Abstract
Transcriptional repressor proteins play essential roles in controlling the correct temporal and spatial patterns of gene expression in Drosophila melanogaster embryogenesis. Repressors such as Knirps, Krüppel, and Snail mediate short-range repression and interact with the dCtBP corepressor. The mechanism by which short-range repressors block transcription is not well understood; therefore, we have undertaken a detailed structure-function analysis of the Knirps protein. To provide a physiological setting for measurement of repression, the activities of endogenous or chimeric Knirps repressor proteins were assayed on integrated reporter genes in transgenic embryos. Two distinct repression functions were identified in Knirps. One repression activity depends on dCtBP binding, and this function maps to a C-terminal region of Knirps that contains a dCtBP binding motif. In addition, an N-terminal region was identified that represses in a CtBP mutant background and does not bind to the dCtBP protein in vitro. Although the dCtBP protein is important for Knirps activity on some genes, one endogenous target of the Knirps protein, the even-skipped stripe 3 enhancer, is not derepressed in a CtBP mutant. These results indicate that Knirps can utilize two different pathways to mediate transcriptional repression and suggest that the phenomenon of short-range repression may be a combination of independent activities.
Transcriptional repression is a critical component of genetic regulation during development, and the Drosophila melanogaster embryo has served as an important model for elucidation of basic repression mechanisms (7, 19). Differential gene expression in the early embryo is controlled in large part by the activity of repressor proteins encoded by gap, pair-rule, and other genes (42, 45). Repression of transcription can involve reactions occuring off the DNA, such as the formation of inactive heteromeric complexes. Another mechanism involves competition between activators and repressors for binding sites on DNA. DNA-binding repressors that function by mechanisms other than competitive binding have been termed active repressors (24).
An active repressor can repress basal promoters or enhancer elements over a short range (<100 bp) or, alternatively, over long ranges (>1,000 bp) (7, 17). One model of repression in the embryo suggests that the short-range–long-range distinction results from the recruitment of distinct classes of cofactors (36, 55). Short-range repressors may interact with dCtBP, while long-range repressors interact with Groucho.
Long-range repressors are typified by the Hairy protein, a transcription factor that binds the Groucho cofactor (5, 27, 40). Long-range repression complexes regulating the dpp, tld, and zen genes also recruit Groucho (7, 27), as do Engrailed, Runt, and dTCF, Drosophila repressors whose range of action has not yet been determined (3, 9, 50).
Short-range repressors present in the early Drosophila embryo include Snail, Krüppel, Giant, and Knirps. These proteins are capable of repressing the activity of enhancer elements when bound within ∼100 bp of key activator sites or of a basal promoter element when cognate sites are introduced close to the start of transcription (2, 16, 18, 22). Knirps, Snail, and Krüppel were recently shown to interact physically and genetically with dCtBP, a Drosophila homolog of mammalian CtBP, a factor that binds to and modulates the transcriptional and transforming activity of the adenovirus E1a protein (37, 40, 43). CtBP proteins also function as corepressors in vertebrates (13, 52).
In embryos lacking dCtBP, repression by Knirps, Snail, and Krüppel of endogenous target genes such as even-skipped (eve), hairy, rhomboid (rho), runt, and fushi-tarazu is disrupted (36, 40). In addition, point mutations affecting residues required for dCtBP binding also compromise repression by Knirps, Snail, and Krüppel (36, 37). The expression patterns of Snail, Krüppel, and Knirps are largely intact in a CtBP mutant, consistent with the hypothesis that dCtBP contributes directly to the transcriptional repression activity of these proteins, rather than controlling their expression (36, 40). When fused to heterologous DNA binding domains, dCtBP can directly repress transcription in embryo and cell culture systems, suggesting that the cofactor has a direct role in mediating repression (13, 36, 37, 52). The mechanism by which CtBP proteins repress transcription is unknown, although there have been suggestions of interactions with histone deacetylases (12, 48). Interactions with Polycomb proteins have also been noted (44).
knirps is expressed in the early embryo in presumptive abdominal and head regions, and mutations in the knirps gene are embryonic lethal, showing a characteristic gap phenotype of the larval cuticle (38, 54). knirps also plays important roles in tracheal and wing formation later in development (10, 32). The knirps gene encodes a transcription factor with homology to nuclear hormone receptors, possessing a conserved N-terminal zinc finger DNA binding domain and a C-terminal effector region (2, 35). The C terminus does not resemble the canonical hormone receptor ligand binding domain, however, and Knirps is not known to interact with small molecule ligands. Analysis of knirps mutations revealed that loss of the DNA binding zinc fingers or more C-terminal truncations at codons 145 or 185 have a strong phenotype, but a frameshift after codon 232 creates a weak allele, with less severe cuticular defects (14). Molecular targets of Knirps protein include the eve and hairy genes (29, 47). In transgenic embryo assays, Knirps represses heterologous enhancers and promoters over a short range. This repression function is modular and can be transferred to a heterologous DNA binding domain (2). The repression domain of Knirps includes a motif, PMDLSMK, important for binding of dCtBP. Mutations in this motif significantly impair repression activities of a chimeric Gal4-Knirps 255-429 protein or a full-length Knirps protein (36, 37). However, a portion of Knirps not including the dCtBP binding region was shown to repress transcription in transiently transfected Drosophila cells (14). The hypomorphic mutation kni14F, which retains partial activity, also encodes a protein that lacks a dCtBP binding motif (14).
We have undertaken a molecular analysis of Knirps repression function to identify mechanisms by which this short-range repressor acts. To characterize the activity of Knirps and Gal4-Knirps chimeras in a chromosomal context, known to be important for proper functioning of repressors such as Engrailed (50), we have created transgenic embryos containing integrated reporter genes. Our studies identify two repression regions of the Knirps protein. One portion of Knirps, spanning amino acids 202 to 358, is dependent on the dCtBP binding motif for repression. The other, an N-terminal repression region, is apparently independent of dCtBP activity. This repression domain does not bind to dCtBP, and it mediates effective repression in embryos lacking dCtBP protein. The dual activities of Knirps may provide the flexibility to regulate different target genes through alternative pathways.
MATERIALS AND METHODS
Plasmids.
The following oligonucleotides were used in construction of various plasmids: 5′ CACTCGAGTGACATG3′ (a), 5′AGATCCTCGAGTACAGCATG3′ (b), 5′ACGTGGATACGATTAAGTATGCATG3′ (c), 5′TCCATGATAAACGCG TGCTAGAC TAT TGCAGG TACTGATCGAATGCC TC TGCATG3′ (d), 5′TCGCTAGACGTGAATCTCGTAGCTTCCGTATCCGTACCAAATGCGTATCAGGCATG3′ (e), 5′GGCCGACTACAAGGATGACGATGACAAGCACCATCACCACCATCACGC3′ (f), 5′TTGGCGCGCCAA3′ (g), 5′CGCGGCGCGCCTGGC3′ (h), 5′CATGCAGGCGCGC3′ (i), 5′CGCGATAGTGATAAGTAGAATT3′ (transcription termination codons are shown in boldface type) (j), 5′CGGGGTACCGCTGCCGCTGCAGCGGCTTCTGCTGCCGATGCCGCT3′ (k), 5′GGGGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAATCCACCTCCACTTCTTGATCCTCGGA3′ (l), 5′CGGGGTACCGATGCCGCTTACCGGCAGGAGATGTACAAGCACCGC3′ (m), 5′GGGGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAATCCACCTCCACTTCTTGATCCTCGGA3′ (n), 5′GGGTCGGTACCGCAGCCCTGCCCCCACACCTCCTCTTCCCA3′ (o), 5′GGGAATCTAGACTAAACTAATTACTAGATGGGCGACTGGCGGGCCGAGGA3′ (p), 5′CGGCAGGAGATGTACGTAGAGTCGCAGAACCGC3′ (q), 5′AAGCACCGCCAGAGCGTGGATTCCTCGCCCATCGATGTCTGCCTGGAG3′ (r), 5′ACTCCGACTAGCAGCAGTACTACCAGCGTTGTACCA3′ (s), 5′GTTTCCGCTCAAGAAGTGCACAGCTTCAACGAC3′ (t), 5′GGGTCGGTACCGCAGCCTCGGCCCGCCAGTCGCCCATCGAT3′ (u), 5′CAGAACCGCTTTAGTCCCGCCAGC3′ (v), 5′GGGGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAATCTCCTTCTTGAGCGGAAACGGTGGG3′ (w), 5′GG GGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAAT CATGCAGGAGGCTTGCGGACGACTG3′ (x), 5′GGGTCGGTACCCACGAACAGGCCGCCGCAGCGGCGGGCAAG3′ (y), 5′GGGTCGGTACCGCCGCAGCGGGCTCGCCACACACTCCCGGATTTGGG3′ (z), 5′GGGTCGGTACCCACCACCATCATCAGCAGCAGCAGCAGCAC3′ (aa), 5′GGGTCGGTACCGCCGCAGCGTCCGCCGCCCTGCCCTTCTTCAGC3′ (bb), 5′GGGTCGGTACCCTGCCCCCACACCTCCTCTTCCCAGGCTAC3′ (cc), 5′GGGGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAATCGAACTTCCGGCGCGGAGCCACCTC3′ (dd), 5′GGGGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAATCGAACTTCCGGCGCGGAGCCACCTC3′ (ee), and 5′GGGGAATCTAGACTAACTAATTACTACTTGTCATCGTCATCCTTGTAATCGAACTTCCGGCGCGGAGCCACCTC3′ (ff).
Promoter spacing constructs.
Genes shown in Fig. 1 containing Knirps binding sites at −70, −75, −100, −130, and −180 were created by modification of a gene containing dual Knirps binding sites located at −55 bp (2). This reporter construct contains divergent white and lacZ genes and enhancer elements derived from the rho and twist genes in the vector C4PLZ (53). The original kni-55 construct was modified by insertion of spacing oligonucleotides a (kni-70) or b (kni-75) at the SphI site between the TATA box and the Knirps binding sites. The kni-75 construct was further modified by insertion of a 25-nucleotide spacer (c) or a 55-nucleotide spacer (d) to create kni-100 and kni-130. The kni-180 construct was created by insertion of oligonucleotide e downstream of the spacer sequence of kni-130.
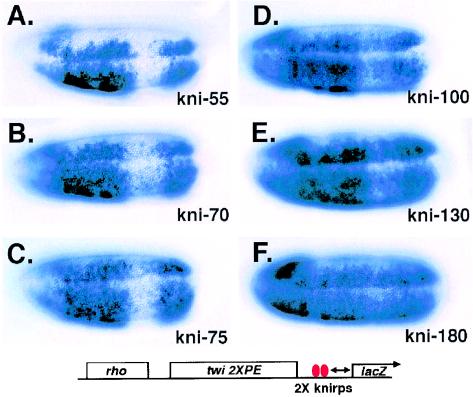
FIG. 1.
Distance-dependent repression of integrated lacZ reporter genes by endogenous Knirps protein. The twi and rho elements normally drive expression in uninterrupted swaths from posterior to anterior. Repression by endogenous Knirps is visible where lacZ staining is attenuated in a broad stripe in the posterior region. Moving the 3′ edge of repressor binding sites from −55 to −180 bp by insertion of short spacers (A to F) gradually compromises transcriptional repression activity. Transgene structure is indicated at the bottom of the figure, where the horizontal arrow indicates variable distances from the Knirps binding sites to the transcriptional start site in the six genes assayed. Gene activation is directed by enhancer elements derived from the rho and the twist genes. Expression of the transgenes was visualized by in situ hybridization with antisense probe to the lacZ gene as described in Materials and Methods. Ventrolateral views of representative embryos are shown, with anterior at the left.
Chimeric Gal4-Knirps constructs.
A transgene containing the open reading frame for the Gal4 DNA binding domain (residues 1 to 93) fused to coding sequence for amino acids 75 to 429 of Knirps was modified with a Flag-hexahistidine tag sequence (f) inserted at a NotI site situated in the linker region between Gal4 and Knirps codons (2). Termination sequences were placed in the knirps coding sequence following codons 189, 254, or 332 by insertion of AscI adapter oligonucleotides g (Kni75-189), h (Kni75-254), or i (Kni75-332) into the PvuII, ClaI, or NcoI restriction sites, respectively, followed by introduction of four stop codons (oligonucleotide j) at the AscI site. Resultant chimeric genes encode proteins truncating with the amino acids (Knirps residues underlined) 75 to 189 (PFQLAR), 75 to 254 (SPIAAR), or 75 to 332 (GPMQAR). An N-terminal deletion removing codons 75 to 187 was created by cleaving at the PvuII site and inserting an oligonucleotide (f) encoding a Flag epitope tag, resulting in the junction sequence (Gal4 and Knirps residues underlined) ALLGTAADYKDDDDKQLP. A gene with an internal deletion was constructed by removing the region between codons 189 and 254, using complementary AscI sites, generating the sequence PFQLARLADVC at the junction.
For constructs 8 to 14 shown in Fig. 2, portions of the knirps cDNA contained in the pCarnegie 20 vector pN741 (G. Struhl, unpublished data) were amplified using PfuI DNA polymerase (Promega). The products were placed between the KpnI and XbaI sites of the pTwiggy vector (2) containing a twi enhancer element (2xPEe-Et) and twist basal promoter. The constructs and the corresponding primers (in parentheses) used were Kni202-358 (k and l) ΔA (m and n), and Kni189-254 (o and p). Additional deletions in this series were generated by oligonucleotide-directed mutagenesis using the Mutagene kit (Bio-Rad) (46). Construction of ΔB, in which codons 202 to 227 are deleted, used the mutagenic oligonucleotide q. Construction of ΔC, in which codons 228 to 251 are deleted, used oligonucleotide r. Construction of ΔD, in which codons 292 to 313 are deleted, used oligonucleotide s. Construction of ΔE, in which codons 330 to 343 are deleted, used oligonucleotide t. The ΔD construct described above was used as a PCR template using oligonucleotides u and l. The product of this amplification was cloned into pTwiggy as above to generate ΔF.
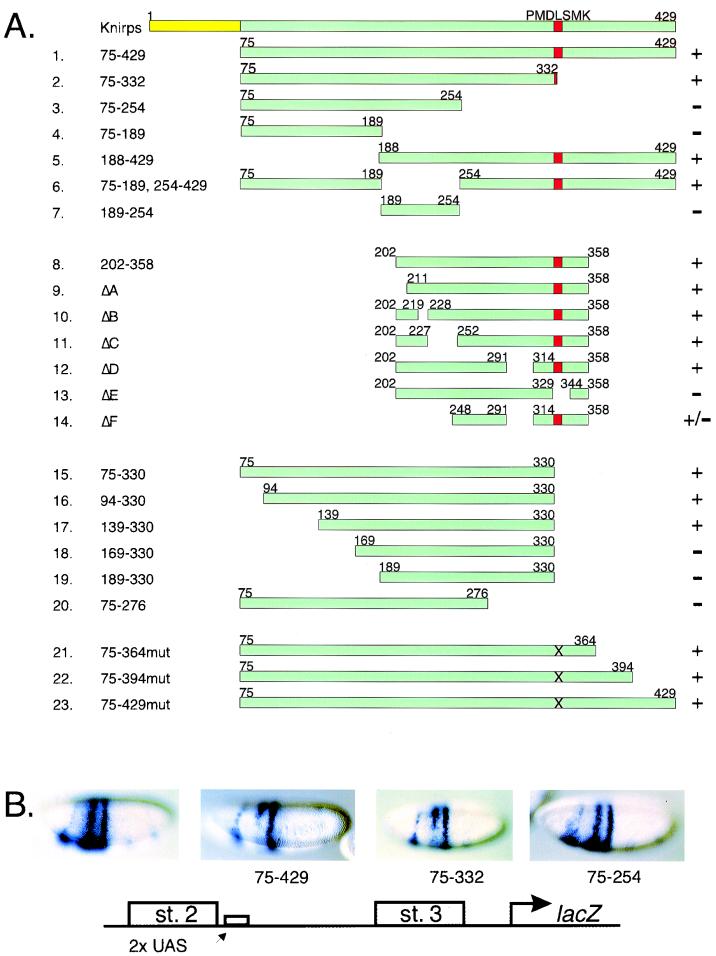
FIG. 2.
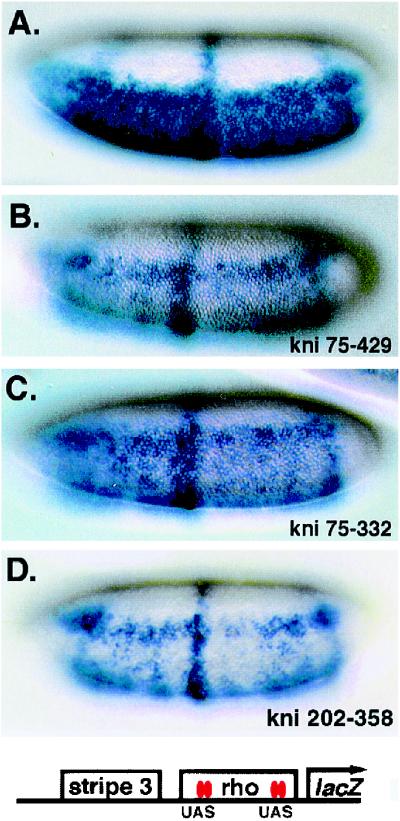
Deletional analysis of the Gal4-Knirps 75-429 repressor. (A) Structure and activities of Gal4-Knirps chimeric proteins. Chimeras numbered 1 to 23 were expressed in transgenic embryos as fusions to the Gal4 protein DNA binding domain (residues 1 to 93). A plus sign signifies at least 6% of embryos showed a clear repression pattern; a minus sign signifies ≤1%. See Table 1 for quantitation. The dCtBP binding motif PMDLSMK is indicated as a red box; an X indicates proteins in which the PMDLSMK motif has been mutated to AAAASMK. (B) Structure and expression pattern of the eve stripe (st.) 2-stripe 3 lacZ reporter gene used to test the activity of Gal4-Knirps repressors shown in panel A. Embryos from left to right: reporter gene in the absence of Gal4-Knirps repressor, repressed pattern generated by crosses to Gal4-Knirps 75-429 and 75-332 lines, and an unrepressed pattern obtained from a cross to a nonfunctional line (Kni75-254). Embryos are shown with anterior at the left and dorsal being at the top.
A portion of the knirps cDNA was amplified from a pBluescript SK(+) clone which includes sequences encoding Knirps residues 75 to 429 using the primers v and either w (Kni75-330) or x (Kni75-276). The amplified fragments were digested with ClaI and XbaI and used to replace the ClaI-XbaI fragment of the knirps cDNA clone in pBluescript SK(+). The KpnI-XbaI fragments of these clones were then inserted into pTwiggy (2). The final constructs encode Knirps amino acids 75 to 330 and 75 to 276 followed by an eight-amino-acid sequence including the Flag epitope, DYKDDDDK.
Portions of the knirps cDNA were amplified as above using the oligonucleotides w and y (Kni94-330), z (Kni124-330), aa (Kni139-330), bb (Kni169-330), or cc (Kni189-330). The amplified products were cloned between the KpnI and XbaI sites of pTwiggy.
A plasmid containing a mutated knirps cDNA, Casper-22FΔKE, was the kind gift of S. Small (36). Portions of this clone were amplified using the oligonucleotides u and dd (75-364mut), ee (75-394mut), or ff (75-429mut). A ClaI/XbaI fragment of the amplified products were cloned into a pBluescript vector containing the Knirps coding sequence 75 to 429, replacing the endogenous C-terminal sequences with the novel sequences. KpnI/XbaI fragments were then subcloned into pTwiggy. The products encoded by these constructs have the dCtBP-binding motif of Knirps, PMDLSMK, replaced by AAAASMK.
Constructs created by PCR were verified by complete sequencing of the open reading frames, and junctions of all clones were confirmed by sequencing. The structures of integrated transgenes for the deletions of Kni202-358 were further confirmed by PCR analysis of genomic DNA.
T7 expression constructs, protein synthesis, and GST interaction assays.
T7 RNA polymerase expression constructs were derived from pET3a (Novagen) in which the XbaI site 5′ of the T7 translational initiation site was destroyed by filling in the site with Klenow fragment and religating the blunted ends. Sequences encoding the Gal4 DNA binding domain (amino acids 1 to 93) from pTwiggy (2) were inserted into the NdeI-BamHI sites of the modified pET3a vector. An XbaI linker, containing the sequence 5′ CTCTAGAG 3′, was inserted into the BamHI site at the 3′ end of the Gal4 encoding sequence. KpnI-XbaI fragments containing Knirps coding regions were isolated from P-element transformation vectors and inserted between the KpnI-XbaI sites 3′ of the Gal4 encoding sequences. Kni 75-340 and Kni 75-340M constructs were a kind gift of Y. Nibu and M. Levine (36). [35S]methionine labeled proteins were prepared by in vitro translation using a coupled rabbit reticulocyte system (TNT; Promega) according to the manufacturer's instructions. Glutathione S-transferase (GST)-dCtBP affinity columns were produced as described (36). Translation products were mixed with GST or GST-dCtBP proteins bound to glutathione agarose (Sigma) in binding buffer (20 mM Tris Cl [pH 7.8], 0.2 mM EDTA, 0.1 M NaCl, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) with 0.2% NP-40 and washed three times in binding buffer with 0.4% NP-40. Proteins retained on the glutathione beads were boiled in Laemmli sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by PhosphorImager analysis (Molecular Dynamics).
P-element transformation, whole-mount in situ hybridization of embryos, and crosses to reporter lines.
P-element transformation vectors were introduced into the Drosophila germ line by injection of y w67 embryos as described (46). For each gene construct, at least three separate lines were tested, and similar results were obtained with all lines except as noted. In situ hybridizations were performed as described (46) using digoxigenin-UTP-labeled antisense RNA probes to lacZ. The Gal4-dCtBP transgenic line used in Fig. 4 expresses the protein under the control of Krüppel regulatory elements (37) and was kindly supplied by Y. Nibu and M. Levine.
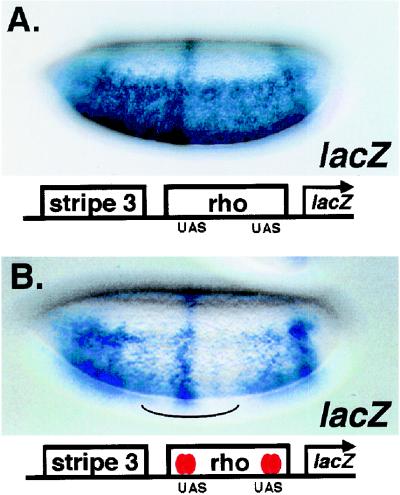
FIG. 4.
The Gal4-dCtBP chimeric protein acts as an autonomously acting repressor within an enhancer. (A) Expression pattern of the eve stripe 3-rho lacZ reporter gene, showing robust ventral expression directed from a rho enhancer element lacking Snail binding sites (2) and a central stripe from the eve stripe 3 enhancer. (B) Repression of rho enhancer activity in the central portion of the embryo mediated by the Gal4-dCtBP chimera (denoted by the curve under embryo). Expression of the repressor is driven by Krüppel regulatory elements (36). Gal4-binding sites were introduced in a 600-bp rho enhancer at the positions previously used for targeting Knirps protein to this gene (22). The most promoter proximal UAS binding site is located at −195 bp (2). Ventrolateral views are shown, with anterior at the left.
Assays of in vivo repression activity.
Transgenic flies carrying chimeric repressor constructs were crossed with reporter lines containing one of three reporters: (i) eve stripe 2 enhancer linked to eve-lacZ (2), (ii) eve stripe 2 and stripe 3 enhancers linked to eve-lacZ (36), or (iii) eve stripe 3 and rho enhancers linked to the transposase-lacZ fusion gene (22). To assay repression activity of Gal4-Knirps chimeras, effector lines were crossed to reporter lines, and several hundred embryos from each cross were collected, aged to 2 to 4 h at room temperature, fixed, and stained as described (46). After mounting on microscope slides, embryos were visually scored in a blinded experiment for evidence of repression. Most functional repressors completely abolished ventral staining in the eve stripe 2 region; embryos exhibiting weakened but not complete repression were scored in a separate category. Typically, a greater number of older (early gastrula) embryos exhibited repression, presumably because of the lag between the activation of the eve or rho enhancer and the production of adequate amounts of the Gal4-Knirps protein after its transcription under the control of the twist enhancer. In general, with heterozygous Gal4-Knirps lines, the maximum percentage of embryos exhibiting repression would be 50%, because only half of the fertilized embryos receive the Gal4-Knirps effector gene from the heterozygous male parent. The observed percentages may be lower than this because all embryos showing reporter gene expression were counted, including younger embryos in which repressors have not yet reached their maximal level of expression.
Analysis of gene expression in embryos lacking maternal dCtBP.
CtBP− germ line clones were produced using the autosomal FLP-DFS technique (11). Females carrying an eve stripe 2-upstream activation sequence (UAS)-lacZ reporter gene on chromosome 1 (2) were crossed to D/TM3, Sb males. Male progeny carrying the lacZ reporter and D were crossed to females carrying a balanced, P-element insertional mutation of CtBP (CtBP03463/TM3, Sb; Bloomington stock no. P1590).
FRT, ovoD1/TM3, Sb males (Bloomington stock no. 2149) were mated to females carrying the Saccharomyces cerevisiae FLP recombinase gene under the control of the hsp70 promoter (hsFLP; D/TM3, Sb; Bloomington stock no. 1970) to generate males with hsFLP on chromosome 1 and ovoD1 over D on chromosome 3. Females carrying the lacZ reporter and the CtBP mutant allele over D were crossed to these hsFLP; ovoD1/D males. Embryos from this cross were collected for 24 h, aged for 48 h and heat shocked for 2 h in a 37°C water bath. The heat shock was repeated 24 h after the first treatment. Females lacking the D marker (hsFLP; FRT, ovoD1/CtBP03463) were mated to males carrying the appropriate Gal4-Knirps transgene. To assay for expression driven by the eve stripe 3 enhancer in a CtBP mutant background, males carrying an eve/lacZ fusion gene containing a 500-bp (−3.8 to −3.3 kbp) or an 800-bp (−3.8 to −3.0 kbp) portion of the eve stripe 3 enhancer (47) were crossed to the females producing oocytes deficient in dCtBP. Embryos were collected, fixed, and stained as described (46). As expected for CtBP mutants, embryos derived from these crosses died before hatching.
RESULTS
Distance dependence of Knirps repression activity at a basal promoter element.
Previous studies indicated that endogenous Knirps, when targeted to a heterologous promoter by introduction of cognate binding sites, was capable of interfering with transcription when the binding sites were situated at −55 bp, but not at −130 bp, consistent with a short range of activity (2). Subsequent analysis of Knirps repression activity on promoters has been limited to reporter genes with binding sites immediately adjacent to the basal promoter element (36, 37). To determine more precisely the distance dependence of Knirps repression and test whether the loss of activity with increasing distance represents a step function or a gradual tapering off of activity, we designed reporter transgenes with tandem Knirps binding sites situated at −55, −70, −75, −100, −130, or −180 bp. These genes were introduced into Drosophila by P-element-mediated germ line transformation, and the embryonic expression patterns of the transgenes were analyzed by in situ hybridization. With these reporter genes, repression by Knirps is most readily seen as weakening of lacZ expression in a broad stripe in the posterior of the embryo where Knirps is expressed (2).
Strong repression was observed in genes with Knirps binding sites situated at −55, −70, or −75 bp (Fig. 1A to C). The Knirps binding sites used in this gene have been shown to confer repression in a Knirps-dependent manner (2), and as expected, the repression was observed only in the presumptive abdomen and ventral anterior regions, where the knirps gene is expressed (35). Repression was less effective in the −100- and −130-bp constructs and almost undetectable in the construct with the sites at −180 bp (Fig. 1D to F). These results indicate that there is a gradual tapering off of repression as Knirps sites are moved away from the promoter region. No differences were observed in the pattern for genes with repressor sites situated at −70 or −75 bp, indicating phasing effects are not important on this reporter gene. The effective distance of repression on this basal promoter is similar to that seen for Knirps action within enhancer elements (2). A previously tested gene with the rho enhancer 4 kbp 3′ of the transcriptional start site showed no repression by a Knirps binding site at −130 bp (2), compared with the weak repression seen here. The more proximal position of the enhancers in reporter genes shown in Fig. 1 may influence the relative effectiveness of Knirps repression.
A repression region identified in cell culture assays does not repress in the embryo.
The experiments with endogenous Knirps protein (Fig. 1) provide information on the general properties of the endogenous repressor, without identifying the regions of the protein necessary for repression. As a first step in identifying the mechanism of repression by Knirps, we carried out a structure-function analysis to define the residues of Knirps important for mediating repression in vivo. Misexpression of full-length Knirps leads to embryonic lethality (37), so we performed our structure-function analysis using Gal4-Knirps fusion proteins.
Genes encoding portions of Knirps fused to the Gal4 DNA binding domain were expressed in ventral regions of transgenic embryos under control of a twist regulatory element (Fig. 2A). To simultaneously measure repression and range of action, these chimeric repressors were tested on a lacZ reporter gene activated by two enhancers. Gal4-binding UAS sequences were placed adjacent to the eve stripe 2 element, which was previously shown to be sensitive to Knirps repression (2). An eve stripe 3 enhancer was placed over 300 bp away, beyond the range of short-range repression. Embryos carrying the stripe 2-stripe 3 lacZ transgene showed a characteristic pattern of two circumferential stripes, with an additional anterior stripe derived from vector sequences (Fig. 2B).
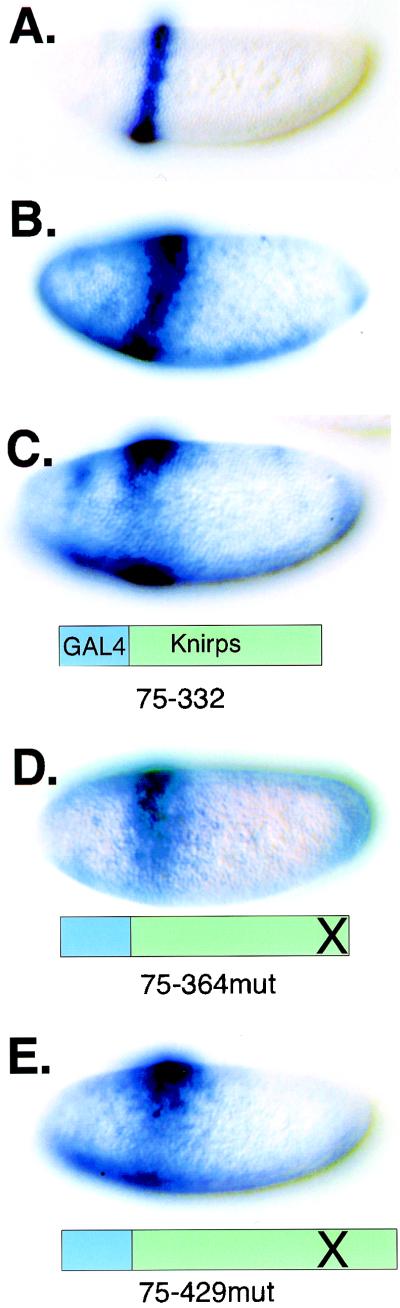
A series of deletions that focused on a region identified as a repression domain in cell culture assays (14) were initially tested (constructs 1 to 7). As expected, a Gal4-Knirps chimera containing most of the knirps open reading frame (codons 75 to 429) showed effective repression of the proximal stripe 2 enhancer, while, consistent with the short range of Knirps activity, the distal stripe 3 enhancer was not affected (Fig. 2). A C-terminal truncation removing residues 333 to 429 did not compromise activity (construct 2, Kni75-332 [Fig. 2]), although it does remove most of the dCtBP interaction region of Knirps (see below). More extensive C-terminal truncations terminating at residues 254 or 189 were inactive (constructs 3 and 4 [Fig. 2]), suggesting that a region of the protein from residue 254 to 332 might be necessary for repression in the absence of the dCtBP binding motif. Effective repression by proteins encoded by constructs 5 and 6 demonstrated that the N-terminal residues 75 to 187 were dispensable for activity in this context, as was the region of the protein including residues 189 to 254 (Fig. 2A). The chimera containing only residues 189 to 254 was not active (Fig. 2 [residues 189 to 254]). Therefore, this region of the protein was neither necessary nor sufficient for repression, in contrast to the result obtained in transient-transfection assays (14). For these chimeras, active constructs showed at least 20% of the embryos repressed in blinded scoring assays, while inactive repressor genes and the reporter construct alone averaged less than 1% embryos scored as repressed (Table 1) (see Materials and Methods).
TABLE 1.
Activity of chimeric Gal4-Knirps repressor proteins in transgenic embryos
| Construct no. | Chimeraf | % Represseda | No. of embryos scored | No. of lines analyzed |
|---|---|---|---|---|
| 1 | 75-429 | 32 ± 8 | 777 | 6 |
| 2 | 75-332 | 21 ± 9 | 754 | 4 |
| 3 | 75-254 | 0.9 ± 0.8 | 471 | 3 |
| 4 | 75-189 | 0.4 ± 0.5 | 691 | 4 |
| 5 | 188-429 | 23 ± 7 | 564 | 2b |
| 6 | 75-189, 254-429 | 24 ± 5 | 995 | 3 |
| 7 | 189-254 | 1 ± 1 | 504 | 7 |
| 8 | 202-358 | 13 ± 4 | 1,258 | 6 |
| 9 | 202-358 (ΔA) | 7 ± 4 | 526 | 6 |
| 10 | 202-358 (ΔB) | 18 ± 6 | 910 | 6 |
| 11 | 202-358 (ΔC) | 14 ± 8 | 303 | 3 |
| 12 | 202-358 (ΔD) | 12 ± 1 | 856 | 4 |
| 13 | 202-358 (ΔE) | 0 | 529 | 5 |
| 14 | 248-291, 314-358 (ΔF) | 1.4–23 | 240 | 4c |
| 15 | 75-330 | 2–9 | 307 | 3 |
| 16 | 94-330 | 32 ± 12 | 725 | 3 |
| 17 | 139-330 | 18 ± 1 | 659 | 3 |
| 18 | 169-330 | 1 ± 1 | 1,272 | 4d |
| 19 | 189-330 | 0 | 1,504 | 4 |
| 20 | 75-276 | 0.9 ± 1 | 1,172 | 3 |
| 21 | 75-364mut | 6 ± 3 | 594 | 3 |
| 22 | 75-394mut | 3–9 | 634 | 2e |
| 23 | 75-429mut | 32 ± 5 | 1,077 | 4 |
The level of embryos scored as repressed in the even-skipped lacZ reporter lines used for these assays was 0.4% ± 0.9%. Values not given as ranges are means ± standard deviations.
Only two lines were obtained.
Range shown. This gene showed large variability between lines.
Approximately 3% of embryos from crosses to these lines showed some weakening of staining in ventral regions.
Range shown. A third line showed no repression activity.
Genes for constructs 1 to 6 contained endogenous kni 3′ UTR sequences.
A minimal dCtBP-dependent repressor.
The activity of the genes described above suggested that a minimal repression region might be localized in the central region of Knirps protein. To further delimit regions important for repression, Gal4 fusion proteins containing residues 202 to 358 and derivatives were tested (constructs 8 to 14 [Fig. 2A]). Deletions of residues 202 to 210 (ΔA), residues 220 to 227 (ΔB), residues 228 to 251 (ΔC), or residues 292 to 313 (ΔD) did not impair repression activity, while deletion of residues 330 to 343 (ΔE) abolished repression completely (Fig. 2A). The region removed in construct ΔE includes the residues PMDLSMK, shown to mediate Knirps interaction with the dCtBP protein (36, 37). Alanine scanning mutations affecting these residues have also been shown to compromise repression activity of a chimeric Gal4-Knirps protein containing residues 255 to 429 and by ectopically expressed Knirps (36, 37). Our results indicate that repression by residues 202 to 358 depends on dCtBP, because the dCtBP binding motif is required for activity. A minimal construct, ΔF, containing only 89 amino acid residues from Knirps (residues 248 to 291 and 314 to 358) was active, consistent with earlier reports that residues N-terminal to 255 were dispensable for dCtBP-dependent activity (37). This chimeric gene showed variation in activity, however, possibly because of line-to-line differences in expression levels.
Characterization of the N-terminal repression region.
Although the Kni75-332 repressor lacks an intact dCtBP binding motif, we considered the possibility that this protein might be able to interact with dCtBP by way of the C-terminal residues (PMQAR) or that some amount of translational readthrough was occurring to produce full-length protein. (The C-terminal truncation mutant genes in constructs 1 to 6 still retain Knirps coding sequence 3′ of the introduced triple stop codons). Therefore, we created a transgene encoding residues 75 to 330, eliminating the remainder of the dCtBP interaction motif and all Knirps coding sequences 3′ of the stop codon, and tested this gene in the in vivo repression assay on the eve stripe 2-stripe 3 lacZ reporter gene (Fig. 2A, construct 15). The Kni75-330 protein was effective in repressing the eve reporter gene, indicating that complete elimination of the dCtBP interaction motif did not compromise transcriptional repression. The number of embryos showing repression was smaller than that seen for other chimeric repressors, perhaps due to lower protein expression levels, but the extent of repression of the lacZ gene in individual embryos was the same.
To further define the N-terminal repression region, additional transgenic lines expressing Gal4 chimeras fused to Knirps residues 94 to 330, 139 to 330, 169 to 330, 189 to 330, or 75 to 276 were tested (Fig. 2A, constructs 16 to 20). The effectiveness of Kni94-330 and Kni139-330 suggests that the residues required for N-terminal repression activity are included within residues 139 to 330. The 169-330 protein had very low activity, while the 75-276 and 189-330 proteins were not active in this assay, suggesting that residues in the regions from residue 139 to 189 and 276 to 330 may be required for the N-terminal repression activity. Difficulties in quantitating levels of the chimeric proteins in embryos by either antibody staining or Western blot analysis precluded a definitive interpretation of inactive constructs. We were, however, able to detect one of the inactive Knirps repressors by gel-shift analysis (ΔE, Fig. 2 [data not shown]), suggesting that at least some of the inactive proteins are expressed.
N-terminal repression activity is not negatively regulated by C-terminal domain of Knirps in Gal4-Knirps chimeras.
Ectopic expression of full-length Knirps protein in an eve stripe 2 pattern causes improper repression of endogenous eve and results in embryonic lethality (36). A mutation in the dCtBP interaction region of the misexpressed protein eliminates lethality and greatly weakens, but does not altogether abolish, repression activity of the ectopically expressed protein (36). This weakly active mutant protein contains the N-terminal repression region that we defined in constructs 15 to 20. We therefore tested whether the activity of the N-terminal repression region might be masked by the presence of the C-terminal regions of the protein (residues 330 to 429), resulting in inhibition of the N-terminal repressor. Three transgenes containing Gal4 fusions to Knirps residues 75 to 364, 75 to 394, and 75 to 429 were generated; each containing the PMDLSMK to AAAASMK mutation that disrupts dCtBP interaction (36). All three of these proteins were effective in mediating transcriptional repression of the stripe 2-stripe 3 lacZ reporter gene (Fig. 2, constructs 21 to 23; Table 1), indicating that the presence of C-terminal residues does not prevent the N-terminal repression region from acting in this context. In fact, the C-terminal residues may enhance protein stability or function; the 75-429mut transgenic lines gave a higher number of repressed embryos than did the 75-394mut and 75-364mut lines (Table 1).
Functional repressors lacking dCtBP binding motifs do not bind dCtBP in vitro.
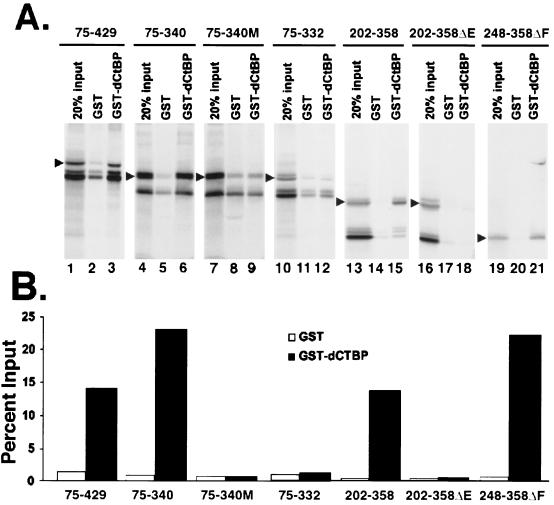
The mutation of DLS to AAA within the PMDLSMK dCtBP binding motif is sufficient to abrogate dCtBP binding to Knirps in vitro (37). The 75-332 chimeric protein contains only the first two residues, PM, of this motif (followed by QAR, introduced during cloning steps). Therefore, it seemed likely that dCtBP would no longer be able to interact with this protein. To test whether there might be some residual binding activity to dCtBP, we measured the ability of 75-332 protein and other proteins to interact directly with GST-dCtBP protein in vitro (Fig. 3). Proteins produced in in vitro transcription-translation reactions were incubated with either GST or GST-dCtBP bound to glutathione agarose (37). The GST-dCtBP protein interacted specifically with Knirps 75-429, 75-340, 202-358, and 248-358ΔF proteins, all of which contain intact PMDLSMK motifs (Fig. 3A, lanes 3, 6, 15, and 21). A mutant 75-340 protein containing PMAAAMK (Fig. 3A, lane 9), the 202-358ΔE protein lacking PMDLSMK (Fig. 3A, lane 18), and the 75-332 protein (Fig. 3A, lane 12) all failed to interact specifically with the dCtBP affinity matrix. Quantitation of the retained proteins (Fig. 3B) indicates the binding of proteins lacking the dCtBP motif was not significantly above background. Thus, in vitro dCtBP binding does depend on an intact interaction motif, but this interaction is apparently not required for activity of the 75-332 protein.
FIG. 3.
dCtBP binding activity of Gal4-Knirps repressors. (A) In vitro-translated Knirps chimeras were tested for interaction with GST-dCtBP. Arrows indicate the sizes of the full-length products, as calculated from mobilities relative to molecular weight standards. The Gal4-Knirps 75-429, 75-340, and 202-358 proteins interact strongly with GST-dCtBP (lanes 3, 6, and 15). Mutations changing the dCtBP binding motif from PMDLSMK to PMAAAMK abolish specific interaction (lane 9). The Gal4-Knirps 75-332 protein, which retains only the PM portion of the dCtBP binding motif, does not demonstrate specific binding to GST-dCtBP (lane 12), although this protein is functional in vivo (Fig. 2). A deletion in the dCtBP interaction domain from residues 202 to 358 abolishes binding (lane 18), while deletions removing residues 202 to 247 and 292 to 313 do not affect binding (lane 21). (B) Quantitation of binding data shown in panel A, comparing protein retained on GST (white bars) to protein retained on GST-dCtBP (black bars), normalized to the input protein. A representative experiment is shown.
Activity of the dCtBP repressor protein from a distal enhancer position.
Our results suggest that in some contexts, binding of the dCtBP repressor protein to Knirps is not required for repression (Fig. 2 and 3). We considered the possibility that dCtBP may only allosterically alter the Knirps protein, allowing the Knirps protein's own repression domain to contact a target in the transcriptional machinery. In this case, dCtBP would not directly mediate transcriptional repression. This picture would contradict earlier studies in which tethering dCtBP (or a murine homologue, CtBP2) to a promoter was sufficient to inhibit gene expression (13, 36, 52). However, due to the design of the reporter genes used in these studies, they might be subject to passive repression caused by steric hindrance of adjacent activators or the basal transcription machinery. Therefore, we tested the activity of the Gal4-dCtBP protein on a reporter gene where the UAS binding sites were over 190 bp from the transcriptional start site and over 50 bp from the nearest Dorsal activator site (Fig. 4). The Gal4-dCtBP chimeric protein, expressed under the control of a Krüppel promoter in the central region of the embryo (36), inhibited the activity of the rho enhancer that contains Gal4-binding UAS sites. Consistent with expected short-range activity of this repressor, the activity of the eve stripe 3 enhancer was not affected (Fig. 4B). Gal4-dCtBP thus mediates short-range repression on a gene where the likelihood of passive blocking is minimized. Therefore, it is likely that dCtBP also directly mediates transcriptional repression when complexed with Knirps.
Gal4-Knirps repressors function comparably to endogenous Knirps on a rho lacZ reporter.
To test whether Gal4-Knirps proteins act similarly to endogenous Knirps, we assayed chimeric proteins on a rho element that had been previously used to measure Knirps activity. Gal4-binding sites were inserted into a rho lacZ reporter gene precisely where Knirps binding motifs had been introduced previously (2). In the absence of Gal4-Knirps, the lacZ reporter gene was expressed as expected in the entire ventral region of the embryo (Fig. 5A). Strong ventral repression was evident in embryos containing the Gal4-Knirps 75-429 protein expressed in ventral regions under the control of a twist regulatory element (Fig. 5B), indicating that Gal4-Knirps has the same activity as endogenous Knirps on the rho element. In ventrolateral regions where the Gal4-Knirps protein is not expressed, rho enhancer activity was apparent as a broad anterior-to-posterior band. In addition, a central circumferential stripe driven by the distal eve stripe 3 element was not repressed, demonstrating that the Gal4-Knirps protein is limited to functioning over a short range, as expected for this class of repressor. The lack of repression of the eve stripe 3 element is not due to inherent insensitivity of this enhancer to Knirps protein, because eve stripe 3 is a direct target of endogenous Knirps protein (47). The 75-332 protein containing the N-terminal repression region and the 202-358 protein with the dCtBP binding motif both mediated repression of the rho element in ventral regions (Fig. 5C and D). Thus, on a comparable lacZ target gene, Gal4-Knirps proteins have the same activity as endogenous Knirps protein.
FIG. 5.
Gal4-Knirps chimeric repressors mimic the activity of endogenous Knirps protein on a rho enhancer element. Previous studies demonstrated that Knirps represses this rho enhancer element when bound 50 bp from the Dorsal 1 and Dorsal 4 activator sites (2). In this reporter gene, UAS binding sites for Gal4-Knirps chimeras have been substituted for Knirps binding sites (22). (A) Expression pattern of the eve stripe 3-rho lacZ reporter gene, showing robust ventral expression directed from a rho enhancer element (lacking endogenous Snail binding sites) (13) and a central stripe from the eve stripe 3 enhancer. (B) Repression in ventral regions mediated by Gal4-Knirps (75-429) repressor protein expressed in ventral regions of the embryo under control of a twist promoter construct. (C) Repression mediated by the Gal4-Knirps (75-332) chimera, lacking the dCtBP interaction motif. (D) Repression mediated by the Gal4-Knirps (202-358) chimera, a protein that contains the dCtBP interaction motif. Ventrolateral views are shown, with anterior at the left.
Repression by Knirps proteins in dCtBP mutant embryos.
We tested whether the N-terminal region of the Knirps protein, which does not directly interact with dCtBP protein (Fig. 3), would mediate transcriptional repression in embryos lacking maternal dCtBP. Such embryos have been shown to be defective for repression by other dCtBP-binding repressor proteins, such as Snail (36), and the embryos show patterning defects reflective of disruption in pair-rule gene expression (40). The CtBP gene is expressed during oogenesis, and the message is deposited in the egg prior to fertilization (40). Therefore, we generated embryos that lacked a maternal contribution of dCtBP by the dominant female sterile FLP-DFS method (39). An eve stripe 2 lacZ reporter (Fig. 6A) was crossed into the dCtBP background, and somatic recombination was induced in females heterozygous for ovoD1 and the CtBP mutant allele to generate oocytes lacking dCtBP. Females were mated to males carrying Gal4-Knirps transgenes, and embryos were analyzed by in situ hybridization. The pattern of expression of the eve stripe 2 transgene is noticeably altered in such a genetic background (Fig. 6B), changing from the normal narrow stripe to a broader band, consistent with the loss of Krüppel repression activity in posterior regions (36) and possibly loss of Giant activity in anterior regions (B. Strunk, unpublished observations). The Gal4-Knirps 75-332 transgene was able to repress reporter gene expression in a significant number of embryos (Fig. 6C), while very few control embryos showed loss of ventral expression. Repression of the transgene in the dCtBP mutant background was also observed when we assayed Gal4-Knirps 75-364mut and 75-429mut proteins that lack the dCtBP interaction motif (Fig. 6D and E). Overall levels of repression were higher than those observed in wild-type embryos, possibly due to changes in Gal4-Knirps protein stability or transcription complex stability.
FIG. 6.
Knirps N-terminal repression activity functions in dCtBP mutant embryos. (A) eve stripe 2 lacZ reporter in a wild-type embryo. (B) eve stripe 2 lacZ reporter in a mutant embryo lacking maternal dCtBP. Anterior and posterior boundaries of the stripe are less well defined. (C) Repression by Gal4-Knirps 75-332 in a dCtBP mutant. Ventral expression of the transgene is repressed. (D) Repression by Gal4-Knirps 75-364mut, lacking the dCtBP binding motif. (E) Repression by Gal4-Knirps 75-429mut, lacking the dCtBP binding motif. Embryos are oriented with anterior being to the left and dorsal being to the top. Lateral views are shown, except for the ventrolateral view in panels C and E. CtBP embryos were generally shorter and broader than wild-type embryos. In the absence of repressor, 4% of embryos showed loss of ventral expression of the eve stripe 2 lacZ stripe in the mutant embryos (versus less than 1% in wild-type embryos [Table 1]). For the repressor shown in panel C, 31% of embryos showed loss of ventral staining (n = 140); for panels D and E, 31% (n = 39) and 75% (n = 65) of embryos, respectively, showed loss of ventral staining.
The endogenous Knirps target eve stripe 3 is repressed in a dCtBP mutant.
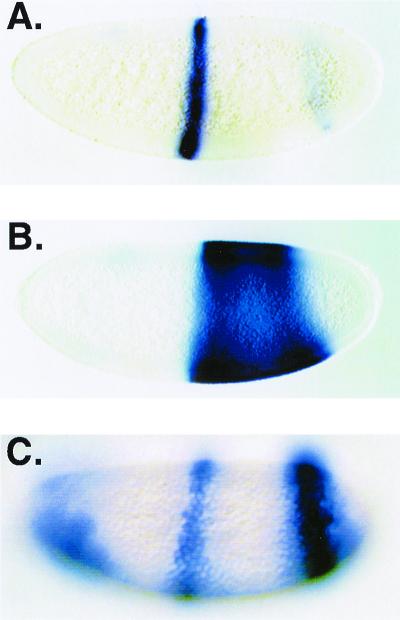
To test whether endogenous targets of the knirps gene might also show repression in a dCtBP mutant embryo, we examined expression of a lacZ reporter gene derived from eve, which is a direct target of Knirps. Five binding sites for the Knirps protein have been identified within the 500-bp eve stripe 3 enhancer (47). Consistent with this picture, a lacZ transgene driven by the eve stripe 3 enhancer shows broad posterior derepression in a kni mutant embryo (Fig. 7A and B) (47). To test whether a similar pattern of derepression would be observed in the absence of dCtBP, we crossed males carrying a 500-bp eve stripe 3 lacZ transgene (47) to females producing dCtBP-deficient oocytes. Expression of the eve stripe 3 transgene was not derepressed in posterior regions of the embryo, suggesting that Knirps is still able to repress this element in the absence of maternal dCtBP (Fig. 7C). The mutant embryos did show other alterations in lacZ expression, including ectopic expression in anterior regions and a broadening and intensifying of the posterior stripe (Fig. 7C). A similar pattern of repression was observed with a stripe 3 lacZ reporter carrying an 800-bp enhancer (−3.8 to −3 kbp) (data not shown). The stronger derepression phenotype of the kni mutant compared to the CtBP mutant suggests that Knirps contains a repression activity separate from dCtBP, consistent with our identification of an additional N-terminal repression region.
FIG. 7.
eve stripe 3 lacZ reporter gene is repressed normally in posterior regions in a CtBP mutant embryo. (A) Expression pattern of a 500-bp eve stripe 3 reporter gene in a wild-type embryo. (B) Posterior derepression of the reporter gene in a knirps mutant embryo. (C) Expression pattern of eve stripe 3 lacZ in a CtBP mutant embryo. Embryos did not show derepression in the posterior region of the embryo but did show consistently stronger staining in eve stripe 7 regions (an activity partially contained within eve stripe 3 enhancer sequence [47]) and ectopic activation in the anterior regions of the embryo. (A and B) Parasagittal view; (C) surface view. Embryos are oriented with anterior at the left and dorsal at the top.
DISCUSSION
Fine-tuning Knirps repression on promoters.
Knirps has been shown to repress transcription when bound adjacent to either basal promoters or activators within enhancer elements (2). Our studies of Knirps activity when the protein binds close to the basal promoter reveals additional properties of the endogenous protein. First, repression by Knirps does not appear to be sensitive to phasing effects, as shown by equivalent activity of constructs with Knirps binding sites offset by 5 bp at −70 and −75 bp (Fig. 1B and C). Second, in this series of genes, the transcriptional repression activity appears to be directed at the basal promoter element, because the repression weakens as the distance from Knirps sites to the basal promoter is increased while the distance to the enhancer element is held constant. Third, while Knirps repression is limited to a relatively short distance, there is a measurable interval (from 100 to 130 bp) over which Knirps activity is attenuated but not entirely abolished.
This intermediate level of repression might be useful in adjusting the amount of repression imposed on a target gene or setting a target gene threshold, as we have recently demonstrated for the Drosophila Giant short-range repressor (22). With Giant, a less-than-twofold difference in posterior versus anterior protein levels is sufficient to switch a gene from on to off. Thus, two features of short-range repressors may allow for flexibility in genetic regulatory circuits: first, short-range repressors allow modular enhancers to act independently, by avoiding regulatory cross talk (16), and second, the exquisite distance dependence may contribute to the differential response of endogenous target genes to repressor gradients (22, 28).
Two distinct repression activities of Knirps.
Our study demonstrates that the Knirps protein contains two functionally distinct repression activities. The C-terminal region appears to mediate repression through recruitment of the dCtBP protein, and based on our and others' results (Fig. 2) (36, 37) it consists of a region contained within residues 202 to 358 (minimally, residues 248 to 291 and 313 to 358 [Fig. 2, construct 14]) including the PMDLSMK dCtBP binding motif. In contrast, the N-terminal repression region (minimally, residues 139 to 330) appears to function independently of dCtBP. Although this region contains some of the amino acid residues that are present in the dCtBP binding constructs, the two activities are clearly distinct based on dCtBP dependence. The N-terminal region does not bind to dCtBP and it can repress in a mutant embryo that lacks maternal dCtBP (Fig. 3 and 6). Any residual amounts of dCtBP from maternal or zygotic expression are likely to be very low, because the loss of maternal dCtBP expression causes a loss of activity of Snail, Knirps, and Krüppel on a number of target genes (36), producing severe embryonic defects and early developmental arrest.
We have defined Knirps repression domains in the context of Gal4 fusion proteins, but several lines of evidence suggest that the native Knirps protein can also repress target genes independently of dCtBP. Most compellingly, an eve stripe 3 lacZ reporter gene that is derepressed in a knirps mutant background is not derepressed in a CtBP mutant (Fig. 7). In addition, a frameshift mutation (kni14F) that produces a protein lacking the dCtBP interaction motif retains partial activity (14), perhaps via the N-terminal repression activity that we have defined. Finally, an earlier study of ectopically expressed Knirps protein that lacks a dCtBP binding motif noted that the protein had weak repression on eve stripe 3 (36).
A region of the Knirps protein containing an alanine-rich tract was identified earlier as a repression domain in cell culture studies (14) but was neither necessary nor sufficient for repression in the embryo (Fig. 2, construct 7; Table 1). The repression function of 189-254 protein may be specific to transfection assays, similar to findings for the non-Groucho binding region of the Engrailed repressor protein (50).
It is not yet clear whether repression by the N-terminal and C-terminal regions of Knirps contribute to quantitative or qualitative differences in repression, or if these two aspects of repression are indeed entirely separable. The eve stripe 3 enhancer is clearly repressed in the region of kni expression in the absence of maternal dCtBP (Fig. 7), yet in previous experiments, ectopically expressed Knirps was able to repress the eve stripe 3 element effectively only when the dCtBP binding motif of the protein was still intact (36). The most likely explanation for these apparently contradictory results is that dCtBP contributes to a portion of the Knirps-mediated repression of stripe 3. Endogenous Knirps is abundant enough to repress expression of eve stripe 3 in dCtBP mutant embryos, but the levels of ectopically produced Knirps protein are apparently insufficient to repress effectively when binding to dCtBP is abolished. dCtBP may also have an effect on Knirps protein stability or targeting, which might contribute to the reduced activity of the mutant protein. Previous studies indicated that in the absence of dCtBP, repression of a synthetic rho lacZ reporter gene by endogenous Knirps was reduced (36). However, close examination of the data indicates that some anterior repression is apparently present (Fig. 3 in reference 36), consistent with the idea that Knirps retains a measurable level of activity in the dCtBP mutant.
Autoinhibition models and activity of C-terminally truncated Knirps proteins.
The Gal4-Knirps chimeras containing only the N-terminal repression domain appear to have higher levels of activity on lacZ reporters than does full-length Knirps protein lacking the dCtBP binding motif (Fig. 2) (36). We tested whether this difference might be attributed to masking of the N-terminal repression region by the C terminus in the absence of dCtBP. Our data indicate that this model is not correct; Gal4-Knirps chimeras containing the N-terminal repression domain linked to a C-terminal region lacking a dCtBP binding activity were highly effective repressors (Fig. 2, constructs 21 to 23). Gal4-Knirps chimeras may be inherently more effective repressors if one role of dCtBP is to facilitate dimerization of Knirps proteins. With chimeras, this function would be provided by the Gal4 DNA binding domain, because Gal4 binds DNA as a dimer (4). Alternatively, autoinhibition of the Knirps DNA binding domain, similar to that seen with Ets-1, AML-1, and Pitx2 (1, 15, 20, 26, 31), may be relieved by dCtBP binding, but Gal4 chimeras would not be subject to such regulation. However, the effective regulation of eve stripe 3 lacZ in a CtBP mutant argues for a simpler quantitative effect model. Loss of dCtBP binding might simply reduce the total repression activity of Knirps protein, so that the low levels of misexpressed Knirps would be unable to effect repression. The Gal4-Knirps repressor utilizing only one repression region might be more functional due to increased effectiveness of dimerized repressor proteins or to a greater sensitivity of the lacZ reporters used.
Qualitative and quantitative effects: repressors with multiple repression activities.
Multiple repression activities in a protein may allow for qualitative or quantitative effects on gene expression. Qualitatively, a repressor may operate selectively in distinct tissue types or on different promoters. Loss of maternal dCtBP protein does not affect eve stripe 3 regulation (Fig. 6), but it does abolish repression of the eve stripe 4+6 enhancer element, suggesting that this element is dCtBP dependent (M. Corado, E. Bajor, M. Fujioka, and S. Small, Abstr. 41st Annu. Drosophila Res. Conf., abstr. 285B, 2000). Quantitatively, dual activities may increase the overall level of repression, much as transcriptional activators have been suggested to employ multiple paths to achieve synergistic activation (8, 51).
Examples of both qualitative and quantitative effects are seen with the ZEB repressor, a protein that contains two repression domains. One domain blocks activation by Myb and Ets factors of lymphocyte-specific promoters (41), while the second domain, which contains a conserved CtBP binding motif (52), blocks the activity of the muscle cell-specific MEF2C factor. In contrast to these activator-specific effects, a quantitative contribution of multiple repression domains was observed with the murine ZEB homolog δEF-1. When CtBP binding residues are mutated in δEF-1, repression of a MyoD-activated promoter is impaired but not abolished (13).
Other repressor proteins may also possess both CtBP-dependent and dCtBP-independent activities. In Drosophila, the Krüppel protein contains a C-terminal dCtBP binding repression domain and an N-terminal repression domain. The latter domain has only been characterized in cell culture assays (21), but genetic evidence indicates that Krüppel can repress hairy in a CtBP mutant, possibly by means of this N-terminal domain (30). The Wnt signalling pathway transcription factor Tcf-3 can interact with both the Groucho and CtBP proteins through separate repression domains in Xenopus laevis, and the CtBP-binding portion of XTcf-3 has potent repression activity in the frog embryo (6). The Rb retinoblastoma protein has been shown to interact with both histone deacetylases and CtBP, although the physiological relevance of the CtBP interactions is not yet clear (34). Net, an Ets protein family member that can repress transcription of the c-fos promoter, has also been shown to possess two independent repression domains, one of which interacts with CtBP1. Loss of the CtBP binding motif from Net reduces the repression activity of the protein in cell culture assays (12). Finally, the BKLF transcription factor, which can interact with CtBP2 to repress transcription in Drosophila cell culture, contains an additional CtBP-independent activity detectable in NIH 3T3 cells (52).
Mechanism of dCtBP protein in transcriptional repression.
dCtBP and its homologs appear to be able to mediate repression directly when recruited to promoters by a heterologous DNA binding domain, both in cell culture systems and in the embryo (Fig. 4) (13, 36, 37). The dCtBP corepressor has homology to α-hydroxy acid dehydrogenases and contains a conserved NAD-binding domain. The protein binds to NAD (R. Jacobson, personal communication), but no dehydrogenase activity has been detected in vitro, and mutation of a conserved histidine in the putative active site did not compromise the repression activity of a chimeric CtBP2 protein in cell culture assays (52). The dCtBP protein may contain other uncharacterized enzymatic activities. Recently it was reported that the Sir2 transcriptional repressor possesses ADP ribosylation activity, and furthermore, that NAD was important for histone deacetylase activity of the protein (23, 49). Some evidence suggests that CtBP may function through histone deacetylase pathways (12, 48), but pair-rule gene repression by gap proteins such as Knirps and Krüppel was not compromised by mutations in the Rpd3 histone deacetylase (33).
The physiological relevance of CtBP binding is not yet known for a number of proteins that were found to interact in yeast two-hybrid assays, but genetic evidence from Drosophila clearly indicates that dCtBP is an important repression cofactor (36, 37, 40). Our data demonstrates that for at least one Knirps target gene, another pathway of repression is also utilized. A considerable body of evidence, including genetic and biochemical data, indicates that repressors may have multiple lines of communication with the transcriptional machinery, just as transcriptional activators have been found to contain multiple activation domains that act on multiple targets (51). Further genetic and biochemical characterization of Knirps will help elucidate the pathways utilized by this short-range repressor.
ACKNOWLEDGMENTS
S. Keller and Y. Mao contributed equally to this work.
G. Attardo generated one of the transgenic lines assayed (Fig. 2). We acknowledge D. Pellek, D. Kalweo, and E. Barnafo for providing technical assistance and Z. Burton, R. W. Henry, L. Kroos, L. Kaguni, S. Small, and S. Triezenberg for helpful comments. We thank Y. Nibu and M. Levine for GST fusion constructs and Gal4-dCtBP fly lines.
C.E.Y., A.R.A., S.B., and R.L.A. were supported by NSF undergraduate research fellowships. This work was supported by an AURIG grant from Michigan State University and grant GM56976 from the National Institutes of Health to D.N.A.
REFERENCES
- 1.Amendt B A, Sutherland L B, Russo A F. Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Mol Cell Biol. 1999;19:7001–7010. doi: 10.1128/mcb.19.10.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnosti D N, Gray S, Barolo S, Zhou J, Levine M. The gap protein knirps mediates both quenching and direct repression in the Drosophila embryo. EMBO J. 1996;15:3659–3666. [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson B D, Fisher A L, Blechman K, Caudy M, Gergen J P. Groucho-dependent and -independent repression activities of Runt domain proteins. Mol Cell Biol. 1997;17:5581–5587. doi: 10.1128/mcb.17.9.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baleja J D, Marmorstein R, Harrison S C, Wagner G. Solution structure of the DNA-binding domain of Cd2-GAL4 from S. cerevisiae. Nature. 1992;356:450–453. doi: 10.1038/356450a0. [DOI] [PubMed] [Google Scholar]
- 5.Barolo S, Levine M. hairy mediates dominant repression in the Drosophila embryo. EMBO J. 1997;16:2883–2891. doi: 10.1093/emboj/16.10.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brannon M, Brown J D, Bates R, Kimelman D, Moon R T. XCtBP is a XTcf-3 co-repressor with roles throughout Xenopus development. Development. 1999;126:3159–3170. doi: 10.1242/dev.126.14.3159. [DOI] [PubMed] [Google Scholar]
- 7.Cai H N, Arnosti D N, Levine M. Long-range repression in the Drosophila embryo. Proc Natl Acad Sci USA. 1996;93:9309–9314. doi: 10.1073/pnas.93.18.9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey M, Lin Y S, Green M R, Ptashne M. A mechanism for synergistic activation of a mammalian gene by GAL4 derivatives. Nature. 1990;345:361–364. doi: 10.1038/345361a0. [DOI] [PubMed] [Google Scholar]
- 9.Cavallo R A, Cox R T, Moline M M, Roose J, Polevoy G A, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 10.Chen C K, Kuhnlein R P, Eulenberg K G, Vincent S, Affolter M, Schuh R. The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development. 1998;125:4959–4968. doi: 10.1242/dev.125.24.4959. [DOI] [PubMed] [Google Scholar]
- 11.Chou T B, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criqui-Filipe P, Ducret C, Maira S M, Wasylyk B. Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J. 1999;18:3392–3403. doi: 10.1093/emboj/18.12.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furusawa T, Moribe H, Kondoh H, Higashi Y. Identification of CtBP1 and CtBP2 as corepressors of zinc finger-homeodomain factor δEF1. Mol Cell Biol. 1999;19:8581–8590. doi: 10.1128/mcb.19.12.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerwin N, La Rosee A, Sauer F, Halbritter H P, Neumann M, Jäckle H, Nauber U. Functional and conserved domains of the Drosophila transcription factor encoded by the segmentation gene knirps. Mol Cell Biol. 1994;14:7899–7908. doi: 10.1128/mcb.14.12.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz T L, Gu T L, Speck N A, Graves B J. Auto-inhibition of Ets-1 is counteracted by DNA binding cooperativity with core-binding factor alpha2. Mol Cell Biol. 2000;20:81–90. doi: 10.1128/mcb.20.1.81-90.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray S, Szymanski P, Levine M. Short-range repression permits multiple enhancers to function autonomously within a complex promoter. Genes Dev. 1994;8:1829–1838. doi: 10.1101/gad.8.15.1829. [DOI] [PubMed] [Google Scholar]
- 17.Gray S, Cai H, Barolo S, Levine M. Transcriptional repression in the Drosophila embryo. Philos Trans R Soc Lond B Biol Sci. 1995;349:257–262. doi: 10.1098/rstb.1995.0111. [DOI] [PubMed] [Google Scholar]
- 18.Gray S, Levine M. Short-range transcriptional repressors mediate both quenching and direct repression within complex loci in Drosophila. Genes Dev. 1996;10:700–710. doi: 10.1101/gad.10.6.700. [DOI] [PubMed] [Google Scholar]
- 19.Gray S, Levine M. Transcriptional repression in development. Curr Opin Cell Biol. 1996;8:358–364. doi: 10.1016/s0955-0674(96)80010-x. [DOI] [PubMed] [Google Scholar]
- 20.Gu T L, Goetz T L, Graves B J, Speck N A. Auto-inhibition and partner proteins, core-binding factor beta (CBFbeta) and Ets-1, modulate DNA binding by CBFalpha2 (AML1) Mol Cell Biol. 2000;20:91–103. doi: 10.1128/mcb.20.1.91-103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna-Rose W, Licht J D, Hansen U. Two evolutionarily conserved repression domains in the Drosophila Krüppel protein differ in activator specificity. Mol Cell Biol. 1997;17:4820–4829. doi: 10.1128/mcb.17.8.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewitt G F, Strunk B S, Margulies C, Priputin T, Wang X D, Amey R, Pabst B A, Kosman D, Reinitz J, Arnosti D N. Transcriptional repression by the Drosophila Giant protein: cis element positioning provides an alternative means of interpreting an effector gradient. Development. 1999;126:1201–1210. doi: 10.1242/dev.126.6.1201. [DOI] [PubMed] [Google Scholar]
- 23.Imai S-I, Armstrong C M, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 24.Jaynes J B, O'Farrell P H. Active repression of transcription by the engrailed homeodomain protein. EMBO J. 1991;10:1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez G, Paroush Z, Ish-Horowicz D. Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed. Genes Dev. 1997;11:3072–3082. doi: 10.1101/gad.11.22.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsen M D, Petersen J M, Xu Q P, Graves B J. Characterization of the cooperative function of inhibitory sequences in Ets-1. Mol Cell Biol. 1996;16:2065–2073. doi: 10.1128/mcb.16.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirov N, Childs S, O'Connor M, Rushlow C. The Drosophila dorsal morphogen represses the tolloid gene by interacting with a silencer element. Mol Cell Biol. 1994;14:713–722. doi: 10.1128/mcb.14.1.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosman D, Small S. Concentration-dependent patterning by an ectopic expression domain of the Drosophila gap gene knirps. Development. 1997;124:1343–1354. doi: 10.1242/dev.124.7.1343. [DOI] [PubMed] [Google Scholar]
- 29.Langeland J A, Carroll S B. Conservation of regulatory elements controlling hairy pair-rule stripe formation. Development. 1993;117:585–596. doi: 10.1242/dev.117.2.585. [DOI] [PubMed] [Google Scholar]
- 30.La Rosée-Borggreve A, Häder T, Wainwright D, Sauer F, Jäckle H. hairy stripe 7 element mediates activation and repression in response to different domains and levels of Krüppel in the Drosophila embryo. Mech Dev. 1999;89:133–140. doi: 10.1016/s0925-4773(99)00219-1. [DOI] [PubMed] [Google Scholar]
- 31.Lim F, Kraut N, Framptom J, Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992;11:643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lunde K, Biehs B, Nauber U, Bier E. The knirps and knirps-related genes organize development of the second wing vein in Drosophila. Development. 1998;125:4145–4154. doi: 10.1242/dev.125.21.4145. [DOI] [PubMed] [Google Scholar]
- 33.Mannervik M, Levine M. The Rpd3 histone deacetylase is required for segmentation of the Drosophila embryo. Proc Natl Acad Sci USA. 1999;96:6797–6801. doi: 10.1073/pnas.96.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meloni A R, Smith E J, Nevins J R. A mechanism for Rb/p130-mediated transcription repression involving recruitment of the CtBP corepressor. Proc Natl Acad Sci USA. 1999;96:9574–9579. doi: 10.1073/pnas.96.17.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauber U, Pankratz M J, Kienlin A, Seifert E, Klemm U, Jäckle H. Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps. Nature. 1988;336:489–492. doi: 10.1038/336489a0. [DOI] [PubMed] [Google Scholar]
- 36.Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. dCtBP mediates transcriptional repression by Knirps, Krüppel and Snail in the Drosophila embryo. EMBO J. 1998;17:7009–7020. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nibu Y, Zhang H, Levine M. Interaction of short-range repressors with Drosophila CtBP in the embryo. Science. 1998;280:101–104. doi: 10.1126/science.280.5360.101. [DOI] [PubMed] [Google Scholar]
- 38.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 39.Perrimon N, Lanjuin A, Arnold C, Noll E. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics. 1996;144:1681–1692. doi: 10.1093/genetics/144.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poortinga G, Watanabe M, Parkhurst S M. Drosophila CtBP: a Hairy-interacting protein required for embryonic segmentation and Hairy-mediated transcriptional repression. EMBO J. 1998;17:2067–2078. doi: 10.1093/emboj/17.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postigo A A, Dean D C. Independent repressor domains in ZEB regulate muscle and T-cell differentiation. Mol Cell Biol. 1999;19:7961–7971. doi: 10.1128/mcb.19.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera-Pomar R, Jäckle H. From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends Genet. 1996;12:478–483. doi: 10.1016/0168-9525(96)10044-5. [DOI] [PubMed] [Google Scholar]
- 43.Schaeper U, Boyd J M, Verma S, Uhlmann E, Subramanian T, Chinnadurai G. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc Natl Acad Sci USA. 1995;92:10467–10471. doi: 10.1073/pnas.92.23.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sewalt R G, Gunster M J, van der Vlag J, Satijn D P, Otte A P. C-terminal binding protein is a transcriptional repressor that interacts with a specific class of vertebrate Polycomb proteins. Mol Cell Biol. 1999;19:777–787. doi: 10.1128/mcb.19.1.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small S. Mechanisms of segmental pattern formation in Drosophila melanogaster. In: Adiyodi K G, Adiyodi R G, editors. Reproductive biology of invertebrates. VII. New York, N.Y: John Wiley and Sons; 1997. pp. 137–178. [Google Scholar]
- 46.Small S, Blair A, Levine M. Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J. 1992;11:4047–4057. doi: 10.1002/j.1460-2075.1992.tb05498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Small S, Blair A, Levine M. Regulation of two pair-rule stripes by a single enhancer in the Drosophila embryo. Dev Biol. 1996;175:314–324. doi: 10.1006/dbio.1996.0117. [DOI] [PubMed] [Google Scholar]
- 48.Sundqvist A, Sollerbrant K, Svensson C. The carboxy-terminal region of adenovirus E1A activates transcription through targeting of a C-terminal binding protein-histone deacetylase complex. FEBS Lett. 1998;429:183–188. doi: 10.1016/s0014-5793(98)00588-2. [DOI] [PubMed] [Google Scholar]
- 49.Tanny J C, Dowd G J, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- 50.Tolkunova E N, Fujioka M, Kobayashi M, Deka D, Jaynes J B. Two distinct types of repression domain in engrailed: one interacts with the groucho corepressor and is preferentially active on integrated target genes. Mol Cell Biol. 1998;18:2804–2814. doi: 10.1128/mcb.18.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triezenberg S J. Structure and function of transcriptional activation domains. Curr Opin Genet Dev. 1995;5:190–196. doi: 10.1016/0959-437x(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 52.Turner J, Crossley M. Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 1998;17:5129–5140. doi: 10.1093/emboj/17.17.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wharton K A, Jr, Crews S T. CNS midline enhancers of the Drosophila slit and Toll genes. Mech Dev. 1993;40:141–154. doi: 10.1016/0925-4773(93)90072-6. [DOI] [PubMed] [Google Scholar]
- 54.White R A, Lehmann R. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell. 1986;47:311–321. doi: 10.1016/0092-8674(86)90453-8. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Levine M. Groucho and dCtBP mediate separate pathways of transcriptional repression in the Drosophila embryo. Proc Natl Acad Sci USA. 1999;96:535–540. doi: 10.1073/pnas.96.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]