Abstract
BACKGROUND:
Myocardial infarction with nonobstructive coronary arteries (MINOCA) occurs in 6% to 15% of myocardial infarctions (MIs) and disproportionately affects women. Scientific statements recommend multimodality imaging in MINOCA to define the underlying cause. We performed coronary optical coherence tomography (OCT) and cardiac magnetic resonance (CMR) imaging to assess mechanisms of MINOCA.
METHODS:
In this prospective, multicenter, international, observational study, we enrolled women with a clinical diagnosis of myocardial infarction. If invasive coronary angiography revealed <50% stenosis in all major arteries, multivessel OCT was performed, followed by CMR (cine imaging, late gadolinium enhancement, and T2-weighted imaging and T1 mapping). Angiography, OCT, and CMR were evaluated at blinded, independent core laboratories. Culprit lesions identified by OCT were classified as definite or possible. The CMR core laboratory identified ischemia-related and nonischemic myocardial injury. Imaging results were combined to determine the mechanism of MINOCA, when possible.
RESULTS:
Among 301 women enrolled at 16 sites, 170 were diagnosed with MINOCA, of whom 145 had adequate OCT image quality for analysis; 116 of these underwent CMR. A definite or possible culprit lesion was identified by OCT in 46.2% (67/145) of participants, most commonly plaque rupture, intraplaque cavity, or layered plaque. CMR was abnormal in 74.1% (86/116) of participants. An ischemic pattern of CMR abnormalities (infarction or myocardial edema in a coronary territory) was present in 53.4% (62/116) of participants undergoing CMR. A nonischemic pattern of CMR abnormalities (myocarditis, takotsubo syndrome, or nonischemic cardiomyopathy) was present in 20.7% (24/116). A cause of MINOCA was identified in 84.5% (98/116) of the women with multimodality imaging, higher than with OCT alone (P<0.001) or CMR alone (P=0.001). An ischemic cause was identified in 63.8% of women with MINOCA (74/116), a nonischemic cause was identified in 20.7% (24/116) of the women, and no mechanism was identified in 15.5% (18/116).
CONCLUSIONS:
Multimodality imaging with coronary OCT and CMR identified potential mechanisms in 84.5% of women with a diagnosis of MINOCA, 75.5% of which were ischemic and 24.5% of which were nonischemic, alternate diagnoses to myocardial infarction. Identification of the cause of MINOCA is feasible and has the potential to guide medical therapy for secondary prevention.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02905357.
Keywords: coronary vessels, magnetic resonance imaging, myocardial infarction, tomography, optical coherence, women
Myocardial infarction with nonobstructive coronary artery disease (MINOCA) accounts for 6% to 15% of patients with spontaneous myocardial infarction (MI) and disproportionately affects women.1 The 4-year rate of major adverse cardiac events after MINOCA is ≈25%, 5-year mortality is ≈11%, and some cases are identified at autopsy.2–4 Pathogenesis of MINOCA is varied and may include atherosclerotic plaque rupture, plaque erosion with thrombosis, vasospasm, embolization, dissection, or a combination of mechanisms. Nonvascular causes including acute myocarditis, takotsubo syndrome, and various nonischemic cardiomyopathies may mimic the clinical presentation. MINOCA is a distinct entity from stable chest pain attributed to microvascular disease, affecting a different group of patients.1 Incomplete understanding of mechanisms of MINOCA leads to variable use of diagnostic testing and medical therapy in the hospital and of secondary prevention measures.5 There is uncertainty about which post-MI treatment recommendations should apply to patients with MINOCA. Optimal treatment is likely to differ substantially by mechanism, and, on the basis of expert opinion, recent European Society of Cardiology guidelines and an American Heart Association Scientific Statement recommend treating according to an underlying diagnosis.1,6 Future clinical research and treatment recommendations require a greater understanding of the pathophysiology of MINOCA. The goal of the Women’s Heart Attack Research Program was to implement a comprehensive imaging protocol to identify underlying causes of MINOCA to guide clinical practice and future intervention trials.
METHODS
The Women’s Heart Attack Research Program was a multicenter observational study using standardized diagnostic imaging protocols in women with MINOCA. The primary objective was to determine the prevalence of vascular causes of MINOCA, as identified by optical coherence tomography (OCT), and the prevalence of myocardial abnormalities in MINOCA, whether ischemic or nonischemic in nature, as identified by cardiac magnetic resonance (CMR) imaging. A secondary objective was to determine the proportion of patients for whom an underlying cause of MI could be determined after combining OCT and CMR imaging results. The data that support the findings of this study are available from the corresponding author on reasonable request.
Women were enrolled at the time of referral for diagnostic angiography to evaluate clinically diagnosed MI. Participants with <50% stenosis in all major epicardial vessels by coronary angiography were eligible to undergo coronary OCT at the time of diagnostic angiography, with the goal of imaging the 3 major epicardial arteries, and CMR within 1 week of the acute presentation. The CMR protocol included imaging for cardiac function, late gadolinium enhancement (LGE), and T1 mapping and T2-weighted imaging for myocardial edema.
OCT, CMR, and angiography images were interpreted by independent core laboratories blinded to the results of the other imaging techniques and to detailed clinical, ECG, and laboratory information. OCT culprit lesions were characterized as plaque rupture, thrombus without plaque rupture (plaque erosion), intraplaque cavity, layered plaque, spontaneous dissection, or intimal bumping interpreted according to published recommendations, using definitions provided in the Expanded Methods (Appendix in the Data Supplement), by the core laboratory at Cardiovascular Research Foundation.7,8 CMR findings were characterized by the core laboratory at Brigham and Women’s Hospital using the standard 16-segment left ventricular model.9 CMR findings were categorized as ischemic in nature if there was evidence of infarction or regional injury (eg, myocardial edema in a coronary territory as evidenced by prolonged native T1, T2 signal hyperintensity, or increased extracellular volume or regional wall motion abnormality colocalizing with LGE in a pattern consistent with infarction) or as nonischemic abnormalities (eg, LGE in a nonischemic pattern, particularly with associated edema suggesting myocarditis, or takotsubo syndrome). Previous studies have provided histologic evidence that a regional pattern of injury as defined in this manner is consistent with myocardial edema in the area at risk after ischemia and reperfusion.10,11 Detailed definitions for CMR are in the Expanded Methods (Appendix in the Data Supplement). Findings of OCT and CMR were combined to define the underlying mechanism of MINOCA. The study was approved by the New York University Grossman School of Medicine Institutional Review Board and by the local institutional review board or ethics committee of each site. All participants provided informed consent.
Participant Eligibility
Clinical Eligibility
Women ≥21 years of age with MI meeting the fourth universal definition12 who were referred for clinically indicated coronary angiography were considered for enrollment. Exclusion criteria were as follows: alternate explanation for troponin elevation (eg, acute heart failure, tachyarrhythmia, hypertensive urgency, pulmonary embolism, or renal failure); previous history of obstructive coronary artery disease (CAD) including history of percutaneous coronary intervention or coronary artery bypass grafting; recent use of vasospastic agents, such as cocaine, triptans, or ergot alkaloids (≤1 month); estimated glomerular filtration rate <45 mL/min or contraindication to additional contrast needed for OCT imaging; pregnancy; and fibrinolytic therapy for the qualifying MI event. Patients with MI who were thought clinically to be attributed to supply–demand mismatch were not enrolled. Fibrinolysis was an exclusion criterion because it would not be possible to know the degree of angiographic stenosis had this treatment not been administered. Some patients were enrolled after OCT was performed during diagnostic angiography for MINOCA on the basis of clinical routine.
Angiographic Eligibility
Transradial or transfemoral coronary angiography was performed as per clinical routine. Patients with <50% stenosis in all major coronary arteries with no contraindication to OCT on the basis of the diagnostic angiography were eligible to complete study imaging. Individuals with angiographic evidence of coronary dissection were excluded from research imaging, as were those with excessive coronary arterial tortuosity that, in the opinion of the operator, was not amenable to OCT.
OCT Image Acquisition
OCT imaging was performed using a commercially available frequency-domain OCT system (ILUMIEN OPTIS, Abbott Vascular, Santa Clara, CA). Anticoagulation and intracoronary nitroglycerin were administered before imaging. The goal was to image at least the proximal 60 mm of the left anterior descending, left circumflex, and right coronary arteries. Imaging was performed using automated pullback (75-mm length, 0.2-mm frame interval). If an artery could not be imaged safely on the basis of size or tortuosity, operators were advised to image a large branch vessel instead (eg, an obtuse marginal if the right coronary artery was small or nondominant).
CMR Image Acquisition
A 1.5- or 3.0-T MRI system with phased-array receiving coils was used, with cardiac gated pulse sequences. The study protocol included cine imaging for left ventricular function, LGE with phase-sensitive inversion recovery reconstruction, and, where available, T2-weighted, fat-suppressed, fast-spin echo imaging and T1 mapping. T1 mapping was acquired serially before and after contrast injection using the modified look-locker inversion recovery sequence or a similar sequence.13 T2-weighted, short-τ inversion recovery black-blood images were acquired before contrast administration in standard long- and short-axis planes. T1-weighted LGE images were acquired 10 to 15 minutes after 0.15- to 0.2-mmol/kg injection of contrast,14 using a standard inversion recovery gradient-echo sequence with inversion time set to null normal myocardial signal intensity, aiming for signal intensity <10 anteriorly.15
Assignment of an Underlying Diagnosis Based on the Combination of OCT and CMR
OCT and CMR findings were integrated to determine the most likely underlying cause of MINOCA for each participant. When a culprit lesion was identified on OCT, a diagnosis of MI was assigned if CMR identified infarction or regional injury in a territory attributed to the culprit coronary artery or was normal. In the small number of cases with a definite or possible coronary culprit lesion in which CMR showed myocarditis, LGE, or myocardial edema in a segment not subtended by the culprit artery, the CMR and OCT core laboratories reviewed all of the images together to arrive at a consensus final diagnosis.
MI was considered definite if there was ischemic LGE with associated myocardial edema, if OCT demonstrated plaque rupture or thrombus without plaque rupture, or if OCT demonstrated intraplaque cavity without thrombus, layered plaque, or intimal bumping and CMR identified regional injury in a coronary territory subtended by the OCT culprit artery. MI was otherwise considered probable.
Statistical Analyses
We computed descriptive statistics of baseline clinical characteristics, laboratory values, ECG findings, and OCT and CMR findings, which are presented as median and interquartile range (IQR), or frequency and percentage, as appropriate. We evaluated differences in study variables between patients with and without OCT culprit lesions and with or without CMR abnormalities using the χ2 or Fisher exact test for categorical variables and Mann–Whitney U test for continuous variables. The likelihood of establishing a diagnosis of an ischemic or nonischemic cause of MINOCA by OCT and CMR was compared with the likelihood of making such a diagnosis by OCT alone and by CMR alone using the McNemar test. Multivariable logistic regression modeling was performed to assess the odds of an OCT-defined culprit lesion, adjusting for demographics, clinical characteristics, and laboratory values. Variables with P values of <0.25 in univariate analyses were selected as candidate predictors for entry into multivariable models. Purposeful variable selection was used to select the variables.16 A separate multivariable logistic regression model was similarly generated to assess the odds of having abnormal CMR, adjusting for demographics, clinical characteristics, and laboratory values. All analyses were performed in R (version 3.5.1; R Foundation for Statistical Computing). A 2-sided P value <0.05 was considered to be statistically significant.
Power was projected on the basis of the precision of the estimated proportion of women with an OCT-defined culprit lesion. A total of 125 participants provided sufficient power to detect a 35% prevalence of OCT culprit lesions with a 95% CI from 26% to 43%.
RESULTS
Patient Characteristics
A total of 301 women were enrolled at 16 sites (Figure 1). The final study cohort included 145 women with a diagnosis of MINOCA who had interpretable OCT imaging. The median age was 60 years; 50% were non-Hispanic White, and 97% presented with a provisional diagnosis of non-ST segment myocardial infarction. A segmental wall motion abnormality was present in 49 (44%) of 111 women who underwent echocardiography. The ECG was abnormal in 95 (65%) of 145. Clinical, laboratory, and angiographic characteristics of the study cohort are shown in the Table. Sites rated the coronary angiogram as normal in 77 participants (53.1%). The angiographic core laboratory reported normal angiography (ie, no stenosis >10%) in 5 women (3.4%). Characteristics of excluded women who had MI with obstructive CAD as compared with the study cohort with MINOCA are shown in Table I in the Data Supplement. Women with MI and obstructive CAD had a greater burden of CAD risk factors and similar degrees of troponin elevation.
Figure 1. Study flow.
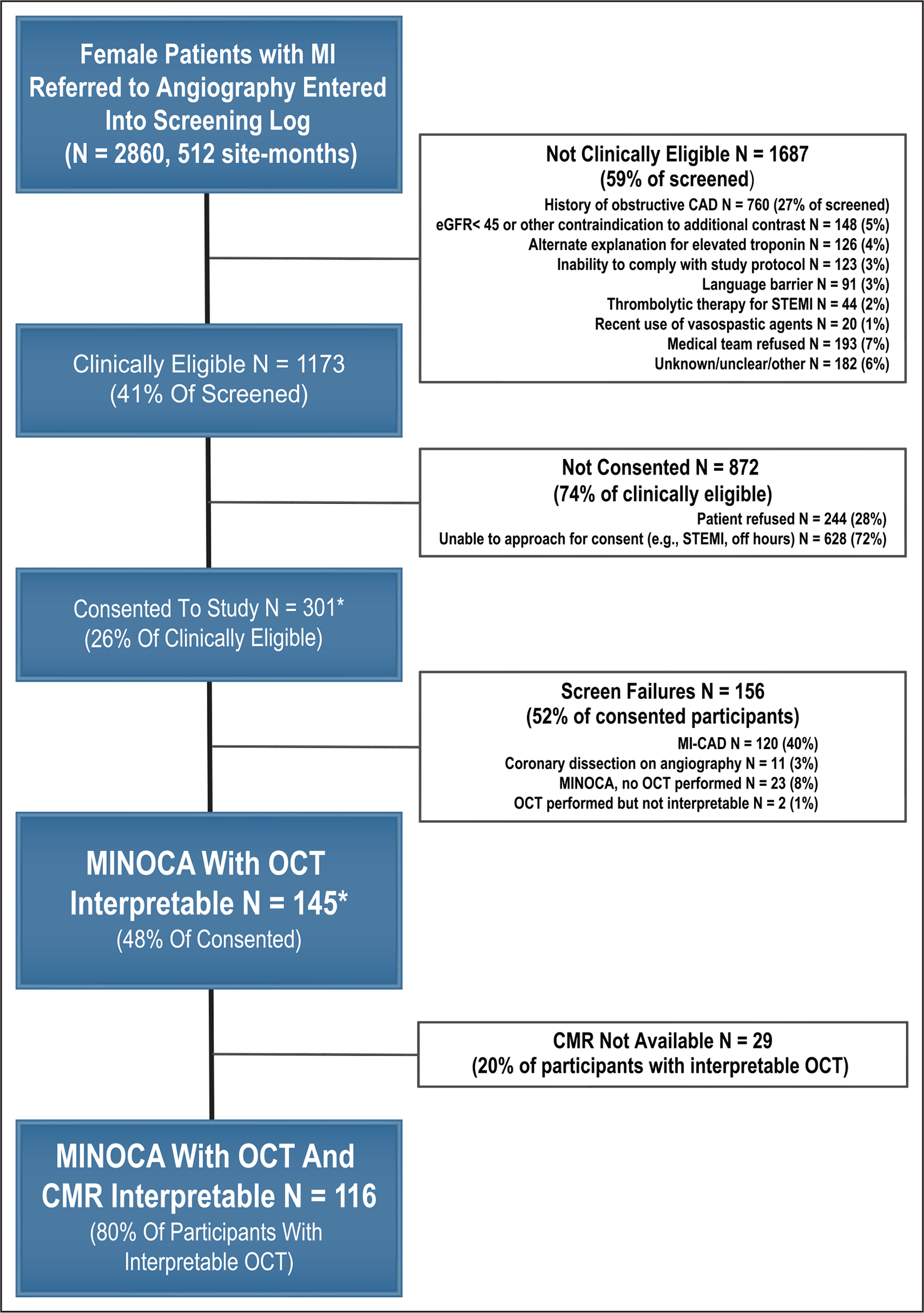
Sites were instructed to enter all consecutive women with MI who were referred for cardiac catheterization into the screening log. A total of 512 site-months of screening logs were submitted between September 2016 and March 2020. Reasons for ineligibility for preangiography consent are shown. One site screened exclusively after angiography and enrolled patients who had OCT conducted for clinical indications. History of coronary artery disease typically included history of percutaneous coronary intervention or coronary artery bypass grafting. OCT was aborted in 1 participant with MINOCA as a result of coronary spasm before images were acquired. Other reasons OCT was not completed in patients with MINOCA were: interventional cardiologist’s assessment that risk of OCT was elevated because of small arteries or excessive tortuosity (n=4), risk of additional contrast (eg, high-contrast load, allergy, or volume overload; n=5), or angiographic evidence of coronary spasm (n=1); cath laboratory scheduling concerns (n=4); ventricular septal defect (n=1); and unexpected technical difficulties (n=1). An additional 3 patients with nonobstructive CAD were determined by the interventional cardiologist to have an alternate explanation for troponin elevation and OCT was not performed (eg, hemodynamically significant aortic stenosis). CMR in the 2 patients with uninterpretable OCT showed infarction in both cases. Reasons that CMR was not available included: participant refused CMR after initial consent (n=16), claustrophobia in scanner (n=3), scheduling issues (n=4), pacemaker (n=1), and CMR performed but not interpretable (n=5; 3%). *Includes 10 pilot patients recruited at New York University, during a time when screening logs were not maintained. CAD indicates coronary artery disease; cath, cardiac catheterization; CMR, cardiac magnetic resonance; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; MI-CAD, myocardial infarction with ≥stenosis in a major epicardial vessel; MINOCA, myocardial infarction with nonobstructive coronary arteries (<50% stenosis in all major epicardial vessels); OCT, optical coherence tomography; and STEMI, ST-segment elevation myocardial infarction.
Table.
Demographics, Clinical Characteristics and Imaging Findings in Women With MINOCA Overall and in Relation to OCT and CMR Findings
| Variable | MINOCA participants with OCT interpretable (N=145) | OCT culprit present (N=67) | No OCT culprit (N=78) | P value | CMR abnormal (N=86) | CMR normal (N=30) | P value |
|---|---|---|---|---|---|---|---|
| Age, median [IQR], y | 59.6 [52.4, 69.4] |
66.05 [57.14, 72.61] |
55.96 [48.55, 64.36] |
<0.001 | 59.51 [49.83, 71.21] |
58.38 [53.38, 67.23] |
0.97 |
| Female sex, n (%) | 145 (100) | 67 (100) | 78 (100) | NA | 86 (100) | 30 (100) | NA |
| Race, n (%) | 0.185 | 0.122 | |||||
| American Indian or Alaska Native | 2 (1.4) | 2 (3.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) | ||
| Asian | 12 (8.3) | 3 (4.5) | 9 (11.5) | 5 (5.8) | 5 (16.7) | ||
| Black or African American | 17 (11.7) | 10 (14.9) | 7 (9.0) | 9 (10.5) | 4 (13.3) | ||
| Native Hawaiian or other Pacific Islander | 1 (0.7) | (1.5) | 0 (0.0) | 0 (0.0) | 1 (3.3) | ||
| Other | 17 (11.7) | 9 (13.4) | 8 (10.3) | 11 (12.8) | 1 (3.3) | ||
| White | 96 (66.2) | 42 (62.7) | 54 (69.2) | 60 (69.8) | 19 (63.3) | ||
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 35 (24.1) | 16 (23.9) | 19 (24.4) | >0.99 | 21 (24.4) | 5 (16.7) | 0.534 |
| Medical history, n (%) | |||||||
| Hypertension | 66 (45.5) | 35 (52.2) | 31 (39.7) | 0.181 | 39 (45.3) | 13 (43.3) | >0.99 |
| Diabetes | 23 (15.9) | 18 (26.9) | 5 (6.4) | 0.002 | 12 (14.0) | 6 (20.0) | 0.621 |
| Dyslipidemia | 51 (35.2) | 33 (49.3) | 18 (23.1) | 0.002 | 31 (36.0) | 10 (33.3) | 0.963 |
| Smoking status | n=144 | n=66 | n=78 | 0.115 | n=86 | n=29 | 0.013 |
| Current smoker, n (%) | 16 (11.1) | 8 (12.1) | 8 (10.3) | 9 (10.5) | 6 (20.7) | ||
| Former smoker, n (%) | 31 (21.5) | 19 (28.8) | 12 (15.4) | 13 (15.1) | 10 (34.5) | ||
| Never smoker, n (%) | 97 (67.4) | 39 (59.1) | 58 (74.4) | 64 (74.4) | 13 (44.8) | ||
| Family history of MI, n/N (%) | 34/132 (25.8) | 18/59 (30.5) | 16/73 (21.9) | 0.357 | 16/80 (20.0) | 9/27 (33.3) | 0.249 |
| Prior MI, n (%) | 13 (9.0) | 8 (11.9) | 5 (6.4) | 0.384 | 7 (8.1) | 3 (10.0) | 0.717 |
| Depression, n/N (%) | 34/143 (23.8) | 14 (20.9) | 20/76 (26.3) | 0.573 | 20 (23.3) | 7/28 (25.0) | >0.99 |
| Anxiety, n/N (%) | 31/143 (21.7) | 15 (22.4) | 16/76 (21.1) | >0.99 | 17 (19.8) | 8/28 (28.6) | 0.475 |
| Symptoms at MI presentation | |||||||
| Chest pain, n (%) | 130 (89.7) | 60 (89.6) | 70 (89.7) | >0.99 | 77 (89.5) | 26 (86.7) | 0.736 |
| Arm pain, n (%) | 59 (40.7) | 28 (41.8) | 31 (39.7) | 0.936 | 38 (44.2) | 0.982 | |
| Duration of symptoms on the day of presentation, median [IQR], h | (n=137) 3.0 [1.0, 10.0] |
(n=61) 5.0 [1.0, 15.0] |
(n=76) 2.0 [1.0, 6.0] |
0.032 | (n=80) 3.5 [1.0, 10.0] |
(n=29) 2.0 [1.0, 8.0] |
0.287 |
| ST-elevation myocardial infarction presentation, n (%) | 5 (3.5) | 2/66 (3.2) | 3/77 (3.7) | >0.99 | 5/84 (6.0) | 0/30 (0.0) | 0.323 |
| Electrocardiogram findings (N=143), n (%) | n=67 | n=76 | n=86 | n=29 | |||
| ST depression | 15 (10.5) | 8 (11.9) | 7 (9.2) | 0.796 | 9 (10.5) | 3 (10.3) | >0.99 |
| T-wave inversion | 65 (45.5) | 30 (44.8) | 35 (46.1) | >0.99 | 44 (51.2) | 9 (31.0) | 0.096 |
| Left bundle-branch block | 2 (1.4) | 1 (1.5) | 1 (1.3) | >0.99 | 2 (2.3) | 0 (0.0) | 0.994 |
| Q waves | 27 (18.9) | 15 (22.4) | 12 (15.8) | 0.428 | 15 (17.4) | 4 (13.8) | 0.788 |
| Prolonged QTc interval | 40 (28.0) | 22 (32.8) | 18 (23.7) | 0.303 | 26 (30.2) | 9 (31.0) | >0.99 |
| Normal electrocardiogram | 50 (35.0) | 18 (26.9) | 32 (42.1) | 0.083 | 28 (32.6) | 11 (37.9) | 0.763 |
| Blood pressure (n=139) | |||||||
| Systolic blood pressure, median [IQR], mm Hg | 132.00 [121.00, 152.50] |
132.00 [121.25, 150.75] |
133.00 [121.00, 154.00] |
0.931 | 134.00 [121.00, 151.00] |
127.00 [113.00, 148.00] |
0.464 |
| Diastolic blood pressure, median [IQR], mm Hg | 78.00 [68.50, 87.00] |
76.00 [69.25, 86.00] |
78.00 [67.00, 87.00] |
0.846 | 79.00 [70.00, 87.00] |
70.00 [63.00, 78.00] |
0.009 |
| Echocardiography (N=111) | |||||||
| Segmental wall motion abnormality on echocardiogram, n (%) | 49/111 (44.1) | 25/53 (47.2) | 24/58 (41.4) | 0.121 | 35/66 (53.0) | 6/20 (30.0) | 0.121 |
| Left ventricular ejection fraction, median [IQR], % | (n=110) 59.5 [50.0, 63.0] |
(n=53) 60.0 [52.0, 62.0] |
(n=57) 59.0 [50.0, 64.0] |
0.945 | (n=67) 57.0 [45.5, 62.0] |
(n=18) 60.0 [55.3, 65.0] |
0.107 |
| Laboratory data | n=67 | n=78 | |||||
| Peak troponin, median [IQR], ng/mL | 0.94 [0.34, 4.38] |
0.75 [0.31, 3.54] |
1.18 [0.40, 4.55] |
0.398 | 1.79 [0.66, 6.53] |
0.52 [0.19, 0.92] |
<0.001 |
| Peak troponin, multiple of local upper limit of normal for the laboratory assay, median [IQR] | 17.25 [7.00, 61.00] |
17.25 [5.42, 57.92] |
17.25 [10.01, 73.19] |
0.526 | 37.95 [11.65, 112.14] |
9.47 [4.34, 14.61] |
<0.001 |
| Coronary angiography | n=67 | n=78 | n=86 | n=30 | |||
| Coronary angiogram reported as normal by site, n (%) | 77 (53.1) | 23 (34.3) | 54 (69.2) | <0.001 | 47 (54.7) | 12 (40.0) | 0.242 |
| Maximal diameter stenosis by core laboratory, median [IQR] | (n=142) 30% [26, 37] |
(n=67) 31% [26, 36] |
(n=75) 29% [26, 38] |
0.724 | (n=85) 29% [25, 38] |
(n=28) 33% [27, 38] |
0.348 |
| Maximal stenosis by visual estimation at core laboratory, n (%) | |||||||
| 0% | 0 (0) | 0 (0) | 0 (0) | 0.245 | 0 (0) | 0 (0) | 0.430 |
| 1% to 10% | 5 (3.4) | 1 (1.5) | 4 (5.1) | 5 (5.8) | 0 (0) | ||
| 11% to 30% | 90 (62.1) | 39 (58.2) | 51 (65.4) | 51 (59.3) | 17 (56.7) | ||
| 31% to 49% | 50 (34.5) | 27 (40.3) | 23 (29.5) | 30 (34.9) | 13 (43.3) | ||
| Study imaging (N=145) | |||||||
| Days from MI to OCT, median [IQR] | 2 [1, 3] | 2.00 [1.00, 3.00] | 1.00 [1.00, 3.00] | 0.03 | (n=86) 1.00 [1.00, 2.75] |
(n=30) 2.00 [1.00, 2.75] |
0.274 |
| OCT number of vessels imaged, n (%) | 0.017 | 0.845 | |||||
| 1 | 12 (8.3) | 3 (4.5) | 9 (11.5) | 6 (7.0) | 3 (10.0) | ||
| 2 | 47 (32.4) | 16 (23.9) | 31 (39.7) | 29 (33.7) | 10 (33.3) | ||
| 3 | 86 (59.3) | 48 (71.6) | 38 (48.7) | 51 (59.3) | 17 (56.7) | ||
| OCT culprit lesion present, n (%) | 67 (46.2) | 67 (100) | 78 (0) | NA | 39/86 (45.3) | 12/30 (40.0) | 0.673 |
| CMR completed and interpretable, n (%) | 116 (80) | 51 (76.1) | 65 (83.3) | 0.382 | 86/86 (100) | 30/30 (100) | NA |
| CMR abnormal, n (%) | 86/116 (74.1) | 39/51 (76.5) | 47/65 (72.3) | 0.768 | 86/86 (100) | 0/30 (0) | NA |
| Days from MI to CMR, median (IQR) | 6.00 [3.50, 9.00] |
(n=50) 6.00 [4.00, 9.75] |
(n=65) 6.00 [3.00, 8.00] |
0.665 | 6.00 [3.00, 7.50] |
8.00 [6.00, 11.50] |
0.002 |
CMR indicates cardiac magnetic resonance; IQR, interquartile range; MI, myocardial infarction; MINOCA, myocardial infarction with nonobstructive coronary arteries; NA, not applicable; and OCT, coronary optical coherence tomography.
Findings on Coronary OCT Imaging
Among 145 participants with interpretable OCT images in at least 1 vessel, OCT was performed in all 3 major coronary arteries in 86 (59.3%), 2 arteries (32.4%) in 47, and 1 artery (8.3%) in 12. The median total vessel length imaged was 148.0 mm (IQR, 105.0–176.6 mm). A definite or possible culprit lesion was identified in 67 (46.2%) of 145 of participants undergoing OCT (Figure 2). The most common culprit lesions were plaque rupture, intraplaque cavity, and layered plaque, all of which represent atherosclerotic plaque disruption (58/145, 40%). An additional 5 (3.4%) had thrombus without plaque rupture (eg, thrombus overlying an intact fibrous cap, or lone thrombus). Three patients (2.1%) had intimal bumping suggesting coronary artery spasm. One had spontaneous coronary artery dissection. There was no calcified nodule identified. Five patients had more than 1 culprit lesion. Representative OCT culprit lesions are shown in Figure 3 and Figure I in the Data Supplement. The OCT findings of this cohort were subtle compared with prior series evaluated by the OCT core laboratory with significant atherosclerotic disease on angiography (Figure II in the Data Supplement). A total of 17 patients had transient slow flow that resolved by the end of imaging. In addition, 46 patients had transient coronary spasm during OCT imaging, 12 of whom also had transient slow flow. There were no coronary dissections in association with OCT. Additional representative cases are shown in Figures III through V in the Data Supplement.
Figure 2. Findings of OCT and CMR in women with MINOCA.
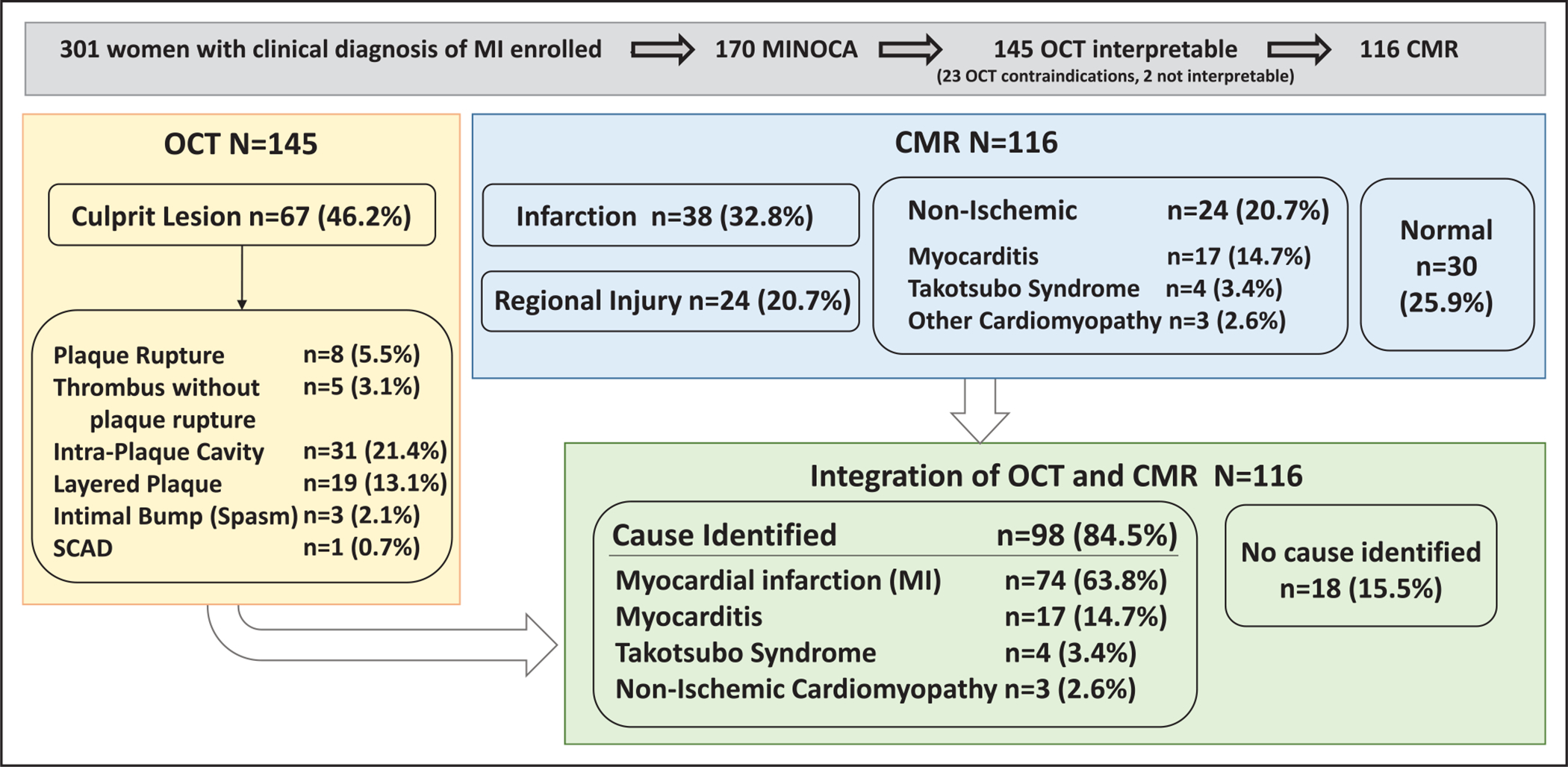
Findings of each core laboratory and the final diagnosis are shown. Note that calcified nodule was not observed. Some patients did not undergo CMR despite eligibility after angiography and OCT (see Figure 1 for reasons CMR was not performed). CMR indicates cardiac magnetic resonance; MINOCA, myocardial infarction with nonobstructive coronary arteries (<50% stenosis in all major epicardial vessels); and OCT, optical coherence tomography.
Figure 3. Representative OCT images demonstrating the various types of culprit lesions in women with MINOCA.
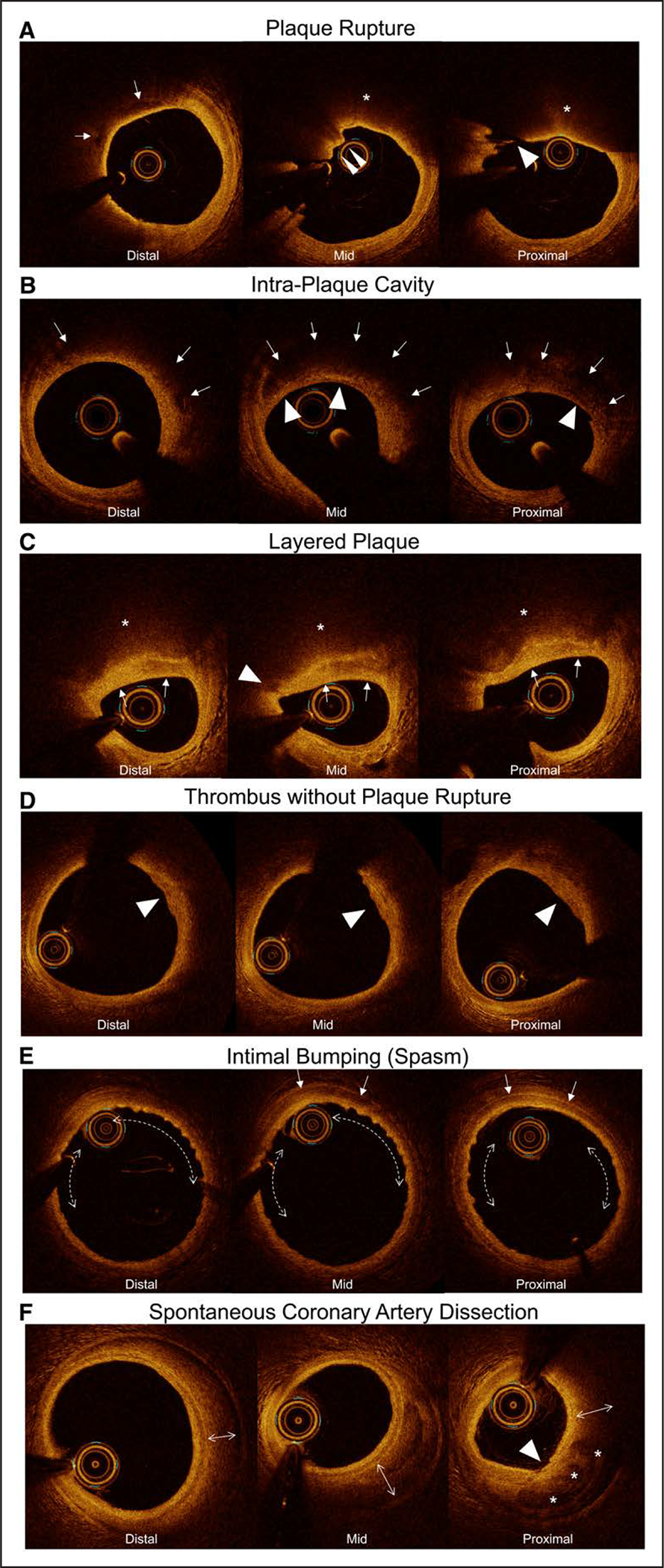
In all OCT figures (Figure 3 and Figures II through V in the Data Supplement), the most proximal frame of OCT is shown in the right panel, the middle frame of OCT is shown in the center panel, and the most distal frame of OCT is shown in the left panel. A, Plaque rupture. In the proximal frame, there is discontinuity of a thin fibrous cap (>), indicating a plaque rupture, although it includes motion artifact. There is red thrombus superimposed on the rupture site in the mid frame (>>). In the distal frame, there are focal low-intensity regions indicating injected contrast within the ruptured cavity (→). The underlying plaque is a lipidic plaque (*). B, Intraplaque cavity. Intraplaque cavity: in the proximal, mid, and distal frames, there are low-intensity regions with limited attenuation indicating organized thrombus and/or injected contrast in the ruptured cavity (→) overlaying a high-backscattered fibrous cap (>). The fibrous cap (>) in the proximal frame looks thin, but there was no discontinuity of the fibrous cap, implying previous rupture with sealing. C, Layered plaque. There is a heterogeneous layer (→) overlaying a lipidic plaque (*) indicating healing of a recent plaque rupture event. In the mid frame, there is a focal low-intensity region (>) at the edge of the luminal interface of the layered plaque, indicating a potential site of previous rupture of the fibrous cap. D, Mural thrombus without plaque rupture. There is a homogeneous region with irregular surface indicating platelet-rich mural thrombus (>). Within the underlying lipidic plaque, no clear rupture was observed. E, Intimal bumping consistent with coronary artery spasm. Intimal bumping: there is diffuse intimal wrinkling (←—→) along with thickening of the arterial media (→) indicating spasm. F, Spontaneous coronary artery dissection. There is a dissection plane causing hematoma (←→) exterior to the arterial media, within the adventitia. There is an intimal tear (>, proximal frame) along with contrast flow into the false lumen (*). MINOCA indicates myocardial infarction with nonobstructive coronary arteries (<50% stenosis in all major epicardial vessels); and OCT, optical coherence tomography.
The finding of a culprit lesion on OCT was associated with older age, history of diabetes, and history of dyslipidemia (Table). Women with an OCT culprit lesion had a longer reported duration of symptoms at the time of presentation (median, 5 [IQR, 1–15 hours] versus 2 hours [IQR, 1–6 hours]; P=0.032). The presence of a culprit lesion was not associated with peak troponin or left ventricular ejection fraction. Sites reported normal coronary angiography less frequently in women with an OCT-defined culprit lesion (34.3% versus 69.2% normal angiography in women without an OCT-defined culprit lesion; P<0.001), but there was no difference in the type of OCT culprit lesion (Table II in the Data Supplement). There was no difference in the core laboratory-determined maximal diameter stenosis among women with versus without a culprit lesion on OCT (median, 31% versus 29%; P=0.724). On vessel-level analysis of OCT findings and coronary stenosis as determined by the angiography core laboratory, the likelihood of finding an OCT culprit was not different on the basis of severity of angiographic stenosis in the same vessel (12/82 [14.6%] for vessels with 0% to 10% stenosis, 44/227 [19.4%] with 11% to 30% stenosis, and 14/55 [25.9%] with 31% to 50% stenosis; P=0.263). Women with more vessels imaged were more likely to have a culprit lesion identified. Characteristics of women with definite, possible, or no culprit lesion are presented in Table III in the Data Supplement.
On multivariable analysis, only older age (OR, 1.05 per year [95% CI, 1.02–1.09]), site designation that angiography was abnormal (OR, 5.43 [95% CI, 2.50–12.41]), and history of diabetes (OR, 5.41 [95% CI, 1.77–19.20]) were independently associated with the presence of an OCT culprit lesion (Table IV in the Data Supplement).
Findings on CMR Imaging
CMR was interpretable in 116 of the 145 participants with interpretable OCT. CMR was performed a median of 6 days from MI onset (IQR, 3.5–9.0). T2-weighted imaging was completed and interpretable in 114 patients (98.3% of CMR). T1 mapping was completed and interpretable in 76 (65.5% of CMR). An ischemic pattern of LGE (ie, infarction) was identified in 38 women (32.8%), 95% of whom had evidence of myocardial edema. Regional injury (ie, increased T2-weighted signal and/or abnormal increased extracellular volume in a single coronary territory) was observed in an additional 24 women (20.7%). CMR evidence of myocarditis was present in 17 women (14.7%), 4 women had findings of takotsubo syndrome (3.4%), and 3 had nonischemic cardiomyopathy (2.6%). The remaining 30 (25.9%) had normal CMR. Representative CMR images are shown in Figure 4. Additional representative cases are shown in Figures VI through VII in the Data Supplement. Among women with a CMR diagnosis of infarction, the median infarct size was 3.8 g (IQR, 2.0–5.4). Among women with a CMR diagnosis of MI or regional injury, the median percentage of myocardium with T2 signal hyperintensity was 3.6% (IQR, 0.2–11.1). Native T1 values appear in Table V in the Data Supplement.
Figure 4. Representative CMR images in women with MINOCA.
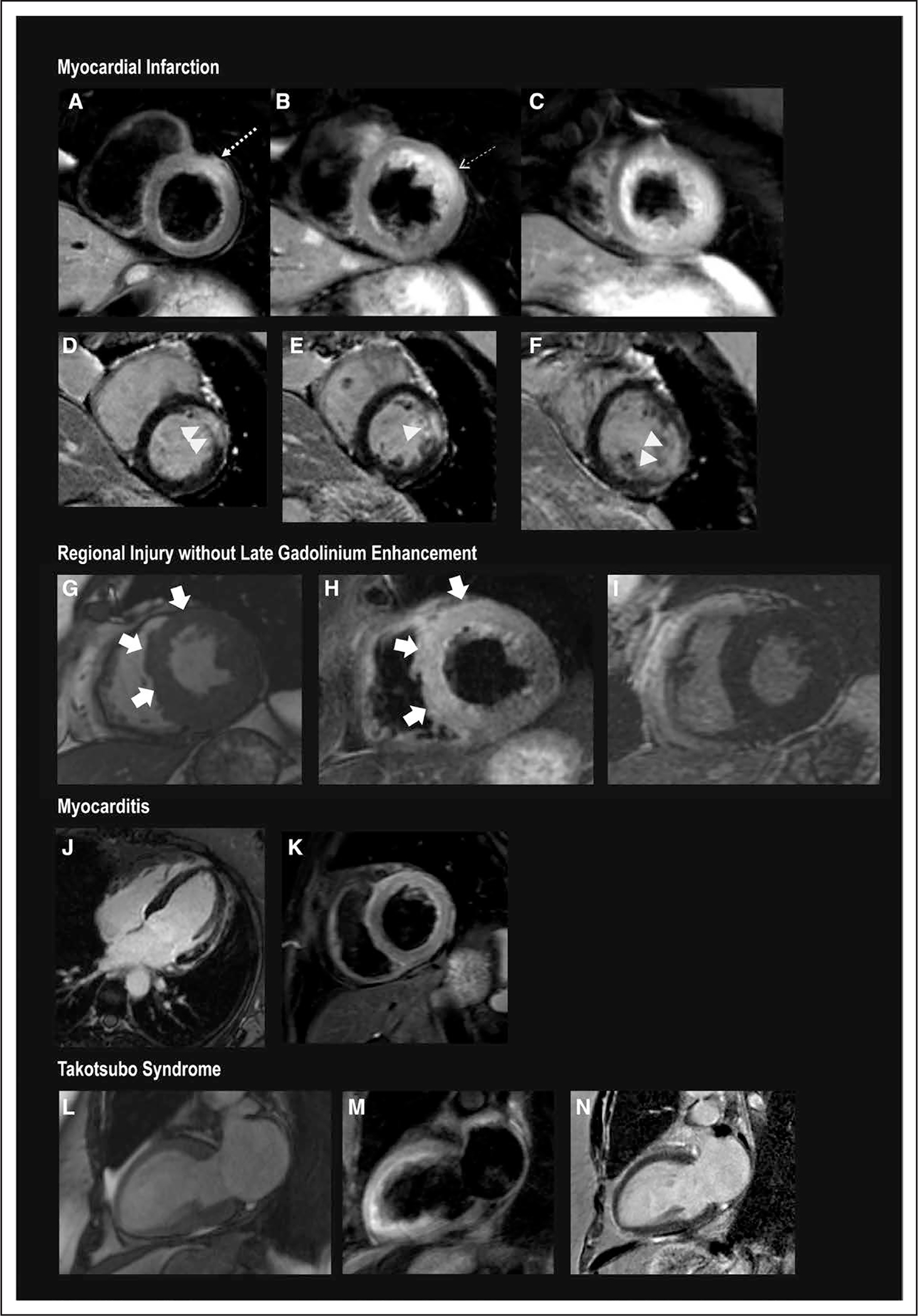
A through F, Myocardial infarction. A through C, T2-weighted images. D through F, Late gadolinium enhanced images, arranged from basal (left) to apical (right). There is subendocardial-to-transmural late gadolinium enhancement of the basal anterior and anteroseptal, mid anterior, and the apical anterolateral wall (>). T2-weighted imaging demonstrates increased signal within and extending beyond the area of late gadolinium enhancement (—→), affecting the LAD territory, including all apical segments. G through I, Regional edema without late gadolinium enhancement. The anterior and anteroseptal walls were hypokinetic (C, systolic frame). This wall motion abnormality was matched by evidence of T2 signal enhancement in the matching regions (D). There was no evidence of infarction on late gadolinium-enhanced imaging (E). J and K, Myocarditis. Multiple foci of late gadolinium enhancement of the anterolateral wall (F), which is matched by T2 hyperintensity in the matching anterolateral wall and anterior wall (G). On cine imaging, there was diffuse hypokinesis of the left ventricle. L and M, takotsubo syndrome. H, Systolic frame demonstrates hypokinesis of the anterior wall, apex, and apical-to-mid inferior wall with preserved contraction at the base. I, T2-weighted imaging with hyperintensity in the anterior wall, apex, and apical-to-mid inferior wall. N, Absence of late gadolinium enhancement. CMR indicates cardiac magnetic resonance; LAD, left anterior descending; and MINOCA, myocardial infarction with nonobstructive coronary arteries (<50% stenosis in all major epicardial vessels).
Abnormal CMR was associated with higher peak troponin (1.79 ng/mL [IQR, 0.66–6.53 ng/mL] versus 0.52 ng/mL in those with normal CMR [IQR, 0.19–0.92 ng/mL]; P<0.001; Table). Abnormal CMR was also associated with higher diastolic blood pressure, current smoking, and shorter time from MI onset to CMR but not with the presence of an OCT culprit lesion or nonobstructive CAD rather than normal angiography (Table IV in the Data Supplement). We were not able to identify a peak troponin threshold below which the likelihood of abnormal CMR was <15%. On multivariate analysis, peak troponin (OR, 1.61 per log increase [95% CI, 1.20–2.27]), creatinine (OR, 0.52 per log increase [95% CI, 0.31–0.86]), and higher diastolic blood pressure (OR, 1.05 [95% CI, 1.00–1.10]) were associated with CMR abnormalities.
Combined Imaging Findings: OCT and CMR
Among 116 women who had both interpretable OCT and CMR, 98 had an abnormality on one or both studies (84.5%). The mechanism of the MINOCA presentation was determined to be vascular (ie, final diagnosis remained MI) in 74 (63.8%), myocarditis in 17 (14.7%), takotsubo syndrome in 4 (3.4%), and nonischemic cardiomyopathy in 3 (2.6%). No mechanism was identified in the remaining 18 (15.5%; Figure 2). MI was definite in 76% and probable in 24% of cases with MI as the mechanism (Table VI in the Data Supplement).
The prevalence of abnormalities indicating a cause of the MINOCA presentation after the combination of OCT and CMR was 84.5% (95% CI, 76.3–90.3), significantly higher than OCT alone (51/116, 44.0% [95% CI, 34.9–53.5]; P<0.001) or CMR alone (86/116, 74.1% [95% CI, 65.0–81.6]; P=0.001).
The association between OCT and CMR findings is illustrated in Figure 5. There was CMR-detected infarction or regional injury in 38 women with an OCT culprit lesion identified (74.5% of 51 women with an OCT culprit lesion undergoing CMR, including 28.6% of women with plaque rupture, 100% of women with thrombus without plaque rupture, 76.2% of women with intraplaque cavity, and 66.7% of women with layered plaque). The only correlate of CMR abnormalities among patients with an OCT culprit was higher peak troponin (median, 23.3 times the upper limit of normal with abnormal CMR [IQR, 13.5–126.0] versus 10.7 times upper limit of normal with normal CMR [IQR, 6.7–15.5]; P=0.021; Table VII in the Data Supplement).
Figure 5. OCT and CMR findings in women with MINOCA (n=116).
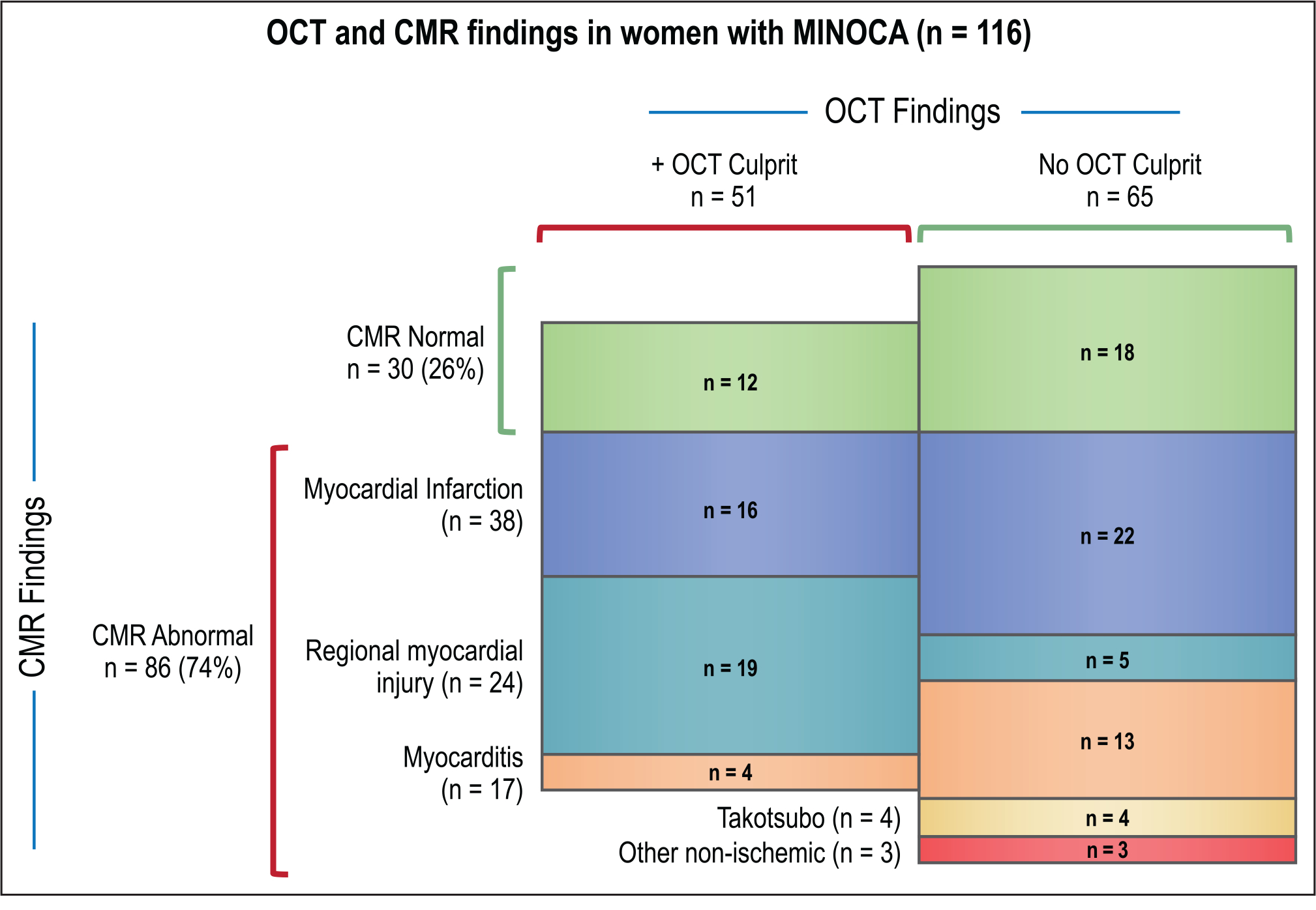
Specific OCT culprit lesions were related to CMR findings as follows (number of cases with CMR completed, findings): plaque rupture n=7 (2 infarct-pattern LGE, 5 normal); thrombus without plaque rupture n=5 (2 infarct-pattern LGE, 3 regional injury without LGE); intraplaque cavity n=21 (6 infarct-pattern LGE, 10 regional injury without LGE, 2 normal, 3 myocarditis); layered plaque n=15 (5 infarct-pattern LGE, 5 regional injury without LGE, 5 normal); intimal bumping n=2 (1 regional injury without LGE, 1 myocarditis); spontaneous coronary artery dissection n=1 (1 infarct-pattern LGE). CMR indicates cardiac magnetic resonance; LGE, late gadolinium enhancement; MINOCA, myocardial infarction with nonobstructive coronary arteries (<50% stenosis in all major epicardial vessels); and OCT, optical coherence tomography.
In contrast, when considering women with CMR-detected infarction or regional injury, 16 (42%) of 38 women with infarct pattern LGE had an OCT culprit lesion and 19 (79%) of 24 women with the CMR finding of regional injury in the absence of LGE had an OCT culprit lesion. In 6 cases with CMR-detected infarct pattern LGE or regional injury, OCT of the coronary artery supplying the affected myocardium was not performed.
Figure 6 shows 2 representative cases with matching of OCT and CMR diagnoses, and Figure 7 shows 1 case with discordant findings. Figure 6A shows proximal plaque rupture associated with a small infarction at the terminus of the affected artery, whereas Figure 6B illustrates atherosclerotic plaque disruption associated with a larger territory of acute infarction.
Figure 6. Representative cases of plaque disruption causing MINOCA.
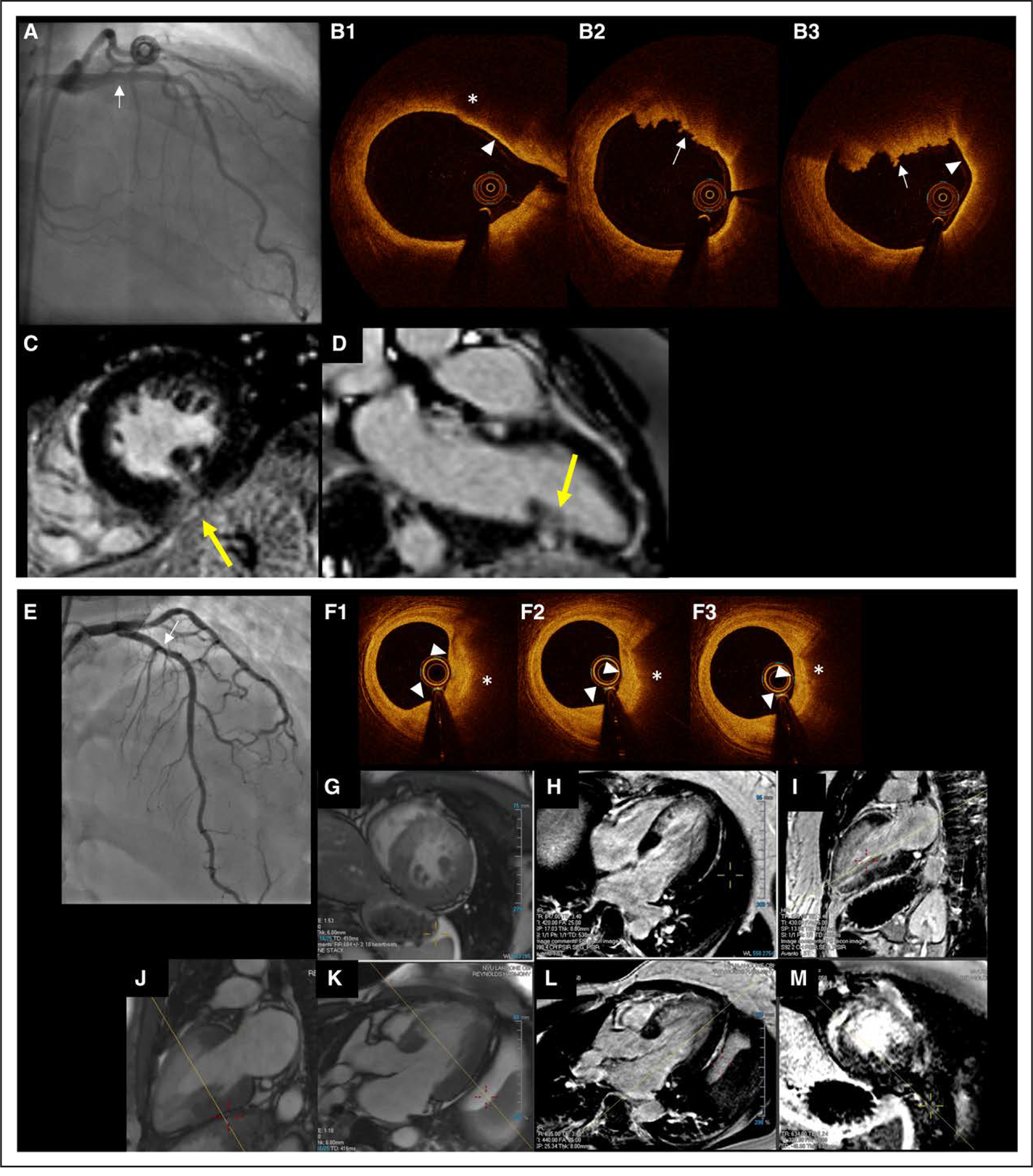
Top, Representative case of plaque rupture with red thrombus in a wraparound LAD coronary artery with associated apical inferior wall infarction. A 44-year-old woman with no coronary artery disease risk factors who presented with chest pain persisting after transfusion in the context of heavy menstrual bleeding, hemoglobin 7 mg/dL. Peak troponin I was 3.25 ng/mL. A, Angiography of the LAD coronary artery demonstrated a lucency in the proximal LAD with <50% angiographic stenosis. Note that the LAD terminates well past the apex at the inferior wall of the left ventricle. B, OCT image showed a protruding mass with irregular surface indicating mural red thrombus (→). Proximally, there is a low intensity region (* in B1) with clear demarcation indicating cavity in the lipidic plaque. Adjacent thin cap fibroatheroma was observed (▻). C and D, CMR late gadolinium enhanced images, short (C) and long axis (D). There is a small, transmural infarction in the mid-to-apical inferior wall, corresponding with the terminus of the LAD. Bottom, Representative case of layered plaque in LAD coronary artery with associated anterior wall infarction. A 41-year-old woman with hypertension and history of cerebral sinus venous thrombosis who presented with 4.5 hours of midsternal chest pain at rest. The presenting ECG showed equivocal ST elevation in the anterior precordial leads, and cardiac troponin I was 5.1 ng/mL. She was taken urgently for cardiac catheterization showing mild-moderate LAD stenosis after intracoronary nitroglycerin; no intervention was performed. Four days later, because of nonsustained ventricular tachycardia, the patient was referred for repeat coronary angiography (E), showing 20% to 30% LAD stenosis. F1 through F3, OCT images corresponding with proximal mild stenosis showed a layered plaque (white triangles) underlying lipidic plaque (*); images are arranged from distal to proximal, left to right. G through M, The myocardial extent of infarction (H, I, L, and M) was matched by regional wall motion abnormality and evidence of edema on the postcontrast cine SSFP (T2/T1-weighted) imaging (G, J, and K). CMR indicates cardiac magnetic resonance; LAD, left anterior descending; MINOCA, myocardial infarction with nonobstructive coronary arteries (<50% stenosis in all major epicardial vessels); OCT, optical coherence tomography; and SSFP, steady-state free precession.
Figure 7. Case with myocarditis on CMR affecting the lateral wall and intimal bumping in left circumflex coronary artery on OCT.
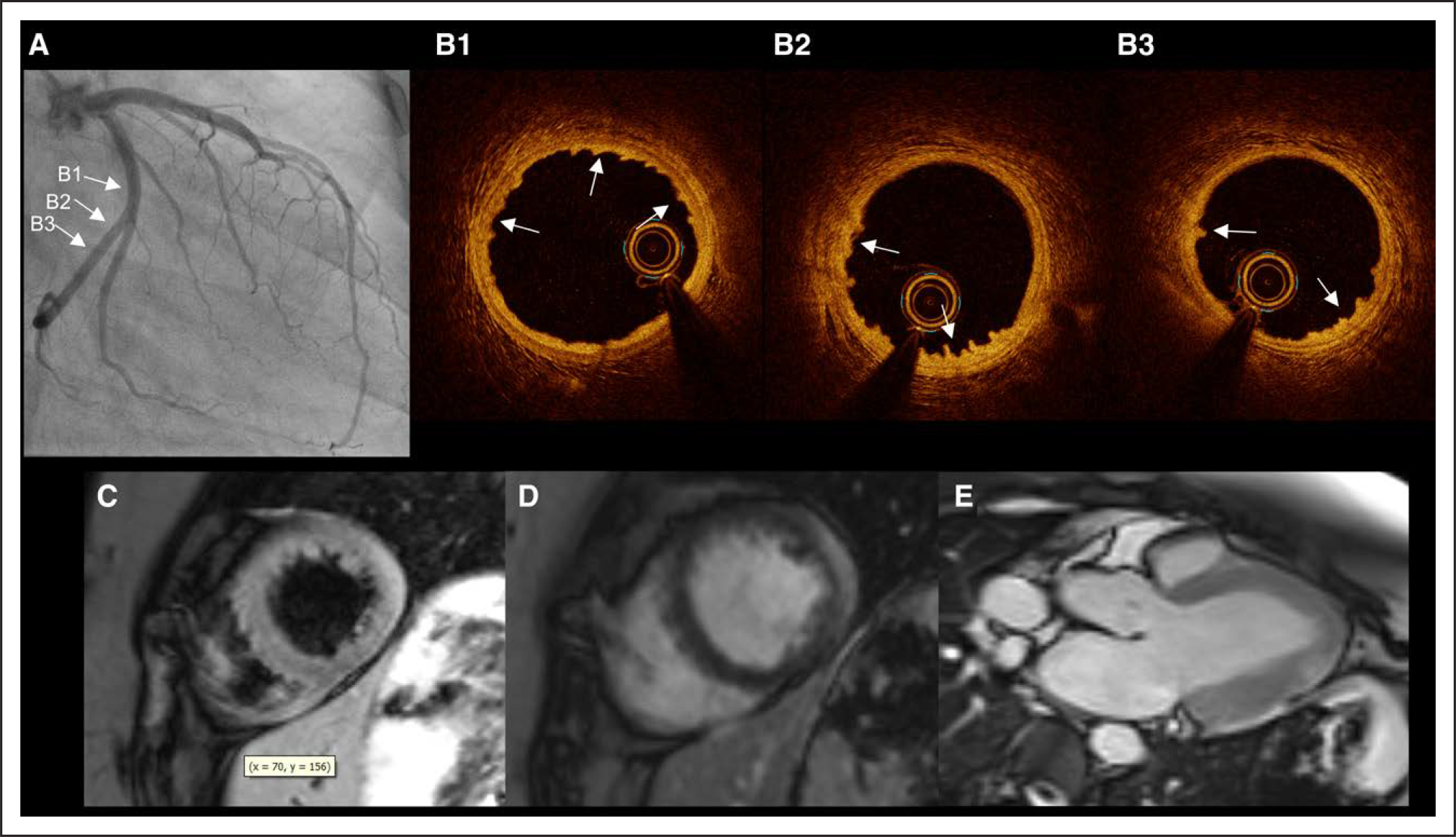
A 29-year-old woman with no cardiac risk factors, history of anxiety, who presented with 2 days of midsternal chest pain and normal ECG; there was no fever. An echocardiogram reportedly demonstrated normal left ventricular wall motion. Troponin peaked at 4.57 ng/mL. A, Coronary angiography showed normal coronary arteries. B1 through B3, Left circumflex artery OCT showed intimal bumping (→) consistent with recent coronary artery spasm (panels are arranged from distal in B1 to proximal in B3). C through E, Postcontrast cine SSFP images (T2/T1-weighted) showed enhancement in the apical lateral wall in short- (C) and long-axis (D) views. Epicardial LGE in the same area of the distal lateral wall (E). The final diagnosis was determined to be myocarditis on the basis of consensus review. CMR indicates cardiac magnetic resonance; LGE, late gadolinium enhancement; and OCT, optical coherence tomography.
There were 4 cases with myocarditis on CMR and a culprit lesion on OCT (7.8% of culprit lesions). Three had intraplaque cavities, rated as possible. The other case showed definite intimal bumping in the artery adjacent to an area of epicardial LGE and edema, suggesting that spasm might have been secondary to nearby myocarditis (Figure 7). After consensus review, the final diagnosis was myocarditis in all 4 cases.
DISCUSSION
In this multicenter study of women with MINOCA referred for coronary angiography to evaluate MI, a cause of MINOCA could be identified in 85% with the combination of intracoronary OCT imaging and CMR. The majority of patients had an ischemic mechanism (64% overall). Approximately 1 in 5 women with MINOCA was diagnosed with a nonischemic process mimicking an MI presentation, such as myocarditis. Only 15.5% had no imaging findings to illustrate the cause of the clinical presentation. Most OCT-defined culprit lesions in women with MINOCA reflected atherosclerotic plaque disruption. Correlation of OCT culprit lesions with CMR findings demonstrated that nonobstructive culprit lesions are frequently associated with MI or ischemic injury. The 2 imaging modalities provided useful diagnostic information, independently and in combination.
The proportion of participants with an identifiable culprit lesion on intracoronary imaging in our study, 46%, was somewhat higher than in earlier studies of MINOCA using intravascular ultrasound and smaller studies with limited, less extensive OCT coronary imaging protocols.17–19 This observation reflects a higher resolution of OCT imaging compared with intravascular ultrasound and improved sensitivity of routine multivessel OCT used in the present study. The proportion of women with an OCT culprit lesion in our study was lower than that reported in a recent 2-center study using OCT in patients with MINOCA, because the screening process in that study in many cases used CMR to exclude myocarditis and provocative testing to exclude coronary artery spasm before OCT, enriching the sample for cases with atherosclerotic plaque disruption.20
Culprit plaques were smaller than typical for MI with obstructive CAD, but the morphology of plaque rupture and its early sequelae after MI, intraplaque cavity, and layered plaque21 was otherwise typical. Intraplaque cavity is a common finding at autopsy in cases of plaque rupture causing sudden cardiac death, particularly when the underlying plaque does not cause severe stenosis.22 A layered appearance of plaque represents healing, which occurs early after rupture and erosion events in pathologic studies.23 Healing thrombus at autopsy commonly has a smooth surface, similar to that observed on OCT in our cases with layered plaque.23 The close correspondence between the location of culprit lesions and the myocardial territory affected by regional injury or infarction in our study, as interpreted by 2 separate core laboratories while blinded to other imaging findings, provides strong evidence that these culprit lesions were the direct cause of the acute MI presentation in these women.
Although the largest plaques are more likely to be vulnerable and to rupture, disruption of smaller, nonobstructive plaques may also lead to coronary thrombosis and acute MI.24 Studies of thrombus aspiration for MI demonstrate that 31% of residual lesions show <50% stenosis by coronary angiography.25 When MI is caused by rupture or erosion of a previously nonobstructive plaque, the size of the superimposed thrombus may determine the angiographic severity of coronary stenosis at presentation. Persistent, large thrombi result in severe narrowing or total occlusion rather than MINOCA, whereas endogenous partial or complete thrombolysis may lead to a nonobstructive angiographic appearance. Smaller thrombi do not result in significant stenosis but may embolize to small terminal vessels or branch vessels, causing relatively small, well-circumscribed infarctions. We hypothesize that larger areas of regional injury or infarction in MINOCA may result from transient severe coronary stenosis or complete occlusion because of some combination of coronary artery spasm and thrombosis. Coronary spasm may occur independent of plaque rupture or erosion, but it is likely that at least some cases of MINOCA are multifactorial on the basis of the frequency of erosive thrombosis or disruption of nonlipidic plaque observed on OCT at spasm sites in cases of MI or out-of-hospital cardiac arrest.26
A small percentage of women with MINOCA in this study had an OCT appearance consistent with coronary spasm, described previously as intimal bumping or luminal irregularity. The OCT appearance in these cases was similar to characteristic patterns described at locations where coronary artery spasm was provoked in previous studies in which a wavy appearance of the intima suggested compression by a thickened arterial media.8,26 We did not perform provocative testing for coronary spasm because of time limitations in combination with 3-vessel OCT and concerns by our participating investigators about the potential risks of provocative testing in patients with acute MI. However, multiple groups have reported on provocative testing soon after MINOCA, with spasm provoked in approximately one quarter to two thirds of patients.20,27–29 The frequency of transient, OCT procedure-induced spasm in our study as reported during angiographic core laboratory review was higher than in previously published OCT series,30 which could suggest a reactive vasculature. It is possible that we observed relatively few cases of intimal bumping in this study because intracoronary nitroglycerin was administered before OCT imaging to reduce the risk of procedure-induced coronary spasm, and arterial vasodilators were administered for transradial cases.
Only 1 patient was found to have coronary dissection on OCT imaging. We excluded patients with angiographic evidence of coronary dissection from study participation. It appears that dissection is an infrequent cause of MINOCA on the basis of our and earlier OCT studies.19,20
An OCT culprit lesion was less likely when the site reported normal coronary angiography, but the likelihood of a culprit lesion in a vessel was not significantly correlated with the severity of nonobstructive stenosis according to the angiographic core laboratory. This is consistent with our previous finding that culprit lesions in women with MINOCA were not usually located at the worst narrowing in that vessel31 and suggests that targeting of OCT to specific vessels on the basis of angiographic features may not be useful. Clinicians should not dismiss patients with smaller increases in troponin, who are as likely as those with larger increases to have an OCT-defined culprit lesion.
CMR was abnormal in approximately 75% of women with MINOCA, most of whom had LGE or myocardial edema in a regional pattern, consistent with infarction or ischemic injury. The proportion of cases with myocarditis on CMR in our study (15% of those undergoing CMR) was lower than in a meta-analysis of CMR in patients with MINOCA (33%).32 This is most likely because of enrollment of patients who were referred for coronary angiography on the basis of a clinical diagnosis of MI, exclusion of patients with clinical myocarditis, the age of our cohort, and restriction to women, who are less likely to have myocarditis.32 Identification of myocarditis by CMR is critically important in patients with a working diagnosis of MINOCA, because patients with myocarditis require none of the usual post-MI secondary prevention medications after recovery. This is the basis for the recent class I recommendation by the European Society of Cardiology to perform CMR in “all MINOCA patients without an obvious underlying cause.”6 The proportion of patients with takotsubo syndrome in our study was lower than might be expected in a sample of women with MI, perhaps because patients with STEMI were rarely enrolled because of inadequate time to obtain research consent before angiography at sites that did not use OCT in such cases as a clinical routine.
Peak troponin levels were higher among women with abnormalities on CMR, perhaps because of limitations of CMR in detection of injury when the duration of ischemia was short or the territory was small. We were unable to determine a threshold below which the likelihood of CMR abnormalities was very low. This suggests that CMR should be considered in all patients with MINOCA.
Approximately half of the women who had CMR evidence of infarction or regional injury did not have an OCT culprit lesion identified. There are several potential reasons for this. We believe that most of these patients had type 2 MI, specifically, coronary spasm or thromboembolism as the mechanism of MI. Coronary spasm can often be provoked in patients with MINOCA and can lead to MI as confirmed by CMR.33 Those with smaller areas of infarction or regional ischemia on CMR may have had thromboembolism. Culprit lesions may have been present in more distal regions of the arteries or in branch vessels that were not imaged, and some participants did not have all 3 major arteries imaged. OCT was technically limited in some patients because of residual blood in the lumen despite intracoronary contrast administration or for other reasons. Alternatively, some patients with regional injury on CMR may have had regional myocarditis rather than ischemic injury.
Although most cases in which there was an OCT culprit lesion and abnormal CMR had myocardial regional injury or infarction localized to the same coronary territory, a small number of cases with an OCT culprit lesion had myocarditis on CMR; all were deemed to have myocarditis as the final diagnosis on consensus review. In a few more cases, the location of MI or regional injury did not correspond with the culprit lesion coronary artery; in these, we speculate that there may have been multiple culprit lesions, with the true culprit not imaged, perhaps because it was too distal or located in a branch vessel. Normal CMR in patients with an OCT culprit lesion may relate to imperfect sensitivity of CMR or a shorter duration of ischemic injury. Alternatively, cases with a lack of correlation between OCT and CMR findings could have resulted from overestimation of the prevalence of abnormalities because core laboratory review was blinded. However, blinding of OCT and CMR core laboratory readers to key clinical information and to the other imaging modality was important to avoid bias in interpretation toward agreement.
Our results provide support to the recent European Society of Cardiology recommendation to follow a diagnostic algorithm to identify the underlying cause of a MINOCA presentation to facilitate treatment according to the underlying cause or causes of MI.6 OCT and CMR provided complementary, independent information about mechanisms of MI in women with MINOCA in this study. The finding that 40% of women with normal CMR had an OCT culprit lesion illustrates the potential for OCT findings to change management of patients with MINOCA. However, clinical trials are needed to investigate the effects of specific therapies on outcomes.
Our study has several limitations. Although the largest of its kind to date, the sample size is relatively limited. Because of the need for research consent before angiography at sites where OCT was not performed per clinical routine, very few patients with STEMI presentation were included. Three-vessel OCT was not completed in all cases, which may have resulted in underestimation of the prevalence of culprit lesions. Some patients did not complete CMR, and some did not have both T1 mapping and T2-weighted imaging to maximize sensitivity for myocardial edema. Imaging was technically limited in some cases. We did not use high-resolution LGE imaging, which may be more sensitive. We considered myocardial edema on CMR in a single coronary territory to be evidence of ischemic injury, but we cannot exclude regional myocarditis in such cases. We did not perform provocative testing for spasm in these patients already planned for 3-vessel OCT. We may have underestimated the prevalence of intimal bumping on OCT because intracoronary nitroglycerin was given before OCT imaging. Not all sites reported all consecutive women with MI on the screening log. A control group was not included because of the potential risk of multivessel OCT. On the basis of the short duration of follow-up and limited sample size, we cannot assess the prognosis of each subtype of MI. We included only women and thus cannot comment on sex differences; this is the subject of our ongoing multicenter imaging study.
In conclusion, in this prospective study of women with MINOCA, multimodality imaging identified an underlying cause of MI in the large majority of patients. Nearly two-thirds had evidence of a vascular cause of the MI presentation, whether an OCT culprit lesion, infarction or regional injury on CMR, or both. An alternate diagnosis to MI was identified in 1 in 5 women with MINOCA on CMR, most commonly myocarditis. Our data suggest that mechanisms of MINOCA are often similar to mechanisms of MI with obstructive CAD, namely, atherothrombosis with possible contribution of coronary artery spasm, and have implications for secondary prevention in these patients.
Supplementary Material
Clinical Perspective.
What Is New?
In this study of 145 prospectively enrolled women with myocardial infarction with nonobstructive coronary arteries (MINOCA), a definite or possible culprit lesion was identified on optical coherence tomography in 67 (46.2%), and cardiac magnetic resonance imaging was abnormal in 86 (74.1%) of 116.
Among 116 women with MINOCA who had both optical coherence tomography and cardiac magnetic resonance imaging, 84.5% had an identifiable cause.
Most patients diagnosed with MINOCA had a final diagnosis of myocardial infarction (74, 63.8% overall).
Nearly two-thirds of our women with a diagnosis of MINOCA had evidence of a vascular mechanism of the myocardial infarction presentation, with an alternate diagnosis to myocardial infarction identified in 1 in 5.
What Are the Clinical Implications?
The mechanisms of MINOCA are similar to myocardial infarction with obstructive coronary artery disease, namely, atherothrombosis with possible contribution of coronary artery spasm.
The findings have implications for secondary prevention in women with MINOCA, which may be targeted to the most likely underlying causes as demonstrated in our study.
Acknowledgments
The authors thank the participants and site staff in the Women’s Heart Attack Research Program study, Abbott Vascular for donation of optical coherence tomography catheters for use in the study, and Siemens for donation of the cardiac magnetic resonance interpretive software for use at the New York University site.
Sources of Funding
The study was funded by American Heart Association Go Red for Women Strategically Focused Research Network grant 16SFRN27810006. The pilot phase enrollment was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR001445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Optical coherence tomography catheters were provided for use in the study by Abbott Vascular.
Footnotes
Disclosures
Dr Reynolds reports nonfinancial support from Abbott Vascular and Siemens related to the submitted work and nonfinancial support from BioTelemetry, outside the submitted work. Dr Maehara has received an institutional research grant from and is a consultant for Abbott Vascular and Boston Scientific. Dr Saw has received unrestricted research grant support (Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, National Institutes of Health, Michael Smith Foundation of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, Boston Scientific, and Abbott Vascular), consultancy and advisory board memberships (AstraZeneca, Abbott Vascular, Boston Scientific, Gore, Baylis, and FEops), and proctorship honoraria (Abbott Vascular and Boston Scientific). Dr Smilowitz reports consulting and advisory board membership for Abbott Vascular. Dr Mahmud reports for Abbott Vascular Clinical trial support as site principal investigator. Dr Wei reports being a consultant for an Abbott advisory board on coronary microvascular dysfunction (paid to institution). Mr. Matsumura reports being a consultant for Terumo. Dr Toma reports consulting for Phillips/Volcano. Dr Shah serves on the advisory board for Philips Volcano. Dr Attubato is a consultant for Medtronic, Boston Scientific, and Philips. Dr Bangalore is on an advisory board for Abbott Vascular, Biotronik, Pfizer, Amgen, and REATA and receives grant support from Abbott Vascular and REATA. Dr Ali has institutionally directed grant support from the National Institutes of Health/National Heart, Lung, and Blood Institute, Abbott, Boston Scientific, and Cardiovascular Systems Inc; receives speaker fees from AstraZeneca; is an advisor to Amgen and Boston Scientific; and holds equity in Shockwave Medical. Dr Merz reports honorarium and consulting (paid to N.B.M.) from iRhythm (board director) and Med Intelligence (Caladrius lecture) and also honorarium and consulting (paid to Barbra Streisand Women’s Heart Center) from Bayer Advisory Board (advisory board). Dr Hochman is the principal investigator for the ISCHEMIA trial (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) for which, in addition to support by National Heart, Lung, and Blood Institute grant, there were in-kind donations for participating sites from Abbott Vascular; Medtronic, Inc; St Jude Medical, Inc; Volcano Corporation; Arbor Pharmaceuticals; AstraZeneca Pharmaceuticals; Merck Sharp & Dohme; Omron Healthcare; and Amgen, as well as financial donations from Arbor Pharmaceuticals and AstraZeneca Pharmaceuticals. The other authors have nothing to disclose.
Supplemental Materials
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.119.045158.
Contributor Information
Harmony R. Reynolds, Sarah Ross Soter Center for Women’s Cardiovascular Research, New York University Grossman School of Medicine, NY; Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Akiko Maehara, Cardiovascular Research Foundation, New York, NY; Columbia University, New York, NY.
Raymond Y. Kwong, Brigham and Women’s Hospital, Boston, MA.
Tara Sedlak, Vancouver General Hospital, British Columbia, Canada.
Jacqueline Saw, Vancouver General Hospital, British Columbia, Canada.
Nathaniel R. Smilowitz, Sarah Ross Soter Center for Women’s Cardiovascular Research, New York University Grossman School of Medicine, NY; Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Ehtisham Mahmud, University of California San Diego Health, La Jolla.
Janet Wei, Barbra Streisand Women’s Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
Kevin Marzo, New York University Winthrop Hospital, New York University Long Island School of Medicine, Mineola.
Mitsuaki Matsumura, Cardiovascular Research Foundation, New York, NY.
Ayako Seno, Brigham and Women’s Hospital, Boston, MA.
Anais Hausvater, Sarah Ross Soter Center for Women’s Cardiovascular Research, New York University Grossman School of Medicine, NY; Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Caitlin Giesler, Ascension Medical Group, Austin, TX.
Nisha Jhalani, Columbia University, New York, NY.
Catalin Toma, University of Pittsburgh Department of Medicine, PA.
Bryan Har, University of Calgary, Alberta, Canada.
Dwithiya Thomas, St Luke’s University Healthcare, Bethlehem, PA.
Laxmi S. Mehta, Ohio State University Wexner Medical Center, Powell, OH.
Jeffrey Trost, Johns Hopkins Medical Center, Baltimore, MD.
Puja K. Mehta, Emory Women’s Heart Center, Atlanta, GA.
Bina Ahmed, Santa Barbara Cardiovascular Medical Group, CA.
Kevin R. Bainey, Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Canada.
Yuhe Xia, Department of Population Health, New York University Grossman School of Medicine, NY..
Binita Shah, Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Michael Attubato, Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Sripal Bangalore, Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Louai Razzouk, Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
Ziad A. Ali, Cardiovascular Research Foundation, New York, NY; Columbia University, New York, NY.
Noel Bairey Merz, Barbra Streisand Women’s Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
Ki Park, University of Florida, Gainesville.
Ellen Hada, Sarah Ross Soter Center for Women’s Cardiovascular Research, New York University Grossman School of Medicine, NY.
Hua Zhong, Department of Population Health, New York University Grossman School of Medicine, NY..
Judith S. Hochman, Sarah Ross Soter Center for Women’s Cardiovascular Research, New York University Grossman School of Medicine, NY; Leon H. Charney Division of Cardiology, Department of Medicine, New York University Grossman School of Medicine, NY..
REFERENCES
- 1.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, et al. ; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 2.Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjöld A, Gard A, Jernberg T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336 [DOI] [PubMed] [Google Scholar]
- 3.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J. 2011;161:681–688. doi: 10.1016/j.ahj.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 4.Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, Brass N, Armstrong PW, Kaul P. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): insights from the Alberta Contemporary Acute Coronary Syndrome Patients Invasive Treatment Strategies (COAPT) study. Int J Cardiol. 2018;264:12–17. doi: 10.1016/j.ijcard.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, Reynolds HR. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes. 2017;10:e003443. doi: 10.1161/CIRCOUTCOMES.116.003443 [DOI] [PubMed] [Google Scholar]
- 6.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020:ehaa575. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 7.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, et al. ; International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Shimada K, Tearney GJ, Kitabata H, Taguchi H, Fukuda S, Kashiwagi M, Kubo T, Takarada S, Hirata K, et al. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol. 2011;58:1608–1613. doi: 10.1016/j.jacc.2011.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- 10.Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by precontrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025 [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 13.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110 [DOI] [PubMed] [Google Scholar]
- 14.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992 [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Lee VS, Chung YC, Babb JS, Simonetti OP. Myocardial infarction: optimization of inversion times at delayed contrast-enhanced MR imaging. Radiology. 2004;233:921–926. doi: 10.1148/radiol.2333032004 [DOI] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Model-Building Strategies and Methods for Logistic Regression. (2000). In: Shewhart WA, Wilks SS, eds. Applied Logistic Regression, 2nd ed. Wiley Online Library; 2000:91–142. [Google Scholar]
- 17.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouldzein H, Elbaz M, Roncalli J, Cagnac R, Carrié D, Puel J, Alibelli-Chemarin MJ. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris). 2012;61:20–26. doi: 10.1016/j.ancard.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 19.Opolski MP, Spiewak M, Marczak M, Debski A, Knaapen P, Schumacher SP, Staruch AD, Grodecki K, Chmielak Z, Lazarczyk H, et al. Mechanisms of myocardial infarction in patients with nonobstructive coronary artery disease: results from the Optical Coherence Tomography Study. JACC Cardiovasc Imaging. 2019;12(11 pt 1):2210–2221. doi: 10.1016/j.jcmg.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 20.Gerbaud E, Arabucki F, Nivet H, Barbey C, Cetran L, Chassaing S, Seguy B, Lesimple A, Cochet H, Montaudon M, et al. OCT and CMR for the diagnosis of patients presenting with MINOCA and suspected epicardial causes. J AM COLL CARDIOL. Cardiovasc Imaging. 2020;13:2619–2631. doi: 10.1016/j.jcmg.2020.05.045 [DOI] [PubMed] [Google Scholar]
- 21.Fracassi F, Crea F, Sugiyama T, Yamamoto E, Uemura S, Vergallo R, Porto I, Lee H, Fujimoto J, Fuster V, et al. Healed culprit plaques in patients with acute coronary syndromes. J Am Coll Cardiol. 2019;73:2253–2263. doi: 10.1016/j.jacc.2018.10.093 [DOI] [PubMed] [Google Scholar]
- 22.Falk E Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis: characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J. 1983;50:127–134. doi: 10.1136/hrt.50.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–132. doi: 10.1016/j.jacc.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. ; PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 25.De Araújo Gonçalves P, Brito J, Sousa PJ, Carvalho MS, Dores H, Teles RC, Raposo L, Gabriel HM, Ferreira J, Almeida M, et al. Nonobstructive coronary disease leading to STEMI: assessment of residual stenosis after thrombus aspiration. Coron Artery Dis. 2013;24:154–159. doi: 10.1097/MCA.0b013e32835c46bd [DOI] [PubMed] [Google Scholar]
- 26.Shin ES, Ann SH, Singh GB, Lim KH, Yoon HJ, Hur SH, Her AY, Koo BK, Akasaka T. OCT-defined morphological characteristics of coronary artery spasm sites in vasospastic angina. JACC Cardiovasc Imaging. 2015;8:1059–1067. doi: 10.1016/j.jcmg.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 27.Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F. Patients with acute myocardial infarction and nonobstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. doi: 10.1093/eurheartj/ehx667 [DOI] [PubMed] [Google Scholar]
- 28.Pirozzolo G, Seitz A, Athanasiadis A, Bekeredjian R, Sechtem U, Ong P. Microvascular spasm in non-ST-segment elevation myocardial infarction without culprit lesion (MINOCA). Clin Res Cardiol. 2020;109:246–254. doi: 10.1007/s00392-019-01507-w [DOI] [PubMed] [Google Scholar]
- 29.Choo EH, Chang K, Lee KY, Lee D, Kim JG, Ahn Y, Kim YJ, Chae SC, Cho MC, Kim CJ, et al. ; KAMIR-NIH Investigators. Prognosis and predictors of mortality in patients suffering myocardial infarction with nonobstructive coronary arteries. J Am Heart Assoc. 2019;8:e011990. doi: 10.1161/JAHA.119.011990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Sijde JN, Karanasos A, van Ditzhuijzen NS, Okamura T, van Geuns RJ, Valgimigli M, Ligthart JM, Witberg KT, Wemelsfelder S, Fam JM, et al. Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. Eur Heart J Cardiovasc Imaging. 2017;18:467–474. doi: 10.1093/ehjci/jew037 [DOI] [PubMed] [Google Scholar]
- 31.Iqbal SN, Feit F, Mancini GB, Wood D, Patel R, Pena-Sing I, Attubato M, Yatskar L, Slater JN, Hochman JS, et al. Characteristics of plaque disruption by intravascular ultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am Heart J. 2014;167:715–722. doi: 10.1016/j.ahj.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 32.Hausvater A, Smilowitz NR, Li B, Redel-Traub G, Quien M, Qian Y, Zhong J, Nicholson JM, Camastra G, Biere L, et al. Myocarditis in relation to angiographic findings in patients with provisional diagnoses of MINOCA. JACC Cardiovasc Imaging. 2020;13:1906–1913. doi: 10.1016/j.jcmg.2020.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gue YX, Prasad S, Isenberg D, Gorog DA. A case of repetitive myocardial infarction with unobstructed coronaries due to Churg–Strauss syndrome. Eur Heart J Case Rep. 2019;3:ytz041. doi: 10.1093/ehjcr/ytz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usui E, Mintz GS, Lee T, Matsumura M, Zhang Y, Hada M, Yamaguchi M, Hoshino M, Kanaji Y, Sugiyama T, et al. Prognostic impact of healed coronary plaque in non-culprit lesions assessed by optical coherence tomography. Atherosclerosis. 2020;309:1–7. doi: 10.1016/j.atherosclerosis.2020.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


