Abstract
This study aimed to systematically review the current literature on studies using negative pressure wound therapy (NPWT) or dressings following fracture-related infection (FRI) in internal osteosynthesis of the extremity. Articles were analyzed on fracture and wound healing and included when comparing or describing the use of either NPWT or dressings in FRI. We conducted a systematic literature search in four electronic databases: Embase, Medline, the Cochrane Library, and Scopus. The studies were screened by two authors using Covidence.org and evaluated for risk of bias. A total of 8576 records were identified. No articles compared NPWT to dressings. Seven case reports and three case series included a total of 115 patients treated for FRI. Fracture healing was achieved in 21 out of 67 patients treated with NPWT (4 amputations and 46 not described) and all 48 patients in the dressing group (4 patients needed additional sequestrectomy procedures). Five studies did not describe fracture healing. In 57 out of 67 patients treated with NPWT, the wounds were described as healed, closed, or requiring soft tissue reconstruction (4 amputations and six lacking description). The dressing group had complete wound coverage in 18 patients and partial coverage in 30 patients. Studies were generally at high risk of bias because of insufficient descriptions of both patient demographics and outcomes. No studies compared NPWT to dressings, and the existing literature is at high risk of bias. The included studies were of low-level evidence. NPWT can be neither recommended nor advised against to cover infected osteosynthesis.
Abbreviations: NPWT, negative pressure wound therapy; FRI, fracture-related infection
Keywords: NPWT, FRI, Dressing, Osteosynthesis
1. Introduction
Fracture-related infection (FRI) in internal osteosynthesis of the extremity is a limb-threatening complication of fracture treatment.1 Treatment options for FRI are versatile, including antibiotic suppression while the bone heals, debridement and antibiotic treatment, debridement and reosteosynthesis, or implant removal with antibiotic suppression. Removing the implant before healing can cause an unstable fracture, increase the risk of nonunion, and accelerate the ongoing infection.1 This raises the question of the possibilities in FRI when retaining the implant is desired.
Fracture healing requires vital tissue, but the most important step in infection control is debridement of the soft tissue. Thorough debridement potentially causes soft tissue defects and thus exposes the fracture and osteosynthesis material. This causes delayed wound healing and increased risks of contamination and reinfection, as well as accelerated development of bone and soft tissue necrosis.2 Applying a physical barrier between the osteosynthesis material and the surrounding environment is crucial to prevent bacteria from entering. Reconstructive surgery with flap coverage is a well-known method to cover wounds,3 but not all patients are candidates for this treatment. Reconstructive surgery is also not available in all hospitals. Another well-known and simpler barrier is applying a dressing to covering the defect.4 Numerous treatment options for dressings have been suggested, but none with any superior results.4 Another treatment option gaining increasing interest is negative pressure wound therapy (NPWT).5 NPWT ensures that the wound is closed with an airtight dressing from which excessive fluid is actively drained.5 It has not been shown to improve the healing of traumatic open wounds or fractures but has been demonstrated to yield faster wound healing in the treatment of chronic foot ulcers.2,6 NPWT has resulted in fewer reconstruction flaps7 and potentially fewer amputations in open tibia–fibula fractures. This study aimed to systematically evaluate the current literature on studies using NPWT or dressings in the treatment of FRI in internal osteosynthesis of the extremity. The primary objective was to investigate the effect on fracture healing of NPWT in comparison to dressings following FRI in internal osteosynthesis of the extremity. The secondary objective was to investigate the fracture healing time, wound healing, implant removal, rate of amputation, time in hospital, quality of life, rate of reconstructive surgery, and cost-effectiveness.
2. Materials and methods
2.1. Protocol and registration
This systematic review is reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statements.8 The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) before data extraction (registration number CRD42020199605).9
2.2. Eligibility criteria
The PICO model was used to create the research question: (P)articipants were patients with fracture related infections following internal osteosynthesis of fracture of the extremity; (I)ntervention was wound cover by NPWT; (C)omparator was all types of dressings; (O)utcome was fracture healing. Dressings were defined as all dressings other than NPWT, such as plain gauze, bandages and medicated bandages. The definition of fracture related infections is based on the algorithm described by Metsemakers et al. from 2018 for fractures treated with internal osteosynthesis.10 The secondary outcomes were fracture healing time, wound healing, implant removal, rate of amputation, quality of life, rate of reconstructive surgery, and cost-effectiveness. The inclusion criteria were published studies and patients over 15 years of age with a fracture related infection following internal osteosynthesis of a fracture of the extremity treated with NPWT, which include all infections needing coverage of dressings or NPWT with or without surgical debridement. The exclusion criteria were animal and cadaver studies; fractures treated with prostheses; face, head, neck, spine, and thoracic fractures; tumor or cancer surgery; external fixation; arthrodesis; and languages other than English, German, or Danish.
2.3. Information sources
We conducted a systematic literature search in four electronic databases: Embase, Medline, the Cochrane Library, and Scopus. The European Bone and Joint Infection Society11 and European Wound Management Association homepages were also searched for studies, but no further studies were included.12
2.4. Search
The search strategy was developed in collaboration with a scientific research librarian from the University of Southern Denmark. The search was made on both MeSH terms and free-text words in three blocs with synonyms for NPWT, dressing, osteosynthesis, and infection. The Boolean operator “AND” was used to combine the three blocs: “NPWT AND osteosynthesis AND infection” or “dressing AND osteosynthesis AND infection.” The Boolean operator “OR” was used between synonyms in each bloc. See Appendices A and B for the complete search string.
The search limitations were publications until 2021 in Scopus, April 2020 in the Cochrane Library, and April 17th, 2020, in Medline and Embase. The last search was performed on February 5th, 2021.
2.5. Study selection
The initial plan was to evaluate studies comparing NPWT to dressings following FRI in internal osteosynthesis of the extremity to perform a meta-analysis. In the initial literature screening, no such studies were found. We therefore decided to change the direction of the study towards any studies describing the use of either NPWT or dressings following FRI in internal osteosynthesis of the extremity.
The records were imported to EndNote to search for duplicates, then imported to Covidence (Veritas Health Innovation, Melbourne, Australia; available at www.covidence.org) for screening. The records were screened based on title and abstract, independently and blinded by the two main authors. The included studies were then full-text screened by the same two authors. Any disagreements were resolved by consultation with the senior author.
2.6. Data collection process
The data were extracted into an Excel sheet (Microsoft® Excel for Mac, Office 365 version 16.44) by one author and verified by another author. Any disagreements were resolved by a senior author.
For additional data, five authors were contacted,13, 14, 15, 16 and one17 responded with an anonymized datasheet. The data extracted resulted in an additional 51 patients for inclusion.
2.7. Data items
The variables registered for each study were title, author, year, patients in study, patients for inclusion, fracture type, age of patients (years), fracture healing, osteosynthesis, intervention, NPWT vacuum, vacuum flow, duration of NPWT, period of changes in NPWT, wound outcome, time to wound healing, time to reconstructive surgery, time to fracture healing, amputations, bacteria, type of antibiotics, days with antibiotics, wound and infection description, implant management, health-related quality of life, cost-effectiveness, and definition of FRI.
2.8. Risk of bias in individual studies
The risk of bias was assessed using the Critical Appraisal Checklist for Case Series and Case Reports from The Joanna Briggs Institute.18 Outcomes were presented as Yes, Unclear, or No in accordance with the checklist. Data were plotted into an Excel sheet (Microsoft® Excel for Mac, Office 365 version 16.44) by one author and verified by another author.
2.9. Statistics and synthesis of results
No summary measures or meta-analysis could be performed because the data presented great heterogeneity.
3. Results
3.1. Study selection
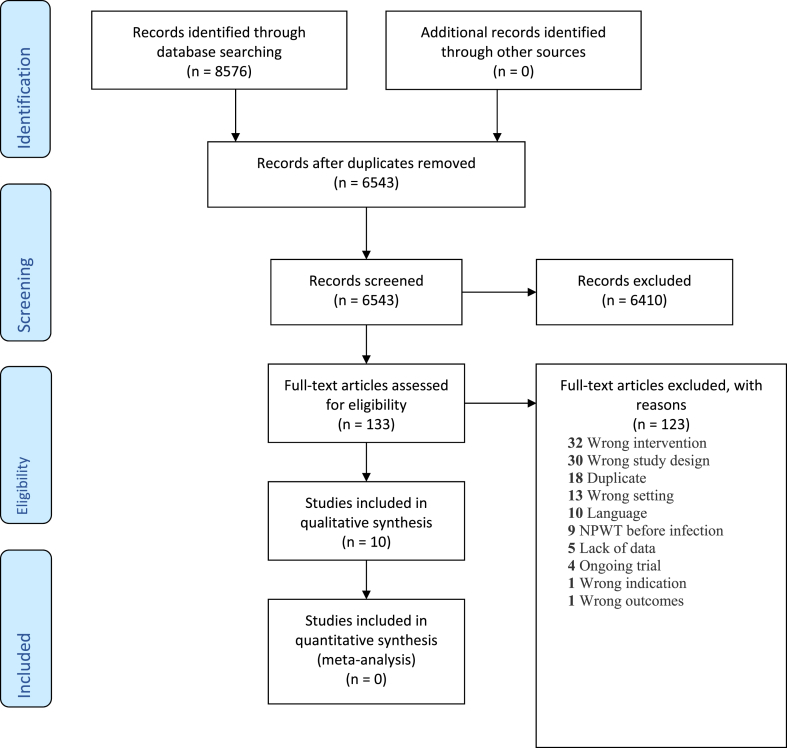
A total of 8576 records were identified; after removal of duplicates, 6543 studies were screened. No articles compared NPWT to dressings following FRI in internal osteosynthesis of the extremity.
Ten studies included either NPWT or dressings following FRI in internal osteosynthesis of the extremity and could therefore be included in the review (Fig. 1).
Fig. 1.
PRISMA flow diagram.
3.2. Included studies
The studies comprised seven case reports19, 20, 21, 22, 23, 24, 25 and three case series.17,26,27 Patient ages ranged from 12 to 83 years (Table 1). One study group had an age range of 12–61 years, and only three patients met the inclusion criteria, but the ages of these patients were not described further. One study included fractures of the upper extremity,17 and eight focused on lower leg fractures.19, 20, 21, 22, 23, 24, 25, 26 One study did not specify the anatomical region but included solely shaft fractures.27 The ten studies had a total of 201 patients. However, 86 patients in the ten studies did not meet the inclusion criteria (e.g., external fixation, no fracture, no infection, no osteosynthesis, prosthesis, and spine fracture), so only data on 115 patients could be analyzed (Table 1), 67 patients treated with NPWT and 48 patients treated with dressings.
Table 1.
Study demographics.
| Author | Year | Study design | Age (years)b | No. in study | No. for inclusion | Patient exclusion reasons |
|---|---|---|---|---|---|---|
| Anagnostakos et al.19 | 2006 | Case report | 58 | 6 | 1 | Prosthesis, spine fracture, no fracture |
| Grecu et al.20 | 2017 | Case report | 59 | 1 | 1 | None excluded |
| Izadpanah et al.17 | 2017 | Case series | 20–83 | 106 | 51 | External fixation, no fracture, spine fracture |
| Kollrack et al.21 | 2012 | Case report | 58–67 | 7 | 6 | External fixation |
| Marinovic et al.22 | 2014 | Case report | 35 | 1 | 1 | None excluded |
| Rawicki et al.26 | 2015 | Case series | 12-61a | 17 | 3 | No infection |
| Roth et al.27 | 1997 | Case series | Not described | 48 | 48 | None excluded |
| Sharp et al.23 | 2013 | Case report | 33, 83 | 10 | 2 | No osteosynthesis, Amputation before NPWT, no fracture, prosthesis, external fixation |
| Wijewardena et al.24 | 2011 | Case report | 24 | 4 | 1 | NPWT after reconstructive surgery, no osteosynthesis |
| Windhofer et al.25 | 2009 | Case report | 46 | 1 | 1 | None excluded |
The study group had an age range of 12–61 years. Only three patients met the inclusion criteria, but the age of these patients were not described.
Total age and range.
3.3. Study intervention
NPWT was described differently depending on the device available. Five studies described NPWT on wound defects, one study on wound breakdown, one on fistulas, one on unspecified deep wound infection, and one on swelling, redness, and pain (Table 2).
Table 2.
Wound treatment.
| Author | Wound outcome | Time for wound healinga | Wound and infection description | Time for infection after osteosynthesisa |
|---|---|---|---|---|
| Anagnostakos et al.19 | Secondary closure | Not described | Infected wound defect with visible implant | 3 weeks |
| Grecu et al.20 | Sural fasciocutaneous flap 28 days after NPWT | 31 days | Infected wound defect with visible implant | 4 days |
| Izadpanah et al.17 | Granulation in 1, secondary wound closure in 24, plastic reconstruction in 17, amputation in 4, unknown in 5 | Not described | Persistent infection or insufficient soft tissue coverage following open reduction and internal fixation, local infection signs, leukocytes and CRP | 2–341 days |
| Kollrack et al.21 | Mesh graft | 16.3 days | Sepsis with infected osteosynthesis | 38–51 days |
| Marinovic et al.22 | Wound healed 10 days after NPWT | 10 days | Skin defect, visible implant, secretion, fever, CRP, leukocytes, SR, pain | 2 months |
| Rawicki et al.26 | Wound healed within 2.5 months | Not described | Deep wound infection, not described if implant was visible | Not described |
| Roth et al.27 | Implant cover in 18 patients, and partial implant cover in 30 patients | Not described | Fever, pain, swelling, redness | From 3 days to several months |
| Sharp et al.23 | NPWT discontinued in one patient and wound healed in the other patient | Not described and 2 months | Wound breakdown, not described if implant was visible | 1 month |
| Wijewardena et al.24 | Skin graft | 108 days | Infected wound defect with visible implant | Not described |
| Windhofer et al.25 | Tensor fascia lata flap | 2 months | Purulence throughout fistula, local and systemic infections signs | 29 days |
Total days/months and range.
Vacuum pressure ranged from 80 mmHg to 150 mmHg, with continuous, intermittent, and combination flow modes. One study used NPWT for periods from zero to more than 50 days, whereas the other studies used NPWT for four to 108 days. The NPWT was changed somewhere between every two to seven days (Table 3).
Table 3.
Wound intervention.
| Author | Intervention described | NPWT vacuum | Vacuum flow | Period with NPWT | Period between exchange of NPWT |
|---|---|---|---|---|---|
| Anagnostakos et al.19 | Debridement, NPWT, antibiotics | 125–150 mmHg | Continuous | 30–50 days | 3–5 days |
| Grecu et al.20 | Debridement, Platelet Rich Plasma, NPWT, antibiotics | 140 mmHg | Continuous, then intermittent | 28 days | 48 h |
| Izadpanah et al.17 | Debridement, NPWT, antibiotics | Not described | Not described | From 0 to more than 50 days | 4–5 days |
| Kollrack et al.21 | Debridement, NPWT | 125 mmHg | Continuous, then intermittent | 53–57 days | 3–4 days |
| Marinovic et al.22 | Debridement, NPWT, antibiotics | 125 mmHg | Continuous, then intermittent | 10 days | 5 days |
| Rawicki et al.26 | Debridement, NPWT, antibiotics | Not described | Not described | 2.5 months | Not described |
| Roth et al.27 | Debridement, antiseptic dressing | – | – | – | – |
| Sharp et al.23 | 2 NPWT, 1 antibiotics | 80 mmHg | Not described | 1 and 3 weeks | 7 days |
| Wijewardena et al.24 | NPWT, activated protein C | 125 mmHg | Intermittent, 5 min on, 2 min off | 3 months and 18 days | Not described |
| Windhofer et al.25 | Debridement, NPWT, antibiotics | Not described | Not described | 4 days | Not described |
Fifty-seven out of 67 patients were treated with debridement, NPWT, and antibiotics. Six patients were treated solely with debridement and NPWT, and one patient was treated with NPWT and antibiotics. One patient was treated with debridement, platelet-rich plasma, NPWT, and antibiotics. One patient was treated with NPWT alone, and one patient was treated with NPWT and activated protein C (Table 3). Of the 67 patients treated with NPWT, osteosynthesis was retained in 25, exchanged in 14, and removed in 27; information was missing for one (Table 4).
Table 4.
Fracture and osteosynthesis management.
| Author | Fracture type | Osteosynthesis | Osteosynthesis management | Fracture healing | Fracture healing time |
|---|---|---|---|---|---|
| Anagnostakos et al.19 | Fibula | Plate, screws, unknown locking | Retained | Not described | Not described |
| Grecu et al.20 | Tibial malleolus | Plate, screws, unknown locking | Retained | Not described | Not described |
| Izadpanah et al.17 | Clavicle, humerus, forearm, femur, patella, tibia, calcaneus | Plate, nail, wires | 23 removed, 14 exchanged, 14 retained | 13 healed, 4 amputated, 34 not described | Not described |
| Kollrack et al.21 | Ankle | Plate, screws, unknown locking | 2 removed, 4 retained | Yes, all fractures healed | 8 weeks |
| Marinovic et al.22 | Tibia | Plate, screws, locking | Retained | Yes | 12 months |
| Rawicki et al.26 | Intraarticular calcaneal | Internal fixation | 2 removed, 1 retained | Not described | Not described |
| Roth et al.27 | Shaft fracture | Plate, screws, unknown locking | 48 removed | 44 spontaneous, 4 sequestrotomy | Not described |
| Sharp et al.23 | Tibia, Tibial plafond | Internal fixation | 1 not described, 1 retained | Not described | Not described |
| Wijewardena et al.24 | Metatarsal | Plate, screws, unknown locking | Retained | Not described | Not described |
| Windhofer et al.25 | Infracondylar tibia | Plate, screws, locking | Retained | Yes | 170 days |
The study by Roth et al. was the only study using dressings.27 In all 48 patients, the wounds were debrided and covered with gauze strips moistened with antiseptic 0.1% hexamethylene biguanide solution (Lavasept®).27 Eight out of 48 patients were treated with systemic antibiotics because of fever or signs of infection. Wound and infection descriptions included fever, pain, swelling, and redness.
3.4. Fracture healing
Fracture healing was achieved in 21 out of 67 patients in four studies with NPWT, four patients were amputated,17,21,22,25 and 42 patients had no description of fracture healing.17 Five studies did not describe fracture healing.19,20,23,24,26 In the dressing group, fracture healing was described as spontaneous in 44 cases, and four required additional sequestrectomy procedures.27
The time to fracture healing was only described in eight out of 67 patients treated with NPWT.21,22,25 Fractures healing time was described after 170 days for one patient in one study,25 8 weeks in six patients in another study,21 after 12 months for one patient in a third study.22 However, the time for fracture healing was not described in the remaining six studies. The time to fracture healing was not described for patients with dressing treatment [26] (Table 4).
The time from osteosynthesis to infection was 2–341 days and was described in eight studies.17,19, 20, 21, 22, 23,25,27 We cannot confirm whether all fractures were unhealed before infection since this was not described for all patients. For NPWT, implant management was not described for one patient, retained in 25 patients, removed in 75 patients, and exchanged in 14 patients.17,19, 20, 21, 22, 23, 24, 25, 26 In the dressing study, all 48 patients had their implants removed.27
3.5. Wound healing
Wound breakdown and protruding metal were the most frequently used descriptions of wound healing failure. In 57 out of 67 patients treated with NPWT, the wounds were described as healed, closed, or requiring additional soft tissue reconstruction (Table 2). Of the remaining ten patients, four were amputated, and wound healing was not described for six. Seventeen out of 67 patients treated with NPWT had additional reconstructive surgery. In 40 out of 50 patients (80%) treated with NPWT, the wounds were described as healed or covered without reconstructive surgery. One patient discontinued NPWT after 7 days due to a lack of regression of the wound bed. In the study with antiseptic dressings by Roth et al. complete wound coverage was achieved in 18 patients and partial coverage in 30 patients; thus, 18 out of 48 patients (38%) had complete wound coverage. The degree of coverage was evaluated at the time of implant removal and not described further.27
Five NPWT studies described the time to wound healing or closure ranging from ten to 108 days.20, 21, 22, 23, 24 Two NPWT studies described the time to reconstructive surgery after 14 and 21 days.20,25 Two patients treated with NPWT were described as diabetic.26 In the dressing study, only complete or partial wound coverage of osteosynthesis was described at removal. Two out of 48 patients had persistent fistulas after removal of the osteosynthesis at three and 11 years of follow-up, respectively.
3.6. Secondary outcomes
Staphylococcus aureus was the most frequently occurring bacterial strain, although not all studies described the strain (Table 5). The type of antibiotics used varied, and the treatment period ranged from 10 days to 7 weeks. Not all studies described the type of antibiotics or route of administration.
Table 5.
Bacterial strains and antibiotics.
| Author | Bacteria | Type of antibiotics | Period of antibiotics |
|---|---|---|---|
| Anagnostakos et al.19 | S. marcescens, S. aureus | Flucloxacillin, clindamycin, levofloxacin, unknown administration path | Not described |
| Grecu et al.20 | Not described | Ceftriaxone 2 g/day | 10 days |
| Izadpanah et al.17 | MRSA, pseudomonas aeroguinosa, staphylococcus aureus, Enterococcus faecium, Bacillus species, staphylococcus epidermidis, streptococcus equisimilis, enterobacter cloacae, stentrophomonas maltoplillae, acinetobacter baumannii, VRE, Peptostreptococcus Species, Streptococcus agalagticae | cephalosporin or bacteria-specific | Not described |
| Kollrack et al.21 | S. aureus, Enterococcus | Not described | Not described |
| Marinovic et al.22 | Staphylococcus spp. | Azithromycin | Not described |
| Rawicki et al.26 | Staphylococcus aureus or Serratia marcescens | Zosyn and Rifampin or Ceftriaxone, via peripherally inserted central catheter | Not described |
| Roth et al.27 | Not described | 8 patients treated with systemic antibiotics if fever or generalized infection signs | Not described |
| Sharp et al.23 | Not described | Intra venous for one patient | Up to 6 weeks |
| Wijewardena et al.24 | Not described | None | None |
| Windhofer et al.25 | Staphylococcus aureus and epidermidis | 1. stage (Clindamycin and vancomycin), 2. stage (levofloxacin and doxycyclin) | Stage 1 for 4 weeks and stage 2 for 3 weeks |
No studies described the health-related quality of life, cost-effectiveness, or definition of FRI.
3.7. Risk of bias
The included studies were critically assessed using the Critical Appraisal Checklist for Case Series and Case Reports from The Joanna Briggs Institute.18 The majority had a high risk of bias, as presented in Table 6, Table 7. No case reports described the demographic characteristics of the patients, and only two case reports included a sufficient description of the diagnostic tests used, methods, and results. No case series described their outcomes in a standard, valid, and reliable way.
Table 6.
Risk of bias case reports.
| Author | Year | Question 1 | Question 2 | Question 3 | Question 4 | Question 5 | Question 6 | Question 7 | Question 8 |
|---|---|---|---|---|---|---|---|---|---|
| Anagnostakos et al.19 | 2006 | Unclear | No | Unclear | Unclear | Unclear | Unclear | Unclear | No |
| Grecu et al.20 | 2017 | Unclear | Yes | Unclear | Yes | Yes | Unclear | Yes | No |
| Kollrack et al.21 | 2012 | Unclear | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Marinovic et al.22 | 2014 | Unclear | Yes | Yes | Unclear | Yes | Yes | No | Unclear |
| Sharp et al.23 | 2013 | Unclear | Unclear | Unclear | No | Unclear | Unclear | Unclear | Yes |
| Wijewardena et al.24 | 2011 | Unclear | Unclear | Unclear | No | Unclear | Unclear | Yes | Unclear |
| Windhofer et al.25 | 2009 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | No | No |
Data plotted as Yes (green) shows low risk of bias, Unclear (yellow) as moderate risk of bias, and No (red) as high risk of bias. Question 1–8 is described in Appendix C.
Table 7.
Risk of bias case series.
| Author | Year | Question 1 | Question 2 | Question 3 | Question 4 | Question 5 | Question 6 | Question 7 | Question 8 | Question 9 | Question 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Izadpanah et al.17 | 2017 | Yes | No | No | Yes | Yes | Unclear | Unclear | Unclear | No | Yes |
| Rawicki et al.26 | 2015 | Unclear | No | No | Yes | Yes | No | No | No | No | No |
| Roth et al.27 | 1997 | Unclear | No | No | Unclear | Unclear | No | No | Unclear | No | No |
Data plotted as Yes (green) shows low risk of bias, Unclear (yellow) as moderate risk of bias, and No (red) as high risk of bias. Question 1–10 is described in Appendix D.
4. Discussion
This is the first systematic review on the use of NPWT and dressings following FRI in internal osteosynthesis of the extremity. We did not find any studies comparing the use of NPWT with dressings following FRI. We found ten studies with a total of 115 patients with FRI treated with either NPWT or dressings. Fracture healing was described in less than one third of the patients, and the time to fracture healing was only described in seven out of 115 patients. The data were therefore too small to show an effect of NPWT versus dressings. Debridement was used in eight out of ten studies, which shows that this is a common step in the treatment of FRI.
Fracture healing is one of the main issues related to FRI and therefore the primary outcome in this review. The fracture location and type of osteosynthesis varied substantially in the studies, which could influence both fracture and wound healing. Osteosynthesis with plate and screws was described in eight out of ten studies, which could influence fracture healing since periosteal stripping might occur and thereby disturb the blood supply.28 Bones surrounded with vital tissue such as muscles have a better blood supply and thereby better fracture healing.28 Large wound defects at the fracture site may increase the risk of infection and compromised fracture healing, and therefore wound healing was our secondary outcome.
A limitation of this review is that the majority of the studies are case reports and therefore present low-level evidence. Case reports are mostly published with positive data or data that presents an effect of treatment and seldom negative data or no effect of treatment. This increases the risk of publication bias. The internal validity of this review is low because of the lack of transparency in the included studies, which is reflected by the poorly described patient demographics and confounders in the case reports. Smoking and diabetes are known risk factors for fracture healing29,30 but were only described in one study for two patients.
Most studies used a vacuum force of 125 mmHg, but this ranged from 80 to 150 mmHg and was not discussed in any studies. Further, some studies used both continuous and intermittent vacuum. Studies have described the risk of patient discomfort with the use of intermittent vacuum.31,32 These studies did not show increased wound contraction with vacuum higher than 75 mmHg, but more fluid drainage was noticed at 125 mmHg.31,32 Therefore, the vacuum force should be adjusted in accordance with the desired result. Animal studies have shown both no difference and more granulation tissue when comparing continuous versus noncontinuous NPWT.31,33,34 Consensus on this is therefore difficult. Additionally, studies using healthy young swine with well-defined clean wounds,31,33 which would normally heal on their own, are difficult to extrapolate to humans with infected irregular wounds with unhealed fractures and metal implants. Therefore, comparable studies are needed with different vacuum forces and continuous versus noncontinuous vacuum on infected human wounds.
The European Wound Management Association has published a compendium on the use of NPWT in visible osteosynthesis postoperatively.35 The recommendations include that NPWT can be used when covering the exposed metalwork is otherwise not possible.35 In addition, they state that NPWT should be used as a last attempt to prevent amputation.35 These recommendations rely on two studies: one on NPWT with exposed bone36 and one on an experimental model with porcine wounds.31 Thus, they do not rely on research on fractures with exposed metalwork. This amplifies the need for further studies on the use of NPWT following FRI with exposed internal osteosynthesis.
None of the included studies clearly defined FRI following internal osteosynthesis. Overall, a consensus is lacking on the definition, which has been described previously.10 A clear definition would enable easier comparison between studies. In addition, a definition of FRI would help surgeons decide whether an osteosynthesis is infected and when to surgically intervene, similar to the procedure for infected arthroplasties.37 In 2019, Govaert et al. published an FRI consensus definition that offered a guideline for surgeons to improve the comparison and quality of published literature.38
Although we included as many studies as possible given the language skills of our authors, many studies in Chinese were rejected, contributing to selection bias. Therefore, studies that compare NPWT to dressings may exist in other languages than those included in our study.
The use of the PRISMA statement makes this study systematic and transparent. All literature and data extraction was systematically and critically reviewed and evaluated for risk of bias by two authors, which strengthens the study. To find additional grey literature, we searched The European Bone and Joint Infection Society homepage11 and The European Wound Management Association homepage.12
We found few studies overall, with few participants, generally inferior quality, no identical definition on the indication for surgery, and no comparison of NPWT to dressings. This systematic review clarifies the need for studies to answer these questions. To raise the level of evidence, multicenter randomized clinical trials on a larger scale are desired with a clear definition and indication for surgery comparing NPWT with dressings to treat FRI in internal osteosynthesis of the extremity. This could include more patients and thereby stratify these, contributing to more transparency in which patients might benefit from these different treatment options.
Regarding the external validity and real-life applicability of this review, it is difficult to extrapolateing the findings into general recommendations is difficult because of the few included studies with low level of evidence and high risk of bias.
5. Conclusions
This study aimed to systematically evaluate the current literature on studies comparing NPWT with dressings following fracture-related infection in internal osteosynthesis of the extremity. No articles compared the two treatment methods. Few studies were found, with few patients and a low level of evidence. Fracture healing was rarely described, and wound healing was described more frequently but not enough to make an adequate comparison. No scientific evidence exists to recommend or advise against the use of NPWT to cover infected internal osteosynthesis materials, based on this systematic review.
CRediT authorship contribution statement
Niels Martin Jensen: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Signe Steenstrup: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. Christen Ravn: Conceptualization, Methodology, Validation, Supervision, Writing – review & editing. Hagen Schmal: Conceptualization, Methodology, Validation, Supervision, Writing – review & editing. Bjarke Viberg: Conceptualization, Methodology, Validation, Supervision, Writing – review & editing.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2021.101710.
Contributor Information
Niels Martin Jensen, Email: niels.martin.jensen@rsyd.dk.
Signe Steenstrup, Email: signe.steenstrup.jensen2@rsyd.dk.
Christen Ravn, Email: christen.ravn@rsyd.dk.
Hagen Schmal, Email: hagen.schmal@uniklinik-freiburg.de.
Bjarke Viberg, Email: bjarke.viberg@rsyd.dk.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Fang C., Wong T.M., Lau T.W., To K.K., Wong S.S., Leung F. Infection after fracture osteosynthesis - Part I. J Orthop Surg. 2017;25(1) doi: 10.1177/2309499017692712. 2309499017692712. [DOI] [PubMed] [Google Scholar]
- 2.Iheozor-Ejiofor Z., Newton K., Dumville J.C., Costa M.L., Norman G., Bruce J. Negative pressure wound therapy for open traumatic wounds. Cochrane Database Syst Rev. 2018;7:CD012522. doi: 10.1002/14651858.CD012522.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen U.K., Juul R., Bonde C.T., et al. A review of forty five open tibial fractures covered with free flaps. Analysis of complications, microbiology and prognostic factors. Int Orthop. 2015;39(6):1159–1166. doi: 10.1007/s00264-015-2712-z. [DOI] [PubMed] [Google Scholar]
- 4.Vermeulen H., Ubbink D.T., Goossens A., de Vos R., Legemate D.A. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005;92(6):665–672. doi: 10.1002/bjs.5055. [DOI] [PubMed] [Google Scholar]
- 5.Moues C.M., Vos M.C., van den Bemd G.J., Stijnen T., Hovius S.E. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12(1):11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong D.G., Lavery L.A., Diabetic Foot Study C. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 7.Parrett B.M., Matros E., Pribaz J.J., Orgill D.P. Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures. Plast Reconstr Surg. 2006;117(4):1315–1322. doi: 10.1097/01.prs.0000204959.18136.36. discussion 23-4. [DOI] [PubMed] [Google Scholar]
- 8.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.PROSPERO 27-04-2021 [available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020199605.
- 10.Metsemakers W.J., Morgenstern M., McNally M.A., et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49(3):505–510. doi: 10.1016/j.injury.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 11.European bone and joint infection Society 17-04-2021 [available from: https://ebjis.org.
- 12.The European wound management Association 17-04-2021 [available from: https://ewma.org.
- 13.Liu X., Liang J., Zhao J., et al. Vacuum sealing drainage treatment combined with antibiotic-impregnated bone cement for treatment of soft tissue defects and infection. Med Sci Mon Int Med J Exp Clin Res. 2016;22:1959–1965. doi: 10.12659/MSM.896108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollrack Y., Mollenhoff G. Ankle osteosynthesis infection : VVVacuum therapy as the treatment of choice. [German] Unfallchirurg. 2009;112(4):433–438. doi: 10.1007/s00113-008-1521-x. [DOI] [PubMed] [Google Scholar]
- 15.Pesch S., Hanschen M., Greve F., et al. Treatment of fracture-related infection of the lower extremity with antibiotic-eluting ceramic bone substitutes: case series of 35 patients and literature review. Infection. 2020;48:333–344. doi: 10.1007/s15010-020-01418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullner T., Mrkonjic L., Kwasny O., Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg. 1997;50(3):194–199. doi: 10.1016/s0007-1226(97)91369-2. [DOI] [PubMed] [Google Scholar]
- 17.Izadpanah K., Hansen S., Six-Merker J., Helwig P., Sudkamp N.P., Schmal H. Factors influencing treatment success of negative pressure wound therapy in patients with postoperative infections after Osteosynthetic fracture fixation. BMC Muscoskel Disord. 2017;18(1) doi: 10.1186/s12891-017-1607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moola S.M.Z., Tufanaru C., Aromataris E., et al. In: JBI Manual for Evidence Synthesis. Aromataris E., Munn Z., editors. 2020. Chapter 7: systematic reviews of etiology and risk. [Google Scholar]
- 19.Anagnostakos K., Kelm J., Schmitt E. Indications for use of the V.A.C.-system in the orthopedic surgery. Zentralblatt Chirurgie, Suppl. 2006;131(1):S87–S92. doi: 10.1055/s-2006-921514. [German] [DOI] [PubMed] [Google Scholar]
- 20.Grecu A., Nica O., Marinescu D., Vintila D., Ciurea M. Case presentation of soft tissue defect after bimalleolar fracture osteosynthesis. Against Curr. 2017;43(4):381–384. doi: 10.12865/CHSJ.43.04.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollrack Y.B., Moellenhoff G. Infected internal fixation after ankle fractures--a treatment path. J Foot Ankle Surg. 2012;51(1):9–12. doi: 10.1053/j.jfas.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Marinovic M., Ivandcic A., Spanjol J., et al. Treatment of hardware infection after osteosynthesis of lower leg using negative pressure wound therapy and transforming powder dressing. Coll Antropol. 2014;38(4):1233–1236. [PubMed] [Google Scholar]
- 23.Sharp E. Single-use NPWT for the treatment of complex orthopaedic surgical and trauma wounds. J Wound Care. 2013;22(10 Suppl):S5–S9. doi: 10.12968/jowc.2013.22.Sup10.S5. [DOI] [PubMed] [Google Scholar]
- 24.Wijewardena A., Vandervord E., Lajevardi S.S., Vandervord J., Jackson C.J. Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds. 2011;10(3):146–151. doi: 10.1177/1534734611417342. [DOI] [PubMed] [Google Scholar]
- 25.Windhofer C., Karlbauer A., Papp C. Bone, tendon, and soft tissue reconstruction in one stage with the composite tensor fascia lata flap. Ann Plast Surg. 2009;62(6):665–668. doi: 10.1097/SAP.0b013e31817e9c0f. [DOI] [PubMed] [Google Scholar]
- 26.Rawicki N., Wyatt R., Kusnezov N., Kanlic E., Abdelgawad A. High incidence of post-operative infection after 'sinus tarsi' approach for treatment of intra-articular fractures of the calcaneus: a 5 year experience in an academic level one trauma center. Patient Saf Surg. 2015;9(1) doi: 10.1186/s13037-015-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth B., Willenegger H., Dora C., Bienz R. Infection management with plates in situ. [German] Acta Chir Austriaca. 1997;29(SUPPL. 133):101–105. [Google Scholar]
- 28.Neagu T.P., Tiglis M., Cocolos I., Jecan C.R. The relationship between periosteum and fracture healing. Rom J Morphol Embryol. 2016;57(4):1215–1220. [PubMed] [Google Scholar]
- 29.Sloan A., Hussain I., Maqsood M., Eremin O., El-Sheemy M. The effects of smoking on fracture healing. Surgeon. 2010;8(2):111–116. doi: 10.1016/j.surge.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Jiao H., Xiao E., Graves D.T. Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep. 2015;13(5):327–335. doi: 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lessing M.C., James R.B., Ingram S.C. Comparison of the effects of different negative pressure wound therapy modes-continuous, noncontinuous, and with instillation-on porcine excisional wounds. Eplasty. 2013;13:e51. [PMC free article] [PubMed] [Google Scholar]
- 32.Borgquist O., Ingemansson R., Malmsjo M. The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127(2):551–559. doi: 10.1097/PRS.0b013e3181fed52a. [DOI] [PubMed] [Google Scholar]
- 33.Malmsjo M., Gustafsson L., Lindstedt S., Gesslein B., Ingemansson R. The effects of variable, intermittent, and continuous negative pressure wound therapy, using foam or gauze, on wound contraction, granulation tissue formation, and ingrowth into the wound filler. Eplasty. 2012;12:e5. [PMC free article] [PubMed] [Google Scholar]
- 34.Dastouri P., Helm D.L., Scherer S.S., Pietramaggiori G., Younan G., Orgill D.P. Waveform modulation of negative-pressure wound therapy in the murine model. Plast Reconstr Surg. 2011;127(4):1460–1466. doi: 10.1097/PRS.0b013e31820a63cb. [DOI] [PubMed] [Google Scholar]
- 35.Apelqvist J., Willy C., Fagerdahl A.M., et al. EWMA document: negative pressure wound therapy. J Wound Care. 2017;26(Sup3):S1–S154. doi: 10.12968/jowc.2017.26.Sup3.S1. [DOI] [PubMed] [Google Scholar]
- 36.Horch R.E., Dragu A., Lang W., et al. Coverage of exposed bones and joints in critically ill patients: lower extremity salvage with topical negative pressure therapy. J Cutan Med Surg. 2008;12(5):223–229. doi: 10.2310/7750.2008.07073. [DOI] [PubMed] [Google Scholar]
- 37.Osmon D.R., Berbari E.F., Berendt A.R., et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 38.Glaudemans A., Bosch P., Slart R., FFA I.J., Govaert G.A.M. Diagnosing fracture-related infections: can we optimize our nuclear imaging techniques? Eur J Nucl Med Mol Imag. 2019;46(8):1583–1587. doi: 10.1007/s00259-019-04378-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.