Summary
Background
In Finland, the surveillance of healthcare-associated infections (HAI) became obligatory by the renewed Communicable Diseases Act on the 1st March 2017.
Aim
To introduce HAI surveillance protocol (HALT-2 by ECDC) in primary care hospitals in the largest hospital district in Finland, and to measure the burden of HAIs and antimicrobial use patterns for improvement.
Methods
Two identical point prevalence surveys (PPS) were organized in autumn 2015 and in spring 2017. The infection control persons (ICP) in the hospitals were inducted to the HAI definitions and the study protocol to collect the data with questionnaires on the study days. The data were checked and analyzed by the areal infection control unit. The hospitals were provided feedback of the results and HAI prevention methods.
Findings
In 2015, 2218 patients from 22 hospitals and in 2017, 2343 patients from 25 hospitals were studied. The prevalence of HAI was 11% in both surveys (ranges per hospital 4–24% and 4–31%, respectively). Of all HAIs, 37% originated from referring hospitals. Respiratory tract, urinary tract and skin were the most frequent sites of infection. One fourth of all patients received at least one systemic antimicrobial. The process showed that recognition of HAIs may be difficult for non-experienced ICPs.
Conclusions
The HALT-2 protocol proved useful in introducing HAI surveillance and prevention in primary care hospitals with active patient transfer from other hospitals and relatively high prevalence of HAI and antimicrobial use. For annually repeated surveys, slightly shorter electronic questionnaires are essential.
KEYWORDS: Healthcare-associated infections, Antimicrobial use, Point prevalence survey, HALT-2, Surveillance, Primary healthcare wards
Introduction
In a few EU countries and US states, surveillance of healthcare-associated infections (HAI) and public reporting is mandatory [1,2]. Earlier, surveillance of HAIs in all hospitals or long-term care facilities in Finland was not routine (although recommended). The renewed Communicable Diseases Act of Finland came into force the 1st March 2017 [3]. In this law, the surveillance and control of HAIs became obligatory for all healthcare and social institutions, but methods are optional. A point prevalence survey (PPS) is a useful way to quantify HAIs and provide data for policymakers [4].
In Finland, the healthcare system is mainly public, i.e. the great majority of Finnish hospitals are owned by municipal authorities. There are 21 hospital districts with five tertiary care (university) hospitals, 16 secondary care hospitals and over 50 primary care hospitals, which are often located at municipal healthcare centres [5]. These primary care hospitals treat patients with acute diseases, rehabilitation patients, and long-term or terminal care patients requiring nursing care [6], but there are no operation theatres, intensive care or dialysis units in these hospitals. They also serve as step-down units for patients at secondary and tertiary care hospitals e.g. after surgery or as holding positions for patients applying for separate rehabilitation or long-term care facilities.
The hospital district of Helsinki and Uusimaa (HUS) is the largest healthcare district in Finland, serving a population of 1.6 (of 5.5) million. In HUS, the surveillance of HAIs in the tertiary care hospitals and in few other secondary and acute care hospitals is well-organized. They notify their HAI rates in the voluntary national HAI surveillance program (SIRO) at the Finnish institute for health and welfare for benchmarking [7]. However, in most of the primary healthcare wards there has been no routine surveillance so far. In some other hospital districts, PPS has been introduced in primary care hospitals [8].
The first standardized European-wide PPS of HAI and antimicrobial use in long-term care facilities (LTCF) and nursing homes (HALT-1) organized by the European Centre for Disease Prevention and Control (ECDC) was performed in 2010 [9]. It developed a sustainable European methodology for PPSs. HALT-2 took place in 2013 and six LTCFs in Finland participated [10]. In 2016–2017 ECDC organized the third PPS in LTCF [11]. In order to implement the surveillance of HAIs obliged in the renewed Communicable Diseases Act, we introduced surveillance activities in the primary healthcare hospitals by two consequent PPSs based on the HALT-2 protocol. This protocol was easy to use for units that had never done HAI surveillance before. We also aimed to demonstrate the burden of HAIs and frequency of risk factors to enhance infection prevention and the resources needed for this.
Methods
HUS Areal Infection Control Unit (AICU) invited all primary care and two or three private rehabilitation hospitals located at the hospital district to participate in two voluntary PPSs. In 2015, 27 and in 2017, 25 hospitals were invited. The chief executive officers and physicians of the hospitals were asked to allocate the infection control person (ICP), either an infection control practitioner (ICPr) or an infection control link nurse (ICL), for the PPS. Before the study period, we organized an online training session on the survey protocol and methodology for the ICPs.
Two types of questionnaires were to be completed: an institutional and a residential. The residential questionnaire was filled in for all patients present at 8.00 a.m. and who stayed for longer than one day. The ward staff and the trained ICPs collected the data on paper forms. A help desk for possible inquiries regarding filling in the questionnaires was provided by phone or email by AICU. In 2015 the data were collected in September/October and in 2017 in March/April. Each ward was surveyed on a single weekday chosen by the ward itself (excluding Mondays and Fridays).
The identification of active (symptomatic or under treatment) HAIs was based on decision algorithms which were integrated in the questionnaire. According to the Surveillance Report of HALT-2 by ECDC, these algorithms were based on case definitions of the US Centers for Diseases Control and Prevention (CDC) and the Society for Healthcare Epidemiology of America (SHEA) Long-Term Care Special Interest Group (LTCSIG) [12]. As a modification, surgical site infection was added as one of the subclasses of healthcare-associated skin infections.
In addition to the HAI data, we collected demographic data, use of antimicrobials and risk factors for the acquisition of HAIs: urinary and vascular catheter use, pressure sores, other wounds and surgery 30 days prior to the PPS. The level of care for each patient was categorized into acute, rehabilitation, long-term or terminal and included in the resident questionnaire. The institutional questionnaire included questions on prevention, control and antimicrobial stewardship strategies of the hospitals.
Completed questionnaires were posted to AICU. In some cases, the ICP that had filled in the questionnaire was contacted because of a misconception or mistake. After checking and correcting missing or unclear data in the questionnaires, the data were entered electronically in an Excel file. The data were analyzed using IBM SPSS, version 22.0 (IBM Corp., Armonk, NY, USA). Categorial variables were analyzed with the Chi-squared test and p<0.05 was considered statistically significant. After both PPSs the results were reported to the infection prevention and control (IPC) teams of the hospitals and the 2015 PPS report was published in the Finnish Medical Journal [13].
Between the two surveys, we provided the hospitals material on infection prevention in practice. This included written guidelines and an educative PowerPoint presentation for prevention of catheter-associated urinary tract infections, vascular catheter-associated infections and pneumonia. The five moments for hand hygiene by WHO and a guide for rational use of antimicrobials were also included. This material was sent by email to the participating hospitals before the second PPS.
Results
In 2015, 22 hospitals (81% of all primary care hospitals in the hospital district) with 2218 patients and in 2017, 25 (all hospitals) with 2343 patients participated in the PPS. The median number of patients per hospital in 2015 was 71 (range: 20–359) and in 2017 76 (range: 16–342). Majority of the hospitals were public, in 2015 two of the 22 hospitals and in 2017 three of the 25 hospitals were private rehabilitation hospitals.
The patients had multiple chronic diseases and disabilities and they used catheters (Table I). Overall, almost half of all study patients were provided acute care, one third rehabilitation, one fifth long-term care and only a minority terminal care. More than half of all patients had hospital admissions during the previous three months. The primary care hospital served as a step-down unit for 41% of the patients coming from secondary and tertiary care hospitals for acute care or rehabilitation.
Table I.
Patient characteristics in both PPSs (n=4561)
| n | % | Range (%) by hospital | 95 % CI | |
|---|---|---|---|---|
| Female sex | 2633 | 57.7 | 31.3–80.0 | 56.3–59.1 |
| Previous location of the patient | ||||
| Home | 1732 | 38.0 | 5.3–86.3 | 36.6–39.4 |
| Long-term care facility | 268 | 5.9 | 0.0–35.0 | 5.2–6.6 |
| Secondary/tertiary hospital in HUS | 1856 | 40.7 | 1.3–87.9 | 39.3–42.1 |
| Other hospital | 705 | 15.5 | 0.0–48.9 | 14.4–16.6 |
| Level of care | ||||
| Acute | 2015 | 44.2 | 0.0–100.0 | 42.8–45.6 |
| Rehabilitation | 1608 | 35.3 | 0.0–84.8 | 33.9–36.7 |
| Long-term | 806 | 17.7 | 0.0–82.2 | 16.6–18.8 |
| Terminal | 132 | 2.9 | 0.0–16.2 | 2.4–3.4 |
| Hospital admission in the last 3 months | 2820 | 61.9 | 18.9–97.4 | 60.5–63.3 |
| Surgery in the previous 30 days | 545 | 11.9 | 0.0–26.5 | 11.0–12.8 |
| McCabe | ||||
| 1 | 1498 | 32.8 | 2.2–88.0 | 31.4–34.2 |
| 2 | 2841 | 62.3 | 10.5–95.7 | 60.9–63.7 |
| 3 | 222 | 4.9 | 0.0–18.9 | 4.3–5.5 |
| Isolation | 314 | 6.9 | 0.0–19.7 | 6.2–7.6 |
| Urinary catheter | 613 | 13.4 | 2.2–30.3 | 12.4–14.4 |
| Vascular catheter | 822 | 18.0 | 0.0–85.7 | 16.9–19.1 |
| Urinary and/or faecal incontinence | 2891 | 63.4 | 15.5–88.0 | 62.0–64.8 |
| Pressure sores | 283 | 6.2 | 0.0–17.4 | 5.5–6.9 |
| Other wounds | 906 | 19.9 | 9.8–30.8 | 18.7–21.1 |
| Dementia or disorientation | 2505 | 54.9 | 26.2–73.9 | 53.5–56.3 |
| Mobility | ||||
| Walking with/without support | 2582 | 56.6 | 26.2–87.0 | 55.2–58.0 |
| Wheelchair | 1230 | 27.0 | 7.0–53.0 | 25.7–28.3 |
| Bedridden | 749 | 16.4 | 0.0–37.8 | 15.3–17.5 |
| Level of care | |
| Acute | The patient has arrived at the primary care hospital via emergency department or because of scheduled investigations or treatment. The patient will probably be discharged to home or another health care facility. |
| Rehabilitation | The patient has arrived at the ward for some weeks or months for active rehabilitation investigations or treatment. The patient will probably be discharged to home or another health care facility. |
| Long-term | The patient's condition requires permanent long-term care. |
| Terminal | The patient is critically ill and may pass away during the stay at the ward. There are no active plans to discharge the patient. |
| McCabe classification [14] | |
| 1 | Non-fatal |
| 2 | Ultimately fatal |
| 3 | Rapidly fatal |
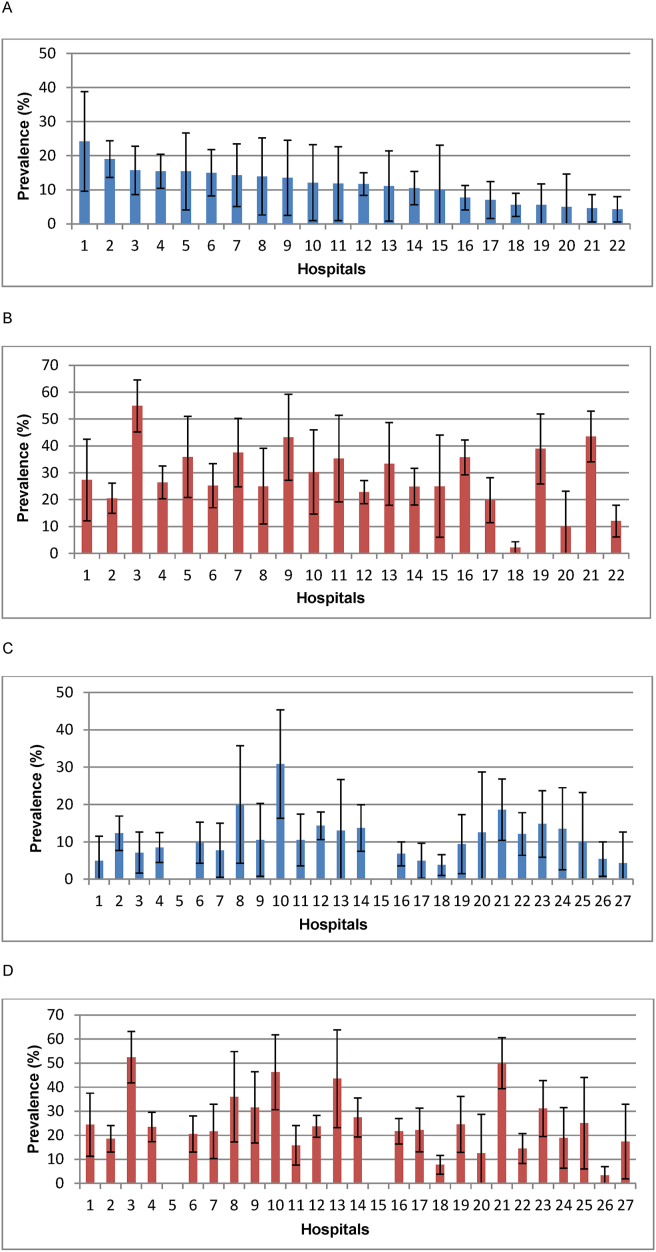
A total of 271 HAIs were confirmed in 251 patients in the first PPS, HAI prevalence being 11% (range by hospital: 4–24%). In the second PPS, there were 259 HAIs in 247 patients (11%; 4–31%). Figure 1 shows the prevalences of patients with at least one HAI and one antimicrobial by hospitals.
Figure 1.
Prevalences (%) and 95 % confidence intervals of patients with HAI and at least one antimicrobial by hospitals. Patients with (A) HAI in 2015, (B) antimicrobial in 2015, (C) HAI in 2017 and (D) antimicrobial in 2017. Most of the hospitals were the same and have the same numbers in both surveys. Two hospitals (5 and 15) merged into hospital 16 after 2015. Five hospitals (23–27) that missed the survey in 2015, participated in 2017.
The patients with HAI were not significantly older than patients without HAI (the median of age 80 versus 79 years). The HAIs were more prevalent in patients whose background diseases were more severe (McCabe score 1: 8%; score 2: 12%; score 3: 17%; p<0.001), who were bedridden vs. ambulant (19% vs. 8%, p<0,001), who had pressure sores (23% vs. 10%, p<0.001) or had been hospitalized over the previous three months (13% vs. 7%, p<0.001). Proportions of patients with HAI were higher in patients receiving acute care, rehabilitation or terminal care than among those receiving long term care (11–13% vs. 8%; p=0.002).
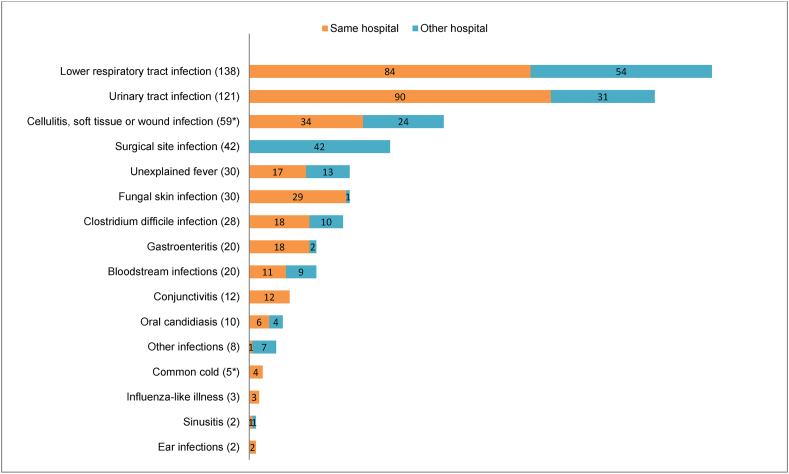
Of all HAIs detected in the PPSs, 37% originated from other, mainly the tertiary care or other hospitals or nursing homes, where the patient stayed before admission to the study ward (Figure 2). All surgical site infections originated in tertiary care hospitals. The HAI acquired elsewhere was often the actual reason for treatment at the primary care hospital.
Figure 2.
Types of HAI and their origins (numbers of cases in parentheses). The origin of two infections (marked with ∗) remained unknown.
The prevalence of at least one HAI among patients who had come to the study ward from the university hospital was 14%. Of the university hospital patients, a little less than one third (28%) had been operated in 30 days prior to the surveys. The patients who had been operated had a higher HAI prevalence compared with patients without previous operation (20% vs. 12%, p<0,001).
Types of HAI
Lower respiratory tract infection (LRTI) was the most prevalent HAI in both PPSs (Figure 2) affecting 3% of all patients in both 2015 and 2017, followed by urinary tract infection (UTI) (3%) and cellulitis, soft tissue or wound infection (2% in 2015 and 1% in 2017). Clostridium difficile infection was the fourth most common in 2015, affecting 1%. In 2017 the amount of C. difficile infection was lower (0.3%) and it was in 11th place. The proportions of HAI types were quite similar for different levels of care.
The presence of urinary catheter (13%) was associated, p<0.001, with the presence of UTIs in this study. The most common indication for a urinary catheter were urinary retention (43%), volume measuring (16%), skin breaks (9%) and incontinence (8%). In one fourth of the cases, the indication was “other/unknown”.
Antimicrobial use
Altogether, 1122 patients (25%) received at least one systemic antimicrobial on the dates of the PPSs. Of these 13% received simultaneously two and 1% three antimicrobials. Of all acute, rehabilitation and long-term patients, 37%, 19% and 8% received at least one antimicrobial. In 87% of all prescriptions, the indication was treatment of an infection (45% of these were used for HAI treatment and 51% for community-acquired infections) and in 8%, prophylaxis. In 5% of the prescriptions, the indication was unclear.
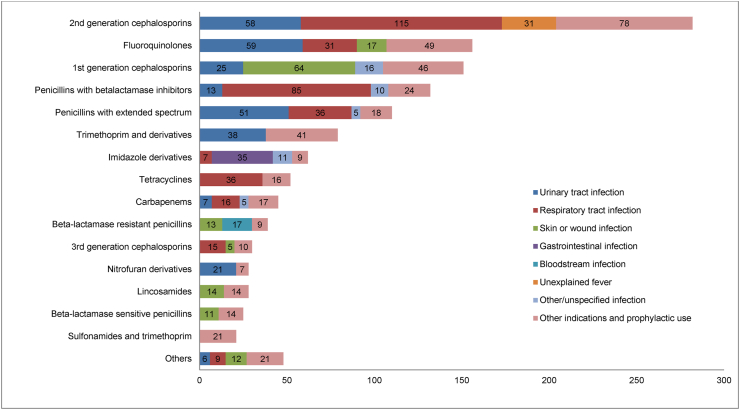
The most frequently prescribed systemic antimicrobial groups and their indications are displayed in Figure 3. The largest group, the 2nd generation cephalosporins (which consisted of only one antimicrobial, intravenously administered cefuroxime), was used for respiratory tract infections and UTIs. The second most frequently prescribed were the fluoroquinolones, used mostly for UTIs and respiratory tract infections. The five most frequently prescribed antimicrobials to treat respiratory tract infections were cefuroxime, combinations of penicillins with beta-lactamase inhibitors (amoxicillin/clavulanic acid 65%, piperacillin/tazobactam 35%), tetracyclines (doxycycline 100%), penicillins with extended spectrum (amoxicillin 100%) and fluoroquinolones (levofloxacin 55%, moxifloxacin 29%, ciprofloxacin 16%). The five most frequently prescribed antimicrobials to treat UTIs were fluoroquinolones (ciprofloxacin 86%, levofloxacin 12%, norfloxacin 2%), cefuroxime, penicillins with extended spectrum (pivmecillinam 84%, amoxicillin 16%), trimethoprim and 1st generation cephalosporins (cephalexin 100%).
Figure 3.
Most frequently prescribed systemic antimicrobials and their most usual indications. The numbers of indications are presented in the columns of the histogram.
Of all antimicrobials, 31% had been prescribed before the patient arrived at the primary care hospital, including 31% of fluoroquinolones, 24% of cefuroxime and 31% of carbapenems.
Microbiological results were available for 368 patients, which was one third of patients with antimicrobial treatment for either healthcare or community-associated infections. The five most frequently isolated bacteria were Escherichia coli (26%), Staphylococcus aureus (15%), Pseudomonas aeruginosa (8%), Clostridium difficile (8%) and Enterococcus faecalis (7%). One infection was caused by carbapenem-resistant E. coli and six by meticillin-resistant S. aureus. No infections caused by vancomycin-resistant Enterococci were detected. Of all enterobacteriaceae 14% were resistant against 3rd generation cephalosporins and 14% of P. aeruginosa against carbapenems. One third of HAIs were microbiologically confirmed.
Results of the process of filling in the questionnaires
The ICPs who were responsible for collecting the data were largely the same persons in both PPSs. In the first PPS, 350 resident questionnaires contained illogical answers or errors and needed to be corrected. Misconceptions concerning the definition of a HAI were frequent. In several cases a community-acquired infection was reported as a HAI. There were errors in questions regarding the patient's demographics and nursing care load indicators. HAI risk factors were in some cases unanswered. In the second PPS the amount of errors decreased in number to 100.
Infection prevention and antimicrobial stewardship resources and practices
In both surveys, all hospitals offered influenza vaccination to the personnel and all but one hospital in 2015 offered it to the patients. Some hospitals did not have an infection control practitioner (ICPr). The proportion of hospitals without ICPr increased from 18% to 27% and the proportion of hospitals with regular supervision of disinfection or sterilization from 82% to 96%. Only one third of the hospitals in 2015 and one fourth in 2017 provided their physicians data and training on antimicrobial use. An alert system for the carriage of multidrug-resistant microbes was used by 73% and 65% of the hospitals, respectively.
Discussion
With these two PPSs we introduced and launched the surveillance of healthcare-associated infections in primary healthcare hospitals in Helsinki and Uusimaa area, the largest hospital district in Finland. The participation rates of these voluntary PPSs were high: 81% and 100%, respectively. The prevalence of HAIs in the two PPSs was 11%, but 37% of them originated from the departure hospitals. One fourth of the patients used antimicrobials, most frequently cephalosporins and fluoroquinolones.
However, more than half of all HAIs originated from the primary care hospitals. Therefore, it is important that the primary care hospitals have a feasible method and frequent routine for HAI surveillance and enough know-how for infection prevention and control. Repeated PPSs can help to reduce the burden of HAIs in hospitals and nursing homes [15,16]. The study also demonstrates that in our health care system, the patients move over between hospitals and bring their infections and microbes along. It is known that patient transfers may also include medical risks, e.g. flaws in patient data transmission. Inadequate plans of antimicrobial and catheter use in patient charts may lengthen their use in the step-down unit. On the other hand, surgical units are not always able to trace their surgical site infections if they manifest after discharge to primary care hospitals.
In the European-wide PPS of LTCFs, HALT-2, the protocol of which was used in our PPSs, the overall prevalence of HAI was 3.4% and the prevalence of antimicrobial use 4.4% [10], thus markedly lower compared to our surveys. The same protocol was used in the PPS of Irish intellectual disability LTCF in 2013. The prevalence of HAI in this survey was 4.3% and the prevalence of antimicrobial use 10% [17]. These numbers are higher compared to HALT-2, but still lower than ours. The Finnish primary care hospitals have a different patient case-mix compared to LTCFs mentioned above and therefore the HAI figures should not be compared. In our survey, the HAI risk varied according to the severity of background diseases and being bedridden or in hospital care within three months. For the same reason, we did not encourage the hospitals to compare their figures but provided them confidence intervals and interpretation of their results. We also stressed the role of chance in these results, especially in hospitals with the smallest number of patients.
The only earlier published PPS of Finnish primary healthcare wards, Oulu University Hospital district in 2006, showed HAI prevalence of 10.1% and antimicrobial prevalence of 36% with half for treatment and half for prophylaxis [8]. These prevalence rates were as high as ours probably due to the typical high proportion of patients receiving acute care in Finnish primary care hospitals. However, the protocol and HAI definitions in the Oulu study were different from ours, which were designed for LTCFs.
In our study, the lower respiratory tract infections, especially pneumonia, was the most frequent HAI type, but no influenza cases were detected. About 60% of pneumonias originated at the primary care hospitals. After the renovation of one hospital and the building of one new hospital with more single-person rooms there were less Clostridium infections in the latter PPS.
There were very few carriers of multidrug resistant bacteria and infections caused by them compared to other European countries. This reflects the low frequency of antibiotic resistance in Finland [18]. However, the results of the antimicrobial use showed not only high prevalence but also scarce use of penicillins and wide use of broad-spectrum antibiotics, especially cephalosporins and fluroquinolones. This is a common trend in the Finnish healthcare system compared with European countries in general [19,20]. There is room for improvement in this culture which is also encouraged in the national Current Care Guidelines [21].
The PPSs were welcomed with interest by the infection control teams of the hospitals. The high participation rate in these surveys showed that the chief executive officers and physicians were interested in finding out the rate of HAIs and antimicrobial use in their hospitals. Furthermore, the renewed Communicable Diseases Act with its obligation for systematic surveillance of HAIs had come into force only some weeks before the initiation of the second PPS, which was one of the factors that increased the participation rate of the hospitals to 100 %.
The high amount of illogical answers and misconceptions in the first PPS showed that the concept of HAI was not adequately understood by the ICPs. When a professional inexperienced with the concept of HAI, is searching information about an infection in the medical record, he/she often ends up seeking any infection, not only HAI. The definition of HAI is challenging because it includes all infections that are initiated in healthcare and not all of them are preventable. To our pleasure the misconceptions decreased in number during the second PPS, which showed the benefit of the training sessions and the existence of a help desk service. The whole process and collecting the data by themselves, taught the IPC teams how to recognize a HAI and gave a closer view to the situation in their own hospital. This advantage would not have been observed if the collecting work was done by someone else, e.g. the researchers of these PPSs.
One weakness in our prevalence process was the paper form of the questionnaires, necessitating arduous hand work when transferring the data in electronic form after first checking each one of them. Therefore, we later continued collecting the annual prevalences with a shorter protocol and electronic forms in Excel and letting the IPC staff of the hospitals analyze their own results and sending them to the HUS AICU.
Regarding the renewed Infectious Diseases Act of Finland, we found this kind of encompassing PPS a practical way to initiate HAI surveillance in hospitals and engage their IPC teams in the process. Repeating PPS often enough makes it easier and more familiar for staff, while also improving the quality of information received. In units where ICP is not part of professional education of the staff, the implementation of prevalence surveys requires lobbying of managers. With help of the PPS results IPC teams were able to show their managers the burden of HAIs and the need for enhanced resources for prevention. The data may increase the managers' interests in a new tool that can be used as an indicator of quality and benchmarking. PPS results can also be used for educating the rest of the personnel. Thus, the whole process of PPS has a political and educational significance.
CRediT author statement
Marjaana Pitkäpaasi: Software, Formal analysis, Investigation, Resources, Data curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Funding acquisition.
Jaana-Marija Lehtinen: Methodology, Validation, Investigation, Resources, Data Curation, Writing – Review & Editing.
Mari Kanerva: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Writing – Original Draft, Writing – Review & Editing, Supervision, Project administration.
Acknowledgements
We owe our warmest gratitude to the IPC staff of the participating hospitals. We would also like to thank statistician Jukka Ollgren for advice.
Conflict of interest statement
None declared.
Funding source
This work was supported by the Inflammation Center of the Hospital District of Helsinki and Uusimaa.
References
- 1.Núnez-Núnez M., Navarro M.D., Palomo V., Rajendran N.B., del Toro M.D., Voss A., et al. The methodology of surveillance for antimicrobial resistance and healthcare-associated infections in Europe (SUSPIRE): a systematic review of publicly available information. Clin Microbiol Infect. 2018;24:105–109. doi: 10.1016/j.cmi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Haustein T., Gastmeier P., Holmes A., Lucet J.-C., Shannon R.P., Pittet D., et al. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis. 2011;11:471–481. doi: 10.1016/S1473-3099(10)70315-7. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Social Affairs and Health, Finland . 2016. Communicable diseases Act.https://finlex.fi/en/laki/kaannokset/2016/en20161227 [Google Scholar]
- 4.Saleem Z., Godman B., Hassali M.A., Hashmi F.K., Azhar F., Rehman I.U. Point prevalence surveys of health-care-associated infections: a systematic review. Pathog Glob Health. 2019;113(4):191–205. doi: 10.1080/20477724.2019.1632070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Social Affairs and Health, Finland Hospitals and specialized medical care. https://stm.fi/en/hospitals-and-specialised-medical-care
- 6.Ministry of Social Affairs and Health, Finland Primary health care. https://stm.fi/en/primary-health-care
- 7.Finnish institute for health and welfare Surveillance of healthcare-associated infections. https://thl.fi/en/web/infectious-diseases-and-vaccinations/surveillance-and-registers/surveillance-of-healthcare-associated-infections
- 8.Puhto T., Ylipalosaari P., Ohtonen P., Syrjala H. Point prevalence and risk factors for healthcare-associated infections in primary healthcare wards. Infection. 2011;39(3):217–223. doi: 10.1007/s15010-011-0123-7. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2014. Point prevalence survey of healthcare-associated infections and antimicrobial use in European long-term care facilities. May–September 2010.https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-consumption-point-prevalence-survey-long-term-care-facilities-2010.pdf [Google Scholar]
- 10.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2014. Point prevalence survey of healthcare-associated infections and antimicrobial use in European long-term care facilities. April–May 2013.https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/healthcare-associated-infections-point-prevalence-survey-long-term-care-facilities-2013.pdf [Google Scholar]
- 11.Suetens C., Latour K., Kärki T., Ricchizzi E., Kinross P., Moro M.L., et al. The Healthcare-Associated Infections Prevalence Study Group. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46):1800516. doi: 10.2807/1560-7917.ES.2018.23.46.1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone N.D., Ashraf M.S., Calder J., Crnich C.J., Crossley K., Drinka P.J., et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965–977. doi: 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitkäpaasi M., Kanerva M., Lehtinen J.M. Hoitoon liittyvien infektioiden prevalenssi HUS-alueen terveyskeskussairaaloissa 2015. Suomen Lääkärilehti (Finnish Medical Journal) 2018;73(16):999–1005. [Google Scholar]
- 14.McCabe W.R., Jackson C.G. Gram-negative bacteremia. I. Etiology and ecology. Arch Int Med. 1962;110(6):847–853. [Google Scholar]
- 15.Arnoldo L., Smaniotto C., Celotto D., Brunelli L., Cocconi R., Tignonsini D., et al. FVG Regional ‘Safety Care’ Group. Monitoring healthcare-associated infections and antimicrobial use at regional level through repeated point prevalence surveys: what can be learnt? J Hosp Infect. 2019;101(4):447–454. doi: 10.1016/j.jhin.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Eikelenboom-Boskamp A., Saris K., van Loosbroek M., Drabbe M.I.J., de Jongh F., de Jong J.W.D., et al. Prevalence of healthcare-associated infections in Dutch nursing homes: follow-up 2010-2017. J Hosp Infect. 2019;101:49–52. doi: 10.1016/j.jhin.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Roche F.M., Donlon S., Burns K. Point prevalence survey of healthcare-associated infections and use of antimicrobials in Irish intellectual disability long-term care facilities: 2013. J Hosp Infect. 2016;93(4):410–417. doi: 10.1016/j.jhin.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2018. Surveillance of antimicrobial resistance in Europe – annual report of the European antimicrobial resistance surveillance Network (EARS-Net) 2017.https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf [Google Scholar]
- 19.Plachouras D., Kärki T., Hansen S., Hopkins S., Lyytikäinen O., Moro M.L., et al. The Point Prevalence Survey Study Group. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23(46):1800393. doi: 10.2807/1560-7917.ES.23.46.1800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricchizzi E., Latour K., Kärki T., Buttazzi R., Jans B., Moro M.L., et al. The Halt Study Group. Antimicrobial use in European long-term care facilities: results from the third point prevalence survey of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23(46):1800394. doi: 10.2807/1560-7917.ES.2018.23.46.1800394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Finnish Medical Society Duodecim . 2021. Current care guidelines.https://www.kaypahoito.fi/en/ [Google Scholar]