Abstract
Purpose
Menopause is accompanied by a decline in estrogen and progesterone. Several studies have demonstrated that upper airway patency decreases in women after menopause, while morphology changes are still a lack of evidence. This study aimed to explore upper airway morphology changes in perimenopausal and postmenopausal women.
Methods
This retrospective cross-sectional study included 367 consecutive Chinese female patients over 25 years old who had routinely taken large-field cone beam computed tomography in the imaging library of Peking University School and Hospital of Stomatology from October 2016 to September 2020. A total of 283 males were screened as sex controls according to the same age group. Upper airway morphology, hyoid position and facial pattern were measured. The association between perimenopausal and postmenopausal years and upper airway morphology in both sexes was analyzed.
Results
Perimenopausal women (aged 45–54 years) showed a significant decrease in the volume (3172.91mm3, 95% CI = 653.86–5691.96) and minimum cross-sectional area (37.08 mm2, 95% CI = 5.36–68.80), and a significant increase in the length (−1.96mm, 95% CI = −3.62 to −0.29) of upper airway compared to adjacent reproductive years (aged 35–44), while this difference was neither seen in other adjacent two reproductive age groups of females nor in the same age groups of males. In postmenopausal women (55 years and older), hyoid position was significantly lower (−2.74mm, 95% CI = −4.42 to −1.07) than either age group, while no similar changes were seen in men.
Conclusion
Women had smaller airway volume, reduced upper airway cross-sectional area and longer airway length in perimenopausal years, and a significantly lower hyoid position in postmenopausal years. These changes may be related to menopause itself and independent of the changes associated with aging.
Keywords: perimenopause, postmenopause, upper airway morphology, cone beam computed tomography
Introduction
Menopause, the permanent cessation of menstrual cycles results from reduced secretion of the ovarian hormones estrogen and progesterone, marks the ending of reproductive life in women.1–3 The transition to menopause is a complex physiological process. Symptoms like hot flashes and night sweats, insomnia, and mood instability may be the first signs of ovarian function failure before menstruation stops.4
Menopause brings various unfavorable changes. Many chronic diseases are thought to occur mainly in men, including cardiovascular diseases, diabetes and stroke.5–9 However, due to the lack of protection from sex hormones, the gender differences of prevalence seem to be attenuated, even postmenopause women have higher risk of hypertension and heart disease than men.9,10 Studies have shown that it may be menopause itself rather than aging account for the pronounced increased risk.2,3,11 Each year of earlier menopause is associated with a 2% increase in all-cause mortality.12,13
In the reproductive years, sex hormones exert a profound protective effect on upper airway patency in women compared with men of a similar age.14–16 Progesterone is a known respiratory stimulant that increases upper airway muscle tone, but progesterone levels decline after menopause, and respiratory drive decreases during menopausal transition.17–19 Fat distribution also changes and is more likely to be distributed in the upper body and trunk area in postmenopausal years.20 The activity of genioglossus muscle decreases in menopausal women, while hormone replacement therapy increases genioglossus muscle activity significantly.21 All of these changes are detrimental to upper airway patency in women after menopausal years.
Physiological changes in upper airway may influence morphology. However, the morphology of upper airway in perimenopausal and postmenopausal women are still a lack of evidence. Cone beam computed tomography (cone beam CT) is an advancement in CT imaging, which uses low-dose cross-sectional technique and provides more rapid acquisition of a data set. It can successfully discriminate borders of soft and hard tissue structures and has been widely used to evaluate upper airway morphology and craniofacial anatomy.22–24 In this study, we collected cone beam CT images to study upper airway morphology in different stages of women and hypothesized that compared with reproductive years, there is a significant deterioration in the upper airway morphology in perimenopausal and postmenopausal women.
Materials and Methods
The present study was a retrospective cross-sectional study conducted at Peking University School and Hospital of Stomatology, Beijing. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Peking University Stomatology School and Hospital [PKUSSIRB-202054026].
Participants
In this study, we retrospectively collected image data of all patients who had routinely taken large-field cone beam CT in the imaging library of the Department of Radiology, Peking University School and Hospital of Stomatology from October 2016 to September 2020. We excluded patients from the department of traumatology, oncology, orthognathic surgery or cleft lip and palate according to the application form. Other exclusion criteria were as follows: 1) history of oral maxillofacial fractures, 2) severe craniofacial destruction and defects, 3) severe Class II and Class III craniofacial deformities, and 4) edentulousness or incorrect occlusal position.
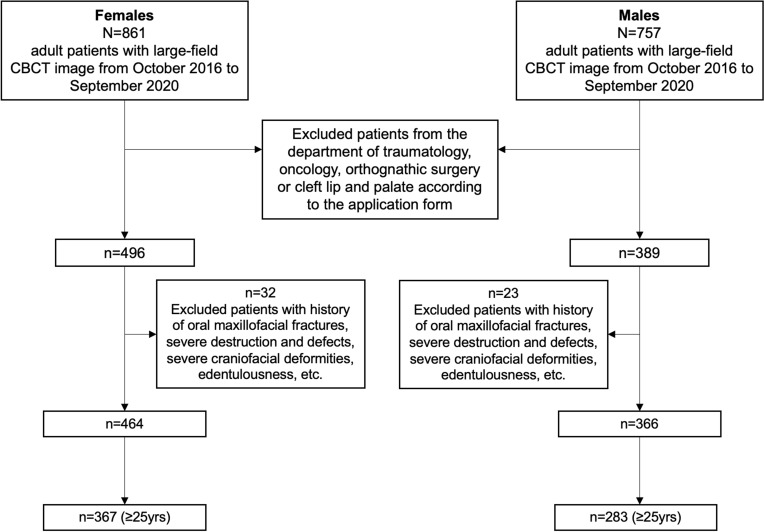
Recent study has suggested that the mean age at natural menopause in Chinese women is 49.3 ± 3.3 years (median 50.0 years),9 while other studies have found similar conclusion.25,26 Thus, we took 45–54 years as the perimenopausal group and ≥55 years as the postmenopausal group. Two groups of women in reproductive age, 25–34 years and 35–44 years, served as age controls. A total of 367 women meet the criteria above, and 283 men were screened as sex controls according to the same age group (Figure 1).
Figure 1.
Screening process of the samples included in the analysis.
Abbreviation: CBCT, cone beam computed tomography.
Imaging Analysis
All cone beam CT scans were performed in routine procedure. Patients were kept Frankfort plane (FH plane, where the uppermost point of the external auditory canal and the lowest point of the infraorbital rim are located) parallel to the horizontal plane in the seated position during scanning. Subjects were asked to stay awake, with the posterior teeth in occlusal contact and not chewing or swallowing.
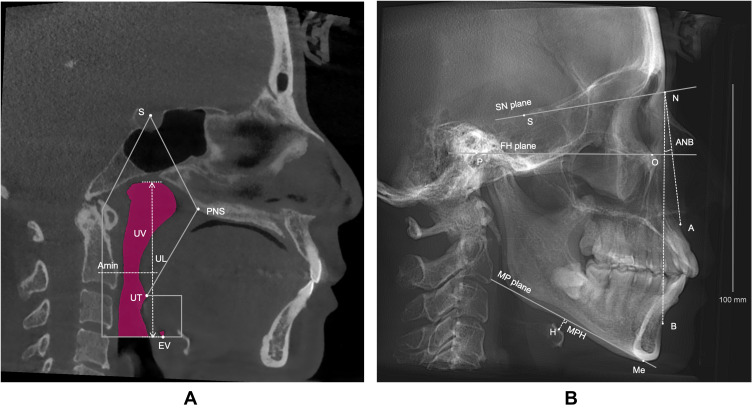
The morphology of the upper airway and hyoid bone position were measured using Dolphin 11.95 (Patterson Dental Supply Inc, Chatsworth, CA). The upper boundary of the total upper airway was the line connecting the point of the sella turcica (S) and the point of the posterior nasal spine (PNS), the lower boundary was the plane parallel to the FH plane where the valley of the epiglottis (EV) was located. The volume (UV), length (UL), minimum cross-sectional area (Amin) of upper airway were measured in the mid-sagittal plane (Figure 2A).
Figure 2.
Measurements of the upper airway morphology and hyoid bone position. (A) Airway measurements in the median sagittal plane. (B) Reconstructed lateral cephalogram.
Abbreviations: S, central point of the sella turcica; PNS, posterior nasal spine; UT, uvula tip; EV, epiglottis valley; UV, upper airway volume; UL, upper airway length; Amin, minimum cross-sectional area of upper airway between PNS and EV; N, nasion, anterior-most point of nasofrontal suture; P, porion, uppermost point of external auditory canal; O, orbitale, lowest point of infraorbital rim; A, subspinale, most concave point of maxilla; B, supramental, most concave point of mandible; Me, menton, lowermost point of the chin; H, the superior anterior point of the hyoid bone; SN plane, S and N points lie in the line, representing the anterior cranial base plane; FH plane, Frankfort plane; MP plane, mandibular plane; ANB, the angle formed by the points A, N and B; MPH, Distance between H and MP plane.
In the reconstructed lateral cephalogram (Figure 2B), we used the angle of ANB to represent sagittal facial pattern, while the angle between FH plane (Frankfort plane) and MP plane (mandibular plane) and the angle between SN plane and MP plane were measured to represent vertical facial pattern. Distance between the superior anterior point of the hyoid bone (H) and MP plane was used to represent hyoid bone position (MPH). All data were measured three times repeatedly.
Statistical Analysis
Analysis was performed using SPSS 26.0 (Armonk, New York, USA). Kolmogorov–Smirnov test was used to test normality. One-way analysis of variance (ANOVA) was used for data processing between four age groups if the homogeneity of variance assumptions were satisfied; otherwise, Kruskal–Wallis analysis was employed. Independent samples t-test was used to compare between two age groups. Statistical significance was determined using a threshold p-value of 0.05.
Results
All patients were measured for craniofacial characteristics as facial pattern may affect upper airway morphology. As is shown in Table 1, no difference was found in sagittal and vertical facial pattern between age groups.
Table 1.
Craniofacial Characteristics in Age Groups
| Females | Age Groups (Mean ± SD) | Statistics | P value | |||
| [25–34] y (n=115) | [35–44] y (n=85) | [45–54] y (n=77) | [≥55] y (n=90) | |||
| ANB (°) | 4.04±2.62 | 4.13±2.32 | 4.08±2.26 | 4.08±2.25 | F=0.023 | 0.995 |
| MP-FH (°) | 24.65±6.09 | 24.62±7.23 | 24.87±5.77 | 24.40±6.38 | F=0.081 | 0.971 |
| MP-SN (°) | 34.30±6.23 | 34.46±8.36 | 35.44±5.99 | 35.33±6.96 | F=0.666 | 0.573 |
| Males | Age Groups (Mean ± SD) | Statistics | P value | |||
| [25–34] y (n=67) | [35–44] y (n=90) | [45–54] y (n=69) | [≥55] y (n=57) | |||
| ANB (°) | 3.96±2.04 | 4.08±2.71 | 4.16±2.11 | 3.98±2.52 | χ2=1.184 | 0.757 |
| MP-FH (°) | 21.71±6.96 | 21.97±7.56 | 22.88±6.53 | 21.76±6.46 | χ2=1.198 | 0.753 |
| MP-SN (°) | 29.86±6.31 | 30.31±8.14 | 31.90±6.11 | 30.91±6.46 | F=1.127 | 0.339 |
Notes: Compared by ANOVA or Kruskal–Wallis analysis according to normality test results.
Abbreviations: ANB, the angle formed by the points A, N and B; MP, mandibular plane; FH, Frankfort plane; SN plane, the anterior cranial base plane.
Table 2 shows the differences in upper airway morphology for different age groups in men and women, respectively. Among four reproductive stages of women, significant differences were found in UV, Amin, UL and MPH. In the four age groups of males, there were significant differences in Amin, UL and MPH, while there were no significant differences in UV.
Table 2.
Comparison of Upper Airway Morphology Between Age Groups in Females and Males
| Age Groups (Mean ± SD) | F | P value | P value of Age Groups Compared | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25–34 (y) | 35–44 (y) | 45–54 (y) | ≥55 (y) | 25–34 to 35–44 (y) | 25–34 to 45–54 (y) | 25–34 to ≥55 (y) | 35–44 to 45–54 (y) | 35–44 to ≥55 (y) | 45–54 to ≥55 (y) | |||
| Females | ||||||||||||
| UV (mm3) | 29,041.3±7665.6 | 26,895.6±8995.8 | 23,722.9±6995.7 | 23,312.5±7468.1 | 11.90 | 0.000** | 0.071 | 0.000** | 0.000** | 0.014* | 0.005** | 0.716 |
| Amin (mm2) | 243.6±100.1 | 215.6±108.4 | 178.6±94.6 | 159.9±87.2 | 14.44 | 0.000** | 0.061 | 0.000** | 0.000** | 0.022* | 0.000** | 0.188 |
| UL (mm) | 70.7±5.3 | 71.1±5.7 | 73.1±4.9 | 73.4±6.5 | 5.31 | 0.001** | 0.641 | 0.002** | 0.002** | 0.022* | 0.016* | 0.724 |
| MPH (mm) | 8.4±4.8 | 9.5±4.7 | 9.4±4.8 | 12.2±5.9 | 9.52 | 0.000** | 0.128 | 0.167 | 0.000** | 0.944 | 0.001** | 0.001** |
| Males | ||||||||||||
| UV (mm3) | 33,262.9±9525.2 | 30,375.5±8930.2 | 29,760.4±10,726.8 | 31,270.7±8623.8 | 1.80 | 0.147 | – | – | – | – | – | – |
| Amin (mm2) | 269.9±125.7 | 217.7±111.8 | 209.3±140.1 | 215.2±109.9 | 3.58 | 0.014* | 0.007** | 0.009** | 0.012* | 0.674 | 0.893 | 0.796 |
| UL (mm) | 79.3±4.94 | 81.7±6.6 | 82.6±6.9 | 83.1±5.6 | 4.90 | 0.002** | 0.014* | 0.002** | 0.000** | 0.422 | 0.177 | 0.615 |
| MPH (mm) | 13.0±5.6 | 14.5±5.7 | 16.5±5.5 | 16.7±5.4 | 6.43 | 0.000** | 0.09 | 0.000** | 0.000** | 0.034* | 0.024* | 0.820 |
Notes: Compared by independent samples t-test. *Indicates statistical significance (P< 0.05), **Indicates statistical significance (P< 0.01).
Abbreviations: UV, upper airway volume; UL, upper airway length; Amin, minimum cross-sectional area of upper airway between PNS and EV; MPH, distance between H and MP plane.
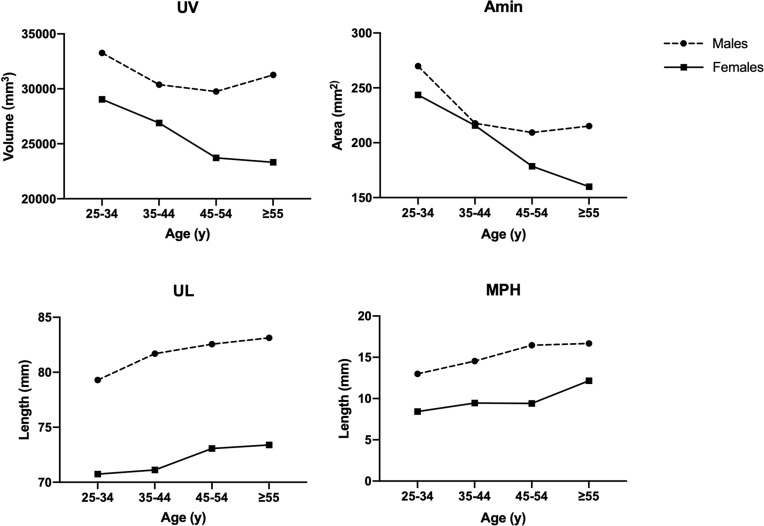
As the differences in upper airway morphology between the two adjacent age groups are more clinically significant, in Table 3 we further studied the absolute differences and 95% confidence intervals for each measurement between the three adjacent groups, 25–34 years versus 35–44 years, 35–44 years versus 45–55 years, and 45–55 years versus ≥55 years. In Figure 3, we showed the aging changes of upper airway morphology and hyoid position in males and females, respectively. In combination with Tables 2 and 3 and Figure 3, we found that upper airway morphology changed differently in men and women at different ages:
Table 3.
Changes of Upper Airway Morphology Between Adjacent Age Groups
| Mean Change (95 CI%) | |||
|---|---|---|---|
| [25–34] – [35–44] y | [35–44] – [45–54] y | [45–54] – [≥55] y | |
| Upper airway volume (mm3) | |||
| Females | 2145.71(−183.13, 4474.54) | 3172.91(653.86, 5691.96) | 410.17(−1813.31, 2633.65) |
| Males | 2887.38(−41.33, 5816.10) | 615.09(−2466.15, 3696.33) | −1510.28(−4993.76, 1973.20) |
| Minimum cross-sectional area of upper airway (mm2)a | |||
| Females | 27.98(−1.27, 57.24) | 37.08(5.36, 68.80) | 18.61(−9.18, 46.41) |
| Males | 52.16(14.59, 89.74) | 8.43(−31.02, 47.87) | −5.90(−51.00, 39.21) |
| Upper airway length (mm) | |||
| Females | −0.37(−1.91, 1.18) | −1.96(−3.62, −0.29) | −0.32(−2.11, 1.47) |
| Males | −2.39(−4.29, −0.50) | −0.86(−2.99, 1.26) | −0.57(−2.82, 1.67) |
| Distance between H and MP plane (mm) | |||
| Females | −1.04(−2.38, 0.30) | 0.05(−1.42, 1.53) | −2.74(−4.42, −1.07) |
| Males | −1.55(−3.34, 0.24) | −1.92(−3.69, −0.15) | −0.22(−2.16, 1.71) |
Notes: Comparing the mean change between adjacent age groups by independent samples t-test and representing the absolute change with 95% CI. aMinimum cross-sectional area of upper airway is an area between PNS and EV.
Abbreviations: CI, confidence interval of the difference; MP plane, mandibular plane.
Figure 3.
Upper airway morphology and hyoid position change between age groups in males and females.
Abbreviations: UV, upper airway volume; UL, upper airway length; Amin, minimum cross-sectional area of upper airway between PNS and EV; MPH, distance between H and MP plane.
Reproductive Years and Upper Airway Morphology
When comparing females aged 25–34 and 35–44 years, no differences were found in UV, Amin, UL, and MPH. While among males, Amin was significantly smaller (52.16mm2, 95% CI=14.59–89.74) in 35–44 years old than in the 25–34 years old group and UL was significantly larger (−2.39mm, 95% CI=−4.29 to −0.50) than in the 25–34 years old group.
Perimenopausal Years and Upper Airway Morphology
Compared to 35–44 years, men aged 45–54 years did not show significant changes in UV, Amin, and UL, but MPH increased (−1.92mm, 95% CI=−3.69 to −0.15). However, unlike men, women aged 45–54 years showed a decrease in UV (3172.91mm3, 95% CI=653.86–5691.96) and Amin (37.08mm2, 95% CI=5.36–68.80) compared to 35–44 years, and UL increases (−1.96mm, 95% CI=−3.62 to −0.29). Besides, gender differences in UV, Amin, and MPH appeared to be increasing in perimenopausal women compared to men of the same age in Figure 3.
Postmenopausal Years and Upper Airway Morphology
In the age of ≥55 years, men did not show significant changes in upper airway morphology compared to 45–54 years. Although women showed a similar trend in upper airway morphology, the hyoid bone position was significantly lower (−2.74mm, 95% CI=−4.42 to −1.07) after menopause, while no significant difference was seen in the hyoid bone position in reproductive and perimenopausal years. In addition, gender differences in UV and UL seem to be greater, while decrease in MPH in postmenopausal years in Figure 3.
Discussion
The aim of this retrospective cross-sectional study was to explore whether there is a difference in upper airway morphology in perimenopausal and postmenopausal women compared to reproductive years. After careful matching of craniofacial morphological characteristics, there were two main findings from our study: 1) Compared to women of reproductive age, perimenopausal women experienced significant changes in upper airway morphology, including a significant reduction in upper airway volume, an increase in upper airway length and a reduced upper airway cross-sectional area; 2) In postmenopausal years, hyoid position was significantly lower than either age group, while no similar changes were seen in men.
Several studies have demonstrated that sex hormones have a profound protective effect on upper airway patency during reproductive years.14–16 Progesterone has a respiratory stimulatory effect. It is commonly believed that women have enhanced ventilatory drive during the luteal phase of the menstrual cycle and pregnancy due to increased progesterone levels.17,21 Another sex hormone called estrogen can also increase respiratory excitatory effects by upregulating progesterone receptors.27 Therefore, the decline in estrogen and progesterone after menopause can compromise upper airway ventilation.28 Studies have shown that postmenopausal women have decreased ventilatory responsiveness to hypercapnia.29 In addition, estrogen deficiency is associated with systemic changes in fat distribution. With lower estrogen after menopause, fat is more likely to be distributed in the upper body and trunk area,20 which may increase fat deposition around the upper airway and do harm to the upper airway morphology.
In our findings, postmenopausal women showed a decrease in hyoid bone position, which may also be associated with the decline in sex hormones. Progesterone increases the activity of the upper airway dilator muscles. Studies have suggested that progesterone improves strength and reduces fatigue of genioglossus muscle,27,30 while hormone replacement therapy increases genioglossus muscle activity significantly.21
Age-induced changes in upper airway morphology may also contribute to changes in airway morphology after menopause, which affecting our judgement of the effect of menopause itself on changes in airway morphology. In this study, we screened men of the same age group as sex controls. Men and women inherently have morphological differences in upper airway due to physique factors.18,31 For example, airway dimensions were normally larger in males than those in females, and healthy men had a larger upper airway cross-sectional area than women while awake and seated.32–34 And the length of upper airway was also found to be higher in males than in females.18,35 Comparing gender differences alone would bias the results. Therefore, we only compared the age-related changing trends in upper airway morphology and hyoid position separately by gender. As men of the same age did not show same changes in this study, we can speculate that these differences were due to menopause itself rather than to aging in females.
The present study contains both limitations and strengths. The limitations are primarily due to retrospective study which have posed difficulties for deep analysis. Because of the lack of information on other possible influences on upper airway morphology such as BMI in the sample population, the results are potentially biased. However, the sample size of this study was large and patients in dental hospital rarely have serious systemic diseases compared to general hospitals, so the findings of this study might reflect a population trend and provide evidence on the effects of menopause on upper airway morphology.
Smaller airway volume, reduced upper airway cross-sectional area, longer airway length and lower hyoid position are all associated with increased upper airway collapsibility.36–40 Hypoventilation of the upper airway contributes to an increased incidence of obstructive sleep apnea (OSA).41–44 Studies have found that the prevalence of OSA doubles in females after menopause independently of age and BMI, which was associated with changes in hormone levels.45–47 Future subgroup analyses based on accurate hormone levels, BMI, sleep breathing events and other factors are still needed to further elucidate the effect of hormone levels on upper airway morphology and their association with the occurrence of sleep breathing events in perimenopausal and postmenopausal women.
Conclusion
In summary, our findings suggested that women had smaller airway volume, reduced upper airway cross-sectional area and longer airway length in perimenopausal years, and a significantly lower hyoid position in postmenopausal years. These changes may be related to menopause itself and independent of the changes associated with aging under the male as background.
Ethics Statement and Consent to Participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Peking University Stomatology School and Hospital [PKUSSIRB-202054026]. Written informed consents have been provided before patients’ routine clinical examinations which informed the examination might use for teaching and researches in such a teaching hospital. However, all data were anonymously used and maintained with confidentiality.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nelson HD. Menopause. Lancet. 2008;371(9614):760–770. doi: 10.1016/S0140-6736(08)60346-3 [DOI] [PubMed] [Google Scholar]
- 2.Davis SR, Lambrinoudaki I, Lumsden M, et al. Menopause. Nat Rev Dis Primers. 2015;1:15004. doi: 10.1038/nrdp.2015.4 [DOI] [PubMed] [Google Scholar]
- 3.Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause - global prevalence, physiology and implications. Nat Rev Endocrinol. 2018;14(4):199–215. doi: 10.1038/nrendo.2017.180 [DOI] [PubMed] [Google Scholar]
- 4.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou L, Ji BT, Blair A, et al. Body mass index and colon cancer risk in Chinese people: menopause as an effect modifier. Eur J Cancer. 2006;42(1):84–90. doi: 10.1016/j.ejca.2005.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen L, Song L, Li H, et al. Association between earlier age at natural menopause and risk of diabetes in middle-aged and older Chinese women: the Dongfeng-Tongji cohort study. Diabetes Metab. 2017;43(4):345–350. doi: 10.1016/j.diabet.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 7.Shen L, Song L, Liu B, et al. Effects of early age at natural menopause on coronary heart disease and stroke in Chinese women. Int J Cardiol. 2017;241:6–11. doi: 10.1016/j.ijcard.2017.03.127 [DOI] [PubMed] [Google Scholar]
- 8.He L, Tang X, Li N, et al. Menopause with cardiovascular disease and its risk factors among rural Chinese women in Beijing: a population-based study. Maturitas. 2012;72(2):132–138. doi: 10.1016/j.maturitas.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 9.Song L, Shen L, Li H, et al. Age at natural menopause and hypertension among middle-aged and older Chinese women. J Hypertens. 2018;36(3):594–600. doi: 10.1097/HJH.0000000000001585 [DOI] [PubMed] [Google Scholar]
- 10.Du Z, Zhang B, Lin M, et al. The epidemiology of atrial fibrillation in Chinese postmenopausal women and its association with age of menopause. Maturitas. 2021;143:151–156. doi: 10.1016/j.maturitas.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 11.Soares CN. Depression in peri- and postmenopausal women: prevalence, pathophysiology and pharmacological management. Drugs Aging. 2013;30(9):677–685. doi: 10.1007/s40266-013-0100-1 [DOI] [PubMed] [Google Scholar]
- 12.Ossewaarde ME, Bots ML, Verbeek AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4 [DOI] [PubMed] [Google Scholar]
- 13.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162(11):1089–1097. doi: 10.1093/aje/kwi324 [DOI] [PubMed] [Google Scholar]
- 14.Heinzer R, Marti-Soler H, Marques-Vidal P, et al. Impact of sex and menopausal status on the prevalence, clinical presentation, and comorbidities of sleep-disordered breathing. Sleep Med. 2018;51:29–36. doi: 10.1016/j.sleep.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 15.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med. 1995;152(2):725–731. doi: 10.1164/ajrccm.152.2.7633734 [DOI] [PubMed] [Google Scholar]
- 16.Wimms A, Woehrle H, Ketheeswaran S, Ramanan D, Armitstead J. Obstructive sleep apnea in women: specific issues and interventions. Biomed Res Int. 2016;2016:1764837. doi: 10.1155/2016/1764837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saaresranta T, Aittokallio T, Polo-Kantola P, Helenius H, Polo O. Effect of medroxyprogesterone on inspiratory flow shapes during sleep in postmenopausal women. Respir Physiol Neurobiol. 2003;134(2):131–143. doi: 10.1016/s1569-9048(02)00208-2 [DOI] [PubMed] [Google Scholar]
- 18.Bonsignore MR, Saaresranta T, Riha RL. Sex differences in obstructive sleep apnoea. Eur Respir Rev. 2019;28:154. doi: 10.1183/16000617.0030-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perger E, Mattaliano P, Lombardi C. Menopause and sleep apnea. Maturitas. 2019;124:35–38. doi: 10.1016/j.maturitas.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 20.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55(5):950–954. doi: 10.1093/ajcn/55.5.950 [DOI] [PubMed] [Google Scholar]
- 21.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84(3):1055–1062. doi: 10.1152/jappl.1998.84.3.1055 [DOI] [PubMed] [Google Scholar]
- 22.Eslami E, Katz ES, Baghdady M, Abramovitch K, Masoud MI. Are three-dimensional airway evaluations obtained through computed and cone-beam computed tomography scans predictable from lateral cephalograms? A systematic review of evidence. Angle Orthod. 2017;87(1):159–167. doi: 10.2319/032516-243.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obelenis Ryan DP, Bianchi J, Ignacio J, Wolford LM, Goncalves JR. Cone-beam computed tomography airway measurements: can we trust them? Am J Orthod Dentofacial Orthop. 2019;156(1):53–60. doi: 10.1016/j.ajodo.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 24.Feng X, Li G, Qu Z, Liu L, Nasstrom K, Shi XQ. Comparative analysis of upper airway volume with lateral cephalograms and cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2015;147(2):197–204. doi: 10.1016/j.ajodo.2014.10.025 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yu Q, Ma L, Sun Z, Yang X. Prevalence of depression and anxiety symptoms and their influence factors during menopausal transition and postmenopause in Beijing city. Maturitas. 2008;61(3):238–242. doi: 10.1016/j.maturitas.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 26.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol. 2014;43(5):1542–1562. doi: 10.1093/ije/dyu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, Liu Y, Li Y. Comparison of natural estrogens and synthetic derivative on genioglossus function and estrogen receptors expression in rats with chronic intermittent hypoxia. J Steroid Biochem Mol Biol. 2014;140:71–79. doi: 10.1016/j.jsbmb.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 28.Edwards BJ, Li J. Endocrinology of menopause. Periodontol. 2013;61(1):177–194. doi: 10.1111/j.1600-0757.2011.00407.x [DOI] [PubMed] [Google Scholar]
- 29.Preston ME, Jensen D, Janssen I, Fisher JT. Effect of menopause on the chemical control of breathing and its relationship with acid-base status. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R722–R727. doi: 10.1152/ajpregu.90865.2008 [DOI] [PubMed] [Google Scholar]
- 30.Li W, Liu YH. Effects of phytoestrogen genistein on genioglossus function and oestrogen receptors expression in ovariectomized rats. Arch Oral Biol. 2009;54(11):1029–1034. doi: 10.1016/j.archoralbio.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 31.Brown IG, Zamel N, Hoffstein V. Pharyngeal cross-sectional area in normal men and women. J Appl Physiol. 1986;61(3):890–895. doi: 10.1152/jappl.1986.61.3.890 [DOI] [PubMed] [Google Scholar]
- 32.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10(9):2087–2090. doi: 10.1183/09031936.97.10092087 [DOI] [PubMed] [Google Scholar]
- 33.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7(5):377–389. doi: 10.1053/smrv.2002.0260 [DOI] [PubMed] [Google Scholar]
- 34.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120(5):1442–1447. doi: 10.1378/chest.120.5.1442 [DOI] [PubMed] [Google Scholar]
- 35.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166(10):1388–1395. doi: 10.1164/rccm.2112072 [DOI] [PubMed] [Google Scholar]
- 36.Edwards BA, O’Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618–633. doi: 10.1055/s-0030-1265902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131(6):1702–1709. doi: 10.1378/chest.06-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119(1):72e9–14. doi: 10.1016/j.amjmed.2005.01.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13(1):197–205. doi: 10.1034/j.1399-3003.1999.13a36.x [DOI] [PubMed] [Google Scholar]
- 40.Segal Y, Malhotra A, Pillar G. Upper airway length may be associated with the severity of obstructive sleep apnea syndrome. Sleep Breath. 2008;12(4):311–316. doi: 10.1007/s11325-008-0191-9 [DOI] [PubMed] [Google Scholar]
- 41.Vicini C, De Vito A, Iannella G, et al. The aging effect on upper airways collapse of patients with obstructive sleep apnea syndrome. Eur Arch Oto-Rhino-L. 2018;275(12):2983–2990. doi: 10.1007/s00405-018-5163-5 [DOI] [PubMed] [Google Scholar]
- 42.Ma MA, Kumar R, Macey PM, Yan-Go FL, Harper RM. Epiglottis cross-sectional area and oropharyngeal airway length in male and female obstructive sleep apnea patients. Nat Sci Sleep. 2016;8:297–304. doi: 10.2147/NSS.S113709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449. doi: 10.1056/NEJMcp1816152 [DOI] [PubMed] [Google Scholar]
- 44.Genta PR, Schorr F, Eckert DJ, et al. Upper airway collapsibility is associated with obesity and hyoid position. Sleep. 2014;37(10):1673–1678. doi: 10.5665/sleep.4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anttalainen U, Saaresranta T, Aittokallio J, et al. Impact of menopause on the manifestation and severity of sleep-disordered breathing. Acta Obstet Gynecol Scand. 2006;85(11):1381–1388. doi: 10.1080/00016340600935649 [DOI] [PubMed] [Google Scholar]
- 46.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064 [DOI] [PubMed] [Google Scholar]
- 47.Dancey DR, Hanly PJ, Soong C, Lee B, Hoffstein V. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120(1):151–155. doi: 10.1378/chest.120.1.151 [DOI] [PubMed] [Google Scholar]