Structured abstract
Context:
Americans express a strong preference for participating in decisions regarding their medical care, yet they are often unable to participate in decision-making regarding their end-of-life care.
Objective:
To examine determinants of end-of-life planning; including, the effect of an individual’s ageing and dying process, health status and socio-economic and racial/ethnic background.
Methods:
US observational cohort study, using data from the Health and Retirement Study (1992 – 2014) including 37,494 individuals. Random-effects logistic regression analysis was used to examine the relationship between the presence of a living will and a range of individual time-varying characteristics, including time to death, and several time-invariant characteristics.
Results:
End-of-life planning depends on several patient characteristics and circumstances, with socio-economic and racial/ethnic background having the largest effects. The probability of having a living rises sharply late in life, as we would expect, and is further modified by the patient’s proximity to death. The dying process, exerts a stronger influence on end-of-life planning than does the aging.
Conclusions:
Understanding differences that increase end-of-life planning is important to incentivize patients’ participation. Advance planning should be encouraged and accessible to people of all ages as it is inevitable for the provision of patient-centered and cost-effective care.
Trial registration number:
Not applicable.
Keywords: End-of-life, living will, end-of-life planning, advance care planning
Editorial Note: David Casarett MD MA
This is a nicely done national study that highlights the impact of socio-economic factors and race/ethnicity on advance care planning.
Introduction
Americans express a strong preference for being involved in decisions regarding their medical care1. However, evidence suggests that they are often unable to participate in decision making related to their end-of-life care2. Around 70% of decedents over 60 surveyed in the Health and Retirement Study (HRS) were not able to participate in those decisions3. Additionally, terminally ill individuals are often subjected to undesired and unhelpful treatments advocated by patients’ carers or family members4,5. This demonstrates the importance of prospectively recording patients’ wishes regarding intensive care in order to achieve goal-concordant care.
An advance directive is a legal document describing a patient’s preferences regarding their medical care in case of incapacity, and is a key mechanism through which patients express their end-of-life care wishes. In the United States (US), this document can take two forms, either a living will or a durable power of attorney. The living will is a written statement containing people’s preferences regarding future medical treatment in situations when they can no longer provide informed consent1. In contrast, a durable power of attorney gives decision-making power to a designated person who will decide on the patient’s behalf, when the latter is incapable of making such decision1. Although written instructions about preferences for end-of-life care can go by various names, we use the term “living will” to refer to all such sets of instructions and consider it as a form of advance care planning. Evidence suggests that having an advanced directive is associated with better quality end-of-life care6-8.
Advance care planning can empower patients and assist physicians in providing goal-concordant care9. In the context of end-of-life care provision, the traditional doctor-patient relationship becomes more complicated as terminally ill patients may experience high levels of dependency and frailty which can limit their sovereignty and exaggerate the problem of asymmetric information10. Information asymmetry implies that the physician is better informed about the patient’s health status and possible treatment pathways11. Often family members and carers become proxy decision-makers to represent the patients’ interests and preferences, which additionally complicates the decision-making process, especially if the patient’s preferences are unrecorded. Therefore, an advance care plan provides an opportunity to align the interests of the physician and the patient, making understanding factors that affect advance care planning critical9,12. Advance planning can be viewed as a type of health behaviour, for which different individuals may have different motivation, facilitators and barriers13. Recently, however, scepticism about the value of advance care planning is increasing, since uptake remains low and evidence for advance care planning influencing patient outcomes is limited.14 Therefore, it is important to understand determinants of advance care planning which could determine the design and implementation of policies that encourage the uptake and engagement in such activities across various patient groups. A patient’s prognosis or their subjective expectations regarding the imminence of death is one factor that could raise awareness of and encourage end-of-life planning. Thus, the aim of this paper is to examine the impact of socio-demographic factors, health status, age and proximity to death on advance care planning.
Methods
Sample
Our analysis uses a sample of 37,494 individuals from the Health and Retirement Study (HRS), surveyed biennially from 1992 through 2014. On average, each sample member appears about 6 times in the dataset. The HRS is a nationally representative longitudinal study of individuals over 50 years of age, designed to assess retirement and health among the elderly in the US15. It is a rich source of data organised into 4 major section: health, work and retirement, social connections and income and wealth.
The surveys include “exit interviews,” questionnaires completed by proxy respondents after a participant’s death, identified from the social network of the deceased. The exit interview provides detailed information on the respondent’s final year of life and death circumstances.
The first cohort of HRS participants was interviewed in 1992; since then 5 additional cohorts have been included in the panel study in order to replenish the study sample. Our data include any age-eligible individual interviewed at least once. The target population for study sample initially included all US residents aged 51 - 61 who live in households, later expanded (by 1998) to include the entire population aged 51 and older. Following conventional practice for population surveys, institutionalized individuals (prisons, jails, nursing homes, long-term or dependent care facilities) are excluded from the initial survey population, although they are retained if in subsequent interviews they have moved to a nursing home. Baseline interviews are conducted face-to-face, while follow-up interviews are mostly conducted via telephone. Since 2006, at each wave, half of the respondents complete the face-to-face while the other half complete the core interview by telephone. The half-samples alternate waves so there is an in-person interview for each respondent every four years.
Analysis
The presence of a living will is our outcome measure and it is used as an indicator of advance care planning. Beginning in 2002 a question regarding whether the deceased had written end-of-life instructions was included in the exit interview. Because the timing of end-of-life instructions was included in the questionnaire, we were able to reconstruct the presence of these for deceased participants prior to their death. For deceased participants without written end-of-life instructions we assumed that these were also not available at the times of previous interviews. Further, from 2012, the core HRS questionnaire included a question regarding the presence of the living will. If a living participant gave a negative response, then we assumed that the participant did not have a living will in all previous waves. In contrast, if a living participant confirmed the existence of a living will, we coded data in all previous waves as missing, as we were not sure when the end-of-life instructions were written. About 33% of deceased participants and 20% of living participants had a living will.
Ageing was represented using both: chronological age and remaining lifetime, with the latter serving as proxy for biological age. As people age, they are more aware that the end of their life is approaching. The aging process can be characterized through chronological age, which refers to amount of time the person has been alive, or through biological age, which refers to how old the person seems and which is related to genetic, behavioural, and environmental conditions16. Biological age can be characterized using various biomarkers of ageing, but these are not routinely measured in population-level studies. On the other hand, if individuals are followed longitudinally, some will die during a follow up period. Remaining lifetime, or time to death (TTD) can be used as a proxy for biological age17. Research has shown that individuals’ expectations regarding their own future survival agree with actual experience, demonstrating that biomarkers were predictive of TTD regardless of age.18-21 The idea of using TTD in describing patients trajectories first appeared in 1970s and focused on hospital care of terminally ill patients22. Later, few other studies considered TTD in assessing disability and classifying dying patients17,23. Despite that, most studies take into account only chronological age, while ignoring the impact of TTD on late life events.
A problem with TTD in panel studies is that for living participants it remains unknown, because only in rare occasions the sample will be followed up until all individuals die, removing the right-censoring problem17. Still, it can be estimated. Information on participants’ month and year of birth, death (if applicable), and month and year of interviews was used to establish measures of age and TTD at each interview. All age variables were expressed relative to age 75. For those who died during the follow up period, TTD is known and can never exceed 22 years. Participants that remain alive at the end of the follow up period have an unobserved value of TTD at each interview. We used interval regression to model remaining lifetime and to provide a basis for imputing the unobserved (censored) values of TTD. For cases with unknown an value of TTD, we constructed variables for lower and upper bounds of TTD. For cases with censored TTD, the lower bound of TTD is always known. For example, for someone still alive in 2014, we know that TTD is greater than 22 in 1992, and TTD is greater than 20 in 1994, and so on. So, at each interview the lower bound of TTD presents a difference between the age at final interview and the age at the current interview. We also assumed that the maximum possible age of participant is 112 years, as this was the age of the oldest deceased participant in the sample. The upper bound of TTD at each wave is defined as the difference between the maximum possible age and the current age of participant at each interview. Our interval regression for remaining lifetime imposes these bounds; when TTD is known (among uncensored cases) the upper and lower bounds are identical. Explanatory variables used in the regression include age, gender, indicator for racial and ethnicity status (Non-Hispanic White, Non-Hispanic Black, Hispanic White and Other), education level (below high-school level, high-school level, degree level), total assets, marital status (married or partnered, not-married or not-partnered), smoking and drinking status, self-reported health, census region (Midwest, Northwest, South, East and Other), and indicators for the following medical conditions: high blood pressure, diabetes, cancer, lung disease, heart disease, stroke, arthritis and psychiatric problems. These conditions are the most common comorbidities among the elderly 24. The estimated regression was used to impute TTD to the censored observations, with the known lower- and upper-bound conditions imposed. Because we are using a linear model of remaining lifetime, it produces an unbiased estimate of the expected value of remaining lifetime among those for whom TTD is not observed.
We used random-effects logistic regression to estimate the relationship between the probability of having a living will and a set of time-varying and time-invariant control variables. To account for the uncertainty present in the TTD imputation process, we performed 5 independent sets of imputations, and used multiple imputation estimation and inference techniques in the analysis. Explanatory variables included age and TTD in linear and quadratic form, gender, indicator for racial and ethnicity status, education level, marital status, income and assets, census region, and indicators for excellent self-reported health (health status is very good or excellent), and the presence of the eight medical conditions listed above. All analyses were performed using the statistical software STATA (Version 14).
Results
On average, individuals were 66 years old over the 12 survey waves (Table 1). Average estimated TTD for the analysed sample was 16.3 years, while average observed TTD for individuals with observed deaths was 7.2 years. Majorities of the sample were non-Hispanic Whites (72.8%), female (60.3%) and had a high-school degree or more (73.2%). Arthritis was the most common chronic condition reported by 49.3% of participants, while stroke was the least common condition, reported by only 5.7% of the sample.
Table 1:
Sample characteristics
| Sample mean / % |
Standard deviation | |
|---|---|---|
| Time-variant characteristics | ||
| Age | 66.29 | 11.37 |
| TTD (imputed) | 16.32 | 6.16 |
| TTD (observed) | 7.16 | 5.01 |
| Income | ||
| Total income | $67,853 | $162.17 (S.E.)* |
| Missing | 6.70% | |
| Assets | ||
| Total assets | $483,137 | $856.45 (S.E.)* |
| Missing | 4.32% | |
| High blood pressure | ||
| Yes | 49.43% | - |
| Missing | 2.84% | - |
| Diabetes | ||
| Yes | 15.34% | - |
| Missing | 3.43% | - |
| Cancer | ||
| Yes | 9.87% | - |
| Missing | 2.76% | - |
| Lung disease | ||
| Yes | 7.34% | - |
| Missing | 4.54% | - |
| Heart disease | ||
| Yes | 16.51% | - |
| Missing | 2.45% | - |
| Stroke | ||
| Yes | 5.65% | - |
| Missing | 3.55% | - |
| Psychiatric problems | ||
| Yes | 13.12% | - |
| Missing | 6.71% | - |
| Arthritis | ||
| Yes | 49.31% | - |
| Missing | 4.76% | - |
| Having difficulties with ADLs | ||
| Yes | 11.12% | - |
| Missing | 3.21% | - |
| Self-reported health excellent | ||
| Yes | 42.25% | - |
| Missing | 4.68% | - |
| Time-invariant characteristics | ||
| Gender | ||
| Female | 60.25% | - |
| Male | 39.75% | - |
| Missing | 0.00% | |
| Race/ethnicity | ||
| Non-Latino White | 72.83% | - |
| Non-Latino Black | 15.89% | - |
| Latino White | 7.45% | - |
| Other race/ethnicity | 3.83% | - |
| Missing | 0.00% | - |
| Cohabitation status | ||
| Living with partner | 63.39% | - |
| Not living with partner | 34.27% | - |
| Missing | 2.34% | - |
| Education | ||
| Lower than high school level | 26.73% | - |
| High school level | 51.57% | - |
| Graduate level | 21.60% | - |
| Missing | 0.10% | |
| Region | ||
| Northeast region | 16.01% | - |
| Midwest region | 24.09% | - |
| South region | 40.49% | - |
| West region | 12.27% | - |
| Other region | 0.14% | - |
| Missing | 0.00% | - |
Notes: TTD denotes “time-to-death”. ADL denotes “activities of daily living”.
S.E. denotes standard error.
Variables “Total income” and “Total assets” are highly skewed, so standard error is a more appropriate measure of dispersion. The values are averaged over the pooled sample of 37,494 individuals and 226,545 person-wave observations.
A range of individual characteristics affected the presence of the living will (Table 2). Age [OR (Age)=1.85, 95%CI (1.81 – 1.90), p<0.001; OR (Age^2)=0.99, 95%CI (0.986 – 0.988), p<0.001], TTD [OR (TTD)=0.73, 95%CI (0.69 – 0.76), p<0.001; OR (TTD^2)=1.01, 95%CI (1.008 – 1.012), p<0.001] and racial and ethnic background [OR (Non-Latino Black)= 0.002, 95%CI (0.002 – 0.004), p<0.001; (OR (Latino White)= 0.002, 95%CI (0.001 – 0.005), p<0.001] are significantly associated the probability of having a living will. Non-Hispanic Whites had significantly higher probability of engaging in end-of-life planning activities, compared to individuals of other racial and ethnic background. Moreover both educational attainment [OR (High school level)=30.49, 95%CI (19.3 – 48.1), p<0.001; OR (Graduate level)=165.2, 95%CI (103.3 – 264.2), p<0.001] and economic well-being [OR (linearized income)=1.65, 95%CI (1.47 – 1.85), p<0.001], were important factors in having a living will. In general, individuals of higher social status were more likely to have a living will. Also, the probability was higher for individuals of poorer health [OR(Difficulties with ADLs)=1.42, , 95%CI (1.32 – 1.53), p<0.001] and females [OR=3.41, 95%CI (2.42 – 4.78), p<0.001], but lower for those living with a partner [OR=0.45, 95%CI (0.35 – 0.59), p<0.001]. Cancer [OR=1.77, 95%CI (1.46 – 2.14), p<0.001] in particular had a strong effect on the probability on having a living will. Other chronic conditions such as high-blood pressure [OR=1.20, 95%CI (1.06 – 1.34), p=0.004], lung disease [OR=1.20, 95%CI (1.01 – 1.41), p=0.034] were also associated with having a living will. However, the presence of diabetes, stroke and psychiatric problems were not associated with having a living will.
Table 2:
Random-effects logistic regression analysis of determinants of planning at the end-of-life
| Independent variable (N= 33,172) |
Presence of the living will Odds Ratios (95% CI) |
P-value |
|---|---|---|
| Intercept | 0.010 (0.000 – 0.020) | <0.001*** |
| TTD | 0.725 (0.693 - 0.758) | <0.001*** |
| TTD^2 | 1.010 (1.008 - 1.012) | <0.001*** |
| Age | 1.853 (1.808 - 1.899) | <0.001*** |
| Age^2 | 0.987 (0.986 - 0.988) | <0.001*** |
| Gender (Ref: Male) | 3.410 (2.431 - 4.782) | <0.001*** |
| Race/ethnicity (Ref: Non-Latino White) | ||
| Non-Latino Black | 0.002(0.002 – 0.004) | <0.001*** |
| Latino White | 0.002 (0.001 – 0.005) | <0.001*** |
| Other | 0.004 (0.001 – 0.012) | <0.001*** |
| Living with partner | 0.452 (0.348 – 0.589) | <0.001*** |
| Linearized total income | 1.647 (1.469 – 1.847) | <0.001*** |
| Total assets | 1.003 (1.002 – 1.004) | <0.001*** |
| Education level (Ref: Lower than high school level) | ||
| High school level | 30.487 (19.330 - 48.083) | <0.001*** |
| Graduate level | 165.181 (103.276 - 264.191) | <0.001*** |
| High blood pressure | 1.199 (1.060 - 1.355) | 0.004** |
| Diabetes | 1.143 (0.966 - 1.353) | 0.119 |
| Cancer | 1.766 (1.458 - 2.139) | <0.001*** |
| Lung disease | 1.195 (1.013 - 1.409) | 0.034** |
| Heart disease | 0.902 (0.808 - 1.008) | 0.068* |
| Stroke | 1.071 (0.883 - 1.299) | 0.487 |
| Psychiatric problems | 1.094 (0.957 - 1.251) | 0.190 |
| Arthritis | 1.113 (0.988 - 1.254) | 0.078* |
| Number of difficulties with ADLs | 1.421 (1.320 - 1.530) | <0.001*** |
| Self-reported health excellent (Ref: Self-reported health is below excellent) | 0.945 (0.790- 1.129) | 0.532 |
| Region (Ref: North-east) | ||
| Midwest | 2.491 (1.541 - 4.027) | <0.001*** |
| South | 1.205 (0.780 - 1.861) | 0.400 |
| West | 4.545 (2.643 - 7.815) | <0.001*** |
| Other | 0.950 (.028 - 32.388) | 0.977 |
Notes: Presented results are from random effects logistic regression analysis. Results are presented as odds ratios. Odds ratio indicates percentage odds change for a unit increase in the observed variable, holding other variables constant.
P<0.1
P<0.05
P<0.001.
N denotes sample size. For categorical variables, the reference category is stated in the row label, otherwise the reference is the complementary category. Total income values and total asset values were divided by 100,000 to aid interpretation.
As individual end-of-life planning trajectories depend both on age and TTD, there are numerous pathways in progress within the general population at any moment. We illustrate selected scenarios in Figure 1 and show how they are modified by various background factors in Figures 2 and 3. In all cases, the fitted probabilities reflect the mix of sample characteristics in all other respects. Figure 1 illustrates the pattern of having a living will for individuals who die at age 80, 90 and 100, depending on current age. Individuals who die at a very old age (e.g. 100 years) will almost certainly have a living will immediately prior to death (97.5%), while the analogous probability for individuals who die at age 80 is much lower (38.1%). Lastly, most individuals don’t initiate a living will until age 75 or older.
Figure 1: Probability of having a living will, by current age and age at death.
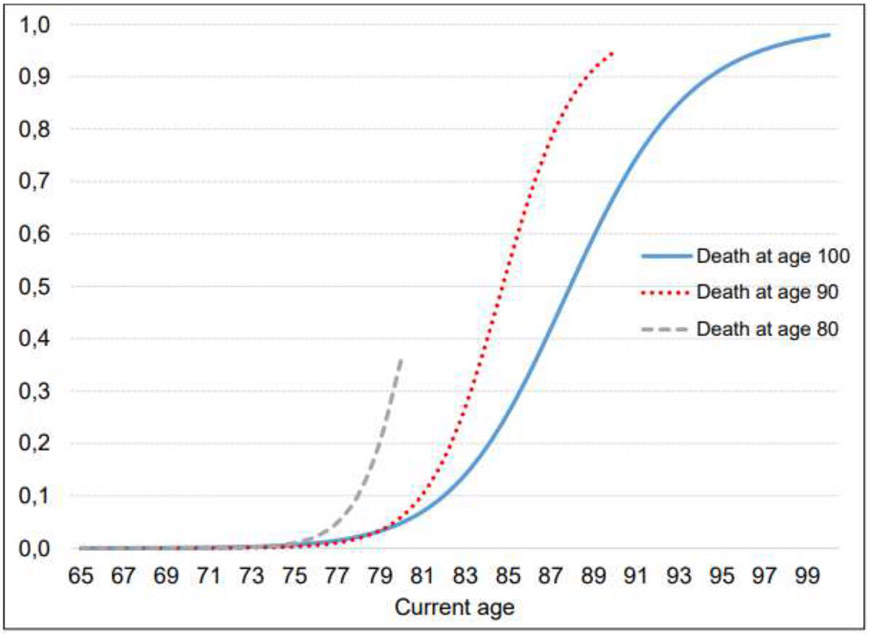
Notes: The graph depicts an average sample population for different ages at death.
Figure 2: Probability of having a living will, by current age and age at death for individuals of different ethnical and racial background.
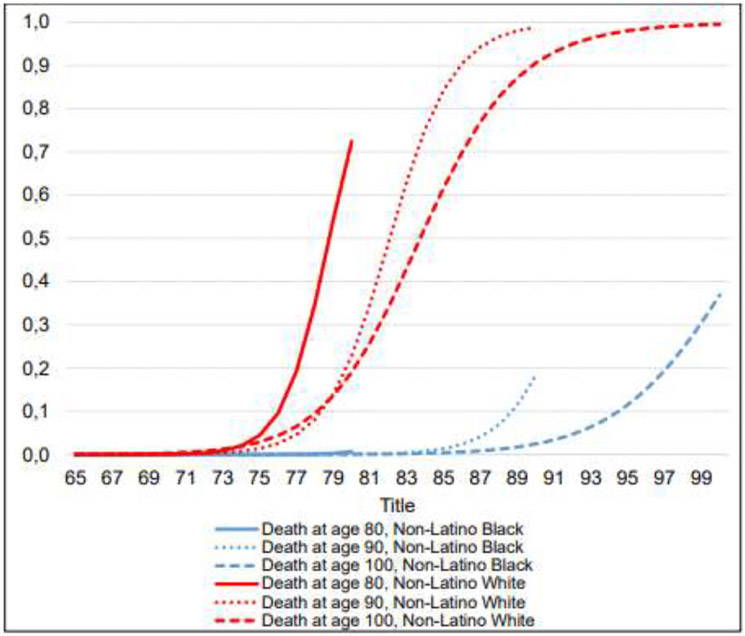
Notes: The graph depicts an average sample population of different ethnical and racial background for different ages at death. Effect of Non-Latino Black is similar to the effect of Latino White and therefore trajectories of Non-Latino Black are indicative of those of Latino White.
Figure 3: Probability of having a living will, by current age and age at death for different educational levels.
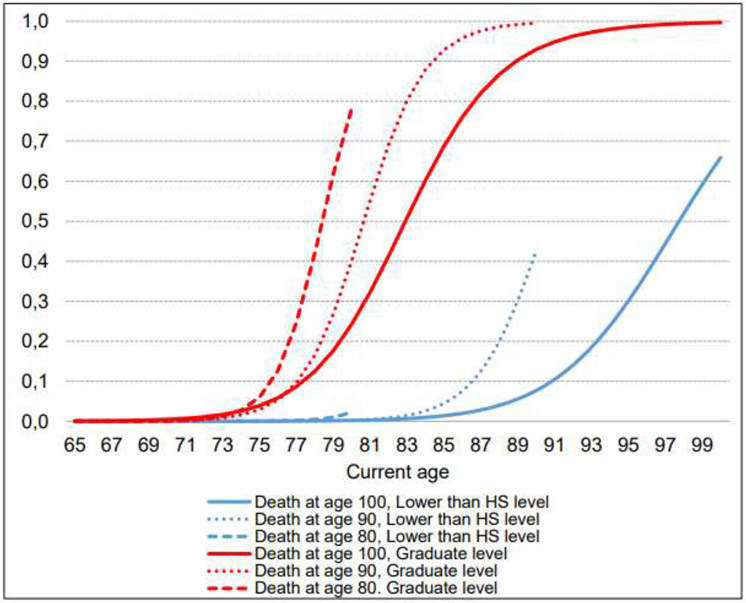
Notes: HS denotes “high-school”. The graph depicts an average sample population that has different levels of educational attainment for different ages at death.
Non-Hispanic Whites initiate end-of-life planning much earlier compared to those of other ethnic and racial backgrounds, and also end up with much higher levels or participation (Figure 2). For example, Non-Hispanic Whites dying at age 100 will almost certainly have a living will (99.2%), while the same probability among Non-Hispanic Blacks dying at the same age is much lower (37.5%). The differences are even larger for individuals who die younger, indicating the importance of ethnicity and race for end-of-life planning.
More educated individuals are more likely to participate in end-of-life planning and, on average, do so earlier compared to their less-educated counterparts (Figure 3). The differences are particularly high for individuals who die younger. For example, a college-educated individual who dies at age 80 years has 79.1% probability of having a living will, while someone with only a basic education dying at the same age has only a 2.4% probability of having a living will.
Discussion
The study provides insights into factors that affect advance care planning. Our results, based on a nationally representative sample of Americans, provide key insights into factors that indicate variation in how and when Americans perform advance care planning. We show that despite concerted efforts to push advance care planning upstream, few individuals undertake advance care planning before the age of 75, and only about a third of patients who die at age 80 adopt an advance care plan. Furthermore, while patients with cancer are most likely to have an advance care plan, patients with heart disease, the most common cause of death in the US, are least likely to have an advance care plan.
Our results provide unique insights into how biological and chronological age are associated with advance care planning. If TTD is viewed as a proxy for biological age, our results indicate that it is chronological age, and not biological age, that exerts a stronger influence on the likelihood of having a living will17. Further, worsened health status is also positively correlated with participation in end-of-life planning. However, even though aging and health status matter, an individual’s socio-economic and racial/ethnic circumstances appear to have a strong association with the propensity to plan for the end-of-life. Non-Hispanic Whites and more educated Americans have considerably higher rates of end-of-life planning participation.
Serious illnesses significantly increase someone’s likelihood of having a living will though not all diseases have a similar impact. A cancer diagnosis is often presented to a patient as a “death sentence,” and may be accompanied by a prognosis regarding remaining lifetime likely prompting patents without living wills to consider adopting one. Additionally, as cancer patients tend to have better access to palliative services, they may also have a better awareness of end-of-life planning options and their significance25. However, patients with heart disease, the most common cause of death in the US, are least likely to have an advance care plan, suggesting opportunities to improve care and communication for this large group of patients. Prior work has shown that both patients with heart disease and their physicians are likely to underestimate their risk of mortality compared to cancer, which could reduce their likelihood of documenting an advance care plan since it alters their perceived time to death26,27.
Our results are consistent with recent studies on end-of-life planning28,29. Older individuals and those in poorer health are more aware that death is approaching and are more inclined to engage in care planning activities that could relieve pain and discomfort in their last moments of life1. However, few studies consider both age and remaining lifetime simultaneously, suggesting that the dying process is more influential than aging process as a determinant of end-of-life planning17. Our results suggest that proximity to death, and not just chronological age, is one of the strongest drivers of health care utilisation at the end-of-life30,31.
Having an advance care plan affects patient outcomes. Individuals with recorded end-of-life preferences are less likely to utilise intensive, out-of-hospital institutional care at end of their lives38. Evidence also suggests that having an advance plan increases the likelihood of fulfilling the patients' preferences regarding their end-of-life care8 and improves their satisfaction and quality of life in their final weeks of life7. End-of-life planning may help to align the interests and minimise disputes between the principal (patient) and the agent (physician). It can prevent overutilization, and diminish futile treatments at the end-of-life ensuring that resources are used in more cost-effective ways39.
In the US, advance care planning has been widely promoted as a tool for communicating end-of-life preferences40. Despite these efforts, completion rates remain low. According to a recent systematic review, only about one third of Americans has completed some form of advance care planning40. Since 2016 Medicare reimburses physicians for having end-of-life conversations, and early evidence suggests that these conversations are associated with less intensive end-of-life care42. Also, advance care planning includes several legal conditions, required for executing advance directives, such as qualified witnesses and notarization40. Even though these restrictions are put in place to protect the patients, these can also be barriers for those that might not fully understand the legal system and cannot afford legal counselling.
This study has several limitations. The analysis utilises exit interviews which are conducted with a proxy respondent, which might lead to missing or erroneous information regarding the existence and content of living wills. Nevertheless, most proxy-respondents (88%) are close family members and they are likely aware of care planning activities of their loved ones. Further, family and health care professionals may have an important role in individual’s end-of-life decision-making, but unfortunately the used data does not allow investigations of their impact to end-of-life decisions and living will content. Also, it was not possible to assess the patient's quality of end-of-life or family satisfaction with the patient death quality and to investigate the relationship between having a living will and the end-of-life care model (e.g. ICU or palliative care). Another potential weakness is the imputation of TTD. While we used an extensive set of possible predictors, we cannot exclude a possibility of unobserved heterogeneity that could bias our estimates. Finally, even though our random-effects logistic model controls for a wide range of individual characteristics that may affect advance care planning, some important determinants might remain unobserved. Therefore, our findings should be interpreted as associations and not as causal effects.
Advance care planning is an important step for the provision of patient-centred and cost-effective care43. Since the US is racially and ethnically diverse, initiatives should also be culturally tailored to acknowledge different attitudes and motivation towards advance planning. In addition, advance care planning can serve as an instrument to accelerate a change in attitudes towards death and dying, moving away from traditional medical paternalism44. Also, this can assist in reducing information asymmetry in the doctor-patient relationship and facilitate informed decision-making. Planning for the end-of-life can have a significant effect on the care patients receive in their last moments3. Policies should be aimed not only at patients and healthcare professionals, but also at close family members and caregivers to incentivize patients to express and record their end-of-life preferences. This may reduce decision-making conflicts and minimise the risk of overtreatment as well as the provision of unwanted and futile care. It can also contribute to a more rational use of scarce healthcare resources and lower societal burden.
Patient autonomy is increasingly becoming a central ethical principle in healthcare decision-making. Policy makers need to favour policies that empower patients and increase their participation in advance care planning. These policies should be aimed at patients of all ages, not only to older adults, as proximity to death and not just chronological age is an important factor in end-of-life planning.
Conclusion
This study presents robust findings on the factors affecting advance care planning in the US and suggests that planning depends on a range of patient characteristics and circumstances, with socio-economic and racial/ethnic background, TTD and health status being the most significant ones. The chances of having a living will rise sharply late in life, as we would expect, but are further modified by the patient’s proximity to death. Providing the right care, at the right moment, and according to patients’ preferences is the ultimate goal of high-quality end-of-life care and advance care planning is a step forward in the achievement of that goal.
Key message.
The study provides insight into the factors affecting of end-of-life planning based on a nationally representative longitudinal dataset of Americans, demonstrating that End-of-life planning depends on a range of patient characteristics and circumstances, with socio-economic and racial/ethnic background being the most important.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The authors have no financial or any other kind of personal conflicts with this paper.
References
- 1.Institute of Medicine (US). Committee on Approaching Death: Addressing Key End-of-Life Issues. Dying in America: improving quality and honoring individual preferences near the end of life. Washington, D.C.: National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.Nelson JE, Angus DC, Weissfeld LA, et al. End-of-life care for the critically ill: A national intensive care unit survey. Critical Care Medicine. 2006;34(10):2547–2553. [DOI] [PubMed] [Google Scholar]
- 3.Silveira MJ, Kim SYH, Langa KM. Advance Directives and Outcomes of Surrogate Decision Making before Death. New England Journal of Medicine. 2010;362(13):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AA, Keating NL, Ayanian JZ, et al. Family Perspectives on Aggressive Cancer Care Near the End of Life. Jama-Journal of the American Medical Association. 2016;315(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes-Hallett T, Cleary J, Grant L, Harding R, Jadad A. Dying healed: Transforming end-of-life Care Through innovation. Qatar: 2013. [Google Scholar]
- 6.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: A national study. Journal of the American Geriatrics Society. 2007;55(2):189–194. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-Life Discussions, Goal Attainment, and Distress at the End of Life: Predictors and Outcomes of Receipt of Care Consistent With Preferences. Journal of Clinical Oncology. 2010;28(7):1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. Journal of the American Geriatrics Society. 2014;62(4):706–710. [DOI] [PubMed] [Google Scholar]
- 10.Eika KH, Kjølsrød L. The difference in principle between the poorly informed and the powerless: a call for contestable authority. Nordic Social Work Research. 2013;3(1):78–93. [Google Scholar]
- 11.Arrow KJ. Uncertainty and the welfare economics of medical care. The American Economic Review. 1963;53(5):941–973. [Google Scholar]
- 12.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR. Regional Variation in the Association Between Advance Directives and End-of-Life Medicare Expenditures. Jama-Journal of the American Medical Association. 2011;306(13):1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried TR, Redding CA, Robbins ML, Paiva A, O'Leary JR, Iannone L. Stages of change for the component behaviors of advance care planning. J Am Geriatr Soc. 2010;58(12):2329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sean Morrison R Advance Directives/Care Planning: Clear, Simple, and Wrong. J Palliat Med. 2020;23(7):878–879. [DOI] [PubMed] [Google Scholar]
- 15.Bugliari D, Campbell N, Chan C, et al. RAND HRS Data Documentation, Version P. Social Security Administration, the National Institute on Aging;2016. [Google Scholar]
- 16.Couoh LR. Differences between biological and chronological age-at-death in human skeletal remains: A change of perspective. American journal of physical anthropology. 2017;163(4):671–695. [DOI] [PubMed] [Google Scholar]
- 17.Wolf DA, Freedman VA, Ondrich JI, Seplaki CL, Spillman BC. Disability Trajectories at the End of Life: A "Countdown" Model. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2015;70(5):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler RN, Sprott R, Warner H, et al. Biomarkers of aging: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci. 2004;59(6):B560–567. [DOI] [PubMed] [Google Scholar]
- 19.Goldman N, Turra CM, Glei DA, Seplaki CL, Lin YH, Weinstein M. Predicting mortality from clinical and nonclinical biomarkers. J Gerontol A Biol Sci Med Sci. 2006;61(10):1070–1074. [DOI] [PubMed] [Google Scholar]
- 20.Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGarry KM. Perceptions of Mortality: Individual Assessments of Longevity Risk. Wharton Pension Research Council Working Paper. 2020(2020-09). [Google Scholar]
- 22.Glaser BG, Strauss AL. Time for dying. AldineTransaction; 1968. [Google Scholar]
- 23.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. Jama. 2003;289(18):2387–2392. [DOI] [PubMed] [Google Scholar]
- 24.Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66(3):301–311. [DOI] [PubMed] [Google Scholar]
- 25.Hawley P Barriers to access to palliative care. Palliative Care: Research and Treatment. 2017;10:1178224216688887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen LA, Yager JE, Funk MJ, et al. Discordance Between Patient-Predicted and Model-Predicted Life Expectancy Among Ambulatory Patients With Heart Failure. JAMA. 2008;299(21):2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warraich HJ, Allen LA, Mukamal KJ, Ship A, Kociol RD. Accuracy of physician prognosis in heart failure and lung cancer: comparison between physician estimates and model predicted survival. Palliative medicine. 2016;30(7):684–689. [DOI] [PubMed] [Google Scholar]
- 28.McAfee CA, Jordan TR, Sheu JJ, Dake JA, Kopp Miller BA. Predicting Racial and Ethnic Disparities in Advance Care Planning Using the Integrated Behavioral Model. Omega (Westport). 2017:30222817691286. [DOI] [PubMed] [Google Scholar]
- 29.Lovell A, Yates P. Advance Care Planning in palliative care: a systematic literature review of the contextual factors influencing its uptake 2008-2012. Palliat Med. 2014;28(8):1026–1035. [DOI] [PubMed] [Google Scholar]
- 30.Zweifel P, Felder S, Meiers M. Ageing of population and health care expenditure: a red herring? Health Econ. 1999;8(6):485–496. [DOI] [PubMed] [Google Scholar]
- 31.Hazra NC, Rudisill C, Gulliford MC. Determinants of health care costs in the senior elderly: age, comorbidity, impairment, or proximity to death? Eur J Health Econ. 2018;19(6):831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerst K, Burr JA. Planning for end-of-life care - Black-White differences in the completion of advance directives. Research on Aging. 2008;30(4):428–449. [Google Scholar]
- 33.Koss CS, Baker TA. Race differences in advance directive completion: the narrowing gap between White and African American older adults. Journal of aging and health. 2017;29(2):324–342. [DOI] [PubMed] [Google Scholar]
- 34.Bullock K Promoting advance directives among African Americans: A faith-based model. Journal of Palliative Medicine. 2006;9(1):183–195. [DOI] [PubMed] [Google Scholar]
- 35.Kermel-Schiffman I, Werner P. Knowledge regarding advance care planning: A systematic review. Archives of gerontology and geriatrics. 2017;73:133–142. [DOI] [PubMed] [Google Scholar]
- 36.Kagawa-Singer M, Blackhall LJ. Negotiating cross-cultural issues at the end of life: "You got to go where he lives". JAMA. 2001;286(23):2993–3001. [DOI] [PubMed] [Google Scholar]
- 37.Sudore RL, Landefeld CS, Barnes DE, et al. An advance directive redesigned to meet the literacy level of most adults: A randomized trial. Patient Education and Counseling. 2007;69(1-3):165–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degenholtz HB, Rhee Y, Arnold RM. Brief communication: The relationship between having a living will and dying in place. Annals of Internal Medicine. 2004;141(2):113–117. [DOI] [PubMed] [Google Scholar]
- 39.Noah BA, Feigenson N. Avoiding Overtreatment at the End of Life: Physician-Patient Communication and Truly Informed Consent. 2016. [Google Scholar]
- 40.Yadav KN, Gabler NB, Cooney E, et al. Approximately One In Three US Adults Completes Any Type Of Advance Directive For End-Of-Life Care. Health Affairs. 2017;36(7):1244–1251. [DOI] [PubMed] [Google Scholar]
- 41.Griffin S, Cubanski J, Neuman T, Jankiewicz A, Rousseau D, Kaiser Family F. Medicare and End-of-Life Care. JAMA. 2016;316(17):1754. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Jin G, Reich A, et al. Association of Billed Advance Care Planning with End-of-Life Care Intensity for 2017 Medicare Decedents. J Am Geriatr Soc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boerner K, Carr D, Moorman S. Family relationships and advance care planning: do supportive and critical relations encourage or hinder planning? J Gerontol B Psychol Sci Soc Sci. 2013;68(2):246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon-Lorda P, Tamayo-Velazquez MI, Barrio-Cantalejo IM. Advance directives in Spain. Perspectives from a medical bioethicist approach. Bioethics. 2008;22(6):346–354. [DOI] [PubMed] [Google Scholar]


