Abstract
Background/Aim: Oral cavity cancer is a major health problem worldwide. The herpes zoster vaccine is an effective method to protect against herpes zoster infection. In this study we aimed to determine the relationship between herpes zoster and oral cavity cancer. Patients and Methods: The Longitudinal Generation Tracking Database in Taiwan was used to select oral and non-oral cavity cancer patients. The primary endpoint was herpes zoster. Results: We included 3131 oral cavity cancer patients and 3131 non-oral cavity cancer patients. Patients with oral cavity cancer [adjusted hazard ratio (HR)=1.66, 95% confidence interval (CI)=1.27-2.16] had a significantly higher risk of herpes zoster compared to the control group. The oral cavity patients who received radiotherapy (adjusted HR=1.79, 95%CI=1.12-2.86) had a significantly higher risk of herpes zoster compared to the oral cavity patients who did not receive radiotherapy. Conclusion: Radiotherapy increases the incidence of herpes zoster infection in oral cavity cancer patients.
Keywords: Oral cavity cancer, radiotherapy, herpes zoster
Oral cavity cancer is a major health problem worldwide. In the report from Global Cancer Statistics in 2020, the number of newly developed oral cavity and lip cancer patients was approximately 370,000 worldwide (1).
The treatment of oral cavity cancer depends on the stage of disease. When a patient’s cancer is operable, then the standard treatment is radical operation. The adjuvant treatment can be observation, radiotherapy or chemoradiation, which is determined by the surgical pathology team (2). In unresectable or metastatic oral cavity cancer, chemotherapy and radiotherapy are often given as first line therapy (2).
Herpes zoster, also called shingles, is caused by the reactivation of the latent varicella-zoster virus. This virus can induce chickenpox when first infected, and then remain dormant in the neuron of a dorsal root ganglion or a cranial nerve (3). Later in life, or under a state of compromised immunity, the virus can re-emerge and trigger a unilateral, painful, vesicular rash along the distribution of a dermatome. In European countries and the US, the annual incidence of herpes zoster is estimated at approximately 1-6.2 per 1,000 persons (4-7).
In Taiwan, the herpes zoster annual incidence is approximately 5 per 1,000 persons (8). Thus far, the herpes zoster vaccination is regarded as the most effective measure to prevent its occurrence and reduce postherpetic neuralgia (9,10).
Given these facts, we were interested in investigating the relationship between herpes zoster and oral cavity cancer. Additionally, we aimed to determine whether surgery, radiotherapy or chemotherapy increases the risk of herpes zoster infection or not. The result will be useful in the recommendation of herpes zoster vaccination in oral cavity cancer patients.
Patients and Methods
This study was approved by the Central Regional Research Ethics Committee, China Medical University, Taichung, Taiwan R.O.C. (CMUH109-109-REC2-031).
Data source. We used the database of the Health and Welfare Data Science Center (HWDC), which stores electronically over 99% of the 23 million Taiwanese residents’ medical records. We conducted a nationwide retrospective cohort study using i) the Longitudinal Generation Tracking Database (LGTD 2005), ii) the Registry for catastrophic illness patients (HV), and iii) the Registry for Beneficiaries. The LGTD contains the data of two million individuals randomly selected from the whole population. The International Classification of Diseases, Clinical Modification ICD-9-CM, and the International Classification of Diseases, Tenth Revision, Clinical Modification ICD-10-CM, were used to identify patients with oral cavity cancer or herpes zoster.
Study population. Oral cavity cancer patients newly diagnosed (codes: ICD-9-CM code 140, 142-145, 149 ICD-10-CM C00, C03.0, C03.1, C03.9, C04.0, C04.1, C04.8, C04.9, C05.0, C05.1, C05.2, C05.8, C05.9, C06.0, C06.1, C06.2, C06.80, C06.89, C06.9, C07, C08.0, C08.1, C08.9, C14.0, C14.2, C14.8, Z51.12) from the HV were defined as the oral cavity cancer cohort between 1st of January 2000 to 31st of December 2016. In the oral cavity cancer cohort, the index date was defined as the date of oral cavity cancer diagnosis.
The non-oral cavity cancer cohort consisted of patients randomly selected from the LGTD 2005 without a history of oral cavity cancer or other types of cancer. We randomly selected one non-oral cavity cancer control per oral cavity cancer patient. For the non-oral cavity cancer cohort, the index date was a random date between 2000 and 2016. Exclusion criteria in the control cohort were a prior history of herpes zoster before the index date. Both cohorts were matched 1:1 based on age, gender, and index year, so as to minimize selection bias.
Main outcome and covariates. The primary study endpoint was the herpes zoster ICD-9-CM code 053 ICD-10-CM B02.0, B02.1, B02.21, B02.22, B02.29, B02.23, B02.24, B02.30, B02.31, B02.32, B02.33, B02.34, B02.39, B02.8, B02.7, and B02.9. With regard to the importance of considering confounding factors, we selected patients whose history of comorbidities had been identified before the index date, with either two outpatient medical records or at least one hospitalization record. The list of comorbidities included: i) hypertension, ii) diabetes, iii) hyperlipidemia, iv) chronic obstructive pulmonary disease (COPD), and v) chronic kidney disease (CKD). Oral cavity cancer-related treatments, including radiotherapy, chemotherapy, and surgery, were also considered.
Statistical analysis. A Chi-square test was used to determine if two categorical variables showed differences in distribution. The difference between two continuous variables was tested by the student’s t-test to determine their relationship. In the Cox proportional hazards regression model, we estimated the risk of herpes zoster between the oral cavity cancer cohort and the non-oral cavity cancer cohort using hazard ratios (HRs) and 95% confidence intervals (CIs). The Kaplan-Meier survival curve was plotted using the R studio (3.5.2) and was evaluated using the log-rank test. SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) was used to perform all statistical analyses. A p-Value of 0.05 was used as the level of significance for all analyses.
Result
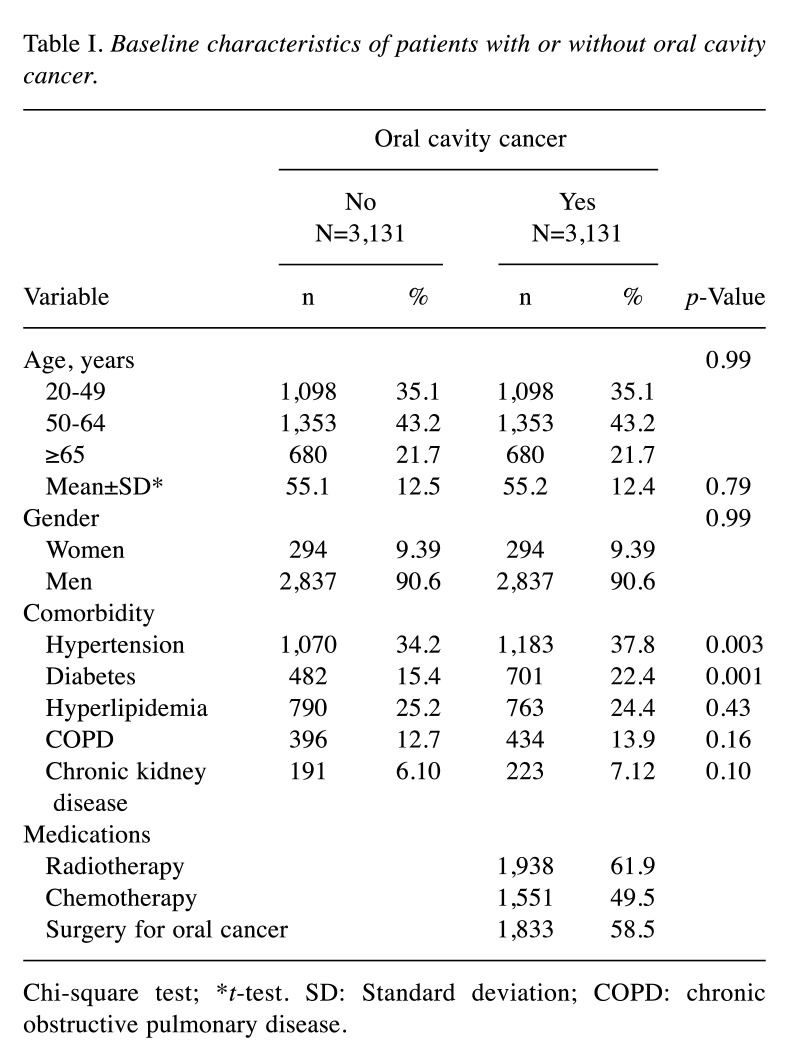
Presented in Table I, we included 3,131 oral cavity cancer patients and 3,131 non-oral cavity cancer patients. Regarding gender, men represented the majority in both the oral cavity cancer (90.6%) and the non-oral cavity cancer cohort (90.6%). The mean age was 55.2 years old at diagnosis in the oral cavity cancer cohort, and the mean age of the non-oral cavity cancer cohort was 55.1 years old. Hypertension and diabetes were more frequent in the oral cavity cancer cohort than in individuals who did not have oral cavity cancer. Of the 3,131 oral cavity cancer patients 61.9% received radiotherapy, 58.5% underwent surgery, and 49.5% received chemotherapy.
Table I. Baseline characteristics of patients with or without oral cavity cancer.
Chi-square test; *t-test. SD: Standard deviation; COPD: chronic obstructive pulmonary disease.
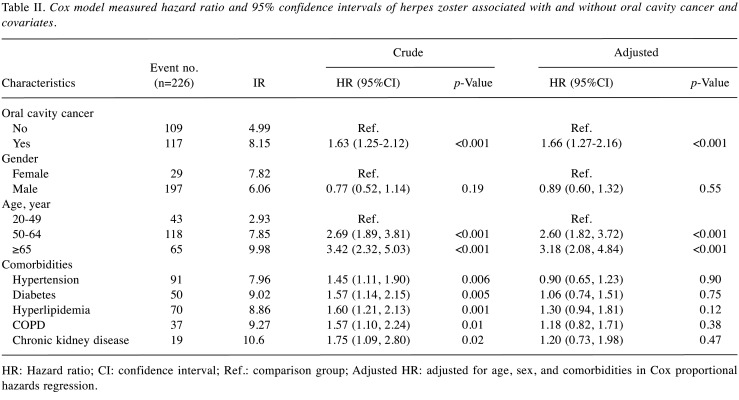
Table II shows the risk of herpes zoster in patients with and without oral cavity cancer and covariates. Patients with oral cavity cancer (adjusted HR=1.66, 95%CI=1.27-2.16) had a significantly higher risk of herpes zoster than the control group. There is no difference in the incidence rate (IR) of herpes zoster between women (IR=7.82) and men (IR=6.06) (p=0.55).
Table II. Cox model measured hazard ratio and 95% confidence intervals of herpes zoster associated with and without oral cavity cancer and covariates.
HR: Hazard ratio; CI: confidence interval; Ref.: comparison group; Adjusted HR: adjusted for age, sex, and comorbidities in Cox proportional hazards regression.
Patients in the 50-64-year old group (adjusted HR=2.60, 95%CI=1.82-3.72) and those in the >64-year old group (adjusted HR=3.18, 95%CI=2.08-4.84) had a significantly higher risk of herpes zoster compared to the 20-49-year old group. Following adjustment for sex, age, and comorbidities, there was no statistical correlation between herpes zoster and any comorbidities.
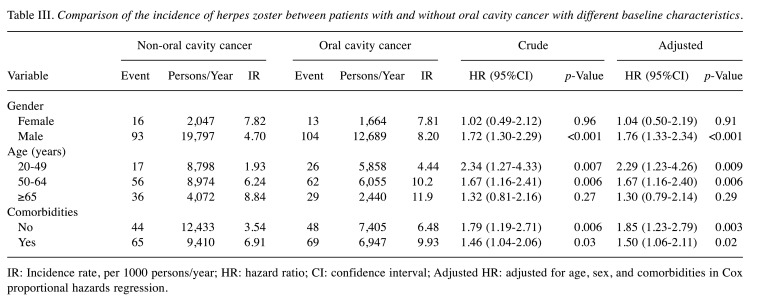
The stratification analysis results are presented in Table III. When targeting gender, male patients (adjusted HR=1.76, 95%CI=1.33-2.34) had a significantly greater risk of herpes zoster in the case group compared to the control group, but this was not the case in females. When stratification targeted age in the 20-49-year old group and comorbidities in the 50-64-year old group, oral cavity cancer patients had a significantly higher risk of herpes zoster compared to non-oral cavity cancer patients, but this was not found to be significant in the >64-year old group.
Table III. Comparison of the incidence of herpes zoster between patients with and without oral cavity cancer with different baseline characteristics.
IR: Incidence rate, per 1000 persons/year; HR: hazard ratio; CI: confidence interval; Adjusted HR: adjusted for age, sex, and comorbidities in Cox proportional hazards regression.
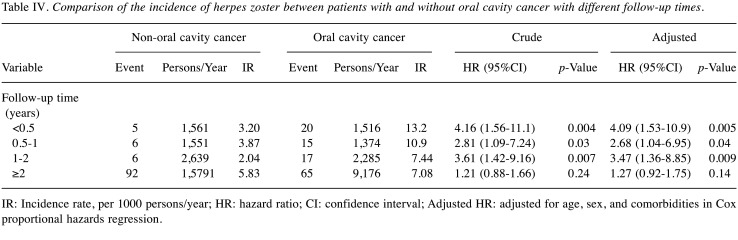
The analysis of the stratification at different follow-up times is shown in Table IV. In the time-stratified-cox regression model, less than a 6-month follow up (adjusted HR=4.09, 95%CI=1.53-10.9), a 6-month to one year follow up (adjusted HR=2.68, 95%CI=1.04-6.95) and a one to two years follow up (adjusted HR=3.47, 95%CI=1.36-8.85) of herpes zoster risk in the oral cavity cancer group were significantly higher compared to the control group. In contrast, follow up of over two years was not found to be statistically significant.
Table IV. Comparison of the incidence of herpes zoster between patients with and without oral cavity cancer with different follow-up times.
IR: Incidence rate, per 1000 persons/year; HR: hazard ratio; CI: confidence interval; Adjusted HR: adjusted for age, sex, and comorbidities in Cox proportional hazards regression.
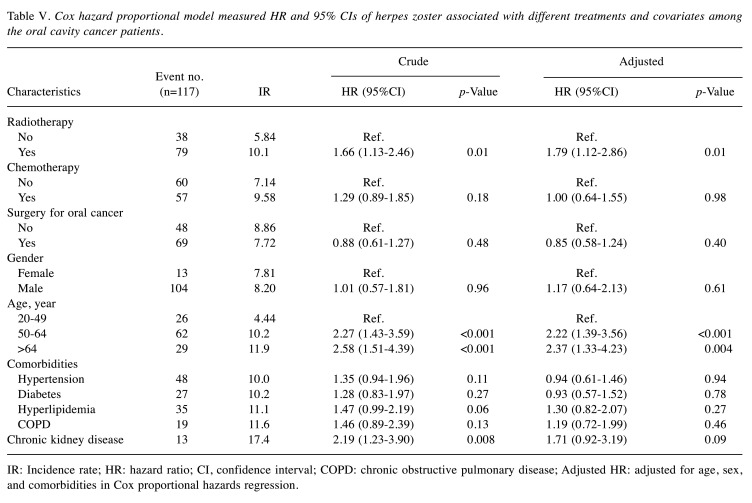
The Cox regression analysis of the different treatment strategies and covariates in oral cavity cancer patients are shown in Table V. When the patients received radiotherapy (adjusted HR=1.79, 95%CI=1.12-2.86), they had a significantly higher risk of herpes zoster. Neither patients receiving chemotherapy nor those undergoing surgery for oral cancer were found to have a significant risk of herpes zoster. The 50-64-year old group (adjusted HR=2.22, 95%CI=1.39-3.56) and the over 64-year old group (adjusted HR=2.37, 95%CI=1.33-4.23) were at a significantly higher risk of herpes zoster compared to the 20-49-year old group. Male and all comorbidities were not risk factors of herpes zoster.
Table V. Cox hazard proportional model measured HR and 95% CIs of herpes zoster associated with different treatments and covariates among the oral cavity cancer patients.
IR: Incidence rate; HR: hazard ratio; CI, confidence interval; COPD: chronic obstructive pulmonary disease; Adjusted HR: adjusted for age, sex, and comorbidities in Cox proportional hazards regression.
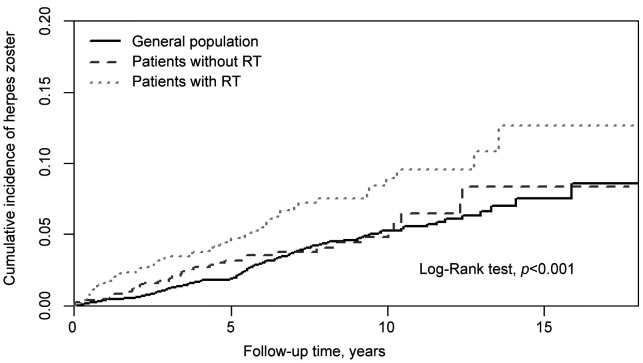
The cumulative incidence of herpes zoster was significantly higher in the radiotherapy group and the non-radiotherapy group in patients with oral cavity cancer compared to the general population (log-rank test, p<0.001) (Figure 1).
Figure 1. Cumulative incidence of herpes zoster in individuals with and without radiotherapy (RT) among patients with oral cavity cancer compared to general population.
Discussion
To the best of our knowledge, this is the first population-based study to investigate the relationship between radiotherapy and the incidence of herpes zoster in oral cavity cancer patients. In 2020 and 2021, there were two articles that investigated the relationship between head and neck cancer and herpes zoster (11,12); however, these studies did not perform a subgroup analysis for oral cavity cancer. The clinical behavior is different in each type of head and neck cancer. Our study used a pure cohort composed of oral cavity cancer patients alone, thus, providing clinical value for this specific type of cancer.
The advantage of our study is that we enrolled a large patient cohort composed of 3,131 oral cavity cancer patients. Additionally, the Taiwanese health insurance covers more than 99% of the Taiwanese population, which avoids the selection bias. A validation study concerning the Taiwanese health insurance database has shown that the accuracy of the database is high (13). Thus, the results we obtained through the database are trustworthy.
Our study demonstrates that oral cavity cancer is associated with herpes zoster. In 2017, a study based on the database of the United Kingdom’s primary care showed that many types of cancer are associated with herpes zoster, including oral cavity cancer (14). In this study approximately 102 patients were included, with an odds ratio around 1.4. Our study used a hazard ratio, and the result was approximately 1.6, which is similar to the one of the UK study. Thus, we demonstrate that oral cavity cancer is associated with herpes zoster not only in European people, but also in Asian people.
The gender may also be an important parameter related to the infection of herpes zoster. According to a meta-analysis published in 2017, females are more likely to develop a herpes zoster infection (15). Our study, however, found no difference between men and women with oral cavity cancer.
Many studies have shown that diabetes mellitus and hypertension are associated with herpes zoster infection (16-18). The proportions of diabetes mellitus and hypertension were the most prevalent pathologies in the oral cavity patient group. Interestingly, after controlling for comorbidities, oral cavity cancer is still associated with a higher incidence of herpes zoster infection, compared to the general population without oral cavity cancer.
Age is an important factor in infectious diseases (19). Our study found that there is a significantly higher risk of herpes zoster in oral cavity cancer patients when compared with non-oral cavity cancer in the population younger than 65 years of age. The Advisory Committee on Immunization Practices (ACIP) guidelines suggest that the herpes zoster vaccine should be given to people aged 50 and above (20). Importantly, our study indicates that if an oral cavity cancer patient is younger than 50, the herpes zoster vaccine should be offered due to the increased risk of infection.
Another issue with herpes zoster that arose in our infection analysis is time. Only within two years after the diagnosis of oral cavity cancer, the incidence of herpes zoster is significantly higher in these patients compared to non-oral cavity cancer patients. This phenomenon indicates that the herpes zoster vaccine must be provided to the oral cavity cancer patients as soon as possible after their diagnosis for a timely protection.
In the Cox regression analysis, the oral cavity patients receiving radiotherapy had a significantly higher incidence rate compared to the non-oral cavity cancer patients. This phenomenon is linked to radiation treatment. When a patient receives radiotherapy, then the radiation oncologist should recommend the patient to receive the herpes zoster vaccine.
The limitation of our study is that the details of radiotherapy were not reported. The design of the radiotherapy field poses a clinical dilemma in many clinical situations (21). The field size influences both the clinical outcome as well as the side effects. If we would be able to determine the radiation field size, we could perform a specific analysis for the radiotherapy field size and the incidence of herpes zoster. The result could, then, give a more personalized prediction of herpes zoster infection.
Another limitation of the study was that patients with tongue cancer were not incorporated, therefore findings may not be applicable or generalizable to individuals specifically affected by tongue cancer. Our objective is to undertake a comparable study in the future that includes participants diagnosed with tongue cancer, allowing for a more comprehensive understanding of the implications and outcomes specific to this population.
In conclusion, radiotherapy appears to increase the incidence of herpes zoster infection in oral cavity cancer patients.
Conflicts of Interest
We declare no conflicts of interest.
Authors’ Contributions
The project was conceptualized by YSK, the manuscript was written was by YSK and YH, administration was done by CYH.
Acknowledgements
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004). We would like to show gratitude to the Health Data Science Center, China Medical University Hospital, for providing administrative, technical and funding support.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Head and Neck Cancer. Version 3. 2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/headand-neck.pdf. [Last accessed on June 23th, 2021]
- 3.Freer G, Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41(2):95–105. [PubMed] [Google Scholar]
- 4.Mareque M, Oyagüez I, Morano R, Casado MA. Systematic review of the evidence on the epidemiology of herpes zoster: incidence in the general population and specific subpopulations in Spain. Public Health. 2019;167:136–146. doi: 10.1016/j.puhe.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Caputo M, Horn J, Karch A, Akmatov MK, Becher H, Braun B, Brenner H, Castell S, Fischer B, Giani G, Günther K, Hoffmann B, Jöckel KH, Keil T, Klüppelholz B, Krist L, Leitzmann MF, Lieb W, Linseisen J, Meisinger C, Moebus S, Obi N, Pischon T, Schipf S, Schmidt B, Sievers C, Steinbrecher A, Völzke H, Mikolajczyk R. Herpes zoster incidence in Germany - an indirect validation study for self-reported disease data from pretest studies of the population-based German National Cohort. BMC Infect Dis. 2019;19(1):99. doi: 10.1186/s12879-019-3691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundström K, Weibull CE, Söderberg-Löfdal K, Bergström T, Sparén P, Arnheim-Dahlström L. Incidence of herpes zoster and associated events including stroke—a population-based cohort study. BMC Infect Dis. 2015;15:488. doi: 10.1186/s12879-015-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes zoster and post-herpetic neuralgia: Changing incidence rates from 1994 to 2018 in the United States. Clin Infect. 2020;Dis:ciaa1185. doi: 10.1093/cid/ciaa1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin YH, Huang LM, Chang IS, Tsai FY, Lu CY, Shao PL, Chang LY, Varicella-Zoster Working Group, Advisory Committee on Immunization Practices Taiwan Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine. 2010;28(5):1217–1220. doi: 10.1016/j.vaccine.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Oxman MN, Levin MJ, Shingles Prevention Study Group Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008;197 Suppl 2:S228–S236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, Vanden Abeele C, Vesikari T, Watanabe D, Zahaf T, Ahonen A, Athan E, Barba-Gomez JF, Campora L, de Looze F, Downey HJ, Ghesquiere W, Gorfinkel I, Korhonen T, Leung E, McNeil SA, Oostvogels L, Rombo L, Smetana J, Weckx L, Yeo W, Heineman TC, ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 11.Lee PY, Lai JN, Chiu LT, Wei YT. Incidence and time trends of herpes zoster among patients with head and neck cancer who did and did not undergo radiotherapy: A population-based cohort study. PLoS One. 2021;16(5):e0250724. doi: 10.1371/journal.pone.0250724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizuguchi T, Sekiya N, Hara K, Taguchi A, Nakajima Y, Miyake Y, Shibata Y, Taguchi K, Ogawa H, Ito K, Karasawa K. Radiation therapy and the risk of herpes zoster in patients with cancer. Cancer. 2020;126(15):3552–3559. doi: 10.1002/cncr.32926. [DOI] [PubMed] [Google Scholar]
- 13.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 14.Hansson E, Forbes HJ, Langan SM, Smeeth L, Bhaskaran K. Herpes zoster risk after 21 specific cancers: population-based case-control study. Br J Cancer. 2017;116(12):1643–1651. doi: 10.1038/bjc.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai K, Yawn BP. Risk factors for herpes zoster: A systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–1821. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Lai SW, Lin CL, Liao KF. Real-world database investigating the association between diabetes mellitus and herpes zoster in Taiwan. Medicine (Baltimore) 2019;98(18):e15463. doi: 10.1097/MD.0000000000015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz-Quiles C, López-Lacort M, Ampudia-Blasco FJ, Díez-Domingo J. Risk and impact of herpes zoster on patients with diabetes: A population-based study, 2009-2014. Hum Vaccin Immunother. 2017;13(11):2606–2611. doi: 10.1080/21645515.2017.1368600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39(6):537–544. doi: 10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: A comprehensive review. Indian J Dermatol Venereol Leprol. 2018;84(3):251–262. doi: 10.4103/ijdvl.IJDVL_1021_16. [DOI] [PubMed] [Google Scholar]
- 20.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–108. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grégoire V, Grau C, Lapeyre M, Maingon P. Target volume selection and delineation (T and N) for primary radiation treatment of oral cavity, oropharyngeal, hypopharyngeal and laryngeal squamous cell carcinoma. Oral Oncol. 2018;87:131–137. doi: 10.1016/j.oraloncology.2018.10.034. [DOI] [PubMed] [Google Scholar]