Abstract
Poor fear extinction learning and recall are linked to the development of fear-based disorders, like posttraumatic stress disorder, and are associated with aberrant activation of fear-related neural circuitry. This includes greater amygdala activation during extinction learning and lesser hippocampal and ventromedial prefrontal cortex (vmPFC) activation during recall. Emerging data indicate that genetic variation in fatty acid amide hydrolase (FAAH C385A; rs324420) is associated with increased peripheral endocannabinoid (eCB) levels and lesser threat-related amygdala reactivity. Preclinical studies link increased eCB signaling to better extinction learning and recall, thus FAAH C385A may protect against the development of trauma-related psychopathology by facilitating extinction learning. However, how this FAAH variant affects fear extinction neural circuitry remains unknown. In the present study, we used a novel, immersive-reality fear extinction paradigm paired with functional neuroimaging to assess FAAH C385A effects on fear-related neural circuitry and conditioned fear responding (US expectancy ratings, subjective units of distress, and skin conductance responding) in healthy adults from an urban area (Detroit, MI; N = 59; C/C = 35, A-carrier = 24). We found lesser amygdala activation in A-allele carriers, compared to C/C homozygotes, during early extinction recall. Likewise, we found lesser dorsal anterior cingulate cortex and greater hippocampus activation in early extinction learning in A-carriers compared to C/C homozygotes. We found no effects of FAAH C385A on vmPFC activation or behavioral fear indices. These data support and extend previous findings that FAAH genetic variation, associated with increased eCB signaling and subsequent enhanced fear extinction, may predict individual differences in successful fear learning.
Keywords: amygdala, aversive, endocannabinoids, genetic polymorphism, magnetic resonance imaging, prefrontal cortex
1 |. INTRODUCTION
Maladaptive fear processing, particularly the inability to overcome learned fear responses to aversive memories or stimuli, hallmarks stress- and fear-related symptomology (e.g., exaggerated hyperarousal, avoidance) and is thought to underlie the risk of fear-based disorders, like posttraumatic stress disorder (PTSD). Successful fear extinction learning and its later recall is characterized by reductions in conditioned fear responses (i.e., skin conductance responding) following non-reinforced presentations of a previously conditioned stimulus (CS). Repeatedly presenting a CS in the absence of an aversive stimulus (fear extinction learning) leads to the formation of a new, safe memory, resulting in a gradual decrease in fear responding over time that differentiates early versus late learning phases (see Quirk & Mueller, 2008 for a review). Impaired fear extinction learning and recall (Graham & Milad, 2011) has been suggested as an important mechanism in the pathogenesis of fear-based disorders that present high levels of disease burden and are not uncommon. Indeed, as much as 12% of trauma-exposed civilians and 24% of veteran populations will develop PTSD (Hoppen & Morina, 2019; Kessler et al., 2005; Shalev et al., 2019; Spottswood et al., 2017). Further, extinction learning forms the basis of exposure-based therapies (e.g., prolonged exposure therapy) that aim to facilitate the extinction of fear memories and are highly effective for treating fear-based disorders. However, individual responses to treatment vary and therapeutic gains can be difficult to maintain (Foa et al., 1999; Hembree et al., 2003). Current knowledge of neurobiological factors impacting fear extinction in humans is limited. Continued research into individual differences contributing to the risk of developing fear-based disorders and variation in treatment response is essential to inform the development of more targeted, effective, evidence-based interventions.
Emerging preclinical and translational studies demonstrate the critical role of the endocannabinoid (eCB) system in fear extinction learning. The eCB system is a key neuromodulatory system involved in various psychophysiological processes including emotional learning and memory, adaptive responses to stress, and fear responding (Marsicano & Lafenêtre, 2009; Marsicano & Lutz, 2006; Marsicano et al., 2002; Ruehle et al., 2012). Preclinical studies have linked chronic stress exposure to altered eCB signaling within the corticolimbic regions involved in fear learning and anxiety, including the amygdala, hippocampus, and medial prefrontal cortex (mPFC) (Gray et al., 2015; Hill et al., 2013; Rademacher et al., 2008). Mounting evidence indicates that the eCB anandamide (AEA) promotes fear extinction learning and protects against anxiogenic effects of stress via its actions on the cannabinoid type-1 receptor (CB1R) (Gray et al., 2015; Hill & McEwen, 2010; Patel et al., 2005; Yasmin et al., 2020). CB1Rs are highly expressed in brain regions involved in stress and fear responding, including the amygdala, hippocampus, mPFC, and anterior cingulate cortex (Glass et al., 1997; Herkenham et al., 1990; Katona et al., 2001).
Fatty acid amide hydrolase (FAAH) enzymatically degrades AEA and is therefore the primary regulator of AEA levels in the brain by modulating its effects on CB1Rs (Basavarajappa, 2007). Indeed, in preclinical models, pharmacological blockade of FAAH increases brain AEA levels in key fear regions (e.g., basolateral amygdala) and prevents stress-induced anxiety-like behaviors and increases AEA levels (Bluett et al., 2014; Duan et al., 2017; Gunduz-Cinar, Hill, et al., 2013; Haller et al., 2009; Hill et al., 2013; Kathuria et al., 2003; Yasmin et al., 2020). FAAH inhibition has also been shown to facilitate fear extinction learning and its later recall in rodents (Gunduz-Cinar, Hill, et al., 2013), suggesting that increased AEA signaling can enhance fear learning. Recent translational studies have demonstrated that robust increases in peripheral AEA levels following FAAH inhibition can buffer negative psychophysiological effects of stress, attenuate activation of fear neural circuitry in response to threat, and lead to improved extinction recall in healthy adults (Mayo, Asratian, Lindé, Morena, et al., 2020; Paulus et al., 2020). Together these studies implicate the eCB system–specifically FAAH–as a potential target for pharmacological interventions to address extinction deficits and a novel target for the treatment of fear-based disorders.
A common functional, single-nucleotide polymorphism (SNP) in FAAH–C385A (rs324420)–leads to the replacement of an evolutionary preserved amino acid (proline) in the FAAH gene, making the FAAH protein more vulnerable to degradation. The A-allele of the FAAH polymorphism, which is present in roughly 37% of individuals of European descent and 54% of individuals of African descent (https://www.snpedia.com/index.php/Rs324420), is linked to reduced cellular expression and lower enzymatic activity of FAAH (Chiang et al., 2004). This decreased activity in FAAH leads to increased peripheral AEA levels (Dincheva et al., 2015; Mayo, Asratian, Lindé, Holm, et al., 2020) and has been associated with decreased FAAH enzymatic binding in the brain, as measured by a PET radiotracer in vivo (Boileau et al., 2015). Parallel studies in humans and a knock-in mouse model of the FAAH C385A variant indicate that FAAH A-allele carriers exhibit elevated basal circulating AEA and are less susceptible to stress-induced anxiety and negative affect, compared to C/C homozygotes (Dincheva et al., 2015; Gee et al., 2016; Mayo, Asratian, Lindé, Holm, et al., 2020; Spagnolo et al., 2016). Interestingly, genetic variation in FAAH, which promotes AEA activation of CB1R, mimics the effects of CB1R activation via exogenous cannabinoids (e.g., delta-9-tetrahydrocannabinol [THC]; Hammoud et al., 2019; Rabinak et al., 2014) that has been shown to enhance fear extinction learning. Likewise, emerging translational evidence in healthy adults suggests that the FAAH A-allele is associated with improved extinction learning as indexed by decreased physiological fear responses (i.e., skin conductance) during late extinction trials (Dincheva et al., 2015). Recent work indicates that A-carriers exhibit elevated peripheral AEA as well as facilitated extinction learning and recall using a Pavlovian fear-potentiated startle paradigm (Mayo, Asratian, Lindé, Holm, et al., 2020). Clinical studies also implicate the FAAH A-allele in lower risk and severity of anxiety disorders, as it is linked to lower trait anxiety in healthy adults (Dincheva et al., 2015) and lower posttraumatic stress symptoms in patients with fear-based disorders (Spagnolo et al., 2016). In all, convergent evidence from preclinical and clinical models suggests that the FAAH A-allele may protect against vulnerability to fear-based disorders via enhanced AEA signaling and fear extinction learning (e.g., Gee et al., 2016).
While the effects of genetic variation in FAAH on fear extinction learning and its purported role in the pathogenesis of anxiety disorders have been well characterized, the underlying neurobiological mechanisms are less well understood. FAAH C385A knock-in mice show increased connectivity from infralimbic prefrontal cortex (IL)-basolateral amygdala (BLA) descending projections and lower anxiety behaviors/phenotype (Dincheva et al., 2015). These findings are consistent with recent translational studies reporting heightened frontolimbic (e.g., mPFC-amygdala, human analog of IL-BLA) structural and resting-state functional connectivity in A-allele carriers being related to lower anxiety levels in human adults and adolescents (Dincheva et al., 2015; Gärtner et al., 2019; Gee et al., 2016). Further, A-allele carriers exhibit greater habituation of, and lesser threat-related amygdala reactivity during, a face processing task, lower trait stress reactivity, and a weaker correlation between amygdala reactivity and trait anxiety (Gunduz-Cinar, Hill, et al., 2013; Hariri et al., 2009). It is unknown whether observed FAAH C385A genotype-related differences in behavioral measures during fear extinction learning and recall are underscored by concurrent differences in activation of fear neural circuitry.
The present study examines the effects of the FAAH C385A variant on fear extinction learning and recall, and activation of fear-relevant brain regions. We extend prior behavioral and neuroimaging research in healthy, primarily Caucasian adults by testing the effects of the FAAH C385A variant on extinction of conditioned fear responses in a sample of racially diverse, trauma-exposed individuals. Further, we used a novel adaptation of a well-established Pavlovian fear extinction paradigm that couples immersive reality with functional magnetic resonance imaging scanning (fMRI) and concurrent psychophysiological recording. Given evidence of facilitated fear extinction and recall and dampened amygdala reactivity, we predict that A-carriers in our sample will exhibit enhanced extinction learning via lesser dorsal anterior cingulate cortex (dACC) and amygdala activation. Likewise, we predict that A-carriers will display facilitated extinction recall and this will be exhibited by lesser amygdala activation and greater vmPFC and hippocampus activation. The present study seeks to further elucidate the neural and genetic mechanisms by which the eCB system modulates successful fear extinction in humans–an essential step toward the development of more individualized, biology-based approaches to preventing and treating disorders characterized by aberrant fear processing like PTSD.
2 |. METHODS AND MATERIALS
2.1 |. Participants
A total of 158 adults were recruited from the Detroit area (MI, USA) via online and community advertisements. Eligible participants were right-handed, between the ages of 18 and 60, fluent in English, medically and neurologically healthy, and had at least a high school diploma or equivalent. Participants were also free of any major psychiatric diagnoses, pervasive developmental disorders, history of traumatic brain injury with cognitive impairment, MRI contraindications (e.g., metal in the body, claustrophobia), and current treatment with medication that would interfere with task performance. Participants who were pregnant or breastfeeding, posed a risk of harm to themselves or others, or had participated in another fear conditioning study in the past 30 days were excluded. All participants were required to pass a urine drug screen and alcohol breathalyzer test prior to MRI scanning and were compensated for their time. Fifty-eight of the 158 potential participants were excluded due to failure to meet inclusion criteria (e.g., presence of psychiatric diagnoses, MRI contraindications, failed drug screen; n = 42), lack of useable genetic (n = 7) or fMRI data (e.g., brain abnormality, high motion during scanning, incomplete scan; n = 6), and noncompliance with the study protocol (e.g., marijuana use; n = 3). An additional 13 participants did not wish to complete the study and 28 participants were lost to follow-up, leaving a final sample of N = 59 (40 females, ages 18–51 years).
Power calculations used to determine the sample sizes proposed here have consistently found that an n of 14–16 healthy controls and an n of 14–16 PTSD patients provide adequate power to observe: (a) SCR differences and amygdala, prefrontal, and hippocampal signal differences between groups during fear extinction and/or extinction recall; and (b) cannabinoid’s effects on extinction recall and amygdala, prefrontal, and hippocampal activation in healthy controls. Given that the effect sizes tested within and between groups in previous fMRI studies have ranged between moderate to large (Cohen d = 0.7–1.5) with the anticipated cohort of 25 participants per group (C/C and A-allele), who complete the entire protocol with usable data, will confer >80% power to detect an effect size 0.72 or higher after correcting for mulitple comparisons (G*Power version 3.1.9.7; Faul et al., 2007). The study protocol was approved by the Wayne State Institutional Review Board, and all participants provided written informed consent prior to completing study procedures.
Participants varied in sociodemographic makeup, with 40.7% Caucasian, 22% African American, 28.8% Asian, 1.7% Hispanic, and 6.8% other. These rates are broadly consistent with the racial/ethnic composition of the recruitment catchment area (Detroit, MI, USA).
The vast majority (98.3%) of participants had at least some college education, with at least 93.2% earning annual household incomes above the national poverty line (for single person households) (Table 1).
TABLE 1.
Participant demographics
|
FAAH C385A genotype |
|||
|---|---|---|---|
| C/C (n = 35) | A-carrier (n = 24) | p value | |
| Age (mean years, SD) | 24.74 (7.38) | 26.38 (8.71) | 0.441 |
|
| |||
| Age range (years) | 18-49 | 18-51 | |
|
| |||
| Sex (n, % female) | 24 (68.6) | 16 (66.7) | 0.878 |
|
| |||
| Race (n, %) | 0.708 | ||
| White | 13 (37.1) | 11 (45.8) | |
| Black | 7 (20.0) | 6 (25.0) | |
| Asian | 12 (34.3) | 5 (20.8) | |
| Hispanic | 1 (2.9) | 0 (0.0) | |
| Other | 2 (5.7) | 2 (8.3) | |
|
| |||
| Highest level of education (n, %) | 0.269 | ||
| High school diploma/ GED | 1 (2.9) | 0 (0.0) | |
| Part college | 12 (34.3) | 9 (37.5) | |
| Graduated 2-year college | 1 (2.9) | 2 (8.3) | |
| Graduated 4-year college | 8 (22.9) | 1 (4.2) | |
| Part graduate/professional school | 10 (28.6) | 7 (29.2) | |
| Completed graduate/professional training | 3 (8.6) | 5 (20.8) | |
|
| |||
| Annual household income (n, %) | 0.555 | ||
| Less than $15,000 | 3 (8.6) | 1 (4.2) | |
| $15–39,999 | 12 (34.3) | 10 (41.7) | |
| $40–69,999 | 12 (34.3) | 5 (20.8) | |
| $70,000+ | 8 (22.9) | 8 (33.3) | |
|
| |||
| Trauma exposure (mean, SD) | 6.20 (4.34) | 6.79 (4.26) | 0.606 |
|
| |||
| Posttraumatic stress symptoms (mean, SD) | 9.17 (9.92) | 8.37 (8.58) | 0.750 |
|
| |||
| State anxiety (mean, SD) | 31.51 (10.09) | 30.21 (7.66) | 0.594 |
|
| |||
| Trait anxiety (mean, SD) | 35.77 (10.18) | 35.37 (7.53) | 0.871 |
|
| |||
| Depressive symptoms (mean, SD) | 6.83 (6.94) | 6.62 (6.39) | 0.909 |
|
| |||
| Frequency of alcohol consumption (n, %) | 0.563 | ||
| 0 times (nondrinker) | 11 (31.4) | 9 (37.5) | |
| Less than once per week | 2 (5.7) | 1 (4.2) | |
| 1–3 times per week | 17 (48.6) | 8 (33.3) | |
| 1–2 times per month | 4 (11.4) | 3 (12.5) | |
| 1–6 times per year | 1 (2.9) | 3 (12.5) | |
|
| |||
| Average number of drinks per occasion (n, %) | 0.397 | ||
| 0 (nondrinker) | 11 (31.4) | 9 (37.5) | |
| 2 or less | 15 (42.9) | 10 (41.7) | |
| 3 or less | 3 (8.6) | 4 (16.7) | |
| 3–5 | 6 (17.1) | 1 (4.2) | |
|
| |||
| Lifetime occasions of cannabis use (n, %) | 0.046 | ||
| 0 (never) | 26 (74.3) | 11 (45.8) | |
| 1–10 | 7 (20.0) | 7 (29.2) | |
| 11–50 | 2 (5.7) | 1 (4.2) | |
| 51–100 | 0 (0.0) | 2 (8.3) | |
| >100 | 0 (0.0) | 3 (12.5) | |
|
| |||
| Lifetime tobacco use (n, %) | 0.770 | ||
| Never (nonsmoker) | 26 (74.3) | 17 (70.8) | |
| Current or past user | 9 (25.7) | 7 (29.2) | |
2.2 |. Questionnaire measures
Participants completed self-report assessments of depressive symptoms (Beck Depression Inventory-Second Edition, BDI-II; Beck et al., 1996), anxiety symptoms (State-Trait Anxiety Inventory for Adults, Spielberger, 1983), lifetime trauma exposure (Life Events Checklist for DSM-5, LEC-5; Weathers et al., 2013a), and posttraumatic stress symptoms (PTSS) (The PTSD Checklist for DSM-5, PCL-5; Weathers, Litz, Keane, et al., 2013). Following our prior work (Rabinak et al., 2020), the total number of traumatic event types (e.g., natural disaster, physical assault, sexual assault) experienced and witnessed was used as an index of trauma exposure. Of note, the stressful, potentially traumatic life events endorsed on the LEC-5 were not assessed against Criterion A which is required for the diagnosis of PTSD (Clinician Administered PTSD Scale for DSM-5; CAPS-5; Weathers et al., 2013b; Weathers, Litz, Keane, et al., 2013). While not a requirement for inclusion, 93% of all participants endorsed prior trauma exposure, which is consistent with prior studies by our group and others in urban areas, including Detroit (Gillespie et al., 2009; Rabinak et al., 2020).
2.3 |. Genotyping
Buccal DNA was extracted on a Qiagen Qiacube machine with the DNA Blood Mini Kit according to the manufacturer’s instructions. The concentrations of extracted DNA were measured on a Qubit fluorometer. The FAAH rs324420 variant was analyzed on a Qiagen Q48 Autoprep pyrosequencer. Primers were designed to analyze rs324420 with Qiagen Assay Design 2.0 to amplify an 87-basepair region by polymerase chain reaction with a forward primer (ACCAACTGTGTGACCTCCTATCTG) and biotinylated reverse primer (CACAGGGACGCCATAGAGCA). The sequencing primer (AGACTCAGCTGTCTCAGG) was utilized with the generated sequence to analyze (CMCAAGGCAGGGCCTGCTCTATGGCGT) on the Q48 Autoprep to determine the rs324420 genotype. All samples were analyzed in duplicate, and FAAH rs324420 was found to be in Hardy–Weinberg equilibrium within our sample χ2 = 0.280, p = 0.597). Participants were classified as either A-allele carriers (i.e., A/A homozygotes and A/C heterozygotes; n = 24) or C/C homozygotes (n = 35), following prior work (e.g., Hariri et al., 2009). Independent samples t-tests and chi-square statistics revealed that A-carriers and C/C homozygotes did not differ significantly (α = 0.05) in age, depressive symptoms, trait or state anxiety, trauma exposure, PTSS (p’s > 0.4), nor in distribution of sex, race, income, or education (p’s > 0.2). Genotype groups also did not differ in frequency/quantity of alcohol consumption or lifetime tobacco use (p’s > 0.3). While there was a significant difference in lifetime cannabis use (p = 0.046), the group difference was no longer significant when excluding the three participants (all A-alleles) who reported lifetime cannabis >100 times (p = 0.15). All reported results remained significant when excluding those three participants (see Supporting Information).
2.4 |. Fear conditioning task
Participants completed an adapted version of a well-validated Pavlovian fear extinction paradigm developed by Milad, Wright, and colleagues (2007), which manipulates context using an ABB design. In this paradigm, the fear conditioning context (“danger” context; CXT+ or A) is separate from the “safety” context where fear is extinguished (CXT− or B). A test of extinction recall also occurred in the safety context (CXT−). All phases occurred during fMRI scanning, and the task was displayed using Vizard (WorldViz Version 5.0, https://www.worldviz.com/releases/vizard5) on a screen viewed behind the scanner using a mirror affixed to the head coil.
Two different 3D environments consisted of different colors, textures, sounds (soft ambient noise), and background scenes, constituted the contexts (Figure 1a). The CSs were messenger bags in three colors, chosen for maximum discriminability by color vision-deficient individuals (Figure 1b). The unconditioned stimulus (US) was a 3D virtual snake darting out of the bag and striking toward the participant’s viewpoint, paired with a snake hiss (800 ms, 95 dB, at 60% reinforcement), chosen as a more evocative US than traditional paradigms (Öhman, 2005).
FIGURE 1.
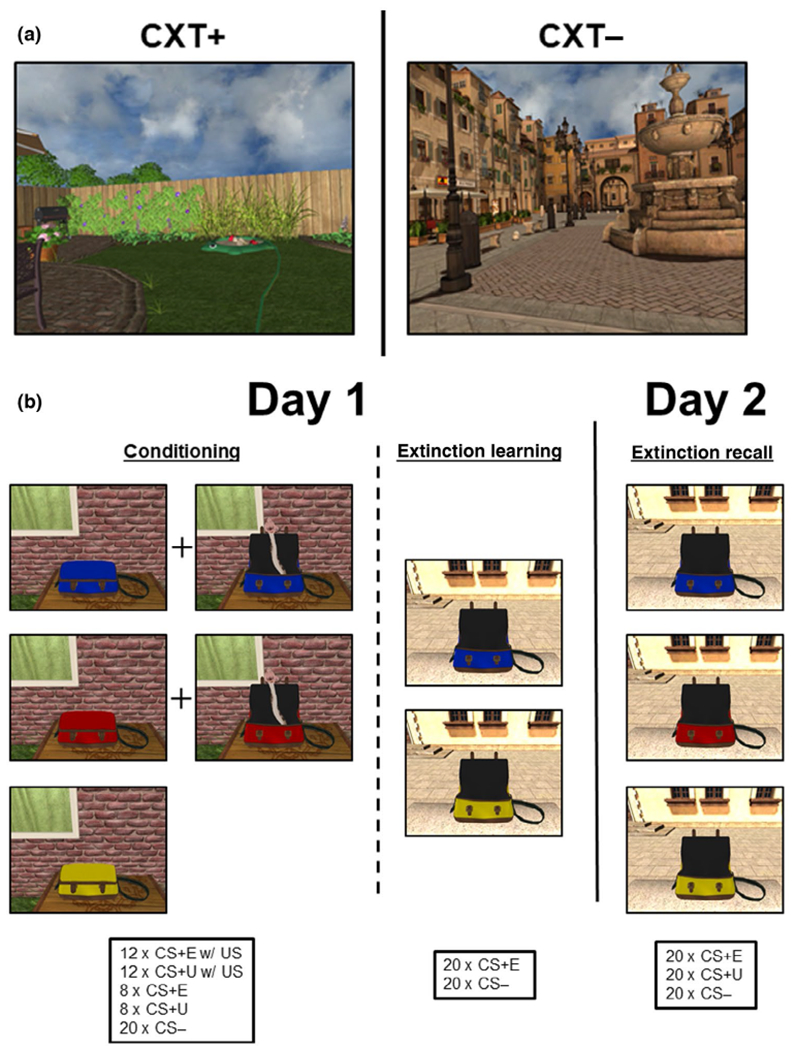
Fear conditioning task. A novel Pavlovian fear conditioning paradigm within a 3D virtual environment using naturally fear-invoking stimuli. (a)Two contexts were utilized, a backyard garden scene during fear conditioning (CXT+) and a public fountain plaza scene during extinction learning and extinction recall (CXT−). (b) Three colored bags (CS) were shown during fear conditioning. Two of the CSs were paired with an aversive stimulus (US), which consisted of a virtual snake and hissing sound (CS+). The third CS was never paired with the aversive stimulus (CS−). Ten minutes later, extinction learning began, with two colored bags (CSs) shown, including one of the CS+ and the CS−. The CS+ was no longer paired with the US and served as the extinguished cue (CS+E). Participants underwent a test of extinction recall 24 hr later. During extinction recall, all three CSs were shown again in the absence of the US, including the CS+E, the CS−, and the unextinguished CS+ (CS+U)
During fear conditioning (~13.8 min), participants were presented with two CSs (CS+; e.g., blue bag and yellow bag) on a screen within the MRI that opened to reveal the snake and hissing sound during reinforced trials. A third CS (e.g., red bag) was presented during fear conditioning but was never paired with the US (CS−), that is the bag opened but no US occurred. An assignment of the CSs to the three colored bags was randomized for each participant. Fear conditioning consisted of 12 reinforced and eight non-reinforced presentations of each of the two CS+’s, and 20 presentations of the CS−. All stimuli were presented within the conditioning context (CXT+) during fear conditioning (Figure 1b). After an approximately 10-min break, participants completed an extinction learning phase (~9.2 min) wherein one of the CS+s was subsequently extinguished, that is, presented in the absence of the US (CS+E). Extinction learning consisted of 20 CS+E and 20 CS− trials presented in the extinction context (CXT−; Figure 1b). Twenty-four hours later, participants returned to the MRI for an extinction recall test phase (~13.8 min), where 20 presentations of the CS+E and CS− each were again completed in the CXT−, with the addition of 20 presentations of the unextinguished CS+ (CS+U; Figure 1b) interspersed. Of note, a subset of the N = 59 participants (n = 14) completed fear conditioning outside of the scanner, and 24 hr prior to the extinction learning phase. The subset completed fear conditioning in the laboratory using a VR head-mounted Oculus Rift. Follow-up analyses were ran in the larger group (n = 45) and results remained consistent with those reported here.
2.5 |. Skin conductance responses
We recorded skin conductance responding (SCR; see Methods in the Supporting Information); however, due to poor data quality we did not have enough data to perform group-level analyses.
2.6 |. Unconditioned stimulus expectancy
Before each phase, participants were instructed to make a rating immediately when they saw the colored bags to indicate whether they believed it would contain a snake or not. Possible responses were “Yes, it will contain a snake,” “No, it will not contain a snake,” and “I don’t know,” and were registered using a MR-compatible 4-button response device using the hand without SCR electrodes attached. Across participants, chi-square statistics assessed differences in US expectancy responses per stimulus per phase versus chance (one third of the responses dedicated to “Yes,” “No,” and “I don’t know”). Early extinction learning and extinction recall were defined as the first trials of each stimulus per phase. Late fear acquisition, extinction learning, and extinction recall were defined as the last trials of each stimulus per phase. Due to >10% of participants missing the first trial of each stimulus during fear acquisition (missing CS+E = 15; CS+U = 8; CS− = 12), the first and second trials for fear acquisition were combined (new missing: CS+E = 4; CS+U = 4; CS− = 3). All other trials presented had less than five missing responses and were therefore presented without pooling across an adjacent trial. Additional chi-square statistics assessed differences in US expectancy responses between phases (i.e., extinction learning vs. extinction recall), within phases (i.e., early extinction learning vs. late extinction learning), and between stimuli within phases (i.e., CS+E late extinction learning vs. CS− late extinction learning) (see Table S1 for pairwise comparisons). Genotype differences were also assessed with chi-square, with C/C-carriers used as the reference for expected spread of responses.
2.7 |. Subjective units of distress
Participants reported on their subjective distress at three points: (a) before starting the task, (b) during the middle of (after 20 trials for extinction learning and 30 trials for extinction recall), and (c) after completing extinction learning and extinction recall. In the scanner, participants used the response device to report how they were feeling at that moment on a scale from 0 to 100 (increments of 10). 0 = “No Anxiety,” 20 = “Mild anxiety, Alert, able to cope,” 50 = “Moderate anxiety, Some trouble concentrating,” 80 being “Severe anxiety, Thoughts of leaving,” and 100 = “Very severe anxiety, Worst ever experienced.” A time (early, mid, late) × genotype (C/C, A-carriers) RM-ANOVA was performed to assess for time and genotype effects on stress. A secondary analysis comparing mid to latefear extinction to early to midextinction recall was also performed [genotype (C/C, A-carriers) × time (mid, late extinction learning; early, mid-extinction recall) RM-ANOVA].
2.8 |. fMRI
2.8.1 |. Data collection
Blood-oxygen level-dependent (BOLD) fMRI data were collected from 66 axial, 2-mm-thick slices (set to the manufacturer-minimum gap size) using a T2*-sensitive gradient echo EPI acquisition sequence (repetition time = 2,000 ms; echo time = 30 ms; 128 × 128 × 66 matrix; field of view = 256 mm; flip angle = 73°; 2.0 × 2.0 × 2.0 mm voxels; multiband acceleration factor = 3). A T1-weighted anatomical image (repetition time = 1,820 ms; echo time = 3.52 ms; 256 × 256 × 120 matrix; field of view = 240 mm; flip angle = 8°; 0.94 × 0.94 × 1.5 mm voxels) and field map image (repetition time = 2,412 ms; echo time = 51 ms; 128 × 128 × 66 matrix; field of view = 256 mm; flip angle = 90°; multiband acceleration factor = 3) were collected within the same scan session for coregistration and distortion correction.
2.8.2 |. Preprocessing
fMRI data were analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). The following preprocessing steps were applied, in order: (a) distortion correction, (b) realignment to the first image, (c) slice timing correction, (d) coregistration, (e) normalization to MNI space, (f) reslicing, and (g) spatial smoothing (6-mm FWHM Gaussian kernel).
2.8.3 |. Motion-related artifact and quality control
Preprocessed data were submitted to quality control analyses, and data from 59 participants who met criteria for high quality and scan stability with minimum motion correction were included in the analyses. For images identified as containing high movement (≥3 mm displacement in any one direction), we censored frames with excessive motion (framewise displacement > 0.8 mm), as well as one frame before and two frames after the high-motion frame, from the first-level analyses to reduce the impact of spurious participant movement. See the Supporting Information for more details regarding subject motion.
2.8.4 |. First-level model
Following preprocessing, a general linear model was applied to the time series, convolved with the canonical hemodynamic response function (HRF) and with a 128-s high-pass filter (Worsley & Friston, 1995). Individual statistical parametric maps were calculated using this general linear model for each participant for each condition type (CS+, CS− for conditioning; CS+E, CS− for extinction learning; CS+E, CS+U, CS− for extinction recall). To reduce the impact of motion-related artifact, the six movement parameters obtained during realignment were included in the first-level models as nuisance regressors, along with their derivatives and the quadratic terms of both the motion and motion derivatives. Volumes with excess motion (i.e., >0.8 mm framewise displacement) were also added as nuisance regressors in the models.
2.8.5 |. Second level model
First-level contrasts were subsequently submitted to second-level analyses in a random-effects statistical model in SPM8 (Friston et al., 1998), for every phase. For each a priori ROI during fear acquisition, a genotype (C/C, A-carrier) × stimulus (CS+, CS−) × time (early, late) flexible ANOVA was performed. For fear extinction learning, a genotype (C/C, A-carrier) × stimulus (CS+E, CS−) × time (early, late) flexible ANOVA was performed. For extinction recall, a genotype (C/C, A-carrier) × stimulus (CS+E, CS+U, CS−) × time (early, late) flexible ANOVA was performed. Previous animal work suggests that activation of fear-related brain regions is strongest during early phases of learning (i.e., early extinction learning and extinction recall (see Milad & Quirk, 2002; Milad, Wright, et al., 2007). Therefore, each phase was split into early and late, defined as the first and second halves of each phase, respectively (10 trials per stimulus for early and 10 trials per stimulus for late for each phase).
Results were considered significant at pFWE < 0.05. Follow-up t-tests were also conducted in SPM8 to detect the direction of F-test main effects and interactions. Variables that contained outliers (z-scores > ± 3.29) were winsorized.
2.8.6 |. Regions of interest (ROIs)
We tested anatomically defined amygdala, hippocampus, and vmPFC, using AAL atlas-based ROIs (Tzourio-Mazoyer et al., 2002). An anatomically defined dACC ROI based on Brodmann area 32 was also used, created with the WFU PickAtlas toolbox version 3.0.5 (Maldjian et al., 2003). The amygdala and hippocampus were assessed unilaterally (left, right), whereas the vmPFC and dACC were assessed bilaterally. Results were considered significant within each ROI using small volume familywise error correction PFWE < 0.05. For each phase, a genotype (C/C, A-carrier) × time (early, late) × stimulus (CS+E, CS+U, CS−) flexible ANOVA was performed in SPM8, and all significant F-tests were followed up by t-tests. Multiple comparison correction in brain space was done via Gaussian random field theory for small volumes, as implemented in SPM8 (Worsley et al., 1996). As follow-up to our flexible ANOVA, it has been noted that CS+E > CS+U in early extinction recall elicits activation in fear extinction brain regions, including the hippocampus and vmPFC (e.g., Fullana et al., 2018). Therefore, we chose to do an additional t-test on CS+E > CS+U in early extinction recall between genotype groups.
2.8.7 |. fMRI power analysis
To detecta medium effect size (d = 0.5) with 80% power and α = 0.05, we would need a total sample of n = 128 with critical t126 = 1.97. To detect a large effect size (d = 0.8), we would need a total sample of n = 52 with critical t50 = 2.01. Cohen’s d and power are reported in the genotype interaction post hoc t-tests within the results section using G*Power (version 3.1.9.7; Faul et al., 2007). All significant results with a genotype interaction have greater than 0.80 power level (recommended by Cohen, 1988).
2.8.8 |. Whole-brain analysis
For completeness, we also analyzed whole-brain results at a combined voxel-level (p < 0.001) and cluster-level (69 voxel) whole-brain correction. This threshold was achieved by computing the spatial autocorrelation of the data via AFNI’s 3dFWHMx and subsequently performing Monte Carlo simulations (10,000 iterations) using 3dClustSim (compile date July 22, 2016; National Institute of Mental Health, Bethesda, MD; https://afni.nimh.nih.gov).
3 |. RESULTS
3.1 |. US expectancy ratings
3.1.1 |. Fear conditioning
Across participants, the last US expectancy ratings for stimuli (CS+E, CS+U, CS−) significantly deviated from chance [, p < 0.001; see Figure 2a], such that CS+E and CS+U had a majority of “Yes” responses, while CS− had less than 25% “Yes” responses (see graphs for spread of responses). Only the first CS+E trial deviated from chance (p < 0.001; CS+U and CS− p’s > 0.05). No statistically significant differences were detected between FAAH genotype groups using Pearson chi-square test [C/C-carriers as reference; , p’s > 0.05].
FIGURE 2.
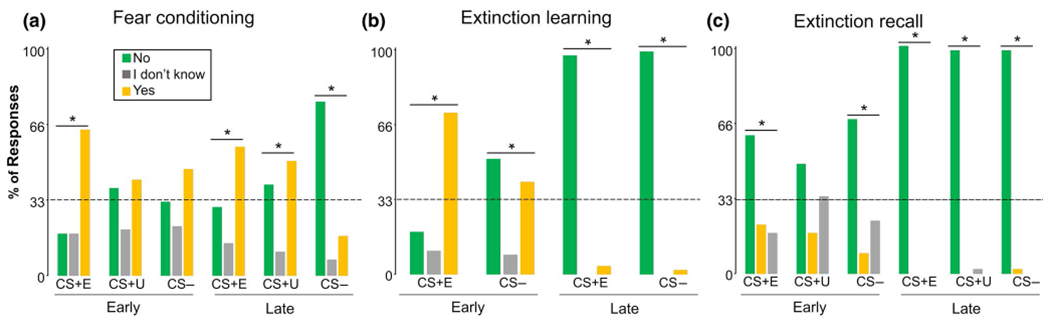
US expectancy ratings across participants and phases. Vertical axis indicates the percentage of responses. The dashed horizontal line indicates chance and the * indicates a significant divergence from chance (33.3%, indicates divergence in response frequency of “Yes,” “I don’t know,” or “No”). Panels a–c show fear conditioning, extinction learning, and extinction recall, respectively. *p < 0.001
3.1.2 |. Extinction learning
Across participants, US expectancy ratings for stimuli significantly deviated from chance , p < 0.003] (see Figure 2b for spread of responses). The first CS+E trial had a majority “Yes” responses, while the last CS+E trial had less than 25% “Yes” responses. The first CS− trial had a majority “Yes” and “No” responses, while the last trial had nearly 100% “No” responses. No statistically significant differences were detected between FAAH genotype groups using Pearson chi-square test [C/C-carriers as reference; , p’s > 0.05].
3.1.3 |. Extinction recall
Across participants, the first US responses for CS+E and CS− and the last US response for each stimulus (CS+E, CS+U, CS−) were significantly different than chance [, p < 0.001] (see Figure 2c for spread of responses). All significant responses were a majority “No.” No statistically significant differences were detected between FAAH genotype groups using Pearson chi-square test [C/C-carriers as reference; , p’s > 0.05].
3.2 |. SUDs ratings
3.2.1 |. Extinction learning
There were no main effects or interactions detected in the RM-ANOVA for SUDs ratings (p’s > 0.05).
3.2.2 |. Extinction recall
A time (mid extinction; late extinction; early recall; mid recall) × genotype (C/C, A-carrier) RM-ANOVA revealed a main effect of time. The main effect of time was driven by higher SUD ratings during mid-late fear extinction as compared to during early-mid extinction recall ratings (see Supporting Information for full results). There were no main effects or interactions for genotype in the RM-ANOVA (p’s > 0.05).
3.3 |. FMRI analyses
3.3.1 |. Fear conditioning
A time (early, late) × stimulus (CS+, CS−) × genotype (C/C, A-carrier) flexible ANOVA revealed a main effect of stimulus type in the dACC, which was driven by higher dACC activation to the CS+ relative to the CS− (Figure 3a). Additionally, a time × stimulus interaction was detected for the right hippocampus such that right hippocampal activation was higher to the CS+ (vs. CS−) during early acquisition (Figure 3b). See Supporting Information for full statistical results.
FIGURE 3.
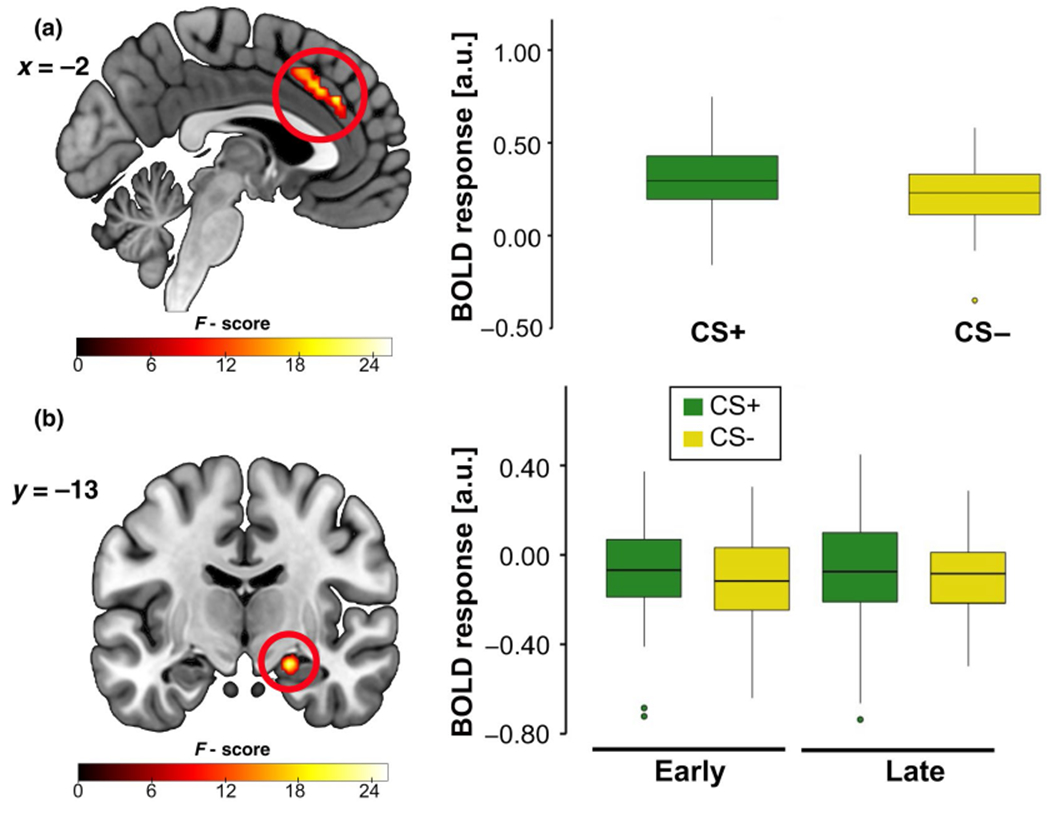
fMRI during fear acquisition showing a (a) main effect of stimulus in the dACC and (b) stimulus × time interaction in the right hippocampus. Across participants, (a) dACC activation was greater to the cue paired with the unconditioned stimulus (CS+) than the safety cue (CS−). (b) Right hippocampus activation was greater to the CS+ (> CS−) during early fear acquisition (> late). Results significant at pFWE < 0.05; both images shown at p < 0.005. Boxplots show the median and upper and lower quartiles
3.3.2 |. Extinction learning
A time (early, late) × stimulus (CS+E, CS−) × genotype (C/C, A-carrier) flexible ANOVA revealed a main effect of time in the dACC, left hippocampus, and right hippocampus. Briefly, dACC activation was greater during early extinction learning as compared to late extinction learning (Figure 4a); however, a smaller cluster in the ventral anterior cingulate cortex (vACC) was more active during late extinction learning as compared to early extinction learning (Figure 4b). Left and right hippocampal activation was greater during late extinction learning as compared to early (see Figure 4c,d and Supporting Information for full results). There was also a significant genotype × time interaction in the right hippocampus (21 voxels, F = 15.59, Z = 3.68, xyz = 32, −30, −4, pFWE = 0.024; see Figure 5a) that was driven by greater right hippocampal activation in A-allele carriers as compared to C/C homozygotes during early extinction learning (26 voxels, t = 3.86, Z = 3.62, xyz = 32, −30, −4, PFWE = 0.030, d = 1.02, power = 0.97). There was also a significant genotype × stimulus × time interaction in the dACC (16 voxels, F = 20.45, Z = 4.24, xyz = −14, 22, 40, pFWE = 0.007; see Figure 5b). Follow-up t-tests revealed that C/C homozygotes had greater dACC activation to the CS+E (> CS−) in early extinction learning (vs. late) compared to A-carriers (17 voxels, t = 5.19, Z = 4.67, xyz = −14, 24, 38, pFWE = 0.001, d = 1.37, power = 0.99; Figure 5b).
FIGURE 4.
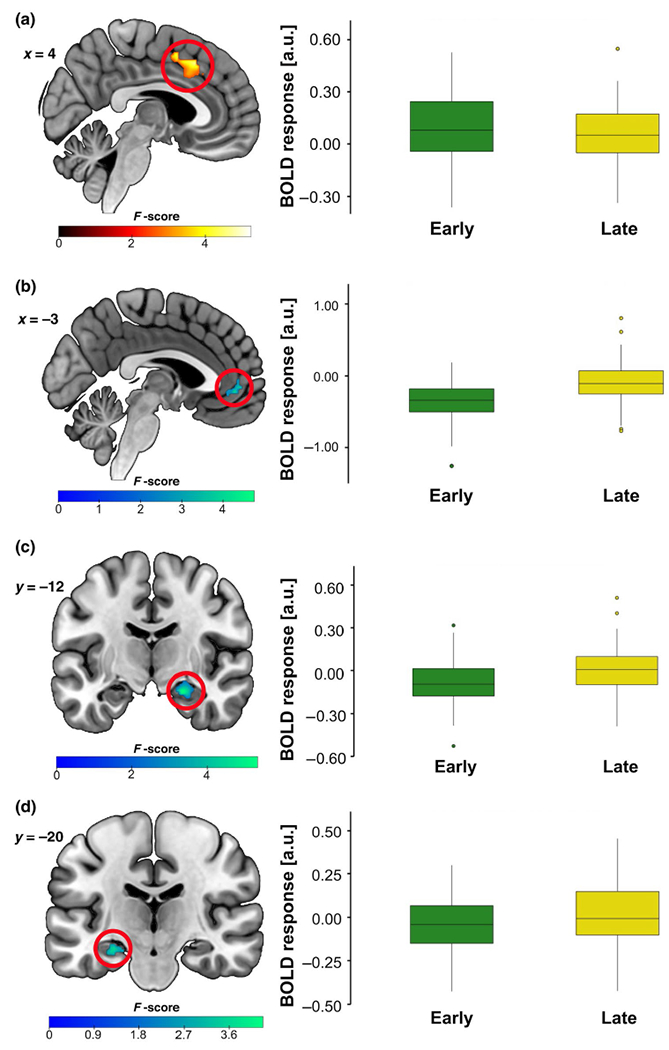
fMRI during fear extinction learning showing a main effect of time in the (a) dACC, (b) vACC, (c) right hippocampus, and (d) left hippocampus. Across participants, (a) dACC activation was greater during early fear extinction (vs. late). In contrast, (b) a cluster within the vACC was activated more during late fear extinction (early < late). Likewise, (c) right and (d) left hippocampus activation is lesser during early fear extinction than late (early < late) across participants. Results significant at pFWE < 0.05; all images shown at p < 0.005. Boxplots show the median and upper and lower quartiles
FIGURE 5.
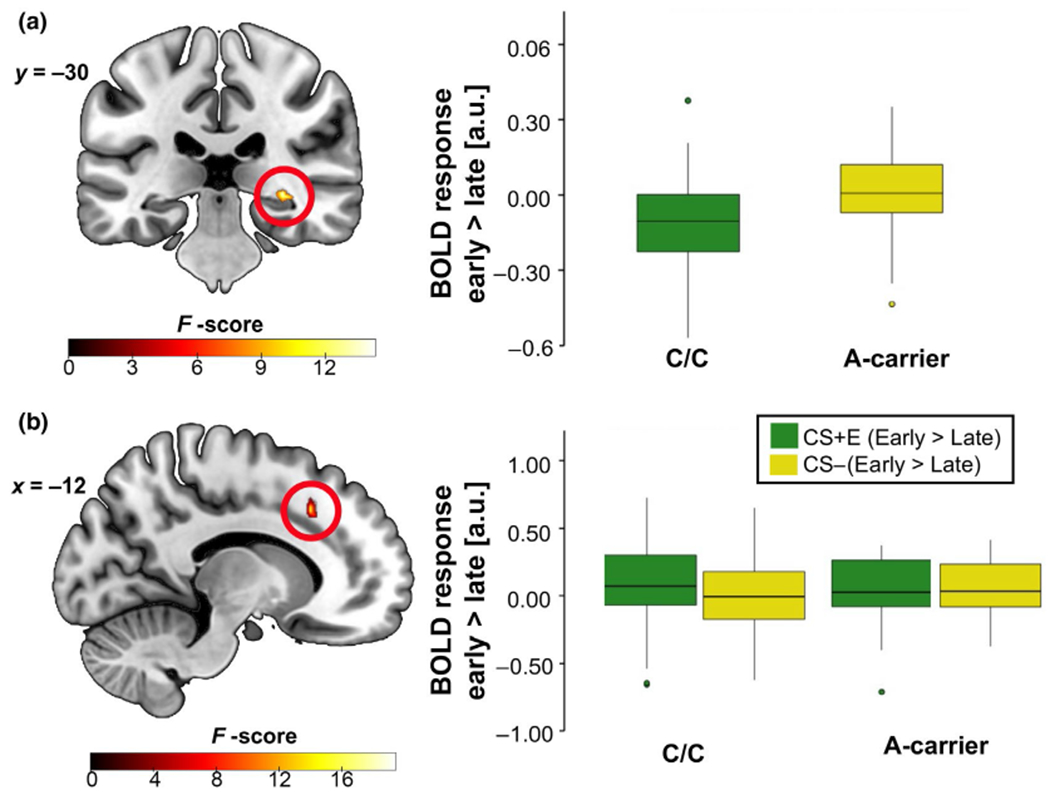
fMRI during extinction learning showing an interaction effect of (a) genotype × time in the right hippocampus and a (b) genotype × stimulus × time interaction in the dACC. (a) A-carriers exhibited greater early (> late) right hippocampus activation versus C/C counterparts. (b) A-carriers exhibited lesser dACC activation to CS+E (> CS−) during early extinction learning (> late). Results significant at PFWE < 0.05; right hippocampus image shown at p < 0.005 while the dACC image shown at p < 0.05 for display purposes only. Boxplots show the median and upper and lower quartiles
3.3.3 |. Extinction recall
A time (early, late) × stimulus (CS+E, CS+U, CS−) × genotype (C/C, A-carrier) flexible ANOVA detected a main effect of time in the dACC. Briefly, dACC activation was greater in early (vs. late) extinction recall (see Figure 6 and Supporting Information for full results). We also found that A-carriers had less right amygdala activation to CS+E (> CS+U, early) than C/C homozygotes (15 voxels, t = 3.62, Z = 3.42, xyz = 28, −28, −16, pFWE = 0.017, d = 0.96, power = 0.95; Figure 7).
FIGURE 6.
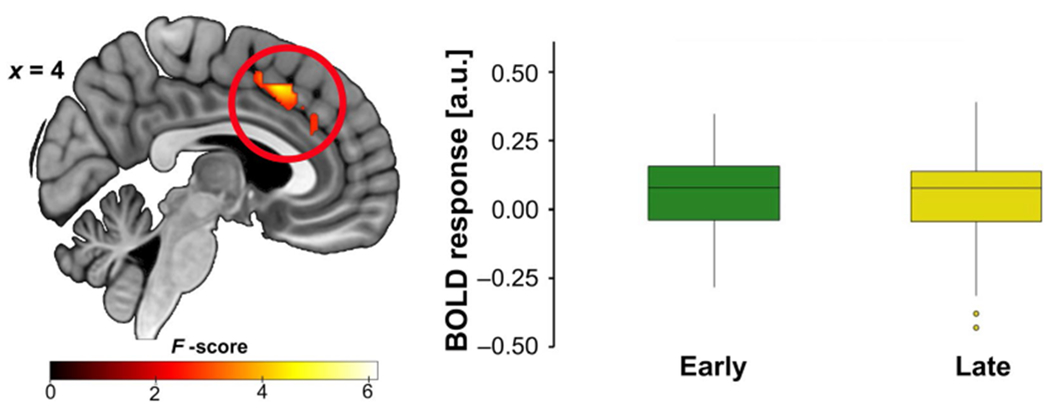
fMRI during fear extinction recall showing a main effect of time in the dACC. Across participants, dACC activation was greater during early extinction recall (> late). Results significant at pFWE < 0.05; image shown at p < 0.005. Boxplots show the median and upper and lower quartiles
FIGURE 7.
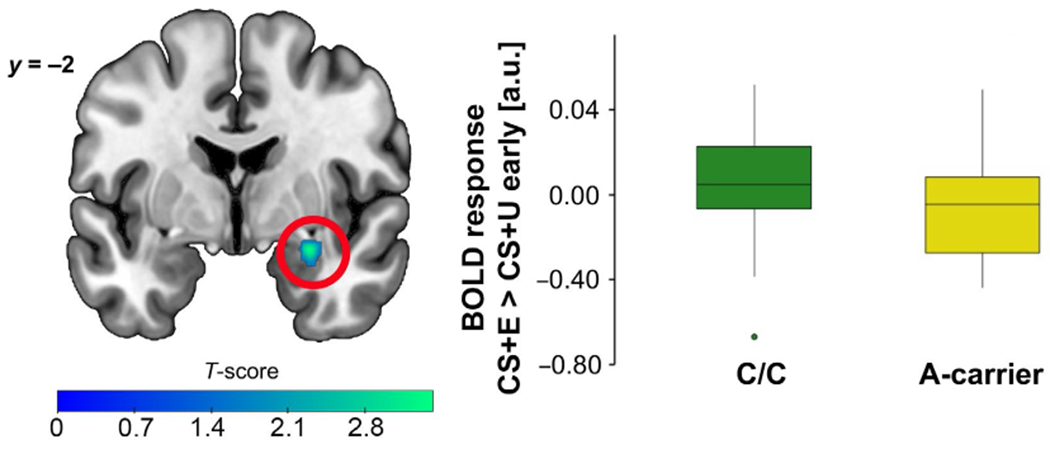
fMRI during extinction recall showing FAAH C385A effect on the right amygdala activation. A-carriers displayed lesser right amygdala activity to a previously extinguished cue (CS+E > CS+U) during early extinction recall, compared to C/C counterparts. Results significant at pFWE < 0.05; right amygdala image shown at p < 0.05 for display purposes only. Boxplots show the median and upper and lower quartiles
3.4 |. FMRI whole-brain analyses
No significant results were noted across phases, stimuli, and time points at the whole-brain corrected threshold.
4 |. DISCUSSION
To our knowledge, this study is the first to assess the effect of FAAH C385A on fear-related neural circuitry during fear extinction learning and recall. We used a novel, immersive-reality fear extinction recall paradigm paired with fMRI to examine the effects of the FAAH C385A variant on fear-related neural circuitry in a racially diverse sample of healthy adults. We found that A-carriers displayed greater activation in the hippocampus and lesser activation in the dACC during extinction learning, as compared to their C/C counterparts. Consistent with previous neuroimaging findings of dampened threat-related amygdala reactivity in healthy adults (Gunduz-Cinar, MacPherson, et al., 2013; Hariri et al., 2009), we found that A-carriers exhibited lesser amygdala activation to the previously extinguished cue (CS+E > CS+U) during early extinction recall, as compared to C/C homozygotes. However, we did not find any effects of FAAH C385A on vmPFC activation or subjective measures of conditioned fear or distress (i.e., US expectancy or SUDs ratings).
The hippocampus is essential for encoding of contextual information during fear extinction learning (Anagnostaras et al., 1999; Kim & Fanselow, 1992; Liu et al., 2012). In rodents, inactivating the hippocampus (i.e., through lesions) inhibits context encoding of fear extinction (Corcoran et al., 2005; Phillips & LeDoux, 1992; Tronson et al., 2012). Importantly, the hippocampus stores both the original fear memory and the new safety (extinction) memory in separate neural engrams (Bernier et al., 2017; Bouton et al., 2006; Lacagnina et al., 2019; Tronson et al., 2009). We found greater hippocampus activation during early extinction learning in FAAH A-carriers. A prior study of spatial memory in rodents found that increasing the expression of FAAH–and thus increasing the breakdown of eCBs–in hippocampal CA1-3 neurons decreased the rate of extinction (Varvel et al., 2007; Zimmermann et al., 2019). Likewise, the inhibition of FAAH is shown to enhance the extinction in the same studies (Varvel et al., 2007; Zimmermann et al., 2019). Functional imaging studies in healthy adults implicate the hippocampus in the recall of extinction learning (Kalisch et al., 2006; Milad, Wright, et al., 2007). The FAAH C385A allele increases eCB levels peripherally and in fear-related brain regions, and thus mimics exogenous activation of CB1R via THC (Mayo, Asratian, Lindé, Holm, et al., 2020; Spagnolo et al., 2016). In a similar Pavlovian conditioning paradigm in healthy adults, oral administration of THC increased hippocampus activation during extinction recall compared to placebo (Rabinak et al., 2014). These effects of CB1R agonism on hippocampus activation were maintained a week later in a follow-up study (Hammoud et al., 2019). While we expected greater hippocampus activation to occur during early extinction recall, the activation we report in our study may reflect earlier encoding of contextual information in FAAH C385A allele carriers, and this effect may be due to increased neural eCB signaling.
ECB signaling in the amygdala is critical for the consolidation and retrieval of extinction learning (Hill & McEwen, 2010; Patel et al., 2005). Prior research shows that eCBs, including 2-arachidonoylglycerol (2-AG) and AEA, are elevated in the BLA after extinction learning in rodents (Marsicano et al., 2002). The systemic inhibition of FAAH before extinction training in rodents causes an increase in basolateral AEA levels and subsequently decreases fear during extinction recall (Gunduz-Cinar, Hill, et al., 2013). We found that FAAH A-allele carriers had lesser amygdala activation to a previously extinguished stimulus during extinction recall, compared to C/C counterparts. These data compliment previous behavioral work suggesting this FAAH genetic variation enhances fear extinction learning and its recall (Dincheva et al., 2015; Mayo, Asratian, Lindé, Holm, et al., 2020). FAAH inhibition within the amygdala is thought to increase CB1R signaling, thus promoting fear extinction via neuronal plasticity (Azad et al., 2004; Gunduz-Cinar, Hill, et al., 2013). In humans, FAAH C385A is associated with lesser amygdala reactivity to threat, greater amygdala habituation to threatening faces, and enhanced extinction recall (Gunduz-Cinar, MacPherson, et al., 2013; Hariri et al., 2009; Mayo, Asratian, Lindé, Holm, et al., 2020). Likewise, the inhibition of FAAH in healthy adult males attenuates amygdala activation during an emotional face processing task (Paulus et al., 2020). Thus, it is likely that lowered expression of FAAH may contribute to enhanced extinction learning, and therefore extinction recall, via increased basal levels of eCBs. Our results compliment and extend previous neuroimaging findings in humans by replicating lesser amygdala reactivity in A-allele carriers.
We found greater dACC activation during early extinction learning in C/C homozygotes, as compared to A-carriers. Milad, Quirk, et al. (2007) identified the dACC as a putative region of threat appraisal and the human analog of the prelimbic cortex in rodents (Maier et al., 2012). Likewise, the dACC activation is associated with return of fear after extinction learning (Levar et al., 2017) and adults with PTSD demonstrate greater dACC activation during extinction recall, as compared to trauma-exposed healthy adults (Milad et al., 2009). DACC activation has been linked to negative emotion processing and fear expression during Pavlovian conditioning paradigms (see review by Etkin et al., 2011). In humans, FAAH inhibition is associated with lesser ACC activation to threatening faces (Paulus et al., 2020). Individuals with the FAAH C385A variant also display greater amygdala-dACC connectivity (Gärtner et al., 2019). Further, while we predicted differential effects in the vmPFC between FAAH genotype groups, we did not find evidence for that in our sample. Null findings in the vmPFC may relate to increased resting-state “default mode network” activity–as the vmPFC resides within the default mode network (Fullana et al., 2018; Harrison et al., 2008, 2011; Raichle et al., 2001). Previous work assessing FAAH C385A effects on threat processing did not find differential vmPFC activation, and similar null results have been reported when inhibiting FAAH in adult males (Gärtner et al., 2019; Paulus et al., 2020). Together, our findings indicate heightened threat-processing in C/C homozygotes, as exhibited by dACC activation during extinction learning.
Across participants, our behavioral index of fear, US expectancy ratings, indicated that participants acquired differential fear to the CS+ (vs. CS−) and extinguished fear to the CS+E. However, we did not find an effect of genotype on US expectancy or distress (SUDs) ratings. This is in agreement with other studies assessing genotype effects on cognitive assessments of stress and arousal (Conzelmann et al., 2012; Gärtner et al., 2019). Differential effects of genotype may have been reflected in our psychophysiological data (SCR); however, due to poor data quality we were unable to assess it. Prior work in healthy adults demonstrates FAAH A-allele carriers having decreased skin conductance and startle response during late extinction learning and early extinction recall (Dincheva et al., 2015; Mayo, Asratian, Lindé, Holm, et al., 2020). FAAH genetic variation may have a more significant impact on biological measures of fear (i.e., SCRs, startle response) compared to cognitive assessments of fear (i.e., US expectancy and SUDs ratings). It is possible that this differential effect of FAAH C385A on physiological and cognitive measures of fear may be partially explained by differences in frontolimbic connectivity that impact expression of conditioned fear, such as heightened vmPFC-amygdala connectivity observed in A-allele carriers (Dincheva et al., 2015; Gunduz-Cinar, MacPherson, et al., 2013).
Limitations of this study should be considered. First, we were unable to conduct analyses using SCR data due to poor data quality, hindering our ability to examine the impact of FAAH C385A on physiological responding during extinction learning and recall, as previously reported (Mayo, Asratian, Lindé, Holm, et al., 2020). We were, however, able to demonstrate that overall, participants were engaged and able to acquire, differentiate, and extinguish fear to the stimuli presented using a cognitive index of fear responding (i.e., US expectancy). Further, our findings represent a novel addition to the existing literature by characterizing the activity of neural circuitry critically involved in fear extinction learning and recall that may underlie potential stress-buffering effects of the FAAH C385A variant. Second, we were unable to examine similar gene by dose-dependent effects of FAAH C385A on peripheral AEA levels (Mayo, Asratian, Lindé, Holm, et al., 2020), providing a more direct link between functional differences in eCB signaling and alterations in fear-related neural circuitry. Future studies will be needed to assess peripheral eCB changes as they relate to extinction learning and recall, as well as how FAAH C385A may impact these changes in humans. Third, our sample size was relatively small, as data were derived from a preliminary study assessing FAAH-related genetic effects on fear-related neural activation in healthy adults. Although we excluded individuals with significant psychopathology (e.g., anxiety disorders, PTSD), there may be unique neural and behavioral effects of a trauma-resilient sample. For example, our lack of genotype effects on activation in the vmPFC may reflect the unique neurobiology of resilience to fear-based disorders in trauma-exposed individuals. Similarly, while self-reported levels of anxiety were relatively low and consistent with other studies of FAAH C385A in healthy and trauma-exposed adults (e.g., Dincheva et al., 2015; Lazary et al., 2016), we do not report an effect of genotype on symptom burden in this trauma-resilient sample. Given recent findings suggesting that A-allele carriers with a history of childhood trauma may be at increased risk of anxiety and depression (Lazary et al., 2016), it is possible that prior trauma and stress exposure within our sample may impact the purported “protective effect” of the A-allele, resulting in symptom levels similar to C/C homozygotes. However, our results replicate and extend previous findings that FAAH C385A is predictive of enhanced extinction learning and recall via fear-related neural circuitry in healthy adults (i.e., lesser amygdala and dACC activation and greater hippocampus activation; Gärtner et al., 2019; Hariri et al., 2009). In addition to trauma and stress exposure reported in our sample, we also report previous substance use—specifically use of alcohol, nicotine, and cannabis. These substances in particular warrant mention as both acute and chronic use can alter eCB functioning. For example, AEA levels in the corticolimbic brain regions (i.e., amygdala and frontal cortex) increase after chronic alcohol (Piggott et al., 2021), nicotine (González et al., 2002), and cannabis exposure in rodents (Di Marzo et al., 2002). While we found a significant difference between genotype groups in lifetime cannabis use, eliminating those who used the most (>100 uses) from our analyses did not change our results. Importantly, CB1Rs and AEA levels return to baseline (or healthy control) levels after only 2 days of cannabis abstinence (D’Souza et al., 2016; Thieme et al., 2014) and cannabinoid metabolites are eliminated from the body within 3–30 days of abstinence (Verstraete, 2004). Since all participants were required to pass a urine drug-screening test, it is unlikely that previous cannabis use significantly impacted the genotype-related differences in brain activation that we report. Last, the enrichment of the A-allele that is typically seen in black Americans was not replicated in our sample. Given our relatively low number of black individuals (n = 13) and similar genotype distribution in other imaging genetics studies (e.g., Dincheva et al., 2015; n < 50), we were not surprised. While our small sample size may have limited our ability to detect population-level allele enrichment, the allele distribution is in Hardy–Weinberg equilibrium across all racial and ethnic groups in our sample (p’s > 0.4). The inclusion of multiple racial and ethnic groups, including black Americans, is a strength of the present study, as existing research examining the effects of FAAH C385A on fear-related learning and reactivity has focused primarily on Caucasian individuals (e.g., Gunduz-Cinar, MacPherson, et al., 2013; Hariri et al., 2009).
Together, the findings of the present study suggest that FAAH C385A contributes to functional alterations in key brain regions underlying facilitated fear extinction learning and its subsequent recall. Importantly, our results suggest that this genetic variant could help to identify individual differences in fear-related neural activation that may mitigate the risk of development of fear- and trauma-related disorders, like PTSD. Likewise, in those who do develop PTSD, genetic variation within the eCB system may be used to identify individuals who might benefit most from treatments targeting impaired fear extinction learning, such as exposure-based therapies.
Supplementary Material
TABLE S1 US expectancy ratings
TABLE S2 SUDs ratings
Transparent Science Questionnaire for Authors
Transparent Peer Review Report
Significance.
The present study is the first to examine the impact of genetic variation in the neuromodulatory endocannabinoid (eCB) system on fear-related neural circuitry during fear extinction learning and recall–a critical mechanism underlying the risk of developing fear-based disorders–in healthy adults. We report that a common functional polymorphism in fatty acid amide hydrolase is associated with lesser dorsal anterior cingulate cortex and greater hippocampus activation during extinction learning, and lesser amygdala activation during extinction recall. We extend prior research linking this genetic variant with enhanced eCB signaling, suggesting a neural mechanism that may predict differential development of fear-based disorders.
ACKNOWLEDGMENTS
The authors thank Shelley Paulisin for initiating this project over a year ago and for their hard work presenting the original data and analysis at scientific conferences. They thank Dr. Ana M. Daugherty for guiding the initial project’s statistical analyses and providing feedback on reporting methods. They also thank all of the individuals who generously shared their time and effort to participate in this research.
Funding information
Research reported in this publication was supported by the Brain and Behavior Research Foundation (2016 NARSAD Young Investigator Grant # 24811) and the National Institute of Mental Health (Award # R61MH111935), awarded to Dr. Rabinak. Dr. Marusak is supported by the National Institute of Mental Health Award #K01MH119241. Dr. Burghardt is supported by the National Institute of Diabetes and Digestive Diseases Award #K23DK118199 (and supplement S1) and L30 DK110823. Nicole Zabik is supported by the National Institute of Mental Health Award #F31H124279.
Footnotes
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
CONFLICT OF INTEREST
The authors do not have any conflict of interest to declare.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
A subset of the data reported in this manuscript is accessible through the National Institute of Mental Health Data Archive (NDA), with collection title Effects of THC on Retention of Memory for Fear Extinction Learning in PTSD: https://nda.nih.gov/edit_collection.html?id=2680. De-identified data from this subset are available to qualified researchers, while summary data are available to all. The remaining subset of data that are not included in the NDA are available from the corresponding author upon reasonable request.
REFERENCES
- Anagnostaras SG, Maren S, & Fanselow MS (1999). Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. Journal of Neuroscience, 19(3), 1106–1114. 10.1523/jneurosci.19-03-01106.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W, & Rammes G (2004). Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. Journal of Neuroscience, 24(44), 9953–9961. 10.1523/JNEUROSCI.2134-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa B (2007). Critical enzymes involved in endocannabinoid metabolism. Protein & Peptide Letters, 14(3), 237–246. 10.2174/092986607780090829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory®-II (BDI®-II). Pearson. [Google Scholar]
- Bernier BE, Lacagnina AF, Ayoub A, Shue F, Zemelman BV, Krasne FB, & Drew MR (2017). Dentate gyrus contributes to retrieval as well as encoding: Evidence from context fear conditioning, recall, and extinction. Journal of Neuroscience, 37(26), 6359–6371. 10.1523/JNEUROSCI.3029-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, & Patel S (2014). Central anandamide deficiency predicts stress-induced anxiety: Behavioral reversal through endocannabinoid augmentation. Translational Psychiatry, 4(7), e408. 10.1038/tp.2014.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Tyndale RF, Williams B, Mansouri E, Westwood DJ, Le Foll B, Rusjan PM, Mizrahi R, De Luca V, Zhou Q, Wilson AA, Houle S, Kish SJ, & Tong J (2015). The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [11C] CURB. Journal of Cerebral Blood Flow and Metabolism, 35(8), 1237–1240. 10.1038/jcbfm.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, & Maren S (2006). Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry, 60(4), 352–360. 10.1016/j.biopsych.2005.12.015 [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, & Cravatt BF (2004). Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: Evidence for a link between defects in the endocannabinoid system and problem drug use. Human Molecular Genetics, 13(18), 2113–2119. 10.1093/hmg/ddh216 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Lawrence Erlbaum Associates, 10.4324/9780203771587 [DOI] [Google Scholar]
- Conzelmann A, Reif A, Jacob C, Weyers P, Lesch K-P, Lutz B, & Pauli P (2012). A polymorphism in the gene of the endocannabinoid-degrading enzyme FAAH (FAAH C385A) is associated with emotional-motivational reactivity. Psychopharmacology, 224(4), 573–579. 10.1007/s00213-012-2785-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, & Maren S (2005). Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience, 25(39), 8978–8987. 10.1523/JNEUROSCI.2246-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Berrendero F, Bisogno T, González S, Cavaliere P, Romero J, Cebeira M, Ramos JA, & Fernández-Ruiz JJ (2002). Enhancement of anandamide formation in the limlbic forebrain and reduction of endocannabinoid contents in the striatum of Δ9-tetrahydrocannabinol-tolerant rats. Journal of Neurochemistry, 74(4), 1627–1635. 10.1046/j.1471-4159.2000.0741627.x [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, & Lee FS (2015). FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Communications, 6, 6395. 10.1038/ncomms7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Cortes-Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T, Planeta B, Neumeister A, Pittman B, Normandin MD, Kapinos M, Ropchan J, Huang Y, Carson RE, & Skosnik PD (2016). Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(1), 60–67. 10.1016/j.bpsc.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Duan T, Gu N, Wang Y, Wang F, Zhu J, Fang Y, Shen Y, Han J, & Zhang X (2017). Fatty acid amide hydrolase inhibitors produce rapid anti-anxiety responses through amygdala long-term depression in male rodents. Journal of Psychiatry and Neuroscience, 42(4), 230–241. 10.1503/jpn.160116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. 10.1016/j.tics.2010.ll.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, & Street GP (1999). A comparison of exposure therapy, stress inoculation training, and their combination for reducing post-traumatic stress disorder in female assault victims. Journal of Consulting and Clinical Psychology, 67(2), 194–200. 10.1037/0022-006X.67.2.194 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, & Turner R (1998). Event-related fMRI: Characterizing differential responses. Neuroimage, 7(1), 30–40. 10.1006/nimg.1997.0306 [DOI] [PubMed] [Google Scholar]
- Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, Radua J, & Harrison BJ (2018). Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neuroscience & Biobehavioral Reviews, 88, 16–25. 10.1016/j.neubiorev.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Gärtner A, Dörfel D, Diers K, Witt SH, Strobel A, & Brocke B (2019). Impact of FAAH genetic variation on fronto-amygdala function during emotional processing. European Archives of Psychiatry and Clinical Neuroscience, 269(2), 209–221. 10.1007/S00406-018-0944-9 [DOI] [PubMed] [Google Scholar]
- Gee DG, Fetcho RN, Jing D, Li A, Glatt CE, Drysdale AT, Cohen AO, Dellarco DV, Yang RR, Dale AM, Jernigan TL, Lee FS, & Casey BJ. (2016). Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proceedings of the National Academy of Sciences of the United States of America, 113(16), 4500–4505. 10.1073/pnas.1600013113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, & Ressler KJ (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry, 31(6), 505–514. 10.1016/j.genhosppsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, & Faull RLM (1997). Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience, 77(2), 299–318. 10.1016/S0306-4522(96)00428-9 [DOI] [PubMed] [Google Scholar]
- González S, Grazia Cascio M, Fernández-Ruiz J, Fezza F, Di Marzo V, & Ramos JA (2002). Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Research, 954(1), 73–81. 10.1016/S0006-8993(02)03344-9 [DOI] [PubMed] [Google Scholar]
- Graham BM, & Milad MR (2011). The study of fear extinction: Implications for anxiety disorders. American Journal of Psychiatry, 168(12), 1255–1265. 10.1176/appi.ajp.2011.11040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Vecchiarelli HA, Morena M, Lee TTY, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, & Hill MN (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. Journal of Neuroscience, 35(9), 3879–3892. 10.1523/JNEUROSCI.2737-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, & Holmes A (2013). Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends in Pharmacological Sciences, 34(11), 637–644. 10.1016/j.tips.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie TS, Gorka AX, Alapafuja SO, Nikas SP, Makriyannis A, Poulton R, Patel S, Hariri AR, Caspi A, Moffitt TE, Kunos G, & Holmes A (2013). Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular Psychiatry, 18(7), 813–823. 10.1038/mp.2012.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, & Goldberg S (2009). Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology, 204(4), 607–616. 10.1007/s00213-009-1494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud MZ, Peters C, Hatfield JRB, Gorka SM, Phan KL, Milad MR, & Rabinak CA (2019). Influence of Δ9-tetrahydrocannabinol on long-term neural correlates of threat extinction memory retention in humans. Neuropsychopharmacology, 44(10), 1769–1777. 10.1038/s41386-019-0416-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, & Manuck SB (2009). Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological Psychiatry, 66(1), 9–16. 10.1016/j.biopsych.2008.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Contreras-Rodríguez O, Soriano-Mas C, López-Solà M, Deus J, Ortiz H, Blanco-Hinojo L, Alonso P, Hernández-Ribas R, Cardoner N, & Menchón JM (2011). Task-induced deactivation from rest extends beyond the default mode brain network. PLoS ONE, 6(7), e22964. 10.1371/journal.pone.0022964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, López-Solà M, Hernández-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yücel M, Pantelis C, & Cardoner N (2008). Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Sciences of the United States of America, 105(28), 9781–9786. 10.1073/pnas.0711791105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, &Tu X. (2003). Do patients drop out prematurely from exposure therapy for PTSD? Journal of Traumatic Stress, 16(6), 555–562. 10.1023/B:JOTS.0000004078.93012.7d [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, & Rice KC (1990). Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America, 87(5), 1932–1936. 10.1073/pnas.87.5.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, & McEwen BS (2013). Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Molecular Psychiatry, 18(10), 1125–1135. 10.1038/mp.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, & McEwen BS (2010). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(5), 791–797. 10.1016/j.pnpbp.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppen TH, & Morina N (2019). The prevalence of PTSD and major depression in the global population of adult war survivors: A meta-analytically informed estimate in absolute numbers. European Journal of Psychotraumatology, 10(1), 1578637. 10.1080/20008198.2019.1578637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, & Dolan RJ (2006). Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience, 26(37), 9503–9511. 10.1523/JNEUROSCI.2021-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M,Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, & Piomelli D (2003). Modulation of anxiety through blockade of anandamide hydrolysis. Nature Medicine, 9(1), 76–81. 10.1038/nm803 [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsády L, Ledent C, Mackie K, Hájos N, & Freund TF (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. Journal of Neuroscience, 21(23), 9506–9518. 10.1523/jneurosci.21-23-09506.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science, 256(5057), 675–677. 10.1126/science.1585183 [DOI] [PubMed] [Google Scholar]
- Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, Lim SC, Santos SL, Denny CA, & Drew MR (2019). Distinct hippocampal engrams control extinction and relapse of fear memory. Nature Neuroscience, 22(5), 753–761. 10.1038/s41593-019-0361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J, Eszlari N, Juhasz G, & Bagdy G (2016). Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. European Neuropsychopharmacology, 26(6), 1020–1028. 10.1016/j.euroneuro.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Levar N, van Leeuwen JMC, Puts NAJ, Denys D, & van Wingen GA (2017). GABA concentrations in the anterior cingulate cortex are associated with fear network function and fear recovery in humans. Frontiers in Human Neuroscience, 11, 202. 10.3389/fnhum.2017.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB,Govindarajan A, Deisseroth K, Tonegawa S (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature, 484(7394), 381–385. 10.1038/naturell028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, Blechert J, Philipsen A, van Elst LT, Kalisch R, & Tüscher O (2012). Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS ONE, 7(11), e50120. 10.1371/journal.pone.0050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Marsicano G, & Lafenêtre P (2009). Roles of the endocannabinoid system in learning and memory. In Kendall D & Alexander S (Eds.), Current topics in behavioral neurosciences (Vol. 1, pp. 201–230). Springer Verlag. 10.1007/978-3-540-88955-7_8 [DOI] [PubMed] [Google Scholar]
- Marsicano G, & Lutz B (2006). Neuromodulatory functions of the endocannabinoid system. Journal of Endocrinological Investigation, 29(3), 27–46. [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, & Lutz B (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418(6897), 530–534. 10.1038/nature00839 [DOI] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, Holm L, Nätt D, Augier G, Stensson N, Vecchiarelli HA, Balsevich G, Aukema RJ, Ghafouri B, Spagnolo PA, Lee FS, Hill MN, & Heilig M (2020). Protective effects of elevated anandamide on stress and fear-related behaviors: Translational evidence from humans and mice. Molecular Psychiatry, 25(5), 993–1005. 10.1038/s41380-018-0215-l [DOI] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, Augier G, Hill MN, & Heilig M (2020). Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: A randomized, controlled experimental medicine trial. Biological Psychiatry, 87(6), 538–547. 10.1016/j.biopsych.2019.07.034 [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, & Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in post-traumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420(6911), 70–74. 10.1038/nature01138 [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, & Rauch SL (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry, 62(10), 1191–1194. 10.1016/j.biopsych.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, & Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62(5), 446–454. 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Öhman A (2005). The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology, 30(10), 953–958. 10.1016/j.psyneuen.2005.03.019 [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, & Hillard CJ (2005). Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. European Journal of Neuroscience, 21(4), 1057–1069. 10.1111/j.1460-9568.2005.03916.x [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB, Simmons AN, Risbrough VB, Halter R, & Chaplan SR (2020). The effects of FAAH inhibition on the neural basis of anxiety-related processing in healthy male subjects: A randomized clinical trial. Neuropsychopharmacology, 46(5), 1011–1019. 10.1038/s41386-020-00936-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. 10.1037/0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- Piggott VM, Lloyd SC, Matchynski JI, Perrine SA, & Conti AC (2021). Traumatic stress, chronic ethanol exposure, or the combination, alter cannabinoid system components in reward and limbic regions of the mouse brain. Molecules, 26(7), 2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, & Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology, 33(1), 56–72. 10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, & Phan KL (2014). Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiology of Learning and Memory, 113, 125–134. 10.1016/j.nlm.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Blanchette A, Zabik NL, Peters C, Marusak HA, ladipaolo A, & Elrahal F (2020). Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: A preliminary study. Psychopharmacology, 237(6), 1813–1826. 10.1007/s00213-020-05499-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Vanessa Ho WS, Jarrahian A, & Hillard CJ (2008). Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology, 54(1), 108–116. 10.1016/j.neuropharm.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle S, Rey AA, Remmers F, & Lutz B (2012). The endocannabinoid system in anxiety, fear memory and habituation. Journal of Psychopharmacology, 26(1), 23–39. 10.1177/0269881111408958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, van der Mei WF, Qi W, Lowe S, Lai BS, Bryant RA, Delahanty D, Matsuoka YJ, Olff M, Schnyder U, Seedat S, deRoon-Cassini TA, Kessler RC, Koenen KC, Errera-Ankri Y, Barbano AC … van Zuiden M (2019). Estimating the risk of PTSD in recent trauma survivors: Results of the International Consortium to Predict PTSD (ICPP). World Psychiatry, 18(1), 77–87. 10.1002/wps.20608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Kwako LE, George DT, Mayo LM, Hillard CJ, & Heilig M (2016). FAAH gene variation moderates stress response and symptom severity in patients with posttraumatic stress disorder and comorbid alcohol dependence. Alcoholism: Clinical and Experimental Research, 40(11), 2426–2434. 10.1111/acer.13210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the state-trait anxiety inventory STAI (form Y) (“self-evaluation questionnaire”). Consulting Psychologists Press, Inc. [Google Scholar]
- Spottswood M, Davydow DS, & Huang H (2017). The prevalence of posttraumatic stress disorder in primary care: A systematic review. Harvard Review of Psychiatry, 25(4), 159–169. 10.1097/HRP.0000000000000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme U, Schelling G, Hauer D, Greif R, Dame T, Laubender RP, Bernhard W, Thieme D, Campolongo P, & Theiler L (2014). Quantification of anandamide and 2-arachidonoylglycerol plasma levels to examine potential influences of tetrahydrocannabinol application on the endocannabinoid system in humans. Drug Testing and Analysis, 6(1-2), 17–23. 10.1002/dta.1561 [DOI] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, & Radulovic J (2012). Fear conditioning and extinction: Emotional states encoded by distinct signaling pathways. Trends in Neurosciences, 35(3), 145–155. 10.1016/j.tins.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, & Radulovic J (2009). Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. Journal of Neuroscience, 29(11), 3387–3394. 10.1523/JNEUROSCI.5619-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, & Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, & Lichtman AH (2007). Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology, 32(5), 1032–1041. 10.1038/sj.npp.1301224 [DOI] [PubMed] [Google Scholar]
- Verstraete AG (2004). Detection times of drugs of abuse in blood, urine, and oral fluid. Therapeutic Drug Monitoring, 26(2), 200–205. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013a). The life events checklist for DSM-5 (LEC-5). National Center for PTSD, 5. 10.1177/1073191104269954 [DOI] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013b). The clinician-administered PTSD scale for DSM-5 (CAPS-5). www.ptsd.va.gov, 10.1037/t00072-000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP (2013). The PTSD checklist for DSM-5 (PCL-5). National Center for PTSD, 5, 2002. [Google Scholar]
- Worsley KJ, & Friston KJ (1995). Analysis of fMRI time-series revisited–Again. Neuroimage, 2(3), 173–181. 10.1006/nimg.1995.1023 [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, & Evans AC (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4(1), 58–73. [DOI] [PubMed] [Google Scholar]
- Yasmin E, Colangeli R, Morena M, Filipski S, van der Stelt M, Pittman QJ, Hillard CJ, Campbell Teskey G, McEwen BS, Hill MN, & Chattarji S (2020). Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proceedings of the National Academy of Sciences of the United States of America, 117(1), 650–655. 10.1073/pnas.1910322116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T, Bartsch JC, Beer A, Lomazzo E, Guggenhuber S, Lange MD, Bindila L, Pape HC, & Lutz B (2019). Impaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning, and emotional responses. Neuropsychopharmacology, 44(8), 1377–1388. 10.1038/s41386-018-0274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 US expectancy ratings
TABLE S2 SUDs ratings
Transparent Science Questionnaire for Authors
Transparent Peer Review Report
Data Availability Statement
A subset of the data reported in this manuscript is accessible through the National Institute of Mental Health Data Archive (NDA), with collection title Effects of THC on Retention of Memory for Fear Extinction Learning in PTSD: https://nda.nih.gov/edit_collection.html?id=2680. De-identified data from this subset are available to qualified researchers, while summary data are available to all. The remaining subset of data that are not included in the NDA are available from the corresponding author upon reasonable request.


