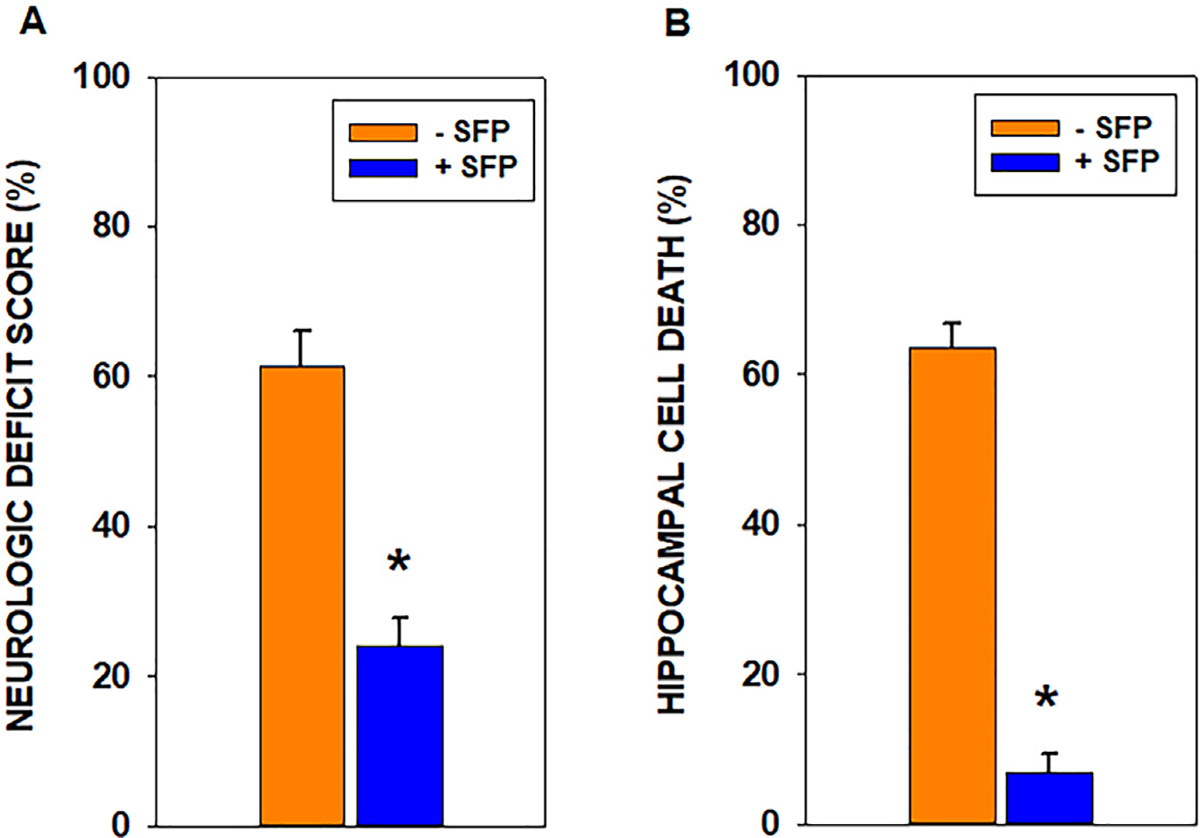
Neurologic deficit scores and hippocampal neuronal death at 23–24 hr post-cardiac arrest and resuscitation. Neurologic deficit scores (NDS) range from zero (normal) to 100 (brain dead). Sulforaphane treatment significantly reduced the NDS by more than 50% (*p < 0.001; n=4–7). Sulforaphane also reduced dead or dying CA1 neurons by almost 90% (p < 0.001; n=4–7).
11.6. Discussion
These results provide preliminary evidence strongly suggesting that one dose of intravenously-administered sulforaphane to young, healthy, adult female beagles following 10 min ventricular fibrillation cardiac arrest provides dramatic neuroprotection in a highly clinically translational model of global cerebral ischemia and resuscitation. This conclusion is, however, limited by several factors. First, the dogs that did not receive sulforaphane also did not receive drug vehicle, and were previously used in another study, albeit with identical experimental methods (Balan et al., 2006). Second, the differences in neurologic characteristics and histopathology between treatment groups was limited to the very short outcome time of 23–24 hr, which is restricted by the guidelines for maintaining lab animals that meet criteria for euthanasia. Other results obtained with the canine cardiac arrest model have been reproduced in a rat global cerebral ischemia model at 30 days post-injury (Hazelton et al., 2010), which was possible because the rats did not meet euthanasia criteria. Third, only females were used in this study and therefore similar tests should be conducted in males. Fourth, the therapeutic potential of sulforaphane was tested using a hyperoxic resuscitation paradigm that generates much greater oxidative stress and neurologic injury than when the animals are maintained under normoxic conditions (Liu et al., 1998; Richards et al., 2007; Richards et al., 2006; Vereczki et al., 2006). Therefore, sulforaphane should also be tested in normoxic resuscitation paradigms that reduce oxidative stress.
11.7. Conclusions
Regulation of Nrf2 activity is mediated by the redox-dependent release of Nrf2 from KEAP1, the phosphorylation state of Nrf2, proteasomal degradation of Nrf2, and the distribution of Nrf2 between cytosolic, nuclear, and possibly mitochondrial compartments. When activated, Nrf2 has important effects on mitochondria, including stimulation of mitochondrial biogenesis and mitophagy, increased mitochondrial levels of antioxidant enzymes, and resistance to redox-regulated mitochondrial permeability transition pore opening.
Nrf2-mediated gene expression moderates several pathogenic processes (e.g. inflammation, oxidative stress, and mitochondrial bioenergetic dysfunction) that contribute to neuronal death and neurologic injury following both acute and chronic neurodegeneration. While we show that sulforaphane reduces hippocampal neuronal death and improves neurologic outcomes in a highly translatable large animal model of cardiac arrest and resuscitation, very few of the pharmacologic agents that stimulate Nrf2-mediated cytoprotective gene expression have been rigorously tested for effectiveness and potential toxicity in clinical trials or even clinically-relevant models of acute neurologic disorders and chronic neurodegenerative diseases. Future research on Nrf2-activating agents should emphasize translatability in model and dosing, as well as investigate potential effects of sex and age on Nrf2-mediated gene expression and neuroprotection.
Acknowledgments
The preparation of this manuscript and the experimental results were funded by NIH R01 NS034152 and NIH R01 NS091099.
Footnotes
Declaration of Competing Interest
The authors have nothing to declare.
References
- Abdullah A, Kitteringham NR, Jenkins RE, Goldring C, Higgins L, Yamamoto M, Hayes J, Park BK, 2012. Analysis of the role of Nrf2 in the expression of liver proteins in mice using two-dimensional gel-based proteomics. Pharmacol. Rep. 64, 680–697. [DOI] [PubMed] [Google Scholar]
- Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA, 2015. Sex differences in glia reactivity after cortical brain injury. Glia 63, 1966–1981. [DOI] [PubMed] [Google Scholar]
- Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW, 2012. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Tr 132, 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, 2013. Protective effects of curcumin against lithium-pilocarpine induced status epilepticus, cognitive dysfunction and oxidative stress in young rats. Saudi J Biol Sci 20, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X, 2017. Nrf2 signaling pathway: pivotal roles in inflammation. BBA-Mol Basis Dis 1863, 585–597. [DOI] [PubMed] [Google Scholar]
- Ahuja M, Ammal Kaidery N, Yang L, Calingasan N, Smirnova N, Gaisin A, Gaisina IN, Gazaryan I, Hushpulian DM, Kaddour-Djebbar I, Bollag WB, Morgan JC, Ratan RR, Starkov AA, Beal MF, Thomas B, 2016. Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson’s-like disease. J. Neurosci. 36, 6332–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AMK, Cook JL, 1999. Nrf2, a cap’n’collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 274, 26071–26078. [DOI] [PubMed] [Google Scholar]
- Almeida S, Alves MG, Sousa M, Oliveira PF, Silva BM, 2016. Are polyphenols strong dietary agents against neurotoxicity and neurodegeneration? Neurotox. Res. 30, 345–366. [DOI] [PubMed] [Google Scholar]
- Andreyev A, Tamrakar P, Rosenthal RE, Fiskum G, 2018. Calcium uptake and cytochrome c release from normal and ischemic brain mitochondria. Neurochem. Int. 117, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anedda A, López-Bernardo E, Acosta-Iborra B, Saadeh Suleiman M, Landázuri MO, Cadenas S, 2013. The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress. Free Radic. Biol. Med. 61, 395–407. [DOI] [PubMed] [Google Scholar]
- Back T, Hemmen T, Schuler OG, 2004. Lesion evolution in cerebral ischemia. J. Neurol. 251, 388–397. [DOI] [PubMed] [Google Scholar]
- Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE, 2006. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke 37, 3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan IS, Saladino AJ, Aarabi B, Castellani RJ, Wade C, Stein DM, Eisenberg HM, Chen HH, Fiskum G, 2013. Cellular alterations in human traumatic brain injury: changes in mitochondrial morphology reflect regional levels of injury severity. J. Neurotrauma 30, 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza I, Tucci A, Galli F, Grottelli S, Mierla AL, Pilolli F, Minelli A, 2012. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J. Nutr. Biochem. 23, 1583–1591. [DOI] [PubMed] [Google Scholar]
- Benedict AL, Knatko EV, Dinkova-Kostova AT, 2012. The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress. Carcinogenesis 33, 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand HC, Schaap M, Baird L, Georgakopoulos ND, Fowkes A, Thiollier C, Kachi H, Dinkova-Kostova AT, Wells G, 2015. Design, synthesis, and evaluation of triazole derivatives that induce Nrf2 dependent gene products and inhibit the Keap1–Nrf2 protein–protein interaction. J. Med. Chem. 58, 7186–7194. [DOI] [PubMed] [Google Scholar]
- Bhatti JS, Bhatti GK, Reddy PH, 2017. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. basis Dis. 1863, 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircher N, Safar P, 1985. Cerebral preservation during cardiopulmonary resuscitation. Crit. Care Med. 13, 185–190. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H, 2012. The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H, 2015. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab. Brain Dis. 30, 1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Vaibhav K, Saad NM, Fatima S, Vender JR, Baban B, Hoda MN, Dhandapani KM, 2017. White matter damage after traumatic brain injury: a role for damage associated molecular patterns. Biochim. Biophys. Acta Mol. basis Dis. 1863, 2614–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni F, Polosa PL, Gadaleta MN, Cantatore P, Roberti M, 2010. Nuclear respiratory factor 2 induces the expression of many but not all human proteins acting in mitochondrial DNA transcription and replication. J. Biol. Chem. 285, 3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, Reeves MJ, Rundek T, 2018. Sex differences in stroke: Challenges and opportunities. J. Cereb. Blood Flow Metab. 38, 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolo M, Casili G, Biundo F, Crupi R, Cordaro M, Cuzzocrea S, Esposito E, 2017. The neuroprotective effect of dimethyl fumarate in an MPTP-mouse model of Parkinson’s disease: involvement of reactive oxygen species/nuclear factor-kappab/nuclear transcription factor related to NF-E2. Antioxid. Redox Signal. 27, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Zheng A, Xu J, Li H, Liu J, Peng Y, Long J, Zou X, Li Y, Chen C, Liu J, Feng Z, 2014. AMPK activation prevents prenatal stress-induced cognitive impairment: modulation of mitochondrial content and oxidative stress. Free Radical Bio Med 75, 156–166. [DOI] [PubMed] [Google Scholar]
- Caplan HW, Cox CS, Bedi SS, 2017. Do microglia play a role in sex differences in TBI? J. Neurosci. Res. 95, 509–517. [DOI] [PubMed] [Google Scholar]
- Carrasco-Pozo C, Tan KN, Borges K, 2015. Sulforaphane is anticonvulsant and improves mitochondrial function. J. Neurochem. 135, 932–942. [DOI] [PubMed] [Google Scholar]
- Caruana M, Cauchi R, Vassallo N, 2016. Putative role of red wine polyphenols against brain pathology in Alzheimer’s and Parkinson’s disezase. Front Nutr 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casili G, Campolo M, Paterniti I, Lanza M, Filippone A, Cuzzocrea S, Esposito E, 2018. Dimethyl fumarate attenuates neuroinflammation and neurobehavioral deficits induced by experimental traumatic brain injury. J. Neurotrauma 35, 1437–1451. [DOI] [PubMed] [Google Scholar]
- Chen S, Wu H, Tang J, Zhang J, Zhang JH, 2015. Neurovascular events after subarachnoid hemorrhage: focusing on subcellular organelles. Acta Neurochir. Suppl. 120, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Zhu GY, Su XH, Wang R, Liu J, Liao K, Ren R, Li T, Liu L, 2017. 7-deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO-1 signaling. Oncotarget 8, 55051–55063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang H, Zhou M, Li X, Fang Z, Gao H, Li Y, Hu W, 2018. Valproic acid attenuates traumatic brain injury-induced inflammation in vivo: involvement of autophagy and the Nrf2/ARE signaling pathway. Front. Mol. Neurosci. 11, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang X, Yang Y, Zhang L, Cui L, Zhang C, Chen R, Xie Y, He J, He W, 2019. Tert-butylhydroquinone enhanced angiogenesis and astrocyte activation by activating nuclear factor-E2-related factor 2/heme oxygenase-1 after focal cerebral ischemia in mice. Microvasc. Res. 126, 103891. [DOI] [PubMed] [Google Scholar]
- Cheng ZG, Zhang GD, Shi PQ, Du BS, 2013. Expression and antioxidation of Nrf2/ARE pathway in traumatic brain injury. Asian Pac J Trop Med 6, 305–310. [DOI] [PubMed] [Google Scholar]
- Cheng T, Wang W, Li Q, Han X, Xing J, Qi C, Lan X, Wan J, Potts A, Guan F, Wang J, 2016. Cerebroprotection of flavanol (−)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic. Biol. Med. 92, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X, Yao W, Xia H, Jin Y, Li X, Cai J, Hei Z, 2015. Elevation of HO-1 expression mitigates intestinal ischemia-reperfusion injury and restores tight junction function in a rat liver transplantation model. Oxidative Med. Cell. Longev. 2015, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MP, Reisman SA, Bakris GL, O’Grady M, Linde PG, McCullough PA, Packham D, Vaziri ND, Ward KW, Warnock DG, Meyer CJ, 2014. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am. J. Nephrol. 39, 499–508. [DOI] [PubMed] [Google Scholar]
- Choi YG, Lim S, 2010. N(varepsilon)-(carboxymethyl)lysine linkage to alpha-synuclein and involvement of advanced glycation end products in alpha-synuclein deposits in an MPTP-intoxicated mouse model. Biochimie 92, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Choi H-I, Kim H-J, Park J-S, Kim I-J, Bae EH, Ma SK, Kim SW, 2017. PGC-1α attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation mediated by activated p38 in HK-2 Cells. Sci. Rep. 7, 4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD, 2013. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32, 3765–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BH, Lundberg L, Yli-Karjanmaa M, Martin NA, Svensson M, Alfsen MZ, Flaeng SB, Lyngso K, Boza-Serrano A, Nielsen HH, Hansen PB, Finsen B, Deierborg T, Illes Z, Lambertsen KL, 2017. Fumarate decreases edema volume and improves functional outcome after experimental stroke. Exp. Neurol. 295, 144–154. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S, 2001. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2, 769–776. [DOI] [PubMed] [Google Scholar]
- Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T, 2005. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J. Neurochem. 93, 493–501. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA, 2003. Nrf2 Is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA, 2004. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24, 8477–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Wang H, Fang J, Zhu Y, Zhou J, Wang X, Zhou Y, Zhou M, 2018a. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 140, 65–71. [DOI] [PubMed] [Google Scholar]
- Dai Y, Zhang H, Zhang J, Yan M, 2018b. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-kappaB pathway. Chem. Biol. Interact. 284, 32–40. [DOI] [PubMed] [Google Scholar]
- Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN, 2009. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, 2013. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest TG, McCarthy MM, 2015. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J. Bioenerg. Biomembr. 47, 173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVience SJ, Lu X, Proctor J, Rangghran P, Melhem ER, Gullapalli R, Fiskum GM, Mayer D, 2017. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-(13)C]pyruvate. Sci. Rep. 7, 1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB, 2018. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 1–18. [DOI] [PubMed] [Google Scholar]
- Ding H, Wang H, Zhu L, Wei W, 2017a. Ursolic acid ameliorates early brain injury after experimental traumatic brain injury in mice by activating the Nrf2 pathway. Neurochem. Res. 42, 337–346. [DOI] [PubMed] [Google Scholar]
- Ding H, Wang X, Wang H, Zhu L, Wang Q, Jia Y, Wei W, Zhou C, Wu H, Ding K, 2017b. Nrf2-ARE signaling provides neuroprotection in traumatic brain injury via modulation of the ubiquitin proteasome system. Neurochem. Int. 111, 32–44. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Abramov AY, 2015. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 88, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Yang B, Wang L, Li B, Guo X, Zhang M, Jiang Z, Fu J, Pi J, Guan D, Zhao R, 2018. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharmacol. 346, 28–36. [DOI] [PubMed] [Google Scholar]
- Dong W, Sun Y, Cheng H, Yang B, Wang L, Jiang Z, Li B, Wen S, Guo X, Guan D, Zhao R, 2019. Dynamic cell type-specific expression of Nrf2 after traumatic brain injury in mice. Eur. J. Neurosci. 50, 1981–1993. [DOI] [PubMed] [Google Scholar]
- East DA, Fagiani F, Crosby J, Georgakopoulos ND, Bertrand H, Schaap M, Fowkes A, Wells G, Campanella M, 2014. PMI: a ΔΨm independent pharmacological regulator of mitophagy. Chem. Biol. 21, 1585–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggler Aimee L., Small E, Hannink M, Mesecar Andrew D., 2009. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem. J. 422, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Scarpulla RC, 1990. NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 4, 1023–1034. [DOI] [PubMed] [Google Scholar]
- Fivenson EM, Lautrup S, Sun N, Scheibye-Knudsen M, Stevnsner T, Nilsen H, Bohr VA, Fang EF, 2017. Mitophagy in neurodegeneration and aging. Neurochem. Int. 109, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, Pesce A, Pinchi E, Santurro A, Fraschetti F, Fineschi V, 2017. Diffuse axonal injury and oxidative stress: a comprehensive review. Int. J. Mol. Sci. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friling RS, Bensimon A, Tichauer Y, Daniel V, 1990. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. P Natl Acad Sci USA 87, 6258–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard GO, Collette B, Anderson J, Chao J, Scannevin RH, Huss DJ, Fontenot JD, 2015. DMF, but not other fumarates, inhibits NF-kappaB activity in vitro in an Nrf2-independent manner. J. Neuroimmunol. 283, 74–85. [DOI] [PubMed] [Google Scholar]
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT, 2012. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. New Engl J Med 367, 1098–1107. [DOI] [PubMed] [Google Scholar]
- Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KJ, Odelberg SJ, Firpo MA, Paine R 3rd, Hoidal JR, Abel ED, Rajasekaran NS, 2012. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One 7, e45697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN, Kvietys PR, 2015. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 6, 524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T, Fiskum G, 2010. Brain mitochondria from rats treated with sulforaphane are resistant to redox-regulated permeability transition. J. Bioenerg. Biomembr. 42, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T, Shafer J, Fiskum G, 2011. Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic. Biol. Med. 51, 2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R, Brooks W, Vukas R, Pierce J, Harris J, 2019. Sex differences in traumatic brain injury: what we know and what we should know. J. Neurotrauma 36 (22), 3063–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureev AP, Shaforostova EA, Popov VN, 2019. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang T, Su J, Zhao Y, Chenchen Wang, Li X, 2017. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 40, 157–162. [DOI] [PubMed] [Google Scholar]
- Handy DE, Loscalzo J, 2012. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 16, 1323–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S, 2009. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 46, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi G, Jasoliya M, Sahdeo S, Saccà F, Pane C, Filla A, Marsili A, Puorro G, Lanzillo R, Brescia Morra V, Cortopassi G, 2017. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 26, 2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M, 2000. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 28, 33–41. [DOI] [PubMed] [Google Scholar]
- Hayes John D., Chowdhry S, Dinkova-Kostova Albena T., Sutherland C, 2015. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem Soc T 43, 611–620. [DOI] [PubMed] [Google Scholar]
- Hazelton JL, Balan I, Elmer GI, Kristian T, Rosenthal RE, Krause G, Sanderson TH, Fiskum G, 2010. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J. Neurotrauma 27, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J, 2006. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation 113, 2425–2434. [DOI] [PubMed] [Google Scholar]
- Hiebert JB, Shen Q, Thimmesch AR, Pierce JD, 2015. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci 350, 132–138. [DOI] [PubMed] [Google Scholar]
- Higgins LG, Hayes JD, 2011. The cap’n’collar transcription factor Nrf2 mediates both intrinsic resistance to environmental stressors and an adaptive response elicited by chemopreventive agents that determines susceptibility to electrophilic xenobiotics. Chem. Biol. Interact. 192, 37–45. [DOI] [PubMed] [Google Scholar]
- Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA, 2016. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J. Neurotrauma 33, 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström KM, Baird L, Zhang Y, Hargreaves I, Chalasani A, Land JM, Stanyer L, Yamamoto M, Dinkova-Kostova AT, Abramov AY, 2013. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2, 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Yan W, Chen S, Sun CR, Zhang JM, 2010. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 31, 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y, Zhao J, 2018. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 336, 32–39. [DOI] [PubMed] [Google Scholar]
- Hu Q, Ren J, Li G, Wu J, Wu X, Wang G, Gu G, Ren H, Hong Z, Li J, 2018. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 9, 403. 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-C, Nguyen T, Pickett CB, 2000. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. U. S. A. 97, 12475–12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D-D, Fan S-D, Chen X-Y, Yan X-L, Zhang X-Z, Ma B-W, Yu D-Y, Xiao W-Y, Zhuang C-L, Yu Z, 2019. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol. 119, 61–73. [DOI] [PubMed] [Google Scholar]
- Hurst S, Hoek J, Sheu SS, 2017. Mitochondrial Ca(2+) and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 49, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniaghe LO, Krafft PR, Klebe DW, Omogbai EKI, Zhang JH, Tang J, 2015. Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol. Dis. 82, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Ishii T, Wakabayashi N, Yamamoto M, 1999. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 31, 319–324. [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM, 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 104, 12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A, 2011. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 14, 2347–2360. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Xu L, Wang X, Zhao X, Yang X, Chen G, Ji Y, 2008. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediat. Inflamm. 2008, 725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y, Tang K, 2009. Role of Nrf2 in protection against traumatic brain injury in mice. J. Neurotrauma 26, 131–139. [DOI] [PubMed] [Google Scholar]
- Jodeiri Farshbaf M, Ghaedi K, 2017. Huntington’s disease and mitochondria. Neurotox. Res. 32, 518–529. [DOI] [PubMed] [Google Scholar]
- Johnson W, Onuma O, Owolabi M, Sachdev S, 2016. Stroke: a global response is needed. Bull. World Health Organ. 94 634–634a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG, 2016. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 36, 1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansanen E, Kuosmanen SM, Leinonen H, Levonen A-L, 2013. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 1, 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Bal A, Sandhir R, 2014. Curcumin supplementation improves mitochondrial and behavioral deficits in experimental model of chronic epilepsy. Pharmacol. Biochem. Behav. 125, 55–64. [DOI] [PubMed] [Google Scholar]
- Kety SS, 1957. The general metabolism of the brain In vivo. In: Richter D (Ed.), Metabolism of the Nervous System. Pergamon Press, Oxford, England, pp. 221–237. [Google Scholar]
- Keum Y-S, Yu S, Chang PP-J, Yuan X, Kim J-H, Xu C, Han J, Agarwal A, Kong A-NT, 2006. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element–mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 66, 8804–8813. [DOI] [PubMed] [Google Scholar]
- Khalil B, El Fissi N, Aouane A, Cabirol-Pol MJ, Rival T, Liévens JC, 2015. PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease. Cell Death Dis. 6, e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Han J, Dugan L, 2005. Mitochondrial uncoupling proteins in the central nervous system. Antioxid. Redox Signal. 7, 1173–1181. [DOI] [PubMed] [Google Scholar]
- Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M, 2016. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou Y-S, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S-I, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M, 2010. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12, 213–223. [DOI] [PubMed] [Google Scholar]
- Kovac S, Angelova PR, Holmstrom KM, Zhang Y, Dinkova-Kostova AT, Abramov AY, 2015. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 1850, 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer T, Grob T, Menzel L, Hirnet T, Griemert E, Radyushkin K, Thal SC, Methner A, Schaefer MKE, 2017. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. J. Neurochem. 143, 523–533. [DOI] [PubMed] [Google Scholar]
- Kunze R, Urrutia A, Hoffmann A, Liu H, Helluy X, Pham M, Reischl S, Korff T, Marti HH, 2015. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp. Neurol. 266, 99–111. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Garcia-Yague AJ, Scannevin RH, Casarejos MJ, Kugler S, Rabano A, Cuadrado A, 2016. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal. 25, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-S, Jeong G-S, 2014. Arylbenzofuran isolated from Dalbergia odorifera suppresses lipopolysaccharide-induced mouse BV2 microglial cell activation, which protects mouse hippocampal HT22 cells death from neuroinflammation-mediated toxicity. Eur. J. Pharmacol. 728, 1–8. [DOI] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA, 2003. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 278, 12029–12038. [DOI] [PubMed] [Google Scholar]
- Lee RHC, Lee MHH, Wu CYC, Couto E, Silva A, Possoit HE, Hsieh T-H, Minagar A, Lin HW, 2018. Cerebral ischemia and neuroregeneration. Neural Regen. Res. 13, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Tian S, Teng C, Huang L, Liu X, Wang J, Zhang Y, Li B, Shan Y, 2019. Sulforaphane improves lipid metabolism by enhancing mitochondrial function and biogenesis in vivo and in vitro. Mol. Nutr. Food Res. 63, 1800795. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, 2005. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Agrawal M, Singh N, Moore JR, Biswal S, Dore S, 2013. Oral administration of the flavanol (−)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. Eur. J. Neurosci. 38, 3659–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang X, Cui L, Wang L, Liu H, Ji H, Du Y, 2013. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 1497, 32–39. [DOI] [PubMed] [Google Scholar]
- Li W, Suwanwela NC, Patumraj S, 2016a. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 106, 117–127. [DOI] [PubMed] [Google Scholar]
- Li X, Wang H, Gao Y, Li L, Tang C, Wen G, Zhou Y, Zhou M, Mao L, Fan Y, 2016b. Protective effects of quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling Pathway. PLoS One 11, e0164237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen J, Sun S, Zhao J, Dong X, Wang J, 2017a. Effects of estradiol on autophagy and Nrf-2/ARE signals after cerebral ischemia. Cell. Physiol. Biochem. 41, 2027–2036. [DOI] [PubMed] [Google Scholar]
- Li PA, Hou X, Hao S, 2017b. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 95, 2025–2029. [DOI] [PubMed] [Google Scholar]
- Liang D, Huang A, Jin Y, Lin M, Xia X, Chen X, Huang A, 2018. Protective effects of exogenous NaHS against sepsis-induced myocardial mitochondrial injury by enhancing the PGC-1α/NRF2 pathway and mitochondrial biosynthesis in mice. Am. J. Transl. Res. 10, 1422–1430. [PMC free article] [PubMed] [Google Scholar]
- Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R, 2011. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–692. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G, 1998. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke 29, 1679–1686. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu P, Wang Y, Hu R, 2017. Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid. Redox Signal. 26, 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S-C, Hannink M, 2008. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res. 314, 1789–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Wang HD, Xu JG, Ding K, Li T, 2015. Deletion of Nrf2 exacerbates oxidative stress after traumatic brain injury in mice. Cell. Mol. Neurobiol. 35, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtmann Marthe H.R., Angelova Plamena R., Zhang Y, Abramov Andrey Y., Dinkova-Kostova Albena T., 2014. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 457, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DR, Farmer J, Hauser L, Blair IA, Wang QQ, Mesaros C, Snyder N, Boesch S, Chin M, Delatycki MB, Giunti P, Goldsberry A, Hoyle C, McBride MG, Nachbauer W, O’Grady M, Perlman S, Subramony SH, Wilmot GR, Zesiewicz T, Meyer C, 2018. Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia. Ann Clin Transl Neur 6, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LL, Xing GP, Yu Y, Liang H, Yu TX, Zheng WH, Lai TB, 2015. Sulforaphane exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Int. J. Clin. Exp. Med. 8, 17811–17817. [PMC free article] [PubMed] [Google Scholar]
- Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, Brann DW, 2017. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, Piantadosi CA, 2012. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Resp Crit Care 185, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AK, McMahon M, Plummer SM, Higgins LG, Penning TM, Igarashi K, Hayes JD, 2009. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis 30, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkutewicz I, Kurowska E, Podlacha M, Myslinska D, Grembecka B, Rucinski J, Pierzynowska K, Wrona D, 2018. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 1686, 19–33. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S, 2010. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 38, 5718–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang T, Li X, Lei X, Sun Y, Zhao Y, Zhang W, Gao Y, Sun B, Zhang F, 2019. Protective effects of sulforaphane in experimental vascular cognitive impairment: contribution of the Nrf2 pathway. J. Cereb. Blood Flow Metab. 39, 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Huelamo M, Rodriguez-Morato J, Boronat A, de la Torre R, 2017. Modulation of Nrf2 by olive oil and wine polyphenols and neuroprotection. Antioxidants (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T, 2003. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 9, 1062–1068. [DOI] [PubMed] [Google Scholar]
- Maynard ME, Underwood EL, Redell JB, Zhao J, Kobori N, Hood KN, Moore AN, Dash PK, 2019. Carnosic acid improves outcome after repetitive mild traumatic brain injury. J. Neurotrauma 36, 2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhed Y, Daiber A, Steven S, 2015. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 16, 15918–15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Wang JA, Buchanan AK, Hall ED, 2014. Temporal and spatial dynamics of nrf2-antioxidant response elements mediated gene targets in cortex and hippocampus after controlled cortical impact traumatic brain injury in mice. J. Neurotrauma 31, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Singh IN, Wang JA, Hall ED, 2015. Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 264, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H, 2012. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22, 66–79. [DOI] [PubMed] [Google Scholar]
- Mohan S, Gupta D, 2018. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 108, 1866–1878. [DOI] [PubMed] [Google Scholar]
- Mollayeva T, Mollayeva S, Colantonio A, 2018. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 14, 711–722. [DOI] [PubMed] [Google Scholar]
- Molteni M, Gemma S, Rossetti C, 2016. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediat. Inflamm. 2016, 6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni F, Tarozzi A, Sita G, Bolondi C, Zolezzi Moraga JM, Cantelli-Forti G, Hrelia P, 2013. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 36, 63–71. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M, 2004. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10, 549–557. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Siddiqui N, Al-Harbi NO, Al-Harbi MM, Ahmad SF, 2016. TLR-7 agonist attenuates airway reactivity and inflammation through Nrf2-mediated antioxidant protection in a murine model of allergic asthma. Int. J. Biochem. Cell Biol. 73, 53–62. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K, 2003. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 546, 181–184. [DOI] [PubMed] [Google Scholar]
- Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA, 2015. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 46 (6), 1626–1632. 10.1161/STROKEAHA.115.008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen D-F, Youle RJ, 2008. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narne P, Pandey V, Phanithi PB, 2017. Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: an epigenetic connection. Mol. Cell. Neurosci. 82, 176–194. [DOI] [PubMed] [Google Scholar]
- Navarro E, Gonzalez-Lafuente L, Pérez-Liébana I, Buendia I, López-Bernardo E, Sánchez-Ramos C, Prieto I, Cuadrado A, Satrustegui J, Cadenas S, Monsalve M, López MG, 2016. Heme-oxygenase I and PCG-1α regulate mitochondrial biogenesis via microglial activation of Alpha7 nicotinic acetylcholine receptors using PNU282987. Antioxid. Redox Signal. 27, 93–105. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB, 2003. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43, 233–260. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Chan PH, 2009. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 109 (Suppl. 1), 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K, Tavernarakis N, 2012. Mitophagy in neurodegeneration and aging. Front. Genet. 3, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palte MJ, Wehr A, Tawa M, Perkin K, Leigh-Pemberton R, Hanna J, Miller C, Penner N, 2019. November. Improving the gastrointestinal tolerability of fumaric acid esters: early findings on gastrointestinal events with diroximel fumarate in patients with relapsing-remitting multiple sclerosis from the phase 3, open-label EVOLVE-MS-1 study. Adv. Ther. 36 (11), 3154–3165. 10.1007/s12325-019-01085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J, 2007. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 49, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CW, Lee JC, Ahn JH, Lee DH, Cho GS, Yan BC, Park JH, Kim IH, Lee HY, Won MH, Cho JH, 2013. Neuronal damage using fluoro-Jade B histofluorescence and gliosis in the gerbil septum submitted to various durations of cerebral ischemia. Cell. Mol. Neurobiol. 33, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, Pastore A, Pascente R, Liang LP, Villa BR, Balosso S, Abramov AY, van Vliet EA, Del Giudice E, Aronica E, Patel M, Walker MC, Vezzani A, 2019. July 1. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain. 142 (7), e39. 10.1093/brain/awz130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Collins S, Waalkes MP, 2007. Molecular mechanism of human Nrf2 activation and degradation: Role of sequential phosphorylation by protein kinase CK2. Free Radic. Biol. Med. 42, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY, 2010. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J. Biol. Chem. 285, 9292–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA, Suliman HB, 2006. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J. Biol. Chem. 281, 324–333. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Carraway MS, Babiker A, Suliman HB, 2008. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 103, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Lezza AMS, 2015. Regulation of mitochondrial biogenesis through TFAM–mitochondrial DNA interactions: useful insights from aging and calorie restriction studies. Mitochondrion 25, 67–75. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ, 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Z, Liu W, Kang Z, Cai J, Wang Q, Cheng N, Wang S, Wang S, Zhang JH, Sun X, 2010. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 1343, 178–185. [DOI] [PubMed] [Google Scholar]
- Posner GH, Cho CG, Green JV, Zhang Y, Talalay P, 1994. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J. Med. Chem. 37, 170–176. [DOI] [PubMed] [Google Scholar]
- Prestera T, Talalay P, 1995. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. U. S. A. 92, 8965–8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor JL, Fourney WL, Leiste UH, Fiskum G, 2014. Rat model of brain injury caused by under-vehicle blast-induced hyperacceleration. J. Trauma Acute Care Surg. 77, S83–S87. [DOI] [PubMed] [Google Scholar]
- Pu D, Zhao Y, Chen J, Sun Y, Lv A, Zhu S, Luo C, Zhao K, Xiao Q, 2018. Protective effects of sulforaphane on cognitive impairments and ad-like lesions in diabetic mice are associated with the upregulation of Nrf2 transcription activity. Neuroscience 381, 35–45. [DOI] [PubMed] [Google Scholar]
- Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A, 2011. SCF/β-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a keap1-independent manner. Mol. Cell. Biol. 31, 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobón-Velasco JC, Devijver H, García-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A, 2012. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell. Biol. 32, 3486–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian R, Cordeau P Jr., Kriz J, 2019. Brain response to injuries: when microglia go sexist. Neuroscience 405, 14–23. [DOI] [PubMed] [Google Scholar]
- Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP, 2011. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am. J. Respir. Cell Mol. Biol. 45, 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende TJR, Martinez ARM, Faber I, Girotto Takazaki KA, Martins MP, de Lima FD, Lopes-Cendes I, Cendes F, França MC Jr., 2019. Developmental and neurodegenerative damage in Friedreich’s ataxia. Eur. J. Neurol. 26, 483–489. [DOI] [PubMed] [Google Scholar]
- Richards EM, Rosenthal RE, Kristian T, Fiskum G, 2006. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic. Biol. Med. 40, 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC, 2007. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke 38, 1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi F, Shukla S, Kakkar P, 2014. Essential role of PH domain and leucine-rich repeat protein phosphatase 2 in Nrf2 suppression via modulation of Akt/GSK3β/Fyn kinase axis during oxidative hepatocellular toxicity. Cell Death Dis. 5, e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G, 2006a. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp. Neurol. 197, 235–243. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Soane L, Siegel ZT, Fiskum G, 2006b. The potential role of mitochondria in pediatric traumatic brain injury. Dev. Neurosci. 28, 432–446. [DOI] [PubMed] [Google Scholar]
- Rohrer PR, Rudraiah S, Goedken MJ, Manautou JE, 2014. Is nuclear factor erythroid 2-related factor 2 responsible for sex differences in susceptibility to acetaminophen-induced hepatotoxicity in mice? Drug Metab. Dispos. 42, 1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo AI, Pajares M, Garcia-Yague AJ, Buendia I, Van Leuven F, Yamamoto M, Lopez MG, Cuadrado A, 2018. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 18, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RE, Williams R, Bogaert YE, Getson PR, Fiskum G, 1992. Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-L-carnitine. Stroke 23, 1312–1317 discussion 1317–1318. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB, 1990. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 265, 14648–14653. [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB, 1991. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266, 11632–11639. [PubMed] [Google Scholar]
- Ryan BJ, Hoek S, Fon EA, Wade-Martins R, 2015. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem. Sci. 40, 200–210. [DOI] [PubMed] [Google Scholar]
- Safdar A, deBeer J, Tarnopolsky MA, 2010. Dysfunctional Nrf2–Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 49, 1487–1493. [DOI] [PubMed] [Google Scholar]
- Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A, 2006. Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 281, 14841–14851. [DOI] [PubMed] [Google Scholar]
- Salim S, 2017. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 360, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganalmath SK, Gopal P, Parker JR, Downs RK, Parker JC Jr., Dawn B, 2017. Global cerebral ischemia due to circulatory arrest: insights into cellular pathophysiology and diagnostic modalities. Mol. Cell. Biochem. 426, 111–127. [DOI] [PubMed] [Google Scholar]
- Schimmel SJ, Acosta S, Lozano D, 2017. Neuroinflammation in traumatic brain injury: a chronic response to an acute injury. Brain Circ 3, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TJ, Ak M, Mrowietz U, 2007. Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-L-cysteine–preparation of S-substituted thiosuccinic acid esters. Bioorg. Med. Chem. 15, 333–342. [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BA, Sobel RA, Wipke BT, Steinman L, Scannevin RH, Zamvil SS, 2016. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc. Natl. Acad. Sci. U. S. A. 113, 4777–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD, 2012. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 120, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel GS, Horvath TL, 2015. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekh-Ahmad T, Eckel R, Dayalan Naidu S, Higgins M, Yamamoto M, Dinkova-Kostova AT, Kovac S, Abramov AY, Walker MC, 2018. KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy. Brain 141, 1390–1403. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH, 2003. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 23, 3394–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH, 2005. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 25, 10321–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, 2017. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 25, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS, 2009. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J. Neurosci. Res. 87, 1659–1669. [DOI] [PubMed] [Google Scholar]
- Siedler DG, Chuah MI, Kirkcaldie MT, Vickers JC, King AE, 2014. Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments. Front. Cell. Neurosci. 8, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Reeta KH, Sharma U, Jagannathan NR, Dinda AK, Gupta YK, 2019a. Neuro-protective effect of monomethyl fumarate on ischemia reperfusion injury in rats: Role of Nrf2/HO1 pathway in peri-infarct region. Neurochem. Int. 126, 96–108. [DOI] [PubMed] [Google Scholar]
- Singh N, Saha L, Kumari P, Singh J, Bhatia A, Banerjee D, Chakrabarti A, 2019b. Effect of dimethyl fumarate on neuroinflammation and apoptosis in pentylenetetrazol kindling model in rats. Brain Res. Bull. 144, 233–245. [DOI] [PubMed] [Google Scholar]
- Spychala MS, Honarpisheh P, McCullough LD, 2017. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J. Neurosci. Res. 95, 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Alfieri A, Siow RC, Mann GE, Fraser PA, 2013. Temporal and spatial distribution of Nrf2 in rat brain following stroke: quantification of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J. Physiol. 591, 3525–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D, Killeen E, Naquin R, Alam S, Alam J, 2003. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 278, 2396–2402. [DOI] [PubMed] [Google Scholar]
- Strom J, Xu B, Tian X, Chen QM, 2016. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 30, 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Youle RJ, Finkel T, 2016. The mitochondrial basis of aging. Mol. Cell 61, 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D, 2010. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal 3, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M, 2010. Calcium, ischemia and excitotoxicity. Cell Calcium 47, 122–129. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Ikeda Y, Ohta Y, Deguchi K, Tian F, Shang J, Matsuura T, Abe K, 2011. Expression of Keap1-Nrf2 system and antioxidative proteins in mouse brain after transient middle cerebral artery occlusion. Brain Res. 1370, 246–253. [DOI] [PubMed] [Google Scholar]
- Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD, 2015. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 88, 108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S, 2002. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 62, 5196–5203. [PubMed] [Google Scholar]
- Townsend BE, Johnson RW, 2016. Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp. Gerontol. 73, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A, 2017. Mitochondria: a central target for sex differences in pathologies. Clin. Sci. 131, 803–822. [DOI] [PubMed] [Google Scholar]
- Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G, 2006. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J. Cereb. Blood Flow Metab. 26, 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser MM, Yassi N, Campbell BCV, Desmond PM, Davis SM, Spratt N, Parsons M, Bivard A, 2019. White matter degeneration after ischemic stroke: a longitudinal diffusion tensor imaging study. J. Neuroimaging 29, 111–118. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P, 2004. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U. S. A. 101, 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR, 1997. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 28, 2252–2258. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang S, Cheng H, Lv H, Cheng G, Ci X, 2016a. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic. Biol. Med. 101, 401–412. [DOI] [PubMed] [Google Scholar]
- Wang Y, He Z, Deng S, 2016b. Ursolic acid reduces the metalloprotease/anti-metalloprotease imbalance in cerebral ischemia and reperfusion injury. Drug Des Devel Ther 10, 1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li L, Deng S, Liu F, He Z, 2018. Ursolic acid ameliorates inflammation in cerebral ischemia and reperfusion injury possibly via high mobility group box 1/toll-like receptor 4/NFkappaB pathway. Front. Neurol. 9, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha P, Tocharus J, Janyou A, Jittiwat J, Changtam C, Suksamrarn A, Tocharus C, 2017. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS One 12, e0189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T, Kanduri C, Lindahl P, Sayin VI, Bergo MO, 2019. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178 330–345.e322. [DOI] [PubMed] [Google Scholar]
- Wild AC, Moinova HR, Mulcahy RT, 1999. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 274, 33627–33636. [DOI] [PubMed] [Google Scholar]
- Wilkins HM, Swerdlow RH, 2016. Relationships between mitochondria and neuroinflammation: implications for Alzheimer’s Disease. Curr. Top. Med. Chem. 16, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte ME, Geurts JJ, de Vries HE, van der Valk P, van Horssen J, 2010. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10, 411–418. [DOI] [PubMed] [Google Scholar]
- Wruck CJ, Streetz K, Pavic G, Götz ME, Tohidnezhad M, Brandenburg L-O, Varoga D, Eickelberg O, Herdegen T, Trautwein C, Cha K, Kan YW, Pufe T, 2011. Nrf2 induces interleukin-6 (IL-6) expression via an antioxidant response element within the IL-6 promoter. J. Biol. Chem. 286, 4493–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Cui JY, Klaassen CD, 2011. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 123, 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zhao F, Gao B, Tan C, Yagishita N, Nakajima T, Wong PK, Chapman E, Fang D, Zhang DD, 2014. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 28, 708–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ, 2018. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 46, 1650–1667. [DOI] [PubMed] [Google Scholar]
- Xu C, Yuan X, Pan Z, Shen G, Kim J-H, Yu S, Khor TO, Li W, Ma J, Kong A-NT, 2006. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 5, 1918–1926. [DOI] [PubMed] [Google Scholar]
- Xu S, Zhuo J, Racz J, Shi D, Roys S, Fiskum G, Gullapalli R, 2011. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. J. Neurotrauma 28, 2091–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Nakano Y, Imai T, Takagi T, Tsuruma K, Shimazawa M, Iwama T, Hara H, 2016. A novel nuclear factor erythroid 2-related factor 2 (Nrf2) activator RS9 attenuates brain injury after ischemia reperfusion in mice. Neuroscience 333, 302–310. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang X, Fan H, Liu Y, 2009. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 1282, 133–141. [DOI] [PubMed] [Google Scholar]
- Yao Y, Miao W, Liu Z, Han W, Shi K, Shen Y, Li H, Liu Q, Fu Y, Huang D, Shi FD, 2016. Dimethyl fumarate and monomethyl fumarate promote post-ischemic recovery in mice. Transl. Stroke Res. 7, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, He Q, Zheng J, Li LY, Hou YH, Song FZ, 2017. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 45, 74–78. [DOI] [PubMed] [Google Scholar]
- Zawada I, Masternak MM, List EO, Stout MB, Berryman DE, Lewinski A, Kopchick JJ, Bartke A, Karbownik-Lewinska M, Gesing A, 2015. Gene expression of key regulators of mitochondrial biogenesis is sex dependent in mice with growth hormone receptor deletion in liver. Aging (Albany NY) 7, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, Zhang L, Cao G, Gao Y, Leak RK, Sporn MB, Chen J, 2012. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke 43, 1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang J, Fang L, Li X, Zhao Y, Shi W, An L, 2014. Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. Int. J. Mol. Sci. 15, 14396–14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Clifton GL, Dash PK, 2005. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J. Neurosci. Res. 82, 499–506. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK, 2006. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 393, 108–112. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, Hu Z, Cai X, Yang J, Sun X, Lu W, Yan H, Chen J, Ye J, Shen J, Cao P, 2016. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci. Rep. 6, 32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y, 2008. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardovasc Toxicol 8, 115. [DOI] [PubMed] [Google Scholar]
- Zhu W, Ding Y, Kong W, Li T, Chen H, 2018. Docosahexaenoic acid (DHA) provides neuroprotection in traumatic brain injury models via activating Nrf2-ARE signaling. Inflammation 41, 1182–1193. [DOI] [PubMed] [Google Scholar]


