Abstract
The effects of a blend of encapsulated organic acids with essential oils (EOA) as an alternative to antimicrobial growth promoter (AGP) on growth performance and gut health of Eimeria spp./Clostridium perfringens (C. perfringens) in chickens infected with necrotic enteritis (NE) broilers was investigated. A total of 432 male Arbor Acres broilers (1-day-old) were randomly distributed into 6 treatment groups, namely noninfected negative control (A); NE-infected positive control (D); NE-infected broiler chickens fed a basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD) plus 90 mg/kg monensin; and NE-infected broiler chicken fed 200; 500; and 800 mg/kg EOA (E, F, G, and H group). Feeding EOA at 200 and 500 mg/kg considerably improved the feed conversion ratio, reduced gut lesions, serum fluorescein isothiocyanate dextran level, and C. perfringens load in the caecum and liver of the NE-infected broiler chickens. This feed was similar to AGP. Furthermore, the increased villous height-to-crypt depth ratio and goblet cells counts, upregulated claudin-1, glucagon-like peptide-2 (GLP-2), insulin-like growth factor-2 (IGF-2) mRNA gene expression, downregulated occludin, zonula occludens-1 (ZO-1), toll-like receptor (TLR-4), interleukin (IL-1β), interferon γ (IFN-γ), TNF receptor-associated factor 6 (TRAF-6), tumor necrosis factor superfamily member 15 (TNFSF15), and Toll-interacting protein (Tollip) genes expression in the jejunum were observed in the NE-infected broiler chickens that received EOA at 200 and 500 mg/kg compared with those of the single NE-challenged groups without EOA supplementations (P < 0.05). The 16S analysis revealed that EOA supplemented with 200 or 500 mg/kg enriched relative abundance of Lactobacillus, unclassified_Lachnospiraceae, and Enterococcus, and carbohydrate metabolic pathways but suppressed unclassified_Erysipelotrichacease and organismal systems involved in the immune system (P < 0.05). Feeding EOA could alleviate NE-induced gut impairment and growth depression and modulate cecal microbiota composition, which has potential as antimicrobial alternatives.
Key words: encapsulated essential oils and organic acids mixture, necrotic enteritis, intestinal health, broiler chickens
INTRODUCTION
Necrotic enteritis (NE) is a common disease in poultry flocks. This disease typically occurs in broiler chickens and is characterized by reduced weight gains, increased mortality, poor feed conversion ratio (FCR), and considerable economic losses. Critically, contaminated chicken products can cause serious health problems in humans (Timbermont et al., 2011; Wade et al., 2015). In the last decades, antibiotics were added to animal feed to not only promote growth but also diminish intestinal pathogen concentration and incidence of enteric diseases such as NE. Antibiotics as antimicrobial growth promoter (AGP) for poultry have gradually been banned or limited in some countries, including EU, USA, Canada, Mexico, Japan, Hong Kong, and China, because of the increasing antimicrobial resistance and drug residue in poultry products (Salim et al., 2018). However, research data have revealed that the removal of AGPs from poultry feed causes frequent occurrence of enteric disorders, such as NE and reduced performance, in many countries (Kaldhusdal et al., 2016). Therefore, effective and safe alternatives should be proposed to prevent undesirable effects, such as occurrence of NE, resulting from the removal of AGPs from chicken diets.
Essential oils (EOs) have been used as a promising in-feed antibiotics alternatives due to their natural, low-toxicity, and no-residue properties (Bassolé and Juliani, 2012). In vitro and in vivo trials have demonstrated that EOs exhibit different biological functions, including antioxidant status (Chowdhury et al., 2018; Pirgozliev et al., 2019b) and antibacterial, anti-inflammatory (Liu et al., 2019), antiviral (El-Shall et al. 2020), and antiparasitic activities (Bozkurt et al., 2016). Poultry studies have revealed that the inclusion of EOs into chicken diets could not only improve growth performance (Cross et al., 2007; Hashemipour et al., 2015; Pirgozliev et al., 2015, 2019a; Sun et al., 2015; Peng et al., 2016; Liu et al., 2017, 2018; Chowdhury et al., 2018; Wang et al., 2019) and regulate the gut microbiota compositions (Hume et al. 2011) but also considerably reduce growth loss and alleviate negative effects caused by pathogenic Salmonella (Alali et al. 2013), Escherichia coli (Liu et al., 2018), Clostridium perfringens (Mitsch et al., 2004; Jerzsele et al., 2012; Du et al., 2015, 2016; Sun et al., 2015; Yin et al., 2017), and Eimeria spp (Bozkurt et al., 2016; Upadhaya et al., 2019).
Organic acids (OAs) are also termed as fatty acids characterized by aliphatic chains with 4–28 carbons. OAs are chemically classified according to their carbon chain length into short-chain fatty acids (SCFAs; 1–6 carbon atoms), medium-chain fatty acids (MCFAs; 7–12 carbon atoms), or long chain fatty acids (LCFAs; 13-21 carbon atoms) (Ferronato and Prandini, 2020). OAs exhibit numerous physiological effects, including antimicrobial activity, antistress effect, gut development promotion, immune modulation, and energy supply for intestinal cells, which eventually leads to a positive effect on animal health and their productivity. Thus, they are excellent feed additive and promising antibiotic alternatives in livestock (Zentek et al., 2011; Abudabos et al. 2016; Emami et al., 2017; Polycarpo et al. 2017; Song et al. 2017; Dittoe et al. 2018; Nguyen et al. 2020). According to studies on poultry, dietary coated OA administration improves nutrient digestibility, increases the populations of beneficial microflora (Lactobacillus spp.), reduces harmful bacteria counts (C. perfringens, E. coli and Salmonella spp.) in gut contents, allowing OAs to function along the gastrointestinal tract (GIT) (Abudabos and Al-Mufarrej, 2014; Dai et al., 2021) and exhibit antimicrobial activities against poultry pathogen infections, including E. coli (Kazempour and Jahanian, 2017; Khodambashi Emami et al., 2017), Salmonella spp. (Van Immerseel et al., 2006; Abudabos et al., 2016; Kazempour and Jahanian, 2017), and C. perfringens (Geier et al., 2010); thereby improving intestinal immune responses and mucosal integrity and function. For example, MCFA (caproic, caprylic, and capric acid) decreases the number of Salmonella in chicken (Upadhyaya et al., 2015). Adding an OA mixture (comprising 30% lactic acid, 25.5% benzoic acid, 7% formic acid, 8% citric acid, and 6.5% acetic acid) in broiler diets improves growth performance (Fascina et al., 2012). Furthermore, 30% sodium n-butyrate upregulated claudin-1, claudin-4, ZO-1, occludin, LEAP-2, and mucin-2 levels are found in the jejunum (Song et al., 2017).
The combination of lipophilic EOs with hydrophobic OAs in animal and poultry feed as antibiotics alternatives has received considerable research attention because combining EOs and essential OA (EOA) products is efficacious on growth performance, intestinal microbiota community, antimicrobial action, and intestinal health in broiler chickens (Liu et al., 2017; Omonijo et al., 2018; Yang et al., 2018, 2019; Abdelli et al., 2020; Stefanello et al., 2020). For example, several protected combinations of EOs and OAs products have been reported to effectively reduce Salmonella (Cerisuelo et al., 2014; Zhang et al., 2019), E. coli (Basmacioğlu-Malayoğlu et al., 2016; Yang et al., 2019), C. jejuni infection in chickens (Thibodeau et al., 2015). Studies have revealed that dietary supplementation of microencapsulated and targeted-release EOAs blends can improve gut microbiome, morphological traits, and absorptive capacity of the intestine of broiler chickens when exposed to C. perfringens infection (Timbermont et al., 2010; Jerzsele et al., 2012; Abdelli et al., 2020; Stamilla et al., 2020; Stefanello et al., 2020).
However, the efficacy of EOA products for chicken growth performance and gut health was influenced by many factors, such as the structure of EOs or OAs, EOA formula composition, EOA coating and dosage, chicken health status, dietary form and composition, and rearing in environmental and hygienic conditions (Zhai et al., 2018). In this study, we assessed the effects of a novel EO and OA mixture product on the growth performance, gut microbiota composition, immune responses, and gut barrier function of NE-infected broiler chickens and compared the results with findings for in-feed antibiotic bacitracin methylene disalicylate (BMD). We hypothesized that these EOAs could be used as alternatives for AGP to improve the growth and gut health and control NE infection in broiler chickens.
MATERIALS AND METHODS
Broiler Chickens, Diets, and Experimental Design
All the procedures implemented in this study were approved by the Animal Care and Use Committee of China Agricultural University (statement no: SYXK 2019-0026).
A total of 432 male Arbor Acres broiler chickens (1-day-old) procured from a local commercial hatchery were weighed and randomly allocated to 36 floor pens with 6 dietary treatment groups, with 6 replicates and 12 chickens per pen. The experimental treatment proceeded as follows: 1) nonchallenged negative control (A, fed with a basal diet); 2) challenged positive control (D; fed with basal diet + co-challenged with sporulated oocysts of Eimeria and C. perfringens); 3) AGP group (E; infected broiler chickens fed the basal diet with 250 mg/kg BMD plus 90 mg/kg monensin); 4) Three inclusion levels of EOA-treated groups (F, G, H groups; infected broiler chickens received the basal diet with 200, 500, and 800 mg EOA/kg of diet, respectively). The blend of encapsulated OAs and EOAs used in the experiment was provided by a commercial company (Menon Co., Ltd., Shanghai, China). Its active ingredients were 4% carvacrol, 4% thyme, 0.5% hexanoic, 3.5% benzoic, and 0.5 % butyric acid. The ingredient composition of the basal diets refers to Arbor Acres broiler chicken nutrient specifications (Aviagen Arbor Acres broiler Nutrition Specifications, 2019). The experimental basal feed was antibiotic-free and coccidiostat-free corn-soybean pelleted diet. The experiment performed in environment floor pens and temperature-controlled rooms at a Zhuozhou poultry farm (Hebei, China). All the pens were within the same environmentally controlled facility, which was equipped with a nipple drinker and a plastic feeder. Chickens had free access to feed and water. The temperature, lighting program, and relative humidity were set according to the commercial Arbor Acres manual.
NE Challenge
The previously described NE model with minor modifications was used in this study (Shojadoost et al., 2012). At d 14, all the groups in the experiment (except group A) were orally gavaged with the mixed oocysts containing Eimeria maxima and Eimeria necatrix (1.0 × 104 oocysts/chick, 5.0 × 103 oocysts/chick, respectively) provided by Dr. Suoxun, College of Veterinary Medicine, China Agricultural University. Four d after mixing Eimeria oocysts, infected chickens were continuously inoculated through the crop with C. perfringens type A CVCC52 (1 mL/chick/d, 2.2 × 108 CFU/mL, China Veterinary Culture Collection Center, China Institute of Veterinary Drug Control, Beijing, China) on d 18, 19, and 20. Similarly, broiler chickens in the negative control group (unchallenged) received 1 mL/chick of sterile thioglycollate broth. Chickens were starved for 8 h before inoculation.
Measurement of Growth Performance Parameters (Traits)
On d 1, 21, and 42, pen feed intake and chicken body weight were weighed and recorded. All broiler chickens were examined daily, and chicken deaths were recorded. Average body weight gain (BWG), average feed intake (AFI), and FCR were calculated for all the phases.
Gut Lesion Scoring and Samples Collection
On d 7 after d postinfection (7DPI), 2 broiler chickens/pen were randomly selected and weighed individually, and cervical dislocation was performed to euthanize them. The duodenum, jejunum, and ileum were collected and scored for NE lesions for each of the upper and lower gut on a scale of 0–4 by 3 independent observers, as previously described (Gholamiandehkordi et al., 2007). The middle intestinal sections of the jejunum were cut off (approximately 1 cm) and carefully washed with sterile saline and subsequently placed into a sterile tube and snap frozen in a liquid nitrogen solution and stored at −80°C until subsequent analysis for mRNA expression. Another jejunum sample (approximately 2 cm) was rinsed in 0.9% of physiological saline and stored in 4% buffered paraformaldehyde solution at 21°C for subsequent morphological analysis (Wu et al., 2019). Liver samples and cecal samples from each broiler were aseptically collected into sterile tubes, immediately frozen in liquid nitrogen, stored at −80°C, and transported to the laboratory for microbiota culture and microbial 16S rRNA analyses.
Assay of Jejunum Morphology and Goblet Cells
The jejunum morphological structure was determined according to a previously described method (Shao et al., 2013). The jejunal samples were washed with phosphate buffer solution (PBS) and then fixed in a 4% paraformaldehyde solution. The fixed samples were dehydrated, cleared, and infiltrated with paraffin in a tissue processor, and then embedded in paraffin blocks. The blocks were sectioned in 5.0-µm-thick slices, with 2 slices per block. The sections were placed on a glass slide and stained with hematoxylin and eosin. Morphometric analysis was performed using a Nikon phase-contrast microscope coupled with a MicroComp integrated digital imaging analysis system (Nikon Eclipse 80i, Nikon, Tokyo, Japan). At least 5 villi and 5 crypt per section and 2 sections per segments were individually measured for determining the villi height (VH) and crypt depth (CD), and their average values were used for statistical analyses. Average VH, CD, and VH/CD ratios were calculated. Goblet cells (GC) in the jejunum segments were stained with periodic acid-Schiff (PAS) as stated previously (Shao et al., 2013). The number of PAS positive cells per villi was measured and counted by using image J software.
Microbiological Measurements
Approximately 1 g of the collected liver and cecal samples of each replicate were analyzed to determine the number of lactic acid bacteria, Coliform bacteria (caecal samples only), and C. perfringens (both liver and cecal samples) by using culture methods on selective media: C. perfringens on cycloserine supplemented tryptose sulfite cycloserine agar media; E. coli on eosin-methylene blue agar, and Lactobacillus on de Man, Rogosa, Sharpe agar media. These samples were homogenized and diluted from 10−1 to 10−7 with the PBS solution under sterile conditions and subsequently plated on selective agar plates to detect bacteria. The incubation conditions and calculation of the number of colony-forming units were followed according to the conventional methods in microbiology (Wu et al., 2019).
Serum Fluorescein Isothiocyanate Dextran Determination
At 7 DPI, 2 chickens were oral gavaged fluorescein isothiocyanate dextran (FITC-d-mol weight (MW) 3000–5000, Sigma - Aldrich, St. Louis, MO) at 8.32 mg/mL/chick. At 1 and 2.5 h after FITC-d gavage, blood samples were collected through wing veins, and centrifuged at 3000 rpm for 10 min to separate serum and stored at −40°C until measurement of the serum FITC-d level according to the previously described method (Baxter et al., 2017).
mRNA Expression Analysis by Using Quantitative Real-Time Polymerase Chain Reaction
The trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) was used to isolate total RNA of jejunum mucosa (∼60 mg) according to manufacturer's instructions. RNA quality was evaluated on an agarose gel, and the concentration and purity of the extracted RNA were measured using a Nanodrop-2000 spectrophotometer (260 and 260/280 nm) (Thermo Fisher Scientific, Waltham, MA). Next, a Primer ScriptRT reagent Kit (Takara Bio Inc.) was used to reverse transcribed RNA to complementary DNA and stored at −20°C until analysis. The target gene sequences and primers for the reference gene are presented in Tables 2 and 3. The expressions of TLR-2, TLR-4, MyD88, TRAF-6, NF-kB, TNFSF15, IL-1β, IL-8, IFN-γ, IL-10, Tollip, PI3K, A20, SOCS-1, SOCS-6, tight junction proteins genes, growth factors genes (Claudin-1, Occlaudin, ZO-1, Mucin-2, TGF-β3, IGF-2, EGFR, GLP-2), and housekeeping gene β-actin were quantified using SYBR Premix Ex TaqTM kits (TaKaRa) on the Applied Biosystems 7500 Fast Real-Time PCR System. The specificity and efficiency of the primer was determined by conducting melt curve analysis. The 2 −ΔΔCT method was used to calculate the levels of mRNA expressions of target genes using β-actin as the reference gene (Livak and Schmittgen, 2001).
Table 1.
Composition of the experimental basal diet, %.
| Items | Starter (d 1–21) | Grower and finisher d (22–42) |
|---|---|---|
| Composition, % | ||
| Corn (CP 7.8%) | 39.70 | 57.0 |
| Soybean meal (CP 46.0%) | 33.0 | 30.0 |
| Wheat | 19.0 | 0 |
| Soybean oil | 4.00 | 4.40 |
| Wheat middlings | 0 | 5 |
| Limestone | 1.50 | 1.50 |
| Dicalcium phosphate | 1.50 | 1.36 |
| Sodium chloride | 0.30 | 0.30 |
| DL-Methionine, 98% | 0.27 | 0.19 |
| L-Lysine sulfate, 78% | 0.20 | 0.11 |
| Choline chloride, 50% | 0.25 | 0.15 |
| Mineral Premixa | 0.20 | 0.20 |
| Vitamin Premixb | 0.03 | 0.03 |
| Phytase | 0.02 | 0.03 |
| Ethoxyquinoline, 33% | 0.05 | 0.03 |
| Total | 100 | 100 |
| Calculated nutrient | ||
| Metabolizable energy, Mcal/kg | 3.02 | 3.10 |
| Crude protein, % | 21.37 | 19.27 |
| Calcium, % | 0.99 | 0.93 |
| Available phosphorus % | 0.45 | 0.43 |
| Digestible Lysine, % | 1.20 | 1.05 |
| Digestible Methionine, % | 0.57 | 0.46 |
| Digestible Methionine + Cysteine, % | 0.90 | 0.78 |
Composition of vitamin premix provided per kg of complete diet: vitamin A (retinyl acetate), 12,500 IU; vitamin D3 (cholecalciferol), 2500 IU; vitamin E (DL-a-tocopherol acetate), 30 IU; vitamin K3 (menadione sodium bisulfate), 2.65 mg; vitamin B12 (cyanocobalamin), 0.025 mg; biotin, 0.30 mg; folic acid, 1.25 mg; nicotinic acid, 50 mg; d-pantothenic acid, 12 mg; pyridoxine hydrochloride, 6.0 mg; riboflavin, 6.5 mg; thiamine mononitrate, 3.0 mg.
Mineral premix provided per kg of complete diet: iron, 80 mg; copper, 8 mg; manganese, 100 mg; zinc, 80 mg; iodine, 0.35 mg; selenium, 0.15 mg.
Table 2.
Nucleotide sequences of primers (TLR-mediated signaling pathway-related cytokines, chemokines and negative regulators) for quantitative real-time PCR assay.
| Genes | Primer sequence (forward and reverse) | Accession number |
|---|---|---|
| TLR-2 | F: GGGGCTCACAGGCAAAATC | NM_001161650.1 |
| R: AGCAGGGTTCTCAGGTTCACA | ||
| TLR-4 | F:CCACTATTCGGTTGGTGGAC | NM_001030693.1 |
| R:ACAGCTTCTCAGCAGGCAAT | ||
| TNFSF15 | F- CCAAGAGCACACCTGACAGT | NM_001024578.1 |
| R- CACAGGTATCACCAGTGCGT | ||
| MyD88 | F:GGATGGTGGTCGTCATTTCA | NM_001030962.1 |
| R:GAGATTTTGCCAGTCTTGTCCA | ||
| TRAF-6 | F: CACAGAGGAGACGCAGGGATA | XM_001235884.1 |
| R: AACAGATCGGGCACTCGTATTT | ||
| NF-kB | F:TGGAGAAGGCTATGCAGCTT | NM_205134.1 |
| R:CATCCTGGACAGCAGTGAGA | ||
| IL-1β | F-CAGCAGCCTCAGCGAAGAG | NM_204524.1 |
| R-CTGTGGTGTGCTCAGAATCCA | ||
| IL-8 | F-GGCTTGCTAGGGGAAATGA | AJ009800 |
| R-AGCTGACTCTGACTAGGAAACTGT | ||
| IL-10 | F:CGCTGTCACCGCTTCTTCA | NM_001004414.2 |
| R:CGTCTCCTTGATCTGCTTGATG | ||
| IFN-γ | F-AAAGCCGCACATCAAACACA | NM_205149.1 |
| R-GCCATCAGGAAGGTTGTTTTTC | ||
| Tollip | F:CATGGTACCTGTGGCAATACC | NM_001006471 |
| R:GCACTGAGCGGATTACTTCC | ||
| PI3K | F:AACATCTGGCAAAACCAAGG | NM_001004410 |
| R:CTGCAATGCTCCCTTTAAGC | ||
| A20 | F:GAGAACGCAGAGCCTACACC | NM_001277522.1 |
| R:CCAACCTTCTTCCTGCACAT | ||
| SOCS-1 | F:GCTCTCAGGCTCGAGGTTAC | NM_001137648.1 |
| R:GCTTGCTCGAGTGATGCTACT | ||
| SOCS-6 | F: CAGATATCTTTGTGGACCAGGCAGTGAA | NM_001127312 |
| R: GGTAGCAAAGGTGAAAGTGGAGGGACATC |
Abbreviations: A20, protein A20; IFN-γ, interferon γ; IL, interleukin; MyD8, myeloid differential protein-88; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol 3-kinase; SOCS, suppressor of cytokine signaling; TLR, toll-like receptor; TNFSF15, tumor necrosis factor superfamily member 15; Tollip, toll-interacting protein; TRAF-6, TNF receptor-associated factor 6.
Table 3.
Nucleotide sequences of primers (tight junction proteins and growth factors) for quantitative real-time PCR assay.
| Genes | Primer sequence (forward and reverse) | Accession number |
|---|---|---|
| Tight junctions Claudin-1 |
F: AAGTGCATGGAGGATGACCA |
NM_001013611.2 |
| R: GCCACTCTGTTGCCATACCA | ||
| Occlaudin | F:TCATCGCCTCCATCGTCTAC | NM_205128.1 |
| R:TCTTACTGCGCGTCTTCTGG | ||
| ZO-1 | F: TATGAAGATCGTGCGCCTCC | XM_015278981.1 |
| R: GAGGTCTGCCATCGTAGCTC | ||
| Mucin-2 | F: AGCGAGATGTTGGCGATGAT | NM_001318434.1 |
| R: AAGTTGCCACACAGACCACA | ||
| Growth factors TGF-β3 |
F:TGCGGCCAGATGAGCAT |
NM_205454.1 |
| R:TGCACATTCCTGCCACTGA | ||
| IGF-2 | F: TGGCTCTGCTGGAAACCTAC | NM_001030342.2 |
| R: ACTTGGCATGAGATGGCTTC | ||
| EGFR | F: ACCAGCCTGCAGAGAATGTA | NM_205497 |
| R: CACCATGTTAAGCGCAATGA | ||
| GLP-2 | F:AAGCTTCCCAGTCTGAACCA | NM_205260.4 |
| R:ATCCTGAGCTCGTCTGCTGT | ||
| House-keeping genes | ||
| β-actin | F: GAGAAATTGTGCGTGACATCA | NM 205518 |
| R: CCTGAACCTCTCATTGCCA |
Abbreviations: EGFR, epidermal growth factor receptor; GLP-2, glucagon-like peptide-2; IGF-2, insulin-like growth factor-2; TGF- β3, transforming growth factor beta 3; ZO-1, zonula occludens-1.
DNA Extraction, 16S rRNA Gene Sequencing, and Analysis
The microbial genome DNA of the caecal sample (d 28 of age) was extracted using the QIAamp Fast DNA stool mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Before PCR amplification, quantity and quality of DNA were determined using a Nanodrop-2000 spectrophotometer. DNA integrity was analyzed using agarose gel electrophoresis. Subsequently, the qualified DNA was used as a template for the microbial 16S RNA V3-V4 gene region to be amplified with barcoded primer pair: F341 (5’-ACTCCTACGGGAGGCAGCA-3’) and R806 (5’-GGACTACHVGGGTWTCTAAT-3’) (using the KAPA HiFi Hotstart Ready Mix PCR kit - Kapa Biosystems, Wilmington, MA). The PCR amplification process and conditions used were as follows: 94°C for 5 min, 95°C for 30 s (30 cycles), 50°C for 30 s, 72°C for 30 s, and 72°C for 5 min. The PCR amplification products were analyzed by 2% agarose gel electrophoresis and purified with QIA quick Gel Extraction Kit (QIAGEN, Hilden, Germany). The Illumina MiSeq PE250 platform (Illumina, Santa Clara, CA) was used to determine amplicon libraries sequencing with MiSeq Reagent Kit and by following standard protocols (Shanghai Personal Biotechnology Co., Ltd., Shanghai, China).
The raw data and Quantitative Insights into Microbial Ecology (QIIME) were filtered and demultiplexed by using the Illumina MiSeq platform (version 1.8.0-dev). Raw tags were obtained by merging sequence data using FLAST (Magoc and Salzberg, 2011). Forward and reverse sequences were combined after trimming and then uploaded to QIIME to analyze their richness (Caporaso et al., 2011). The sequences and reference operational taxonomic units (OTU 97% similarity) were clustered and classified by using UCLUST and with QIIME software. Next, MOTHUR software was used to conduct α-diversity and β-diversity analysis (Lozupone et al., 2007). The Mann–The Whitney U test was used to compare significant differences between microbiota components, and the principal component was analyzed based on the level of phylum and genus compositional profiles (Ramette, 2007). A Venn diagram was created with R software to visualize the shared and unique OTUs among samples based on Silva taxonomic database (Zaura et al., 2009). Differences of microorganisms between experimental groups were determined using PLS-DA (partial least squares discriminant analysis) through “mix Omics” (Chen et al., 2011). Furthermore, the phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) was used to predict the functionality of cecal microbiota by relying on high-quality sequences (Langille et al., 2013). The functions were deduced using Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations for level 2 pathways.
Statistical Analysis
The one-way analysis of variance (ANOVA) in SPSS 20.0 (SPSS Inc., Chicago, IL) was used to analyze the results for performance, gut lesion scores, level of cecum and liver C. perfringens load, jejunum histomorphology, serum FITC-d concentration, and relative mRNA expressions by using the multivariable and univariable ANOVA to compare the differences between groups. Mean separations were performed by using Tukey's multiple comparison test. The statistical analysis of the number of bacteria converted to log colony-forming units. The Kruskal–Wallis test with Benjamini–Hochberg P-value correction was used to calculate species abundances for comparing groups. In this experiment, P < 0.05 was used as the value for statistical significance, and 0.05 ≤ P ≤ 0.10 was used because these values tend to be reliable.
RESULTS
Growth Performance
Table 4 presents the results of growth performance. During d 1 to 21, NE infection (D) significantly reduced BWG and AFI and increased feed consumption/weight gain ratio (F:G) compared with the noninfected negative group (A). The infected broiler chickens received antibiotics growth promoters (D) (P < 0.05). No difference was observed for aforementioned parameters compared with the other infected broiler chickens fed with different doses of EOA.
Table 4.
Effects of dietary EOA supplementation on growth performances of broiler chickens coinfected with Eimeria maxima and Clostridium perfringens1.
| Items | Experimental design |
SEM2 | P-values3 | |||||
|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | |||
| D1 to 21 | ||||||||
| BWG, g/bird | 870a | 655b | 830a | 657b | 647b | 636b | 16.25 | <0.01 |
| AFI, g/bird | 1249a | 1047b | 1219a | 1042b | 1057b | 1028b | 15.57 | <0.01 |
| FCR, g/g | 1.44b | 1.60a | 1.46b | 1.60a | 1.64a | 1.62a | 0.01 | <0.01 |
| D22 to 42 | ||||||||
| BWG, g/bird | 1904 | 1891 | 2071 | 1929 | 1919 | 1840 | 23.67 | 0.059 |
| AFI, g/bird | 3095 | 3114 | 3275 | 3047 | 3083 | 2976 | 31.75 | 0.120 |
| FCR, g/g | 1.63ab | 1.65a | 1.58bc | 1.56c | 1.60abc | 1.62ab | 0.01 | 0.011 |
| d1 to 42 | ||||||||
| BWG, g/bird | 2774a | 2546b | 2904a | 2596b | 2566b | 2476b | 32.38 | <0.01 |
| AFI, g/bird | 4344ab | 4161bc | 4494a | 4089bc | 4140bc | 4004c | 42.48 | 0.002 |
| FCR, g/g | 1.54bc | 1.63a | 1.52c | 1.57bc | 1.61ab | 1.62ab | 0.01 | <0.01 |
Means within the same row without a common superscript differ significantly (P < 0.05).
Each value represents the mean of 6 replicates. A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA. 2SEM, standard error of the mean. 3P-values represent the interaction between the dietary treatments. Abbreviations: AFI, average feed intake, g/bird; BWG, average body weight gain, g/bird; FCR, feed conversion ratio (g of feed intake/g of BW gain, g/g).
From d 22 to 42, although NE infection did not affect the indicators of BWG and AFI, the infected broiler chickens fed EOA at 200 mg/kg EOA of feed exhibited improved FCR compared with the noninfected negative control group, the infected positive treatment, and the infected broiler chickens that received 800 mg/kg EOA (P < 0.05) treatment, but no significant difference in these performance parameters was observed when compared with the infected broiler chickens supplemented with either AGP or 500 mg/kg EOA.
During the period, infected broiler chickens fed with AGP exhibited the highest BWG and AFI as well as the lowest FCR (P < 0.05) compared with the infected positive control and the EOA-added groups, but were the same as the noninfected and untreated groups. Compared with the NE-infected positive control, only the 200 mg/kg EOA group exhibited improved FCR (P < 0.05), the 500 mg/kg EOA group exhibited a decreasing trend for FCR, and the 800 mg/kg EOA group revealed no effect on FCR. NE-infected broiler chickens fed with different amounts of EOA exhibited no remarkable influence (P < 0.05) on BWG and AFI compared with the infected broiler chicken control.
Concentration of C. perfringens in Liver and Cecum Samples
As listed in Table 5, dietary EOA or AGP treatments did not affect (P > 0.05) cecal E.coli and Lactobacillus (P = 0.100) counts. Single NE infection significantly increased (P < 0.01) the amount of C. perfringens in the liver and caecum compared with noninfected treatment and other infected treatments. Compared with the infected control, infected broiler chickens receiving AGP or different levels of EOA revealed a considerable decrease (P < 0.05) in the number of C. perfringens in the liver and intestine. In addition, AGP addition sharply reduced C. perfringens numbers (P < 0.05) in the cecum of the infected treatment groups compared with that in the infected groups with EOA addition. However, EOA inclusion dosage resulted in no notable difference (P > 0.05) on the amount of C. perfringens in the liver and cecum.
Table 5.
Effect of EOA on intestinal bacterial concentration and liver Clostridium perfringens numbers (at 7 DPI) of broiler chickens challenged with NE1.
| Items | Bacteria | Experimental design |
SEM2 | P-values3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | ||||
| Cecal | |||||||||
| Clostridium perfringens | 0.00c | 4.69a | 0.00c | 2.26b | 2.23b | 1.57b | 0.31 | <0.01 | |
| Escherichia coli | 6.54 | 8.00 | 6.69 | 7.40 | 7.06 | 7.87 | 0.23 | 0.334 | |
| Lactobacillus | 9.55 | 8.92 | 9.61 | 9.64 | 10.60 | 9.61 | 0.16 | 0.100 | |
| Liver | Clostridium perfringens | 0.32b | 1.93a | 0.11c | 0.40bc | 0.42bc | 1.09b | 0.14 | <0.01 |
Means within the same row without a common superscript differ significantly (P < 0.05).
Each value represents the mean of six replicates. A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA. 2SEM, standard error of the mean. 3P-values represent the interaction between the dietary treatments.
Intestinal Lesion Score Observation and Morphological Evaluation
Intestinal lesion scores and morphological observations on 7DPI are presented in Tables 6 and 7. The broiler chickens infected with NE alone exhibited the most severe gut lesions in the duodenum, jejunum, and ileum compared with the uninfected groups and the infected broiler chickens given in-feed antibiotics or EOA treatments (P < 0.05). The infected broiler chickens treated with AGP exhibited the lowest gut lesions (P < 0.05), which was similar to the result of the infected broiler chickens given EOA at 200 mg/kg and 500 mg/kg, compared with the infected broiler chickens. Thus, adding EOA at 200 mg/kg and 500 mg/kg to basal diets could significantly (P < 0.05) reduce intestinal lesions scores of infected broiler chickens. However, infected broiler chickens receiving 800 mg/kg EOA revealed higher gross pathological scores (P < 0.05) than the uninfected groups and infected broiler chickens supplemented with AGP did but had no significant difference (P > 0.05) related with the infected broiler chickens treated with EOA at 200 mg/kg and 500 mg/kg.
Table 6.
Effect of dietary EOA supplementation on gut lesion scores (at 7 DPI) of broiler chickens challenged necrotic enteritis.
| Items | Experimental design |
SEM1 | P-values2 | |||||
|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | |||
| Duodenum | 0.07c | 1.58a | 0.29c | 0.58bc | 0.67bc | 0.83b | 0.09 | <0.01 |
| Jejunum | 0.36b | 1.33a | 0.363b | 0.673b | 0.583b | 0.58b | 0.07 | <0.01 |
| Ileum | 0.00c | 0.83a | 0.29bc | 0.30bc | 0.25bc | 0.67ab | 0.08 | 0.028 |
| Pathological scores | 0.14c | 1.25a | 0.31c | 0.51bc | 0.50bc | 0.723b | 0.22 | <0.01 |
Means within the same row without a common superscript differ significantly (P < 0.05); A: uninfected and untreated control; D: infected and untreated control; E: infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: infected birds fed basal diet supplemented with 200 mg/kg EOA; G: infected birds fed basal diet supplemented with 500 mg/kg EOA; H: infected birds fed basal diet supplemented with 800 mg/kg EOA.
SEM, standard error of the mean.
P-values represent the interaction between the dietary treatments.
Table 7.
Effect of EOA on jejunal morphology and goblet cell numbers of broiler chickens challenged with NE.
| Items | Experimental design |
SEM1 | P-values2 | |||||
|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | |||
| Villous height, μm | 439.66 | 398.69 | 444.83 | 460.95 | 446.39 | 478.87 | 9.72 | 0.302 |
| Crypt depth, μm | 61.93c | 112.52a | 94.48b | 88.04b | 66.69c | 66.48c | 3.65 | <0.01 |
| VH/CD3 | 7.07a | 3.56c | 4.77b | 5.33b | 6.75a | 7.36a | 0.26 | <0.01 |
| GC4 cells | 23.57a | 17.23b | 21.43a | 22.93a | 23.87a | 21.13a | 0.74 | 0.091 |
Means within the same row without a common superscript differ significantly (P < 0.05); A: uninfected and untreated control; D: infected and untreated control; E: infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: infected birds fed basal diet supplemented with 200 mg/kg EOA; G: infected birds fed basal diet supplemented with 500 mg/kg EOA; H: infected birds fed basal diet supplemented with 800 mg/kg EOA.
SEM, standard error of the mean.
P-values represent the interaction between the dietary treatments.
VH/CD = villus height to crypt depth ratio
GC cells = goblet cells numbers per mm2.
Jejunal morphological and goblet cells examination (Table 7) revealed that single NE infection significantly increased the depth of intestinal CD and reduced VH/CD and GC cells numbers compared with other groups (P < 0.05). The addition of AGP or different levels of EOA to the infected broiler chickens significantly declined CD and remarkably improved VH/CD and GC cell counts compared with the single NE-infected positive control (P < 0.01). In addition, the jejunal VH/CD ratio of the infected broiler chickens given EOA at 200 mg/kg was closed to that of the AGP-treated group, except that of the other EOA-treated broiler chickens (P < 0.05).
Serum FITC-d Levels
Table 8 reveals the results of the serum FITC-d concentration. Compared with the positive control groups, infected chickens given AGP or different levels of EOA exhibited significantly reduced concentrations of serum FITC-d at 1 h after administering FITC-d (P < 0.05), and the lowest concentration of serum FITC-d was observed in the infected broiler chickens receiving dietary addition with 800 mg EOA/kg of feed, whereas no notable difference was observed between several EOA-treated and the AGP-treated groups (P > 0.05). Moreover, no significant difference was observed on serum FITC-d levels among all the treatments at 2.5 h post administration of FITC-d (P > 0.05).
Table 8.
Effects of dietary supplemental with EOA on intestinal permeability (serum fluorescein isothiocyanate dextran (FITC-d) concentration, ng/mL) broiler chickens challenged with NE.
| Items | Experimental design |
SEM1 | P-values2 | |||||
|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | |||
| 1 h | 9.74ab | 9.88a | 9.29bc | 9.12bc | 9.22bc | 9.01c | 0.09 | 0.030 |
| 2.5 h | 9.84 | 10.00 | 9.81 | 9.93 | 9.83 | 9.99 | 0.03 | 0.387 |
Means within the same row without a common superscript differ significantly (P < 0.05); A: uninfected and untreated control; D: infected and untreated control; E: infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: infected birds fed basal diet supplemented with 200 mg/kg EOA; G: infected birds fed basal diet supplemented with 500 mg/kg EOA; H: infected birds fed basal diet supplemented with 800 mg/kg EOA.
SEM, standard error of the mean. 2P-values represent the interaction between the dietary treatments.
Results of mRNA Gene Expression in the Jejunum Samples
As presented in Table 9, compared with the uninfected negative control, NE infection alone significantly downregulated ZO-1 mRNA levels (P < 0.05) and numerically downregulated claudin-1, mucin-2 and EGFR mRNA in jejunum. Compared with the NE-infected and untreated broiler chickens, the infected broiler chickens’ diet supplemented with antibiotic AGP significantly reduced ZO-1 mRNA but significantly increased mucin-2, TGF-β, and EGFR gene expression in jejunum (P < 0.05). The challenged broiler chickens given different doses of EOA upregulated (EOA 200 mg/kg feed) or tended to increase (500 mg/kg and 800 mg/kg feed) claudin-1 mRNA levels (P < 0.05), but no remarkable effect was observed on the level of mucin-2, TGF-β3, and EGFR (P > 0.05). The dietary administration of 500 or 800 mg/kg EOA downregulated ZO-1 and occludin mRNA levels and upregulated IGF-2 (P < 0.05) gene expression in the jejunum of the infected broiler chickens. Additionally, the infected broiler chickens given EOA at 200 mg/kg or 500 mg/kg exhibited an increase in the levels of GLP-2 mRNA compared with the infected broiler chicken control. Compared with the infected broiler chickens given AGP, the inclusion of EOA at 500 mg/kg in the diets of the infected broiler chickens considerably (P < 0.05) enhanced the levels of IGF-2 and GLP-2 mRNA in jejunum, whereas EOA supplements with 200 mg/kg reduced jejunal mucin-2, TGF-β, and EGFR mRNA levels (P < 0.05).
Table 9.
Effects of dietary supplemental with EOA on gene expressions of tight junction proteins, growth factors, and mucin-2 in the jejunum of broiler chickens challenged with NE.
| Items | Experimental design |
SEM1 | P-values2 | |||||
|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | |||
| Claudin-1 | 0.82ab | 0.56b | 0.51b | 1.26a | 1.02ab | 0.97ab | 0.09 | 0.036 |
| Occludin | 1.02a | 0.93a | 0.80ab | 0.76ab | 0.57b | 0.68b | 0.05 | 0.068 |
| ZO-1 | 1.07a | 0.66b | 0.31c | 0.43bc | 0.21c | 0.23c | 0.06 | <0.01 |
| Mucin-2 | 1.05ab | 0.71bc | 1.35a | 0.64bc | 0.55c | 0.72zbc | 0.07 | <0.01 |
| TGF- β3 | 1.03b | 0.89b | 1.61a | 0.81b | 1.17b | 1.06b | 0.06 | <0.01 |
| IGF-2 | 1.04c | 1.34c | 1.79bc | 1.31c | 2.47a | 2.33ab | 0.12 | <0.01 |
| EGFR | 1.02ab | 0.86bc | 1.26a | 0.74bc | 0.60c | 0.80bc | 0.06 | 0.009 |
| GLP-2 | 1.04c | 1.05c | 1.33bc | 1.58ab | 1.84a | 1.27bc | 0.08 | 0.010 |
Means within the same row without a common superscript differ significantly (P < 0.05); A: uninfected and untreated control; D: infected and untreated control; E: infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: infected birds fed basal diet supplemented with 200 mg/kg EOA; G: infected birds fed basal diet supplemented with 500 mg/kg EOA; H: infected birds fed basal diet supplemented with 800 mg/kg EOA.
SEM, standard error of the mean. 2P-values represent the interaction between the dietary treatments.
As summarized in Table 10, NE infection alone significantly upregulated TLR-4 and IFN-γ mRNA levels and considerably downregulated the PI3K gene expression (P < 0.05) in jejunum compared with the negative control. The infected broiler chickens fed with AGP significantly reduced TLR-4, NF-κB, and IFN-γ mRNA levels but significantly increased the SOCS-6 mRNA level and tended to exhibit a higher PI3K mRNA than that of the positive NE-challenged control. The infected chickens that were given different levels of EOA exhibited lower TLR-2 and TLR-4 mRNA levels (P < 0.05) than the positive control groups. The inclusion of 500 mg/kg and 800 mg/kg EOA into the diet of the NE-infected chickens reduced TRAF6 and Tollip gene expression (P < 0.05) compared with the NE-infected control. Different supplemental levels of EOA exhibited a decreasing trend for NF-κB, IFN-γ, and TNFSF15 mRNA levels. Moreover, IFN-γ (P < 0.05) was significantly downregulated in the infected broiler chickens given 200 mg/kg EOA. The decreased expression of NF-kB and TNFSF15 (P < 0.05) was observed in the 500 mg/kg EOA-treated broiler chickens but higher IL-8 gene expression was found in the 800 mg/kg EOA-treated broiler chickens (P < 0.05) than in the positive broiler chickens. Furthermore, Compared with the infected broiler chickens administrated AGP, downregulated TLR-2, TRAF6, Tollip, and SOSC-6 mRNA level were found in the infected broiler chickens fed different concentrations of EOA (P < 0.05). Moreover, the EOA group (500 mg/kg) significantly declined (P < 0.05) the TNFSF15 gene expression and 800 mg/kg EOA significantly decreased the TLR4 mRNA level (P < 0.05) in the jejunum of the infected broiler chickens. No changes were observed in the levels of IL-1β, NF-κB, IL-10, SOCS-1, MyD88, A20, and PI3K mRNA between the AGP-treated broiler chickens and infected groups supplemented with different levels of EOA.
Table 10.
Effects of dietary supplemental with EOA on TLR signaling pathway-related genes expressions in the jejunum of broiler chickens challenged with NE.
| Items | Experimental design |
SEM1 | P-values2 | |||||
|---|---|---|---|---|---|---|---|---|
| A | D | E | F | G | H | |||
| TLR2 | 1.01b | 1.26ab | 1.50a | 0.9b | 0.91b | 0.75b | 0.07 | 0.005 |
| TLR-4 | 1.07b | 1.66a | 0.90bc | 0.88bc | 0.59bc | 0.46c | 0.09 | <0.001 |
| TRAF6 | 1.02ab | 0.96ab | 1.12a | 0.759b | 0.36c | 0.46c | 0.06 | <0.001 |
| MyD88 | 1.02 | 1.01 | 1.13 | 0.79 | 0.87 | 1.13 | 0.05 | 0.292 |
| NF-κB | 1.06a | 0.90ab | 0.50c | 0.57c | 0.39c | 0.69bc | 0.06 | 0.003 |
| IL-1β | 1.05 | 1.14 | 0.93 | 0.80 | 0.62 | 0.85 | 0.06 | 0.125 |
| IL-8 | 1.38b | 0.63c | 0.48c | 0.45c | 1.02b | 1.97a | 0.18 | 0.079 |
| IL-10 | 1.07 | 1.37 | 1.38 | 0.82 | 1.24 | 0.53 | 0.10 | 0.091 |
| IFN-γ | 1.01c | 3.89a | 2.39abc | 2.06bc | 3.13ab | 2.90ab | 0.25 | 0.009 |
| TNFSF15 | 1.00abc | 1.08ab | 1.03abc | 1.14a | 0.79c | 0.85bc | 0.04 | 0.034 |
| Tollip | 1.02a | 0.84ab | 0.97a | 0.73bc | 0.57c | 0.53c | 0.04 | <0.001 |
| SOCS-1 | 1.06 | 1.23 | 1.32 | 1.19 | 1.19 | 1.11 | 0.09 | 0.979 |
| SOCS-6 | 1.02b | 0.92bc | 1.41a | 0.97bc | 0.67c | 0.67c | 0.06 | <0.001 |
| A-20 | 1.02 | 1.06 | 1.33 | 1.10 | 1.43 | 0.93 | 0.09 | 0.558 |
| PI3K | 1.04a | 0.73b | 0.96ab | 0.80ab | 0.73b | 0.69b | 0.04 | 0.046 |
Means within the same row without a common superscript differ significantly (P < 0.05); A: uninfected and untreated control; D: infected and untreated control; E: infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: infected birds fed basal diet supplemented with 200 mg/kg EOA; G: infected birds fed basal diet supplemented with 500 mg/kg EOA; H: infected birds fed basal diet supplemented with 800 mg/kg EOA.
SEM, standard error of the mean. 2P-values represent the interaction between the dietary treatments.
Result of Cecal Microbiota Analyses
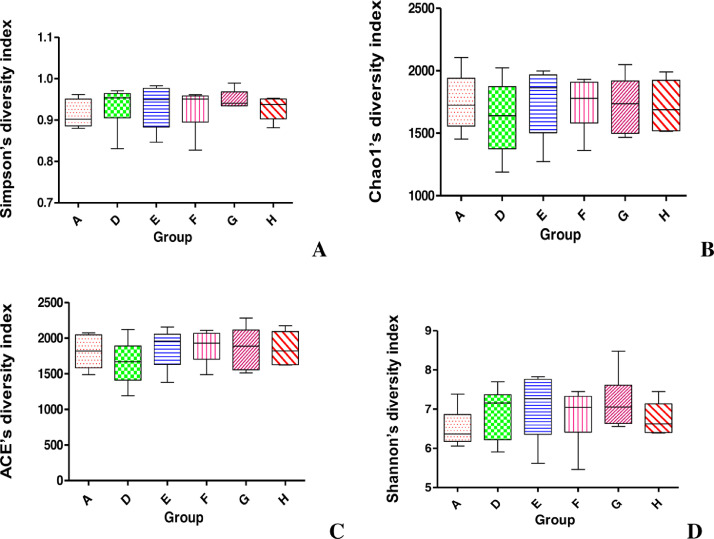
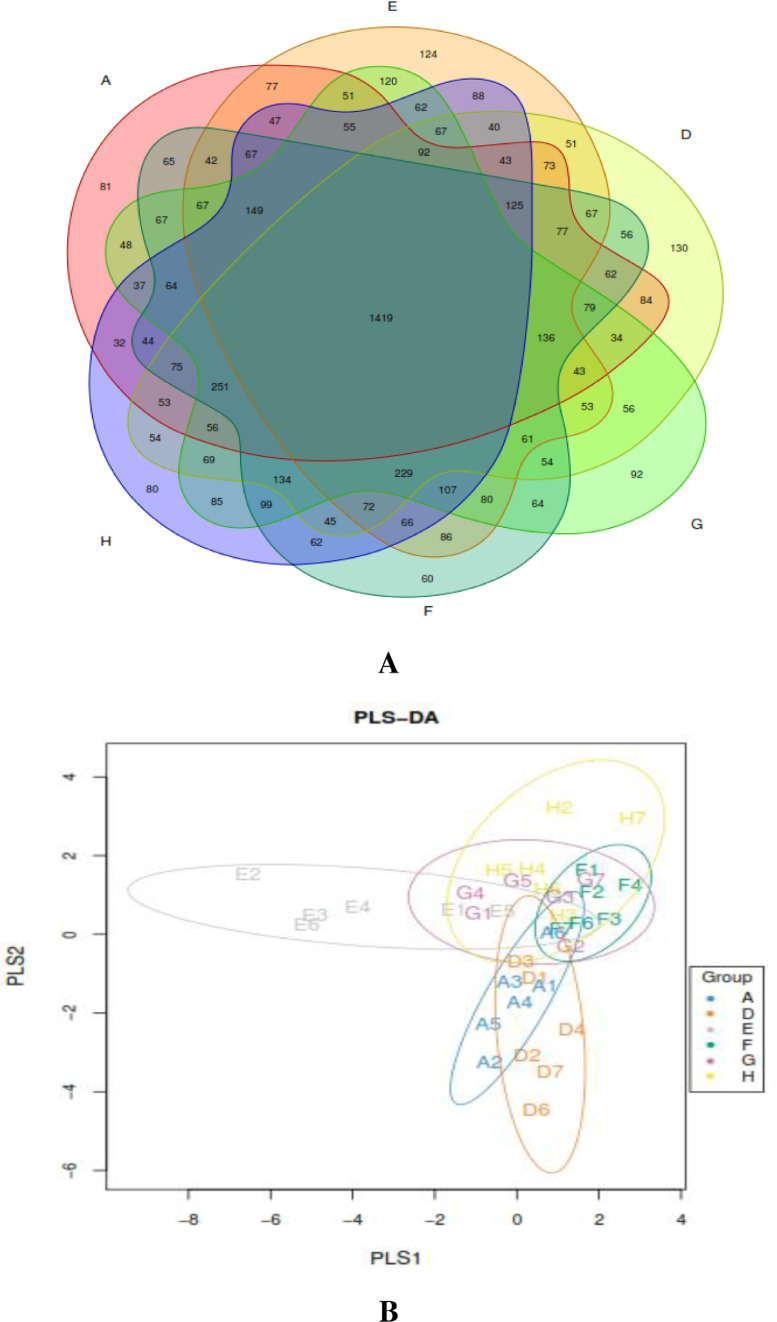
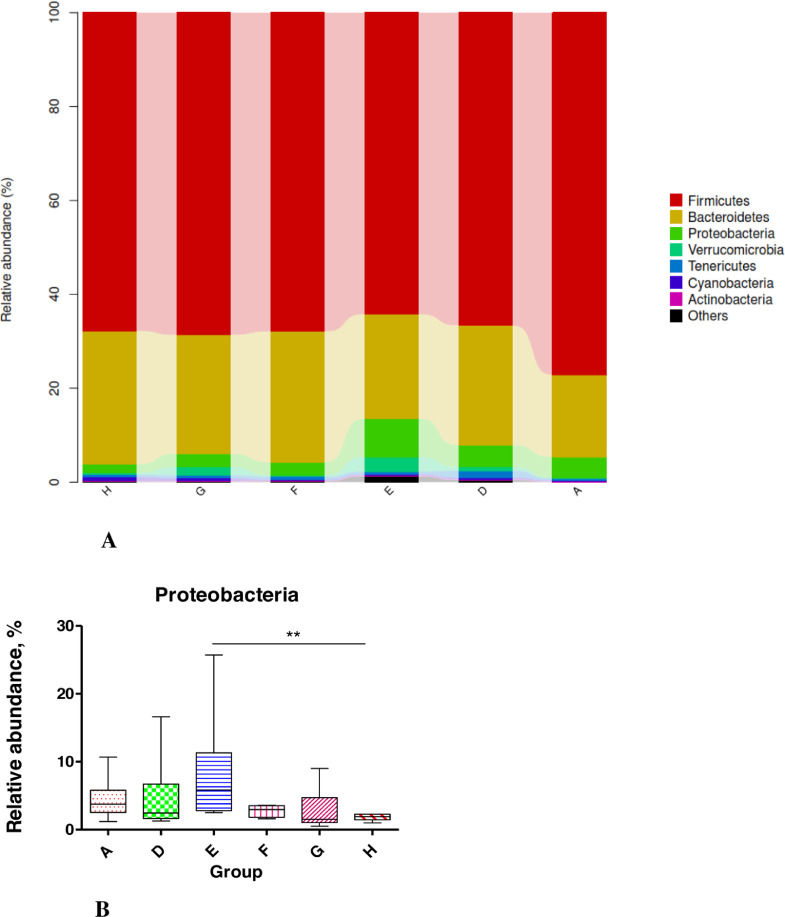
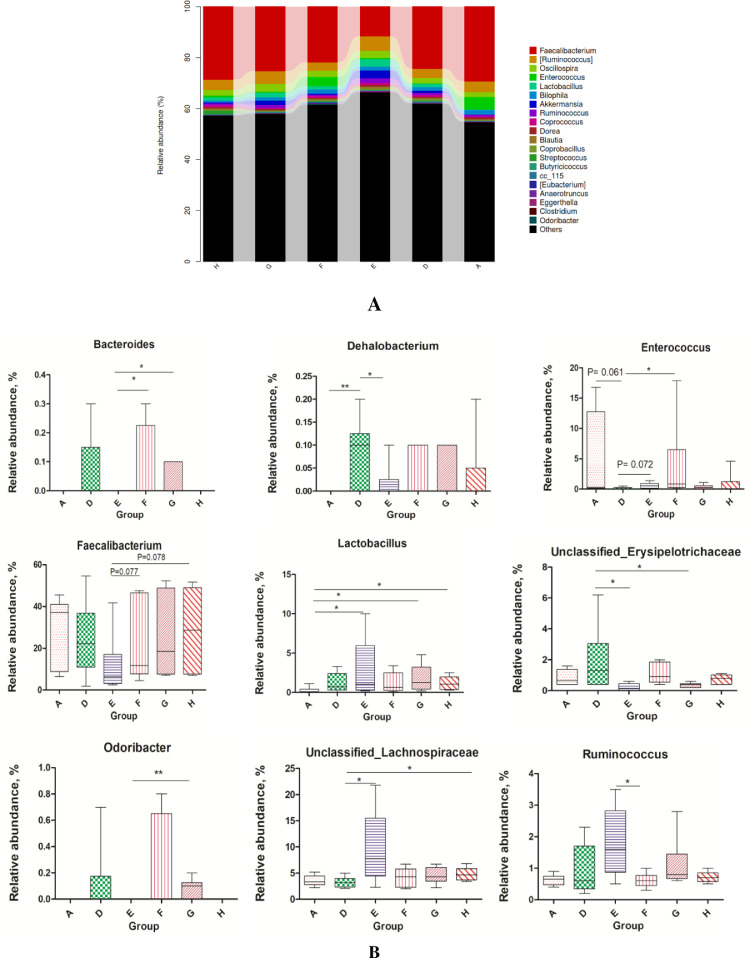
We obtained 769,274 effective tags (an average of 45,685 reads from each sample) from 36 samples (6 samples per group) through 16S rRNA sequencing analyses. The alpha diversity indices of cecal feces revealed no significant difference between groups (P > 0.05; Figure 1) and suggested that the alpha diversity of gut microbiota was not altered by NE infection, EOA, or AGP treatment. Venn diagrams revealed that the number of common core OTUs were 1,419 OTUs, whereas 81, 124, 130, 92, 60, and 80 OTUs were unique to one of the 6 groups (Figure 2A). Principal coordinate analysis indicated that microbes from the ceca of the infected broiler chickens administrated with different levels of EOA (F, G, and H groups) formed distinct clusters, which differed from microbes from the ceca of the D group (Figure 2B). The phylum level analysis revealed that dietary EOA treatment significantly reduced (P < 0.05) the percentages of Proteobacteria (P < 0.01) compared with the AGP groups, but no significant differences (P > 0.05) were observed in the relative abundances of Firmicutes, Bacteroidetes, Actinobacteria, or other bacterial phyla (Figure 3). Figure 4 displays cecal microbiota difference among the 6 groups at the genus level. The NE-infected and untreated control broiler chickens exhibited lower abundances of Enterococcus (0.05 < P < 0.10) and higher abundances of Dehalobacterium (P < 0.01) than the uninfected control group. The infected broiler chickens fed with AGP exhibited higher relative abundances of Unclassified_Lachnospiraceae (P < 0.05) and Enterococcus (0.05 < P <0.10) and lower abundances of Dehalobacterium and unclassified_ Erysipelotrichacease (P < 0.05) than the single NE-infected group. For the NE-infected and untreated broiler chickens, the infected broiler chickens fed different levels of EOA exhibited elevated Unclassified_Lachnospiraceae population (P < 0.05) and reduced abundance of unclassified_ Erysipelotrichacease. Additionally, the infected broiler chickens fed with EOA 200 mg/kg exhibited elevated relative abundances of Enterococcus (P < 0.05), the infected broiler chickens fed with EOA 500 mg/kg exhibited the lowest relative proportion of unclassified_ Erysipelotrichacease (P < 0.05). The infected broiler chickens fed with EOA (200 or 500 mg/kg) exhibited higher relative proportion of Bacteroides (P < 0.05), Odribacter (P < 0.05), and Faecalibacterium (0.05 < P <0.10) and lower abundances of Ruminococcus (P < 0.05) than the infected and AGP-treated broiler chickens. In addition, relative distributions of Lactobacillus were higher in the challenged broiler chickens fed with EOA than in the uninfected group (Figure 4).
Figure 1.
Effect of EOA on alpha diversity indices in cecum from different groups. Panel (A) represents differences in bacterial community diversity (Shannon) among the 6 groups. Panel (B) represents differences in bacterial community richness (Chao 1) among the 6 groups. Panel (C) represents differences in bacterial community diversity (ACE) among the 6 groups. Panel (D) represents differences in bacterial community diversity (Shannon) among the 6 groups. A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA.
Figure 2.
(A) Venn diagram showing the unique and shared OTUs in the samples from different groups. (B) Partial least squares discriminant analysis (PLS-DA) of cecal microbial community structure of broiler chickens from different groups. A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA.
Figure 3.
(A) Relative abundance of cecal microbiota from different groups at the phylum level. (B) Different groups of differential microbiota at the phylum levels. A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA. Asterisk shows significant differences between groups (**P < 0.01, *P < 0.05, Mann-Whitney U test).
Figure 4.
(A) Relative abundance of cecal microbiota from different groups at the genus level. (B) Differential gut microbiota at the genus level among different groups. Asterisk shows significant differences between groups (**P < 0.01, *P < 0.05, Mann-Whitney U test). A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA.
Analysis of the Function of Cecal Microbiota Using PICRUSt
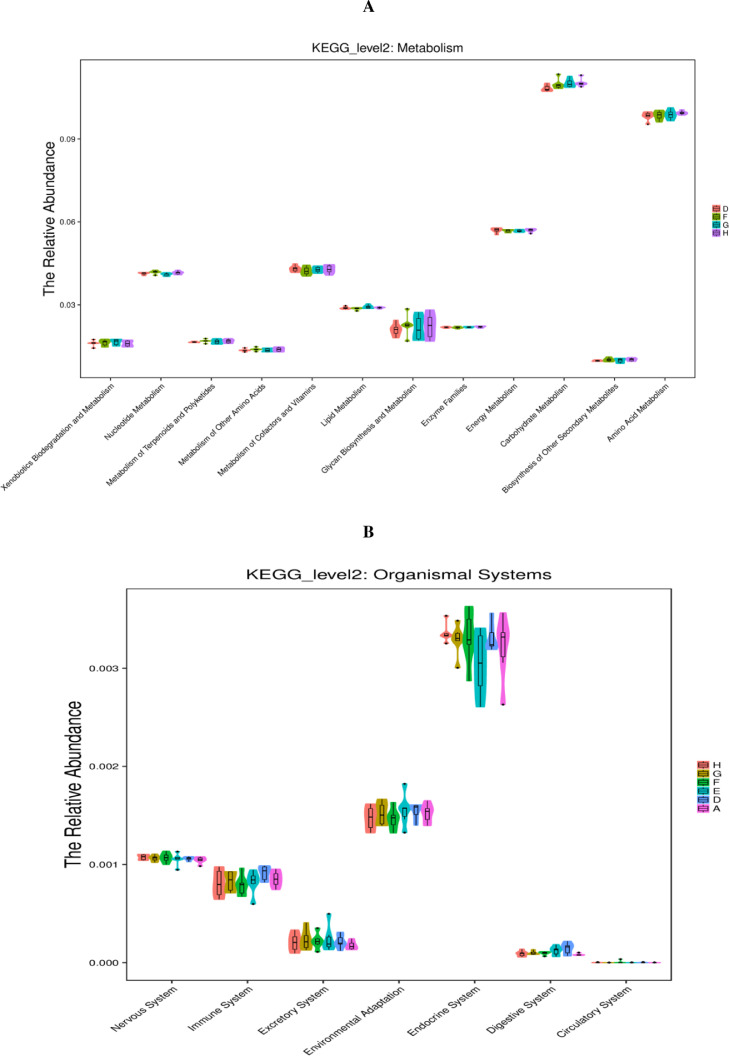
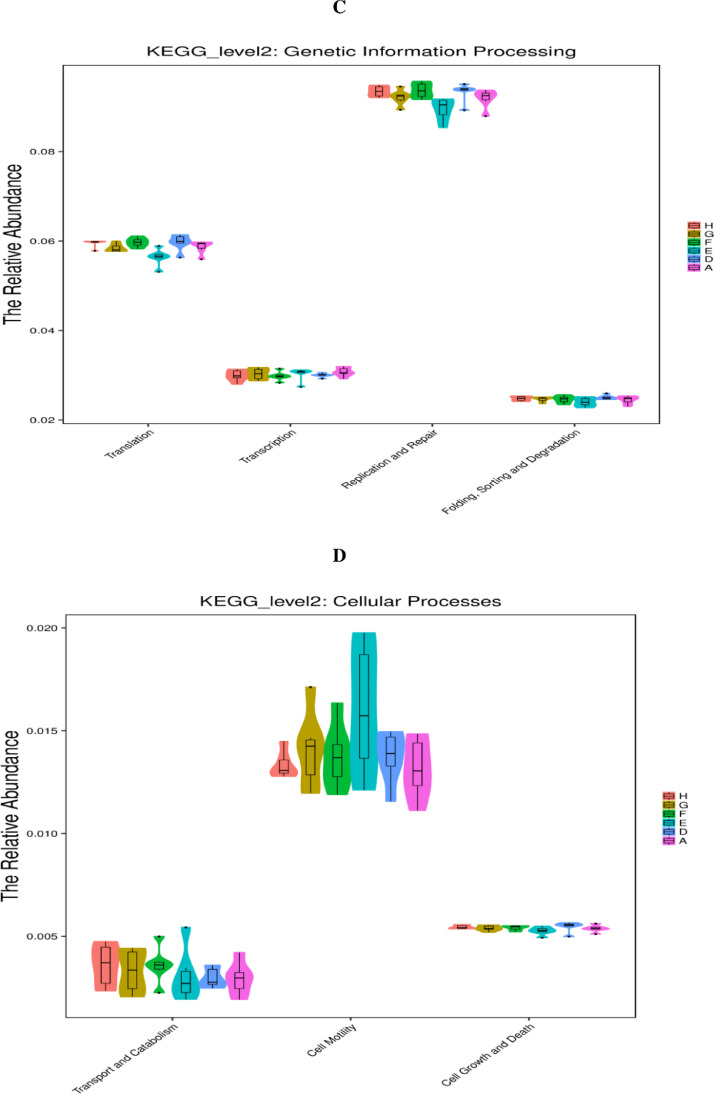
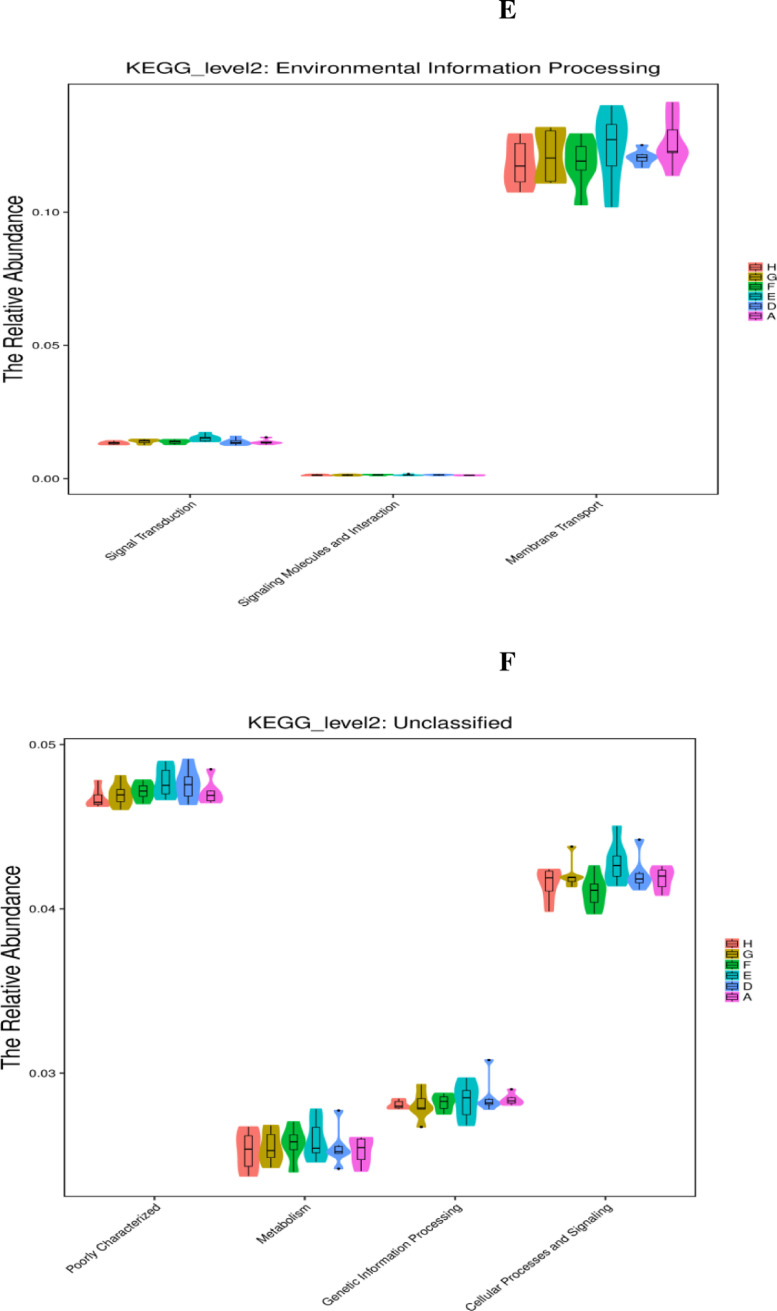
As displayed in Figure 5, the PICRUSt analysis of the functional pathways of caecal microbiota at the KEGG level 2 revealed that carbohydrate metabolism downregulated (P < 0.05) in the single NE-infected group compared with that in the noninfected negative group. Compared with the single NE-infected group, the abundance of the metabolic pathway functions, including nucleotide metabolism and genetic information processing (folding, sorting, and degradation; signal transduction as well as replication and repair), were notably suppressed (P < 0.05) in the cecal microbiota in the NE-infected broiler chickens given AGP. Moreover, AGP administration tended to increase (0.05 < P < 0.10) the abundance of the metabolic pathway related to carbohydrate metabolism, and xenobiotics biodegradation and metabolism in the cecal microbiota of the NE-infected broiler chickens. Similarly, NE-infected broiler chickens given different levels of EOA tended to increase carbohydrate metabolism, and supplemental of EOA at 200 mg/kg tended to reduce the relative abundance of the organismal systems, namely the immune system in the cecal microbiota, compared with the single NE-infected broiler chickens (0.05 < P <0.10).
Figure 5.
KEGG pathways of gut microbiome (PICRUSTs). Predictive functional profiles generated from 16S rRNA marker gene sequences using PICRUST. Functional profiles were generated based on KEGG ortholog prediction and collapsed into higher pathways (level 2), according to the KEGG pathway database. A: the uninfected and untreated control; D: the infected and untreated control; E: the infected birds fed basal diet supplemented with 250 mg/kg bacitracin methylene disalicylate (BMD, 15% purity) plus 90 mg/kg monensin; F: the infected birds fed basal diet supplemented with 200 mg/kg EOA; G: the infected birds fed basal diet supplemented with 500 mg/kg EOA; H: the infected birds fed basal diet supplemented with 800 mg/kg EOA.
DISCUSSION
The development of safe and effective anti-bacterial substances to replace in-feed antibiotics is critical. The continuous feeding of a new blend of the EOA at 200 mg/kg or 500 mg/kg improved BWG and feed efficiency when confronted with NE infection during the whole study period compared with the NE-infected positive broiler chickens. However, the degree of improvement in BWG and feed efficiency in the NE-infected broiler chickens fed with a diet supplemented with EOA was less than that in the broiler chickens fed with an AGP diet. Consistent with our findings, studies have revealed that the inclusion of the mixture of EOA improved the growth performance and feed utilization in chickens (Liu et al. 2017). Similarly, the FCR of chickens fed a mixture of EO (thymol) + OA (fumaric and sorbic acid) decreased on d 42 (Yang et al., 2018, 2019). Furthermore, the EOs (thymol, vanillin, and eugenol) combined with OAs (fumaric, sorbic, malic, and citric acids) improved growth performance, nutrient digestibility, and intestinal health in the NE-challenged broiler chickens (Stefanello et al., 2020). The combination of MCFA (capric-caprylic; caproic and lauric acid) + AC (cinnamaldehyde, carvacrol, and thymol) + OA (calcium butyrate + fumaric and citric acid) improved the growth efficiency and intestinal histomorphology of NE-challenged chickens on d 42 (Abdelli et al., 2020). Therefore, the results of the study revealed that adding EOAs at 200 mg/kg or 500 mg/kg could be beneficial to BWG and feed efficiency in the broiler chickens with NE infection. By contrast, the other results revealed that no significant difference was observed in the growth performance and FCR in chickens given a combination of OAs and EOs (Fascina et al., 2012). The results differed from the effects of EOAs on growth performance with factors, such as the chemical composition of EOs, formulated diet, breed, animal age, health status, experimental environments, and hygienic conditions (Zhai et al., 2018). Additionally, trials should be performed in the future to confirm our observation.
Gut morphology and serum FITC-d are critical markers to indirectly assess the extent of intestinal damage and intestinal permeability, respectively (Vicuna et al., 2015; Celi et al. 2017). NE gross gut lesions and C. perfringens burden in the gut and liver are common parameters used to assess the preventive efficacy of various antibiotics alternatives on NE infection in chickens. In this study, single NE infection caused gut injury, as indicated by severe gut lesions, increased C. perfringens load in the cecum and liver, decreased VH/CD and GC cell numbers, and increased serum FITC-D levels. These data proved that the experimental NE model was successfully established in our study, and its results are consistent with our previous studies (Song et al., 2017; Wu et al., 2018, 2019; Zhen et al., 2018). However, these parameters and gut injury caused by NE challenge decreased by either EOA (200 mg/kg and 500 mg/kg) or AGP addition, which was similar to those of the noninfected groups. Furthermore, the protective efficacy of AGP on gut injury induced by NE infection was better or not higher than that of different doses of EOA. Similarly, studies have demonstrated that the EOA blend could restore intestinal homeostasis of chickens subjected to pathogens challenge by suppressing intestinal pathogen (C. perfringens, Salmonella, E. coli and/or Campylobacter jejuni) proliferation (Basmacioğlu-Malayoğlu et al. 2016; Yang et al. 2019), thus decreasing gross pathological and/or histopathological lesion scores (Abdelli et al. 2020; Stefanello et al. 2020) and improving intestinal morphological structure (Jerzsele et al., 2012). Therefore, our results indicated that adding appropriate doses of EOA into the NE-infected broiler diet could result in protective effects on the growth performance and gut health of the broiler chickens against the mixed coccidian/C. perfrigens and exhibit potential to replace antibiotic growth promoters. A decrease in the level of injury caused by NE may be directly related to improved gut health and improved growth performance because of the antimicrobial and/or anti-inflammatory activity of EOs (Sun et al. 2015; Chowdhury et al. 2018; Eid et al. 2018) or OAs (Polycarpo et al. 2017; Song et al. 2017).
The intestinal epithelium and tight junction proteins (TJPs) significantly contribute to maintain the integrity of the intestinal mucosa barrier, immune hemostasis, and intestinal health (Gil-cardoso et al., 2016; Lee et al., 2018). In this study, enhanced goblet cell density and elevated claudin-1 mRNA were observed in the NE-infected chickens given different doses of EOA, indicating that EOA could alleviate intestinal barrier damage caused by NE. Consistent with our findings, studies have revealed that upregulated claudin-1 mRNA and enhanced intestinal barrier function when C. perfringens-infected broiler chickens fed EOs (Du et al., 2016; Liu et al., 2018), OAs (Song et al., 2017; McKnight et al., 2019), or EOAs (Yang et al., 2019). We revealed that dietary administration of EOA at 500 or 800 mg/kg significantly downregulated ZO-1 and occludin mRNA levels, but the mucin-2 mRNA level was not influenced compared with the positive challenged control. However, the changes in mucin-2 and these TJ (ZO-1 and occludin) protein gene expressions in the NE-infected broiler chickens that received EOA at 500 or 800 mg/kg did not cause any significant increase in alteration in intestinal permeability and did not reduce serum FITC-d levels and liver C. perfringens counts. In contrast to our findings, the expression of mucin-2 and TJs (Claudin-1, ZO or occludin) in the intestinal mucosa was elevated or unaffected in the EOs-treated (Liu et al., 2018), OAs-treated (Song et al., 2017), and EOA-supplemented broiler chickens subjected to C. perfringens (Stefanello et al., 2020). These variations indicated that EOA treatment modulated differentially TJP expression and distribution in the gut. The causes for EOA downregulating ZO-1 and occludin gene expression must be further investigated.
To evaluate the protective effect of EOA supplementation against NE, we examined the gene expressions of pro- and anti-inflammatory cytokines and repair proteins of gut tissue injury in jejunum. Modulating the epithelial barrier integrity and inflammation of the intestine were related to Toll-like receptor mediated signaling pathways (Nighot et al. 2017; Wu et al. 2019). NE infection significantly lowered the expression of ZO-1 and PI3K genes but significantly upregulated TLR-4 and IFN-γ genes expression and tended to downregulate claudin-1, mucin-2, and EGFR mRNA levels compared with the noninfected control broiler chickens. The results indicated that single NE infection led to intestinal inflammation by activating the TLR-mediated signal pathway and differentially modulating immune-related genes and growth factor genes expression, which resulted in damaged intestinal barrier function. These results were consistent with other reports (Wu et al., 2019). However, infected broiler chickens supplemented with different levels of EOA showed remarkably increased IGF-2 and GLP-2 mRNA levels, reduced TLR-2 and TLR-4 mRNA levels, and a decreasing trend for NF-κB, IFN-γ, and TNFSF15 mRNA levels, similar to the change trend of AGP administration. Moreover, compared with the infected positive control, jejunal mucosa IFN-γ mRNA levels were significantly downregulated in the infected broiler chickens given 200 mg/kg EOA; TRAF6, NF-κB, TNFSF15, and Tollip mRNA levels were remarkably downregulated in the 500 mg/kg EOA-treated infected broiler chickens. TRAF6 and Tollip gene expression decreased following 800 mg/kg EOA supplementation. However, chemokine IL-8 (CXCLi2) gene expression was strongly elevated in the NE-infected broiler chickens that received 800 mg/kg EOA. These findings agree with the results of previous studies, in which the expressions of TLR and its downstream signal molecules, such as some pro-inflammatory cytokines in the gut of broiler chickens subjected to C. perfrigens or Eimeria spp. challenge was downregulated by EOs (Du et al. 2016) or OAs (Liu et al., 2019). These results suggested that supplementing appropriate levels of EOA could alleviate NE-induced intestinal inflammation possibly by suppressing the activation of the TLR-NF-κB signaling pathway in the broiler chickens, whereas high levels of EOA possibly induced excessive immune responses in the gut but without severe damage to the intestine but revealed decreasing trend for the feed efficiency. The anti-inflammatory activity of the EOA may be associated with the antimicrobial activity of EOs or OAs (Zeng et al., 2015). Reduced intestinal inflammation in the NE-infected broiler chickens that received appropriate level of EOA resulted in a strengthened intestinal barrier function, which supports our observation.
Gut microbiota affect health, disease, and poultry production (Broom and Kogut 2018). To investigate the underlying action mechanisms of the EOA on the gut health, we investigated cecal microbial composition. The results revealed that EOA administration, NE challenge, or both did not alter α-diversity, which is similar with previous reports or results (Bortoluzzi et al., 2017). However, EOA treatment remarkably modified β-diversity, which indicated that EOA administration, NE challenge, or both significantly disturbed intestinal bacterial community profiles. Additionally, in the infected broiler chickens administered with different levels of EOA, percentage of Proteobacteria decreased compared with that in the AGP groups. An enrichment in the amount of Proteobacteria in the cecal microbiota is a potential diagnostic signature of gut dysbiosis, which indicated inflammatory response and epithelial dysfunction in the gut (Hiippala et al. 2016; Litvak et al. 2017). A low proportion of Proteobacteria was observed in EOA treatment, indicating that EOA treatment could inhibit the growth of phylum Proteobacteria in intestine, thus reducing intestinal inflammation and improving gut health compared with AGP administration.
Furthermore, in the NE-infected and untreated broiler chickens, the supplementation of EOA at 200 mg/kg increased the amount of the Unclassified_Lachnospiraceae and Enterococcus, but EOA supplementation at 500 mg/kg reduced abundance of the unclassified_Erysipelotrichacease in the cecum. Additionally, feeding 200 mg/kg of EOA enriched Lactobacillus population compared with the uninfected control broiler chickens. Reports have revealed that EO or EOA mixture increased the proportion of Lactobacillus spp. in pigs (Diao et al., 2015; Li et al., 2018) and chickens (Yin et al., 2017; Yang et al., 2019). The results of the study indicated that modification in intestinal microbiota profiles induced by EOA was affected by EOA the supplemental dose. Some Enterococcus strains and Lactobacillus spp. exhibit beneficial effects on intestinal health (Braiek and SlimSmaoui, 2019; Nascimento et al., 2019; Wu et al., 2019). Lachnospiraceae family such as Coprococcus, Anaerostipes, Roseburia spp., and Eubacterium rectale could produce bacteriocins and n-butyrate, which could protect patients against intestinal inflammatory disease (Koh et al., 2016; Medvecky et al., 2018) and is positively correlated with the feed conversion efficiency and growth in broiler chickens (Stanley et al., 2016). The abundance of Erysipelotrichaceae was inversely correlated with BFW, colonic butyrate concentrations, and gut health (Hu et al., 2019). Increased population of Lactobacillus, Enterococcus, and Unclassified_Lachnospiraceae and declined abundance of Erysipelotrichaceae were observed in the EOA-treated broiler chickens irrespective of NE challenge, which indicated that EOA addition could improve gut microbial communities. Additionally, in the infected broiler chickens, EOA administration (200 mg/kg or 500 mg/kg) elevated relative abundances of Bacteroides, Odribacter, and Faecalibacterium but reduced proportions of Ruminococcus of compared with AGP administration. Bacteroides species, the most predominant anaerobes in the gut, was reported to have many functions, including the most effective degradation of complex and indigestible carbohydrates, bile acid metabolism, transformation of toxic or mutagenic compounds, and weight loss in obese humans (Wang and Jia, 2016), positively influence the host immunity, or limit the colonization of the GIT by pathogens (Stanley et al., 2013; Hiippala et al., 2018). Odoribacter, the mucin-degrading bacteria, can metabolize healthy lipid (Brahe et al., 2015). Faecalibacterium belongs to Ruminococcaceae, an obligate anaerobe, which contains numerous bacteria-producing butyric acid and the other SCFAs, and ferments the fiber in the host's digestive tract (Martín et al., 2017) in addition to significantly contributing to increasing ADG and improving FCR in the broiler chickens (Stanley et al., 2016). Faecalibacterium are related health-related bacteria, which are correlated with reduction in the expansion of regulatory T-cell and stimulate the production of anti-inflammatory cytokines, exhibit anti-inflammatory properties in the gut (Shaufi et al., 2015; Chang et al., 2016; Dubin et al., 2016). Ruminococcaceae can break down complex carbohydrates. The abundance of Ruminococcus was positively associated with the absorption capacity, immune function, and self-repair function of gut (Duncan et al., 2002; Lee et al., 2013). BWG in chickens after E. tenella infection is closely related to a decrease in Ruminococcaceae, which affects carbon metabolism (Zhou et al., 2020). Increasing the relative abundances of Bacteroides, Odoribacter, and Faecalibacterium in NE-infected broiler chickens by feeding 200 mg/kg EOA may be positively associated with the restoration of intestinal microbiota balance, decreased gut inflammation, and healthy gut because of its effectiveness in controlling NE infection. However, lower proportions of Ruminococcus in the NE-infected broiler chickens after EOA treatment than those after AGP treatment revealed the reason why AGP displayed the improvement in FCR compared with the EOA treatment when subjected to NE. Additionally, the infected broiler chickens fed with AGP exhibited growth-promoting effect possibly because of the abundance of Unclassified_Lachnospiraceae and low proportion of Dehalobacterium and unclassified_Erysipelotrichacease relative to the single NE-infected group. Therefore, the current study indicated that EOA-improved FCR and gut health and alleviated gut inflammation of the NE-infected broiler chickens probably because of beneficial modulation on intestinal microflora composition.
The PICRUSt analysis revealed that the carbohydrate metabolism functions of cecal microbiota increased, whereas the function related to immune system was suppressed in the EOA treatment, which was similar to AGP administration in the NE-infected broiler chickens. Similarly, Yin et al. reported that EO administration inhibited the abundance of immune pathway in the ileal microbiota of the C. perfringens-challenged broiler chickens (Yin et al., 2017). Carbohydrate could be metabolized using hindgut microflora into SCFAs, which can protect the host against inflammation, fight intestinal diseases, and improve the gut barrier function (Fukuda et al., 2011). The suppressed immune pathway could insinuate an anti-inflammatory gut environment associated with the supplementation of EOA in the NE-infected broiler chickens. Our results revealed that similar to AGP treatment, the supplement-appropriate dose of EOA could improve gut microbiological composition and their metabolic function and reduce intestinal inflammation when the broiler chickens were confronted with NE challenge, resulting in improved intestinal health and FCR. The effects of EOA on fecal metabolite profiles should be analyzed to explain the causal associations between EOA, metabolites, and intestinal function.
CONCLUSIONS
The results of our study revealed that the addition of appropriate doses of EOA (200 mg/kg and 500 mg/kg) could effectively ameliorate gut injury caused by NE infections, which was evidenced by the decreased gut lesion scores, concentration of serum FITC-D, and number of C. perfringens in liver; increased VH/CD and GC cells numbers; and remarkably upregulated jejunal claudin-1, GLP-2 and IGF-2 mRNA levels and downregulated jejunal TLR-NF-κB signaling pathway immune-related genes, TNFSF15, TLR-4, TRAF-6, IL-1β, IFN-γ, and Tollip mRNA levels in the NE-infected broiler chickens. Feeding EOA at 200 mg/kg enhanced or tended to elevate the abundance of Lactobacillus, Unclassified_Lachnospiraceae, Enterococcus, Bacteroides, Odribacter, and Faecalibacterium and decreased the abundance of unclassified_ Erysipelotrichacease, and Ruminococcus in the cecal microbiota. The enhancement of carbohydrate metabolic pathways and the reduction of the immune system in the intestinal microbiota of the NE-infected broiler chickens given EOA were observed, which improved the overall feed efficiency, similar to AGP administration. The results of this study indicated that adding this EOA product at 200 mg/kg or 500 mg/kg into broiler chickens’ diet results in beneficial effects on the growth and gut health of the broiler chickens infected with NE and can replace antibiotic growth promoters.
ACKNOWLEDGMENTS
The authors acknowledge Menon Animal Nutrition Technology Co. Ltd., Shanghai, China provided EOA product for this experiment. We'd like to thank the Zhuozhou Poultry Experimental Base with limited liability for animal management.
Author contribution: Zhong Wang conceived and designed the experiment; Van Hieu Pham, Waseem Abbas, Qiang He, Wenrui Zhen and Jinyu Huang carried out the research; Van Hieu Pham analyzed the data and wrote the manuscript; Zhong Wang, Waseem Abbas and Yuming Guo participate in the draft editing process manuscript. All authors read and approved the final manuscript.
DISCLOSURES
No competing or previously published interests are reported by the authors.
REFERENCES
- Abdelli N., Pérez J.F., Vilarrasa E., Luna I.C., Melo-Duran D., D'angelo M., Solà-Oriol D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals. 2020;10:1–31. doi: 10.3390/ani10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudabos A.M., Al-Mufarrej S.I. Effects of organic acid supplementation on antioxidant capacity and immune responses of broilers challenged orally with Salmonella enterica subsp. enterica Typhimurium. South African J. Anim. Sci. 2014;44:342–349. [Google Scholar]
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler broilers challenged with Salmonella typhimurium. Environ. Sci. Pollut. Res. 2016;23:24151–24157. doi: 10.1007/s11356-016-7665-2. [DOI] [PubMed] [Google Scholar]
- Alali W.Q., Hofacre C.L., Mathis G.F., Faltys G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poult. Sci. 2013;92:836–841. doi: 10.3382/ps.2012-02783. [DOI] [PubMed] [Google Scholar]
- Avigen, Arbor Acces Broiler Nutrition Specifications., 4, 2019, Avigen, 1–8, 0419-AVNAA-043.
- Basmacioğlu-Malayoğlu H., Ozdemir P., Bağriyanik H.A. Influence of an organic acid blend and essential oil blend, individually or in combination, on growth performance, carcass parameters, apparent digestibility, intestinal microflora and intestinal morphology of broilers. Br. Poult. Sci. 2016;57:227–234. doi: 10.1080/00071668.2016.1141171. [DOI] [PubMed] [Google Scholar]
- Bassolé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017;4:1–6. doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017;96:3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Ege G., Aysul N., Akşit H., Tüzün A.E., Küçükyllmaz K., Borum A.E., Uygun M., Akşit D., Aypak S., Simşek E., Seyrek K., Koçer B., Bintaş E., Orojpour A. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 2016;95:1858–1868. doi: 10.3382/ps/pew077. [DOI] [PubMed] [Google Scholar]
- Brahe L.K., Chatelier E.Le, Prifti E., Pons N., Kennedy S., Hansen T., Pedersen O., Astrup A., Ehrlich S.D., Larsen L.H. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes. 2015;5:e159. doi: 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiek O.B., SlimSmaoui A. Enterococci : between emerging pathogens and potential probiotics. Biomed Res. Int. 2019;2019:1–13. doi: 10.1155/2019/5938210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018;204:44–51. doi: 10.1016/j.vetimm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Jeremy E., Ley R.E., Lozupone C.A., Mcdonald D., Muegge B.D., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2011;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017;234:88–100. [Google Scholar]
- Cerisuelo A., Marín C., Sánchez-Vizcaíno F., Gómez E.A., De La Fuente J.M., Durán R., Fernández C. The impact of a specific blend of essential oil components and sodium butyrate in feed on growth performance and Salmonella counts in experimentally challenged broilers. Am. Hist. Rev. 2014;119:599–606. doi: 10.3382/ps.2013-03528. [DOI] [PubMed] [Google Scholar]
- Chang C.L.T., Chung C., Kuo C., Kuo T. Beneficial effect of bidens pilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang F., Lu H., Wang B., Chen Y., Lei D., Wang Y., Zhu B., Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K., Kumar P., Samanta I., Pradhan S., Samanta A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018;236:39–47. [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Dai D., Qiu K., Zhang H., Wu S., Han Y., Wu Y., Qi G., Wang J. Organic acids as alternatives for antibiotic growth promoters alter the intestinal structure and microbiota and improve the growth performance in broilers. Front. Microbiol. 2021;11:1–14. doi: 10.3389/fmicb.2020.618144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Zheng P., Yu B., He J., Mao X., Yu J., Chen D. Effects of benzoic acid and thymol on growth performance and gut characteristics of weaned piglets. Asian Australas. J. Anim. Sci. 2015;28:827–839. doi: 10.5713/ajas.14.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018;5:1–12. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Gan L., Li Z., Wang W., Liu D., Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015;6:1–12. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin K., Callahan M.K., Ren B., Khanin R., Viale A., Ling L., No D., Gobourne A., Littmann E., Huttenhower C., Pamer E.G., Wolchok J.D. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016;7:1–8. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid N.M., Dahshan A.M., Shalaby B. Anticlostridial activity of the thyme and clove essential oils against experimentally induced necrotic enteritis in commercial broiler chickens. Vet. Sci. Res. Rev. 2018;4:25–34. [Google Scholar]
- El-Shall N.A., Shewita R.S., Abd El-Hack M.E., AlKahtane A., Alarifi S., Alkahtani S., Abdel-Daim M.M., Sedeik M.E. Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poult. Sci. 2020;99:2944–2954. doi: 10.1016/j.psj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Daneshmand A., Naeini S.Z., Graystone E.N., Broom L.J. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: Performance, microbiology, intestinal morphology, and immune response. Poult. Sci. 2017;96:3254–3263. doi: 10.3382/ps/pex106. [DOI] [PubMed] [Google Scholar]
- Fascina V.B., Sartori J.R., Gonzales E., De F.B., Mailinch I., Pereira G., Polycarpo V., Stradiotti A.C., Pelícia V.C., Veterinária D.M. Phytogenic additives and organic acids in broiler chicken diets. Rev. Bras. Zootec. 2012;41:2189–2197. [Google Scholar]
- Ferronato G., Prandini A. Dietary supplementation of inorganic, organic, and fatty acids in pig: a review. Animals. 2020;10:1–27. doi: 10.3390/ani10101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Toh H., Hase K. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Letter. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Geier M.S., Mikkelsen L.L., Torok V.A., Allison G.E., Olnood C.G., Boulianne M., Hughes R.J., Choct M. Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 2010;109:1329–1338. doi: 10.1111/j.1365-2672.2010.04758.x. [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi A.R., Timbermont L., Lanckriet A., Den Broeck V., Pedersen K., Dewulf J., Pasmans F., Ducatelle R., Van Immerseel F., Den Broeck V., Pedersen K., Dewulf J., Pasmans F., Haesebrouck F., Gholamiandehkordi A.R., Timbermont L., Lanckriet A. Quantification of gut lesions in a subclinical necrotic enteritis model Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol. 2007;36:375–382. doi: 10.1080/03079450701589118. [DOI] [PubMed] [Google Scholar]
- Gil-cardoso K., Ginés I., Ardévol A., Blay M., Terra X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016;29:234–248. doi: 10.1017/S0954422416000159. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Khaksar V., Rubio L.A., Veldkamp T., van Krimpen M.M. Effect of feed supplementation with a thymol plus carvacrol mixture, in combination or not with an NSP-degrading enzyme, on productive and physiological parameters of broilers fed on wheat-based diets. Anim. Feed Sci. Technol. 2015;211:117–131. [Google Scholar]
- Hiippala K., Jouhten H., Ronkainen A., Hartikainen A., Kainulainen V., Jalanka J., Satokari R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10:1–23. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiippala K., Kainulainen V., Kalliomäki M., Arkkila P., Satokari R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front. Microbiol. 2016;7:1–13. doi: 10.3389/fmicb.2016.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Li F., Duan Y., Yin Y., Kong X. Function Glutamic acid supplementation reduces body fat weight in fi nishing pigs when provided solely or in combination with arginine and it is associated with colonic propionate and butyrate concentrations. Food Funct. 2019;10:1039. doi: 10.1039/c9fo00520j. [DOI] [PubMed] [Google Scholar]
- Hume M.E., Barbosa N.A., Dowd S.E., Sakomura N.K., Nalian A.G., Martynova-Van Kley A., Oviedo-Rondón E.O. Use of pyrosequencing and denaturing gradient gel electrophoresis to examine the effects of probiotics and essential oil blends on digestive microflora in broilers under mixed Eimeria infection. Foodborne Pathog. Dis. 2011;8:1159–1167. doi: 10.1089/fpd.2011.0863. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Russell J.B., Flythe M.D., Gantois I., Timbermont L., Pasmans F., Haesebrouck F., Ducatelle R. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J.J., Galfi P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Benestad S.L., Løvland A. Epidemiologic aspects of necrotic enteritis in broiler chickens – disease occurrence and production performance. Avian Pathol. 2016;45:271–274. doi: 10.1080/03079457.2016.1163521. [DOI] [PubMed] [Google Scholar]
- Kazempour F., Jahanian R. Effects of different organic acids on performance, ileal microflora, and phosphorus utilization in laying hens fed diet deficient in non-phytate phosphorus. Anim. Feed Sci. Technol. 2017;223:110–118. [Google Scholar]
- Khodambashi Emami N., Daneshmand A., Zafari Naeini S., Graystone E.N., Broom L.J. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: performance, microbiology, intestinal morphology, and immune response. Poult. Sci. 2017;96:3254–3263. doi: 10.3382/ps/pex106. [DOI] [PubMed] [Google Scholar]
- Koh A., Vadder F.D., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Kim S., Kim E., Ha E., You H., Kim B., Kim M., Kwon Y., Ryu J., Lee W. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Lee B., Moon K.M., Kim C.Y. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018;2018:1–11. doi: 10.1155/2018/2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu X., Ma X., Geng S., Jiang X., Huang Q., Hu C., Han X. Intestinal microbiome-metabolome responses to essential oils in piglets. Front. Microbiol. 2018;9:1–13. doi: 10.3389/fmicb.2018.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y., Byndloss M.X., Tsolis R.M., Bäumler A.J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017;39:1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019;98:2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Lee C.H., Kwak W.G., Han N.S., Kim H.B., Cho J.H. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 2018;102:1257–1265. doi: 10.1111/jpn.12944. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real- time quantitative PCR and the 2 Ϫ ⌬⌬ C T Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lozupone C.A., Hamady M., Kelley S.T., Knight R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R., Miquel S., Benevides L., Bridonneau C. Functional characterization of novel faecalibacterium prausnitzii strains isolated from healthy volunteers : a step forward in the use of F. prausnitzii as a next-generation probiotic isolation of novel extremely oxygen. Front. Microbiol. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight L.L., Peppler W., Wright D.C., Page G., Han Y. A blend of fatty acids, organic acids, and phytochemicals induced changes in intestinal morphology and inflammatory gene expression in coccidiosis-vaccinated broiler chickens. Poult. Sci. 2019;98:4901–4908. doi: 10.3382/ps/pez241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvecky M., Cejkova D., Polansky O., Karasova D., Kubasova T., Cizek A., Rychlik I. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genomics. 2018;19:1–15. doi: 10.1186/s12864-018-4959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsch P., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004;83:669–675. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- Mohd Shaufi M.A., Sieo C.C., Chong C.W., Gan H.M., Ho Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015;7:1–12. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento L.C.S., Casarotti S.N., Todorov S.D., Penna A.L.B. Probiotic potential and safety of enterococci strains. Ann. Microbiol. 2019;69:241–252. [Google Scholar]
- Nguyen D.H., Seok W.J., Kim I.H. Organic acids mixture as a dietary additive for pigs—a review. Animals. 2020;10:952. doi: 10.3390/ani10060952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighot M., Al-sadi R., Guo S., Rawat M., Nighot P., Watterson M.D. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4 /myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am. J. Pathol. 2017;187:2698–2710. doi: 10.1016/j.ajpath.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonijo F.A., Ni L., Gong J., Wang Q., Lahaye L., Yang C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q.Y., Li J.D., Li Z., Duan Z.Y., Wu Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 2016;214:148–153. [Google Scholar]
- Pirgozliev V., Bravo D., Mirza M.W., Rose S.P. Growth performance and endogenous losses of broilers fed wheat-based diets with and without essential oils and xylanase supplementation. Poult. Sci. 2015;94:1227–1232. doi: 10.3382/ps/peu017. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Lillehoj H.S., Bravo D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 2019;98:3443–3449. doi: 10.3382/ps/pey472. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Mackenzie A.M., Beccaccia A., Karadas F., Ivanova S.G., Staykova G.P., Oluwatosin O.O., Bravo D. Dietary essential oils improve feed efficiency and hepatic antioxidant content of broiler chickens. Animal. 2019;13:502–508. doi: 10.1017/S1751731118001520. [DOI] [PubMed] [Google Scholar]
- Polycarpo G.V, Andretta I., Kipper M., Cruz-Polycarpo V.C., Dadalt J.C., Rodrigues P.H.M., Albuquerque R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017;96:3645–3653. doi: 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007;62:142–160. doi: 10.1111/j.1574-6941.2007.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H.M., Huque K.S., Kamaruddin K.M., Beg M.A.H. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018;101:52–75. doi: 10.3184/003685018X15173975498947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Guo Y., Wang Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013;92:1764–1773. doi: 10.3382/ps.2013-03029. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:1. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Li H., Wu Y., Zhen W., Wang Z., Xia Z., Guo Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed Sci. Technol. 2017;232:6–15. [Google Scholar]
- Stamilla A., Messina A., Sallemi S., Condorelli L., Antoci F., Puleio R., Loria G.R., Cascone G., Lanza M. Effects of microencapsulated blends of organics acids (OA) and essential oils (EO) as a feed additive for broiler chicken. a focus on growth performance, gut morphology and microbiology. Animals. 2020;10:442. doi: 10.3390/ani10030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Geier M.S., Denman S.E., Haring V.R., Crowley T.M., Hughes R.J., Moore R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013;164:85–92. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Geier M.S., Moore R.J., Moore R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion : challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 2016;7:1–13. doi: 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanello C., Rosa D.P., Dalmoro Y.K., Segatto A.L., Vieira M.S., Moraes M.L., Santin E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 2020;6:1–10. doi: 10.3389/fvets.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Liu D., Guo S., Chen Y., Guo Y. Effects of dietary essential oil and enzyme supplementation on growth performance and gut health of broilers challenged by Clostridium perfringens. Anim. Feed Sci. Technol. 2015;207:234–244. [Google Scholar]
- Magoc T., Salzberg S.L. FLASH : fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau A., Fravalo P., Yergeau É., Arsenault J. Chicken caecal microbiome modifications induced by campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F., Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers : an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., Ducatelle R., van Immerseel F. Control of clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010;39:117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- Upadhaya S.D., Cho S.H., Chung T.K., Kim I.H. Anti-coccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from Eimeria tenella and Eimeria maxima. Poult. Sci. 2019;0:1–8. doi: 10.3382/ps/pez040. [DOI] [PubMed] [Google Scholar]
- Upadhyaya I., Upadhyay A., Kollanoor-Johny A., Mooyottu S., Baskaran S.A., Yin H.B., Schreiber D.T., Khan M.I., Darre M.J., Curtis P.A., Venkitanarayanan K. In-feed supplementation of trans-cinnamaldehyde reduces layer- chicken egg-borne transmission of Salmonella enterica serovar enteritidis. Appl. Environ. Microbiol. 2015;81:2985–2994. doi: 10.1128/AEM.03809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicuna E.A., Kuttappan V.A., Tellez G., Hernandez-Velasco X., Seeber-Galarza R., Latorre J.D., Faulkner O.B., Wolfenden A.D., Hargis B.M, Bielke L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler broilers. Poult. Sci. 2015;94:1353–1359. doi: 10.3382/ps/pev111. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A.L., Seemann T., Rood J.I., Moore R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015;180:299–303. doi: 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Wang J., Jia H. Metagenome-wide association studies: fine-mining the microbiome. Microbiology. 2016;14:508–522. doi: 10.1038/nrmicro.2016.83. [DOI] [PubMed] [Google Scholar]
- Wang H., Liang S., Li X., Yang X., Long F., Yang X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poult. Sci. 2019;98:6751–6760. doi: 10.3382/ps/pez391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y., Shahid M.S., Nie W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Sci. Biotechnol. 2018;9:1–14. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019;9:1–17. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Yang X., Xin H., Yang C., Yang X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Du E., Yuan J., Gao J., Wang Y.L., Aggrey S.E., Guo Y. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-07420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E., Keijser B.J.F., Huse S.M., Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9:1–12. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 2015;6:1–10. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Ferrara F., Vahjen W., Van Kessel A.G., Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011;12:83–93. doi: 10.1017/S1466252311000089. [DOI] [PubMed] [Google Scholar]
- Zhai H., Liu H., Wang S., Wu J., Kluenter A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018;4:179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Shen Y.R., Wu S., Xiao Y.Q., He Q., Shi S.R. The dietary combination of essential oils and organic acids reduces Salmonella enteritidis in challenged broilers. Poult. Sci. 2019;98:6349–6355. doi: 10.3382/ps/pez457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen W., Shao Y., Gong X., Wu Y., Geng Y., Wang Z., Guo Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult. Sci. 2018;97:2654–2666. doi: 10.3382/ps/pey119. [DOI] [PubMed] [Google Scholar]
- Zhou B., Jia L., Wei S., Ding H., Yang J., Wang H. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken caecum. Poult. Sci. 2020;99:1297–1305. doi: 10.1016/j.psj.2019.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]