Abstract
Histoplasmosis is one of the most common endemic mycoses affecting immunocompromised individuals in the United States and Latin America. Involvement of the central nervous system carries higher mortality rates and worse prognosis, given its resemblance to stroke, vasculitis, and meningitis of other etiologies. The diagnosis is challenging, due to its subtle clinical presentation and the poor sensitivity of the cerebrospinal fluid culture. Herein the authors present a case of a middle-aged man with HIV, who presented with intermittent headaches exacerbated by an oculomotor nerve palsy, concerning for acute stroke. A diagnosis of central nervous system histoplasmosis was made, and his neurological deficits subsided after initiation of treatment. The stroke-like syndrome in this scenario may be secondary to granulomatous vasculitis of small caliber cerebral blood vessels. Histoplasmosis of the central nervous system remains a challenging diagnosis, which requires a high index of suspicion by the clinician for an early institution of therapy in order to improve outcomes.
Keywords: Mycoses, Fungal lung diseases, Stroke, Lacunar stroke, Central nervous system, Vasculitis, Acquired immunodeficiency syndrome, HIV
Introduction
Histoplasma capsulatum is a thermally dimorphic soil fungus endemic in river valleys of the Midwest and Southeastern United States, as well as in many Latin American countries [1], [2], [3]. Primary infection occurs through inhalation of mycelial fragments and microconidia that are engulfed by the alveolar macrophages and then transform into yeast intracellularly [3]. These yeasts subsequently migrate into the lymph nodes and hematogenously spread to distant organs, predominantly to those rich in mononuclear cells, such as the reticuloendothelial system. Under normal conditions, adaptive cellular immunity is critical for the host defense and yeast clearance [3]. Hence, subjects with defective T cells, such as those infected with the human immunodeficiency virus (HIV) and organ transplant recipients on immunosuppressive therapy, may develop a progressive disseminated form, either by fungal reinfection or from reactivation of a dormant source [4], [5]. Involvement of the central nervous system (CNS) can occur as part of disseminated disease or as an isolated syndrome [6]. Additionally, CNS compromise carries particularly high mortality rates and worse outcomes, being often overlooked during the initial evaluation given its resemblance to stroke, vasculitis, meningitis of other etiologies, and difficult microbiological diagnosis [4], [7], [8]. CNS histoplasmosis with visual disturbances on presentation is rare, and the incidence of associated brain infarctions in the largest case series was 2% [7], which indicates a challenging diagnosis without a high index of suspicion by the clinician.
Herein the authors describe a case of CNS histoplasmosis in a middle-aged man with HIV and acquired immunodeficiency syndrome (AIDS), who presented with chronic headaches, exacerbated by what appeared to be an acute cerebrovascular event. A review of the pertinent literature was also performed.
Methods
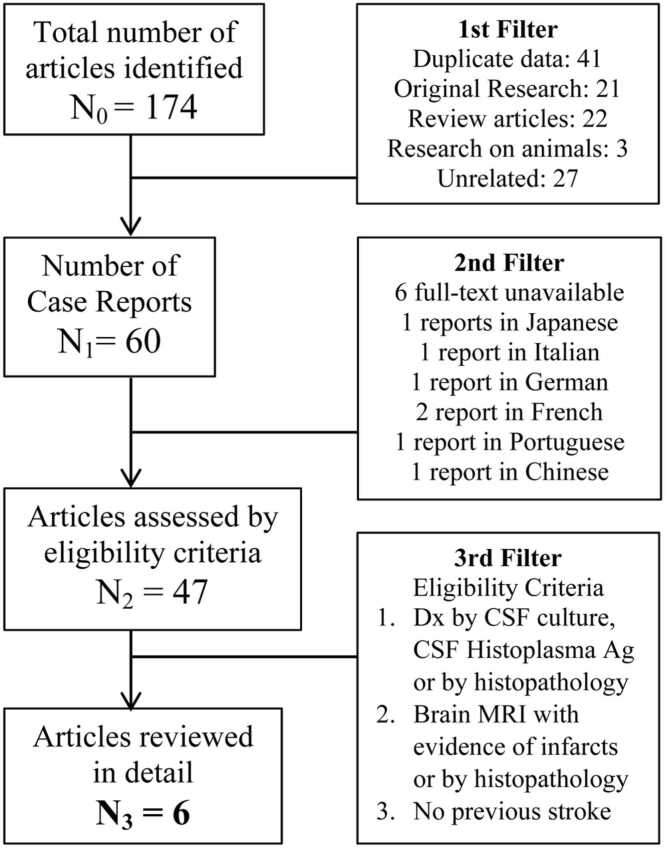
During January 2021, three independent investigators (RPR, IRG, AL) performed a literature search with PubMed to identify case reports of disseminated histoplasmosis with CNS involvement presenting with focal neurological deficits and brain infarcts. The eligibility criteria were [6]: [1] diagnosis of CNS histoplasmosis either by cerebrospinal fluid (CSF) study and culture, CSF Histoplasma antigen, antibody, or histopathology; and [2] neuroimaging with findings suggestive of infarction, or infarctions demonstrated on histopathology examination. Individuals with history of previous strokes, or with significant risk factors for cerebrovascular disease were excluded. The terms used were<CNS histoplasmosis>,<histoplasmosis AND stroke>,<disseminated histoplasmosis AND neurological deficit>,<disseminated histoplasmosis AND cerebrovascular accident>; and the MeSH terms<“Histoplasmosis” AND “Stroke”>,<“Histoplasmosis” AND “Cranial nerve diseases”>, and<“Histoplasmosis” AND “Brain infarction”>. In addition, a secondary search of the literature through Google/Google Scholar was performed, including references from the previous articles. Duplicate data, articles other than case reports, and those unrelated to the topic of interest were excluded. Reports in languages other than English or Spanish, and those without a full-text version readily available were also excluded. Fig. 1 summarizes the screening process. Reports meeting the eligibility criteria were analyzed in detail. The authors (RPR, IRG, AL and MH) evaluated these cases and any discrepancies were solved by consensus. Data was stored in a spreadsheet.
Fig. 1.
Literature review screening process.
Results
Case presentation
A 46-year-old man presented to the Emergency Department complaining of acute onset diplopia for one day, as well as worsening intermittent occipital headaches over the past 3 months. His medical history was significant for HIV/AIDS with most recent CD4 count 100 cells/μL and viral load 38,541 copies/mL, with poor adherence to his antiretroviral therapy regimen for the past 6 months. He also described occasional subjective fevers and drenching night sweats but denied dyspnea, cough, chest pain, or other respiratory symptoms. He denied known drug allergies, and his current medications were abacavir, dolutegravir, and lamivudine. He denied tobacco smoking or drug use and reported drinking 1–2 beers during the weekends. He worked as a truck driver and had trips across the Midwest and Southern regions of the US. Six days prior to admission he was discharged from another hospital, where he was hospitalized for chronic headaches. During that hospitalization, a lumbar puncture revealed a CSF with mild pleocytosis of monocytic predominance, normal CSF protein and glucose and negative Gram stain. He was treated with ampicillin, ceftriaxone and acyclovir empirically. CSF Herpes Simplex PCR was negative. Neuroimaging, including magnetic resonance imaging (MRI) and MR angiography of the brain were reported as unremarkable. His symptoms were attributed to an aseptic meningitis of probable viral etiology and he was discharged home off antimicrobials.
On arrival to our hospital, the patient was afebrile and hemodynamically stable and in no acute distress, with oxygen saturation 100% on room air. Cardiopulmonary and abdominal exam were unremarkable. Neurological examination revealed binocular diplopia with left ptosis, exotropia and inability to adduct the left eye. Laboratory exams were remarkable for mild leukopenia with white blood cell (WBC) count of 2.6 × 103/μL, absolute neutrophil count of 1.4 × 103/μL, and normocytic anemia. Transaminases were mildly elevated at ALT 63, AST 45. Initial computed tomography (CT) scan of the head without contrast was unremarkable. Despite concern for ischemic stroke, the patient was not a candidate for thrombolysis due to onset of symptoms being 24 h prior. A transthoracic echocardiogram was negative for thrombi or vegetations.
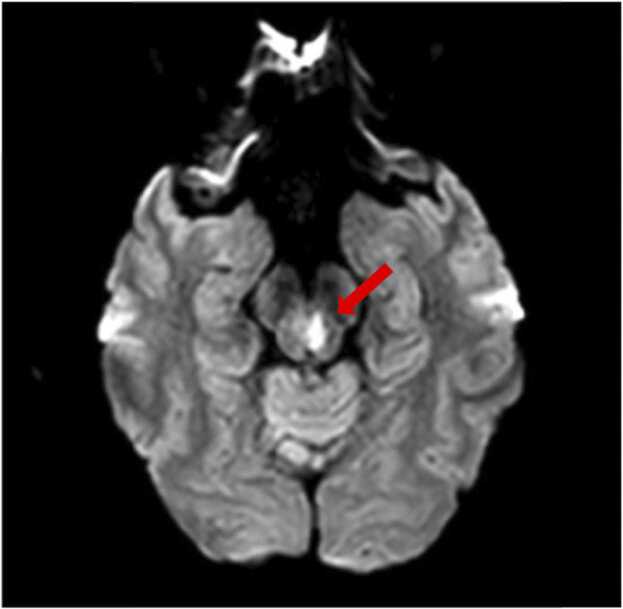
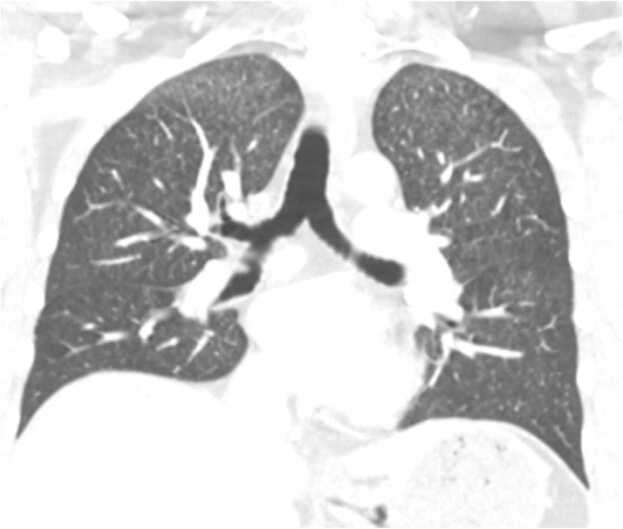
Given the history of headaches, subjective fevers, and AIDS, a lumbar puncture was performed on day 1, to assess for chronic meningitis. CSF study showed WBC of 8/μL and 13/μL red blood cells, with 88% lymphocytes and 10% monocytes. CSF protein was 45 gm/dL and glucose was 52 mg/dL. Gram stain was negative for any organisms. Also on day 1, a brain MRI revealed an acute lacunar infarct with restricted diffusion in the left paramidline midbrain, in the expected position of the oculomotor nerve nucleus (Fig. 2). At this point, studies were sent on serum and CSF to evaluate the suspected conditions in the differential diagnosis, including tuberculosis, endemic mycoses (histoplasmosis, coccidioidomycosis, blastomycosis), and syphilis. On further testing, a chest CT scan with contrast revealed bilateral numerous tiny miliary nodules of apical predominance, concerning for miliary tuberculosis versus fungal or malignant etiologies (Fig. 3). A bronchoscopy showed normal airways and the bronchoalveolar lavage (BAL) Gram stain revealed rare budding yeasts. The oculomotor nerve palsy resolved spontaneously over the next 4 days only with supportive therapy. Consecutive acid-fast bacillary sputum and BAL smears were negative. Eventually the results of serum, urine and CSF Histoplasma antigens returned as positive. Intravenous liposomal amphotericin B was initiated for disseminated histoplasmosis with CNS involvement for 6 weeks and the patient was then transitioned to oral itraconazole. He continued to have regular follow-ups at the infectious disease clinic after a year of treatment and had no relapses to date.
Fig. 2.
Brain MRI on day 1 of admission showing an acute lacunar infarct with restricted diffusion in the left oculomotor nerve nucleus (red arrow).
Fig. 3.
Chest CT scan with contrast revealing bilateral micronodules predominantly in the upper lung fields in a “miliary pattern” (coronal view).
Review of the literature
Six other cases of CNS histoplasmosis with features of acute stroke or cerebrovascular disease were identified (Table 1) [9], [10], [11], [12], [13], [14]. The median age was 46 years (IQR 16.5), and the male-to-female ratio was 5:2. Two individuals had comorbidities of dyslipidemia and hypertension (cases 2 and 3), and one had rheumatoid arthritis on chronic corticosteroids (case 5). Two patients were considered immunocompromised: the current case (HIV/AIDS) and the one on chronic steroids. Most cases (5/7, 71.4%) were reported in the USA while the remaining ones were from Brazil. Contact with endemic areas was found in four cases (57.1%). Among clinical manifestations, all presented with a syndrome of subacute to chronic meningitis with additional neurological deficits. Interestingly, 3/7 subjects had diplopia on initial presentation (42.8%). The disease duration at the time of diagnosis had a mean of 3.21 months (SD 2.03). In the CSF analysis, four cases exhibited pleocytosis of lymphocytic predominance. Six of the seven cases exhibited significantly elevated CSF protein levels, median 137 mg/dL (IQR 117.5). Most infarcts appeared to be lacunar, localized primarily in areas supplied by the vertebrobasilar system (3/7 pons, 1/7 midbrain, 1/7 in the cerebellar peduncle). Two patients were reported deceased. The remaining five (71.4%) reported significant improvement in neurological deficits after initiation of treatment.
Table 1.
Literature analysis of cases of CNS histoplasmosis complicated by cerebrovascular events.
| Author and year | Age/Sex | Geography | Risk factors | Clinical presentation | Disease duration at time of diagnosis | Diagnosis | CSF findings | Infarct location | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Carod-Artal et al. 2008 [9] | 34/M | Brasilia, Brazil | Endemic area | Right hemiparesis, quadriparesis, chronic meningitis | At least 2 months | CSF culture | WBC 33, Lymph 89%, Mono 9%, PMN 2%, Prot 96, Glu 32 | Pons | FLU, AMB, ITR | Improved deficits |
| 2 | Franzoi et al. 2018[13] | 55/M | Santa Catarina, Brazil | Endemic area | Diplopia, chronic meningitis | 6 months | Brain biopsy, serology | WBC 67, Lymph 70%, Prot 168, Glu 25 | Cerebellar peduncle | AMB, ITR | Deceased |
| 3 | Nguyen et al. 2013[10] | 35/M | Texas, USA | None | Dysarthria, ataxia, weakness, subacute meningitis | 1 month | CSF & urine Ag, CSF culture | WBC 330, Lymph 25%, Mono 16%, PMN 56%, RBC 2 Prot 320, Glu 2 | Pons | AMB, VOR, ITR | Improved deficits |
| 4 | Wilson et al. 2018[11] | 10/F | Midwest USA | Endemic area | Left facial droop, subacute meningitis, paraplegia | 6 months | CSF culture and 18 s rRNA PCR | WBC 68, PMN 33%, Mono 30%, RBC 98, Glu < 20, Prot 292 | Pons | N/A | Improved deficits |
| 5 | Zalduondo et al. 1996 [14] | 47/F | North Carolina, USA | None | Chronic meningitis, diplopia; Left 3rd, 5th & 7th nerve palsies | At least 1.5 months | Brain biopsy and culture | WBC 155, Prot 137 | Left thalamus | AMB | Deceased |
| 6 | Zetir et al. 2017 [12] | 61/M | Florida, USA; Lived in China for 1 year | None | Memory loss, ataxia, chronic meningitis | 3 months | CSF antibody | WBC 38, RBC 4, Lymph 87%, PMN 2%, Mono 11%, Prot 129, Glu 10 | Periventricular white matter | AMB, ITR | Improved deficits |
| 7 | Present case, 2021 | 46/M | Florida, USA | HIV/AIDS, travel to endemic areas | Acute onset diplopia, chronic meningitis | 3 months | CSF, serum & urine Ag | WBC 8, RBC 13, Lymph 88%, Mono 10%, Prot 45, Glu 52 | Oculomotor nerve nucleus (midbrain) | AMB, ITR | Improved deficits |
AMB amphotericin B; Ag antigen; CSF cerebrospinal fluid; FLU fluconazole; Glu glucose; ITR itraconazole; Lymph lymphocytes; Mono monocytes; N/A not available; PCR polymerase chain reaction; PMN polymorphonuclears; Prot protein; RBC red blood cells; VOR voriconazole; WBC white blood cells.
Discussion
The clinical presentation of CNS histoplasmosis encompasses multiple neurological syndromes that tend to overlap. Those include: chronic meningitis with or without hydrocephalus, cerebritis, cerebral or spinal cord histoplasmoma, CNS vasculitis with ischemic infarcts of small caliber arteries, and rarely, cardioembolic stroke syndromes secondary to Histoplasma endocarditis [6], [15], [16], [17]. According to early studies, the rates of CNS involvement among subjects with disseminated histoplasmosis approach 10% [6], [15], however, the diagnosis is often obscured when neurological manifestations are the only presenting signs/symptoms and there are no specific epidemiological risk factors. Moreover, visual disturbances in CNS histoplasmosis are rare, with an incidence below 10%, as stated in the largest multicenter retrospective study to date by Wheat et al. [7] Most neurological manifestations were reported to be transient, lasting for less than one week in a quarter of the cases. Therefore, high index of suspicion and a prompt diagnosis are critical, as the CNS compromise not only increases morbidity, mortality and the risk of relapse, but also dictates a more aggressive and longer course of treatment [4], [7], [8], [18].
The pathophysiology of cerebrovascular disease in CNS histoplasmosis is not fully understood. Evidence to date suggests that brain infarctions can occur in two different settings: [1] granulomatous small vessel vasculitis with lacunar infarcts predominantly in the brainstem and deep white matter [15], [16] and [2] mycotic emboli and microemboli to arteries of various calibers secondary to Histoplasma endocarditis [19], [20]. These syndromes may coexist in severe disseminated disease [20]. Perivascular and vascular infiltrates have been described in histopathology studies of the brain, involving the adventitia and media layers of blood vessel walls, with endothelial proliferation and granuloma encasing fungal structures [15], [20], [21]. Fibrinoid necrosis of cerebral arteries associated with obstruction of the vasa vasorum, as well as perineuritis of cranial nerves were also noted [15]. These findings appear to be one phase of a continuum spectrum of disease, that may lead to cerebritis with rim-enhancing lesions on neuroimaging and CNS histoplasmoma [14] On the other hand, Histoplasma endocarditis is rare and it is estimated to represent 1% of all endocarditis cases. The characteristic clinical presentation is a syndrome of culture-negative endocarditis, mostly affecting individuals with prosthetic valves in the contemporary era [19], [22], [23]. A multicenter case series describing 14 cases from 2003 to 2012 reported 4/14 (28.5%) incidence of embolisms to different organs including the CNS [22]. The intertwinement of all these phenomena in a prolonged illness would explain some of the multiple neurological manifestations in CNS histoplasmosis.
In the case described by the authors, a middle-aged man with HIV/AIDS presented with a chronic course of illness characterized by intermittent headaches, that were exacerbated by an acute palsy of the oculomotor nerve. The clinical key in this scenario was the presence of miliary pulmonary infiltrates in the context of HIV/AIDS and chronic meningitis, which narrowed the differential diagnosis. Most experts recommend at least 10 mL of CSF for culture and antigen/antibody tests [6]. The CSF antigen has significantly better sensitivity in immunocompromised individuals (67–81% vs. 31–38%) [6], [7], in severe cases compared to non-severe cases (93% vs. 55%), and is more specific than CSF antibody tests (96% vs. 83%) [7]. False positives and cross-reactions can happen with blastomycosis, paracoccidioidomycosis, cryptococcosis, and penicilliosis [24], [25]. Additionally, fungal culture of the CSF is a lengthy and poorly sensitive process and is therefore best used as a confirmatory test rather than diagnostic [6], [8].
The standard of care per IDSA guidelines is 4–6 weeks of liposomal amphotericin B followed by itraconazole to complete 1 year of therapy at minimum [18]. Improved mortality outcomes have been observed with the liposomal and deoxycholate amphotericin B, as compared with the lipid complex formulation [7]. Importantly, most reports indicate improvement and resolution of neurological deficits after institution of treatment.
Conclusion
CNS histoplasmosis remains a challenging diagnosis that affects both immunocompromised and immunocompetent individuals. Ischemic cerebrovascular events in this condition are rare, mostly secondary to CNS vasculitis with granulomatous arteritis of small vessels or, less frequently, embolic phenomena due to Histoplasma endocarditis. Awareness of this condition and its various clinical presentations among clinicians and a prompt diagnosis are crucial, as the neurological deficits and outcomes may improve with the institution of appropriate therapy. Subacute-to-chronic meningitis with infarcts involving the brainstem and deep white matter could act as a clinical hint. Chest imaging can be a useful strategy to narrow down the differential diagnosis in specific cases.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Revision by Ethics Committee was not necessary for this Case Report. Informed consent was signed by the patient
Author contributions
RPR: Conception and analysis design, collection of data, data analysis, drafting of the manuscript, preparation of the final version of the manuscript. IRG: Collection of data, data analysis, drafting of the manuscript. AL: Collection of data, data analysis. MH: Conception and analysis design, data analysis, critical revision of the manuscript, preparation of the final version of the manuscript.
Funding
No specific funding was obtained for this study.
Disclaimer
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Conflicts of interest
The authors have no competing interests to declare.
References
- 1.Bennett J.E., Dolin R., Blaser M.J.Mandell. Douglas, and Bennett’s principles and practice of infectious diseases. Ninth edition. Elsevier; Philadelphia: 2020. [Google Scholar]
- 2.Hage C.A., Azar M.M., Bahr N., Loyd J., Wheat L.J. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med. 2015;36(5):729–745. doi: 10.1055/s-0035-1562899. [DOI] [PubMed] [Google Scholar]
- 3.Myint T., Leedy N., Villacorta Cari E., Wheat L.J. HIV-associated histoplasmosis: current perspectives. HIV AIDS. 2020;12:113–125. doi: 10.2147/HIV.S185631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyalakonda H., Albuerne M., Suazo Hernandez L.P., Sarria J.C. Central nervous system histoplasmosis in acquired immunodeficiency syndrome. Am J Med Sci. 2016;351(2):177–186. doi: 10.1016/j.amjms.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Assi M., Martin S., Wheat L.J., Hage C., Freifeld A., Avery R., et al. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013;57(11):1542–1549. doi: 10.1093/cid/cit593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheat L.J., Musial C.E., Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis. 2005;40(6):844–852. doi: 10.1086/427880. [DOI] [PubMed] [Google Scholar]
- 7.Wheat J., Myint T., Guo Y., Kemmer P., Hage C., Terry C., et al. Central nervous system histoplasmosis: multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine. 2018;97(13) doi: 10.1097/MD.0000000000010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddell J., Wheat L.J. Central nervous system infection with Histoplasma capsulatum. J Fungi. 2019 doi: 10.3390/jof5030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carod-Artal F., Venturini M., Gomes E., de Mello M. Chronic central nervous system histoplasmosis in an immunocompetent patient. Neurologia. 2008;23(4):263–268. [PubMed] [Google Scholar]
- 10.Nguyen F.N., Kar J.K., Zakaria A., Schiess M.C. Isolated central nervous system histoplasmosis presenting with ischemic pontine stroke and meningitis in an immune-competent patient. JAMA Neurol. 2013;70(5):638–641. doi: 10.1001/jamaneurol.2013.1043. [DOI] [PubMed] [Google Scholar]
- 11.Wilson M.R., O’Donovan B.D., Gelfand J.M., Sample H.A., Chow F.C., Betjemann J.P., et al. Chronic meningitis investigated via metagenomic next-generation sequencing. JAMA Neurol. 2018;75(8):947–955. doi: 10.1001/jamaneurol.2018.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zetir M., Imam J., Bhattacharya T., Reza M., Bosch W.. Pulmonary Histoplasmosis Causing Stroke-Like Symptoms. American Thoracic Society International Conference; Washington, DC: American Journal of Respiratory and Critical Care Medicine; 2017.
- 13.Franzoi A.E.A., Monfredini N.H., Pope L.Z.B., dos Reis F.I., Tironi F.A. Cerebral histoplasmosis in non-immunosuppressed patient - case report. Int J Neurol Neurother. 2018;5(2) [Google Scholar]
- 14.Zalduondo F.M., Provenzale J.M., Hulette C., Gorecki J.P. Meningitis, vasculitis, and cerebritis caused by CNS histoplasmosis: radiologic-pathologic correlation. AJR Am J Roentgenol. 1996;166(1):194–196. doi: 10.2214/ajr.166.1.8571874. [DOI] [PubMed] [Google Scholar]
- 15.Wheat L.J., Batteiger B.E., Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine. 1990;69(4):244–260. doi: 10.1097/00005792-199007000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Bolen R.D., Bushnell C.D., Reynolds P.S. Acute ischemic strokes from small vessel vasculitis due to disseminated histoplasmosis infection. J Neurol Sci. 2015;352(1–2):125–126. doi: 10.1016/j.jns.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Rangel-Castilla L., Hwang S.W., White A.C., Zhang Y.J. Neuroendoscopic diagnosis of central nervous system histoplasmosis with basilar arachnoiditis. World Neurosurg. 2012;77(2) doi: 10.1016/j.wneu.2011.06.016. 399.E9-13. [DOI] [PubMed] [Google Scholar]
- 18.Wheat L.J., Freifeld A.G., Kleiman M.B., Baddley J.W., McKinsey D.S., Loyd J.E., et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 19.Adigun R.O., Baddour L.M., Geske J.B. A case report of Histoplasma capsulatum prosthetic valve endocarditis: an extremely rare presentation with characteristic findings. Eur Heart J Case Rep. 2019;3(3) doi: 10.1093/ehjcr/ytz127. ytz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerber H.J., Schoonmaker F.W., Vazquez M.D. Chronic meningitis associated with Histoplasma endocarditis. N Engl J Med. 1966;275(2):74–76. doi: 10.1056/NEJM196607142750204. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro J.L., Lux J.J., Sprofkin B.E. Histoplasmosis of the central nervous system. Am J Pathol. 1955;31(2):319–335. [PMC free article] [PubMed] [Google Scholar]
- 22.Riddell J., Kauffman C.A., Smith J.A., Assi M., Blue S., Buitrago M.I., et al. Histoplasma capsulatum endocarditis: multicenter case series with review of current diagnostic techniques and treatment. Medicine. 2014;93(5):186–193. doi: 10.1097/MD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatti S., Vilenski L., Tight R., Smego R.A. Histoplasma endocarditis: clinical and mycologic features and outcomes. J Infect. 2005;51(1):2–9. doi: 10.1016/j.jinf.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hage C.A., Ribes J.A., Wengenack N.L., Baddour L.M., Assi M., McKinsey D.S., et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53(5):448–454. doi: 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 25.Bloch K.C., Myint T., Raymond-Guillen L., Hage C.A., Davis T.E., Wright P.W., et al. Improvement in diagnosis of histoplasma meningitis by combined testing for histoplasma antigen and immunoglobulin G and immunoglobulin M anti-histoplasma antibody in cerebrospinal fluid. Clin Infect Dis. 2018;66(1):89–94. doi: 10.1093/cid/cix706. [DOI] [PMC free article] [PubMed] [Google Scholar]