Abstract
Background
The estimated likelihood of lower limb amputation is 10 to 30 times higher amongst people with diabetes compared to those without diabetes. Of all non‐traumatic amputations in people with diabetes, 85% are preceded by a foot ulcer. Foot ulceration associated with diabetes (diabetic foot ulcers) is caused by the interplay of several factors, most notably diabetic peripheral neuropathy (DPN), peripheral arterial disease (PAD) and changes in foot structure. These factors have been linked to chronic hyperglycaemia (high levels of glucose in the blood) and the altered metabolic state of diabetes. Control of hyperglycaemia may be important in the healing of ulcers.
Objectives
To assess the effects of intensive glycaemic control compared to conventional control on the outcome of foot ulcers in people with type 1 and type 2 diabetes.
Search methods
In December 2015 we searched: The Cochrane Wounds Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; EBSCO CINAHL; Elsevier SCOPUS; ISI Web of Knowledge Web of Science; BioMed Central and LILACS. We also searched clinical trial databases, pharmaceutical trial databases and current international and national clinical guidelines on diabetes foot management for relevant published, non‐published, ongoing and terminated clinical trials. There were no restrictions based on language or date of publication or study setting.
Selection criteria
Published, unpublished and ongoing randomised controlled trials (RCTs) were considered for inclusion where they investigated the effects of intensive glycaemic control on the outcome of active foot ulcers in people with diabetes. Non randomised and quasi‐randomised trials were excluded. In order to be included the trial had to have: 1) attempted to maintain or control blood glucose levels and measured changes in markers of glycaemic control (HbA1c or fasting, random, mean, home capillary or urine glucose), and 2) documented the effect of these interventions on active foot ulcer outcomes. Glycaemic interventions included subcutaneous insulin administration, continuous insulin infusion, oral anti‐diabetes agents, lifestyle interventions or a combination of these interventions. The definition of the interventional (intensive) group was that it should have a lower glycaemic target than the comparison (conventional) group.
Data collection and analysis
All review authors independently evaluated the papers identified by the search strategy against the inclusion criteria. Two review authors then independently reviewed all potential full‐text articles and trials registry results for inclusion.
Main results
We only identified one trial that met the inclusion criteria but this trial did not have any results so we could not perform the planned subgroup and sensitivity analyses in the absence of data. Two ongoing trials were identified which may provide data for analyses in a later version of this review. The completion date of these trials is currently unknown.
Authors' conclusions
The current review failed to find any completed randomised clinical trials with results. Therefore we are unable to conclude whether intensive glycaemic control when compared to conventional glycaemic control has a positive or detrimental effect on the treatment of foot ulcers in people with diabetes. Previous evidence has however highlighted a reduction in risk of limb amputation (from various causes) in people with type 2 diabetes with intensive glycaemic control. Whether this applies to people with foot ulcers in particular is unknown. The exact role that intensive glycaemic control has in treating foot ulcers in multidisciplinary care (alongside other interventions targeted at treating foot ulcers) requires further investigation.
Keywords: Humans; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 1/complications; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Diabetic Foot; Diabetic Foot/etiology; Diabetic Foot/therapy; Hyperglycemia; Hyperglycemia/complications; Hyperglycemia/therapy
Plain language summary
Controlling blood glucose in treating diabetic foot ulcers (sores)
Background: People with diabetes can develop foot ulcers (sores) for a number of reasons. This includes nerve damage and reduced blood flow to the feet and legs. Having high blood glucose may affect the ability of foot ulcers to heal and therefore intensively controlling blood glucose may be beneficial.
Review question: This Cochrane review aimed to answer the question; how does controlling blood glucose more intensively compared to conventional blood glucose control influence foot ulcer healing in people with diabetes?
What we found: We did not find any trials which have been completed on this topic with available results. The only trial which met our criteria for inclusion had been terminated due to encountering difficulties with recruiting participants. Therefore we cannot be sure whether controlling blood glucose intensively when people have diabetic foot ulcers is beneficial or harmful. The lack of evidence however should not deter efforts to achieve optimal glycaemic control in people with diabetic foot ulcers to encourage healing as is current practice. We believe there are currently two trials underway which may provide some evidence on this topic once completed.
This Plain Language Summary is up to date as of 7 December 2015.
Background
Description of the condition
In 2011, 366 million people worldwide (8.3% of adults) were estimated to have diabetes mellitus (IDF 2012). It is expected that this figure will reach 552 million (10% of adults) by 2030 (IDF 2012). Diabetes mellitus is a metabolic disorder characterised by dysregulation of blood glucose levels. Type 1 diabetes (previously known as insulin‐dependent, juvenile or childhood‐onset) is characterised by deficient insulin production and requires daily administration of insulin (IDF 2012). The cause of type 1 diabetes is not known and it is currently not preventable (IDF 2012). Type 2 diabetes (formerly known as non‐insulin‐dependent or adult‐onset) results from the body’s ineffective use of insulin. Ninety per cent of people with diabetes, worldwide, have type 2 diabetes (IDF 2012). One of the major complications of diabetes is diabetic foot ulceration (Boulton 2004). A diabetic foot ulcer (an ulcer which occurs due to diabetes) has been defined as either a full‐thickness wound below the ankle in people with diabetes, irrespective of duration (Apelqvist 1999), or a lesion of the foot penetrating through the dermis (Schaper 2004). The prevalence of foot ulceration in people diagnosed with diabetes is 4% to 10%; the annual population incidence is 1% to 4%, and the lifetime incidence is as high as 25% (Singh 2005). In a recent multi‐centre study, poor glycaemic (blood glucose) control was evident in nearly half of the participants who had foot ulcers, with 49% having an HbA1c (glycaemic measure) level above 8.4% (Schaper 2012).
Foot ulceration is caused by the interplay of several factors, most notably diabetic peripheral neuropathy (DPN, i.e. loss of sensation to the foot), peripheral arterial disease (PAD, i.e. lack of blood‐flow) and changes in foot structure (Clayton 2009; Shenoy 2012). These factors have been linked to chronic hyperglycaemia and the altered metabolic state associated with diabetes (Ikem 2010; Ogbera 2008; Tesfaye 2012).The prevalence of DPN ranges from 16% to 66% in people with diabetes (Cook 2012). The prevalence rates of PAD are as high as 50% in people with diabetic foot ulcers (Hinchliffe 2012). What is most notable is that within one year of an ulcer healing, up to 60% of patients will develop another foot ulcer (Wu 2007), and often the end point is lower limb amputation.
It is currently estimated that there is an amputation every 30 seconds, somewhere in the world that is due to diabetes (Game 2012). The estimated likelihood of amputation is 10 to 30 times higher amongst people with diabetes compared to those without diabetes and 85% of all amputations in people with diabetes are preceded by a foot ulcer (Boulton 2004; Singh 2005). The five‐year mortality rate after the onset of a foot ulcer ranges from 43% to 55%, and is up to 74% for patients with lower limb amputation (Robbins 2008).
Description of the intervention
Chronic hyperglycaemia appears to be one of the most important factors in the development and healing of diabetic foot ulcers (Christman 2011; Falanga 2005). Current guidelines recommend that treatment of diabetic foot ulcers should involve a multidisciplinary team, as well as utilising several interventions (Table 1). This review was performed to clarify the effect of intensive glycaemic control on the healing of foot ulcers in people with diabetes.
1. Diabetic foot management guidelines and levels of evidence.
| Guideline and management recommendations |
Level of evidence (According to Oxford Centre for Evidence‐based Medicine ‐ Levels of Evidence (March 2009)) |
Glycaemic target |
National Health and Medical Research Council (NHMRC): Prevention, identification and management of foot complications in diabetes mellitus 2011
Note: as per NHMRC levels of evidence |
Expert opinion Grade B Grade B Grade B Expert opinion Grade C Grade B Grade B Grade C Grade B Grade D |
Not reported |
National Clearinghouse Guidelines 2011
|
Not reported | HbA1c < 7% Level B |
|
National Clearinghouse guidelines 2012 (treatment of neuropathic wounds)
|
Grade C |
|
National Health Service (NHS): Type 2 diabetes: prevention and management of foot problems 2004
|
Grade D Grade D Grade D Grade D Grade C Grade D Grade D Grade B Grade B Grade D Grade B |
Not reported |
National Health Service (NHS): 2011 National Institute for Health and Care Excellence (NICE) clinical guideline. Developed by the Centre for Clinical Practice at NICE: Diabetic foot problems: inpatient management of diabetic foot problems
|
Not reported |
Not reported |
2012 International Working Group on Diabetic Foot (IWGDF): Global guideline for type 2 diabetes
|
Not reported |
< 8 mmol/l |
Australian diabetic foot Network: Management of diabetes related foot ulceration ‐ a clinical update
|
Not reported |
Not reported |
American College of Foot and Ankle surgeons 2006 (revision): Diabetic foot disorders – a clinical practice guideline
|
Not reported |
Not reported |
Scottish Intercollegiate Guidelines Network (SIGN) Guidelines 2010
|
Grade C Grade B Grade B Grade B Grade B |
Not reported |
American Diabetes Association Standards of Medical Care in Diabetes 2012
|
Grade B Not reported |
As per position Statement for optimal Control |
The management of diabetes includes glycaemic control (Table 2) (Daroux 2010; Geraldes 2010; Giacco 2010; Inzucchi 2012). A common list of glycaemic control medications used in diabetes management is shown in Table 3. Most guidelines recommend a glycaemic control target of 7% or lower for HbA1c (glycated haemoglobin) (Table 2). The revised guidelines of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend individualisation, with more stringent (6.5% or lower) or less stringent (8% or lower) HbA1c targets as appropriate for individuals (ADA 2012; Cheung 2009; Inzucchi 2012). There is marked variation in the definition of intensive glycaemic control between guidelines and trials (Hemmingsen 2011a). For the purposes of this review we included trials where an intervention has been performed with the aim of achieving improved glycaemic control in comparison to a conventional control group.
2. HbA1c targets recommended by different international guidelines ª.
| Country | Guideline | Year | Hba1c targets in adults |
Level of Evidence (According to Oxford Centre for Evidence‐based Medicine ‐ Levels of Evidence (March 2009)) |
| Australia | National Health and Medical Research Council/Diabetes Australia | 2009 |
≤ 7% | Grade A |
| Australian Paediatric Endocrine Group/ Australian Diabetes Society | 2011 | ≤ 7% | Grade D | |
| UK | National Institute for Health and Care Excellence (NICE) ‐ Managing type 1 DM diabetes in adults ‐ Blood glucose lowering therapy for type 2 DM |
2012 2012 |
≤ 7.5% if increased arterial risk ≤ 6.5% Between 6.5% and 7.5% |
Grade B Not reported Not reported |
| Scottish Intercollegiate Guidelines Network (SIGN) ‐ Type 1 Diabetes ‐ Type 2 Diabetes |
2010 | No set figure < 7% |
Not reported Grade A |
|
|
USA |
National Clearinghouse |
2012 | < 7% or individualize to a goal of < 8% | Grade B |
| American Diabetes Association |
2012 | ≤ 7% or individualise to a goal: < 6.5% < 8% |
Grade B Grade C Grade B |
|
| American Association of Clinical Endocrinologists | 2011 | ≤ 6.5% | Grade D | |
| International Diabetes Federation (IDF) | International Diabetes Federation ‐ Global Guideline for type 2 Diabetes | 2012 | < 7.0% | U/K |
| Canada | Canadian Diabetes Association |
2008 | ≤ 7% ≤ 6.5% (may be considered to lower risk of nephropathy further) |
Grade C, Level 3 Grade A, Level 1A |
| Europe | European Association for the Study of Diabetes (EASD) and American Diabetes Association (ADA) | 2012 | < 7% or individualise to a goal of: 6% to 6.5% (patients with short disease, duration, long life expectancy, no significant CVD) 7.5% to8.0% (history of severe hypoglycaemia, limited life expectancy, advanced complications, extensive comorbid conditions and those in whom the target is difficult to attain) |
Not reported |
| New Zealand | New Zealand Group Guidelines | 2003 | ≤ 7% | Grade D |
ª Adapted from Australian Electronic Therapeutic Guidelines (Electronic Therapeutic Guidelines Australia 2012)
Abbreviations
CVD = cerebrovascular disease DM = diabetes mellitus U/K = unknown
3. Commonly used medications in diabetes mellitus (type 1 and type 2) for the management of hyperglycaemia.
| Class/Drug | Expected decrease in HbA1c |
| ORAL ANTIDIABETIC THERAPY | |
| Metformin | 1% to 2% |
Sulfonylureas
|
1% to 2% |
DPP‐4‐inhibitors
|
0.5% to 0.8% |
| Acarbose | 0.5% to 0.8% |
Thiazolidinedione (glitazones)
|
0.5% to 1.4% |
| PARENTERAL THERAPY | |
GLP‐analogues
|
0.5% to 1.0% |
| Insulin | 1.5% to 3.5% |
| Insulin | Generic name |
| Very‐short‐acting (rapid) | Aspart Glulisine Lispro |
| Short‐acting | Neutral |
| Intermediate‐acting | Isophane (protamine suspension) |
| Long‐acting | Determir Glargine |
| Biphasic | Neutral/isophane Lispro/lispro protamine Aspart/aspart protamine |
| Methods of insulin delivery | |
| |
Most of the current glycaemic targets for diabetes are based on several landmark trials that investigated the effects of intensive glycaemic control compared to conventional treatments (Table 2) (Cheung 2009; Hemmingsen 2011b; Macisaac 2011; Mazzone 2010). The findings from these studies also illustrate the benefits and risks associated with intensive glycaemic control. Therefore, when investigating intensive glycaemic control as a potential intervention for diabetic foot ulcers, it is important to take into account the present literature underpinning current glycaemic management.
Intensive glycaemic control implemented in the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) led to a reduction in the progression and development of microvascular (small vessel) complications including DPN (Mattila 2010). The UKPDS demonstrated a 37% reduction in the risk of microvascular complications for each 1% decrease in HbA1c (95% confidence interval: 33% to 41%) (UKPDS 1998; Stratton 2000). Similarly, the ADVANCE trial found a 14% relative risk reduction for major microvascular events in the intensive control group when compared to the standard control group (9.4% versus 10.9%; hazard ratio (HR) 0.86; 95% CI: 0.77 to 0.97), although mainly in terms of reduced incidence of nephropathy (kidney disease) (ADVANCE 2008).
A recent Cochrane review concluded that intensive glucose control reduced the risk of amputation by 36% in type 2 diabetes (relative risk (RR) 0.64, 95% CI: 0.43 to 0.95; 6960 participants in eight trials) (Hemmingsen 2011b). In addition there was an 11% relative risk reduction (RR 0.89, 95% CI: 0.83 to 0.95; 25,760 participants in four trials) and a 1% to 2% absolute risk reduction in composite microvascular outcomes in favour of intensive glycaemic control for all included trials (Hemmingsen 2011b). A number of meta‐analyses have demonstrated that the incidence of hypoglycaemia (low blood glucose) was increased during intensive glycaemic control, making this a significant adverse outcome (Hemmingsen 2011b; Ma 2009; Mattila 2010). It must be noted that the beneficial effects on microvascular complications from using intensive glycaemic control took more than five years to emerge, and the benefits were less pronounced for people with advanced type 2 diabetes compared to those with new‐onset type 2 diabetes (Hemmingsen 2011b; Mattila 2010). Despite this, data on retinopathy (disease of the retina) suggest that people with the advanced stages of type 2 diabetes may also benefit from intensive glycaemic control (Hemmingsen 2011a). The effects of intensive glycaemic control in people with type 1 diabetes demonstrated in the DCCT were still evident after 14 years of follow‐up (i.e. long after the intervention was completed), and that phenomenon had been termed 'glycaemic memory' (Giacco 2010). More recent data suggested that glycaemic memory also occurred in people with type 2 diabetes, where it is termed the 'legacy effect', whereby the benefits of earlier interventions were still evident later on in the disease course (Giacco 2010).
While intensive therapy, with the goal of achieving near normal HbA1c levels (7%), has altered the clinical course of nephropathy, neuropathy and retinopathy, the majority of studies have not examined the benefits of intensive therapy when implemented after the onset of late diabetes complications, such as diabetic foot ulcers (Nathan 2012).
How the intervention might work
Optimum healing of a foot ulcer requires a well‐orchestrated integration of molecular and biological events including cell migration, proliferation, extracellular matrix deposition and remodelling, which is hindered by the effects of hyperglycaemia (Falanga 2005; Rafehi 2010). Hyperglycaemia has been associated with delayed healing of foot ulcers (Burakowska 2006; Christman 2011; D'Souza 2009; Falanga 2005; Rafehi 2010). Interventions that target improvements in glycaemic control are thus of potential benefit. Delayed healing of foot ulcers appears to be the net result of both microvascular and macrovascular disease (Burakowska 2006; Dinh 2005). Well‐orchestrated wound healing is essential for tissue replacement and restoration, and generally involves three main phases: acute inflammation, proliferation, and remodelling (Rafehi 2010). In contrast, diabetic foot ulcers do not follow the orderly process of wound healing and differ at a molecular level in terms of expression of growth factors, cytokines and proteins (Dinh 2005; Rafehi 2010). These processes are known to be affected by hyperglycaemia.
Several proposed pathogenic pathways exist to explain the adverse effects of hyperglycaemia (Geraldes 2010). These include: 1) activation of the polyol pathway; 2) non‐enzymatic glycosylation and formation of advanced glycation end products (AGEs); 3) activation of the diacylglycerol‐ (DAG) protein kinase C pathway; and 4) overactivity of the hexosamine pathway (Brownlee 2004; Geraldes 2010; Giacco 2010; Gupta 2010). All four mechanisms have been linked to a single, unified preceding event, namely mitochondrial overproduction of reactive oxygen species (ROS) (Brownlee 2004). ROS are known to promote cellular dysfunction through damage to DNA synthesis, oxidation of lipids and amino acids and inactivation of key enzymes involved in metabolic function, which are implicated in the formation of diabetic foot ulcers. Hyperglycaemia also promotes endothelial dysfunction, vascular leakage and impaired angiogenesis (formation of new blood vessels) originating from the above mentioned pathways, and leads to activation of the inflammatory response via activation of nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) (D'Souza 2009; Giacco 2010). The incidence of infection is also increased in people with diabetes and immunological disturbances, such as deficiencies in polymorphonuclear leukocyte, monocyte and macrophage (types of white blood cell) function have been noted during hyperglycaemia (Delamaire 1997; Stegenga 2008). All these factors, which are a consequence of hyperglycaemia, may play a role in delayed healing of foot ulcers.
A recent observational study showed that HbA1c was an important clinical predictor of the rate of wound healing; with each 1% increase in HbA1c level associated with a decrease in the wound healing rate of 0.028 cm² per day (95% CI: 0.003 to 0.054) (Christman 2011). Despite this, the effects of short‐term reduction in HbA1c did not appear to have any effect on endothelial function in patients with type 2 diabetes with a history of poor glycaemic control (Bagg 2001). Therefore, there remains a clear need to document benefits associated with improved glycaemic control in people with diabetic foot ulcers. While chronic complications of diabetes such as DPN and PAD maybe difficult to reverse, it can be postulated that aspects of ulcer healing relating to immunological and connective tissue function may be more amenable to improvement if normoglycaemia (normal level of glucose in blood) is achieved (Jeffcoate 2004).
Why it is important to do this review
Foot ulcers continue to be a significant burden for people with diabetes, their caregivers and the healthcare system (Schaper 2012). The outcome of a foot ulcer in people with diabetes should not only be viewed from a clinical perspective (e.g. healing or amputation), but also from an individual and socioeconomic perspective. Health‐related quality of life (HRQoL) is significantly reduced in people with diabetes, and further impaired by the presence of foot disease, whilst it is improved with foot ulcer healing (Hogg 2012). Healthcare costs associated with foot ulcers and amputations contribute significantly to the financial burden of diabetes (Jones 2008). In the United States in 2008, the total number of discharges attributed to diabetes‐related amputations was 45,000. The average length of stay was 10.1 days and the in‐hospital mortality proportion was 1.29% (Cook 2012). The mean hospital charges were USD 56,216 per patient and the estimated aggregate cost for the year 2008 was USD 2,548,319,965 (Cook 2012).
Therefore, foot ulceration in people with diabetes has substantial socioeconomic, quality‐of‐life, and healthcare implications, and it is imperative that all efforts be made to prevent and treat the burden of foot ulceration in order to reduce amputation rates ‐ as highlighted by the St Vincent Declaration in 1989 (Game 2012). Advances in the treatment of diabetic foot ulcers are promising, however the intrinsic pathophysiological abnormalities of hyperglycaemia that lead to ulceration and delayed ulcer healing cannot be ignored (Falanga 2005).
Objectives
To assess the effects of intensive glycaemic control compared with conventional control on the outcome of foot ulcers in people with type 1 and type 2 diabetes.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) for inclusion where they investigated the effects of intensive glycaemic control on the outcome of active foot ulcers in people with diabetes. We excluded non‐randomised and quasi‐experimental trials.
Types of participants
Men and women (over 18 years) diagnosed with type 1 or type 2 diabetes by clearly‐defined, accepted standards relevant to the time of the study, with an active foot ulcer that had any of the following aetiologies (causes):
neuropathic, or
neuro‐ischaemic, or
ischaemic, with or without
infection (as clinically or diagnostically documented by laboratory analysis).
For the purposes of this review, venous ulcers, malignant ulcers and post‐surgical ulcers were excluded.
Types of interventions
We planned to include trials that had assessed any intervention that aimed to achieve a lower glycaemic target in a diabetes group (i.e. near normal glycaemic levels) when compared to a control group with a higher glycaemic target. The latter group was defined as the 'conventional' group. Therefore the intensive group would have had a lower glycaemic target level compared to the conventional group in the trial.
Therefore, we sought to include any intervention that had:
attempted to maintain or control blood glucose levels and measured changes in markers of glycaemic control (HbA1c or fasting, random, mean plasma glucose, home capillary or urine glucose), and
documented the effect of these interventions on active foot ulcer outcomes.
Allowable interventions included subcutaneous insulin administration, continuous subcutaneous insulin infusion using an insulin pump or oral anti‐diabetes agents or a combination of both, or any lifestyle interventions or both (Table 4). The overall definition of the interventional (intensive) group was that it should have a lower glycaemic target than the comparison (conventional) group.
4. Alternative treatments for lowering blood glucose in people with diabetic foot ulcers.
| Nature of intervention | ||
| Exercise | Psychological and behavioural | Dietary |
| Any exercise intervention that has the primary aim of improving glycaemic control in people with diabetes, where the impact of the intervention on glycaemic control and changes in an active foot ulcer has been documented | Any psychological or behavioural intervention that has the primary aim of improving glycaemic control in people with diabetes, where the impact of the intervention on glycaemic control and the resultant changes in a foot ulcer has been documented | Any dietary or nutritional intervention that has the primary aim of improving glycaemic control in people with diabetes, where the changes in glycaemic control have been correlated with changes in active foot ulcer outcome |
| Examples | ||
| Exercise programmes of any intensity and duration that had the primary aim of improvement in glycaemic control | Frequent checking of blood glucose levels, interventions aimed at good pharmaceutical practice (i.e. improving compliance with medication) | Healthy eating programmes, dietary or nutritional supplements |
Pharmaceutical treatments included any route of administration, dose, duration or frequency of insulin or other pharmaceutical agents, or both.
Types of outcome measures
Primary outcomes
Number of ulcers healed.
Time to complete ulcer healing.
Change in ulcer severity reported as a change in an ulcer grading score using a well‐defined validated ulcer grading scale e.g. University of Texas Wound Classification System (UTWCS) that measures the depth, presence of infection and ischemia of an ulcer (Armstrong 1998).
Incidence of amputation related to foot ulcers (identified on International Classification of Disease (ICD) codes (NCCH 2006).
Secondary outcomes
New ulcer development (recurrence of an ulcer or initiation of a new ulcer).
Proportion of infected ulcers at study completion.
Adverse events: adverse events were to be noted from each individual trial, and, where trial reports were based on a sound methodology with standardised approach to detect and assess adverse events, these were to be included in any potential analysis and judged on a case‐by‐case basis. Treatment‐focused examples included: adverse drug reaction requiring hospitalisation; weight gain; and hypoglycaemia. Disease‐focused examples included: worsening of neuropathy (clinically or using a validated neuropathy score); development or worsening of PAD (clinically or by diagnostic measurement such as ankle brachial index (ABI); gangrene; congestive heart failure; chronic kidney disease (CKD) (stages 1‐5); dialysis; retinopathy and documented diabetic ketoacidosis (DKA); hyperosmolar hyperglycaemic state, hyperglycaemia; and lactic acidosis.
Effect on HRQoL: As measured by a validated quality of life (QOL) measurement tool that is disease‐specific to foot ulcers or generic to QOL or both.
Cost of intervention compared to conventional treatment, including: direct medical costs; direct non‐medical costs (e.g. transport, assistive devices); indirect costs (e.g. sick leave, reduced productivity, early retirement and premature death); disability‐adjusted life years (DALY); and years of life lost (YLL).
All‐cause mortality.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant randomised clinical trials:
The Cochrane Wounds Specialised Register; (searched 7 December 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 11);
Ovid MEDLINE (1946 to 7 December 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 7 December 2015);
Ovid EMBASE (1974 to 7 December 2015);
EBSCO CINAHL Plus (1937 to 7 December 2015);
Elsevier SCOPUS (1960 to 13 December 2015);
ISI Web of Knowledge Web of Science (1965 to 13 December 2015);
BioMed Central (1997 to 13 December 2015);
LILACS (1995 to 13 December 2015).
We used the following search strategy in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Blood Glucose] explode all trees #2 MeSH descriptor: [Hypoglycemic Agents] explode all trees #3 MeSH descriptor: [Hyperglycemia] explode all trees #4 MeSH descriptor: [Hypoglycemia] explode all trees #5 MeSH descriptor: [Insulin] explode all trees #6 MeSH descriptor: [Metformin] explode all trees #7 MeSH descriptor: [Thiazolidinediones] explode all trees #8 MeSH descriptor: [alpha‐Glucosidases] explode all trees #9 MeSH descriptor: [Glucagon‐Like Peptide 1] explode all trees #10 MeSH descriptor: [Acarbose] explode all trees #11 (blood glucose):ti,ab,kw #12 (((glycaemic or glycemic) next control) or "intensive glucose control"):ti,ab,kw #13 ((hypoglycaemi* or hypoglycemi*) next (agent* or drug*)):ti,ab,kw #14 (oral next (hypoglycaemi* or hypoglycemi*)):ti,ab,kw #15 ("fasting glucose" or "glucose target"):ti,ab,kw #16 ((anti‐diabetes next medication*) or (diabetes next medication*) or insulin* or sulphonyureas or metformin or thiazolidinedione* or DPP‐4 inhibitor* or glitinide or (glucosidase next inhibitor*) or biguinide or "GLP‐1 agonist" or acarbose or (incretin next enhancer*) or (incretin next mimetic*) or HbA1c):ti,ab,kw #17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #13 or #14 or #15 or #16 #18 MeSH descriptor: [Foot Ulcer] explode all trees #19 MeSH descriptor: [Diabetic Foot] explode all trees #20 (diabet* near/3 ulcer*):ti,ab,kw #21 (diabet* near/3 (foot or feet)):ti,ab,kw #22 (diabet* near/3 wound*):ti,ab,kw #23 (diabet* near/3 defect*):ti,ab,kw #24 ("foot gangrene" or amputat*):ti,ab,kw #25 #18 or #19 or #20 or #21 or #22 or #23 or #24 #26 #17 and #25
The search strategies were adapted for Ovid MEDLINE, Ovid EMBASE, EBSCO CINAHL and can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2012). We did not restrict studies with respect to language, date of publication or study setting.
Searches of the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE, EMBASE and CINAHL were carried out at Cochrane Wounds editorial base. We modified the original search strategy shown above to search the SCOPUS, Biomed Central, Web of Science and LILACS databases.
We also searched the following ongoing trial databases for relevant published, non‐published, ongoing and terminated clinical trials:
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/index.html) (last searched 7 December 2015);
ClinicalTrials.gov (http://www.clinicaltrials.gov/); (last searched 7 December 2015);
WHO International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/) (last searched 7 December 2015);
Current Controlled Trials (http://www.controlled‐trials.com) (last searched 7 December 2015).
We searched the pharmaceutical trials databases listed below (known pharmaceutical companies involved in manufacturer of diabetes medication) for relevant published, non‐published, ongoing and terminated clinical trials:
AstraZeneca Clinical Trials web site (www.astrazenecaclinicaltrials.com) (last searched 7 December 2015);
Eli Lilly and Company Clinical Trial Registry (www.lillytrials.com) (last searched 7 December 2015);
Novartis (https://www.novartisclinicaltrials.com/TrialConnectWeb/home.nov) (last searched 7 December 2015);
Novo Nordisk (http://www.novonordisk‐trials.com/WebSite/Search/Default.aspx) (last searched 7 December 2015);
MSD (http://www.msd‐australia.com.au/research/discoveryanddevelopment/pages/clinicaldevelopment/l) (last searched 7 December 2015);
Servier (http://www.servier.co.uk/content/clinical‐trials) (last searched 7 December 2015).
For completeness, we searched through any clinical guidelines produced by the Joanna Briggs Institute, the National Institute for Health and Care Excellence (NICE), the National Health Service (NHS), the National Health and Medical Research Council (NHMRC) Australia, the Scottish Intercollegiate Guidelines Network (SIGN), National Clearinghouse and the International Working Group on the Diabetic Foot for any studies or publications of relevance that had not been identified through other search options.
Where translation(s) were required, we contacted the original trial authors first to acquire an English language version of the manuscript. If the authors were not able to provide an English version, or where we did not receive any correspondence back from the authors, the articles were translated to English using translation services from our institution and then the authors were contacted again to clarify our understanding of the study. If the authors were unable to clarify the data, we planned to still attempt to use the trial.
Searching other resources
We checked the reference lists of all full‐text articles considered for inclusion for any further studies of relevance.We contacted key local and international pharmaceutical groups regarding any unpublished trials. We also contacted several leading academics, clinicians and researchers in the area of diabetes management for information about any prospective or past studies not identified by the literature searches.
Data collection and analysis
Selection of studies
All review authors participated in the title and abstract screening of the search results. Two review authors (MF and RS) retrieved and assessed articles for inclusion independently using the following selection criteria. Included studies needed to:
investigate changes in the glycaemic state of participants with type 1 or type 2 diabetes via changes in markers of glycaemic control (HbA1c or fasting, random, mean plasma glucose, home capillary or urine glucose), and
report foot ulcer outcomes.
We obtained full‐text publications of all articles meeting these selection criteria, or abstracts where these criteria could not be determined, and excluded any articles that we deemed not to be suitable (exclusion after screening of full text). Three third parties (JG, KS, YT) resolved any differences in opinion regarding whether to include or exclude a study. If no resolution was achieved, we contacted the original authors of the study for further clarification. We held team meetings when there was a need to make a final decision on inclusion. The PRISMA flow diagram in Figure 1 shows the study selection process (Moher 2009), and the Excluded studies table shows the reasons for exclusion for all the excluded trials. All citations were managed using Endnote version 5.1 (Endnote 2012).
1.
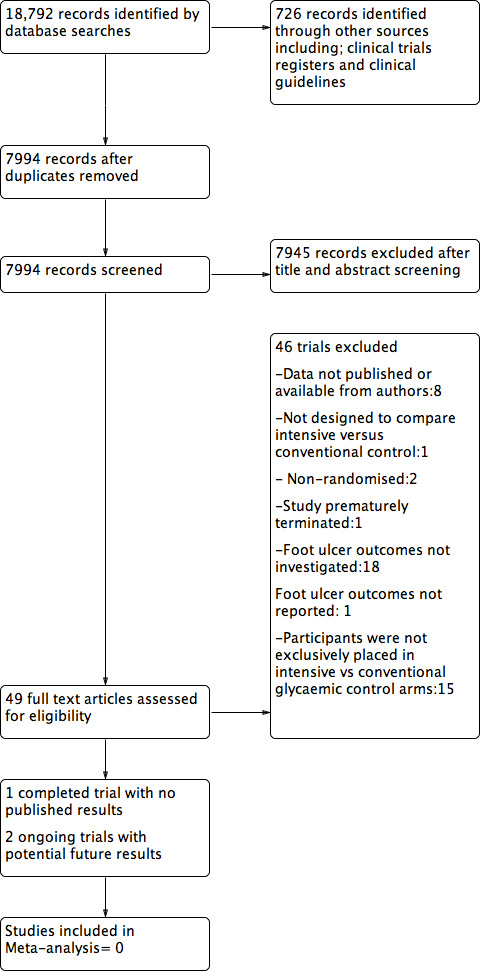
Flow diagram.
Data extraction and management
Two review authors (MF and RS) would have independently extracted data, with a third and fourth review author (MC and PB) resolving any disagreements. We planned to enter data into a structured electronic data format, using the Cochrane Wounds extraction form to collect and organise data.This included information concerning:
general information about the study (i.e. location, setting, aims);
study eligibility;
characteristics of study methods;
participants;
intervention groups;
outcomes;
'risk of bias' assessment;
subgroup analyses
The data to be extracted also included information on participant characteristics, study design, interventions utilised, outcomes assessed, and adverse events. MF and RS independently extracted all ongoing trial information and reported and compared it in appropriate Tables.
Dealing with duplicate publications
When more than one publication was found for a study, we evaluated all publications together to extract the maximum amount of relevant information. We resolved any discrepancies between the studies by contacting the study authors. If there were repeated observations of the same participants, we planned to use the longest follow‐up period for defining outcome measures of the study.
Assessment of risk of bias in included studies
We planned to assess risk of bias using the guidelines provided in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011a). We would have rated risk of bias as low, high or unclear in nature (Higgins 2011a), and planned to include a 'risk of bias' graph and 'risk of bias' summary. Two review authors (MF and RS) were to assess each study independently looking at he following criteria:
sequence generation (confounding);
allocation concealment (information bias): we planned to summarise how allocation sequences were generated and to report any attempts to conceal allocation of assigned intervention, along with any judgements concerning the risk of bias that may have arisen from the methods used;
blinding for participants, personnel and outcome assessment (performance and detection bias): a brief summary of who was blinded or masked during the conduct and analysis of the studies;
incomplete outcome data (attrition or selection bias): review authors' concerns over exclusion of participants and excessive (or differential) drop‐out rates;
selective reporting (reporting bias): we would have summarised any concerns over the selective availability of data, including evidence of selective reporting of outcomes, time‐points, subgroups or analyses;
other bias(es) identified.
We planned to present a 'risk of bias' summary figure, which represents all bias assessment points in a table format.
Measures of treatment effect
For dichotomous data, we planned to present results as summary risk ratios (RR) with 95% confidence intervals (CI). For continuous data, when outcomes were measured the same way in different trials, we planned to use the mean difference (MD). We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods of measurement (SMD). Time to complete wound healing was time‐to‐event data. It was proposed that the most appropriate way of summarising it was to use methods of survival analysis and to express the intervention effect as a hazard ratio. It is not appropriate to analyse time‐to‐event data using methods used for continuous outcomes (e.g. using mean times‐to‐event), as the relevant times were only known for the subset of participants who have had the event. Censored participants were to be excluded, which, almost certainly, would have introduced bias. We planned to discuss time‐to‐event data that had been presented incorrectly as continuous data narratively, rather than as an analysis (Deeks 2011).
Unit of analysis issues
We planned to identify the unit of analysis used in each individual study in relation to a wound, a foot, a participant or as multiple wounds on the same participant. We would have recorded if studies had incorrectly treated multiple wounds on a participant as being independent, rather than using within‐patient analysis methods, in the risk of bias assessment. For wound healing and amputation, unless otherwise stated, where the number of wounds appeared to equal the number of participants, we would have treated the wound as the unit of analysis. We planned to treat these studies with caution. We planned to include them in the systematic review, but to conduct meta‐analysis with and without them in sensitivity analyses, to assess the effect they would have had on the results. We also planned to assess the level of randomisation of each trial to see whether the number of observations matched the number of units randomised. Where the unit of analysis was unclear, we would have contacted the trial author for results per person.
We planned to assess the unit of analysis for adverse event data on a trial‐by‐trial basis to establish whether the data were at participant level, or whether multiple events per participant were possible. Where the latter was the case, although the data could be reported on a trial by trial basis, they could not be analysed further without violating assumptions of independence. We planned to discuss the method of data collection, potential risks of measurement and performance biases, as well as the unit of analysis of adverse event data in detail in the review.
If multiple treatment arms were reported, we planned to carry out multiple meta‐analyses. If more than one control group was used or where a single 'conventional' control group was not recognisable, we planned to combine all control group results and carry out pooled analyses of all control groups against the intervention group.
In relation to the inclusion of cluster RCTs, we planned to attempt analysis where relevant information was available (i.e. the number, or mean size, of clusters, outcome data for total individuals with events, and an estimate of the intra‐cluster/intra‐class correlation coefficient (ICC). A more reliable analysis was then going to be conducted by reducing the size of each trial to its effective sample size using the design effect of a cluster RCT, and the standard error obtained from confidence intervals, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Potential meta‐analysis could therefore have been performed using the inflated variances.
Dealing with missing data
We planned to seek missing information from the original trial authors by emailing the contact person for the published studies. In particular, we would have contacted the authors for the relevant data where the reported findings of a study extended beyond foot ulcers and it would have been difficult to determine which, if any, findings related to foot ulcers specifically. When responses were not received, we were to contact additional authors from the publication. To avoid overly positive answers and the risk of false information, we planned to use open‐ended questions for contacting authors (Higgins 2011b). If information relating to outcomes (according to outcome measures) was missing, we deemed the article unsuitable for the review.
Multiple efforts were made to acquire any missing data from authors. We planned to inspect factors such as attrition rates, drop‐out rates, randomised and included subject numbers, as well as numbers for intention‐to‐treat, treated‐per‐protocol and losses to follow‐up carefully. We would have appraised these critically and assessed their impact on the data in the light of the results of the review.
We acknowledged that sometimes measures of dispersion are not recorded in trials. Where the standard error (SE) or the t‐statistic was reported, we planned to calculate standard deviations with statistical assistance from review author, PB. Where the authors did not report the aetiology of ulcers, we planned to contact them for details. Where the authors were unable to confirm aetiology, we would have excluded the study.
Assessment of heterogeneity
Clinical heterogeneity
We planned to determine potential reasons for heterogeneity by exploring individual study and subgroup characteristics such as age and gender of participants, risk factors for foot ulceration, duration of disease, initial size of ulcer, type of treatment, duration of follow‐up, presence or absence of infection, history of ulceration, history of significant cardiovascular events, presence or absence of PAD, type of ulcer, location of ulcer, time to ulcer healing, type of medication used, as well as how ulcer healing was defined within the context of the study.
Methodological heterogeneity
We planned to use the formal assessment of bias of each study, as described above, to assist in identifying methodological heterogeneity between studies.
Statistical heterogeneity
We planned to use forest plots, Q and I2 statistics to assess heterogeneity (Higgins 2003). If heterogeneity was present, then we aimed to identify the studies that produced it, and to conduct an analysis without them. The Q‐statistic assessed for the presence of heterogeneity (Higgins 2003). With the I2 statistic, values of 75% or more were taken as indicative of high levels of heterogeneity (Deeks 2011).
Only those studies that were clinically, methodologically and statistically homogenous were to be pooled for meta‐analysis effect‐size calculations. We planned to define subgroups for analysis using the factors we identify above as being responsible for heterogeneity.
Assessment of reporting biases
If there was a sufficient number of studies (10 or more) available, we planned to use funnel plots to assess publication bias. If there were not enough studies in the meta‐analysis for constructing a meaningful funnel plot, we would have discussed the potential for publication bias using available studies (Sterne 2011).
Data synthesis
We consulted the Cochrane Collaboration recommendations and decided to conduct both random‐effects and fixed‐effect models where appropriate for any potential meta‐analysis. For example where clinical, methodological and statistical heterogeneity were not apparent, we planned to pool similar studies in a fixed‐effect model. Where any of the above‐mentioned heterogeneity was evident we would have used a random‐effects model. Where heterogeneity levels were insignificant and no other forms of heterogeneity were evident, we would have used both random‐effects and fixed‐effect models for comparison. We planned to attempt to investigate any significant differences in results and heterogeneity of studies through use of these two statistical models. If there were any vast differences between the two methods, we planned on exploring these differences. If fixed‐effect and random‐effects meta‐analyses gave identical results, then we thought that it was unlikely that there was important statistical heterogeneity, and we believed either method was appropriate for reporting. We planned to include all studies meeting the inclusion criteria and reporting outcome variables of interest in the review and we would have included all studies meeting eligibility criteria for meta‐analysis in a meta‐analysis. We planned to conduct meta‐analysis separately on provided and published data, and also on results from intention‐to‐treat trials. We would have used Review Manager (RevMan 2014)for data analysis.
'Summary of findings' tables
We would have presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We planned to present the following outcomes in the 'Summary of findings' tables:
Number of ulcers healed
Time to complete ulcer healing
Change in ulcer severity
Incidence of amputation related to foot ulcers.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses at several levels in the meta‐analysis. We would have decided the subgroups after consideration of a number of factors, based on:
Follow‐up time: studies stratified as short, medium and long term, where less than one year of follow‐up was to be considered as short term, one to three years as medium term, and more than three years as long term;
Variation in the intervention and control group (e.g. groups who received lifestyle interventions versus anti‐diabetic medication versus insulin).
Sensitivity analysis
We planned to carry out sensitivity analyses by excluding and including studies that caused heterogeneity in the data. We also planned to carry out sensitivity analyses by excluding and including studies that were deemed to be of lower quality (high risk of bias). We planned to discuss the results of the sensitivity analyses.
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
Our final database search identified 18,792 records in total. A further 726 records were identified by searching clinical trials registers, pharmaceutical trials registers and by looking through currently available clinical guidelines (see Figure 1). After the removal of duplicates, the review authors screened 7994 records for inclusion based on the title and abstract matching the review inclusion criteria. This screening process further excluded 7945 articles. MF and RS reviewed 49 trials from full‐text articles or online trial registrations which were considered potentially eligible for inclusion based on the inclusion criteria. The most recent database and clinical trial registry searches were carried out in December 2015.
We found two ongoing registered trials (NCT01472432 and ACTRN12613000418774) which were of potential interest (see Characteristics of ongoing studies). These are both investigating the effect of dipeptidyl peptidase‐4 (DPP‐4) inhibitors (specifically vildagliptin) on foot ulcer outcomes. No results have been published from these trials to date, however the study protocols are published in clinical trials registries.
The first ongoing trial (NCT01472432) is investigating the clinical and humoral effects of vildagliptin on healing of chronic ulcers in people with type 2 diabetes. The experimental group in this trial will receive vildagliptin 50 mg orally twice a day for four months.The placebo group will receive oral antidiabetic therapy titrated for optimal glycaemic control for three months. The primary outcome measure is full epithelisation of the wound within four months and capillary density on punch biopsy before and after the treatments. The second ongoing trial (ACTRN12613000418774) is investigating the effects of vildagliptin on inflammation markers in people with type 2 diabetes with foot ulcers. The intervention group receive oral metformin with the addition of vildagliptin for 12 weeks: the dose being determined by fasting blood glucose levels with the aim of achieving fasting glucose levels < 7 mmol/l and postprandial levels of < 10 mmol/l. The second group receive a similar dose of metformin and receive a placebo instead of vildagliptin. This trial has listed partial and complete wound closure within 12 weeks as a secondary outcome measure.
Included studies
The only eligible study which met the inclusion criteria was a pilot trial which was undertaken to determine the feasibility of performing a definitive trial assessing the effect of close glycaemic control on healing foot ulcers in people with diabetes (Idris 2004). This pilot study was prematurely terminated due to difficulty in participant recruitment. No data was reported and so we were unable to obtain any data from this trial; see Characteristics of included studies.
Excluded studies
A list of all excluded trials can be found here (see Characteristics of excluded studies). Out of the 49 trials screened for inclusion, 46 were excluded (see Characteristics of excluded studies for reasons of exclusion). Eight studies were excluded because we couldn't contact authors to obtain clarification on outcome measures, or where outcome measures were not reported (Abraira 1992; Abraira 2003; Calles‐Escandon 2010; Duckworth 2009; Gaede 2003; Nathan 2009; UK Prospective Diabetes Study (UKPDS) Group 1998; Van Olmen 2013). Another study (Zhang 2011) investigated the effect of normal subcutaneous insulin injections in comparison to injecting half the dose of insulin at the ulcer‐site as a localised treatment and half subcutaneously, over a period of seven days. The trialists found an improvement in ulcer healing in the localised injection group with comparable fasting glucose levels, as the control group receiving the same dose of insulin. This study was published in Chinese and was translated. We excluded this study as it was not designed to compare intensive versus conventional glycaemic control on foot ulcer outcomes, especially foot ulcer healing.
A further two trials were excluded due to non‐randomisation (Sullivan 2009; Kostev 2012) and another trial due to premature termination (ACTRN12606000426583). We contacted the trial author of ACTRN12606000426583 and the authors were able to confirm that this study had been terminated before commencement. Eighteen trials were excluded due to foot ulcer outcomes not being investigated as an outcome measure (see Characteristics of excluded studies). One trial Marso 2013 reported foot ulcers as a tertiary study outcome on their protocol, yet no data has been reported in relation to this outcome. Authors of the trial were unable to provide us with any data on foot ulcer outcomes when contacted. Therefore we also excluded this study. A further fifteen trials were excluded because participants were not exclusively placed in intensive versus conventional glycaemic control arms, as per the requirement for this review (see Characteristics of excluded studies).
Risk of bias in included studies
We could not determine the risk of bias from the prematurely terminated included trial (Idris 2004). See Risk of bias in included studies.
Effects of interventions
One eligible study was identified however this provided no data.
Discussion
This systematic review aimed to assess the effects of intensive glycaemic control compared to conventional control on the treatment of foot ulcers in people with type 1 and type 2 diabetes. We performed an exhaustive search of evidence which consisted of both published and unpublished material. Despite these efforts, we were unable to find any clinical trials which had successfully investigated the impact of intensive versus conventional glycaemic control on foot ulcer outcomes. We found one trial which was completed without any results (Idris 2004) and two ongoing trials (ACTRN12613000418774; NCT01472432) which were investigating intensive versus conventional glycaemic control, and which may report foot outcomes at a later date.
Although Idris 2004 cannot contribute to current evidence due to a lack of results, there were several noteworthy points which were made by the authors. The authors of this study faced challenges in recruiting and allocating patients. A number of patients were frail or thought to be incapable of adhering to intensive glycaemic control. Recruitment is a challenge for any researcher recruiting patients to a trial of intensive diabetes control for a number of reasons.The authors reported that the attitude of people with foot ulcers towards participating in a study involving intensive glycaemic control was negative despite the potential clinical benefit. We believe factors such as the number of additional clinical consultations needed, treatment compliance and participants' ability to endure potential side effects of intensive glycaemic control may have adversely impacted on recruitment. The additional ethical clinical dilemma faced by any modern day study investigating the effects of intensive versus conventional glycaemic control is the strong precedent set by major landmark trials such as the UKPDS (UK Prospective Diabetes Study (UKPDS) Group 1998). These studies demonstrated a strong incentive for optimised glycaemic control. This would be especially important for a trial assessing foot ulcer healing, which is believed to be strongly influenced by hyperglycaemia. Although there remains a clear need to document the benefits associated with attempts to improve glycaemic control in this population, the perceived cost and adverse events of such a treatment needs to be taken into account in an older vulnerable population prone to the effects of hypoglycaemia.
It is interesting to note that many of the trials investigating intensive versus conventional glycaemic control screened in this review included lower limb amputation as an outcome measure (Althouse 2013; Christiansen 2009; Dormandy 2005; Gaede 2003; Nathan 2009; Pedersen 2003; Vaccaro 2012). This outcome was not, however, reported in relation to presentation with, development of, or healing of foot ulceration. Amputation was likely used rather than ulcer healing due to the ease of measurement and definitive nature of such a procedural end‐point. The clinical outcome of ulcer healing has several different definitions available in the literature and is considered more labour intensive to measure than amputation outcomes. Given that ulceration precedes lower limb amputations in up to 85% of people with diabetes who have amputations (Boulton 2004; Singh 2005), it would seem logical that successful ulcer healing could prevent the clinical progression towards amputation and hence be of clinical significance. None of the trials which were screened included amputation in patients who had presented with foot ulcers as a specific outcome. Amputations were reported in relation to aetiological factors, such peripheral artery disease (Gaede 2003) and neuropathy (which was not well‐defined) Nathan 2009, or the cause was entirely undefined (Althouse 2013; Christiansen 2009). One trial reported above‐the‐ankle amputations but skin lesions were not documented (Vaccaro 2012). The authors of the screened trials were unable to provide further information or data on how many amputations occurred subsequent to a foot ulcer. Therefore we were unable to include these trials.
As foot ulceration often precedes amputation, we believe future trials should report on ulcer‐specific outcomes. It has previously been reported that the combination of patient‐specific, limb‐specific and ulcer‐specific measures should be used as outcome measures in trials focusing on diabetic foot complications and clinical care (Jeffcoate 2004). Furthermore, the use of amputation as a stand‐alone outcome measure of ulcer healing has been questioned and needs to be carefully considered (Margolis 2013). From a patient and HRQoL point of view, foot ulcer healing may be seen as a beneficial outcome over a detrimental endpoint such as amputation. For example, in a previous meta‐analysis, intensive glycaemic control reduced the risk of amputation by 36% in people with type 2 diabetes (relative risk (RR) 0.64, 95% CI: 0.43 to 0.95; 6960 participants in eight trials) (Hemmingsen 2011b). Unfortunately this information was based on amputations defined in several different ways. The underlying cause of amputation varied in the trials and included a mix of ischaemic and neuropathic aetiologies. The UKPDS, which contributed almost half of the reported events in this analysis, defined amputation as major limb complications requiring lower limb amputation of a digit or any limb for any reason and included digital amputations which are usually classified as minor amputations (UKPDS 1998). In other trials the definition of amputation has been less clear. For example it has not been clear whether minor (such as digital amputation) were grouped together with major (such as below knee) amputations (Hemmingsen 2011b). Overall the authors of this meta‐analysis concluded that the data provided low level evidence for a significantly reduced risk of amputation of a lower extremity using intensive glycaemic control (Hemmingsen 2011b). This was based on the GRADE scoring system which indicates that further research is very likely to have an important impact on the confidence in the estimate of effect and is likely to change the estimate. Other limitations in this analysis included the inability to stratify the number of reported amputations according to cause of amputation and the overall low number of reported amputations. Although this data provides evidence to support the efficacy of intensive glycaemic control in preventing amputations, its exact relationship to foot ulcer healing remains unanswered. Studies have recommended that outcomes such as ulcer‐free survival time, ulcer recurrence rate and time to ulcer healing should also be documented in clinical studies as these give important information regarding the overall effectiveness of an intervention (Jeffcoate 2006; Margolis 2013; Pound 2004).
There are a plethora of factors which we believe contribute to the difficulty in performing an effective clinical trial on this particular topic and we would like to highlight a few of these which we consider to be important. There are many factors determining ulcer healing (see Table 1) which makes it challenging to investigate the impact of one variable while keeping other factors consistent. The first challenge is thus in effective randomisation with appropriate stratification of these additional risk predictors. Other determinants of ulcer healing need to be kept consistent in the intervention and control groups. Each ulcer typically involves slightly different treatments such as antibiotics, ulcer dressing and offloading, which is challenging to standardise taking into account the participants’ clinical requirements. At best a trial in a group of participants receiving multiple treatments can only provide a measure of the effect of a particular management intention.There is an added issue that some people have more than one ulcer on the same or different legs. These ulcers may respond to treatment differently. Additionally, ulcer recurrence and new ulceration are also a possibility during follow‐up. This needs to be taken into account when defining the outcome measures for such a trial.
The presence of infection further complicates this clinical scenario and may have a profound impact on glycaemic control. Additionally, a trial investigating intensive versus conventional glycaemic control on foot ulcer outcomes needs to be able to observe an immediate effect of glycaemic control which will assist with foot ulcer healing, rather than observing long term glycaemic improvements, which is often the aim of clinical trials investigating glycaemic control. Therefore utilising mean blood glucose instead of HbA1c may be preferable in such a trial due to the ability to observe more immediate changes in glycaemic control.
Lastly and perhaps most importantly, we believe that there is also a major sample size challenge to designing a trial in this area. Previous trials investigating the effect of intensive versus conventional glycaemic control on microvascular end points contained large sample sizes and used multiple study centres for recruitment. For example the ADVANCE trial (ADVANCE 2008) recruited from 215 different study centres and had 11,140 participants, and the UKPDS (UKPDS 1998) recruited from 23 different study centres and had 5102 participants randomised to two groups. Although the difference in glycaemic control needed to demonstrate a significant statistical effect on ulcer healing is unknown, clinical guidelines recommend narrow glucose differences between intensive and conventional arms (see Table 2). This might mean that large sample sizes involving multiple centres may be required to provide adequate statistical power to observe significant differences in ulcer healing. As amputation is a binary outcome measure and as ulcer healing can be a numerical outcome measure (i.e. reduced ulcer area), it would seem plausible that sample sizes adequate for amputation outcomes should be sufficient for investigating foot ulcer healing. Appropriate sample size calculations based on multiple outcomes are however needed to understand the true extent of intensive glycaemic control on foot ulcer outcomes. Whether or not the two trials that are currently in progress (ACTRN12613000418774; NCT01472432) will ultimately have enough statistical power is yet to be observed. One trial (ACTRN12613000418774) has reported a proposed sample size of only 50, which suggests it is likely to be underpowered to assess the effect of the intervention on ulcer outcomes.
The eligibility of these ongoing trials for future inclusion in this review depends on a number of factors. Most importantly this will be based on whether glycaemic levels of the intervention and control groups differ. Successful, partial or complete wound healing also needs to be observed in the trials in order to assess a relationship between glycaemic control and wound healing within the reported time‐frames of the trials. The authors of both trials have been contacted to obtain any data which may become available at a later date.
In conducting this review, we have made exhaustive efforts to contact researchers and manufacturers of pharmaceutical agents who may have investigated foot ulcer outcomes. We do acknowledge the difficulties in identifying unpublished data and accept that some unpublished work may have been missed. It is also possible that literature published in languages other than English may have been missed. We intentionally did not exclude studies on the basis of language. We found two non‐English studies amid the full‐text trials screened for eligibility. One trial was in Chinese (Zhang 2011) and one was in German (Fresenius 2009). These were translated into English for assessment and the original authors were contacted for any clarification.
Authors' conclusions
Implications for practice.
The current review failed to find any randomised clinical trials with results. Therefore we are unable to conclude whether intensive glycaemic control when compared to conventional glycaemic control has a positive or detrimental effect on the treatment of foot ulcers.The exact role and place that intensive glycaemic control may have on treating foot ulcers remains to be resolved.
Implications for research.
Independent, well‐designed, large RCTs may be required to investigate the benefits and adverse effects of intensive versus conventional glycaemic control on diabetic foot ulcer outcomes.Those who are considering research in this area should consider the challenges of conducting a trial on this topic which have been outlined in the discussion. Ideally future RCTs investigating this topic should be specifically designed and tailored to answer the question of superiority of intensive over conventional glycaemic control. In particular future research should aim to:
consider ulcer‐specific outcome measures (i.e. healing) in addition to amputation;
find ways to control, adjust or match for ulcer characteristics, participant characteristics and the various interventions required for optimal ulcer healing using appropriate randomisation, adjustment or stratification;
assess the role of intensive versus conventional glycaemic control in predisposing to diabetic foot ulcer infection as well as foot ulcer healing;
have a sample size that is large enough to test a statistically significant effect and be large enough to allow for subgroup analyses.
Notes
The review authors agreed to change the title to 'Intensive versus conventional glycaemic control for treating diabetic foot ulcers', because of the wider use of the term 'intensive glycaemic control' over 'strict glycaemic control' within the literature, and also because of external reviewers' comments.
Acknowledgements
Firstly we would like to thank the librarians of Townsville Hospital, Queensland Health for assistance with literature searching and sourcing full‐text articles. We especially thank Mrs. Bronia Renison (Senior Librarian, Townsville Hospital Library) for her time, expertise and assistance with literature searches. We also thank Mr. Stephen Anderson (James Cook University Library) for assistance with literature searches. A special thanks to Dr. Hongyou Yu for his assistance with article translation. The review authors wish to thank Cochrane Wounds for the opportunity given to us and for the support provided. We would like to thank the following peer referees for their feedback and suggestions in the construction of the protocol; Jo Dumville, Duncan Chambers, Richard Kirubakaran, David Armstrong, Zena Moore, Nadine Madams and Rachel Richardson and acknowledge Elizabeth Royle who copy edited the protocol. We would like to thank the following peer referees for their feedback and suggestions on the construction of the review; David Margolis, Richard Kirubakaran, David Armstrong and Ankur Barua. Thanks also to and Denise Mitchell who copy edited the review.
Appendices
Appendix 1. Glossary of Terms
Diabetes: a disease caused by reduced production of the hormone insulin, or a reduced response of the liver, muscle, and fat cells to insulin. This affects the body's ability to use and regulate sugars effectively. Diabetic Peripheral Neuropathy (DPN): damage to the peripheral nerves that is characterised by numbness, tingling, pain, or sometimes muscle weakness, particularly in the extremities. Peripheral Arterial Disease (PAD): narrowing or obstruction of the arteries supplying the legs that is characterised by intermittent claudication (numbness, tingling and pain in the legs that occurs on walking, but is relieved by a short rest). Hyperglycaemia: excessive glucose (sugar) in the blood. HbA1c (glycated haemoglobin): a commonly used laboratory measurement that measures average blood glucose levels over the previous two to three months. Microvascular: small blood vessels. Macrovascular: large blood vessel. Nephropathy: disorder of the kidney that includes inflammatory, degenerative and sclerotic (scar forming) conditions. Retinopathy: disease of the small retinal blood vessels in the eye. Growth factors: chemical messengers that induce cell growth. Glycation: binding of a sugar molecule to an amino‐acid. In hyperglycaemia, sugar molecules become attached to cell surface proteins throughout the body; this sugar coating leads to small blood vessel damage in nerves, kidney, and the retina. Polyol pathway: metabolic pathway involved in breakdown of excess glucose. Advanced Glycation End products (AGEs): proteins that have been non‐enzymatically modified by the addition of sugar residues. Reactive Oxygen Species (ROS): molecules and ions of oxygen that have an unpaired electron, which makes them extremely reactive. Many cellular structures are susceptible to damage by reactive oxygen species. DAG‐protein kinase C pathway: metabolic pathway involved in diabetes‐related complications. Hexosamine pathway: metabolic pathway involved in diabetes‐related complications. Mitochondria: involved in respiration and adenosine tri‐phosphate (ATP; energy) production. Endothelial: cells lining the heart, blood vessels and lymph vessels. Angiogenesis: process of forming new blood vessels. NF‐κB: transcription factor involved in activation of genes involved in the inflammatory response. Ulcer grading scale: an ulcer grading system implies any system where the dimensional change in an ulcer has been documented – e.g. the University of Texas Wound Classification System (UTWS), PEDIS system or another.
Appendix 2. Search strategies
Cochrane Wounds Specialised Register
#1 (((blood glucose or (glycaemic or glycemic) next control) or "intensive glucose control" or (hypoglycaemi* or hypoglycemi*) next (agent* or drug*))) AND (INREGISTER) #2 (((oral next (hypoglycaemi* or hypoglycemi*)) or ("fasting glucose" or "glucose target" or anti‐diabetes medication* or diabetes medication* or insulin* or sulphonyureas or metformin or thiazolidinedione* or DPP‐4 inhibitor* or glitinide or glucosidase inhibitor* or biguinide or "GLP‐1 agonist" or acarbose or incretin next enhancer* or incretin next mimetic* or HbA1c))) AND (INREGISTER) #3 #1 OR #2 #4 (((diabet* near3 ulcer*) or (foot near3 ulcer*) or (feet near3 ulcer*) or (foot near3 defect*) or ("foot gangrene" or amputat*))) AND (INREGISTER) #5 #3 AND #4
Ovid MEDLINE
1 exp Blood Glucose/ 2 exp Hypoglycemic Agents/ 3 exp Hyperglycemia/ 4 exp Hypoglycemia/ 5 exp Insulin/ 6 exp Metformin/ 7 exp Thiazolidinediones/ 8 exp alpha‐Glucosidases/ 9 exp Glucagon‐Like Peptide 1/ 10 exp Acarbose/ 11 blood glucose.tw. 12 (((glycaemic or glycemic) adj control) or "intensive glucose control").tw. 13 ((hypoglycaemi* or hypoglycemi*) adj (agent* or drug*)).tw. 14 (oral adj (hypoglycaemi* or hypoglycemi*)).tw. 15 (fasting glucose or glucose target).tw. 16 (anti‐diabetes medication* or diabetes medication* or insulin* or sulphonylureas or metformin or thiazolidinedione* or DPP‐4 inhibitor* or glitinide or glucosidase inhibitor* or biguanide or GLP‐1 agonist or acarbose or incretin enhancer* or incretin mimetic* or HbA1c).tw. 17 or/1‐16 18 exp Foot Ulcer/ 19 exp Diabetic Foot/ 20 (diabet* adj3 ulcer*).tw. 21 (diabet* adj3 (foot or feet)).tw. 22 (diabet* adj3 wound*).tw. 23 (diabet* adj3 defect*).tw. 24 (foot gangrene or amputat*).tw. 25 or/18‐24 26 17 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomi?ed.ab. 30 placebo.ab. 31 clinical trials as topic.sh. 32 randomly.ab. 33 trial.ti. 34 or/27‐33 35 exp animals/ not humans.sh. 36 34 not 35 37 26 and 36
Ovid EMBASE
1 exp glucose blood level/ 2 exp antidiabetic agent/ 3 exp hyperglycemia/ 4 exp hypoglycemia/ 5 exp insulin/ 6 exp metformin/ 7 exp 2,4 thiazolidinedione derivative/ 8 exp alpha glucosidase/ 9 exp glucagon like peptide 1/ 10 exp acarbose/ 11 blood glucose.tw. 12 (((glycaemic or glycemic) adj control) or "intensive glucose control").tw. 13 ((hypoglycaemi* or hypoglycemi*) adj (agent* or drug*)).tw. 14 (oral adj (hypoglycaemi* or hypoglycemi*)).tw. 15 (fasting glucose or glucose target).tw. 16 (anti‐diabetes medication* or diabetes medication* or insulin* or sulphonylureas or metformin or thiazolidinedione* or DPP‐4 inhibitor* or glitinide or glucosidase inhibitor* or biguanide or GLP‐1 agonist or acarbose or incretin enhancer* or incretin mimetic* or HbA1c).tw. 17 or/1‐16 18 exp foot ulcer/ 19 exp diabetic foot/ 20 (diabet* adj3 ulcer*).tw. 21 (diabet* adj3 (foot or feet)).tw. 22 (diabet* adj3 wound*).tw. 23 (diabet* adj3 defect*).tw. 24 (foot gangrene or amputat*).tw. 25 or/18‐24 26 17 and 25 27 Randomized controlled trials/ 28 Single‐Blind Method/ 29 Double‐Blind Method/ 30 Crossover Procedure/ 31 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 32 (doubl$ adj blind$).ti,ab. 33 (singl$ adj blind$).ti,ab. 34 or/27‐33 35 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 36 human/ or human cell/ 37 and/35‐36 38 35 not 37 39 34 not 38 40 26 and 39
EBSCO CINAHL
S39 S26 AND S38 S38 S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 S37 MH "Quantitative Studies" S36 TI placebo* or AB placebo* S35 MH "Placebos" S34 TI random* allocat* or AB random* allocat* S33 MH "Random Assignment" S32 TI randomi?ed control* trial* or AB randomi?ed control* trial* S31 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S30 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S29 TI clinic* N1 trial* or AB clinic* N1 trial* S28 PT Clinical trial S27 MH "Clinical Trials+" S26 S17 AND S25 S25 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S24 TI ( foot gangrene or amputat* ) OR AB ( foot gangrene or amputat* ) S23 TI diabet* N3 defect* or AB diabet* N3 defect* S22 TI diabet* N3 wound* or AB diabet* N3 wound* S21 TI diabet* N3 foot OR diabet* N3 feet* or AB diabet* N3 foot OR diabet* N3 feet S20 TI diabet* N3 ulcer* or AB diabet* N3 ulcer* S19 (MH "Foot Ulcer+") S18 (MH "Diabetic Foot") S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 AB anti‐diabetes medication* or diabetes medication* or insulin* or sulphonyureas or metformin or thiazolidinedione* or DPP‐4 inhibitor* or glitinide or glucosidase inhibitor* or biguinide or GLP‐1 agonist or acarbose or incretin enhancer* or incretin mimetic* or HbA1c S15 TI anti‐diabetes medication* or diabetes medication* or insulin* or sulphonyureas or metformin or thiazolidinedione* or DPP‐4 inhibitor* or glitinide or glucosidase inhibitor* or biguinide or GLP‐1 agonist or acarbose or incretin enhancer* or incretin mimetic* or HbA1c S14 TI ( fasting glucose or glucose target ) OR AB ( fasting glucose or glucose target ) S13 TI ( oral hypoglycaemi* or oral hypoglycemi* ) OR AB ( oral hypoglycaemi* or oral hypoglycemi* ) S12 TI ( hypoglycaemi* agent* or hypoglycemi* agent* or hypoglycaemi* drug* or hypoglycemi* drug* ) OR AB ( hypoglycaemi* agent* or hypoglycemi* agent* or hypoglycaemi* drug* or hypoglycemi* drug* ) S11 TI ( glycaemic control or glycemic control or "intensive glucose control" ) OR AB ( glycaemic control or glycemic control or "intensive glucose control" ) S10 TI blood glucose OR AB blood glucose S9 (MH "Acarbose") S8 (MH "Glucagon‐Like Peptide 1") S7 (MH "Thiazolidinediones+") S6 (MH "Metformin") S5 (MH "Insulin+") S4 (MH "Hypoglycemia+") S3 (MH "Hyperglycemia+") S2 (MH "Hypoglycemic Agents+") S1 (MH "Blood Glucose")
Elsevier SCOPUS
1. (TITLE‐ABS‐KEY(blood PRE/3 glucose) OR TITLE‐ABS‐KEY(hypoglyc*mic PRE/3 agent* OR hyperglyc*mia OR hypoglyc*mia) OR TITLE‐ABS‐KEY(insulin* OR metformin OR thiazolidinedione*) OR TITLE‐ABS‐KEY({alpha‐glucosidases} OR {glucagon‐like peptide 1}) OR TITLE‐ABS‐KEY(acarbose OR {blood glucose}) OR TITLE‐ABS‐KEY(glyc*mic W/3 control OR {intensive glucose control}) OR TITLE‐ABS‐KEY(agent* W/3 hypoglycaemi* OR hypoglycemi*) OR TITLE‐ABS‐KEY(drug* W/3 hypoglycaemi* OR hypoglycemi*) OR TITLE‐ABS‐KEY(oral W/3 hypoglycaemi* OR hypoglycemi*) OR TITLE‐ABS‐KEY(“fasting glucose” OR “glucose target”) OR TITLE‐ABS‐KEY({anti‐diabetes} W/3 medication* OR diabetes W/3 medication*) OR TITLE‐ABS‐KEY(sulphonylureas OR sulfonylureas OR “dpp‐4 inhibitor*”) OR TITLE‐ABS‐KEY(glitinide OR glucosidase W/3 inhibitor*) OR TITLE‐ABS‐KEY(biguanide OR {GLP‐1 agonist} OR incretin W/3 enhancer*) OR TITLE‐ABS‐KEY(incretin W/3 mimetic* OR hba1c))
2. Searched within results from above search for each of the following terms (combined with Boolean "AND" operator):‐
Foot w/3 ulcer Diabet* w/3 foot or feet Diabet* w/3 ulcer* Diabet* w/3 wound* Diabet* w/3 defect* foot gangrene amputat*
ISI Web of Knowledge Web of Science
1. Topic=(blood near/3 glucose) OR Topic=(hypoglyc*mic near/3 agent* or hyperglyc*mia or hypoglyc*mia) OR Topic=(Insulin* or Metformin or Thiazolidinedione*) OR Topic=(alpha‐glucosidases or “glucagon‐like peptide 1”) OR Topic=(Acarbose or “blood glucose”) OR Topic=(Glyc*mic near/3 control or “intensive glucose control”) OR Topic=(Agent* near/3 hypoglycaemi* or hypoglycemi*) OR Topic=(Drug* near/3 hypoglycaemi* or hypoglycemi*) OR Topic=(Oral near/3 hypoglycaemi* or hypoglycemi*) OR Topic=(“fasting glucose” or “glucose target”) OR Topic=(anti‐diabetes near/3 medication* or diabetes near/3 medication*) OR Topic=(Sul*onylureas or “DPP‐4 inhibitor*”) OR Topic=(glitinide or glucosidase near/3 inhibitor*) OR Topic=(Biguanide or “GLP‐1 agonist” or incretin near/3 enhancer*) OR Topic=(Incretin near/3 mimetic* or HbA1c)
2. Results from above search results were combined with each of the following terms:‐
Foot near/3 ulcer* Diabet* near/3 foot or feet Diabet* near/3 ulcer* Diabet* near/3 wound* Diabet* near/3 defect* “foot gangrene” amputat*
3. Duplicates were removed and remaining references were searched for the following terms as [as field contains]:‐
Randomized Randomised Controlled clinical trial Placebo Drug therapy Randomly Trial Groups
BioMed Central
1. Randomized or randomised or “controlled clinical trial” or placebo or drug therapy or randomly or trial or groups (All words) in All fields (full text)
2. Searched within #1 for:
“Foot and ulcer*” or (diabet* and foot or diabet* and feet) or “Diabet* and ulcer*” or “Diabet* and wound*” or “Diabet* and defect*” or “foot and gangrene” or amputat*)
3. Searched within #2 for each of the following terms in All fields (full text):
“blood glucose” or “Hypoglyc*mic agent*” or hyperglyc*mia or hypoglyc*mia
OR
Insulin* or Metformin or Thiazolidinedione* or “alpha‐glucosidases” or “alpha glucosidases”
OR
“glucagon‐like peptide 1” or “glucagon like peptide 1” or Acarbose or “Glyc*mic control”
OR
“intensive glucose control” or “Hypoglyycaemi* agent” or “hypoglycemi* agent” or “hypoglycaemi* drug*” or “hypoglycemi* drug*”
OR
“Oral hypoglycaemi*” or “oral hypoglycemi*” or “fasting glucose” or “glucose target” or “anti‐diabetes medication*” or “diabetes medication*” or Sul*onylureas or “DPP‐4 inhibitor*”
OR
glitinide or “glucosidase inhibitor*” or Biguanide or “GLP‐1 agonist” or “incretin enhancer*” or “Incretin mimetic*” or “HbA1c”
LILACS
1. ( blood glucose ) or "BLOOD GLUCOSE" or "BLOOD GLUCOSE/" or "BLOOD GLUCOSE/AN" or "BLOOD GLUCOSE/BI" or "BLOOD GLUCOSE/BL" or "BLOOD GLUCOSE/CH" or "BLOOD GLUCOSE/DE" or "BLOOD GLUCOSE/DU" or "BLOOD GLUCOSE/GE" or "BLOOD GLUCOSE/IM" or "BLOOD GLUCOSE/ME" or "BLOOD GLUCOSE/MT" or "BLOOD GLUCOSE/PH" or "BLOOD GLUCOSE/RE" or "BLOOD GLUCOSE/ST" [Subject descriptor] and "HYPOGLYCEMIC AGENTS" [Subject descriptor] and "HYPERGLYCEMIA" or "postprandial HYPERGLYCEMIA" [Subject descriptor]
2. "HYPOGLYCEMIA" [Subject descriptor] or "INSULIN" [Subject descriptor] or "METFORMIN" [Subject descriptor]
3. "THIAZOLIDINEDIONES" [Subject descriptor] or "ALPHA‐GLUCOSIDASES" [Subject descriptor] or "GLUCAGON‐LIKE PEPTIDE 1" or "GLUCAGON‐LIKE PEPTIDEs" [Subject descriptor]
4. acarbose [Subject descriptor]
5. "BLOOD GLUCOSE" or "BLOOD GLUCOSE/" [Words] or "GLYCEMICCONTROL" or "GLYCEMICSELF‐CONTROL" [Words]
6. "HYPOGLYCEMIC AGENTS" or "HYPOGLYCEMIC AGENTS/" or "HYPOGLYCEMIC AGENTS/AD" or "HYPOGLYCEMIC AGENTS/AE" or "HYPOGLYCEMIC AGENTS/AN" or "HYPOGLYCEMIC AGENTS/BL" or "HYPOGLYCEMIC AGENTS/CH" or "HYPOGLYCEMIC AGENTS/CL" or "HYPOGLYCEMIC AGENTS/CT" or "HYPOGLYCEMIC AGENTS/DU" or "HYPOGLYCEMIC AGENTS/EC" or "HYPOGLYCEMIC AGENTS/IM" or "HYPOGLYCEMIC AGENTS/IP" or "HYPOGLYCEMIC AGENTS/ME" or "HYPOGLYCEMIC AGENTS/PD" or "HYPOGLYCEMIC AGENTS/PK" or "HYPOGLYCEMIC AGENTS/PO" or "HYPOGLYCEMIC AGENTS/SD" or "HYPOGLYCEMIC AGENTS/TO" or "HYPOGLYCEMIC AGENTS/TU" or "HYPOGLYCEMIC/HYPOTRIGLYCERIDEMIC" or "HYPOGLYCEMICAGENTS" [Words]
7. No words found in the index for: oral hypoglyc[a]emi*
8. No words found in the index for: glucose target
9. "FASTINGGLUCOSE" [Words]
10. "ANTI‐DIABETES" or "ANTI‐DIABETIC" or "ANTI‐DIABETICA" or "ANTI‐DIABETICAS" or "ANTI‐DIABETICO" or "ANTI‐DIABETICOS" or "ANTI‐DIABETICS" [Words] or "INSULIN" [Words] or "SULPHONYLUREA" or "SULPHONYLUREA‐BASED" or "SULPHONYLUREAS" [Words] No words found in the index for: diabetes next medication*
11. "SULFONYLUREAS" or "SULFONYLUREA" or "SULFONYLUREA COMPOUNDS" or "SULFONYLUREA COMPOUNDS/" or "SULFONYLUREA COMPOUNDS/AD" or "SULFONYLUREA COMPOUNDS/AE" or "SULFONYLUREA COMPOUNDS/BL" or "SULFONYLUREA COMPOUNDS/CL" or "SULFONYLUREA COMPOUNDS/CT" or "SULFONYLUREA COMPOUNDS/PD" or "SULFONYLUREA COMPOUNDS/PK" or "SULFONYLUREA COMPOUNDS/PO" or "SULFONYLUREA COMPOUNDS/TO" or "SULFONYLUREA COMPOUNDS/TU" or "SULFONYLUREAS" [Words] or "METFORMIN" or "METFORMIN’S" or "METFORMIN‐INDUCED" or "METFORMIN‐TREATED" or "METFORMIN/" or "METFORMIN/AD" or "METFORMIN/AE" or "METFORMIN/AN" or "METFORMIN/CH" or "METFORMIN/CT" or "METFORMIN/DU" or "METFORMIN/EC" or "METFORMIN/GLIMEPIRIDE" or "METFORMIN/ME" or "METFORMIN/PD" or "METFORMIN/PK" or "METFORMIN/TO" or "METFORMIN/TU" or "METFORMINA" or "METFORMINA‐GLIBEN‐CLAMIDA" or "METFORMINA." or "METFORMINA/" or "METFORMINA/AD" or "METFORMINA/AE" or "METFORMINA/AN" or "METFORMINA/CH" or "METFORMINA/CT" or "METFORMINA/DU" or "METFORMINA/EC" or "METFORMINA/GLIMEPIRIDA" or "METFORMINA/ME" or "METFORMINA/PD" or "METFORMINA/PK" or "METFORMINA/TO" or "METFORMINA/TU" or "METFORMINADE" or "METFORMINAHCL" or "METFORMINAND" or "METFORMINAREVISAO" or "METFORMINCONCLUSIONS" or "METFORMINE" or "METFORMINGROUP" or "METFORMINHCL" or "METFORMYN" [Words] or "THIAZOLIDINEDIONAS" or "THIAZOLIDINEDIONE" or "THIAZOLIDINEDIONES" or "THIAZOLIDINEDIONES/" or "THIAZOLIDINEDIONES/AD" or "THIAZOLIDINEDIONES/AE" or "THIAZOLIDINEDIONES/AG" or "THIAZOLIDINEDIONES/CH" or "THIAZOLIDINEDIONES/EC" or "THIAZOLIDINEDIONES/ME" or "THIAZOLIDINEDIONES/PD" or "THIAZOLIDINEDIONES/TU" or "THIAZOLIDINONE" or "THIAZOLINEDIONES" [Words]
12. "BIGUANIDA" or "BIGUANIDAS" or "BIGUANIDAS/" or "BIGUANIDAS/AD" or "BIGUANIDAS/AE" or "BIGUANIDAS/AN" or "BIGUANIDAS/CH" or "BIGUANIDAS/CT" or "BIGUANIDAS/DU" or "BIGUANIDAS/ME" or "BIGUANIDAS/PD" or "BIGUANIDAS/PK" or "BIGUANIDAS/ST" or "BIGUANIDAS/TU" or "BIGUANIDE" or "BIGUANIDE‐BASED" or "BIGUANIDE‐DERIVATIVE" or "BIGUANIDES" or "BIGUANIDES/" or "BIGUANIDES/AD" or "BIGUANIDES/AE" or "BIGUANIDES/AN" or "BIGUANIDES/CH" or "BIGUANIDES/CT" or "BIGUANIDES/DU" or "BIGUANIDES/ME" or "BIGUANIDES/PD" or "BIGUANIDES/PK" or "BIGUANIDES/ST" or "BIGUANIDES/TU" or "BIGUANIDICO" or "BIGUANIDIL" or "BIGUANIDINS" or "BIGUANIDOS" [Words] or "ACARBOSA" or "ACARBOSA/AD" or "ACARBOSA/AE" or "ACARBOSA/CT" or "ACARBOSA/ME" or "ACARBOSA/PD" or "ACARBOSA/TU" or "ACARBOSE" or "ACARBOSE‐INDUCED" or "ACARBOSE‐TREATED" or "ACARBOSE/AD" or "ACARBOSE/AE" or "ACARBOSE/CT" or "ACARBOSE/ME" or "ACARBOSE/PD" or "ACARBOSE/TU" [Words] or "INCRETIN‐MIMETICS" or "INCRETINA‐MIMETICOS" or "INCRETINMIMETICS" or "INCRETINOMIMETICAS" or "INCRETINOMIMETICOS" [Words]
No words found in the index for: glitinide; glucosidase inhibitor*; incretin next enhancer*
13. "GLP‐1" or "GLP‐1." or "GLP1" or "GLP1:" [Words] and "AGONIST" or "AGONIST’S" or "AGONIST‐‐IN" or "AGONIST‐ANTAGONIST" or "AGONIST‐BINDING" or "AGONIST‐HOLD‐RELAX" or "AGONIST‐INDUCED" or "AGONIST‐LIKE" or "AGONIST‐MEDIATED" or "AGONIST‐RECEPTOR" or "AGONIST‐SPECIFIC" or "AGONIST‐TO‐ANTAGONIST" or "AGONIST‐UINDUCED" or "AGONIST/ANTAGONIST" or "AGONIST/ANTAGONISTAS" or "AGONIST/CHLORIDE" or "AGONISTA" or "AGONISTAS" [Words]
14. "HBA1:C287C" OR "HBA1:C46G" OR "HBA1C" OR "HBA1C>9.5" OR "HBA1C‐PESO" OR "HBA1C10" OR "HBA1C12" OR "HBA1CLEVELS" OR "HBA1CMEASUREMENT" OR "HBA1CMETODOS" [Words]
The results of searches #1–#14 were combined with the "OR" Boolean operator and duplicates were removed.
The results were combined with the "foot ulcer" concept terms, as below. Terms #15‐#23 were individually searched within the results for #1‐#14.
15. Foot [and] ulcer
16. Diabetic foot
17. Diabet* [and] ulcer*
18. Diabet* [and] foot
19. Diabet* [and] feet
20. Diabet* [and] wound*
21. Diabet* [and] defect*
22. Foot gangrene
23. Amputat*
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Idris 2004.
| Methods | Single‐centre, prospective, parallel group randomised trial with two arms, and with clinical assessments performed by assessors blinded to randomisation group. All patients attending a dedicated multidisciplinary clinic for the management of established foot ulcers over a 20‐week period were systematically screened for inclusion in a randomised, single‐blinded study. The primary endpoints were healing and glycaemic control within 12 weeks. Secondary endpoints included those which were related to the ulcer (change in ulcer area, amputation), the person (health‐related quality of life, survival, pre‐ and postprandial glucose concentrations, hypoglycaemia) and the process (withdrawals, costs). | |
| Participants | Intended to randomise a total of 50 patients over six months as a pilot for a definitive randomised controlled trial.
|
|
| Interventions | Participants were to be randomised to either continue with current hypoglycaemic measures (adjusted by the usual carers in accordance with perceived clinical need) or to an intensive effort to achieve tight control. Both groups were to receive equivalent baseline education and be encouraged to perform regular home blood glucose monitoring.Tight control would be attempted through the intervention of a diabetes specialist nurse and dietitian (each with considerable experience of attempting close glycaemic control in pregnancy), using one‐to‐one education linked to the stepped introduction of basal‐bolus insulin therapy, using glargine (Lantus®) and Novorapid® insulins, adjusted according to the results of home blood glucose monitoring. The aim was to achieve mean preprandial glucose concentrations of between 5 mmol/l and 7 mmol/l and mean postprandial concentrations of between 7 mmol/l and 11 mmol/l. |
|
| Outcomes | Out of 200 patients attending the clinic, 188 were ineligible. Of 12 possible recruits, three who had not had a recent HbA1c were excluded when the result was found to be less than 7.5%. Two were judged incapable of complying with an intensive insulin regimen. Four withheld consent, and one was advised by his community nurse to withhold consent. One had an ulcer which became clinically infected on the day before his initial visit, and prior to randomisation. One was successfully recruited and randomised (to the non‐intervention group), and completed the 12 weeks of the study. . |
|
| Notes | The study was completed and it was established that it was not feasible to undertake such an intervention in the group of patients managed at that particular specialist clinic. It is possible that the failure to recruit sufficient numbers was peculiar to the centre, despite the size of the population being managed, and that other centres might have been more successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to be determined |
| Allocation concealment (selection bias) | Unclear risk | Unable to be determined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to be determined |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to be determined |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to be determined |
| Selective reporting (reporting bias) | Unclear risk | Unable to be determined |
| Other bias | Unclear risk | Unable to be determined |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraira 1992 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study, authors were unable to provide additional data for analyses |
| Abraira 2003 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study, authors were unable to provide additional data for analyses |
| ACTRN12606000426583 | Study prematurely terminated/ was not finished |
| Althouse 2013 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. PAD, lower‐extremity revascularization and non‐ulcer‐specific lower extremity amputation were documented, but the two intervention arms did not meet inclusion criteria. All participants were treated with a target HbA1c of 7.0% |
| Bayat 2013 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Bloomgarden 1987 | Foot ulcer outcomes or other outcomes relevant to this review were not investigated in study.The study only investigated incidence of foot ulceration and the impact of ulcer prevention interventions on ulcer incidence |
| Boaz 2009 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. The study assessed the role of prevention and active checking of foot and leg sores |
| Calles‐Escandon 2010 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. The study investigated mortality as an outcome from intensive control verses conventional control and the authors were unable to provide additional data for analyses |
| Chiu 2011 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Christiansen 2009 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. Study reported all‐cause amputation and ulcer specific or related amputation data were unable to be provided |
| Clark 2001 | Participants were not randomised to intensive or conventional glycaemic control arms for comparison |
| Dormandy 2005 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. One secondary outcome was amputation above the ankle however this was not related to ulcers. Authors were unable to provide any additional data |
| Duckworth 2009 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. The authors were unable to provide additional data for analyses. The study investigated the incidence of neuropathy and neuropathic ulcers with intensive versus conventional control as a prevention measure |
| Fresenius 2009 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Gaede 2006 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. Amputation results were based on vascular causes rather than causes related to foot ulceration. Authors were unable to provide additional clarification |
| Gaede 2003 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study and the authors were unable to provide additional data for analyses |
| Garber 2002 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study |
| Gram 2011 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study |
| Griffin 2011 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study and the authors were unable to provide any additional data. This was a population‐level study with the unit of analyses not focused on participants or ulcers |
| Jarnert 2012 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Kawamori 2010 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in study. Only PAD outcomes were reported and composite outcomes in relation to PAD and other outcomes were reported |
| Kostev 2012 | Non‐randomised study design |
| Leung 2012 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Li 2008 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Litzelman 1993 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Marso 2013 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study. The study stated diabetic foot ulcers as a tertiary outcome but did not report data. Authors were unable to provide any additional data |
| Martinez‐Sanchez 2005 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| McMurray 2002 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Meigs 2003 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Nathan 2009 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study and authors were unable to provide additional data for analyses. Study reported outcomes on amputation related to severe neuropathy but not foot ulcers |
| NCT00850798 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study |
| NCT01421966 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| NCT01813305 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Nybo 2011 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study and the authors were unable to provide any additional data |
| Pedersen 2003 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study. Study reported amputations likely related to vascular causes and the authors were unable to provide any additional data |
| Rong 2012 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
| Sullivan 2009 | Non‐randomised study design |
| Timlin 2010 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study |
| UK Prospective Diabetes Study (UKPDS) Group 1998 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study. Authors stated that outcomes in relation to foot ulcers were not collected |
| Vaccaro 2012 | Foot ulcer healing outcomes or other outcomes relevant to this review were not investigated in this study. The study investigated major leg amputations (above the ankle) and these were not in relation to foot ulcers |
| Vadstrup 2009 | Foot ulcer healing outcomes or other outcomes in relation to intensive versus conventional glycaemic control were not reported. Authors could not provide data on ulcer specific outcomes |
| Van Olmen 2013 | Foot ulcer healing outcomes or other outcomes in relation to intensive versus conventional glycaemic control were not reported. The main outcome measure is number of foot ulcers, this study is ongoing currently. No preliminary results published to date. Proportion of patients with good glycaemic control used rather than mean HbA1c to randomise patients. Authors were unable to provide any further information |
| Williams 2012 | Foot ulcer healing outcomes or other outcomes in relation to intensive versus conventional glycaemic control were not reported. Foot inspections were reported however this was not in relation to foot ulcers or ulcer healing outcomes. Authors were unable to provide any further information |
| Zhang 2011 | Localised injection of insulin improved systemic glycaemic control, however foot ulcer healing outcomes or other outcomes in relation to intensive versus conventional glycaemic control were not reported. The author was unable to provide any additional information |
| Zhang 2013 | Foot ulcer healing outcomes or other outcomes in relation to intensive versus conventional glycaemic control were not reported. Outcomes reported were not specific to the impact of glycaemic control |
| Zhenghua 2011 | Participants were not in intensive versus conventional glycaemic control arms for comparison |
Please note that when 'foot ulcer outcomes not investigated in study' was used as a reason for exclusion this was based on whether studies reported outcomes as deemed appropriate as per the review protocol and/or where data were not available from authors or where no primary outcomes were investigated at all.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000418774.
| Trial name or title | Effects of Galvus (vildagliptin) on markers of inflammation in diabetic foot ulcer: a prospective, randomized, double‐blind, placebo‐controlled pilot study |
| Methods | The objective of this trial is to study the effects of vildagliptin therapy on inflammatory markers in subjects with diabetic foot ulcer. The study plans to prospectively enrol 50 patients with proven diabetic foot ulcer and randomize them in a 1:1, ratio to vildagliptin + metformin or placebo + metformin. The study expects to show an improvement (defined as > 20% serum IL‐6 reduction between the baseline and 3‐month assessment) that comprise the primary end point in at least 50% of the patients randomized to vildagliptin and metformin therapy and in < 10% of the patients randomized to metformin and placebo therapy. The proposed sample size was calculated to demonstrate a significant improvement in the intervention group compared to the control group with at least 80% power and a two‐sided 5% type 1 error. The authors have quoted a sample size of 44 patients and a 1:1 randomization design to allow for an approximate dropout rate of 10%. Therefore it is planned that 50 patients will be recruited: this will result in approximately 25 patients in the vildagliptin + metformin ‐ intervention group and in the placebo + metformin ‐ control group. The data from the study is planned to be pooled and summarized with respect to demographic and baseline characteristics and efficacy and safety observations. Exploratory analyses will be performed using descriptive statistics. Data will be presented for the complete intent‐to‐treat population (all patients having taken at least one dose of study medication) as well as the per‐protocol population (all patients who completed the study without major protocol deviations). |
| Participants |
Inclusion criteria 1) Subjects ≥18 years of age diagnosed with diabetes (type 1 or 2) on diet only or any diabetic medication regime. 2) Existing diabetes index foot ulcer grade A1 or higher according to the University of Texas Wound Classification System of Diabetic Foot Ulcers on the day of study inclusion. A foot ulcer will be defined as any full thickness skin defect existing for at least 14 days. In patients with multiple diabetic foot ulcers the index foot ulcer is defined as the foot ulcer with the largest wound area at the time of inclusion. 3) A sub‐optimal HbA1c ≥ 7.0% documented somewhere in the patient source documents within 12 weeks prior to study inclusion or on the day of study inclusion. Exclusion criteria 1. Exclusion criteria will comply with local label 2. Clinical infection at the studied ulcer site (bacterial and fungal) 3. Planned surgical intervention for the ulcer 4. Hypersensitivity to either of the study drug components 5. History of lactic acidosis 6. Type 1 diabetes 7. Current HbA1c < 7 or > 9% 8. Current Insulin treatment. 9. Active treatment with GLP‐1 or other DPP4i medication 10. Use of thiazolidinediones, statins, anti‐inflammatory or anti‐platelet agents 11. Clinically significant lower‐extremity ischemia (as defined by an ankle/brachial index of < 0.65) 12. Significant medical conditions that would impair wound healing will also be excluded from the study. These conditions include hepatic, respiratory or cardiac failures, aplastic anemia, scleroderma and malignancy, treatment with immunosuppressive agents or steroids, myocardial infarcts, stroke, major surgery within six months of the study, usage of tobacco 13. Severe non‐proliferative or proliferative diabetic retinopathy 14. Active Charcot's foot as determined by clinical and radiographic examination 15. Ulcer of a non‐diabetic pathophysiology (e.g. rheumatoid, radiation‐related, and vasculitis‐related ulcers, calciphylaxis or dystrophic calcinosis cutis) 16. Active malignancy other than basal cell carcinoma as well as subjects with cancerous or pre‐cancerous lesions in the ulcer area 17. Renal dysfunction: eGFR < 60 ml/min 18. Chronic inflammation (inflammatory bowel disease, inflammatory or rheumatoid arthritis) 19. Pregnancy, lactation or child‐bearing potential 20. Recent venous thromboembolism 21. Inability to comply with study protocol |
| Interventions | Arm 1: (Intervention group) will be on oral metformin 500 mg to 3000 mg per oral in single or divided dosages (two or three times a day) plus vildagliptin 50 mg to 100 mg per day also to be administered orally for 12 weeks. The dosages of both medications will be determined based on blood glucose levels with the aim of achieving average fasting blood glucose of < 7 mmol/l and post‐prandial blood glucose of 10 mmol/l or below. Improved adherence will be enhanced by weekly clinic visit and drug tablet return as well as monitoring blood glucose control and progress of the diabetic foot ulcer. Arm 2: (Comparator group) will be on given metformin as described above plus placebo comprising lactose 50 mg to 100 mg per day to be taken orally for 12 weeks. The placebo will be identical in appearance to vildagliptin without the active ingredient. |
| Outcomes | Primary outcome: significant (20%) reduction of serum levels of IL‐6. IL‐6 will be quantified using the Multiplex FlowCytomix system (Bendermedsystems). Antibody‐coated beads will be incubated with either patient serum, followed by a biotin‐conjugated secondary antibody and finally streptavidin‐PE. The sample will be run on BD caliber. Analysis will be performed using software provided by the manufacturer. Secondary outcomes: a) Partial or complete closure of foot ulcer b) Worsening of the foot ulcer beyond Wagner grade 2 c) Requirement for limb or toe amputation These will be based on weekly measurement of the ulcer at the start (week 1) and week 12. |
| Starting date | 1/08/2013 |
| Contact information | Assoc. Professor Usman H. Malabu; FRCPI, FRACP. School of Medicine and Dentistry James Cook University & Townsville Hospital 100 Angus Smith Drive Douglas, Townsville QLD 4814,Australia |
| Notes | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364001 *All content above adapted from the published clinical trial record |
NCT01472432.
| Trial name or title | DPP IV inhibition facilitates healing of chronic foot ulcers in type 2 diabetes |
| Methods | This study is a randomized versus placebo trial designed to evaluate the clinical and humoral effects of four months of vildagliptin on healing of chronic ulcers in type 2 diabetes. Both micro and macroangiopathy strongly contribute to development and delayed healing of diabetic wounds, through an impaired tissue feeding and response to ischemia. HIF‐1α and VEGF, as well as the NO production from iNOS, may contribute to limitation of hypoxic injury by promoting angiogenesis and wound healing. Experimental and pathological studies suggest that the incretin hormone glucagon‐like peptide‐1 (GLP‐1) may improve VEGF generation, and promote pancreatic islet viability through the up‐regulation of HIF1α.Therefore, the aim of this study is to evaluate the effect of the augmentation of GLP‐1, by inhibitors of the dipeptidyl peptidase IV (DPP‐4), such as vildagliptin, on HIF‐1α, VEGF and iNOS in diabetic chronic ulcers. |
| Participants | Inclusion Criteria
Exclusion Criteria
|
| Interventions | Experimenta group: vildagliptin The experimental arm will follow the treatment of placebo group, but received also vildagliptin 50 mg per os b.i.d. for four months. Placebo group: the dose of other concomitant hypoglycaemic medication will be changed to obtain a similar profile of metabolic parameters. Additional antidiabetic therapy, including sulphonylurea, metformin, and insulin, was titrated for optimal glycaemic control for three months. All patients will have diabetes and at least one full‐thickness wound below the ankle for > 3 months. All patients will be examined weekly for the first four weeks (day 28) then every other week until day 120 or ulcer closure by any means. At each visit, tracings of the wound margins will be made for computer planimetry to document changes in wound size, and photographs will be taken for a visual record. All patients will be followed up for regular treatment at the multidisciplinary diabetic foot clinic, included treatment of infection, debridement, off‐loading, and metabolic control according to high international standards and standard good medical practice. |
| Outcomes | Primary Outcome Measures
Secondary Outcome Measures:
|
| Starting date | Unknown, last updated November 15, 2011 |
| Contact information | Raffaele Marfella, Assistant Professor, Second University of Naples Second University of Naples Naples, Italy, I‐80100 |
| Notes | https://clinicaltrials.gov/ct2/archive/NCT01472432 *All content above adapted from the published clinical trial record |
The information for the ongoing trials listed in the table above was adapted directly from the clinical trial registration source referenced in the notes section.
Differences between protocol and review
We changed the term diabetes foot ulcer to diabetic foot ulcer to be consistent with other reviews investigating various modalities on foot ulcer healing in people with diabetes. Also please note that Incidence of amputation cited as a primary outcome measure in this review related to amputation as a result of foot ulceration and not "all cause" amputation.
Contributions of authors
Malindu Fernando: Designed and drafted the protocol and review. Approved the final manuscript for submission to the Wounds Group. Searched background information for protocol, organised group meetings to discuss and co‐ordinate protocol development, and wrote the protocol. Carrried out literature searches and abstract screening, and contacted authors of eligible studies for additional information. Approved the final manuscript for submission to Cochrane Wounds. Ridmee Seneviratne: Drafted the protocol and review, assisted in organising group meetings to discuss the protocol. Searched background information, assisted in retrieval of papers, and wrote the protocol. Carried out literature searches and abstract screening, and contacted authors of eligible studies for additional information. Co‐authored final review. Edited and approved the final manuscript for submission to Cochrane Wounds. Yong Mong Tan: Developed and edited the protocol and provided intellectual contributions, advice, and content‐related expertise. Assisted with abstract screening and study inclusion. Edited and approved the final manuscript for submission to Cochrane Wounds. Peter Lazzarini: Edited, developed and made an intellectual contribution to the protocol, as well as providing advice on certain aspects of it and providing content‐related expertise. Assisted with abstract screening and study inclusion. Edited and approved the final manuscript for submission to Cochrane Wounds. Kunwarjit Sangla: Developed and edited the protocol, and provided intellectual contributions, advice, and content‐related expertise. Assisted with abstract screening and study inclusion. Edited and approved the final manuscript for submission to Cochrane Wounds. Margaret Cunningham: Edited, developed and made an intellectual contribution to the protocol, as well as writing parts of it and providing advice on certain aspects. Assisted with abstract screening and study inclusion. Edited and approved the final manuscript for submission to Cochrane Wounds. Petra Buttner: Developed and edited the protocol and provided intellectual contributions and statistical methodological advice. Assisted with abstract screening and study inclusion. Assisted with article translation from German. Edited and approved the final manuscript for submission to Cochrane Wounds. Jonathan Golledge: Developed and edited the protocol, and assisted significantly with the final version for submission. Provided methodological and clinical guidance on the topic. Assisted with abstract screening and study inclusion. Edited and approved the final manuscript for submission to Cochrane Wounds.
Contributions of editorial base
Liz McInnes, Editor: advised on methodology, interpretation and content. Approved the final review prior to submission. Sally Bell‐Syer and Gill Rizzello: co‐ordinated the editorial process. Advised on interpretation and content. Edited the review. Ruth Foxlee: designed and carried out the search strategy. Reetu Child and Rocio Rodriguez‐Lopez ran updated searches.
Sources of support
Internal sources
-
James Cook University, Australia.
Institutional resources and support
-
Queensland Health, Australia.
Institutional resources and support
External sources
This project was supported by the National Institute for Health Research via Cochrane Programme Grant funding. The views and opinions expressed herein are not those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Malindu Fernando: none known. Ridmee Seneviratne: none known. Yong Mong Tan: Over the years I have received grants as principal and co‐investigator of local sites of multicentre and multinational clinical trials, including those sponsored by pharmaceutical companies. I have also received honoraria from pharmeceutical companies for giving talks, and in advisory committees and sponsorship to international scientific meetings. I have no conflict of interest with respect to this project. Peter Lazzarini: The author declares that he is a former board member of the Australasian Podiatry Council that received sponsorship money for footwear companies (New Balance, Clarks and Steel Blue) and a pharmaceutical company (Novartis). He also received payment from a pharmaceutical company (AstraZeneca) to provide lectures to General Practitioners on foot care for people with diabetes and from a wound care company (Urgo Medical) to provide advice on wound care products. Lastly he has received grants to his institution for diabetic foot clinical and research products from the Wound Management Innovation Cooperative Research Centre (Australia). Kunwarjit Sangla: I have received a number of pharmaceutical and non‐pharmaceutical grants as the principal investigator over the years for research in the field of Diabetes and Endocrinology. But this was always as member of a group. No money ever has been paid to me as part of the grants for services rendered. I have received speaker honoraria from pharmaceutical companies over the years for topics unrelated to this Cochrane review. Margaret Cunningham: none known. I have provided consultancy work related to cancer research to University of Stirling, Macmillan Cancer Research and the European Society of Oncology Nursing. The consultancy work had no relationship to any aspect of the submitted work. Petra Buttner: Although I am a director of a company (Tropical Health Solutions), my involvement with this company has nothing to do with this review. I am involved in this review because of my position as Associate Professor at James Cook University which ended in December 2013 and my expertise in statistics and epidemiology. Neither I nor THS will have any financial gain from this review. Jonathan Golledge: I have received support to speak at a number of meetings not related to this project. I have received funding from a number of competitive grants unrelated to this topic.
New
References
References to studies included in this review
Idris 2004 {unpublished data only}
- Idris I, Game F, Jeffocoate W. Does close glycaemic control promote healing in diabetic foot ulcers? Report of a feasibility study. Diabetic Medicine 2004;22:1060‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraira 1992 {published data only (unpublished sought but not used)}
- Abraira C, Emanuele N, Colwell J, Henderson W, Comstock J, Seymor L, et al. Glycemic control and complications in Type II diabetes: design of a feasibility trial. Diabetes Care 1992;15(11):1560‐71. [DOI] [PubMed] [Google Scholar]
Abraira 2003 {published data only (unpublished sought but not used)}
- Abraira C, Duckworth W, McCarren M, Emanuele N, Arca D, Reda D, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. Journal of Diabetes and Its Complications 2003;17:314‐22. [DOI] [PubMed] [Google Scholar]
ACTRN12606000426583 {unpublished data only}
- Ogrin R. Non‐weight bearing exercise in people with diabetes and foot complications. http://www.anzctr.org.au/ (accessed 10 April 2015).
Althouse 2013 {published data only}
- Althouse A, Abbott J, Sutton‐Tyrrell K, Forker A, Lombardero M, Buitron L, et al. Favorable effects of insulin sensitizers pertinent to peripheral arterial disease in Type 2 diabetes: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Cardiovascular and Metabolic Risk 2013;36:3269‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayat 2013 {published data only}
- Bayat F, Shojaeezadeh D, Baikpour M, Heshmat R, Baikpour M, Hosseini M. The effects of education based on extended health belief model in type 2 diabetic patients: a randomized controlled trial. Journal of Diabetes & Metabolic Disorders 2013;12(45):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloomgarden 1987 {published data only (unpublished sought but not used)}
- Bloomgarden Z, Karmally W, Metzger J, Brothers M, Nechemias C, Bookman J, et al. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care 1987;10(3):263‐72. [DOI] [PubMed] [Google Scholar]
Boaz 2009 {published data only}
- Boaz M, Hellman K, Wainstein J. An automated telemedicine system improves patient‐reported well‐being. Diabetes Technology and Therapeutics 2009;11(3):181‐6. [DOI] [PubMed] [Google Scholar]
Calles‐Escandon 2010 {published data only (unpublished sought but not used)}
- Calles‐Escandon J, Lovato L, Simons‐Morton D, Kendall D, Pop‐Busui R, Cohen R, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:721‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiu 2011 {published data only}
- Chiu CC, Huang CL, Weng S‐F, Sun LM, Chang YL, Tsai FC. A multidisciplinary diabetic foot ulcer treatment programme significantly improved the outcome in patients with infected diabetic foot ulcers. Journal of Plastic, Reconstructive & Aesthetic Surgery 2011;64:867‐72. [DOI] [PubMed] [Google Scholar]
Christiansen 2009 {published data only (unpublished sought but not used)}
- Christensen L, Almdal T, Boesgaard T, Breum L, Dunn E, Gade‐Rasmussen B, et al. Study rationale and design of the CIMT trial: The Copenhagen Insulin and Metformin Therapy trial. Diabetes, Obesity and Metabolism 2009;11:315‐22. [DOI] [PubMed] [Google Scholar]
Clark 2001 {published data only}
- Clark C, Snyder J, Meek R, Stutz L, Parkin C. A systematic approach to risk stratification and intervention within a managed care environment improves diabetes outcomes and patient satisfaction. Diabetes Care 2001;24:1079‐86. [DOI] [PubMed] [Google Scholar]
Dormandy 2005 {published data only}
- Dormandy J, Charbonnel B, Eckland D, Erdmann E, Massi‐Benefetti M, Moules I, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366(9493):1279‐89. [DOI] [PubMed] [Google Scholar]
Duckworth 2009 {published data only (unpublished sought but not used)}
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven P, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England Journal of Medicine 2009;360(2):129‐39. [DOI] [PubMed] [Google Scholar]
Fresenius 2009 {published data only (unpublished sought but not used)}
- Fresenius K, Framer I. Implementation and evaluation of pharmaceutical care on the outcomes of patients suffering from diabetic foot syndrome. Krankenhauspharmazie 2009;30(1):2‐10. [Google Scholar]
Gaede 2003 {published data only (unpublished sought but not used)}
- Gaede P, Vedel P, Larsen N, Gunnar V, Jensen G, Parving H, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. The New England Journal Of Medicine 2003;348(5):383‐93. [DOI] [PubMed] [Google Scholar]
Gaede 2006 {published data only}
- Gaede P. Intensified multifactorial intervention in patients with type 2 diabetes and microalbuminuria: rationale and effect on late‐diabetic complications. Danish Medical Bulletin 2006;53:258‐84. [PubMed] [Google Scholar]
Garber 2002 {published data only}
- Garber A, Larsen J, Schneider S, Piper B, Henry D on behalf of The Glyburide/Metformin Initial Therapy Study Group. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes, Obesity and Metabolism 2002;4:201‐8. [DOI] [PubMed] [Google Scholar]
Gram 2011 {published data only}
- Gram J, Henriksen J, Grodum E, Juhl H. Hansen R, Christiansen C, et al. Pharmacological treatment of the pathogenetic defects in type 2 diabetes: the randomized multicenter South Danish diabetes study. Diabetes Care 34;1:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2011 {published data only}
- Griffin S, Knut B, Davies M, Khunti K, Rutten G. Effect of early intensive multifactorial therapy on 5‐year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION‐Europe): a cluster‐randomised trial. Lancet 2011;378:156‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarnert 2012 {published data only}
- Jarnert C, Kalani M, Ryden L, Bohm F. Strict glycaemic control improves skin microcirculation in patients with type 2 diabetes: a report from the Diabetes mellitus And Diastolic Dysfunction (DADD) study. Diabetes & Vascular Disease Research 2012;9(4):287‐95. [DOI] [PubMed] [Google Scholar]
Kawamori 2010 {published data only}
- Kawamori R. Evidence demonstrating the effects of anti‐atherosclerotic actions of pioglitazone ‐ special emphasis on PROactive Study and PERISCOPE Study. Japanese Journal of Clinical Medicine 2010;68(2):235‐41. [PubMed] [Google Scholar]
Kostev 2012 {published data only}
- Kostev K, Dippel FW, Rockel T, Siegmund T. Risk of diabetic foot ulceration during treatment with insulin glargine and NPH insulin. Journal of Wound Care 2012;21:483‐9. [DOI] [PubMed] [Google Scholar]
Leung 2012 {published data only}
- Leung P, Pang S, Wong E, Cheng K. Inflammatory state of Type II diabetic patients with chronic ulcers in response to herbal treatment. The Foot 2012;22(3):181‐5. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Y, Song ZR, Ren HZ. Alprostadil combined with deproteinized calf blood extractives injection for treatment of diabetic foot. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12:9970‐2. [Google Scholar]
Litzelman 1993 {published data only}
- Litzelman D, Slemenda C, Langefeld C, Hays L, Welch M, Bild D, et al. Reduction of lower extremity clinical abnormalities in patients with non‐insulin‐dependent diabetes mellitus. A randomized, controlled trial. Annals of Internal Medicine 1993;119(1):36‐41. [DOI] [PubMed] [Google Scholar]
Marso 2013 {published data only}
- Marso S, Poulter N, Nissen S, Nauck M, Zinman B, Daniels G, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. American Heart Journal 2013;166:823‐30. [DOI] [PubMed] [Google Scholar]
Martinez‐Sanchez 2005 {published data only (unpublished sought but not used)}
- Martinez‐Sanchez G, Al‐Dalain S, Menendez S, Giuliani L, Candelario‐Jalil E, Alvarez H, et al. Therapeutic efficacy of ozone in patients with diabetic foot. European Journal of Pharmacology 2005;523:151‐61. [DOI] [PubMed] [Google Scholar]
McMurray 2002 {published data only}
- McMurray S, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. American Journal of Kidney Diseases 2002;40(3):566‐75. [DOI] [PubMed] [Google Scholar]
Meigs 2003 {published data only}
- Meigs J, Cagliero E, Dubey A, Murphy‐Sheehy P, Gildesgame C, Chueh H. A controlled trial of web‐based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care 2003;26(3):750‐7. [DOI] [PubMed] [Google Scholar]
Nathan 2009 {published data only}
- Nathan D, Zinman B, Cleary P, Blacklund J, Genuth S, Miller R, et al. Modern‐day clinical course of type 1 diabetes mellitus after 30 years' duration: the Diabetes Control and Complications trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience. Archives of Internal Medicine 2009;169(14):1307‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00850798 {unpublished data only}
- Chen HS, Lin H. The benefits of intensive glycemic control in elderly patients with type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00850798 (accessed 10 April 2015).
NCT01421966 {unpublished data only}
- Macrocure Ltd. Evaluation of CureXcell® in treating lower extremity chronic ulcers in adults with diabetes. https://clinicaltrials.gov/ct2/show/NCT01421966 (accessed 10 April 2015).
NCT01813305 {unpublished data only}
- Charsire Biotechnology Corp. CSTC1 for diabetic foot ulcers phase II study. https://clinicaltrials.gov/ct2/show/NCT01813305 (accessed 10th April 2015).
Nybo 2011 {published data only}
- Nybo M, Preil SR, Juhl H, Olesen M, Yderstraede K, Gram J, et al. Rosiglitazone decreases plasma levels of osteoprotegerin in a randomized clinical trial with type 2 diabetes patients. Basic & Clinical Pharmacology & Toxicology 2011;109:481‐5. [DOI] [PubMed] [Google Scholar]
Pedersen 2003 {published data only}
- Pedersen O, Gaede P. Intensified multifactorial intervention and cardiovascular outcome in type 2 diabetes: the Steno‐2 study. Metabolism: clinical and experimental 2003;52(8):19‐23. [DOI] [PubMed] [Google Scholar]
Rong 2012 {published data only}
- Rong L, Xia D, Luo Z, Aimin Z, Cao M. Two‐year foot care program for minority patients with type 2 diabetes mellitus of Zhuang Tribe in Guangxi, China. Canadian Journal of Diabetes 2012;36(1):8‐5. [Google Scholar]
Sullivan 2009 {published data only}
- Sullivan S, Alfonso‐Cristancho R, Conner C, Hammer M, Blonde L. Long‐term outcomes in patients with type 2 diabetes receiving glimepiride combined with liraglutide or rosiglitazone. Cardiovascular Diabetology 2009;8(12):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Timlin 2010 {published data only}
- Timlin L, Beaudet A, WIlson B, Bruhn D, Boye J, Palmer J, et al. Long‐term clinical outcomes of exenatide once‐weekly versus insulin glargine for the treatment of type 2 diabetes projected using the core diabetes model. Value in health 2010 Conference: ISPOR 13th Annual European Congress Prague Czech Republic. Prague Czech Republic.: ISPOR, 2010; Vol. Conference Publication:284‐85.
UK Prospective Diabetes Study (UKPDS) Group 1998 {published data only}
- UK Prospectice Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
Vaccaro 2012 {published data only}
- Vaccaro O, Masulli M, Bonora E, Prato S, Giorda C, Maggioni A P, et al. Addition of either pioglitazone or a sulfonylurea in type 2 diabetic patients inadequately controlled with metformin alone: impact on cardiovascular events. A randomized controlled trial. Nutrition, Metabolism & Cardiovascular Diseases 2012;22:997‐1006. [DOI] [PubMed] [Google Scholar]
Vadstrup 2009 {published data only}
- Vadstrup E, Frolich A, Perrild H, Borg E, Roder M. Lifestyle intervention for type 2 diabetes patients – trial protocol of The Copenhagen Type 2 Diabetes Rehabilitation Project. BMC Public Health 2009;9:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Olmen 2013 {published data only}
- Olmen J, Ku GM, Pelt M, Kalobu J, Hen H, Darras C, et al. The effectiveness of text messages support for diabetes self‐management: protocol of the TEXT4DSM study in the democratic Republic of Congo, Cambodia and the Philippines. BMC Public Health 2013;13:423‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2012 {published data only}
- Williams ED, Bird D, Forbes A, Russell A, Ash S, Friedman R, et al. Randomised controlled trial of an automated, interactive telephone intervention (TLC Diabetes) to improve type 2 diabetes management: baseline findings and six‐month outcomes. BMC Public Health 2012;12:602‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang ZX, Liu X, Lu L, Zhang L, Di DL, Liu LH. Effect of insulin by local injection on the level of systemic blood glucose and granulation tissue formation of wound in patients with diabetic foot ulcer. Chinese Journal of Burns 2011;27(6):451‐5. [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang J, Burridge L, Baxter K, Donald M, Foster M, Hollingworth S, et al. A new model of integrated primary‐secondary care for complex diabetes in the community: study protocol for a randomised controlled trial. Trials 2013;14:382‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhenghua 2011 {published data only}
- Zhenghua X, Dingyu C, Qiling Y, Qian Z, Jin X, Chunling H, et al. Individualised diabetic education can contribute to decrease the incidence of diabetic foot and avoid amputation: results of a 9‐year prospective study. Diabetologia 2011;54:S32. [Google Scholar]
References to ongoing studies
ACTRN12613000418774 {unpublished data only}
- Malabu UH. Effects of Galvus (vildagliptin) on interleukin‐6 in diabetic foot ulcer. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364001 (accessed 10 April 2015).
NCT01472432 {published data only}
- Marfella R. Dipeptidyl peptidase (DPP) IV inhibition facilitates healing of chronic foot ulcers in patients with type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT01472432 (accessed 10 April 2015). [DOI] [PMC free article] [PubMed]
Additional references
ADA 2012
- American Diabetes Association. Standards of medical care in diabetes 2012. Diabetes Care 2012;35(Suppl 1):11‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADVANCE 2008
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560‐72. [DOI] [PubMed] [Google Scholar]
Apelqvist 1999
- Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot?. Diabetes/Metabolism Research and Reviews 1999;16(Suppl 1):75‐83. [DOI] [PubMed] [Google Scholar]
Armstrong 1998
- Armstrong D, Lavery DC, Harkless L. Validation of a diabetic wound classification system ‐ the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21(5):855‐9. [DOI] [PubMed] [Google Scholar]
Bagg 2001
- Bagg W, Whalley GA, Gamble G, Drury PL, Sharpe N, Braatvedt GD. Effects of improved glycaemic control on endothelial function in patients with type 2 diabetes. Internal Medicine Journal 2001;31(6):322‐8. [DOI] [PubMed] [Google Scholar]
Boulton 2004
- Boulton AJM. The diabetic foot: from art to science: the 18th Camillo Golgi lecture. Diabetologia 2004;47(8):1343‐53. [DOI] [PubMed] [Google Scholar]
Brownlee 2004
- Brownlee M. The pathobiology of diabetic complications. A unifying mechanism. Diabetes 2004;54(6):1615‐25. [DOI] [PubMed] [Google Scholar]
Burakowska 2006
- Korzon‐Burakowska A, Edmonds M. Role of the microcirculation in diabetic foot ulceration. International Journal of Lower Extremity Wounds 2006;5(3):144‐8. [DOI] [PubMed] [Google Scholar]
Cheung 2009
- Cheung NW, Conn JJ, D'Emden MC, Gunton JE, Jenkins AJ, Ross GP, et al. Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Medical Journal of Australia 2009;191:339–44. [DOI] [PubMed] [Google Scholar]
Christman 2011
- Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. Journal of Investigative Dermatology 2011;131(10):2121‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clayton 2009
- Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Journal of Clinical Diabetes 2009;27(2):52‐8. [Google Scholar]
Cook 2012
- Cook JJ, Simonson DC. Epidemiology and health care cost of diabetic foot problems. In: Veves A editor(s). The Diabetic Foot: Medical and Surgical Management. Boston: Contemporary Diabetes, 2012:17‐32. [Google Scholar]
D'Souza 2009
- D'Souza DR, Salib MM, Bennett J, Mochin‐Peters M, Asrani K, Goldblum SE, et al. Hyperglycemia regulates RUNX2 activation and cellular wound healing through the aldose reductase polyol pathway. Journal of Biological Chemistry 2009;284(27):17947‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daroux 2010
- Daroux M, Prévost G, Maillard‐Lefebvre H, Gaxatte C, D'Agati VD, Schmidt AM, et al. Advanced glycation end‐products: implications for diabetic and non‐diabetic nephropathies. Diabetes and Metabolism 2010;36:1‐10. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Delamaire 1997
- Delamaire M, Maugendre D, Moreno M, Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Medicine 1997;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
Dinh 2005
- Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. International Journal of Lower Extremity Wounds 2005;4(3):154‐9. [DOI] [PubMed] [Google Scholar]
Electronic Therapeutic Guidelines Australia 2012
- ETG. Type 2 diabetes and cardiovascular disease risk. Australian Electronic Therapeutic Guidelines http://www.tg.org.au/etg_demo/phone/tgc/cvg/951.htm (accessed 10 December 2012).
Endnote 2012
- Endnote. http://endnote.com/. Thomson Reuters, 2012.
Falanga 2005
- Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet 2005;366(9498):1736–43. [DOI] [PubMed] [Google Scholar]
Game 2012
- Game F. Choosing life or limb. Improving survival in the multi‐complex diabetic foot patient. Diabetes/Metabolism Research and Reviews 2012;28(Suppl 1):97‐100. [DOI] [PubMed] [Google Scholar]
Geraldes 2010
- Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Journal of Circulation Research 2010;106(8):1319‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giacco 2010
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation Research 2010;107(9):1058‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2010
- Gupta SK, Panda S, Singh SK. The etiopathogenesis of the diabetic foot: an unrelenting epidemic. The International Journal of Lower Extremity Wounds 2010;9(3):127–31. [DOI] [PubMed] [Google Scholar]
Hemmingsen 2011a
- Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta‐analysis and trial sequential analysis of randomised clinical trials. BMJ 2011;343:d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemmingsen 2011b
- Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008143.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7417):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks J, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinchliffe 2012
- Hinchliffe RJ, Andros G, Apelqvist J, Bakker K, Friederichs S, Lammer J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes/Metabolism Research and Reviews 2012;28(Suppl 1):179‐217. [DOI] [PubMed] [Google Scholar]
Hogg 2012
- Hogg FR, Peach G, Price P, Thompson MM, Hinchliffe RJ. Measures of health‐related quality of life in diabetes‐related foot disease: a systematic review. Diabetologia 2012;55(3):552‐65. [DOI] [PubMed] [Google Scholar]
IDF 2012
- International Diabetes Federation. IDF diabetes atlas update 2012. The global burden. http://www.idf.org/diabetesatlas/5e/the‐global‐burden (accessed 10 December 2012).
Ikem 2010
- Ikem R, Ikem I, Adebayo O, Soyoye D. An assessment of peripheral vascular disease in patients with diabetic foot ulcer. The Foot 2010;20(4):114‐7. [DOI] [PubMed] [Google Scholar]
Inzucchi 2012
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Journal of Diabetes Care 2012;35(6):1364‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffcoate 2004
- Jeffcoate WJ, Price P, Harding KG, International Working Group on Wound Healing and Treatments for People with Diabetic Foot Ulcers. Wound healing and treatments for people with diabetic foot ulcers. Diabetes/Metabolism Research and Reviews 2004;20(Suppl 1):S78‐89. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2006
- Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer‐related and person‐related measures. Diabetes Care 2006;29(1):1784‐7. [DOI] [PubMed] [Google Scholar]
Jones 2008
- Jones RN, Marshall WP. Does the proximity of an amputation, length of time between foot ulcer development and amputation, or glycemic control at the time of amputation affect the mortality rate of people with diabetes who undergo an amputation?. Advances in Skin & Wound Care 2008;21(3):118‐23. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ma 2009
- Ma J, Yang W, Fang N, Zhu W, Wei M. The association between intensive glycemic control and vascular complications in type 2 diabetes mellitus: a meta‐analysis. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 2009;19(9):596‐603. [DOI] [PubMed] [Google Scholar]
Macisaac 2011
- Macisaac RJ, Jerums G. Intensive glucose control and cardiovascular outcomes in type 2 diabetes. Heart, Lung and Circulation 2011;20(10):647‐54. [DOI] [PubMed] [Google Scholar]
Margolis 2013
- Margolis DJ, Jeffcoate W. Epidemiology of foot ulceration and amputation: can global variation be explained?. Medical Clinics of North America 2013;97(1):791‐805. [DOI] [PubMed] [Google Scholar]
Mattila 2010
- Mattila TK, Boer A. Influence of intensive versus conventional glucose control on microvascular and macrovascular complications in type 1 and 2 diabetes mellitus. Drugs 2010;70(17):2229‐45. [DOI] [PubMed] [Google Scholar]
Mazzone 2010
- Mazzone T. Intensive glucose lowering and cardiovascular disease prevention in diabetes reconciling the recent clinical trial data. Circulation 2010;122:2201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG for the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:332‐6. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2012
- Nathan DM. Understanding the long‐term benefits and dangers of intensive therapy of diabetes. Archives of Internal Medicine 2012;172(10):769‐70. [DOI] [PubMed] [Google Scholar]
NCCH 2006
- National Centre for Classification in Health. The Australian Classification of Health Interventions (ACHI) 5th edition: tabular list of Interventions and alphabetic index of interventions. The University of Sydney, Faculty of Health Sciences 2006.
Ogbera 2008
- Ogbera OA, Osa E, Edo A, Chukwum E. Common clinical features of diabetic foot ulcers: perspectives from a developing nation. International Journal of Lower Extremity Wounds 2008;7(2):93‐8. [DOI] [PubMed] [Google Scholar]
Pound 2004
- Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer‐free survival following management of foot ulcers in diabetes. Diabetic Medicine 2005;22(1):1306‐9. [DOI] [PubMed] [Google Scholar]
Rafehi 2010
- Rafehi H, El‐Osta A, Karagiannis TC. Genetic and epigenetic events in diabetic wound healing. International Wound Journal 2010;8(1):12‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robbins 2008
- Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration?. Journal of the American Podiatric Medical Association 2008;98:489‐93. [DOI] [PubMed] [Google Scholar]
Schaper 2004
- Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes/Metabolism Research and Reviews 2004;20(Suppl 1):S90–5. [DOI] [PubMed] [Google Scholar]
Schaper 2012
- Schaper NC. Lessons from Eurodiale. Diabetes/Metabolism Research and Reviews 2012;28(Suppl 1):21‐6. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Shenoy 2012
- Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum Lifelong Learning in Neurology 2012;18(1):192‐8. [DOI] [PubMed] [Google Scholar]
SIGN 2012
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 10 December 2012).
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217‐28. [DOI] [PubMed] [Google Scholar]
Stegenga 2008
- Stegenga ME, Crabben SN, Blümer RM, Levi M, Meijers JC, Serlie MJ, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood 2008;112(1):82‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stratton 2000
- Stratton IM, Adler AI, Neil AW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tesfaye 2012
- Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes/Metabolism Research and Reviews 2012;1(1):8‐14. [DOI] [PubMed] [Google Scholar]
UKPDS 1998
- UKPDS. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837‐53. [PubMed] [Google Scholar]
Wu 2007
- Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Journal of Vascular Health Risk Management 2007;3(1):65‐76. [PMC free article] [PubMed] [Google Scholar]


