Abstract
Background
The analgesic effects of Cannabis sativa are mediated by ∆9 tetrahydrocannabinol (THC), but the contributions of other bioactive complex components, including cannabigerol (CBG) and cannabidiol (CBD), are unclear. We describe the individual and combined effects of CBG, CBD and THC, on blocking capsaicin responses in dorsal root ganglion (DRG) neurons, in an in vitro model of nociceptor hypersensitivity.
Materials and Methods
Adult rat DRG were dissected and enzyme digested to obtain a neuronal suspension in BSF2 medium containing 2% fetal calf serum, and the neurotrophic factors NGF and GDNF. After 48 h, cultured neurons were loaded with Fura-2 AM, to determine the effects of cannabinoids on capsaicin responses using calcium imaging. In control experiments, neurons were treated with vehicle, followed by 1 µM capsaicin. In cannabinoid treated cultures, CBG, CBD or THC were applied individually, or combined (1:1:1 ratio), followed by 1 µM capsaicin. Data from n = 6 experiments were analysed with Student’s t-test and Pearson’s correlation coefficient.
Results
CBG, CBD and THC, applied individually, elicited dose-related calcium influx in a subset of DRG neurons, and a corresponding dose-related reduction of subsequent responses to capsaicin. Maximum inhibition of capsaicin responses was observed at 30 µM CBG, 100 µM CBD, and 100 µM THC individually, and with combined CBD+CBG+THC (1:1:1) at 90 µM. THC+CBD+CBG combined in a 1:1:1 proportion has the potential to enhance the potency of these compounds applied individually. There was a high correlation between cannabinoid-mediated calcium influx and reduction of capsaicin responses: CBG = −0.88, THC = −0.97, CBD = −0.99 and combined CBG + THC + CBD = −1.00.
Conclusion
CBG, CBD and THC demonstrated potent dose-related inhibition of capsaicin responses in DRG neurons when applied individually in vitro, and enhanced when applied in combination, being most effective at 90 μM. Thus, efficacy and tolerability of THC could be improved in combination with CBG and CBD at optimal concentrations, which deserve further studies in vivo.
Keywords: cannabinoid, CBG, CBD, THC, TRPV1, DRG neurons, pain
Plain Language Summary
Chronic pain affects 1 in 15 individuals globally, impacting their quality of life. Current drugs for the treatment of chronic pain have limited efficacy, and undesirable side-effects. The ability of cannabis to provide pain relief is well recognised, as are its many side-effects, which include drowsiness. Cannabis contains hundreds of different compounds, of which tetrahydrocannabinol (THC) is known to be psychoactive, by producing euphoria. Other non-psychoactive components may also have analgesic effects. Here we have tested the ability of THC, and the non-psychoactive cannabidiol (CBD) and cannabigerol (CBG) applied individually and in combination, to block responses in pain-sensing neurons, to determine their most effective concentrations for providing pain relief. This was tested in cultured sensory neurons, which are nerve cells grown in a tissue culture dish in a laboratory, and which respond to painful stimuli such as capsaicin (the hot ingredient of chilli peppers). We found that THC, CBG and CBD applied individually were able to block the capsaicin responses in the sensory neurons, and that THC+CBD+CBG combined in a 1:1:1 proportion was more effective than these compounds applied alone. This combination also made THC more effective at a lower concentration, with the potential to reduce its unwanted psychoactive effects. The findings of our study will enable the testing of these compounds in human studies, to develop more effective cannabis-based drugs for treating chronic pain, and with reduced side-effects.
Introduction
Chronic pain may be defined as “pain which has persisted beyond normal tissue healing time”, which is generally considered to be three months.1 In the UK, 13–50% of adults are reported to be affected by chronic pain, with 10.4–14.3% experiencing moderate to severe disabling pain.2 The aetiology of chronic pain includes postsurgical pain that affects up to 10% of patients undergoing surgery,3 arthritis and neuropathy, and is influenced by factors such as age, obesity and psychosocial aspects.4 Opioids remain a keystone of treatment for severe and persistent pain, in accordance with the analgesic ladder proposed by the World Health Organization (WHO), especially for palliative care.5 However, inadequate relief of chronic pain remains an unmet need, severely compromising the quality of life in a significant proportion of affected individuals. The current ‘opioid crisis’ has accelerated the need for non-opioid treatments of chronic pain.
The identification of the analgesic effects of Δ9 tetrahydrocannabinol (THC), the psychoactive phytocannabinoid derived from the plant Cannabis sativa,6 led to the formulation of novel drugs such as Marinol (the synthetic version of Dronabinol), targeting the cannabinoid subtype 1 (CB1) and subtype 2 (CB2) receptors.7 Elderly patients with chronic pain treated with Dronabinol reported more than 50% pain relief in 10% of patients, and more than 30% pain relief in 52% of patients.8 However, significant central side-effects of synthetic THC derivatives such as dysphoria have limited their usefulness,9 including for chronic neuropathic pain.10,11
The analgesic effects of non-psychotropic ingredients of the cannabis plant have been utilized for developing oral medications such as the licensed Sativex (GW Pharmaceuticals, Cambridge, UK), an oromucosal spray containing THC and CBD in a 1:1 ratio, which is more effective and better tolerated.12 Cannabinoid combination preparations have provided significant dose-related pain relief for postoperative pain,13 and improved pain relief and quality of sleep in patients with chronic neuropathic pain following brachial plexus injury.14 A Phase III placebo-controlled study of Sativex also reported alleviation of central neuropathic pain in multiple sclerosis patients.15
Cannabis sativa contains hundreds of different components, including 200 different terpenes. These components characterize the complex nature of cannabis, and may provide a variety of therapeutic effects in synergy with cannabinoids.16 Recent legalization of medical cannabis supports the development of novel cannabis-based therapeutics, based on emerging information of its less well-known components. These may have synergistic effects similar to the entourage effect described for endocannabinoids,17 via the cannabinoid CB1, CB2 receptors, and transient receptor potential (TRP) ion channels. While THC is well known to provide pain relief, more information about the non-psychoactive cannabinoid CBD has recently been provided from studies especially in the context of analgesia.18
CBD is well known for not being psychoactive or inducing euphoria,7,18 – it can increase the tolerability and therapeutic window of THC to potentiate its beneficial effects,19 which led to the development of Sativex. Another key non-psychoactive cannabis component is cannabigerol (CBG), the precursor of CBD and THC, which modulates cannabinoid receptor subtype 2 (CB2) signalling.20 CBG has more potent analgesic, muscle relaxant, anti-erythema and lipoxygenase blocking activity than THC.12,21,
The effects of cannabinoids are mediated by specific cannabinoid receptors, CB1,22 and CB2,23 to activate downstream effects via adenylyl cyclase inhibition.24 There is evidence for CBD and CBG interaction with other receptors such as the transient receptor potential vanilloid subtype 1 receptor (TRPV1) in transfected HEK cells.25–27 TRPV1 is expressed in small sensory neurons of the DRG, and which detects noxious stimuli such as temperature of 43°C and above, inflammatory mediators, low pH, and capsaicin, the pungent ingredient of chilli peppers.28,29 The endocannabinoid anandamide also reduced hyperalgesia and inflammation via peripheral TRPV1 and CB1 receptors expressed in primary afferent fibres.30
Other members of the TRP ion channel superfamily activated by cannabinoids include TRPA1, which is activated by wasabi and mustard oil,31 and is expressed by nociceptors. TRPV1 and TRPA1 are co-expressed in rodent sensory neurons,32 increased in human DRG neurons after injury, and cross-desensitize in cultured human and rat DRG neurons.33,34 These receptors provide potential targets for the anti-nociceptive effects of cannabinoids. Cannabinoid agonists were previously reported to stimulate TRPV1 and TRPA1 in sensory neurons, leading to calcium influx and desensitization.35 We have recently reported CBD inhibition of TRPV1 in DRG neurons at low physiological doses that did not stimulate calcium influx,36 similar to inhibition of TRPV1 and TRPM8 in transfected HEK cells.27 However, as higher concentrations of cannabinoids induce calcium influx, it is likely that these doses of CBG, CBD and THC would lead to desensitization of TRPV1 in DRG neurons, similar to capsaicin-mediated effects.
In this study, we have used an in vitro model of neuronal hypersensitivity produced by added neurotrophic factors NGF and GDNF, which are increased in clinical chronic pain conditions.37,38 Adult DRG neurons cultured with additional neurotrophic factors NGF and GDNF demonstrate neuronal sensitization, manifested as enhanced responses to capsaicin stimuli.33 We examined the effects of CBD, CBG and THC on TRPV1 activation by capsaicin in cultured adult rat DRG neurons. Our findings show that these cannabinoids induce dose-related calcium influx and inhibit capsaicin responses when applied individually, with greater inhibition when applied in combination.
Materials and Methods
Neuronal Cultures
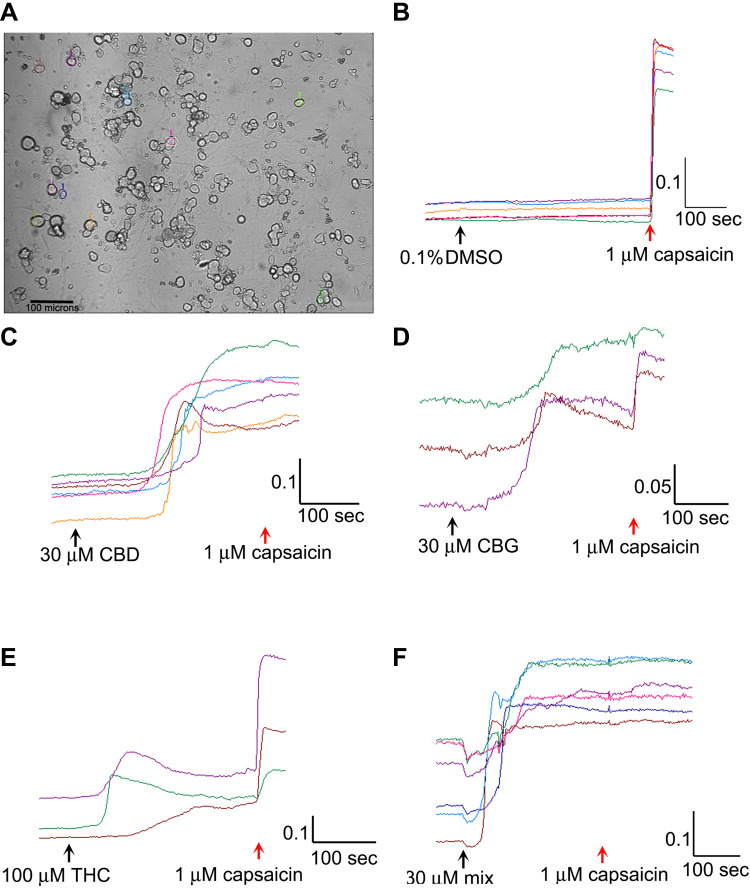
For each experiment, one adult female Wistar rat (Charles River UK Ltd, Margate, Kent, UK), was sacrificed by exposure to rising titres of CO2 followed by cervical dislocation (with approvals from the Animal Welfare Ethical Review Body, Imperial College London, following UK Home Office approved procedures, and in keeping with the 3Rs ARRIVE guidelines, Animal Scientific Procedures Act (ASPA 1986), in conjunction with guidelines from the Laboratory Animal Science Association (LASA) and Federation of European Laboratory Animal Science Association (FELASA)). Bilateral DRG from all levels were harvested in Ham’s F12 medium under sterile conditions, and enzyme digested in 2 mL Ham’s F12 medium containing collagenase (0.2%) and dispase (0.5%), at 37°C for 3 h. The enzyme digested tissue was triturated in 1 mL BSF2 medium containing 100 ng/mL nerve growth factor (NGF-7s, Merck Life Science, UK Ltd), and 50 ng/mL glial cell-line derived neurotrophic factor (GDNF, Merck Life Science, UK Ltd), trypsin inhibitor and DNase, to obtain a neuronal cell suspension. 8000–10000 neurons in 200 µL medium were plated onto each of 20 glass-bottom petri dishes (MatTek Corp, USA), coated with 20 μg/mL poly-l-lysine and 20 μg/mL laminin. The cultures were incubated at 37°C for 45 min to allow the cells to attach before adding 2 mL BSF2 medium. 24 h later 5 µM cytosine arabinoside was added to all dishes to inhibit the growth of non-neuronal cells. Calcium imaging was performed 48 h after plating.
Calcium Imaging
The culture medium was aspirated from each dish, and the neurons rinsed with 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffered Hank’s Balanced salt solution (HBSS), containing 0.1% Bovine Serum Albumin (BSA) (pH 7.4). 1 mL HEPES buffered HBSS containing 2 µMol Fura-2 AM (Life Technologies, Paisley, UK), was added to each dish, and the petri dishes were incubated at 37°C for 40 min. The medium was then replaced with HEPES-HBSS containing 0.1% BSA for 20 min to allow de-esterification in the dark. The intracellular bound/unbound Ca2+ ratio was determined by alternately exciting the neurons at 340 and 380 nm wavelengths (λex) for one min to establish a stable baseline of the 340/380 nm λex ratio. For vehicle control, 0.1% DMSO was added to the dish, followed 5 min later by 1 µM capsaicin. One image was captured every two seconds in each of three channels: brightfield, 340 nm and 380 nm λex as previously described. Regions of interest were highlighted in 10–15 phase bright neurons (Figure 1A), and the change of 340/380 nm λex ratio from baseline to maximum was recorded for each neuron to reveal intracellular Ca2+ changes due to capsaicin or cannabinoid application. In each experiment, the largest calcium responses to the cannabinoid were selected for analysis. Responses were recorded as the difference between baseline (mean 340/380 nm λex ratio just before addition of the cannabinoid or drug) and peak after the addition. Data were recorded for the mean change in 340/380 nm λex ratio in response to added cannabinoid (s), and capsaicin, and expressed as a percentage of the control obtained from the same animal specimen. In separate dishes, following determination of the baseline, THC (1, 10, 30, 100, 150 μM), or CBG (1, 10, 30, 100 μM), CBD (1, 10, 30, 100 μM), or combined CBD/THC/CBG (1:1:1 ratio, concentrations of 3, 30 or 90 μM) were added at the indicated concentrations, followed 5 min later by 1 μM capsaicin (Figure 1B–F).
Figure 1.
Image showing a field of view of cultured rat DRG neurons, with individual cells highlighted for analysis; bar=100 µm (A). Sample traces showing absence of response to vehicle followed by rapid rise in calcium in response to capsaicin (B). Sample traces showing response to 30 µM CBD followed by 1 µM capsaicin (C). Similar traces of response to 30 µM CBG and capsaicin (D). Sample traces of responses to 100 µM THC and capsaicin (E). Sample traces of responses to the 30 µM mix of CBG+CBD+THC (1:1:1), followed by capsaicin (F). Scale bars indicate change in 340/380 ratio on the y axis, and time in seconds on x axis.
Cannabinoid Solutions
Stock solution of CBG (from extract paste, #401100P Curaleaf International, London, UK), was prepared at 316 mM in DMSO. 100 mM CBD stock solution was prepared in DMSO (# 200003, Curaleaf International, London, UK), and ∆9THC (#401100P, Curaleaf International, London, UK) was prepared in DMSO at 317 mM. All stock solutions were aliquoted, stored at −20°C, and freshly thawed prior to use. Intermediate dilutions were freshly prepared at 1000x final concentration, so that the final concentration of vehicle was 0.1%. THC, CBD and CBG were combined in 1:1:1 proportion by adding each cannabinoid at 1, 10 or 30 mM to give a mixture containing 3, 30 or 90 mM total in DMSO. This was diluted 1:1000 so that the final concentration of the added mixture was 3, 30 or 90 µM for the combination. Capsaicin stock solution was prepared in ethanol as a 100 mM solution, aliquoted and stored at −20°C, until use; intermediate dilution of 500 µM was freshly prepared prior to use. All chemicals were obtained from Merck, unless otherwise indicated.
Data Analysis
Cannabinoid and capsaicin responses were averaged for each concentration for each rat, and normalized to controls. The number of rats tested for each concentration, and the total number of neurons for each group, from which the data is derived, is indicated in the results Tables below. Average values for each cannabinoid concentration were compared to the control using a one-tailed Student’s t-test. Pearson’s correlation coefficient was used to determine a correlation between calcium influx in response to the cannabinoid, and calcium influx in response to capsaicin administration in the presence of the cannabinoid. All analyses were carried out using GraphPad Prism software. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Control
Responses to 1 µM capsaicin alone, following vehicle application, were used as control (mean = 100 ± 20.5%). No change in baseline was observed following application of vehicle. Capsaicin responses were observed in phase bright neurons (Figure 1A), within seconds of application, as a rapid and sustained increase in intracellular 340/380 ratio (Figure 1B). Application of the cannabinoids resulted in calcium influx after a long delay, unlike the immediate capsaicin responses (Figure 1C–F).
Effect of CBG
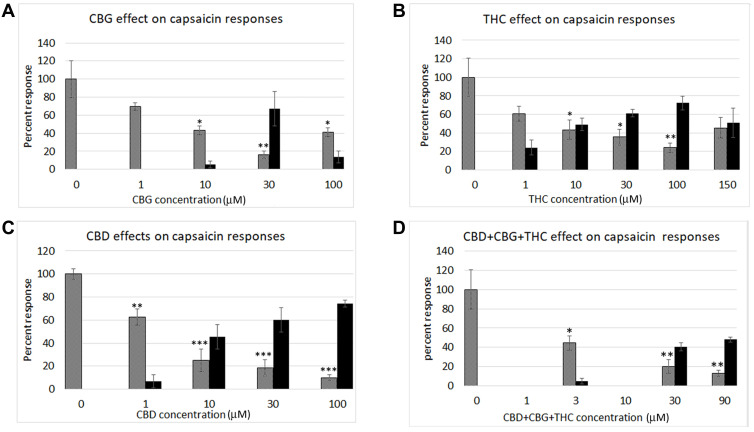
Application of CBG at 1 µM did not elicit calcium influx, but reduced the subsequent capsaicin response (n.s.). Higher concentrations of 10 µM and 30 µM elicited dose-related increases in calcium influx in a subset of neurons, that was reduced at the highest concentration of 100 µM tested, in a bell-shaped distribution. Capsaicin responses showed dose-related reduction, in a U-shaped distribution (Figure 2A). CBG responses were inversely related to capsaicin response reduction, with a Pearson’s coefficient of −0.88 (Table 1).
Figure 2.
Graphs showing dose-related calcium responses to cannabinoids (black bars), CBG (A), THC (B), CBD (C), and combined CBD+CBG+THC (D). Each graph also shows the inhibitory effects of the added cannabinoids on capsaicin responses (grey bars) at the indicated concentrations, expressed as a percentage of calcium influx in response to 1 µMol capsaicin. The inhibition of capsaicin responses was inversely proportional to the cannabinoid concentrations. *P < 0.05, **P < 0.1, ***P < 0.001.
Table 1.
Effect of CBG
| CBG concentration (µM) | 0 | 1 | 10 | 30 | 100 |
|
CBG response Mean ± s.e.m |
0 | 0 | 5.3 ± 4 | 67.2 ± 19 | 13.5 ± 6.6 |
| N (neurons) | 8 (52) | 6 (47) | 6 (60) | 6 (58) | 6 (37) |
| Capsaicin response Mean ± s.e.m. | 100.0 ± 20.5 | 69.8 ± 4.3 | 43.1 ± 5.1* | 16.0 ± 4.2** | 41.2 ± 4.8* |
| N (neurons) | 8 (52) | 6 (47) | 6 (59) | 6 (58) | 6 (44) |
Notes: Data showing dose-related responses to CBG expressed as a percentage of the response to 1 µMol capsaicin, and reduction of capsaicin responses following CBG application. “N” indicates number of rats tested for each concentration, with the total number of neurons in each group, from which the data is derived, in brackets. *P < 0.05, **P < 0.01, Students t-test.
Effect of THC
Dose-related calcium influx was observed in a subset of neurons, following application of THC at 1, 10, 30, 100 and 150 µM in a bell-shaped distribution. Corresponding dose-related reduction of capsaicin responses was observed in the presence of THC, with maximum reduction at 100 µM, in a U-shaped distribution (Pearson’s coefficient = −0.99, Table 2, and Figure 2B).
Table 2.
Effect of THC
| THC concentration (µM) | 0 | 1 | 10 | 30 | 100 | 150 |
|
THC response Mean ± s.e.m |
0 | 24.2 ± 7.9 | 49.2 ± 6.7 | 61.4 ± 4.2 | 72.4 ± 7.4 | 50.7 ± 15.8 |
| N (neurons) | 8 (52) | 6 (40) | 7 (51) | 6 (59) | 6 (69) | 5 (59) |
|
Capsaicin response Mean ± s.e.m. |
100.0 ± 20.5 | 60.8 ± 7.9 | 43.3 ± 10.4 * | 35.6 ± 8.5* | 24 ± 5 ** | 45.5 ± 11.2 |
| N (neurons) | 8 (52) | 6 (41) | 7 (51) | 6 (58) | 6 (63) | 5 (60) |
Notes: Data showing dose related responses to Δ9THC application, and corresponding reduction of capsaicin responses. “N” indicates number of rats and the total number of neurons is in brackets. *P < 0.05, **P < 0.01, Students T-Test.
Effect of CBD
Application of CBD at 1, 10, 30 and 100 µM concentrations elicited dose-related calcium influx, with corresponding dose-related reduction of capsaicin responses. Figure 2C and Table 3.
Table 3.
Effect of CBD
| CBD concentration (µM) | 0 | 1 | 10 | 30 | 100 |
|
CBD response Mean ± s.e.m |
0 | 6.9 ± 5.5 | 45.6 ± 10.6 | 59.9 ± 10.6 | 74.3 ± 2.8 |
| N (neurons) | 6 (78) | 6 (80) | 6 (81) | 6 (71) | 6 (80) |
| Capsaicin response Mean ± s.e.m. | 100.0 ± 4.7 | 62.6 ± 7 ** | 25 ± 9.7 *** | 18.6 ± 7.1 *** | 9.99 ± 2.5 *** |
| N (neurons) | 6 (78) | 6 (80) | 6 (81) | 6 (71) | 6 (80) |
Notes: Data showing dose related responses to applied CBD, and reduction of capsaicin responses after CBD application. Pearson’s coefficient = −0.99. “N” indicates number of rats and the total number of neurons is in brackets. **P < 0.01, ***P < 0.001, Students T-Test.
Effect of Combined CBG, THC and CBD
Combined CBG+THC+CBD applied in a 1:1:1 ratio at 3 µM, 30 µM, and 90 µM elicited dose-related calcium influx, with maximum influx at 90 µM. Capsaicin responses were dose-dependently diminished at 3 µM, 30 µM, and 90 µM CBG+THC+CBD (Figure 2D), and Table 4. Pearson’s coefficient = −1.00.
Table 4.
Effect of Combined CBG+CBD+THC
| CBG + CBD + THC concentration (µM) | 0 | 3 | 30 | 90 |
|
CBG + CBD + THC response Mean ± s.e.m |
0 | 4.6 ± 2.8 | 40.3 ± 4.3 | 48 ± 2.8 |
| N (neurons) | 8 (52) | 6 (47) | 6 (57) | 6 (55) |
|
Capsaicin response Mean ± s.e.m. |
100.0 ± 20.5 | 44.4 ± 7.5 | 20.1 ± 7.1** | 13.0 ± 3.4 ** |
| N (neurons) | 8 (52) | 6 (47) | 6 (62) | 6 (55) |
Notes: Data showing dose related responses to combined CBG, CBD, and THC application, and corresponding reduction of capsaicin responses. Pearson’s coefficient=−1.00. “N” indicates number of rats and the total number of neurons is in brackets. **P < 0.01, Students T-Test.
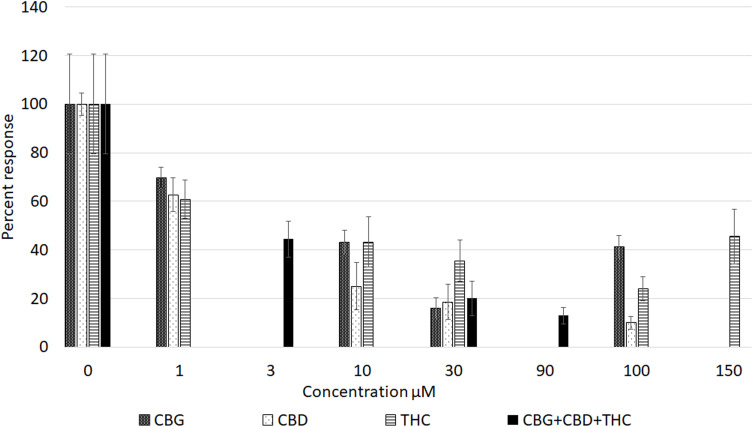
All three cannabinoids, CBG, CBD and THC - had dose-related inhibitory effects on capsaicin responses, whether applied separately or combined, with capsaicin responses distributed in a “U” shaped pattern (Figure 3). Maximum inhibition due to CBG alone was observed at 30 µM, and that due to CBD and THC applied individually was observed at 100 µM. The combined inhibitory effect at 3 µM was equivalent to the individual effects at 10 µM. Inhibition due to 30 µM mix was equivalent to the individually applied cannabinoids at the same concentration. Inhibition due to 90 µM mix was greater than that due to 100 µM CBG and 100 µM THC, but similar to inhibition due to 100 µM CBD (Figure 4). At higher concentrations, the inhibitory effects of CBG, CBD and THC applied individually were diminished, ie, the capsaicin responses were greater, than those in the presence of lower cannabinoid concentrations. Inhibition due to CBG application diminished at the higher concentration of 100 µM. Similarly, inhibition due to THC was less at 150 µM than at 100 µM.
Figure 3.
Graph showing comparison of dose-related reduction of capsaicin responses due to cannabinoids applied individually (patterned bars), or in combination (black bars).
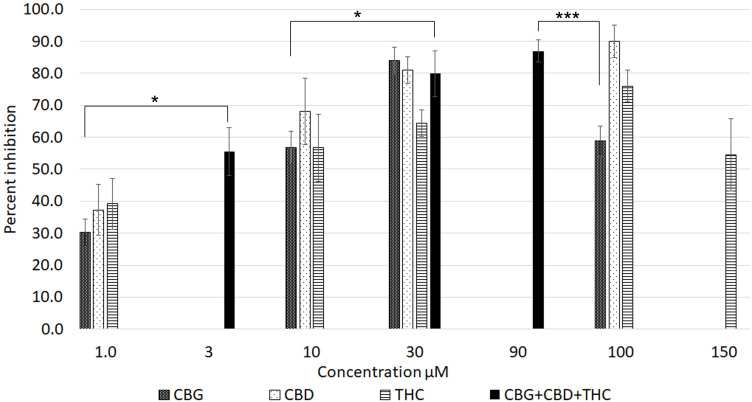
Figure 4.
Graph showing the percentage inhibition of 1 µM capsaicin responses in the presence of CBG, CBD and THC applied individually (patterned bars), and combined (black bars). *P < 0.05, ***P < 0.001.
The comparative inhibitory effects of the cannabinoids applied individually and combined, were distributed in an inverted U pattern, as shown in Figure 4. There was a high degree of correlation between cannabinoid-induced calcium influx and inhibition of capsaicin responses. Inhibition by 3 µM combined application was significantly greater than by 1 µM CBG alone (*P < 0.05), and equivalent to inhibition by individually applied cannabinoids at 10 µM. Inhibition by 30 µMol combined application was significantly greater than by 10 µM CBG (*P < 0.05). Inhibition by 90 µM combined application was significantly greater than by 3 µM mix (**P < 0.01), 30 µM THC (*P < 0.05), and by 100 µM CBG (***P < 0.001).
Discussion
This study aimed to determine the dose-related effects of the phytocannabinoids THC, CBG and CBD, applied individually and in combination, on capsaicin responses, using an in vitro model of neuronal hypersensitivity.33 CBD, CBG and THC induced calcium influx in a pattern characteristic of cannabinoids, resulting in desensitization to capsaicin stimulation. Capsaicin responses were inversely correlated with the magnitude of the cannabinoid response, with a high coefficient of correlation in each case, for CBG, THC, CBD and CBD/THC/CBG combination. This suggests that the cannabinoids activate DRG neurons resulting in desensitisation and consequent anti-nociception, in agreement with previous findings.26,27 These studies reported that CBD, CBG, cannabidivarin (CBDV), and tetrahydrocannabivarin (THCV) act as agonists that desensitize TRPV1 in human recombinant TRPV1 transfected HEK cells. Our study has further shown that the combined 1:1:1 mix of CBG:CBD:THC has the potential to enhance the potency of these compounds applied individually.
CBG showed a maximum effective concentration of 30 μM, with less efficacy at lower and higher concentrations. THC was most effective at 100 μM. The combined THC, CBD and CBG (1:1:1) at 3 µM was equivalent to CBD, CBG or THC applied individually at 10 µM. Maximum inhibition by 90 µM mix was similarly equivalent to inhibition by 100 µM CBD applied alone, and greater than that due to CBG and THC at 100 µM. This enhanced efficacy of combined phytocannabinoids is akin to the combined effects described for the endocannabinoids,17 and recently reviewed.39
While the cannabinoids caused neuronal activation, the calcium influx induced was less than that of the control (1 µM capsaicin). TRPV1 is expressed by nociceptive thin myelinated Aδ and unmyelinated C nerve fibres. It is activated by its highly specific potent stimulant capsaicin,40 resulting in the opening of cation-specific ion channels, with a high permeability to calcium.41 Calcium influx is known to activate the protein phosphatase calcineurin, that dephosphorylates TRPV1, causing its desensitization.42 TRPV1 desensitization using high dose topical capsaicin is an effective treatment for neuropathic pain.43,44 We also observed reduced capsaicin responses in the presence of 1 µM CBG, which did not elicit calcium influx, and significant desensitization at 1 µM CBD, suggesting that mechanisms involving different targets, are likely to be involved. Capsaicin application stimulates calcium influx, and blocks further capsaicin responses by desensitization. It is likely that cannabinoid mediated calcium influx has similar desensitizing effects to subsequent capsaicin stimuli. Investigating the mechanisms of action underlying TRPV1 desensitization by capsaicin and cannabinoids, and their differential effects, will be the focus of our future studies. These may enable optimal doses and formulations of cannabinoids for pain relief, such as specific combinations of cannabinoids taken orally, while minimising their individual side-effects. Elucidation of the pathways involved requires further investigations, including using receptor antagonists to identify their roles. As CBD activates TRPV1 at high concentration, other receptors involved in cannabinoid effects including CB1 and CB2 will require examination, using their respective antagonists. Interaction of cannabinoids with other receptors has recently been reviewed.39
The CB1 and CB2 receptors are G protein coupled receptors (GPCRs) that lead to adenylyl cyclase inhibition and decreased intracellular cAMP upon ligand binding.24 cAMP is a critical regulator of TRPV1 sensitivity, as TRPV1 is sensitized when phosphorylated, and desensitized when dephosphorylated.45,46 Thus, cannabinoid inhibition of capsaicin responses in our study may result from the activation of multiple targets. The mechanism(s) of cannabinoid desensitization may involve several receptors that are co-expressed in subsets of DRG neurons, and lead to their in vivo analgesic effects; these are considered in turn below for CBG, THC, CBD and their combination.
CBG has been shown to bind different receptors including TRPM8,27 TRPV1,27,47 CB1 and CB2.20,48 Experiments carried out in HEK-293 cells expressing the rat recombinant TRPM8 channel showed large decreases in response to the TRPM8 agonist icilin in the presence of increasing concentrations of CBG, with maximum inhibition of icilin at a concentration of around 30 μM CBG. Similar results were obtained in our study, utilising the TRPV1 agonist capsaicin instead of icilin. We found that Ca2+ influx in response to the addition of CBG was the highest at 30µM, with a Pearson’s coefficient value of −0.88. This supports the proposition that 30 μM is the most effective concentration of CBG for inhibiting calcium influx at both TPRM8 and TRPV1 channels. We attempted a study of higher CBG doses, eg, 150 µM; however, solubility issues of this viscous concentration were problematic.
Recent evidence using in vitro studies highlights many potential beneficial effects of CBG. These include treatment for neuroinflammation and oxidative stress,49 with reduced secretion of inflammatory mediators such as TNF-α and IL-6.50 There is, however, very limited evidence that CBG has anti-nociceptive effects. Zagzoog et al48 used the in vivo Tetrad Test with multiple phytocannabinoids, including CBG, to determine their effects on spontaneous activity, catalepsy, hypothermia, and analgesia in mice, as defined by Ben Shabat et al.17 They found that CBG caused a small but statistically significant anti-nociceptive effect at 3 mg/kg, as well as an anxiolytic effect at 10 mg/kg. We have, in this study, provided in vitro evidence to support the anti-nociceptive effect of CBG, reported in vivo previously.
In clinical trials of pain reduction with cannabis administration, adverse events were found to be more likely with high-THC preparations than multi-cannabinoid-containing products.51 Two randomised control trials52,53 found that inhaled cannabis provided significant analgesic effects in neuropathic pain, with increasing concentrations of THC providing greater pain relief. However, both studies reported that the higher THC concentrations were also associated with adverse cognitive side effects, such as memory deficits and impaired performance in neuropsychological tests. The psychoactive effect of THC is well established and limits the licensing of higher concentrations of THC in analgesic medicinal preparations.
It is because of these adverse effects that alternatives to high concentrations of THC are needed. Our study has shown that THC+CBD+CBG combined in a 1:1:1 proportion at 30 µM, was more effective than 100 µM THC applied alone, and 30 µM CBG had an equivalent effect as the combined formulation at 30 µM. 30 μM CBG, 30 µM and 90 μM CBG+CBD+THC combination is more effective at reducing capsaicin-induced calcium influx, than the most effective concentration of 100 μM THC. By using a combination of CBD, THC and CBG, equivalent or greater levels of analgesia could be achieved with lower concentrations of THC, thus minimising its side-effects profile. The effects of cannabinoid combinations excluding THC have not been examined, and will be determined in our future studies. Such studies may guide the rational design of trials, to investigate their clinical efficacy and potential benefits.
A recent study, examining the effects of CBD on capsaicin response in rat DRG neurons, found that administration of 10 μM and 50 μM CBD caused significant calcium influx, but lower concentrations did not.36 In this study, 50 μM CBD was the most effective concentration, in a similar range to CBG (30 μM). Various other receptor targets for CBD have been identified, such as the orphan G-protein coupled receptor GPR18, GPR55, PPARs, 5-HT1a and the α3 and α1 glycine receptors.54–57 A major difference in calcium influx mediated by cannabinoids and by capsaicin is their activation time-course. Capsaicin responses were very rapid (within a few seconds), while the cannabinoid responses were relatively delayed. The activation kinetics of cannabinoids require further investigation to explain this difference. As cannabinoids activate multiple targets, unlike capsaicin, their co-expression and interaction with TRPV1 requires further analysis. This would include use of specific receptor antagonists and elucidation of the signalling pathways involved. Ward et al58 researched the effects of CBD in mice with chemotherapy-induced hyperalgesia, and concluded that CBD successfully prevents the development of cold and mechanical allodynia. These results suggest that CBD could be used to prevent the development of chemotherapy-induced pain in humans. Further clinical investigations into the role of CBD-containing cannabinoid combinations for anti-nociception are also required.
Comelli et al59 used a rat model of neuropathic pain to demonstrate the anti-hyperalgesic effects of a cannabis extract containing multiple cannabinoid and non-cannabinoid fractions. The plant extract, which included CBD, THC and CBG, showed better efficacy than any cannabinoid administered individually. This data is supported by our findings that combined CBD+THC+CBG provides generally greater antinociceptive effects than THC applied alone, especially at 30 uM concentration. Our study found that the most effective concentration of the combination was 90 μM CBD+THC+CBG, which caused only 13% of the calcium influx in response to capsaicin as the control, and similar to inhibition by 100 µM CBD. Similarly, at 30 μM, there was no statistical difference between the CBD+THC+CBG combination treatment and CBG alone. Higher concentration of the CBD+THC+CBG combination treatment than 90 µM could not be used in this study due to limitations in solubility of the stock solutions.
A four-way crossover clinical trial randomised patients with chronic fibromyalgia pain to different ratios of inhaled THC and CBD (high THC, roughly 1:1 THC:CBD and high CBD or placebo).60 Only those receiving the THC/CBD combination treatment had a significant improvement of pain compared to placebo. This trial also found that CBD increased plasma THC concentrations, but decreased THC-induced analgesic effects, highlighting the complex pharmacokinetic and pharmacodynamic interactions of cannabinoids.
In conclusion, this study has determined that the cannabinoids CBG, CBD and THC dose-dependently reduce capsaicin responses in rat DRG neurons, in a model of neuronal hypersensitivity. The combined treatment has the potential to enhance the potency of individually applied cannabinoids. The cannabinoids dose-dependently caused calcium influx, which was inversely correlated to subsequent capsaicin response. These results highlight the potential anti-nociceptive application of phytocannabinoids, particularly in combination.
Acknowledgment
This study was funded by EMMAC Life Sciences, UK.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
This study was funded by EMMAC Life Sciences Ltd. Barbara Pacchetti and Mikael H Sodergren are employees of EMMAC Life Sciences Ltd. Barbara Pacchetti reports personal fees from EMMAC Life Sciences, outside the submitted work. Mikael H Sodergren reports consultancy for and personal fees from Emmac Life Sciences, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed and report no other potential conflicts of interest for this work. No writing assistance was utilized in the production of this manuscript.
References
- 1.Merskey H, Bogduk N, editors, IASP Task Force on Taxonomy, Part III: Pain Terms, a Current List with Definitions and Notes on Usage. Seattle, WA: IASP Press; 1994:209–214. [Google Scholar]
- 2.Fayaz A, Croft P, Langford R, Donaldson J, Jones G. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. doi: 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher D, Stamer UM, Pogatzki-Zahn E. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32:725–734. doi: 10.1097/EJA.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 4.Mills SEE, Nicholson KP, Smith BH. (2019) Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e283. doi: 10.1016/j.bja.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand U. Mechanisms and management of cancer pain. Alison E, editor. The Cancer Handbook; Copyright © 2005 John Wiley & Sons, Ltd. Chapter 310; 2005. [Google Scholar]
- 6.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;1(86):1646–1647. [Google Scholar]
- 7.Pertwee RG. The pharmacology and therapeutic potential of cannabidiol. In: Di Marzo V, editor. Cannabinoids. NY, USA: Kluwer Academic/Plenum Publishers; 2004:32–83. [Google Scholar]
- 8.Wendelmuth C, Wirz S, Torontali M, Gastmeier A, Gastmeier K. Dronabinol bei geriatrischen Schmerz- und Palliativpatienten. Eine retrospektive Auswertung der ambulanten kassenärztlichen Therapie [Dronabinol in geriatric pain and palliative care patients: a retrospective evaluation of statutory-health-insurance-covered outpatient medical treatment]. Schmerz. 2019;33(5):384–391. doi: 10.1007/s00482-019-00408-1. [German]. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol. (Marinol). J Psychoactive Drugs. 1998;30:187–196. doi: 10.1080/02791072.1998.10399689 [DOI] [PubMed] [Google Scholar]
- 10.Clermont-Gnamien S, Atlani S, Attal N, Le Mercier F, Guirimand F, Brasseur L. Utilisation thérapeutique du D9-tétrahydrocannabinol (dronabinol) dans les douleurs neuropathiques réfractaires [The therapeutic use of D9-tetrahydrocannabinol (dronabinol) in refractory neuropathic pain]. Presse Med. 2002;31(39 Pt 1):1840–1845. French. [PubMed] [Google Scholar]
- 11.Attal N, Brasseur L, Guirimand D, Clermond-Gnamien S, Atlami S, Bouhassira D. Are oral cannabinoids safe and effective in refractory neuropathic pain? Eur J Pain. 2004;8(2):173–177. doi: 10.1016/S1090-3801(03)00084-3 [DOI] [PubMed] [Google Scholar]
- 12.Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4(1):245–259. doi: 10.2147/TCRM.S1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holdcroft A, Maze M. Dor´e C Tebbs S, Thompson S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology. 2006;104(5):1040–1046. doi: 10.1097/00000542-200605000-00021 [DOI] [PubMed] [Google Scholar]
- 14.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112(3):299–306. doi: 10.1016/j.pain.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 15.Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260(4):984–997. doi: 10.1007/s00415-012-6739-4 [DOI] [PubMed] [Google Scholar]
- 16.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. BrJ Pharmacol. 2011;(2011)(163):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353(1):23–31. doi: 10.1016/S0014-2999(98)00392-6 [DOI] [PubMed] [Google Scholar]
- 18.Mlost J, Bryk M, Starowicz K. Cannabidiol for Pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21(22):8870. doi: 10.3390/ijms21228870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karniol I, Carlini E. Pharmacological interaction between cannabidiol and 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33(1):53–70. doi: 10.1007/BF00428793 [DOI] [PubMed] [Google Scholar]
- 20.Navarro G, Varani K, Reyes-Resina I. Sánchez de Medina V, et al. Cannabigerol action at Cannabinoid CB1 and CB2 receptors and at CB1–CB2 heteroreceptor complexes. Front Pharmacol. 2018;9:632. doi: 10.3389/fphar.2018.00632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans FJ. Cannabinoids: the separation of central from peripheral effects on a structural basis. Planta Med. 1991;57:S60–7. doi: 10.1055/s-2006-960231 [DOI] [PubMed] [Google Scholar]
- 22.Devane WA, Dysarz Ill FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 23.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- 24.Howlett AC, Johnson MR, Melvin LS, Milne GM. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol. 1988;33:297–302. [PubMed] [Google Scholar]
- 25.Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iannotti FA, Hill CL, Leo A, Alhuseini A, Soubrane C, Mazzarella E. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5(11):1131–1141. doi: 10.1021/cn5000524 [DOI] [PubMed] [Google Scholar]
- 27.De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine J, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 29.Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- 30.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75(1):111–119. doi: 10.1016/S0304-3959(97)00213-3 [DOI] [PubMed] [Google Scholar]
- 31.Jordt SE, Bautista DM, Chuang H, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- 32.Patapoutian A, Peier SM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141 [DOI] [PubMed] [Google Scholar]
- 33.Anand U, Otto WR, Casula MA, et al. The effect of neurotrophic factors on morphology TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons, Neurosci. Lett. 2006;399:51–56. [DOI] [PubMed] [Google Scholar]
- 34.Anand U, Otto WR, Facer P, et al. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat DRG neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 35.Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28(5):1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand U, Jones B, Korchev Y, et al. CBD effects on TRPV1 signaling pathways in cultured DRG neurons. J Pain Res. 2020;11(13):2269–2278. doi: 10.2147/JPR.S258433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492.36. [DOI] [PubMed] [Google Scholar]
- 38.Bar KJ, Saldanha GJ, Kennedy AJ, et al. GDNF and its receptor component Ret in injured human nerves and dorsal root ganglia. Neuroreport. 1998;9(1):43–47. doi: 10.1097/00001756-199801050-00009 [DOI] [PubMed] [Google Scholar]
- 39.Anand U, Pacchetti B. Anand P and Sodergren MH. Cannabis-based medicines and pain: a review of potential synergistic and entourage effects. Pain Manag. 2021;11(4):395–403. doi: 10.2217/pmt-2020-0110 [DOI] [PubMed] [Google Scholar]
- 40.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–20143. [PubMed] [Google Scholar]
- 41.Wood JN, Winter J, James IF, Rang HP, Yeats JC, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Docherty RJ, Yeats JC, Bevan S, Bodekke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431(6):828–837. doi: 10.1007/s004240050074 [DOI] [PubMed] [Google Scholar]
- 43.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;(2011(107):490–502. doi: 10.1093/bja/aer260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sousa-Valente J, Andreou AP, Urban L, Nagy I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol. 2014;171(10):2508–2527. doi: 10.1111/bph.12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/S0896-6273(02)00802-4 [DOI] [PubMed] [Google Scholar]
- 46.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200 [DOI] [PubMed] [Google Scholar]
- 47.Starkus J, Jansen C, Shimoda LMN, Stokes AJ, Small-Howard AL, Turner H. Diverse TRPV1 responses to cannabinoids. Channels. 2019;13(1):172–191. doi: 10.1080/19336950.2019.1619436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zagzoog A, Mohamed KA, Kim HJJ, et al. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Cannabis Sativa Sci Rep. 2020;10(1):20405. doi: 10.1038/s41598-020-77175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gugliandolo A, Pollastro F, Grassi G, Bramanti P, Mazzon E. In vitro model of neuroinflammation: efficacy of cannabigerol, a non-psychoactive cannabinoid. Int J Mol Sci. 2018;19(7):1992. doi: 10.3390/ijms19071992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granja A, Carrillo-Salinas F, Pagani A, et al. A Cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J Neuroimmune Pharmacol. 2012;7(4):1002–1016. doi: 10.1007/s11481-012-9399-3 [DOI] [PubMed] [Google Scholar]
- 51.Haleem R, Wright R, Scoping A. Review on clinical trials of pain reduction with cannabis administration in adults. J Clin Med Res. 2020;12(6):344–351. doi: 10.14740/jocmr4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506–521. doi: 10.1016/j.jpain.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain. 2015;16(7):616–627. doi: 10.1016/j.jpain.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okine BN, Gaspar JC, Finn DP. PPARs and pain. Br J Pharmacol. 2018;176(10):1421–1442. doi: 10.1111/bph.14339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa B, Giagnoni G, Franke C, Trovato EA, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci. 2006;103(30):11393–11398. doi: 10.1073/pnas.0603861103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowin T, Schneider M, Pongratz G. Joints for joints: cannabinoids in the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2019;31:271–278. doi: 10.1097/BOR.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 58.Ward SJ, Ramirez MD, Neelakantan H, Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anaesth Analgesia. 2011;113(4):947–950. doi: 10.1213/ANE.0b013e3182283486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytotherapy Res. 2008;22(8):1017–1024. doi: 10.1002/ptr.2401 [DOI] [PubMed] [Google Scholar]
- 60.van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860–869. doi: 10.1097/j.pain.0000000000001464 [DOI] [PMC free article] [PubMed] [Google Scholar]