Abstract
Background/Aim: Sperm cells are competent to integrate exogenous DNA into their genome. We sought to clarify Human Pappiloma Virus (HPV) internalization in spermatozoa and early preimplantation embryos. Materials and Methods: Sperm was incubated with plasmid vectors containing the complete genome of human HPV 16 and HPV 18 tagged with the green fluorescent protein (GFP) gene, to investigate HPV 16 and HPV 18 integration in mouse spermatozoa. Oocytes were in vitro fertilized with preincubated spermatozoa to investigate HPV 16 and HPV 18 potential transfer to mouse embryos. Results: Spermatozoa were able to internalize constructs of cloned high-risk HPV either as integrated or as episomal DNA. Constructs of cloned HPV can also be transferred to mouse embryos, through in vitro fertilization of the oocytes by mouse spermatozoa. Conclusion: Viral DNA transmission to the early mouse embryo via sperm, highlights the effect of HPV in reproductive cells and preimplantation development.
Keywords: Human Pappiloma Virus, mouse, spermatozoa, embryos
Human Pappiloma Virus (HPV) is a non-enveloped, circular, double stranded DNA virus (1). The viral genome consists of a regulatory non-coding long control region, an early region encoding for E6, E7, E1, E2, E4 and E5 genes and a late region encoding for L1 and L2 genes (2). HPVs are generally classified as low and high risk, according to their potential to cause genital cancer. The different types of papilloma viruses exhibit characteristic tropism and distinct life cycle features (3).
HPV 16 and HPV 18, as oncogenic types, are responsible for cervical cancer, the second most common cause of cancer-related death worldwide (4,5). HPV 16 is considered to be the most frequently identified high-risk HPV genotype, followed by HPV 18. Approximately 70% of cervical cancer cases are linked with these two genotypes (6,7).
Cancer of the uterine cervix is associated with high-risk HPV presence and increased HPV E6/E7 oncogene expression (8-11). Persistent infection with oncogenic HPV genotypes triggers HPV DNA integration into the host genome, eventually leading to chromosomal damage accumulation and genome destabilization in infected cells (12-17). The exogenous DNA insertion into the oocyte by sperm (18-20), as well as the mechanisms involved (21-28), have been extensively studied. In fact, living spermatozoa of almost all species are able to take up spontaneously exogenous DNA and internalize a part of it into their nucleus (24). The exogenous DNA fragments are localized at the postacrosomal and equatorial regions of the sperm head (22,23) with 15-22% internalized into the sperm nuclei (24). A proportion of the internalized DNA is integrated at specific sites in sperm genome, probably at a nucleosomal subfraction of chromatin, suggesting a common site for exogenous DNA insertion (29,30). Sperm has the capacity to actively take up exogenous DNA derived from HPV. In addition to HPV L1 gene, sperm probably take up DNA from HPV types 16 and 18 (31,32).
Taking into account the capability of sperm cells to integrate exogenous DNA into their genome, we sought to clarify HPV internalization into sperm genome. In this study, we incubated mouse spermatozoa with plasmid vectors containing the complete genome of human HPV 16 and HPV 18 virus tagged with the green fluorescent protein (GFP) gene, to investigate HPV 16 and HPV 18 integration in mouse spermatozoa. We also in vitro fertilized oocytes with preincubated spermatozoa to investigate HPV 16 and HPV 18 potential transfer to mouse embryos.
Materials and Methods
Animals. FVB/N male and female mice were provided by the Hellenic Pasteur Institute. Their health was verified by a quality control report. Mice were kept in the Breeding Laboratory Animal Institute (Ioannina University, Ioannina, Greece) under a controlled 12 h light and 12 h darkness life cycle and a standard nutritional program. All research protocols were in compliance with the European Union guidelines for animal use and were approved by the General Directorate of Agricultural Economics and Veterinary Medicine of Epirus Region, Greece, in accordance to principles of laboratory animal handling.
Plasmids. HPV 16 and HPV 18 full genome was cloned in plasmid vectors and tagged with green fluorescence protein (GFP) gene (GFP-HPV 16, GFP-HPV 18 - kindly provided by Professor Vincent C. Lombardi, Department of Microbiology and Immunology, University of Nevada, Reno, USA). A sufficient amount of plasmid vectors containing HPV 16 and HPV 18 genome was isolated form bacterial cell cultures.
Sperm retrieval and preparation. Male fertile mice, non-mated with female mice for 3-7 days, were sacrificed by cervical dislocation. Sperm was collected from cauda epididymis in culture medium (Sydney IVF Sperm Medium, COOK, Limerick, Ireland). Swim Up procedure was employed as to select for the most motile spermatozoa. Sperm cells were subsequently counted in a Neubauer chamber. Mouse spermatozoa were used for in vitro fertilization (IVF) procedure following fluorescence-activated cell sorting (FACS) and confocal microscopy.
In IVF experiments, sperm was placed in fertilization medium (Sydney IVF Fertilization Medium, COOK, Limerick, Ireland) for about 2 h (37˚C, 5% CO2), so as to be capacitated and acquire fertilization ability. Semen samples were divided into a control group and a study group, where sperm was incubated for 30 min with GFP-HPV 16, GFP-HPV 18 plasmid vectors. All sperm samples were finally added to the fertilization plates (200 sperm cells/μl). As for fluorescence-activated cell sorting analysis, spermatozoa were incubated with plasmid vectors, containing HPV 16 and HPV 18 genome (50 ng/106 sperm cells), for 5 h under liquid culture conditions (37˚C, 5% CO2). As for confocal microscopy analysis, sperm samples were placed on microscope slides and screened for GFP fluorescence.
Oocyte retrieval. For superovulation and multiple oocytes development, 8 IU of the follicle stimulating hormone FSH (rec-FSH, Gonal-F, Merck Serono, London, UK) were intraperitoneally injected in 6-8 weeks old female mice. Forty-eight h later, 5 IU of the human chorionic gonadotropin hCG (hCG, Pregnyl, N.V. Organon, Oss, the Netherlands) were also injected to induce oocyte maturation. Female mice were sacrificed by cervical dislocation. About 12 h after hCG treatment, ocytes were collected from the oviducts in culture medium (Sydney IVF Follicle Flush Buffer & Fertilization Medium, COOK, Limerick, Ireland).
In vitro fertilization. Oocytes were placed in fertilization plates and incubated with sperm in suitable fertilization medium (Sydney IVF Fertilization Medium, COOK) for about 5 h. Mouse zygotes were cultured in cleavage and blastocyst medium (Sydney IVF Cleavage Medium & Sydney IVF Blastocyst Medium, COOK) in culture laboratory incubator (37˚C, 5% CO2), up to the blastocyst stage. Fertilization outcome was checked by the second polar body extension and the zygote pronuclei formation. In vitro preimplantation development of mouse embryos was daily observed and recorded.
Fluorescence-activated cell sorting (FACS). Spermatozoa, after being incubated with the plasmid vectors, were washed, resuspended in PBS and analyzed by quantitative flow cytometry using the FACS Calibur cytometer. The measurements were performed on 10,000 spermatozoa per semen sample. Non incubated spermatozoa were used as controls to evaluate background fluorescence setting intensity thresholds. The number of HPV positive cells was determined using fluorescence distributions, characterized by specific fluorescence intensity thresholds, to distinguish positive from negative signal. In each assay, fluorescence limits were set so that 99.6% of the cells were considered as negative and 0.4% as false positive. Sample values above the 0.4% threshold were considered as positive. The percentage of virus-positive cells was calculated subtracting false positive from positive values percentage, after overlapping the distribution curves of the study and the control sample. Data analysis was performed by CellQuest software.
Confocal microscopy. GFP observation in mouse spermatozoa and embryos as well as preimplantation development evaluation, were performed through Leica X10 objective lens, using a Leica TCS SP5 confocal microscope equipped with an argon laser (excitation at 488nm), a 561 solid-state laser line and a helium neon laser (excitation at 633 nm). All images were obtained by LASAF Software.
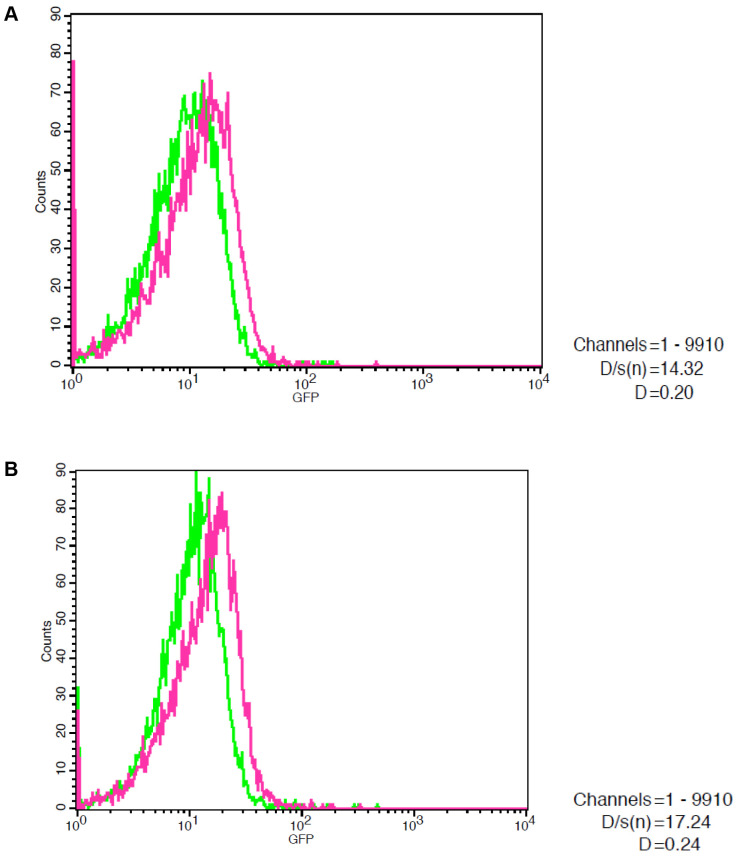
Statistical analysis. FACS analysis was evaluated by the Kolmogorov-Smirnov test. All measurements with p≤0.001, D/s(n) ≥12 and D≥0.12 were set as statistically significant.
Results
HPV 16 and HPV 18 integration in mouse spermatozoa. In order to study HPV 16 and HPV 18 integration to mouse spermatozoa, semen was incubated with plasmid vectors, containing the complete genome of human HPV 16 and HPV 18 virus tagged with the GFP gene. Spermatozoa were incubated with GFP-HPV 16 and GFP-HPV 18 constructs (50 ng/106 sperm cells) for 5 h under liquid culture conditions (37˚C, 5% CO2). HPV 16 and HPV 18 integration in mouse spermatozoa was monitored by flow cytometry (Figure 1). A total of 26 sperm samples were studied. Twelve sperm samples (46.2%) were HPV positive. To further verify HPV 16 and HPV 18 presence, sperm samples were also screened for GFP expression by confocal microscopy. A GFP fluorescent signal was observed in HPV-positive spermatozoa. The results indicate that spermatozoa are able to internalize constructs of cloned high risk HPV viruses either as integrated or as episomal DNA.
Figure 1. Fluorescence-activated cell sorting (FACS) analysis in mouse epididymal spermatozoa after a 5-h incubation with GFP-HPV 16 (A) and GFP-HPV 18 (B) constructs (50 ng/106 sperm cells). Spermatozoa are able to internalize constructs of cloned high risk HPV viruses.
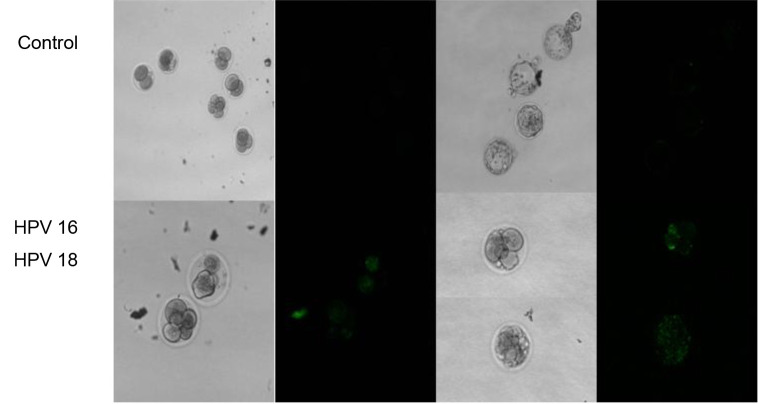
HPV 16 and HPV 18 transfer to mouse embryos. In order to study the HPV 16 and HPV 18 presence in mouse embryos, exploring the effect of HPV DNA transfer to mouse oocytes by sperm during fertilization, oocyte fertilization by preincubated spermatozoa was conducted, as almost half of them were GFP positive. Prior to in vitro fertilization process, spermatozoa were incubated with GFP-HPV 16 and GFP-HPV 18 plasmid vectors (50 ng/106 sperm cells) for 30 min. HPV 16 and HPV 18 presence in mouse embryos was evaluated through green fluorescence detection by confocal microscopy (Figure 2). The control group included 97 embryos. As for the HPV 16 and HPV 18 group, a total of 89 embryos were studied. A total of 36 embryos (40.4%) were HPV positive. The results indicate that constructs of cloned HPV viruses can be transferred to mouse embryos, through oocyte in vitro fertilization by spermatozoa.
Figure 2. Confocal microscopy analysis of control embryos and HPV-positive embryos. HPV 16 and HPV 18 positive embryos present green fluorescence protein signal. Constructs of cloned HPV viruses can be transferred to mouse embryos, through oocyte in vitro fertilization by preincubated spermatozoa.
Discussion
HPVs constitute agents of one of the most common sexually transmitted diseases worldwide, affecting both males and females. Despite the fact that HPV is present in semen (33-36), the role of infected sperm cells in virus transmission remains unknown. In fact, it is not still clear whether HPV positive spermatozoa infect the partner, retain their fertilization ability or possibly interfere with in vitro fertilization procedure, embryo development and assisted reproduction techniques outcome. In the current study, we investigated HPV 16 and HPV 18 integration in mouse epididymal spermatozoa caused by cloned human HPV viruses and their potential transfer to mouse embryos. Taking into account the capability of mammalian sperm cells to bind exogenous DNA or RNA molecules and internalize them into their nucleus (24), we incubated mouse spermatozoa with plasmid vectors containing the complete genome of human HPV 16 and HPV 18 virus tagged with GFP gene.
Generally, the specific sites on the spermatozoon where the foreign DNA is localized have been reported to be at the equatorial and postacrosomal regions of the sperm head (24). The action of sperm cells as vectors or carriers and the integration of HPV DNA into the sperm nucleus are both not well-defined, raising queries on whether viral DNA is just trapped in the membrane, is free in the cytoplasm, or is internalized into the nucleus and maintained as episomal. HPV probably binds to two distinct sites along the equatorial region of the spermatozoon’s head. Glycosaminoglycans and soluble factors of spermatozoon’s surface mediate the interaction between HPV and sperm (37-39). Specifically, glycosaminoglycan syndecan-1 and L1 HPV capsid protein co-localize in the equatorial region of the head (40). Although HPV DNA mainly localizes in the sperm head, its integration into the nucleus is unclear. In this study, HPV 16 and HPV 18 integration in mouse sperm cells was monitored by flow cytometry and verified through GFP expression screening by confocal microscopy, indicating that spermatozoa are able to internalize constructs of cloned high-risk HPV viruses either as integrated or as episomal DNA. Viral DNA integration into the sperm nucleus could not be directly demonstrated, but nevertheless integration is evident by GFP gene expression.
Sperm cells are considered to be transcriptionally and translationally silent, due to their compact DNA structure and their restricted cytoplasm. Keeping in mind spermatozoas’ transcription and translation inactivation, the detection of viral RNA or protein in sperm suggests that HPV could infect the early stages of spermatogenesis. Spermatozoa’ strongly compacted nucleus makes viral DNA penetration a demanding process. However, it was indicated that HPV 16 and HPV 18 E6 gene-specific mRNA is expressed in human sperm, assuming that HPV genes are actively expressed in infected sperm cells (41). It was also demonstrated that HPV is capable of transferring viral DNA into sperm following incubation and that infected cells are able to deliver exogenous DNA to the cumulus cells surrounding ovulated oocytes at the moment of fertilization (42). In this study, HPV presence was confirmed in embryos, indicating that spermatozoa convey the exogenous human viruses to oocytes during in vitro fertilization. The exogenous DNA was possibly integrated into the sperm genome and consequently transferred by sperm in the genome of HPV-positive embryos.
Regarding HPV transmission and embryogenesis, spermatozoa carrying exogenous HPV DNA act potentially as vectors, transmitting HPV to the fetus through fertilized oocytes (43-46). In case that sperm cells carrying foreign DNA are allowed to contact directly oocytes, the DNA is transferred into the oocytes at the time of fertilization (21,47). Additionally, in mouse models, HPV infected sperm successfully fertilized oocytes (48), followed by subsequent gene expression in the inner cell mass and the trophectoderm of preimplantation blastocysts (32). During the hamster egg-human sperm penetration test, the human sperm transferred both E6/E7 genes and L1 major capsid protein to oocytes (40), while increased DNA fragmentation and trophoblast death were observed in blastocysts after E6/E7 genes expression (49-51). Exposure of early mouse embryo to HPV 16 and HPV 18 DNA fragments, demonstrated embryo stage specific effects, showing a decrease in blastocyst formation (HPV 16) and hatching process (HPV 18) (51).
Conclusion
This study highlights the possibility of viral DNA transmission to the early embryo via sperm, opening new perspectives on the effect of HPV in reproductive cells. It is of crucial importance to clarify HPV integration and address the impact of HPV expression in mouse early preimplantation development. HPV persistence may impair sperm parameters, suggesting caution in the use of these cells for assisted reproduction techniques or sperm banking. At present, it is unknown whether there are risks for embryos fertilized by infected sperm; however, HPV-positive spermatozoa injected in the oocyte cytoplasm could possibly interfere with fertilization, implantation, embryo development, premature abortion, and definitively with outcome of assisted reproduction techniques. More work needs to be carried out to refine the transmission process and to improve researchers’ understanding on DNA transfection properties of sperm cells. Of course, further studies are necessary to discuss the possible clinical implications of the experimental findings.
Conflicts of Interest
The Authors declare that they have no conflicts of interest regarding this study.
Authors’ Contributions
E.M., C.K. and I.G. conceived and designed the study; E.M. and C.K. conducted the experiments; E.M. and I.G. wrote the paper; E.M., C.K., T.E., A.Z., N.Z. and I.G. discussed the manuscript and provided important feedback; I.G. supervised the project.
Acknowledgements
The Authors are grateful to Professor Vincent C. Lombardi, Department of Microbiology and Immunology, University of Nevada, Reno, USA. They are also grateful to Associate Professor Thomas Vrekousis and to Dr Georgios Vartholomatos.
References
- 1.zur Hausen H. Papillomavirus infections – a major cause of human cancers. Biochim Biophys Acta. 1996;1288(2):F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110(5):525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 3.Bernard HU, Calleja-Macias IE, Dunn ST. Genome variation of human papillomavirus types: phylogenetic and medical implications. Int J Cancer. 2006;118(5):1071–1076. doi: 10.1002/ijc.21655. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, Franceschi S. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 6.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX, Retrospective International Survey and HPV Time Trends Study Group Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen CL, Münger K. Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1. J Virol. 2009;83(4):1700–1707. doi: 10.1128/JVI.01971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howie HL, Katzenellenbogen RA, Galloway DA. Papillomavirus E6 proteins. Virology. 2009;384(2):324–334. doi: 10.1016/j.virol.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 11.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 12.Hudelist G, Manavi M, Pischinger KI, Watkins-Riedel T, Singer CF, Kubista E, Czerwenka KF. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol. 2004;92(3):873–880. doi: 10.1016/j.ygyno.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Cricca M, Morselli-Labate AM, Venturoli S, Ambretti S, Gentilomi GA, Gallinella G, Costa S, Musiani M, Zerbini M. Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions. Gynecol Oncol. 2007;106(3):549–557. doi: 10.1016/j.ygyno.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Wang W, Si M, Han L, Gao Q, Luo A, Li Y, Lu Y, Wang S, Ma D. The physical state of HPV16 infection and its clinical significance in cancer precursor lesion and cervical carcinoma. J Cancer Res Clin Oncol. 2008;134(12):1355–1361. doi: 10.1007/s00432-008-0413-3. [DOI] [PubMed] [Google Scholar]
- 15.Boulet GA, Benoy IH, Depuydt CE, Horvath CA, Aerts M, Hens N, Vereecken AJ, Bogers JJ. Human papillomavirus 16 load and E2/E6 ratio in HPV16-positive women: biomarkers for cervical intraepithelial neoplasia >or=2 in a liquid-based cytology setting. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2992–2999. doi: 10.1158/1055-9965.EPI-09-0025. [DOI] [PubMed] [Google Scholar]
- 16.Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, Rocco JW, Teknos TN, Kumar B, Wangsa D, He D, Ried T, Symer DE, Gillison ML. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014;24(2):185–199. doi: 10.1101/gr.164806.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsakogiannis D, Bletsa M, Kyriakopoulou Z, Dimitriou TG, Kotsovassilis C, Panotopoulou E, Markoulatos P. Identification of rearranged sequences of HPV16 DNA in precancerous and cervical cancer cases. Mol Cell Probes. 2016;30(1):6–12. doi: 10.1016/j.mcp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Lauria A, Gandolfi F. Recent advances in sperm cell mediated gene transfer. Mol Reprod Dev. 1993;36(2):255–257. doi: 10.1002/mrd.1080360224. [DOI] [PubMed] [Google Scholar]
- 19.Chan PJ. Sperm-mediated DNA transfer to cells of the uterus and embryo. Mol Reprod Dev. 2000;56(2 Suppl):316–318. doi: 10.1002/(SICI)1098-2795(200006)56:2+<316::AID-MRD23>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Spadafora C. Sperm cells and foreign DNA: a controversial relation. Bioessays. 1998;20(11):955–964. doi: 10.1002/(SICI)1521-1878(199811)20:11<955::AID-BIES11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Brackett BG, Baranska W, Sawicki W, Koprowski H. Uptake of heterologous genome by mammalian spermatozoa and its transfer to ova through fertilization. Proc Natl Acad Sci USA. 1971;68(2):353–357. doi: 10.1073/pnas.68.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camaioni A, Russo MA, Odorisio T, Gandolfi F, Fazio VM, Siracusa G. Uptake of exogenous DNA by mammalian spermatozoa: specific localization of DNA on sperm heads. J Reprod Fertil. 1992;96(1):203–212. doi: 10.1530/jrf.0.0960203. [DOI] [PubMed] [Google Scholar]
- 23.Lavitrano M, French D, Zani M, Frati L, Spadafora C. The interaction between exogenous DNA and sperm cells. Mol Reprod Dev. 1992;31(3):161–169. doi: 10.1002/mrd.1080310302. [DOI] [PubMed] [Google Scholar]
- 24.Francolini M, Lavitrano M, Lamia CL, French D, Frati L, Cotelli F, Spadafora C. Evidence for nuclear internalization of exogenous DNA into mammalian sperm cells. Mol Reprod Dev. 1993;34(2):133–139. doi: 10.1002/mrd.1080340204. [DOI] [PubMed] [Google Scholar]
- 25.Mu Y, Jiao M, Zhao Y, Lv J, Wang J, Hao J, Zhang X, Kong Q, Liu Z. A method for tracing exogenous DNA uptake in live spermatozoa and embryos. Pol J Vet Sci. 2018;21(1):193–202. doi: 10.24425/119038. [DOI] [PubMed] [Google Scholar]
- 26.Patil JG, Khoo HW. Nuclear internalization of foreign DNA by zebrafish spermatozoa and its enhancement by electroporation. J Exp Zool. 1996;274(2):121–129. doi: 10.1002/(SICI)1097-010X(19960201)274:2<121::AID-JEZ5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Tsai HJ, Lai CH, Yang HS. Sperm as a carrier to introduce an exogenous DNA fragment into the oocyte of Japanese abalone (Haliotis divorsicolor suportexta) Transgenic Res. 1997;6(1):85–95. doi: 10.1023/a:1018413318223. [DOI] [PubMed] [Google Scholar]
- 28.Huguet E, Esponda P. Foreign DNA introduced into the vas deferens is gained by mammalian spermatozoa. Mol Reprod Dev. 1998;51(1):42–52. doi: 10.1002/(SICI)1098-2795(199809)51:1<42::AID-MRD5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.Zoraqi G, Spadafora C. Integration of foreign DNA sequences into mouse sperm genome. DNA Cell Biol. 1997;16(3):291–300. doi: 10.1089/dna.1997.16.291. [DOI] [PubMed] [Google Scholar]
- 30.Pittoggi C, Zaccagnini G, Giordano R, Magnano AR, Baccetti B, Lorenzini R, Spadafora C. Nucleosomal domains of mouse spermatozoa chromatin as potential sites for retroposition and foreign DNA integration. Mol Reprod Dev. 2000;56(2 Suppl):248–251. doi: 10.1002/(SICI)1098-2795(200006)56:2+<248::AID-MRD7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Chan PJ, Seraj IM, Kalugdan TH, King A. Evidence for ease of transmission of human papillomavirus DNA from sperm to cells of the uterus and embryo. J Assist Reprod Genet. 1996;13(6):516–519. doi: 10.1007/BF02066536. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera M, Chan PJ, Kalugdan TH, King A. Transfection of the inner cell mass and lack of a unique DNA sequence affecting the uptake of exogenous DNA by sperm as shown by dideoxy sequencing analogues. J Assist Reprod Genet. 1997;14(2):120–124. doi: 10.1007/BF02765781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai YM, Lee JF, Huang HY, Soong YK, Yang FP, Pao CC. The effect of human papillomavirus infection on sperm cell motility. Fertil Steril. 1997;67(6):1152–1155. doi: 10.1016/s0015-0282(97)81454-9. [DOI] [PubMed] [Google Scholar]
- 34.Rintala MA, Grénman SE, Pöllänen PP, Suominen JJ, Syrjänen SM. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int J STD AIDS. 2004;15(11):740–743. doi: 10.1258/0956462042395122. [DOI] [PubMed] [Google Scholar]
- 35.Nielson CM, Flores R, Harris RB, Abrahamsen M, Papenfuss MR, Dunne EF, Markowitz LE, Giuliano AR. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1107–1114. doi: 10.1158/1055-9965.EPI-06-0997. [DOI] [PubMed] [Google Scholar]
- 36.Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, Palù G. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril. 2010;93(3):802–806. doi: 10.1016/j.fertnstert.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Andino J, Buck CB, Ribbeck K. Adsorption of human papillomavirus 16 to live human sperm. PLoS One. 2009;4(6):e5847. doi: 10.1371/journal.pone.0005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, Pozzi E, Clementi M, Viganò P, Montanari E, Montorsi F, Salonia A. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod. 2019;34(2):209–217. doi: 10.1093/humrep/dey348. [DOI] [PubMed] [Google Scholar]
- 39.Garolla A, Pizzol D, Foresta C. The role of human papillomavirus on sperm function. Curr Opin Obstet Gynecol. 2011;23(4):232–237. doi: 10.1097/GCO.0b013e328348a3a4. [DOI] [PubMed] [Google Scholar]
- 40.Foresta C, Patassini C, Bertoldo A, Menegazzo M, Francavilla F, Barzon L, Ferlin A. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One. 2011;6(3):e15036. doi: 10.1371/journal.pone.0015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadze R, Chan PJ, Jacobson JD, Corselli JU, King A. Temperature variable and the efficiency of sperm mediated transfection of HPV16 DNA into cells. Asian J Androl. 2002;4(3):169–173. [PubMed] [Google Scholar]
- 42.Lee CA, Huang CT, King A, Chan PJ. Differential effects of human papillomavirus DNA types on p53 tumor-suppressor gene apoptosis in sperm. Gynecol Oncol. 2002;85(3):511–516. doi: 10.1006/gyno.2002.6662. [DOI] [PubMed] [Google Scholar]
- 43.Lai YM, Yang FP, Pao CC. Human papillomavirus deoxyribonucleic acid and ribonucleic acid in seminal plasma and sperm cells. Fertil Steril. 1996;65(5):1026–1030. [PubMed] [Google Scholar]
- 44.Chan PJ, Su BC, Kalugdan T, Seraj IM, Tredway DR, King A. Human papillomavirus gene sequences in washed human sperm deoxyribonucleic acid. Fertil Steril. 1994;61(5):982–985. doi: 10.1016/s0015-0282(16)56719-3. [DOI] [PubMed] [Google Scholar]
- 45.Chan PJ, Kalugdan T, Su BC, Whitney EA, Perrott W, Tredway DR, King A. Sperm as a noninvasive gene delivery system for preimplantation embryos. Fertil Steril. 1995;63(5):1121–1124. [PubMed] [Google Scholar]
- 46.Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Transplacental transmission of Human Papillomavirus. Virol J. 2008;5:106. doi: 10.1186/1743-422X-5-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gagné MB, Pothier F, Sirard MA. Electroporation of bovine spermatozoa to carry foreign DNA in oocytes. Mol Reprod Dev. 1991;29(1):6–15. doi: 10.1002/mrd.1080290103. [DOI] [PubMed] [Google Scholar]
- 48.Chan PJ, Su BC, Tredway DR, Seraj M, Seraj IM, King A. Uptake of exogenous human papilloma virus L1 DNA by oocytes and detection by the polymerase chain reaction. J Assist Reprod Genet. 1992;9(6):531–533. doi: 10.1007/BF01204249. [DOI] [PubMed] [Google Scholar]
- 49.You H, Liu Y, Carey MJ, Lowery CL, Hermonat PL. Defective 3A trophoblast-endometrial cell adhesion and altered 3A growth and survival by human papillomavirus type 16 oncogenes. Mol Cancer Res. 2002;1(1):25–31. [PubMed] [Google Scholar]
- 50.Calinisan JH, Chan SR, King A, Chan PJ. Human papillomavirus and blastocyst apoptosis. J Assist Reprod Genet. 2002;19(3):132–136. doi: 10.1023/a:1014736805127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henneberg AA, Patton WC, Jacobson JD, Chan PJ. Human papilloma virus DNA exposure and embryo survival is stage-specific. J Assist Reprod Genet. 2006;23(6):255–259. doi: 10.1007/s10815-006-9030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]