Abstract
Background:
The striatum supports motivated behavior and impulse control. Altered striatal activation and connectivity has been observed in link with impulse control dysfunction in individuals with drug addiction.
Objectives:
We examined how resting state functional connectivity (rsFC) of the striatum is altered as a result of chronic ketamine misuse.
Methods:
Thirty-six ketamine users (10 women) and 20 healthy controls (9 women) completed an assessment with the Barratt Impulsiveness Scale (BIS-11) and magnetic resonance imaging. In SPM we examined voxel-wise connectivities of the caudate, pallidum, putamen, and ventral striatum in ketamine users (versus healthy controls) and in association with BIS-11 score and duration of use, all at a corrected threshold.
Results:
Compared to controls, ketamine users showed higher connectivity between caudate and dorsal anterior cingulate cortex and between pallidum and bilateral cerebellum. In ketamine users, putamen showed higher connectivity with the left orbitofrontal cortex (OFC) in association with both BIS-11 score and months of ketamine use. Mediation analyses suggest that the connectivity z score mediated the relationship between impulsivity and duration of use.
Conclusions:
These preliminary findings highlighted altered striatal connectivity in chronic ketamine users, and the potential role of putamen OFC connectivity in supporting the correlation between impulsivity and duration of ketamine use. If replicated in a larger sample, these findings may represent neural markers of ketamine misuse.
Keywords: Ketamine, SUD, impulsivity, fMRI, striatum, insula, sex difference
Introduction
Impulsivity, striatum, and addiction
Though not listed as a diagnostic criterion in DSM-5, impulsivity is one of the core characteristics of substance use disorders (SUD). Many studies have pointed out the pivotal role of impulsivity in the initiation and maintenance of drug seeking and consumption (1–6). Impulsivity along with sensation seeking prompted first use of illicit substances in adolescents (7,8). Patients with opioid or cocaine dependence showed higher impulsivity as assessed with the Barratt Impulsivity Scale (BIS-11) (9–12), Eysenck’s I7 Impulsiveness Inventory (13) or delayed discounting tasks (14). Higher impulsivity was associated with more risky behaviors (15,16), and worse prognosis (17,18) in individuals with SUD, and impulse control represents an important target in the treatment of SUD.
The striatum is a critical component of the reward circuit. It has been linked to the drive for immediate reward gratification with robust response during anticipation of monetary reward and reinforcement learning (19). As individuals progress from occasional to compulsive drug use, drug-seeking behavior shifts from being reward to habit driven (20), and the dorsal striatum becomes increasingly involved during this transition (21,22). Selective lesions of the nucleus accumbens core induced persistent impulsive choice in rats (23). Lesion studies in animals showed distinct roles of the lateral and medial dorsal striatum in response selection and inhibition (24). In functional imaging of humans, striatal activation tends to accompany impulsive responding (25). An earlier study showed that intrinsic network connectivity of the striatum was significantly weaker in cocaine users relative to controls, in relation to greater non-planning impulsivity in cocaine users (26). In another study, cocaine-addicted individuals exhibited reduced connectivity between the putamen and posterior insula and right postcentral gyrus, and the reduction in connectivity partially mediated Barratt impulsivity in cocaine-addicted participants (27). Together, a substantial body of evidence suggests that the fronto-striatal circuitry balances impulsive and controlled decision-making (28), and fronto-striatal circuit dysfunction is associated with impulsive behavior (28) and trait impulsivity in individuals with SUD (29,30).
Functions of striatum
The striatum comprised a number of nuclei with distinct anatomical connections and functions (31,32). Anatomically, the cerebral cortex projects widely to the striatum with a topographical organization. The caudate nucleus receives inputs primarily from the medial and lateral prefrontal cortex and both the putamen and pallidum receives inputs from the motor cortex. Striatal output nuclei project to the thalamus, which sends projections back to the cerebral cortex, forming a cortical-striatal-thalamic-cortical circuit for motor and cognitive control. This circuit parallels the cortical-pontine-cerebellar-thalamic-cortical loop and supports large-scale integration of motor and “higher” cognitive functions (33). Dysfunction of these circuits have been implicated in the etiology of many neuropsychiatric conditions (34–36).
Numerous studies have characterized the roles of the striatum in motor and cognitive control and in reward processing (31,32,37). As these processes are all intricately related to impulsivity, we explored the relationship between the functional connectivity of the basal ganglia in relation to individual impulsivity as assessed by the Barratt Impulsivity Scale. Further, cortical projections to the striatum are largely glutamatergic and chronic use of ketamine, an antagonist of N-Methyl-D-aspartate (NMDA) receptor, may influence functional connectivity of the basal ganglia.
Ketamine abuse in Asia
Besides amphetamine-type stimulants, many new psychoactive drugs sneak up in the abused drug scenes involving Asian youth. Among them, ketamine has become one of the major substances of abuse. In Hong Kong, ketamine has been the most common substance of abuse in teenagers since 2005 (38) and ketamine-related events accounted for 7.1% of all toxicology consultation in the year 2010 (39). In Taiwan, in a National Household Survey on health and substance abuse conducted in 2005 by the Department of Health and Welfare, ketamine ranked third (22%), following amphetamine (49%) and MDMA (35%), as the most used illicit substance in the population 12 to 64 years of age. Among high school students who used club drugs, 64.4% reported using ketamine, followed by ecstasy (50%) and methamphetamine (29%). The average age at first ketamine use was 13.95 years, a critical period of adolescent brain development.
Importantly, whereas ketamine is frequently used concomitantly with other illicit drugs in western countries (40–45), use of ketamine as the primary or sole substance is not rare in Asia (46,47). This has created a tremendous public health issue but also provided a unique opportunity to study the long-term consequences of chronic ketamine exposure.
The current study
Despite grave concerns for growing ketamine use, there have been few studies of the neuropsychological consequences of chronic ketamine exposure. Even fewer studies have directly employed brain imaging to investigate neural dysfunction in chronic ketamine users. Ketamine affects NMDA receptor system and has powerful effects on many cognitive functions including impulse control. Ketamine or NMDA antagonists treatment increased impulsive choice dose-dependently in animal models (48–51) and impulsivity in humans (52,53), suggesting that ketamine exposure may contribute to diminished inhibitory control. Importantly, the striatum receives glutamatergic inputs from the prefrontal cortex to support learning and goal-directed behavior (54), and dysfunction of these processes is intricately related to drug addiction (55). Here, we examined resting state functional connectivity (rsFC) of the striatum as a neural metric to investigate how ketamine may alter cerebral circuit functions. Specifically, we contrasted a group of chronic ketamine users with demographically matched non-drug using controls in striatum rsFC. We will explore group differences in trait impulsivity and the neural bases of impulsivity in ketamine users as well as the influences of the duration of ketamine use on striatal connectivity. We would like to note that the current study was not powered to examine sex differences and thus men and women were combined in data analyses.
Experimental procedures
Subjects and clinical assessments
The Research Ethics Committee of the China Medical University Hospital approved the study protocol (CMUH103-REC2-052). Candidates were assured at screening that their decision to participate in the study or not would not affect their right to medical care, that all personal information would be kept confidential, and that they could withdraw from the study at any time. Each participant provided a written informed consent prior to data collection.
Ketamine-using and healthy control participants were recruited through posters at hospitals and online advertisements in the greater area of Taichung City, Taiwan. After consenting to the study, participants completed a clinical interview, questionnaire assessment, behavioral test, and magnetic resonance imaging. Ketamine users met the International Statistical Classification of Diseases and Related Health Problems (ICD) criteria for ketamine use disorders and tested positive for ketamine in urine toxicology. A positive test result for other substances including methamphetamine, opioids, ecstasy, or marijuana, was an exclusion criterion. All healthy control participants denied the use of any illicit substances and showed negative urine test results. None of the ketamine using or healthy control participants had any major medical or neurological illnesses, history of brain concussion that resulted in the loss of consciousness or psychotic disorders. A total of 36 ketamine users and 20 healthy controls participated in this study. Table 1 summarizes the key clinical characteristics of the participants.
Table 1.
Clinical characteristics of the participants.
| KU (M) n = 26 |
KU (W) n = 10 |
HC (M) n = 11 |
HC (W) n = 9 |
group effect p value | |
|---|---|---|---|---|---|
| Age (years) | 25.2 ± 5.8 | 27.5 ± 5.7 | 25.3 ± 4.5 | 25.1 ± 4.2 | 0.45 |
| BIS-11 score | 55.5 ± 7.1 | 60.0 ± 11.0 | 53.0 ± 7.4 | 48.6 ± 3.5 | 0.003 |
| Ketamine use duration* (months) | 59.4 ± 37.0 | 59.0 ± 40.0 | NA | NA | NA |
| Cigarette in 30 days (days) | 24.5 ± 11.1 | 30.0 ± 0.0 | 1.5 ± 2.5 | 0.0 ± 0.0 | 3.6 × 10−16 |
| Cigarette in life (years) | 8.4 ± 4.7 | 12.1 ± 7.7 | 2.5 ± 3.5 | 0.0 ± 0.0 | 4.2 × 10−8 |
| Alcohol in 30 days (days) | 4.8 ± 8.5 | 9.0 ± 11.1 | 3.0 ± 3.8 | 0.4 ± 0.7 | 0.02 |
| Alcohol in life (years) | 4.3 ± 4.3 | 6.7 ± 6.2 | 5.2 ± 6.2 | 1.9 ± 3.4 | 0.18 |
All values are mean ± SD; KU: ketamine users; HC: healthy controls; BIS-11: Barratt Impulsivity Scale; M: men; W: women;
All p values were obtained from ANOVA except for ketamine use duration (*), where KU men and women were compared with a two sample t-test.
Magnetic resonance imaging: procedures and parameters
Participants underwent an MRI scan, consisting of 10 min resting-state fMRI (with eyes closed) and high-resolution structural imaging. MR image data were acquired using a 3-Tesla scanner (Signa HDx, GE, Milwaukee, USA) at the Department of Radiology, China Medical University Hospital, Taichung, Taiwan. The high-resolution structural images were acquired in transverse plane along the AC-PC line. A three-dimensional spoiled gradient-recalled protocol with inversion recovery pulse prepared (3D-SPGR-IrP) sequence was used (parameters: TE = 2.98 ms; prep time = 450 ms; flip angle = 12 degree; image matrix = 224 × 224; FOV = 224 mm × 224 mm; slice thickness = 1 mm; NEX = 1). The resting-state fMRI data were acquired using a gradient echo single-shot echo planar imaging sequence (parameters: TE = 35 ms; TR = 2000 ms; slice thickness = 4.4 mm; slice number = 32; image matrix = 64 × 64; FOV = 240 mm; total scan time = 10 min). Four dummy scans acquired at the beginning of EPI were discarded.
Imaging data pre-processing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 12, Wellcome Department of Imaging Neuroscience, University College London, U.K.). We followed standard procedures in image preprocessing, as in recent work (56–60). Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (61,62). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (63–66). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1 Hz contribute to regionally specific BOLD correlations (67). Thus, we applied a temporal band-pass filter (0.009 < f < 0.08 Hz) to the time course in order to obtain low-frequency fluctuations, as in previous studies (64–66,68). As extensively investigated in Van Dijk et al., 2012, micro head motion (> 0.1 mm) is an important source of spurious correlations in rsFC analysis (69). Therefore, we applied a “scrubbing” method proposed by Power and colleagues (70) and successfully applied in previous studies (70–72) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by where (dx, dy, dz)and (α, β, γ) are the translational and rotational movements, respectively, and r (= 50 mm) is a constant that approximates the mean distance between the center of MNI space and the cortex and transform rotations into displacements (70). The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point that exceeded the head motion limit FD (t) > 0.5 mm or DVARS(t) > 0.5% (70,72). On average, 1% of the time points were removed across subjects.
Seed-based correlation and group analyses
The caudate, putamen, and pallidum masks were obtained from the Automated Anatomical Labeling or AAL atlas (73). The ventral striatum mask is not available from the AAL atlas. Thus, as with our previous studies (74,75), we used a VS mask based on cytoarchitectonic and topographical criteria (76). All masks were in the Montreal Neurological Institute space (Figure 1).
Figure 1.
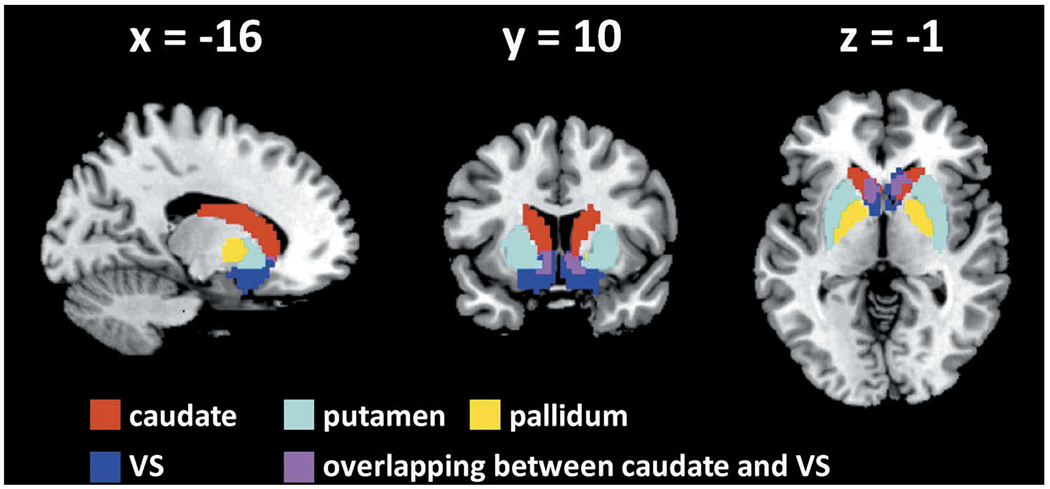
Seed regions: voxels overlapping between the caudate and ventral striatum (VS) were removed from each mask.
The BOLD time courses were averaged spatially across voxels over each striatum seed. For individual subjects, we computed the correlation coefficient between the averaged time course of a seed region and the time courses of all other brain voxels. To assess and compare rsFC, we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (77,78): z = 0.5loge[(1 + r)/(1 − r)]. The Z maps were used in group, random effect analyses. We performed a covariance analysis to compare ketamine users and healthy controls with sex as a covariate. In additional models, we also included variables of nicotine and alcohol use as covariates. All results were examined with a combination of voxel p < .001 uncorrected and cluster p < .05, FWE corrected, on the basis of Gaussian random field theory, in SPM, following current reporting standards (79).
Next, we performed whole-brain simple regression analyses each with BIS-11 score and duration of ketamine use (months) as a regressor for ketamine users, both with sex and age as covariates. For brain regions that showed a significant correlation with both duration of use and BIS-11 score in linear regressions, we derived the connectivity z scores of the regions of interest for individual subjects and followed up with mediation analyses to examine the inter-relationship between BIS-11 score, duration of ketamine use, and functional connectivity.
Mediation analyses
Across ketamine-using participants, the BIS-11 score was positively correlated with duration of ketamine use (months). Further, the putamen showed higher connectivity with the left orbitofrontal cortex (OFC) both in association with BIS-11 score and months of ketamine use (see Results). Thus, we examined in mediation analyses the inter-relationships of impulsivity, connectivity z score, and duration of use with sex and age as covariates. We performed mediation analyses (80), using the toolbox M3, developed by Tor Wager and Martin A. Lindquist (http://wagerlab.colorado.edu/tools).
In a mediation analysis, the relation between the independent variable X and dependent variable Y, i.e. X→Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by employing three regression equations (80):
where a represents X→M, b represents M→Y (controlling for X), c’ represents X→Y (controlling for M), and c represents X→Y. The constants i1, i2, i3 are the intercepts, and e1, e2, e3 are the residual errors. In the literature, a, b, c and c’ were referred to as path coefficients or simply paths (80,81), and we followed this notation. Variable M is said to be a mediator of the correlation X→Y if (c – c’), which is mathematically equivalent to the product of the paths a⋆b, is significantly different from zero (80). If the product a⋆b and the paths a and b are significant, one concludes that X→Y is mediated by M. In addition, if path c’ is not significant, there is no direct connection from X to Y and X→Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and should not be confused with the correlation coefficient between Y and M.
We considered and presented the results of all six models, although the primary goal was to test whether putamen OFC connectivity mediated the influence of impulsivity on the duration of ketamine use.
Results
Clinical assessments
For all clinical measures, we conducted a covariance analysis to compare ketamine users and healthy controls with sex as a covariate (Table 1). Compared to healthy controls, ketamine users showed higher BIS-11 score (p = .003). Ketamine users also showed significantly higher cigarette and alcohol use than healthy controls. Further, linear regression with sex and age as covariates showed that BIS-11 score was correlated with duration of ketamine use (months) (r = 0.34, p = .0478).
Resting-state functional connectivity (RSFC): ketamine users vs. healthy controls
In a covariance analysis of the z maps, we compared ketamine users and healthy controls with sex and age as covariates. We evaluated the results at a threshold of uncorrected voxel p < .001 in combination with cluster p < .05, FWE corrected. For the caudate nucleus, ketamine users showed higher caudate connectivity with the dorsal anterior cingulate cortex (dACC; voxel Z = 4.24, x = −9, y = 26, z = 31, 18,009 mm3), as compared to healthy controls. (Figure 2(a)). For the pallidum, ketamine users showed greater connectivity with bilateral cerebellum (two clusters; voxel Z = 5.28, x = 27, y = −61, z = −23, 6,804 mm3; voxel Z = 4.87, x = −30, y = −61, z = −20, 5,751 mm3) than healthy controls (Figure 2(b)). For the putamen or ventral striatum, there were no significant group differences.
Figure 2.
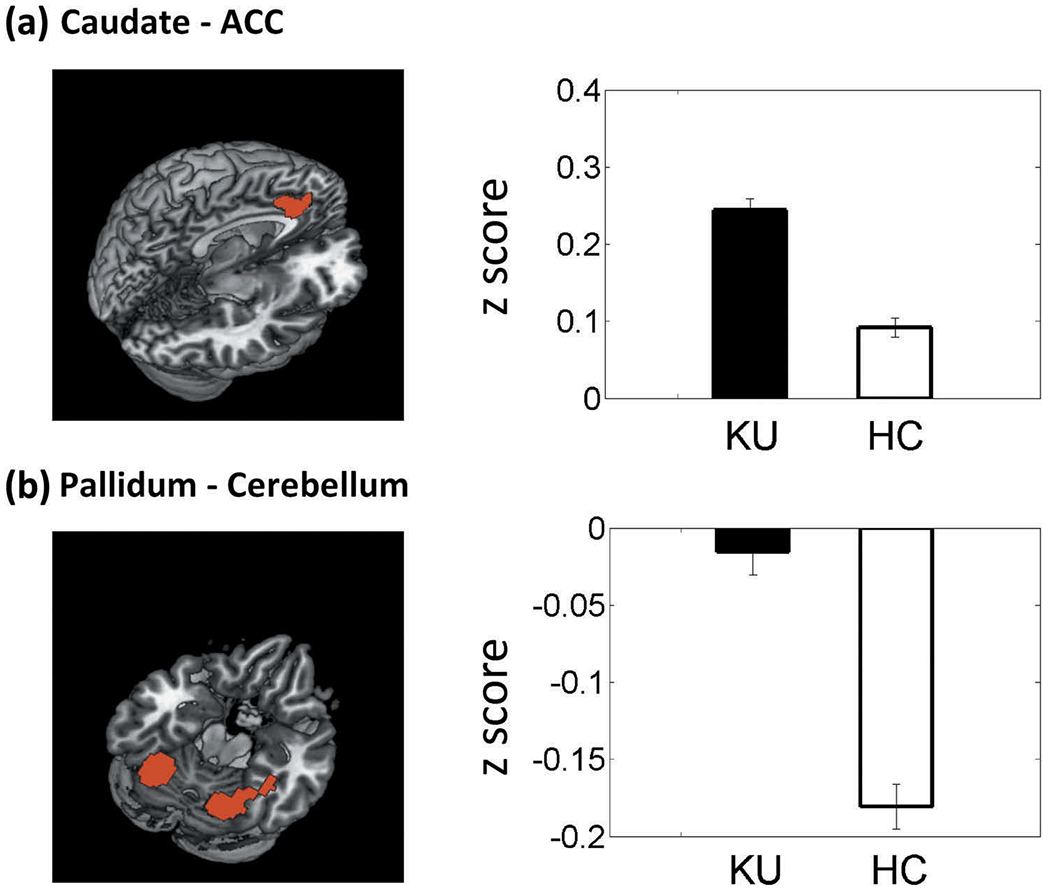
Examined at a threshold of p < .001 uncorrected, combined with cluster p < .05, FWE corrected, the results of whole-brain covariance analysis showed higher (a) connectivity of the caudate with anterior cingulate cortex (ACC) and (b) pallidum connectivity with the cerebellum in ketamine users (KU), as compared to healthy controls (HC). Histograms plot the mean ± S.E. of the connectivity z score of each group.
Ketamine users and healthy controls differed in the extent of cigarette and alcohol use (Table 1). Thus, we examined whether the findings described above were related to cigarette and alcohol use. We cross-correlated the z score of caudate dACC connectivity and pallidum cerebellum connectivity with years of smoking, days of smoking in the prior month, years of drinking, and days of drinking in the prior month across participants each for ketamine users and healthy controls. None of the regressions yielded significant correlations.
Impulsivity, duration of ketamine use, and striatal RSFC
We examined the rsFC of each seed region in relation to BIS-11 score and duration of ketamine use (months) for ketamine-using participants, both with sex and age as covariates. The results are summarized in Table 2. Briefly, putamen showed higher connectivity with the left orbitofrontal cortex (OFC, Figure 3(a)) and the ventral striatum (VS) showed less connectivity with the right superior temporal sulcus (STS) and left superior frontal gyrus (SFG) with higher BIS-11 score. The caudate nucleus showed higher connectivity with the cerebellum, the pallidum showed higher connectivity with the VS and ventromedial prefrontal cortex (vmPFC), and the putamen showed higher connectivity with the left OFC (Figure 3(a)) and vmPFC, all with longer duration of ketamine use.
Table 2.
Regions showing functional connectivity with the seed regions in correlation with impulsivity and duration of ketamine use.
| Seed region |
||||
|---|---|---|---|---|
| Regressor of interest | Caudate | Pallidum | Putamen | VS |
| BIS-11 score | – | – | +L OFC1 | - R STS2 - L SFG3 |
| Duration of use (months) | +Cerebellum4 | +VS5 +vmPFC6 |
+L OFC7 +vmPFC8 |
– |
Note: voxel p < 0.005 uncorrected and cluster-level p < 0.05, FWE corrected; L: left; R: right; OFC: orbitofrontal cortex; STS: superior temporal sulcus; SFG: superior frontal gyrus; VS: ventral striatum; vmPFC: ventromedial prefrontal cortex. + and − each indicates positive and negative correlation with the regressor of interest. – indicates no significant findings.
L OFC: voxel Z = 3.47, x = −24, y = 20, z = −23, 4,023 mm3
R STS: voxel Z = 3.91, x = 54, y = −64, z = 19, 6,669 mm3
L SFG: voxel Z = 4.00, x = −27, y = 23, z = 49, 5,130 mm3
Cerebellum; voxel Z = 3.89, x = −6, y = −55, z = −11, 6,615 mm3
VS: two clusters; voxel Z = 4.69, x = 12, y = 2, z = −11, 2,241 mm3; voxel Z = 3.59, x = −12, y = −1, z = −8, 1,836 mm3
vmPFC: voxel Z = 3.64, x = 0, y = 41, z = −11, 4,833 mm3
L OFC: voxel Z = 4.57, x = −33, y = 20, z = −23, 7,533 mm3
vmPFC: voxel Z = 3.39, x = 0, y = 41, z = −14, 1,863 mm3
Figure 3.
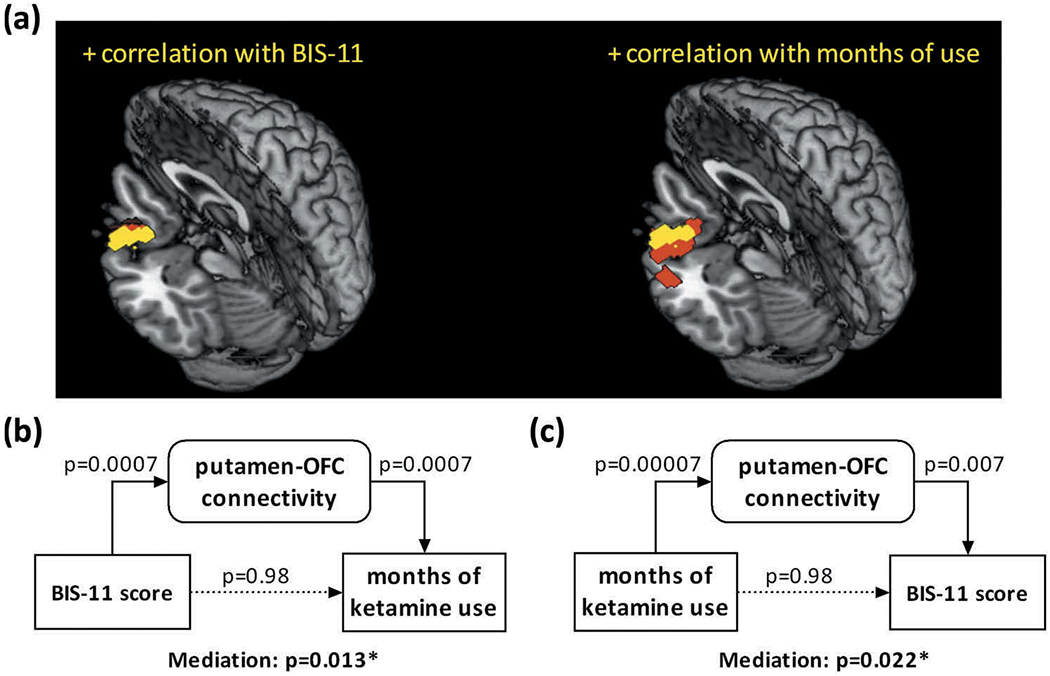
Putamen connectivity with the left lateral orbitofrontal cortex (OFC) was correlated positively with both impulsivity (left) and duration of ketamine use (right). (a) Voxels shown in yellow represent those that overlap between the two regressions. (b) and (c) show the results of significant mediation of the relationship of BIS-11 score and months of ketamine use, bidirectionally, by the connectivity of the overlapping voxels.
Mediation analyses: BIS-11 score, duration of ketamine use, putamen-OFC connectivity
The results of mediation analyses showed that putamen-OFC connectivity z score mediated the correlation bidirectionally between BIS-11 score and months of ketamine use (Figure 3(b, c)). All other models were not significant in the mediation effect. Table 3 summarizes the statistics of all six models. Considering these findings, we also tested whether putamen-OFC connectivity was correlated with BIS-11 score in HC participants. The results of linear regression showed that the putamen-OFC connectivity z score was not correlated with the BIS-score in HC (p = .41, r = 0.19). However, the slopes did not differ significantly between the CD and HC in a slope test (p = .20, t = 1.30 (82)).
Table 3.
Mediation analyses of impulsivity, duration of use, and putamen-left orbitofrontal cortical connectivity.
| p value |
|||||||
|---|---|---|---|---|---|---|---|
| X | M | Y | X → M | M → Y | X → Y | unmediated X → Y | mediated X → Y |
| BIS score | conn z | mo of use | 0.0007 | 0.0007 | 0.047 | 0.98 | 0.013* |
| mo of use | conn z | BIS score | 0.00007 | 0.007 | 0.047 | 0.98 | 0.022* |
| conn z | BIS score | mo of use | 0.0007 | 0.98 | 0.00007 | 0.0007 | 0.99 |
| mo of use | BIS score | conn z | 0.047 | 0.007 | 0.00007 | 0.0007 | 0.10 |
| BIS score | mo of use | conn z | 0.047 | 0.0007 | 0.0007 | 0.007 | 0.08 |
| conn z | mo of use | BIS score | 0.0007 | 0.98 | 0.00007 | 0.0007 | 0.98 |
Note: conn z: connectivity z score; mo of use: months of ketamine use.
p < 0.05
Discussion
Chronic ketamine users showed higher impulsivity than non-drug using healthy controls, as assessed by the Barratt Impulsivity Scale (BIS-11), and higher BIS score was associated with longer duration of ketamine use in ketamine users. In resting state fMRI, compared to healthy controls, ketamine users demonstrated higher caudate connectivity with the dorsal anterior cingulate cortex (dACC) and pallidum connectivity with the cerebellum. Further, in ketamine-using participants, striatal rsFC was altered in relation to both BIS-11 score and duration of ketamine use. These findings are discussed in the below.
Ketamine users vs. controls: caudate – dACC connectivity
Involved in reward-driven behavior (83) and in habit formation (21), the dorsal striatum is widely implicated in drug craving (84–86) and seeking (87–89). The dACC plays a role maintaining working memory, monitoring error, and processing conflict (90), and represents a core region of the saliency circuit (91,92).
Although many studies implicated the caudate and dACC, few have addressed the role of caudate dACC connectivity in the psychological processes related to drug use. Increased rsFC between dACC and caudate were reported in patients with obsessive-compulsive disorder, in positive correlation with symptom severity (93). In a treatment study of individuals with nicotine use disorders, higher functional connectivity between the caudate and dACC significantly predicted worse treatment outcome (94). In a study of neurotypical populations, individuals with less reward dependency as a personality trait rated salient visual stimuli less salient and demonstrated higher caudate dACC connectivity during expectancy of salient stimuli (95). As lower reward dependency reflects psychological distancing from the behavioral outcome, increased caudate dACC connectivity may conduce to non-goal directed or habit-like behavior, as with substance misuse. Thus, higher caudate dACC connectivity may be associated with a compromised capacity in discriminating salient stimuli for goal-directed behavior, and, as a result, compulsive drug use in ketamine users.
Ketamine users vs. controls: pallidal cerebellum connectivity
We also observed greater connectivity between the pallidum and bilateral cerebellum in ketamine users. The cerebellum and basal ganglia are disynaptically interconnected and involved in motor and non-motor functions (see (96) for a review). The cerebellum and pallidum coactivated during appetitive conditioning with a pleasant taste stimulus in healthy subjects (97), suggesting a potential role of pallido-cerebellar connectivity in mediating reward-related processes. The cerebellum responds to reinforcement learning (98), drug cues (99), memory (100) and craving (101). Drug-induced activity-dependent synaptic changes in the cerebellum may be crucial to the transition from recreational to compulsive drug use (see (102) for a review). In other imaging studies, Koehler et al. demonstrated increased rsFC between right striatum and cerebellum in pathological gamblers (103). The findings of increased pallido-cerebellar connectivity may reflect an outcome of drug conditioning in chronic ketamine users.
Striatal connectivity in relation to impulsivity and duration of use
Barratt impulsivity and duration of ketamine use were both associated with increased putamen connectivity with the orbitofrontal cortex (OFC). Mediation analyses showed that the connectivity mediated the relationship, bidirectionally, between impulsivity and duration of use. Other models of mediation were not significant. The results suggested mutual influences between impulsivity and duration of ketamine use via cerebral connectivity. That is, impulsive personality trait may contribute to longer duration of ketamine use via increases in putamen OFC connectivity. It is statistically equally plausible that longer duration of ketamine use may render individuals more impulsive via the changes in connectivity, although Barratt impulsivity has largely been considered as a trait measure and less amenable to environmental influences.
Although no studies to our knowledge have reported alterations of putamen OFC connectivity in individuals with substance use disorders, the roles of both putamen and OFC have been examined in relation to addiction-related behavioral processes. In recordings from behaving primates, both OFC and putamen showed neuronal activities that depended upon the choice of which reward to collect in a spatial-delayed task (104). Both putamen and OFC connectivity have been implicated in self-control during delayed gratification (105). An fMRI study demonstrated a correlation between a fun seeking trait and resting-state connectivity between the OFC and putamen (106). In cigarette smokers engaged in cue reactivity tests, the putamen and OFC showed cue responses each in relation to attentional bias and craving (107). An earlier positron emission tomography study demonstrated a lower level of dopamine D2 receptor availability in the striatum, including the putamen, in association with altered metabolic rate in the OFC in stimulant abusers (108). More broadly, in rodent models, a high level of serotonin in the OFC combined with a low level of dopamine in the putamen predicted the emergence of rigid decision-making (109), a behavior reminiscent of habitual drug taking. Although mediation analyses did not distinguish the directional relationship between impulsivity and duration of use, the current findings add to the literature of putamen and OFC dysfunction in substance misuse.
Limitations of the study and conclusions
A number of limitations are worth considering. First, the sample size is small in this pilot study. In particular, a group of non-substance-abusing individuals with a wider range of impulsivity is needed to confirm whether the current findings are specific to chronic ketamine users or relate more broadly to impulsivity. In particular, the study was not powered to examine sex differences. Thus, these findings are preliminary and will need to be replicated in future work. An additional issue concerns the potential influence of psychiatric comorbidities on the current findings. We did not screen for psychiatric illnesses other than psychosis in the current study. Second, although regression analyses largely ruled out an effect of alcohol and cigarette use on the current findings, it remained unclear how these comorbidities may influence striatal connectivity. That is, while the analyses did not reveal much relationship between imaging findings and smoking/drinking variables, we could not conclude that these findings are specific to ketamine misuse. Third, questionnaires, such as the urgency, premeditation, perseverance, sensation seeking, and positive urgency (UPPS-P) behavioral scale (110), and behavioral tests, such as the stop signal task (111,112), may address impulsivity features not captured by the BIS-11 and reveal other changes in striatal rsFC in ketamine users. Finally, we targeted the striatum in the current study, but other regions of the frontal-limbic circuit need to be investigated in relation to impulsivity (113).
In conclusion, we demonstrated changes in resting state striatal connectivity in chronic ketamine users. Increased caudate connectivity with the anterior cingulate cortex may be related to heightened saliency response to drug cues and habitual drug seeking. Putamen connectivity with orbitofrontal cortex supported the inter-relationship between impulsivity and duration of use. If corroborated in a larger sample, these findings may add to a growing literature of the addiction neuroscience of ketamine misuse.
Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [AA021449, DA023248, DA040032]; Ministry of Science and Technology of Taiwan [MOST 104-2410-H-003-012].
Disclosure statement
The study was supported by grant MOST 104-2410-H-003-012 from the Ministry of Science and Technology of Taiwan and NIH grants AA021449, DA023248, and K25DA040032. The funding agencies are otherwise not involved in the design and execution of the study or in the decision to publish these findings. We declare no financial interests in the current work.
References
- 1.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Jentsch JD, Pennington ZT. Reward, interrupted: inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76 Pt B:479–86. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90. [DOI] [PubMed] [Google Scholar]
- 6.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–18. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerra G, Angioni L, Zaimovic A, Moi G, Bussandri M, Bertacca S, Santoro G, Gardini S, Caccavari R, Nicoli MA. Substance use among high-school students: relationships with temperament, personality traits, and parental care perception. Subst Use Misuse. 2004;39:345–67. [DOI] [PubMed] [Google Scholar]
- 8.Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: further evidence for a dual systems model. Dev Psychol. 2011;47:739–46. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- 9.LoBue C, Cullum CM, Braud J, Walker R, Winhusen T, Suderajan P, Adinoff B. Optimal neurocognitive, personality and behavioral measures for assessing impulsivity in cocaine dependence. Am J Drug Alcohol Abuse. 2014;40:455–62. doi: 10.3109/00952990.2014.939752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen DA, Ho A, Bahl A, Varma P, Kellogg S, Borg L, Kreek MJ. Former heroin addicts with or without a history of cocaine dependence are more impulsive than controls. Drug Alcohol Depen. 2012;124:113–20. doi: 10.1016/j.drugalcdep.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vonmoos M, Hulka LM, Preller KH, Jenni D, Schulz C, Baumgartner MR, Quednow BB. Differences in self-reported and behavioral measures of impulsivity in recreational and dependent cocaine users. Drug Alcohol Depend. 2013;133:61–70. doi: 10.1016/j.drugalcdep.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Zeng H, Lee TMC, Waters JH, So KF, Sham PC, Schottenfeld RS, Marienfeld C, Chawarski MC. Impulsivity, cognitive function, and their relationship in heroin-dependent individuals. J Clin Exp Neuropsyc. 2013;35:897–905. doi: 10.1080/13803395.2013.828022. [DOI] [PubMed] [Google Scholar]
- 13.Robles E, Huang BE, Simpson PM, McMillan DE. Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. J Subst Abuse Treat. 2011;41:354–62. doi: 10.1016/j.jsat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. [DOI] [PubMed] [Google Scholar]
- 15.Dissabandara LO, Loxton NJ, Dias SR, Dodd PR, Daglish M, Stadlin A. Dependent heroin use and associated risky behaviour: the role of rash impulsiveness and reward sensitivity. Addict Behav. 2014;39:71–76. doi: 10.1016/j.addbeh.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MJ, Vassileva J. Neurocognitive and psychiatric dimensions of hot, but not cool, impulsivity predict HIV sexual risk behaviors among drug users in protracted abstinence. Am J Drug Alcohol Ab. 2016;42:231–41. doi: 10.3109/00952990.2015.1121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshier SJ, Ewen M, Otto MW. Impulsivity as a moderator of the intention-behavior relationship for illicit drug use in patients undergoing treatment. Addict Behav. 2013;38:1651–55. doi: 10.1016/j.addbeh.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens L, Verdejo-Garcia A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat. 2014;47:58–72. doi: 10.1016/j.jsat.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R, Adermark L. Dopaminergic regulation of striatal interneurons in reward and addiction: focus on alcohol. Neural Plast. 2015;2015:814567. doi: 10.1155/2015/814567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belin D, Everitt BJ. Cocaine seeking habits depend upon doparnine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Belin-Rauscent A, Everitt BJ, Belin D. Intrastriatal shifts mediate the transition from drug-seeking actions to habits. Biol Psychiatry. 2012;72:343–45. doi: 10.1016/j.biopsych.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 24.Brasted PJ, Robbins TW, Dunnett SB. Distinct roles for striatal subregions in mediating response processing revealed by focal excitotoxic lesions. Behav Neurosci. 1999;113:253–64. [DOI] [PubMed] [Google Scholar]
- 25.Feja M, Hayn L, Koch M. Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:31–42. doi: 10.1016/j.pnpbp.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Wisner KM, Patzelt EH, Lim KO, MacDonald AW. An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am J Drug Alcohol Ab. 2013;39:403–13. doi: 10.3109/00952990.2013.848211. [DOI] [PubMed] [Google Scholar]
- 27.McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39:424–32. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- 28.Economides M, Guitart-Masip M, Kurth-Nelson Z, Dolan RJ. Arbitration between controlled and impulsive choices. NeuroImage. 2015;109:206–16. doi: 10.1016/j.neuroimage.2014.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–04. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 30.Hu YZ, Salmeron BJ, Gu H, Stein EA, Yang YH. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–92. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 31.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 32.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell PT, Shine JM. Subcortical contributions to large-scale network communication. Neurosci Biobehav Rev. 2016;71:313–22. doi: 10.1016/j.neubiorev.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 34.Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haaland KY, Dum RP, Mutha PK, Strick PL, Troster AI. The neuropsychology of movement and movement disorders: neuroanatomical and cognitive considerations. J Int Neuropsychol Soc. 2017;23:768–77. doi: 10.1017/S1355617717000698. [DOI] [PubMed] [Google Scholar]
- 36.Rubia K, Alegria AA, Brinson H. Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev Neurol. 2014;58:S3–16. doi: 10.33588/rn.58S01.2013570. [DOI] [PubMed] [Google Scholar]
- 37.Schultz W Reward functions of the basal ganglia. J Neural Transm. 2016;123:679–93. doi: 10.1007/s00702-016-1510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narcotics Division HK Central Registry of Drug Abuse Sixty-fourth Report, 2015; 7–18. [Google Scholar]
- 39.Cheung YC, Fei LL. Hong Kong poison information centre: annual report. Hong Kong J Emergency Med. 2012;19:110–20. doi: 10.1177/102490791201900206. [DOI] [Google Scholar]
- 40.Curran HV, Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction. 2001;96:749–60. doi: 10.1080/09652140020039116. [DOI] [PubMed] [Google Scholar]
- 41.Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depen. 2003;69:23–28. doi: 10.1016/S0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- 42.Gill JR, Stajic M. Ketamine in non-hospital and hospital deaths in New York City. J Forensic Sci. 2000;45:655–58. [PubMed] [Google Scholar]
- 43.Lankenau SE, Clatts MC. Ketamine injection among high risk youth: preliminary findings from New York City. J Drug Issues. 2002;32:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan MJ. Memory deficits associated with recreational use of “ecstasy” (MDMA). Psychopharmacology. 1999;141:30–36. [DOI] [PubMed] [Google Scholar]
- 45.Parrott AC, Milani RM, Parmar R, Turner JD. Recreational ecstasy/MDMA and other drug users from the UK and Italy: psychiatric symptoms and psychobiological problems. Psychopharmacology. 2001;159:77–82. doi: 10.1007/s002130100897. [DOI] [PubMed] [Google Scholar]
- 46.Chen WJ, Wu SC, Tsay WI, Chen YT, Hsiao PC, Yu YH, Ting TT, Chen CY, Tu YK, Huang JH, et al. Differences in prevalence, socio-behavioral correlates, and psychosocial distress between club drug and hard drug use in Taiwan: results from the 2014 National Survey of Substance Use. Int J Drug Policy. 2017;48:99–107. doi: 10.1016/j.drugpo.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Han E, Kwon NJ, Feng LY, Li JH, Chung H. Illegal use patterns, side effects, and analytical methods of ketamine. Forensic Sci Int. 2016;268:25–34. doi: 10.1016/j.forsciint.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology. 2013;226:127–38. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT2C receptor stimulation and 5-HT2A receptor blockade. Neuropharmacology. 2011;61:468–77. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology. 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- 51.Murphy ER, Fernando ABP, Urcelay GP, Robinson ESJ, Mar AC, Theobald DEH, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABA(A) receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–10. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YC, Wang LJ, Lin SK, Chen CK. Neurocognitive profiles of methamphetamine users: comparison of those with or without concomitant ketamine use. Subst Use Misuse. 2015;50:1778–85. doi: 10.3109/10826084.2015.1050110. [DOI] [PubMed] [Google Scholar]
- 53.Higgins GA, Silenieks LB, MacMillan C, Sevo J, Zeeb FD, Thevarkunnel S. Enhanced attention and impulsive action following NMDA receptor GluN2B-selective antagonist pretreatment. Behav Brain Res. 2016;311:1–14. doi: 10.1016/j.bbr.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–61. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma YY, Cepeda C, Cui CL. The role of striatal NMDA receptors in drug addiction. Int Rev Neurobiol. 2009;89:131–46. doi: 10.1016/S0074-7742(09)89006-5. [DOI] [PubMed] [Google Scholar]
- 56.Kann S, Zhang S, Manza P, Leung HC, Li CR. Hemispheric lateralization of resting-state functional connectivity of the anterior insula: association with age, gender, and a novelty-seeking trait. Brain Connect. 2016;6:724–34. doi: 10.1089/brain.2016.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kline RL, Zhang S, Farr OM, Hu S, Zaborszky L, Samanez-Larkin GR, Li CS. The effects of methylphenidate on resting-state functional connectivity of the basal nucleus of meynert, locus coeruleus, and ventral tegmental area in healthy adults. Front Hum Neurosci. 2016;10:149. doi: 10.3389/fnhum.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomasi DG, Shokri-Kojori E, Volkow ND. Temporal evolution of brain functional connectivity metrics: could 7 min of rest be enough? Cerebral Cortex. 2016. doi: 10.1093/cercor/bhw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S, Hu S, Chao HH, Li CS. Resting-state functional connectivity of the locus coeruleus in humans: in comparison with the ventral tegmental area/substantia nigra pars compacta and the effects of age. Cerebral Cortex. 2016;26:3413–27. doi: 10.1093/cercor/bhv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, Hu S, Fucito LM, Luo X, Mazure CM, Zaborszky L, Li CR. Resting-state functional connectivity of the basal nucleus of meynert in cigarette smokers: dependence level and gender differences. Nicotine Tob Res. 2016. doi: 10.1093/ntr/ntw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friston K, Ashburner J, Frith C, Polone J, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–89. doi: 10.1002/hbm.460030303. [DOI] [Google Scholar]
- 63.Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. NeuroImage. 2003;20:1236–45. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 64.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–78. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 67.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- 68.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 69.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–38. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cerebral Cortex. 2010;20:2852–62. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cerebral Cortex. 2014;24:935–44. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 74.Li CS, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. NeuroImage. 2014;97:321–32. doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang S, Hu S, Chao HH, Li CR. Hemispheric lateralization of resting-state functional connectivity of the ventral striatum: an exploratory study. Brain Struct Funct. 2017;222:2573–83. doi: 10.1007/s00429-016-1358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage. 2008;42:1127–41. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins GM, Watts DG. Spectral Analysis and Its Applications. San Francisco: Holden-Day; 1968. [Google Scholar]
- 78.Berry KJ, Mielke PW Jr. A Monte Carlo investigation of the Fisher Z transformation for normal and non-normal distributions. Psychol Rep. 2000;87:1101–14. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- 79.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–05. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zar JH. Biostatistical Analysis. New Jersey: Prentice-Hall, Inc; 1999. [Google Scholar]
- 83.Fanelli RR, Klein JT, Reese RM, Robinson DL. Dorsomedial and dorsolateral striatum exhibit distinct phasic neuronal activity during alcohol self-administration in rats. Eur J Neurosci. 2013;38:2637–48. doi: 10.1111/ejn.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Gong J, Xie C, Ye EM, Jin X, Song H, Yang Z, Shao Y. Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: evidence from resting state fMRI. Neuroscience. 2015;284:998–1010. doi: 10.1016/j.neuroscience.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, Ming F, Yang Z. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438:322–26. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 87.Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47:242–55. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Schultz W Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 89.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–88. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS. Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci. 2006;23:1658–64. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- 91.Hu S, Ide JS, Zhang S, Li CS. Anticipating conflict: neural correlates of a Bayesian belief and its motor consequence. NeuroImage. 2015;119:286–95. doi: 10.1016/j.neuroimage.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manza P, Hu S, Chao HH, Zhang S, Leung HC, Li CR. A dual but asymmetric role of the dorsal anterior cingulate cortex in response inhibition and switching from a non-salient to salient action. NeuroImage. 2016;134:466–74. doi: 10.1016/j.neuroimage.2016.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Z, Fan Q, Zhu Y, Tan L, Chen Y, Gao R, Zhang H, Li Y, Xiao Z. Intrinsic functional connectivity alteration of dorsal and rostral anterior cingulate cortex in obsessive-compulsive disorder: A resting fMRI study. Neurosci Lett. 2017;654:86–92. doi: 10.1016/j.neulet.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Wilcox CE, Calhoun VD, Rachakonda S, Claus ED, Littlewood RA, Mickey J, Arenella PB, Hutchison KE. Functional network connectivity predicts treatment outcome during treatment of nicotine use disorder. Psychiatry Res. 2017;265:45–53. doi: 10.1016/j.pscychresns.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li S, Demenescu LR, Sweeney-Reed CM, Krause AL, Metzger CD, Walter M. Novelty seeking and reward dependence-related large-scale brain networks functional connectivity variation during salience expectancy. Hum Brain Mapp. 2017;38:4064–77. doi: 10.1002/hbm.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–54. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. [DOI] [PubMed] [Google Scholar]
- 98.Swain RA, Kerr AL, Thompson RF. The cerebellum: a neural system for the study of reinforcement learning. Front Behav Neurosci. 2011;5:8. doi: 10.3389/fnbeh.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2005;31:1318. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- 100.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–45. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreno-Rius J, Miquel M. The cerebellum in drug craving. Drug Alcohol Depend. 2017;173:151–58. doi: 10.1016/j.drugalcdep.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 102.Miquel M, Toledo R, Garcia LI, Coria-Avila GA, Manzo J. Why should we keep the cerebellum in mind when thinking about addiction? Curr Drug Abuse Rev. 2009;2:26–40. [DOI] [PubMed] [Google Scholar]
- 103.Koehler S, Ovadia-Caro S, van der Meer E, Villringer A, Heinz A, Romanczuk-Seiferth N, Margulies DS. Increased functional connectivity between prefrontal cortex and reward system in pathological gambling. PLoS One. 2013;8:e84565. doi: 10.1371/journal.pone.0084565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cromwell HC, Tremblay L, Schultz W. Neural encoding of choice during a delayed response task in primate striatum and orbitofrontal cortex. Exp Brain Res. 2018;236:1679–88. doi: 10.1007/s00221-018-5253-z. [DOI] [PubMed] [Google Scholar]
- 105.Hanggi J, Lohrey C, Drobetz R, Baetschmann H, Forstmeier S, Maercker A, Jancke L. Strength of structural and functional frontostriatal connectivity predicts self-control in the healthy elderly. Front Aging Neurosci. 2016;8:307. doi: 10.3389/fnagi.2016.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Angelides NH, Gupta J, Vickery TJ. Associating resting-state connectivity with trait impulsivity. Soc Cogn Affect Neurosci. 2017;12:1001–08. doi: 10.1093/scan/nsx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang OS, Chang DS, Jahng GH, Kim SY, Kim H, Kim JW, Chung SY, Yang SI, Park HJ, Lee H, et al. Individual differences in smoking-related cue reactivity in smokers: an eye-tracking and fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:285–93. doi: 10.1016/j.pnpbp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 108.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 109.Pittaras E, Callebert J, Chennaoui M, Rabat A, Granon S. Individual behavioral and neurochemical markers of unadapted decision-making processes in healthy inbred mice. Brain Struct Funct. 2016;221:4615–29. doi: 10.1007/s00429-016-1192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–89. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- 111.Farr OM, Hu S, Zhang S, Li CS. Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. NeuroImage. 2012;63:1070–77. doi: 10.1016/j.neuroimage.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hendrick OM, Ide JS, Luo X, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS One. 2010;5:e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang S, Hu S, Hu J, Wu PL, Chao HH, Li CS. Barratt impulsivity and neural regulation of physiological arousal. Plos One. 2015;10:e0129139. doi: 10.1371/journal.pone.0129139. [DOI] [PMC free article] [PubMed] [Google Scholar]


