Abstract
Objective
Patients with Prader-Willi syndrome (PWS) are known to have a high mortality rate. However, little is known about the exact reason for this, particularly in adults, because so few reports have been published. The present study examined cardiovascular abnormalities to determine the cause of death in adults with PWS.
Methods
From September 2017 to April 2019, a total of 18 adults with PWS, and, no history of cardiovascular diseases, were enrolled. We investigated the levels of the cardiovascular biomarkers: high-sensitivity C-reactive protein (hs-CRP) and troponin T (TnT). To estimate the cardiac function, we measured the left ventricular ejection fraction (LVEF), global longitudinal systolic strain (GLS) of the left ventricle, ratio of peak early mitral filling velocity (E) to early diastolic mitral annular velocity (E/e' ratio), mitral annular plane systolic excursion (MAPSE) and tricuspid annular plane systolic excursion (TAPSE) using standard and tissue Doppler echocardiography.
Results
The mean patient age was 28±9 years old. There were 11 men, and the mean body mass index was 45.1 kg/m2. Dyslipidemia (82%), diabetes mellitus (82%) and hypertension (83%) were commonly found as comorbidities. Most patients had elevated levels of hs-CRP (mean 1.007±0.538 mg/dL). The LVEF (mean 61%±5%) showed normal values, while the GLS (mean 15.0%±3.0%) was decreased. The TAPSE was mildly reduced (mean 16±3 mm).
Conclusion
These results suggest that subtle cardiovascular abnormalities have already begun in young adults with PWS. We need to manage obesity and the resultant obesity-related disorders in order to prevent heart failure and coronary atherosclerosis in PWS patients.
Keywords: obesity, global longitudinal strain, high sensitivity C-reactive protein
Introduction
Prader-Willi syndrome (PWS) is a genetic disorder that affects 1 in 25,000 people (1). It results from a lack of gene expression in the PWS region of the paternally inherited chromosome 15q11-13, caused by a deletion, maternal uniparental disomy (maternal UPD), imprinting center defect, or translation.
Patients with PWS have a high incidence of sudden unexpected death, with causes reported to differ between children and adults. Several previous studies have mentioned obesity-related cardiovascular and respiratory diseases as the leading causes of death among adults with PWS (2,3). PWS is usually associated with behavioral and intellectual disabilities. These disabilities are also reported to contribute to the severity of adult obesity and related disorders, leading to an overall increased risk of death. However, the exact cause of death in PWS adults is unknown because of the limited number of reports available.
The present study investigated the cardiac structure and function as well as, vascular disorders in adults with PWS using biomarkers and transthoracic echocardiography (TTE).
Material and Methods
Study population
A total of 18 participants with PWS over 20 years old, who visited our cardiovascular medicine with consultations of the pediatrics between September 2017 and April 2019 at Dokkyo Medical University, Saitama Medical Center were studied. All patients were diagnosed with PWS using fluorescence in situ hybridization or the methylation test at another hospitals. The interval to the diagnosis of PWS ranged from a few months to several years after birth.
Informed consent for cytogenetic and/or molecular-genetic studies was obtained from all individuals: 15 participants with a deletion involving 15q11-13 and 3 with an maternal UPD of chromosome 15. For each patient, the data evaluated at baseline included: laboratory data, echocardiographic data and medication use. This study was approved by the Dokkyo Medical University, Saitama Medical Center Review Board.
Clinical assessments
The body mass index (BMI) was calculated as the body weight (kg) divided by the height squared (m2). We defined the use of antihypertensive drugs or a systolic blood pressure (SBP)>130 mmHg or diastolic blood pressure (DBP)>80 mmHg according to the patient's medical records as hypertension (HT) (4); the use of lipid-lowering therapy or triglyceride levels>150 mg/dL, high-density lipoproteins (HDL) cholesterol<40 mg/dL or low-density lipoproteins (LDL) cholesterol>140 mg/dL as dyslipidemia (DL); a history of a diagnosis of diabetes mellitus (DM) or the use of oral hypoglycemic agents, insulin or hemoglobin A1C (HbA1C)>6.5% as DM; and a glomerular filtration rate (GFR)<60 mL/min/1.73 m2 for ≥3 months, irrespective of the cause, as chronic kidney disease (CKD).
Biochemical assessments
Venous blood for biochemical analyses was obtained on the day when TTE was performed. The serum TG, HDL cholesterol, LDL cholesterol, glucose, HbA1C, creatinine, free thyroxine (FT4), free triiodothyronine (FT3), and thyroid-stimulating hormone (TSH) levels were measured. Cardiac troponin T (TnT), brain natriuretic peptide and high-sensitivity C-reactive protein (hs-CRP) levels were also examined using standard laboratory techniques.
Echocardiography
TTE was performed using a commercially available ultrasound system (Vivid E9; General Electric Medical Systems, Horten, Norway). The left ventricular ejection fraction (LVEF) and left atrial volume were measured using the Simpson biplane method. The left ventricular mass (LVM) was calculated using two-dimensional (2D) -derived linear dimensions according to the Cube formula. The transmitral early (E) and, late (A) diastolic filling velocities and deceleration time (DT) were measured in the apical three-chamber view or four-chamber view using pulsed-wave Doppler. Next, early diastolic (e') velocity of the septal and lateral mitral annulus was measured and the E/e' ratio was calculated. The mitral annular plane systolic excursion (MAPSE) (5) was measured at the septal or lateral mitral annulus in the four-chamber view from its highest position after atrial ascent to the maximal descent during systole, and the tricuspid annular plane systolic excursion (TAPSE) (6) was measured at the free wall of the tricuspid annulus in the four-chamber view through the same method as MAPSE.
The left ventricular global longitudinal strain (GLS) (7) was assessed offline by a single investigator using the EchoPAC version 203 software program (GE Healthcare, Cary, NC, USA). A single sonographer recorded three views: (apical four-chamber, two-chamber and three-chamber), and traced the endocardium of the left ventricle (LV) at end-diastole in each view. The software program detected the movement of the entire myocardial wall and defined the areas of interest, while the sonographer determined whether or not the quality was considered acceptable. In poorly detected segments, the sonographer readjusted the endocardial contour until better detection was obtained. If this was not possible, the cases were excluded from the analysis.
Statistical analyses
Statistical analyses were performed using the SPSS Statistics 26 software (IBM SPSS, USA).
Results
Patient characteristics
The 18 patients, were 11 men and 7 women with a mean age of 28±9 years old. The mean BMI was 45.1±11.6 kg/m2. The majority of patients had HT (83%), DL (82%) and DM (82%). Their HbA1C values (mean 9.0%±2.8%) were very high. The TG values showed a wide range, from normal to high, and the mean white blood cell (WBC) count was increased in most cases (78%). Among the biomarkers, hs-CRP levels were elevated in all but one case, although the mean brain natriuretic peptide (BNP) value was normal.
We examined medications containing cardio-protective agents. The prescription rates of angiotensin receptor blockers (ARBs), calcium channel blockers, and diuretics were 58%, 58%, and 42%, respectively. None of the patients were taking angiotensin-converting enzyme inhibitors (ACEIs) or β blockers. Statins, oral anti-diabetic drugs or insulin and sex hormones were being taken by 67%, 75% and 17% of the patients, respectively (Table 1).
Table 1.
Patients’ Characteristics.
| Variable | PWS (n=18) | |
|---|---|---|
| Age, years | 28±9 | |
| Male/Female | 11/7 | |
| BMI, kg/m2 | 45.1±11.6 | |
| Hypertension, % | 78 | |
| Systolic blood pressure, mmHg | 135±13 | |
| Diastolic blood pressure, mmHg | 77±9 | |
| Diabetes mellitus, % | 82 | |
| HbA1C, % | 9.0±2.8 | |
| Dyslipidemia | 94 | |
| TG, mg/dL | 155 (107-263) | |
| HDL mg/dL | 46±7 | |
| LDL, mg/dL | 125±27 | |
| CKD, % | 0 | |
| TSH, μU/mL | 2.4±1.4 | |
| WBC, μL | 9,150 (8,000-12,000) | |
| hs-CRP, mg/dL | 1.007±0.538 | |
| BNP, pg/mL | 17.4±19.8 | |
| Troponin T, ng/mL | 0.007±0.009 | |
| Medications | ||
| ARB, n (%) | 10 (58) | |
| Calcium channel blocker, n (%) | 10 (58) | |
| Diuretics, n (%) | 8 (42) | |
| Statin, n (%) | 12 (67) | |
| Metformin, n (%) | 11 (61) | |
| SGLT-2 inhibitor, n (%) | 1 (6) | |
| α-GI, n (%) | 4 (22) | |
| DPP-4 inhibitor, n (%) | 9 (50) | |
| Insulin, n (%) | 8 (44) | |
| Sex hormones, n (%) | 3 (17) |
Values are n, mean±SD or median (interquartile range).
PWS: Prader-Willi syndrome, BMI: body mass index, HbA1C: hemoglobin A1C, TG: triglyceride, HDL: high density lipoprotein, LDL: low density lipoprotein, CKD: chronic kidney disease, TSH: thyroxine-stimulating hormone, WBC: white blood cell, hs-CRP: high-sensitive C-reactive Protein, BNP: brain natriuretic peptide, ARB: angiotensin II receptor blocker, SGLT-2 inhibitor: sodium-glucose cotransporter-2 inhibitor, α-GI: α-glucosidase inhibitor, DPP-4 inhibitor: dipeptidyl peptidase-4 inhibitor
Echocardiographic parameters
The echocardiographic findings of the 14 patients available for an analysis are shown in Table 2. The mean LVEF was normal (61%±5%). The mean GLS was decreased (15.0%±3.0%). The mean MAPSE was 12±3 mm, and the mean TAPSE was 16±3 mm. The mean E wave, A wave, E/A, DT and E/e' were normal. The mean left atrial volume index (LAVI) (22±8 mL/m2) and left ventricular mass index (LVMI) (62±14 mg/m2) were also normal.
Table 2.
Echocardiographic Parameters.
| Variable | PWS (n=14) | |
|---|---|---|
| LVDd, mm | 47±5 | |
| LVDs, mm | 32±4 | |
| LAVI, mL/m2 | 22±8 | |
| LVMI, mg/m2 | 62±14 | |
| Left ventricular ejection fraction, % | 61±5 | |
| Stroke volume index, mL/m2 | 34±6 | |
| GLS, % | 15.0±3.0 | |
| MAPSE, mm | 12±3 | |
| TMF : E, cm/sec | 89±16 | |
| A, cm/sec | 56±14 | |
| DT, msec | 152±23 | |
| E/e’ | 9±2 | |
| TAPSE, mm | 16±3 |
Values are n, mean±SD.
PWS indicates Prader-Willi syndrome, LVDd: left ventricular end diastolic diameter, LVDs: left ventricular end systolic diameter, LAVI: left atrial volume index, LVMI: left ventricular mass index, GLS: global longitudinal systolic strain, MAPSE: mitral annular plane systolic excursion, TMF: trans mitral flow, E: early diastolic transmitral flow velocity, A: atrial systolic transmittal flow velocity, DT: deceleration time of E velocity, E/e’: the ratio of mitral peak velocity of E to early diastolic mitral annular velocity, TAPSE: tricuspid annular plane systolic excursion
Enrollment of patients with PWS
The clinical, laboratory and echocardiographic findings of the 18 patients are summarized in Table 3. Sample images of case 10 are shown (Fig. 1 in the Supplemental Material file). This case was a 27-year-old man. His BMI was 43.7 kg/m2, and he had both DM and DL. His hs-CRP level was elevated (0.518 mg/dL), although his BNP (1.2 pg/dL) and TnT (<0.003 ng/mL) levels were normal. His LVEF was 61%, and his TAPSE was 16 mm, although his GLS (16.3%) and MAPSE (10 mm) values were decreased on TTE.
Table 3.
Summary of the Clinical, Laboratory and Echocardiographic Findings in 18 Patients with PWS.
| Case No. | Age (years) | Gender | Genetic subtype | BMI (kg/m2) | HbA1C (%) | hs-CRP (mg/dL) | TnT (ng/mL) | GLS (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 41 | Female | Deletion | 38.1 | 5.4 | 0.122 | <0.003 | 16.2 |
| 2 | 28 | Male | Deletion | 50.9 | 6.1 | 1.078 | 0.008 | 10.5 |
| 3 | 41 | Female | Deletion | 39.2 | 7.2 | 0.974 | 0.026 | - |
| 4 | 50 | Male | Deletion | 46.3 | 5.7 | 0.965 | <0.003 | 20.9 |
| 5 | 23 | Male | Deletion | 41.8 | 12.8 | 1.496 | 0.012 | 14.8 |
| 6 | 26 | Male | UPD | 44.3 | 9.5 | 0.962 | 0.004 | 12.1 |
| 7 | 32 | Female | Deletion | 66.2 | 9.2 | 1.124 | 0.003 | 15.3 |
| 8 | 20 | Male | Deletion | 36.3 | 6.8 | 0.148 | 0.017 | - |
| 9 | 20 | Male | Deletion | 37.6 | 13.7 | 1.429 | 0.003 | 15.7 |
| 10 | 27 | Male | Deletion | 43.7 | 11.4 | 0.518 | <0.003 | 16.3 |
| 11 | 22 | Male | UPD | 38.3 | 11.1 | 1.302 | 0.004 | 14.8 |
| 12 | 28 | Female | Deletion | 66.2 | 10.8 | 2.066 | 0.004 | 17.0 |
| 13 | 29 | Male | Deletion | 37.8 | 12.0 | 0.534 | 0.032 | 10.5 |
| 14 | 20 | Male | Deletion | 36.6 | 12.2 | 1.369 | 0.003 | - |
| 15 | 20 | Female | Deletion | 31.8 | 9.4 | 0.664 | 0.006 | 13.6 |
| 16 | 22 | Female | UPD | 38.7 | 7.1 | 1.521 | <0.003 | 14.4 |
| 17 | 20 | Male | Deletion | 72.2 | 5.7 | 0.307 | 0.009 | 14.6 |
| 18 | 31 | Female | Deletion | 45.0 | 5.9 | 1.546 | <0.003 | - |
PWS: Prader-Willi syndrome, BMI: body mass index, HbA1C: hemoglobin A1C, hs-CRP: high-sensitive C-reactive protein, TnT: troponin T, GLS: global longitudinal strain, maternal UPD: maternal uniparental disomy
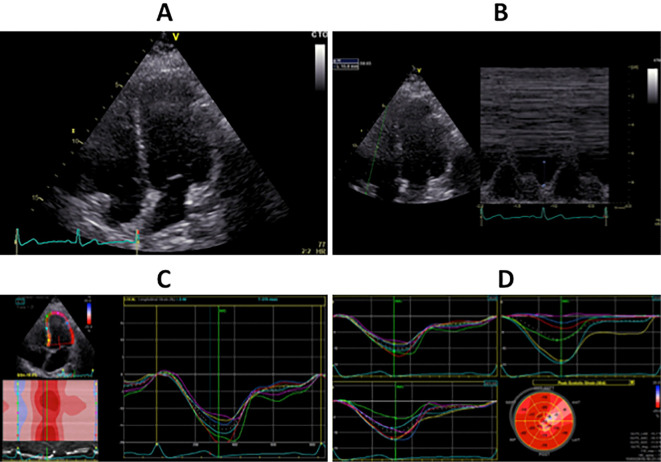
Figure 1.
Transthoracic echocardiography images in a patient with Prader-Willi syndrome. A: Four-chamber view by two-dimensional echocardiography. B: Tricuspid annular plane systolic excursion (16 mm) by M mode echocardiography. C: Left ventricular global longitudinal strain (16.3%) in the 4-chamber view by tissue Doppler echocardiography. D: Mean left ventricular global longitudinal strain (14.6%) in the 4-chamber, 2-chamber and 3-chamber views by tissue Doppler echocardiography.
Correlation of GLS or hs-CRP and other findings
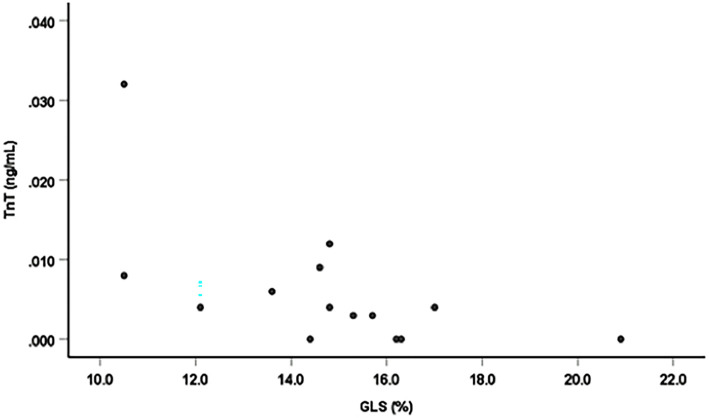
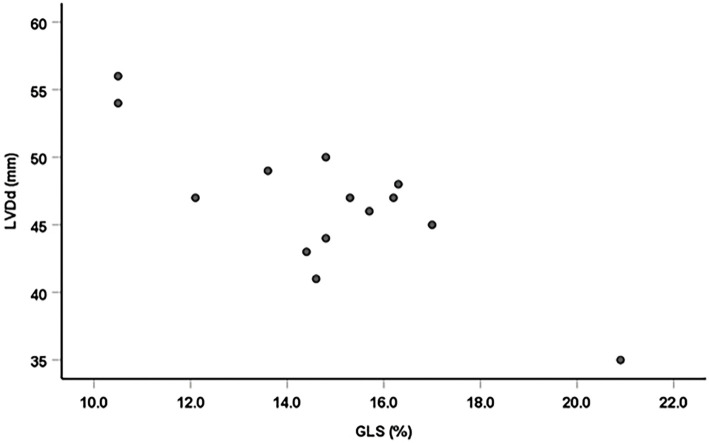
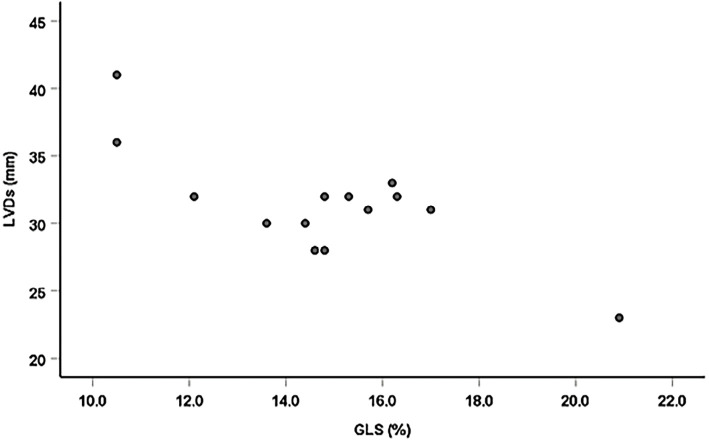
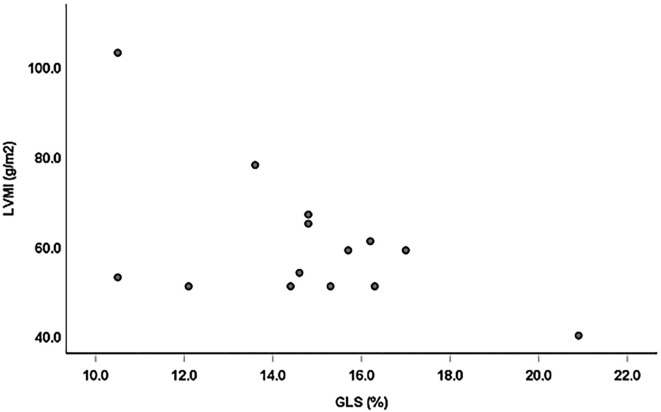
There were significant negative linear correlations between the GLS and TnT, left ventricular end-diastolic dimension (LVDd), left ventricular end-systolic dimension (LVDs) and LVMI (r=-0.61 p=0.026; r=-0.81 p=0.001; r=-0.71 p=0.001; and r=-0.54, p=0.046, n=14, respectively; Figs. 2-5). However, there was no correlation between the GLS or hs-CRP and other findings.
Figure 2.
Correlation between the GLS and TnT. There was a significant negative linear correlation between the GLS and TnT (r=-0.61, p=0.026, n=14).
Figure 3.
Correlation between the GLS and LVDd. There was a significant negative linear correlation between GLS and LVDd (r=-0.81, p=0.001, n=14).
Figure 4.
Correlation between the GLS and LVDs. There was a significant negative linear correlation between GLS and LVDs (r=-0.71, p=0.001, n=14).
Figure 5.
Correlation between the GLS and LVMI. There was a significant negative linear correlation between GLS and LVMI (r=-0.54, p=0.046, n=14).
Discussion
Cardiac evaluations in adult patients with PWS
Our study showed an obvious decrease in the GLS in PWS patients, suggesting that LV dysfunction may have already begun in young adulthood in patients with PWS, even those without a history of heart disease. PWS is a genetic obesity syndrome characterized by hyperphagia, behavioral disturbance and intellectual disability. Obesity has many adverse effects on hemodynamics and the cardiovascular structure and function via various mechanisms (8). It increases the total blood volume and arterial wall and LV wall stress, causes cardiac structural changes and induces LV systolic and diastolic dysfunction. Aside from its direct effects on the heart, obesity worsens most of the major cardiovascular risk factors, including plasma lipids, blood pressure, glucose and inflammation. Obesity-related disorders, such as HT, DM and DL, increase the burden on the heart (9). Marcus et al. (10) also reported that the global peak systolic strain values and global peak systolic strain rate measurements were significantly decreased in children with PWS compared with healthy control subjects.
The major objective of the present study was to show the cardiac structural changes in adult PWS related to heart failure. We conducted this study because heart failure was shown to be the leading cause of cardiac death, and the fourth-leading cause of all-cause death in adult PWS (11). We noted significant correlations of GLS with LVDd, LVDs, and LVMI. In addition, we showed for the first time the biventricular functions using MAPSE and TAPSE in adult PWS. There was also a significant negative linear correlation between the GLS and TnT. Whether or not these findings indicate ongoing cardiac damage is unclear. However, these results supported our notion that subtle cardiac abnormalities exist in adults with PWS.
Nagai et al. (12) reported instances of sudden death in two adult patients with PWS, associated with complications of heart failure secondary to massive obesity and DM. In addition, growth hormone (GH) and Insulin-like growth factor-1 (IGF-1) have been implicated in cardiac structural and functional abnormalities (13), and GH deficiency is known to increase vascular intima-media thickness and atheromatous plaques (14). GH secretion was shown to be reduced in both children and adults with PWS (15), and it can cause cardiovascular diseases. The early detection of cardiac dysfunction is essential for preventing the onset of heart failure. GLS is a sensitive marker for detecting and quantifying subtle disturbances in the LV systolic function. Evidence has shown that the GLS is more sensitive for estimating the LV dysfunction than the LVEF, and provides additional prognostic information (16,17). The GLS might be useful for estimating early LV dysfunction in patients with severe obesity or PWS. However, it is difficult to estimate the GLS in patients with poor acoustic windows; therefore, in such cases, early subtle cardiac dysfunction must be detected using other simple and reliable measurements.
Levels of hs-CRP in adult patients with PWS
Most of the subjects in the present study had high hs-CRP levels. Patel et al. (9) reported that the hs-CRP level was increased in all patients with PWS. In patients with obesity, the secretion of inflammatory cytokines is increased, and the secretion of adiponectin is decreased with the promotion of low-grade chronic inflammation (18). hs-CRP is a marker of inflammation with prognostic importance in atherosclerotic disease (19). In particular, hs-CRP is a strong independent predictor of future myocardial infarction and stroke among healthy men and women (20). Chronic, systemic subclinical inflammation has been identified as a driving force for insulin resistance, metabolic syndrome, and type 2 DM (21). In fact, most of our patients were complicated with poorly controlled DM (mean HbA1C of 9.1%±2.9%). Obesity in patients with high hs-CRP levels needs to be managed, and intervention to prevent atherosclerosis is necessary.
Medications in adult patients with PWS
In this study, the administration of cardio-protective agents and statins had insufficient therapeutic effects. Several potential reasons for this are proposed. First, since adults with PWS continued to be treated by pediatricians, the necessary management may have been inadequate due to the lack of involvement of appropriate specialists. Second, given the rarity of adult patients with PWS, definite medication guidelines for their treatment are currently lacking. The present results highlight the difficulties in the long-term management of PWS patients.
Limitations
The present study identified the circulatory abnormalities in adults with PWS. However, Butler et al. (22) reported that respiratory failure was the most common cause of death in PWS patients with a mean age of 29.5±16.0 years old. In addition, sleep-disordered breathing, which has been linked to significant deficits in the neurocognitive function and excessive daytime sleepiness in patients with PWS, can cause death. Additional respiratory evaluations and sleep-related examinations are thus required in these patients (23).
We used echocardiography to assess cardiac abnormalities. Other imaging modalities, such as CT and MRI, are also useful for estimating not only the cardiac function but also atherosclerosis and myocardial fibrosis. Depending on the degree of obesity, the combination of these imaging modalities can also be used to facilitate the early diagnosis of circulatory abnormalities in PWS.
Conclusion
In our study, the GLS was decreased, and the hs-CRP level was increased in most young adults with PWS. These results suggest that subtle cardiac dysfunction and atherosclerosis might begin in young adulthood in patients with PWS. We need to manage obesity and obesity-related disorders in order to prevent heart failure and ischemic heart disease in patients with PWS.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet 38: 792-798, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scgrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 124: 333-338, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Grugni G, Crinò A, Bosio L, et al. The italian national survey for Prader-Willi syndrome: an epidemiology study. Am J Med Genet A 146: 861-871, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med 30: 160-164, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Wenzelburger FW, Tan YT, Choudhary FJ, Lee ES, Leyva F, Sanderson JE. Mitral annular plane systolic excursion on exercise: a simple diagnostic tool for heart failure with preserved ejection fraction. Eur J Heart Fail 13: 953-960, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Gorter TM, Hoendermis ES, Veldhuisen DJ, et al. Right Ventricular Dysfunction in Heart Failure With Preserved Ejection Fraction: A Systemic Review and Meta-Analysis. Eur J Heart Fail 18: 1472-1487, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Am Soc Echocardiogr 28: 1-39, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases. J Am Coll Cardiol 63: 1345-1354, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Patel S, Harmer JA, Loughnan G, Skilton MR, Steinbeck K, Celemajer DD. Characteristics of cardiac and vascular structure and function in Prade Willi syndrome. Clin Endocrinol 66: 771-777, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Marcus KA, van Alfen-van der Velden JA, Otten BJ, et al. Cardiac evaluation in children with Prader-Willi syndrome. Acta Paediatr 101: 225-231, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Pacoricona DL, Lemoine P, Ehlinger V, et al. Causes of death in Prader-Willi syndrome: lessons from 11 years' experience of a national reference center. Orphanet J Rare Dis 14: 238-247, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai T, Obata K, Tonoki H, et al. Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A 136: 45-48, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Bisi G, Podio V, Valetto MR, et al. Acute cardiovascular and hormone effects of GH and hexarelin, a synthetic GH-releasing peptide, in humans. J Endocrinol Invest 22: 266-272, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF. Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol 50: 457-464, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Burman P, Ritzen EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev 22: 787-799, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 71: 1947-1957, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. J Am Coll Cardiol 11: 260-274, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Park JJ, Park JB, Park JH, Cho GY. Circulating adiponectin levels, body composition and obesity-related variables in Prader-Willi syndrome: comparison with obese subjects. Int J Obes 30: 382-387, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke AP, Tracy RP, Kolodgie F, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death association with different pathologies. Circulation 105: 2019-2023, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 103: 1813-1818, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Pfützner A, Schöndorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol 4: 706-716, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi syndrome association (USA) 40-year mortality survey. Genet Med 19: 635-642, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 38: 1249-1263, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]