Abstract
Gastrointestinal stromal tumors (GISTs) develop in the digestive tract, mainly in the stomach, small intestine, colon, or esophagus. However, primary tumors with the same pathologic features as GISTs have been reported to occur outside of the digestive tract and are called extragastrointestinal stromal tumor (EGIST). We herein report a rare case of EGIST arising from the greater omentum in a patient with abdominal pain caused by intraperitoneal bleeding from the tumor.
Keywords: extragastrointestinal stromal tumor, greater omentum, intraperitoneal bleeding
Introduction
The development of a gastrointestinal stromal tumor (GIST) is generally seen in the digestive tract. This tumor is thought to originate from interstitial cells of Cajal, and the majority reported show the expression of c-KIT and CD34 (1,2). In the digestive tract, GISTs have been found in the stomach (60-70%), small intestine (20-25%), colon (5%), and esophagus (5%) (1). However, a very small number of reports have described GISTs arising from outside of the gastrointestinal tract (3-25), termed extragastrointestinal stromal tumors (EGISTs).
We herein report a rare case of EGIST arising from the greater omentum in a patient affected by severe left lateral abdominal pain caused by intraperitoneal bleeding from the tumor.
Case Report
A 45-year-old woman came to the emergency room of our hospital with severe left lateral abdominal pain and a fever, without any particular medical or drug history.
Upon arrival, her blood pressure was 148/83 mmHg, heart rate was 120 beats per minute, and body temperature was 38.2°C. A physical examination showed compression pain in the left lateral abdominal region, and blood examination results included a white blood cell count of 10,340/μL, hemoglobin level of 11.5 g/dL, platelet count of 222×103/μL, albumin level of 4.6 g/dL, and C-reaction protein level of 2.34 mg/dL.
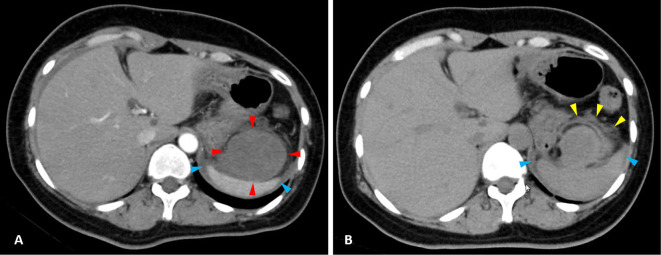
Contrast-enhanced computed tomography (CT) demonstrated a large intraperitoneal mass (maximum diameter 60 mm) between the stomach and spleen, with no enhancement in the arterial phase (Fig. 1A; red arrowheads), as well as the presence of intraperitoneal effusion around the spleen (Fig. 1A; blue arrowheads) and on the surface of the liver. However, no findings of extravasation were detected (Fig. 1A). Results of plain CT revealed the greater omentum on the ventral side near the tumor (Fig. 1B; yellow arrowheads), although whether or not the tumor originated from there was unclear. In addition, the intraperitoneal effusion noted around the spleen was suspected to be bloody ascites due to the level of density on plain CT (Fig. 1B; blue arrowheads). There were no signs of peritoneal irritation observed, and the abdominal symptoms gradually improved, so the patient was hospitalized under conservative treatment and scheduled for further examinations, with semi-urgent surgery considered necessary.
Figure 1.
(A) Contrast-enhanced computed tomography (CT) showing a large intraperitoneal mass (maximum diameter 60 mm) between the stomach and spleen, with no enhancement in the arterial phase (red arrowheads), as well as the presence of intraperitoneal effusion around the spleen (blue arrowheads). (B) Plain CT showing the greater omentum (yellow arrowheads) and intraperitoneal effusion suspected to be bloody ascites (blue arrowheads).
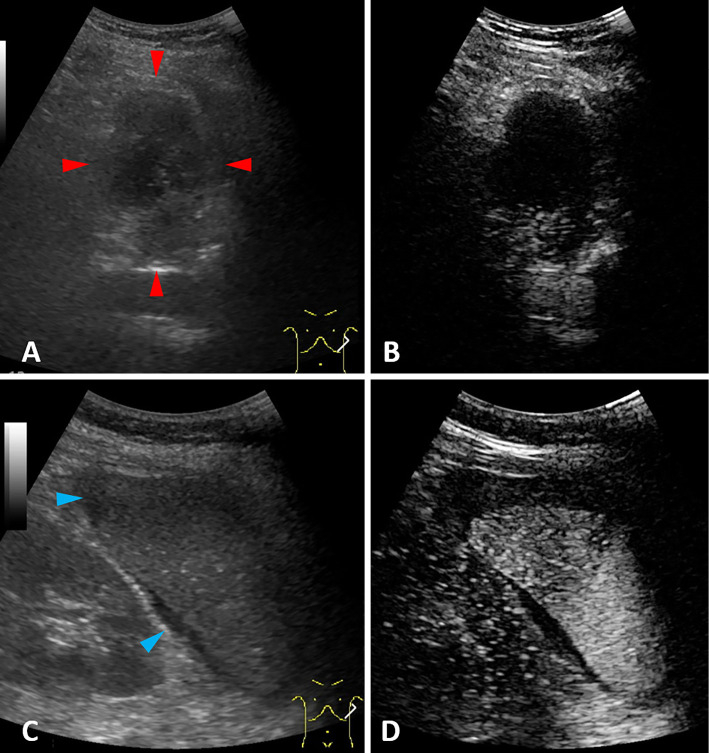
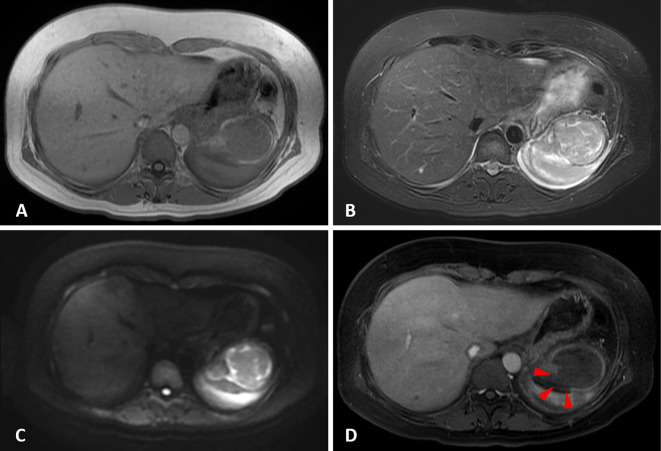
On the day following her hospitalization, the patient underwent abdominal ultrasonography (US), and a hypoechoic heterogeneous lesion between the stomach and spleen was revealed (Fig. 2A; red arrowheads), with no enhancement shown by contrast-enhanced ultrasound (CEUS) with Sonazoid (Fig. 2B). In addition, intraperitoneal effusion was demonstrated around the spleen (Fig. 2C; blue arrowheads). Although CEUS was performed to confirm the presence of extravasation, which would explain the suspected bloody ascites, no extravasation was detected (Fig. 2D). Paracentesis showed bloody ascites and a negative cytology result, so magnetic resonance imaging (MRI) was performed for the further investigation of the lesion. Gadolinium-enhanced MRI revealed a large tumor between the gastric fornix and spleen, which was seen as a low-intensity mass with a high-intensity surface on T1-weighted imaging (T1WI) (Fig. 3A) and as a heterogeneous high-intensity mass on T2WI (Fig. 3B) and diffusion-weighted imaging (Fig. 3C). An area of decreased gadolinium enhancement on the posterior surface of the tumor was suspected to indicate rupture of the capsule, so that site was considered the cause of the intraperitoneal bleeding (Fig. 3D; red arrowheads).
Figure 2.
(A) Ultrasonography (US) findings showing a hypoechoic heterogeneous lesion between the stomach and spleen (red arrowheads). (B) No enhancement was shown by contrast-enhanced ultrasound (CEUS) with Sonazoid. (C) Intraperitoneal effusion around the spleen was demonstrated (blue arrowheads). (D) No extravasation was detected by CEUS.
Figure 3.
Magnetic resonance imaging (MRI). (A) Low-intensity mass between the stomach and spleen with a high-intensity surface on T1-weighted imaging (T1WI). (B) Heterogeneous high-intensity mass on T2-weighted imaging (T2WI). (C) Diffusion-weighted imaging (DWI). (D) A decreased gadolinium enhancement area on the posterior surface of tumor.
Although the origin of the tumor remained unclear even after performing other examinations, GIST arising from the gastric wall, a neuroendocrine tumor of the pancreas, and mesenchymal tumor originating from intraperitoneal tissues were considered for the differential diagnosis. Since a puncture examination under abdominal US guidance for the diagnosis carried a risk of inducing hemorrhaging and dissemination, laparoscopic tumor excision was selected for this case.
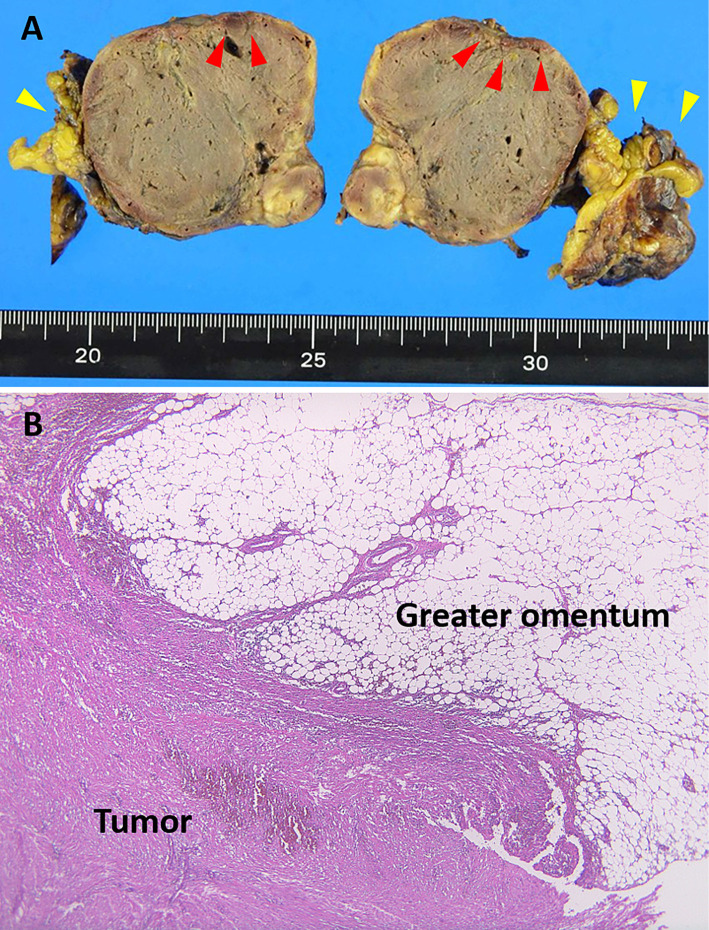
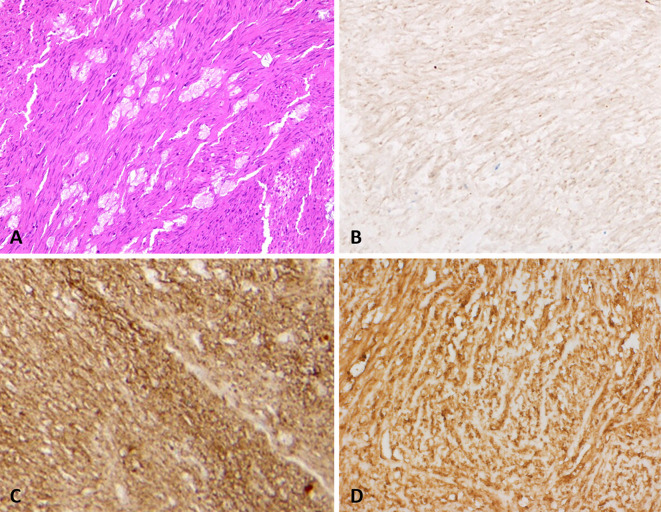
The intraoperative findings showed that the tumor was 60×50×50 mm in size and covered the greater omentum, with adhesion to the spleen and diaphragm. No peritoneal dissemination was detected. The gross pathological findings revealed degeneration and hemorrhaging inside the tumor, partial capsule breakage (Fig. 4A; red arrowheads), and the greater omentum (Fig. 4A; yellow arrowheads). A histological examination (hemotoxin and eosin staining) indicated that the tumor was continuous with the greater omentum (Fig. 4B) and composed of an increased level of spindle cells (Fig. 5A). Histological section immunoreactivity showed positivity for c-kit (Fig. 5B), CD34 (Fig. 5C), and DOG-1 (Fig. 5D), partial positivity for desmin and h-caldesmon, and negativity for platelet-derived growth factor (PDGF)-α, SMA, S100, and calponin. Based on these findings, the diagnosis was EGIST occurring in the greater omentum.
Figure 4.
(A) Gross pathological findings of the resected tumor included degeneration and hemorrhaging inside the tumor and partial capsule breakage (red arrowheads) as well as the greater omentum (yellow arrowheads). (B) Histological (Hematoxylin and Eosin staining) results indicated that the tumor was continuous with the greater omentum.
Figure 5.
Histological examination results. (A) Tumor composition of increased spindle cells (Hematoxylin and Eosin staining). Positive immunoreactivity in histological sections can be seen for (B) c-kit, (C) CD34, and (D) DOG-1.
Although the diameter of the mass was 60 mm and a histological analysis revealed a low level of Ki-67 (MIB-1 index 1.0%), the tumor was shown to have arisen from non-gastric tissue (greater omentum), and the patient was complicated by intraperitoneal bleeding from tumor rupture. As a result, this case was treated as having a high risk of EGIST occurrence (26), and imatinib treatment was given for three years after the operation. At the time of writing, five years have passed since the surgery, and the patient remains alive with no recurrence.
Discussion
An EGIST is a tumor that develops outside of the GI tract and accounts for 1.5-2% of all GIST cases. Previous reports have noted the most frequent EGIST site of occurrence to be the mesentery (29.4%), followed by the retroperitoneum (25.5%) and omentum (15.7%) (1,2). EGISTs are considered to originate from mesenchymal cells in the omentum. Sakurai et al. reported findings indicating the presence of some c-kit-positive mesenchymal cells resembling interstitial cells of Cajal in the omentum and speculated those to be the origin of an EGIST in their patient (27). The present patient is a rare case of EGIST with an origin in the omentum that demonstrated intraperitoneal bleeding, an unusual complication associated with this tumor.
We searched for previous reports of EGIST patients with intraperitoneal bleeding using the PubMed, Ichu-shi, and isho-jp databases with the key words “extragastrointestinal stromal tumor (EGIST),” “greater omentum,” “lesser omentum,” “mesentery,” “intraperitoneal hemorrhage” or “bleeding,” and “hemoperitoneum.” Seven cases (4 men, 3 women; average age: 59.4 years old) with tumors arising from the greater omentum were found (average diameter: 164 mm) (Table). Each of those patients had abdominal pain or distention with ascites suspected of being the cause of intraperitoneal bleeding at the time of the onset and underwent surgical resection for treatment of the tumor. In all cases, the operative and pathological findings revealed that the tumor had originated from the greater omentum. In those 7 cases, each of which had bleeding, the tumor diameter was ≥50 mm and exceeded 100 mm in 6 cases, suggesting that large EGISTs originating from the greater omentum may increase the risk of tumor-associated bleeding. However, the histological findings, including the mitogenic index or MIB-1 index, did not always demonstrate high values, even in the bleeding cases. The present case was affected by abdominal pain and hemorrhagic ascites, symptoms similar to those other cases. A fever was noted upon arrival, which might have indicated peritoneal inflammation caused by EGIST rupture. Our patient was also relatively young, and the tumor size was relatively small compared to those in previous reports.
Table.
Extra Gastrointestinal Stromal Tumor Cases with Intraperitoneal Bleeding.
| Case | Age | Gender | Symptom | Origin of tumor | Maximum diameter(mm) | Treatment | Mitotic index (/50 HPF) |
Mib-1 Index (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | F | Abdominal pain | Greater omentum | 130 | Surgery | 1 | 2 | 3 |
| 2 | 63 | M | Abdominal distension | Greater omentum | 220 | Surgery | 20 | 10 | 15 |
| 3 | 58 | M | Abdominal pain | Greater omentum | 55 | Surgery | <5 | - | 17 |
| 4 | 60 | F | Abdominal distension | Greater omentum | 300 | Surgery | 1 | - | 18 |
| 5 | 43 | M | Abdominal pain | Greater omentum | 190 | Surgery | 6 | - | 19 |
| 6 | 53 | F | Abdominal distension | Greater omentum | 150 | Surgery | 4 | - | 20 |
| 7 | 67 | M | Abdominal pain | Greater omentum | 102 | Surgery | 1 | - | 22 |
| 8 | 40 | M | Abdominal distension | Lesser omentum | 120 | Surgery | <5 | 3 | 16 |
| 9 | 85 | M | Abdominal pain | Mesentery | 80 | Surgery | 0-2 | - | 14 |
| 10 | 70 | M | Abdominal pain | Mesentery | 90 | Surgery | 13 | - | 21 |
| Our case | 41 | F | Abdominal pain | Greater omentum | 60 | Surgery | 3 | 1 |
M: male, F: female
Regarding EGIST cases with intraperitoneal bleeding, one case arising from the lesser omentum and two from the mesentery have been reported, each of which had similar clinical symptoms to cases with tumors arising from the greater omentum (Table).
Apart from with intraperitoneal cases, pancreatic, vaginal, and urinary EGISTs have often been found in those with overt bleeding, such as in the GI, or with postmenopausal vaginal or urinary discharge. Previous reports of three cases with bleeding in the pancreas were diagnosed based on upper or lower GI symptoms due to penetration by a pancreas tumor into the intestinal tract (4-6). In contrast, the main symptoms of an EGIST arising from the vagina are postmenopausal bleeding or brown discharge directly related to vaginal bleeding (8-10). In addition, two previous cases - one with bladder bleeding and the other with prostate bleeding - were diagnosed based on overt urinary bleeding symptoms (11,12). Bleeding related to an EGIST varies depending on the tumor origin, so the clinical symptoms noted in reported cases vary. However, even though the tumor size is well recognized as being significantly associated with GI bleeding frequency, we found no such tendency in previously reported cases of EGIST, although that might be related to the small number of reports available.
Several reports of EGIST cases with intraperitoneal or distant metastasis have been presented. Jacobs et al. (21) and Haba et al. (23) reported EGIST findings in patients with liver metastasis, while others treated patients with pulmonary metastasis or peritoneal seeding (24,25). However, the tendency for metastasis in affected patents is unclear, possibly due to the low number of EGIST cases reported.
A definitive preoperative diagnosis of the tumor in the present case was not obtained. Elevated leukocyte counts and CRP levels were found in a blood test, considered to be due to inflammatory changes associated with intraperitoneal bleeding. Based on the results obtained from the various imaging examinations performed, we detected an intraperitoneal tumor, which resulted in a differential diagnosis of GIST, pancreatic, or mesenchymal tumor. In particular, images of the tumor obtained on CT and MRI were compatible with findings of a previous EGIST case reported by Tokunaga et al. (13), although those findings alone were not adequate to confirm the final diagnosis. Since a preoperative biopsy is difficult due to the bleeding risk in EGIST cases, the final diagnosis is often obtained based on the pathological findings of surgical specimens. Furthermore, several previous reports of EGIST patients have noted that the determination of a definitive preoperative diagnosis was difficult. The accumulation of additional EGIST cases will be required to establish novel biomarkers and specific image findings useful for the diagnosis.
In summary, we encountered a rare case of EGIST originating from the greater omentum with intraperitoneal hemorrhaging. It is important to point out that intraperitoneal bleeding can occur in association with this type of tumor, and EGISTs should be considered for the differential diagnosis when such symptoms are noted.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Yi JH, Park BB, Kang JH, et al. Retrospective analysis of extra-gastrointestinal stromal tumors. World J Gastroenterol 21: 1845-1850, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors - definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438: 1-12, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Nishizaki D, Mizuno K, Takeda R, et al. Laparoscopic resection for PDGFRA-mutant gastrointestinal stromal tumor in the greater omentum presenting with intraabdominal hemorrhage: a case report. Nihon Naishikyou Geka Gakkai Zasshi (J Japan Soc Endosc Surg) 20: 269-275, 2015(in Japanese). [Google Scholar]
- 4.Wegge J, Bartholomew DM, Burke LH, et al. Pancreatic extra-gastrointestinal stromal tumor masquerading as a bleeding duodenal mass. BMJ Case Rep 2012: bcr2012007040, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziret M, Çetinkünar S, Aktaş E, et al. Pancreatic gastrointestinal stromal tumor after upper gastrointestinal hemorrhage and performance of Whipple procedure: A Case Report and Literature Review. Am J Case Rep 16: 509-513, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yol S, Polat S, Duman S, et al. Pancreatic extragastrointestinal stromal tumor invading the duodenum. Turk J Surg 34: 231-233, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harindhanavudhi T, Tanawuttiwat T, Pyle J, et al. Extra-gastrointestinal stromal tumor presenting as hemorrhage pancreatic cyst diagnosed by EUS-FNA. JOP 10: 189-191, 2009. [PubMed] [Google Scholar]
- 8.Hanayneh W, Starr J, George TJ Jr, et al. Exrtagastrointestinal stromal tumors of the pelvic cavity and the vagina: two case reports and review of literature. Gynecol Oncol Rep 25: 3-7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weppler EH, Gaertner EM. Malignant extragastrointestinal stromal tumor presenting as a vaginal mass: report of an unusual case with literature of review. Int J Gynecol Cancer 15: 1169-1172, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Le BH, Nguyen J, Bossert A, Cran dall T, Robinson-Benett B. Surgical enucleated gastrointestinal tumor of the retrovaginal septum. Cureus 11: e5019, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZH, Feng GW, Liu HF, et al. A young man with primary prostatic extra-gastrointestinal stromal tumor: a rare case report and review of the literature. Int J Clin Exp Pathol 7: 1764-1770, 2014. [PMC free article] [PubMed] [Google Scholar]
- 12.He F, Fang Z, Zhu P, et al. Bladder extragastrointestinal stromal tumor in an adolescent patient: a case-based review. Mol Clin Oncol 2: 960-962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokunaga T, Miyake H, Aoyama M, et al. A case of giant gastrointestinal stromal tumor primarily occurred in the greater omentum. Shikoku Acta Medica 68: 139-146, 2012(in Japanese, Abstract in English). [Google Scholar]
- 14.Ogata N, Uehuji K, Takeo H, et al. A case of EGIST originated from the mesentery which ruptured spontaneously and caused intraabdmoinal bleeding. Nihon Rinsho Geka Gakkai Zasshi (J Japan Surg Assoc) 79: 1725-1729, 2018(in Japanese, Abstract in English). [Google Scholar]
- 15.Ito H, Funahashi H, Sakou T, et al. Two cases of giant GIST of the omentum. Nihon Rinsho Geka Gakkai Zasshi (J Japan Surg Assoc) 65: 3307-3311, 2004(in Japanese, Abstract in English). [Google Scholar]
- 16.Gomi K, Shirota H, Shimada KY, et al. A case of pedunculated gastrointestinal stromal tumor of the lesser omentum presenting preoperatively as a hemoperitoneum. Nihon Rinsho Geka Gakkai Zasshi (J Japan Surg Assoc) 71: 828-832, 2010(in Japanese, Abstract in English). [Google Scholar]
- 17.Yamada S, Suguwara H, Takahashi T, et al. A case of primary gastrointestinal stromal tumor of the omentum presenting as an intraabdominal hemorrhage. Nihon Fukubu Kyukyu Igakkai Zasshi (J Abdom Emerg Med) 38: 757-761, 2018(in Japanese, Abstract in English). [Google Scholar]
- 18.Seow-En I, Chiow AK, Tan SS, et al. Primary omental gastrointestinal stromal tumor (GIST) presenting with a large abdominal mass and spontaneous haemoperitoneum. BMJ Case Rep 2014: bcr201420558, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murayama Y, Yamamoto M, Iwasaki R, et al. Greater omentum gastrointestinal stromal tumor with PDGFRA-mutation and hemoperitoneum. World J Gastrointest Oncol 4: 119-124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shingu Y, Terasaki M, Okamoto Y, et al. A case of gastrointestinal stromal tumor (GIST) of the omentum. Nihon Rinsho Geka Gakkai Zasshi (J Japan Surg Assoc) 64: 1246-1250, 2003(In Japanese). [Google Scholar]
- 21.Jacobs K, de Gheldere Ch, Vanclooster P. A ruptured gastrointestinal stromal tumor of the transverse mesocolon: a case report. Acta Chir Belg 106: 218-221, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Islam S, Hosein D, Bheem V, et al. Primary greater omental GIST presenting with acute intra-abdominal haemorrhage. BMJ Case Rep 2017: bcr2017220254, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haba R, Kobayasshi S, Miki Y, et al. A case of malignant gastrointestinal tumor (GIST) of the omentum. Nihon Rinsho Saibou Gakkai Zasshi (J Jpn Soc Clin Cytol) 40: 76-80, 2001(in Japanese, Abstract in English). [Google Scholar]
- 24.Akolkar S, Melitas C, Piper M. Plevic gastrointestinal stromal tumor with pulmonay metastasis. ACG Case Rep J 6: 1-3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang J, Jeon TJ, Yoon SO, et al. An extragastrointestinal stromal tumor in the omentum with peritoneal seeding mimicking an appendiceal mucinous cancer with carcinomatosis. Ann Coloproctol 30: 93-96, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39: 1411-1419, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai S, Hishima T, Takazawa Y. Gasrtiointestinal stromal tumors and KIT-positive mesenchymal cells in the omentum. Pathol Int 51: 524-531, 2001. [DOI] [PubMed] [Google Scholar]