Figure 4: Pharmacokinetics and pharmacodynamics in diabetic rats.
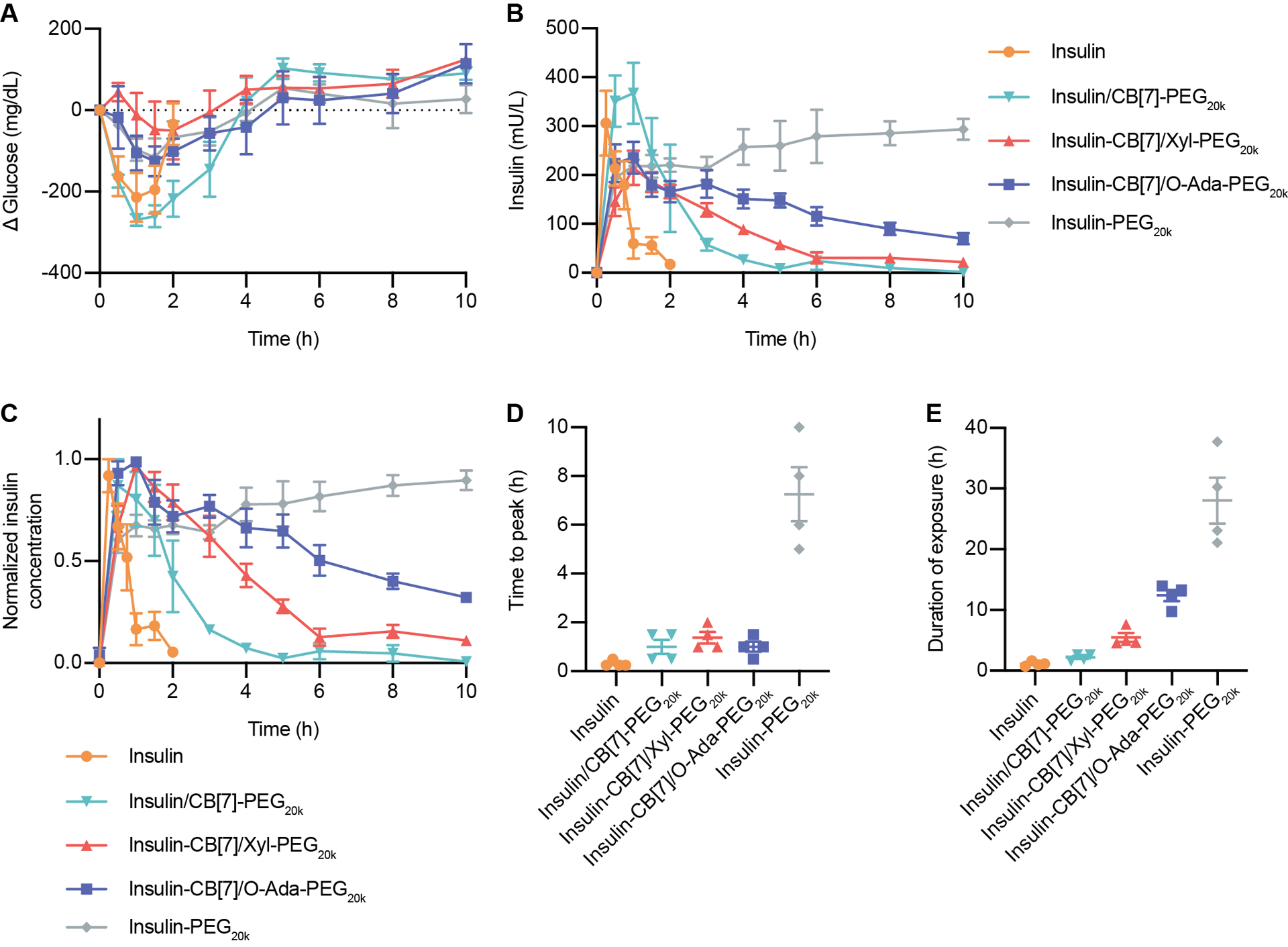
Fasted diabetic rats were administered one of five insulin formulations subcutaneously: (i) insulin (2 U/kg), (ii) insulin & CB[7]-PEG20k (10 U/kg), (iii) insulin-CB[7] & Xyl-PEG20k (10 U/kg), (iv) insulin-CB[7] & O-Ada-PEG20k (10 U/kg), (v) insulin-PEG20k (10 U/kg). Following administration, rats were given access to food. (A) Change in blood glucose levels following insulin administration. (B) Pharmacokinetics in terms of serum insulin concentrations following insulin administration. (C) Pharmacokinetics for each rat was normalized individually, and normalized values were averaged for insulin concentration for each treatment group. (D) Time to reach peak insulin concentrations. (E) Duration of exposure, defined as time to depletion of 25% of insulin peak concentration. Error bars indicate means ± SEM with n = 4 for all groups.
