Abstract
Purpose
Lutetium-177 prostate-specific membrane antigen-617 (177Lu-PSMA-617) in end-stage metastatic castration-resistant prostate cancer (mCRPC) has reported favourable outcomes. In this study, we aimed to prospectively compare the efficacy and safety of 177Lu-PSMA-617 and docetaxel in chemotherapy-naïve mCRPC patients.
Methods
This was a randomized, parallel-group, open-label, phase 2, and non-inferiority trial. Chemotherapy-naïve patients with mCRPC and high PSMA-expressing lesions on 68 Ga-PSMA-11 PET/CT were randomly assigned in 1:1 ratio to 177Lu-PSMA-617 (6.0–7.4 GBq/cycle, every 8 weeks, up to 4 cycles) or docetaxel (75 mg/m2/cycle, every 3 weeks, up to 10 cycles). The primary end-point was best prostate-specific antigen response rate (PSA-RR), defined according to Prostate Cancer Clinical Trials Working Group-3 as proportion of patients achieving ≥ 50% decline in PSA from baseline. Non-inferiority margin of − 15% was pre-specified for PSA-RR.
Results
Between December 2019 and March 2021, 40 of the 45 patients assessed for eligibility underwent randomization. Fifteen of 20 patients in 177Lu-PSMA-617 arm and 20/20 patients in docetaxel arm received treatment per protocol. Of these, best PSA-RR in the 177Lu-PSMA-617 arm was 60% (9/15) versus 40% (8/20) in the docetaxel arm. The difference in the PSA-RRs between the two arms was 20% (95% confidence interval, CI: − 12–47, P = 0.25), meeting the pre-specified criterion for non-inferiority in per-protocol analysis. Further, progression-free survival rates at 6 months were 30% and 20% in the 177Lu-PSMA-617 and docetaxel arms respectively (difference 10%, 95% CI: − 18–38, P = 0.50). Overall, treatment-emergent grade ≥ 3 adverse events occurred less frequently with 177Lu-PSMA-617 than with docetaxel (6/20, 30% versus 10/20, 50%, respectively, P = 0.20). Quality-of-life outcomes improved significantly in 177Lu-PSMA-617 arm compared to docetaxel arm (P < 0.01).
Conclusion
177Lu-PSMA-617 was demonstrated to be safe and non-inferior to docetaxel in the treatment of mCRPC and could, thus, be potentially employed earlier in the disease course rather than being solely reserved for advanced end-stage disease.
Clinical trial registration
Clinical Trials Registry-India, CTRI/2019/12/022282.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-021-05618-3.
Keywords: Castration-resistant prostate cancer, Prostate-specific membrane antigen, 177Lu-PSMA-617, Docetaxel, Randomized controlled trial, Non-inferiority
Introduction
Metastatic prostate cancer (PCa) arising in the hormone-refractory setting remains a therapeutic challenge [1, 2]. So far, only few drugs have shown a survival advantage in metastatic castration-resistant PCa (mCRPC). Docetaxel has most commonly been the first-line treatment for such patients, after two landmark trials, viz. the TAX 327 study and the SWOG 9916 study, demonstrated significantly longer overall survival (OS) with docetaxel compared to their respective control arms [3, 4]. Subsequent successful trials led to the approval of other drugs, viz. cabazitaxel, abiraterone, enzalutamide, radium-223, sipuleucel-T, and olaparib in this setting. While the novel androgen-axis drugs (NAADs), abiraterone and enzalutamide, have shown survival benefit in both taxane-naïve and taxane-treated patients, the other drugs have usually been reserved for more progressive disease [5, 6]. Despite these encouraging results, the relatively high frequency of treatment administration with the chemotherapeutic agents, non-compliance to oral NAADs, the multitude of associated adverse events, resultant deterioration in quality-of-life (QOL), and healthcare-associated costs present potential challenges in the treatment of mCRPC. Thus, there is a need for alternative efficacious and safe therapeutic options that can also improve the QOL for such patients.
Prostate-specific membrane antigen (PSMA) is a 750-amino acid type II transmembrane glycoprotein that is overexpressed in > 90% of the prostate cancer cells, with the expression further increasing dependent on the Gleason score and in CRPC [7]. Gallium (68 Ga)-PSMA-11 positron emission tomography/computed tomography (PET/CT) has been shown to be useful in evaluating the disease burden in patients with mCRPC [8]. Its therapeutic analogue, lutetium-177-PSMA-617 (177Lu-PSMA-617), has been proven to augment survival, when added to standard care, in patients with mCRPC who are positive on 68 Ga-PSMA-11 PET/CT [9]. 177Lu, being a medium-energy beta emitter (maximum energy 497 keV) with a beta-particle range of < 2 mm and a physical half-life of 6.7 days, allows for the delivery of high activities of 177Lu-PSMA-617 to PSMA-expressing prostate cancer cells while minimizing the damage to surrounding normal tissues [10].
Few cohort studies and phase 2/3 trials, using 177Lu-PSMA-radioligand therapy (RLT) in end-stage, taxane-progressive mCRPC, have reported favourable outccomes [9, 11–16]. A recent meta-analysis reported a pooled biochemical response rate of 46% with 177Lu-PSMA-RLT and an excellent safety profile. Grade 3/4 toxicities were uncommon with only 1% of the patients having grade 3/4 nausea and fatigue, only 2% of the patients having xerostomia, and 8% of the patients having anaemia [17]. Given these remarkable results, 177Lu-PSMA-RLT seems to be a promising alternative to existing therapeutic options in mCRPC. The lesser toxicity added to the lesser number and frequency of treatment cycles in RLT can potentially lead to better patient compliance, improved QOL, and lesser financial burden. Hence, there exists a need for prospective studies with head-to-head comparison of 177Lu-PSMA-RLT and other active interventions in the setting of mCRPC. In this study, we aimed to prospectively enrol chemotherapy-naive mCRPC patients to compare the efficacy and safety of 177Lu-PSMA-617 and docetaxel.
Materials and methods
Study design and patient population
This was an investigator-initiated, randomized, parallel group, open-label, and phase 2 non-inferiority trial conducted at a tertiary care institution. Between December 2019 and March 2021, patients with biopsy-proven adenocarcinoma prostate and castration-resistant disease were recruited. Patients were considered eligible if they had metastatic disease on 68 Ga-PSMA-11 PET/CT with significant PSMA expression. Significant PSMA expression was defined as tracer avidity of at least 80% of the lesions being significantly (≥ 1.5 times) greater than that of normal liver with none of the lesions having uptake less than that of liver. Only chemotherapy-naïve patients were considered for inclusion in this trial; however, patients with prior treatment of NAADs were also included. The patients were required to have Eastern Cooperative Oncology Group (ECOG) performance score ≤ 2, and adequate haematological, renal, and liver function reserve (Supplementary Table 1). Patients with histological evidence of sarcomatous, spindle-cell or small-cell differentiation, and Sjogren syndrome were excluded. Informed written consent was obtained from the patients prior to inclusion in the study. The study was approved by the Institute Ethics Committee (INT/IEC/2019/001972) and was conducted in accordance with the guidelines enshrined in the Declaration of Helsinki. The trial was also prospectively registered at the Clinical Trials Registry-India (CTRI/2019/12/022282).
Randomization and masking
The eligible patients were randomly assigned in 1:1 ratio to one of the two treatment arms: experimental arm-177Lu-PSMA-617 or control arm-docetaxel. Random allocation of participants into either arm was accomplished using a computer-generated block randomization sequence. However, the allocation of interventions was not blinded to the patients and to the staff involved in the subject recruitment, administration of intervention, and data analysis.
Intervention procedures
Patients in the experimental arm were administered up to four cycles of 177Lu-PSMA-617 intravenously at eight weekly intervals. Approximately 6.0–7.4 GBq of 177Lu-PSMA-617 was administered per cycle depending on the patient weight, disease burden, renal, and hematological parameters. Adequate hydration (1.5–2 L of oral fluids on the day of administration) and premedication for anti-emesis (intravenous ondansetron and dexamethasone) were ensured. Post-therapy whole-body scans were acquired after 24 h to look for the distribution of 177Lu-PSMA-617 in the lesions and normal tissues.
Patients in the control arm were administered docetaxel, 75 mg/m2 intravenously once every 3 weeks, up to a maximum of 10 cycles, with prednisone 5 mg twice daily orally during the chemotherapy course, and prophylactic pegfilgrastim 6 mg subcutaneously on day two.
Patients received standard supportive care, e.g. blood transfusions, granulocyte colony-stimulating factor injections, bisphosphonates, or denosumab, as clinically indicated. Patients also continued to receive androgen deprivation therapy to maintain castrate levels of testosterone, unless prior orchiectomy was done. Patients who showed biochemical/radiological progression on follow-up or were unable to tolerate either treatment discontinued the study and were provided treatment with alternative approved therapeutic options as per guidelines.
Response outcomes
Patients were followed every 3 weeks with complete hemogram, liver and renal function tests, and serum prostate-specific antigen (PSA). The primary end-point was the best PSA response rate (PSA-RR), which was defined according to the Prostate Cancer Clinical Trials Working Group-3 (PCWG3) as the proportion of patients achieving a ≥ 50% decline in PSA from baseline [18]. Secondary end-points included best objective response rate (ORR), molecular response rate (MRR), progression-free survival (PFS), toxicity, and health-related QOL (HRQOL) outcomes. Radiological response was assessed on the CT images of 68 Ga-PSMA-11 PET/CT as per the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) [19]. An interim 68 Ga-PSMA-11 PET/CT was scheduled at 6 weeks after the 2nd cycle of 177Lu-PSMA-617 and 3 weeks after the 5th cycle of docetaxel. An end-of-treatment 68 Ga-PSMA-11 PET/CT was also performed after 6 weeks of the last treatment cycle. The proportions of patients achieving complete response (CR) and partial response (PR) on RECIST 1.1 were combined as the ORR. Molecular response was assessed on the 68 Ga-PSMA-11 PET/CT as per the adapted PET Response Criteria in Solid Tumors (PERCIST) 1.0, and the proportions of patients achieving CR and PR were combined as the MRR [20]. PFS was estimated from the start of the treatment regimen till documented biochemical progression or radiological progression, or death. Biochemical progression was defined as per the PCWG3 criteria and radiological progression was defined as per RECIST 1.1 [18, 19].
Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. HRQOL outcomes were assessed using the National Comprehensive Cancer Network—Functional Assessment of Cancer Therapy—Prostate Symptom Index—17 (NCCN-FACT-FPSI-17) questionnaire, version 2 (FACIT.org, Ponte Vedra, Florida, USA) at baseline and 12 weeks following the first treatment cycle. The questionnaire includes 17 items under four domains: disease-related symptoms-physical (FPSI-DRS-P), disease-related symptoms-emotional (FPSI-DRS-E), treatment side effects (FPSI-TSE), and function/well-being (FPSI-F/WB) [21]. Response for each question ranged from 0 to 4. A high score was considered to be good with a score of 0 indicating a highly symptomatic patient.
Statistical analysis
The sample size of 40 patients was calculated by assuming 80% power at two-sided alpha level of 5%, best PSA-RR of 65% with 177Lu-PSMA-617 and 35% with docetaxel, non-inferiority margin of − 15% for the absolute difference between the PSA-RRs and attrition rate of 10%. The primary analysis was by intention-to-treat (ITT) and included all patients randomized to either treatment arm. Per-protocol sensitivity analysis was also done by including only those patients who underwent at least half of the allocated treatment, i.e. received at least two cycles of 177Lu-PSMA-617, or at least five cycles of docetaxel. In the analysis of the primary end-point, non-inferiority would be demonstrated if the lower limit of the 95% confidence interval (CI) for the absolute difference between the PSA-RRs of 177Lu-PSMA-617 and docetaxel was not less than − 15%. This non-inferiority margin was arrived at through consensus after detailed inter-departmental discussions, which took into account the expected advantages with 177Lu-PSMA-617, viz. the lesser number and frequency of cycles, potentially better safety profile, and benefits in HRQOL outcomes.
The categorical variables were expressed as numbers and percentages. For inter-group comparison, chi-square test was used for testing significance of any difference. The quantitative variables were expressed as median and range. The Mann–Whitney U test was used for testing significance of difference between the two groups. Survival analysis was done using the Kaplan–Meier curve method and Cox proportional hazards model. The Tarone–Ware test was used to compare the median PFS durations between the groups. All statistical analyses were done using IBM SPSS Statistics for Windows, version 20.0. Armonk, NY; IBM Corp, and MedCalc® Statistical Software version 19.6.1 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020). Graphical plots were made using Microsoft Office 2018 and GraphPad Prism 9 for Windows, version 9.1.2 (226). A two-tailed P value < 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics
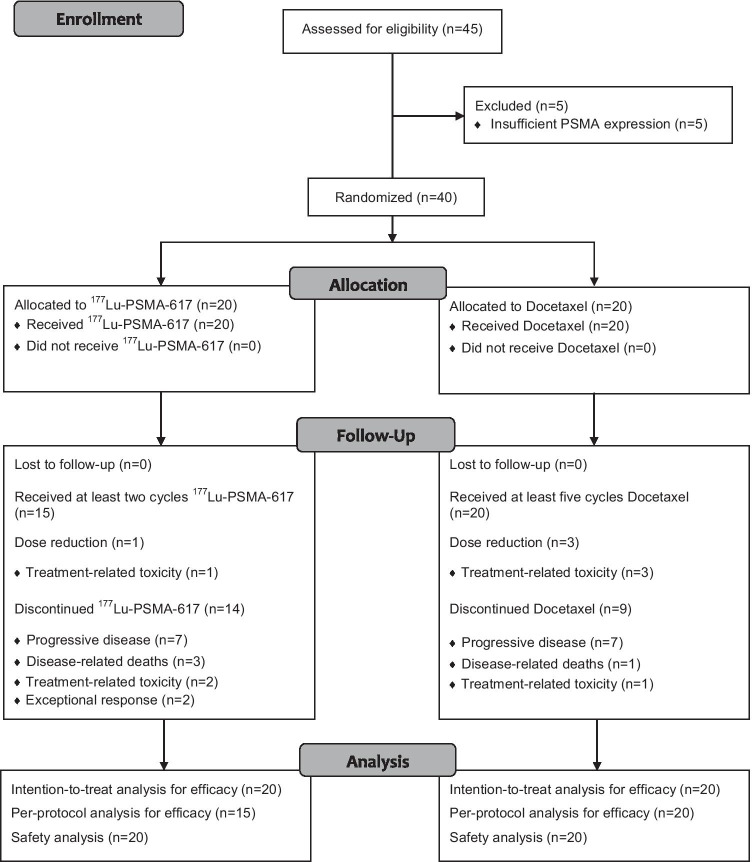
Of the 45 patients assessed for eligibility, 40 patients with chemotherapy-naïve mCRPC were included in this study cohort, comprising 20 patients in each arm (Fig. 1). The demographic and clinical characteristics of the patients were observed to be similar in both the arms (Table 1). Majority of the patients had high-grade prostate cancer with 14 (70%) patients in the 177Lu-PSMA-617 arm and 12 (60%) in the docetaxel arm having Gleason score ≥ 8. Prior treatment with NAADs was noted in 26/40 patients (overall 65%, comprising 14/20 patients in the 177Lu-PSMA-617 arm and 12/20 patients in the docetaxel arm). Hence, 6/20 patients (30%) in the experimental arm and 8/20 patients (40%) in the control arm received treatment with 177Lu-PSMA-617 and docetaxel, respectively, as the first-line modality for mCRPC.
Fig. 1.
CONSORT flow diagram describing the patient enrollment process and follow-up. PSMA, prostate-specific membrane antigen
Table.1.
Patients’ characteristics and treatment details
| Characteristic | 177Lu-PSMA-617 arm (n = 20) | Docetaxel arm (n = 20) |
|---|---|---|
| Age in years, median (range) | 68 (54–85) | 68 (50–84) |
| Time since diagnosis of prostate cancer in years, median (range) | 3 (2–7) | 2 (1–6) |
| Gleason score at diagnosis, No. (%) | ||
| • 6–7 | 6 (30) | 8 (40) |
| • 8 | 6 (30) | 4 (20) |
| • 9 | 7 (35) | 6 (30) |
| • 10 | 1 (5) | 2 (10) |
| ECOG performance status, No. (%) | ||
| • 0 | 8 (40) | 7 (35) |
| • 1 | 7 (35) | 7 (35) |
| • 2 | 5 (25) | 6 (30) |
| Prior treatments, No. (%) | ||
| • ADT | 20 (100) | 20 (100) |
| • Palliative radiotherapy | 5 (25) | 3 (15) |
| • Bisphosphonate and/or denosumab | 20 (100) | 20 (100) |
| • Novel anti-androgens (abiraterone and/or enzalutamide) | 14 (70) | 12 (60) |
| ○ Abiraterone only | 10 (50) | 12 (60) |
| ○ Enzalutamide only | 0 (0) | 0 (0) |
| ○ Both abiraterone and enzalutamide | 4 (20) | 0 (0) |
| Disease extent on baseline 68 Ga-PSMA PET/CT, No. (%) | ||
| • Local nodes | 15 (75) | 15 (75) |
| • Distant nodes | 8 (40) | 10 (50) |
| • Skeletal | 20 (100) | 20 (100) |
| ○ < 10 lesions | 3 (15) | 5 (25) |
| ○ 10–20 lesions | 3 (15) | 7 (35) |
| ○ > 20 lesions | 14 (70) | 8 (40) |
| • Visceral | 5 (25) | 4 (20) |
| ○ Liver | 2 (10) | 1 (5) |
| ○ Lung | 0 (0) | 2 (10) |
| ○ Adrenal | 2 (10) | 0 (0) |
| ○ Others | 1 (5) | 1 (5) |
| Pre-therapy PSA in ng/mL, median (range) | 100.9 (6.5–1230) | 37 (1.7–955) |
| ALP in IU/mL, median (range) | 185 (62–790) | 192 (71–942) |
| Number of cycles (range) | 1–4 | 5–10 |
ADT androgen deprivation therapy (medical or surgical), ECOG Eastern Cooperation of Oncology Group, PSA prostate-specific antigen
A total of forty-nine cycles of 177Lu-PSMA-617 were administered to the 20 patients in the 177Lu-PSMA-617 arm. The patients received a median cumulative activity of 15 GBq (range 6–30 GBq) of 177Lu-PSMA-617 over 1–4 cycles at intervals of 8–16 weeks. Due to availability issues of 177Lu in view of the lockdown measures imposed during the novel coronavirus disease 2019 (COVID-19) pandemic, there was delay in the administration of 177Lu-PSMA-617 as per the set protocol at intervals of 8 weeks in four patients. Nevertheless, all the patients received at least one cycle of 177Lu-PSMA-617, while 15 patients (75%) received at least two cycles: of these 15 patients, two patients (10%) received three cycles and six patients (30%) received four cycles. The reasons for not completing four cycles were disease progression in seven patients (35%), disease-related deaths in three patients (15%), persistent treatment-related myelosuppression (≥ grade 3) in two patients (10%), and exceptional response in two patients (10%). In the docetaxel arm, all the patients received at least five cycles, while 11 patients (55%) completed ten cycles. Seven patients (35%) discontinued further cycles due to progressive disease, while one patient did not complete treatment due to chemotherapy-related interstitial pneumonitis, and another patient died after undergoing eight cycles.
Biochemical response
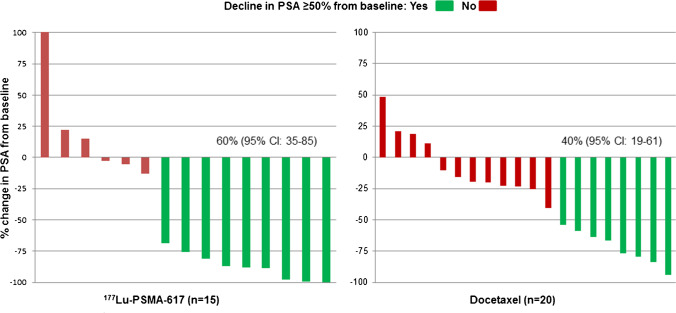
In the ITT analysis, the primary end-point, i.e. the best PSA response was achieved in 10/20 patients (50%, 95% CI: 28–72) in the 177Lu-PSMA-617 arm compared to 8/20 patients (40%, 95% CI: 19–61) in the docetaxel arm (Supplementary Fig. 1). The difference in the best PSA-RRs between the two arms was 10% (95% CI: − 19–37, P = 0.53). However, in the pre-specified per-protocol analysis, 9/15 patients (60%, 95% CI: 35–85) in the 177Lu-PSMA-617 arm achieved a best PSA response in contrast to 8/20 patients (40%, 95% CI: 19–61) in the docetaxel arm (difference 20%, 95% CI: − 12–47, P = 0.25) (Fig. 2). The lower confidence limit of this difference was greater than the specified non-inferiority margin of − 15%, thereby, demonstrating non-inferiority for the primary end-point in the per-protocol analysis (Fig. 3). However, there was no statistically significant difference in the PSA-RRs between the two treatment arms. On subgroup analysis, treatment with 177Lu-PSMA-617 as a first-line agent tended to show higher PSA-RR compared to first-line docetaxel (Supplementary Table 2).
Fig. 2.
Waterfall plots describing the percentage changes in serum PSA values from baseline in per-protocol analysis. CI, confidence intervals; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen
Fig. 3.
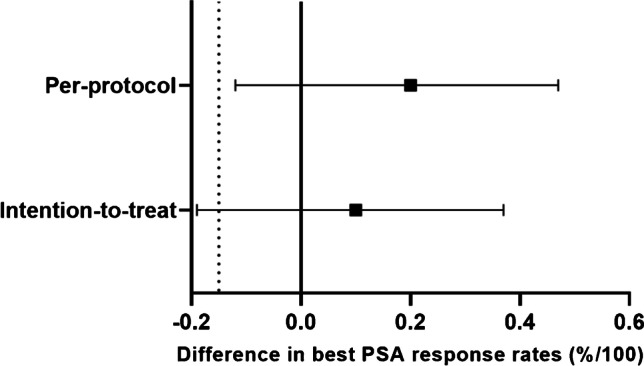
Forest plot depicting difference in PSA response rates of the two arms. Solid square represents absolute difference in the PSA response rates and solid horizontal lines represent the 95% confidence intervals. Solid vertical line is the line of no-effect and the dashed vertical line represents the non-inferiority margin. PSA, prostate-specific antigen
Radiological response
Radiological response assessment using serial 68 Ga-PSMA-11 PET/CT was feasible in 19/20 patients in the docetaxel arm, but in 14/20 patients in the 177Lu-PSMA-617 arm. Four of the patients in the 177Lu-PSMA-617 arm died before their scheduled assessments (three disease-related deaths plus one toxicity-related death), while follow-up 68 Ga-PSMA-11 PET/CT was not possible in two other patients due to the COVID-19 lockdown. Further, all the 19 patients in the docetaxel arm had measurable disease on the CT images of the baseline 68 Ga-PSMA-11 PET/CT as per RECIST 1.1, while one patient in the 177Lu-PSMA-617 arm with purely sclerotic skeletal metastases had non-measurable disease on the baseline CT. Thus, according to RECIST 1.1, in the ITT analysis, the best objective response was observed in 5/13 patients (39%, 95% CI: 12–65) in the 177Lu-PSMA-617 arm in contrast to 6/19 patients (32%, 95% CI: 11–52) in the docetaxel arm (difference 7%, 95% CI: − 24–38, P = 0.69). Further, as per the adapted PERCIST 1.0, the best molecular responses in the 177Lu-PSMA-617 and docetaxel arms were observed in 6/14 (43%, 95% CI: 17–69) and 6/19 (32%, 95% CI: 11–52) patients respectively (difference 11%, 95% CI: − 19–41, P = 0.51). In the per-protocol analysis, the best objective response was seen in 5/11 patients (46%, 95% CI: 16–75) in the 177Lu-PSMA-617 arm versus 6/19 patients (32%, 95% CI: 11–52) in the docetaxel arm (difference 14%, 95% CI: − 19–45, P = 0.45). The corresponding best molecular response rates in the 177Lu-PSMA-617 and docetaxel arms were 50% (95% CI: 22–78) and 32% (95% CI: 11–52) respectively (difference 18%, 95% CI: − 14–48, P = 0.31).
Survival analysis and follow-up
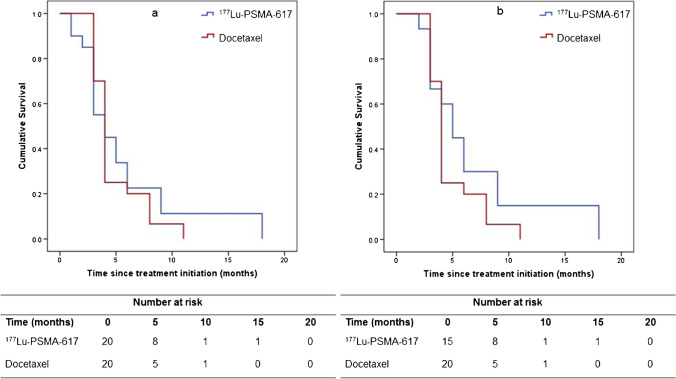
As of 15th of June 2021, a total of 36 events of disease progression or death (whichever was earlier) had occurred in the study cohort: 17 events in the 177Lu-PSMA-617 arm and 19 events in the docetaxel arm. In the ITT analysis, the estimated median PFS was 4.0 months (95% CI: 1.8–6.2 months) in the 177Lu-PSMA-617 arm and 4.0 months (95% CI: 3.6–4.4 months) in the docetaxel arm (P = 0.98) (Fig. 4a). The PFS rate at 6 months was 23% in the 177Lu-PSMA-617 arm compared to 20% in the docetaxel arm (difference 3%, 95% CI: − 22–28, P = 0.82). The univariate hazard ratio (HR) for disease progression or death with 177Lu-PSMA-617 versus docetaxel was 0.90 (95% CI: 0.46–1.77) (Supplementary Table 3). In the per-protocol analysis, the median PFS durations in the 177Lu-PSMA-617 and docetaxel arms were 5.0 months (95% CI: 3.3–6.7) and 4.0 months (95% CI: 3.6–4.4 months) respectively (P = 0.30) (Fig. 4b). The corresponding PFS rate at 6 months was 30% in the 177Lu-PSMA-617 arm versus 20% in the docetaxel arm (difference 10%, 95% CI: − 18–38, P = 0.50). The univariate HR for PFS with 177Lu-PSMA-617 versus docetaxel, in the per-protocol analysis, was 0.68 (95% CI: 0.32–1.44). Further post-study treatments in the 177Lu-PSMA-617 and docetaxel arms included docetaxel (five versus zero patients, respectively), abiraterone (one versus three patients, respectively), enzalutamide (two versus nine patients, respectively), 177Lu-PSMA-617 (zero versus one patient, respectively), and actinium (225Ac)-PSMA-617 (one versus zero patient, respectively) (Supplementary Table 4). Data for OS was not mature at the time of writing this manuscript.
Fig. 4.
Kaplan–Meier curves for progression-free survival: a intention-to-treat analysis, b per-protocol analysis. PSMA, prostate-specific membrane antigen
Toxicity
Overall, treatment-emergent grade 3–5 adverse events occurred in 6/20 patients (30%, 95% CI: 10–50) in the 177Lu-PSMA-617 arm in contrast to 10/20 patients (50%, 95% CI: 28–72) in the docetaxel arm (difference 20%, 95% CI: − 10–45, P = 0.20). The most commonly encountered symptomatic adverse event in the 177Lu-PSMA-617 arm was grade 1/2 dryness of mouth (60%) with other common events being fatigue, loss of appetite, and constipation. Six patients (30%) also experienced transient generalized pain after 177Lu-PSMA-617 therapy, possibly due to flare phenomenon, which was managed with opioid analgesics. Among the patients in the docetaxel arm, the common symptomatic adverse events reported were fatigue, nausea/vomiting, diarrhoea, stomatitis, loss of weight, and loss of appetite. Majority of these toxicities were grade 1/2 events; however, four patients (20%) in the docetaxel arm reported grade 3 symptomatic adverse events. Two patients experienced grade 3 diarrhoea (after one and six cycles, respectively) requiring intravenous fluid replacement and dose reduction of docetaxel to 65 mg/m2. Two patients also developed interstitial pneumonitis-related grade 3 dyspnoea, with one of them requiring treatment discontinuation after five cycles. Grade 3 palmar-plantar erythrodysesthesia syndrome was also observed in one patient in the docetaxel arm after eight cycles, requiring dose reduction to 50 mg/m2.
Among the laboratory parameters, grade 1/2 anaemia was the most commonly observed treatment-related adverse event in the 177Lu-PSMA-617 (45%, 95% CI; 23–67) as well as the docetaxel (55%, 95% CI: 33–77) recipients. The grade 1/2 toxicities pertaining to the laboratory parameters were observed to be transient in most patients with values normalizing spontaneously between 8 and 12 weeks post therapy. The frequencies of the grade 3–5 adverse events concerning laboratory parameters were not markedly different between the two arms. However, dose reduction was necessitated in one patient in the 177Lu-PSMA-617 arm owing to grade 3/4 lymphopenia. Notably, three patients (two in the 177Lu-PSMA-617 arm and one in the docetaxel arm) developed persistent grade 4 thrombocytopenia leading to treatment-related deaths (grade 5 toxicity). The toxicity profiles of both the treatment regimens are summarized in Table 2.
Table.2.
Summary data of adverse events as per CTCAE v5.0
| Type of adverse eventa | 177Lu-PSMA-617 arm (n = 20) | Docetaxel arm (n = 20) | ||
|---|---|---|---|---|
| Any grade, No. (%) | Grade ≥ 3, No. (%) | Any grade, No. (%) | Grade ≥ 3, No. (%) | |
| Nausea/vomiting | 4 (20) | 0 (0) | 7 (35) | 0 (0) |
| Diarrhoea | 0 (0) | 0 (0) | 6 (30) | 2 (10) |
| Constipation | 5 (25) | 0 (0) | 1 (5) | 0 (0) |
| Fatigue | 9 (45) | 0 (0) | 7 (35) | 0 (0) |
| Dryness of mouth | 12 (60) | 0 (0) | 0 (0) | 0 (0) |
| Stomatitis | 0 (0) | 0 (0) | 6 (30) | 0 (0) |
| Palmar-plantar erythrodysesthesia syndrome | 0 (0) | 0 (0) | 2 (10) | 1 (5) |
| Dryness of eyes | 2 (10) | 0 (0) | 0 (0) | 0 (0) |
| Dyspnoea | 0 (0) | 0 (0) | 3 (15) | 2 (10) |
| Pain abdomen | 1 (5) | 0 (0) | 2 (10) | 0 (0) |
| Generalized pain | 6 (30) | 0 (0) | 2 (10) | 0 (0) |
| Peripheral neuropathy | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Loss of weight | 4 (20) | 0 (0) | 6 (30) | 0 (0) |
| Loss of appetite | 8 (40) | 0 (0) | 6 (30) | 0 (0) |
| Hematological toxicity | ||||
| • Anaemia | 14 (70) | 5 (25) | 15 (75) | 4 (20) |
| • Leucopenia | 5 (25) | 1 (5) | 4 (20) | 1 (5) |
| • Febrile neutropenia | 0 (0) | 0 (0) | 1 (5) | 1 (5) |
| • Thrombocytopenia | 5 (25) | 2 (10) | 6 (30) | 1 (5) |
| Nephrotoxicity | 1 (5) | 0 (0) | 4 (20) | 1 (5) |
| Hepatotoxicity | ||||
| • Raised serum bilirubin | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| • Decreased serum albumin | 1 (5) | 0 (0) | 1 (5) | 0 (0) |
| Adverse event leading to dose reduction | 1 (5) | 1 (5) | 3 (15) | 3 (15) |
| Adverse event leading to drug discontinuation | 2 (10) | 2 (10) | 1 (5) | 1 (5) |
| Adverse event leading to death | 2 (10) | 2 (10) | 1 (5) | 1 (5) |
CTCAE Common Terminology Criteria for Adverse Events
aSome patients had more than one type of adverse event
Health-related quality-of-life outcomes
HRQOL outcomes at 12 weeks were evaluable in 15/20 patients in the experimental arm and 20/20 patients in the control arm. Significant improvement was noted in the median total FPSI-17 score in the 177Lu-PSMA-617 arm in comparison to the docetaxel arm (P < 0.01). Separate domain-based analyses revealed statistically significant changes in the FPSI-DRS-P (P = 0.02), FPSI-DRS-E (P = 0.04), and FPSI-TSE (P < 0.01) sub-scores in the 177Lu-PSMA-617 arm compared to the docetaxel arm (Supplementary Table 5).
Discussion
In this randomized phase 2 trial, treatment with at least two cycles of 177Lu-PSMA-617 was demonstrated to be non-inferior to docetaxel for achieving biochemical response and had a comparable PFS status. Moreover, 177Lu-PSMA-617 was tolerated well vis-à-vis docetaxel with less frequent grade 3/4 adverse events. A favourable efficacy and safety profile coupled with the relatively lesser number and frequency of treatment cycles was further reflected in the significant improvement in the HRQOL outcomes with 177Lu-PSMA-617 compared to those with docetaxel. The findings are encouraging and support the use of 177Lu-PSMA-617 as a treatment modality for mCRPC that can be instituted earlier in the disease course.
While a large number of studies have reported favourable outcomes with 177Lu-PSMA-RLT in the taxane-treated population, there exists a lack of prospective phase 2 studies in the taxane-naïve setting. The recently concluded TheraP and VISION trials have reported superior outcomes with 177Lu-PSMA-617 in comparison to their respective active control arms in docetaxel-progressive patients [9, 16]. The current trial is, thus, unique in two aspects: first, it is only one of the few initial prospective studies with head-to-head comparison of 177Lu-PSMA-617 with an approved treatment modality, specifically docetaxel, and, secondly, it includes chemotherapy-naïve patients. Prior chemotherapy has been shown to be a worse predictor of response and survival outcomes with 177Lu-PSMA-RLT [22–24]. In a retrospective study, Barber et al. observed better PSA response outcomes in taxane-naïve patients as compared to taxane-pretreated patients (57% versus 40%, respectively) [22]. Our results of PSA response are, thus, comparable to that reported in the chemotherapy-naïve arm of the aforementioned study.
Though the current trial demonstrated non-inferiority of 177Lu-PSMA-617 to docetaxel, the outcomes are not significantly different between the two arms despite the institution of 177Lu-PSMA-RLT in the chemotherapy-naïve population. One plausible explanation for this could be the relatively higher tumour burden in the 177Lu-PSMA-617 arm. This is evident from the higher median baseline PSA, as well as the higher proportion of patients with > 20 skeletal metastases, and having received both abiraterone and enzalutamide in the 177Lu-PSMA-617 arm. Therefore, the non-inferior treatment outcomes with 177Lu-PSMA-617, despite an unfavourable tumour burden, are quite promising. Further, the institution of 177Lu-PSMA-617 as the first-line treatment option in mCRPC tended to result in bigger treatment effects. Future trials with larger sample sizes should further explore the efficacy of 177Lu-PSMA-617 vis-à-vis other interventions in the first-line setting in mCRPC.
While the median PFS with 177Lu-PSMA-617 was similar to that with docetaxel in our study, it was markedly lower when compared to that reported for 177Lu-PSMA-RLT in the existing literature [9, 14, 22]. These findings could be attributed to certain key differences between the current trial and its contemporaries. The presence of visceral metastases has been previously identified to negatively affect PSMA-RLT outcomes [24, 25]. In comparison to the TheraP and VISION trials, the 177Lu-PSMA-617 arm in our study consisted of a relatively higher proportion of patients with visceral metastases and resultant worse outcomes (Supplementary Table 6) [9, 16]. Notably, the TheraP trial also employed dual 68 Ga-PSMA/18F-FDG-PET/CT for patient selection prior to 177Lu-PSMA-RLT, and the lack of a baseline 18F-FDG-PET/CT in our patients could have missed lesions with neuroendocrine differentiation and an inherent unfavourable prognosis [16, 26, 27].
In the current study, we used an empirical dosing approach of 6.0–7.4 GBq/cycle of 177Lu-PSMA-617 every 8 weeks. The activities of 177Lu-PSMA-617 used as well as the frequency of administration were observed to be lower in comparison to the TheraP and VISION trials [9, 16]. Further, the treatment was delayed in few patients due to the COVID-19 pandemic. Since 177Lu is a beta emitter with considerably lower linear energy transfer, it is possible that the tumour absorbed dose was lower in few of our patients and mostly produced the easy-to-repair single-stranded DNA breaks, thereby inducing radioresistance and early progression [28]. Adopting a de-escalating strategy of administered radioactivity, as followed in the TheraP trial, could potentially mitigate this problem [16]. The presence of germline or somatic mutations in DNA damage repair-associated genes could have also affected our results and needs further research [28].
Apart from the favourable treatment response with 177Lu-PSMA-617, its relatively high safety margin vis-à-vis docetaxel remains a key highlight of this study. However, an exception was grade ≥ 3 thrombocytopenia which occurred in a higher proportion of patients in the 177Lu-PSMA-617 arm. Although the incidence of grade ≥ 3 thrombocytopenia observed in our study was consistent with that reported in literature, it is essential to obtain serial hemograms in order to ensure its early diagnosis and prompt treatment [9, 14, 16]. Nevertheless, the risk of causing more grade ≥ 3 thrombocytopenia presents a potential hurdle in the early institution of PSMA-RLT, as it could compromise further therapies. Since the treatment options for mCRPC are non-curative, the impact of each therapy on the tolerance or candidature for future therapies should also be considered on longer follow-up with larger studies.
Our study has certain limitations, notably, the open-label nature of the study, lack of a baseline 18F-FDG-PET/CT, and the unavoidable delays in the treatment administration and follow-up in few patients during the COVID-19 pandemic. Further, the calculated sample size was adequate for PSA-RR as the primary end-point and not for the other observations. Nevertheless, the current trial remains the first prospective study comparing 177Lu-PSMA-617 with docetaxel in taxane-naïve patients, and its randomized and non-inferiority study design is its major strength.
In conclusion, this phase 2 trial demonstrates 177Lu-PSMA-617 to be non-inferior to docetaxel for achieving PSA response in chemotherapy-naïve mCRPC with an acceptable safety profile. However, further large-scale trials are required to validate our observations and determine the specific sequence of treatment options for these patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
A part of this work with preliminary results was selected for poster presentation at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, 2021. Satapathy S, Mittal B, Sood A, et al. Efficacy and safety of lutetium-177 PSMA radioligand therapy versus docetaxel in metastatic castration resistant prostate cancer-preliminary results of a randomized controlled trial. J Nucl Med. 2021; 62:1311.
Availability of data and material
De-identified participant data can be made available to bona fide researchers affiliated to an appropriate institution on reasonable request to the corresponding author following publication.
Declarations
Ethics approval and consent to participate
The study was approved by the Institute Ethics Committee (INT/IEC/2019/001972) and was conducted in accordance with the guidelines enshrined in the Declaration of Helsinki. Informed written consent was obtained from the patients prior to inclusion in the trial.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Oncology—Genitourinary
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helgstrand JT, Røder MA, Klemann N, et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer—a population-based analysis of 2 national cohorts. Cancer. 2018;124:2931–2938. doi: 10.1002/cncr.31384. [DOI] [PubMed] [Google Scholar]
- 2.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, De Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Ingrosso G, Detti B, Scartoni D, et al. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin Oncol. 2018;45:303–315. doi: 10.1053/j.seminoncol.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Powers E, Karachaliou GS, Kao C, et al. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol. 2020;13:144. doi: 10.1186/s13045-020-00978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright GL, Jr, Haley C, Beckett ML, et al. Expression of prostate-specific membrane antigen innormal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-Y. [DOI] [PubMed] [Google Scholar]
- 8.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emmett L, Willowson K, Violet J, et al. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52–60. doi: 10.1002/jmrs.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–1176. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu] Lu-PSMA-617. Eur J Nucl Med Mol Imag. 2017;44:1448–1454. doi: 10.1007/s00259-017-3716-2. [DOI] [PubMed] [Google Scholar]
- 13.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 14.Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 15.Emmett L, Crumbaker M, Ho B, et al. Results of a prospective phase 2 pilot trial of 177Lu–PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17:15–22. doi: 10.1016/j.clgc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 17.Sadaghiani MS, Sheikhbahaei S, Werner RA, et al. A systematic review and meta-analysis of the effectiveness and toxicities of lutetium-177-labeled prostate-specific membrane antigen-targeted radioligand therapy in metastatic castration-resistant prostate cancer. Eur Urol. 2021;80:82–94. doi: 10.1016/j.eururo.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Seitz AK, Rauscher I, Haller B, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imag. 2018;45:602–612. doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]
- 21.Victorson DE, Beaumont JL, Rosenbloom SK, et al. Efficient assessment of the most important symptoms in advanced prostate cancer: the NCCN/FACT-P Symptom Index. Psychooncology. 2011;20:977–983. doi: 10.1002/pon.1817. [DOI] [PubMed] [Google Scholar]
- 22.Barber TW, Singh A, Kulkarni HR, et al. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60:955–962. doi: 10.2967/jnumed.118.216820. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadzadehfar H, Rahbar K, Baum RP, et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu] Lu-PSMA-617. A WARMTH multicenter study (the 617 trial) Eur J Nucl Med Mol Imaging. 2020;48:113–22. doi: 10.1007/s00259-020-04797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessel K, Seifert R, Schäfers M, et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics. 2019;9:4841–4848. doi: 10.7150/thno.35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satapathy S, Mittal BR, Sood A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate cancer treated with 177Lu-labeled prostate-specific membrane antigen radioligand therapy: a systematic review and meta-analysis. Clin Nucl Med. 2020;45:935–942. doi: 10.1097/RLU.0000000000003307. [DOI] [PubMed] [Google Scholar]
- 26.Parimi V, Goyal R, Poropatich K, et al. Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol. 2014;2:273–285. [PMC free article] [PubMed] [Google Scholar]
- 27.Suman S, Parghane RV, Joshi A, et al. Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort. Br J Radiol. 2019;92:20190380. doi: 10.1259/bjr.20190380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kratochwil C, Giesel FL, Heussel CP, et al. Patients resistant against PSMA-targeting α-radiation therapy often harbor mutations in DNA damage-repair-associated genes. J Nucl Med. 2020;61:683–688. doi: 10.2967/jnumed.119.234559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data can be made available to bona fide researchers affiliated to an appropriate institution on reasonable request to the corresponding author following publication.