Abstract
Introduction
The real-world data evaluating treatment outcomes of atezolizumab plus carboplatin-etoposide chemotherapy (atezolizumab) for extensive-stage SCLC (ESCLC) are lacking. Our objective was to evaluate real-world outcomes of ESCLC treated with atezolizumab.
Methods
A retrospective analysis of provincial patients with ESCLC who started first-line (1L) systemic treatment was conducted. We primarily evaluated the progression-free survival (PFS) and overall survival (OS) outcomes in association with atezolizumab compared with platinum-etoposide chemotherapy (chemotherapy) while adjusting for relevant demographic and clinical factors. Adverse events (AEs) during 1L were evaluated.
Results
A total of 67 patients were identified. Of the 34 patients who received atezolizumab, 24% had Eastern Cooperative Oncology Group performance status greater than or equal to 2, approximately 50% were more than or equal to 65 years, 21% received cisplatin-etoposide chemotherapy before atezolizumab, and 12% had thoracic radiation (tRT).
Within the atezolizumab versus chemotherapy group, the median PFS equals to 6.0 versus 4.3 months (p = 0.03) whereas OS = 12.8 versus 7.1 months (p = 0.01). Relative to chemotherapy, the hazard ratio (95% confidence interval) for PFS was 0.53 (0.28–1.02) and OS was 0.42 (0.20–0.88) with atezolizumab. tRT compared with no tRT receipt correlated with reduced death risk (hazard ratio [95% confidence interval] = 0.33 [0.13–0.88]).
AE-related treatment withdrawal with atezolizumab was 32% and 15% with chemotherapy (p = 0.02). Within the tRT subgroup, 25% versus 20% in atezolizumab versus chemotherapy group, respectively, discontinued 1L owing to AE.
Conclusions
This is the first real-world study revealing comparable survival with that in the IMpower133 trial. Treatment discontinuation from AEs was higher with atezolizumab among Canadian patients with ESCLC. Our data suggest safe use of tRT and chemoimmunotherapy, but its efficacy for ESCLC warrants further study.
Keywords: Extensive stage SCLC, Chemo-immunotherapy, Thoracic radiation, Survival outcomes
Introduction
SCLC accounts for approximately 13% of lung cancers and it is an aggressive cancer associated with early development of metastases. Approximately two-thirds of patients present with extensive-stage SCLC (ESCLC), and only 3% of those with distant metastatic disease are alive at 5 years.1,2 Until recently, minimal progress in ESCLC management had been made in more than two decades. Fortunately, improved survival outcomes for ESCLC have recently been found in clinical trials with the addition of immune checkpoint blockade to chemotherapy.3, 4, 5, 6
Atezolizumab, a programmed death-ligand 1 inhibitor, was studied in the IMpower133 trial in combination with carboplatin and etoposide.3,6 This was found to have an improvement in median overall survival (mOS = 12.3 versus 10.3 mo with chemotherapy). Durvalumab, another programmed death-ligand 1 inhibitor, was also found to have a similar survival benefit in the CASPIAN trial (mOS = 12.9 mo versus 10.5 mo).4,5 The programmed cell death protein-1 inhibitor, pembrolizumab, has also been studied in this setting in the KEYNOTE-604 trial which was found to have an improvement in progression-free survival (PFS) but did not reach significance for OS benefit.7 Overall, approximately a third of those patients treated with the addition of immune checkpoint blockade remain alive at 18 months. This improved outcome is independent of biomarker status so that identifying those most likely to benefit is challenging.3, 4, 5
Although combination systemic therapy consisting of immune checkpoint inhibitors (ICIs) plus chemotherapy is the preferred regimen for ESCLC on the basis of these trials, its adoption has not been universal. In Canada, atezolizumab and durvalumab are both approved for first-line (1L) treatment of ESCLC by the regulatory authority (Health Canada). Although the Pan-Canadian Oncology Drug Review Expert Review Committee recommends that durvalumab should be reimbursed for the treatment ESCLC, atezolizumab is yet to be publicly funded owing to uncertainty on the treatment's long-term benefit and high cost, and it has been accessed through special access programs for those treated with this regimen.10, 8, 9
Radiation treatment also plays important roles in the management of ESCLC. Some guidelines recommend consolidative thoracic radiation (tRT) in patients with ESCLC with a response to initial chemotherapy but with residual thoracic disease, whereas palliative radiation is routinely used for symptomatic control of metastatic SCLC.8,11,12 In the era of immunotherapy, the safety and efficacy of consolidative tRT for ESCLC remain unclear.13,14 Because of the observed synergy between radiotherapy and ICI in the preclinical studies, several clinical trials are now evaluating the safety and efficacy of radiation with immunotherapy in lung cancer, most in the postprogressive disease settings.13, 14, 15, 16, 17, 18, 19 Early studies suggest that tRT and ICI are safe and effective in limited-stage SCLC, but this has not yet been found in extensive-stage disease.13, 14, 15,18,20, 21, 22
The launch of the Special Access Program for 1L atezolizumab in ESCLC (Roche OnCare TECENTRIQ [atezolizumab] Patient Assistance Program) which began on August 12, 2019, and ended on February 1, 2020, in Canada provided an opportunity to study the impact of this new regimen in a real-world setting. The aim of this retrospective analysis was to evaluate the effectiveness and safety of adding atezolizumab to ESCLC treatment in a real-world Canadian population. We evaluated the outcome of the cohort who received atezolizumab on the special access program and compared it with a cohort of ESCLC treated with chemotherapy alone. Of specific interest was how real-world clinical practice might differ in terms of patient heterogeneity and the incorporation of radiation to management strategies for ESCLC.
Material and Methods
Data Source
A retrospective analysis of SCLC data in the Glans-Look Lung Cancer Research (GLR) database was conducted. The GLR is an institutional database approved by the University of Calgary Research Ethics Board (HREBA.CC-16-0574) to capture data of adults diagnosed or treated for lung cancer in Alberta, Canada. Data within the GLR database are managed using the Research Electronic Data Capture tools hosted at the University of Calgary. As a retrospective review, no patient consent was required. Patients were identified through the Alberta Cancer Registry and Alberta Health Services pharmacy system, and their detailed clinical information was retrieved from chart review.
Patient Population
Patients with ESCLC diagnosed between January 1, 2017, and December 31, 2019, who presented at the tertiary or community cancer centers in Alberta and commenced 1L on the atezolizumab special access program were included. Patients with previous curative-intent treatment for limited-stage SCLC were excluded.
For treatment, safety, and survival outcome comparison with the atezolizumab-treated cohort, a random sample of chemotherapy only-treated patients with ESCLC diagnosed in Alberta during 2018 (N = 132) was identified. Using Research Randomizer,23 a random sampling of patients with chemotherapy was performed with the assumption that any confounding baseline characteristics within groups would potentially be adjusted for by the regression modeling.24
Study Design
Patient demographics and clinical, pathologic, treatment, toxicity, and outcome data were collected. Stage was estimated by the chart reviewer using diagnostic imaging, consultation notes, and pathologic reports on the basis of the American Joint Committee on Cancer TNM eighth edition. Comorbidity was captured as documented in patient’s past medical records on the basis of the Charlson Comorbidity Index list and presence of other comorbidities of interest not listed in the Charlson Comorbidity Index, such as follows: autoimmune disorder, weight loss, superior vena cava obstruction, paraneoplastic syndrome including syndrome of inappropriate antidiuretic hormone, Cushing syndrome, Lambert-Eaton syndrome, sensory neuropathy, cerebellar degeneration, and other neurologic disorders at presentation.
AEs were captured by a single reviewer as described by the treating physicians, as identified from flagged laboratory reports (e.g., abnormal laboratory values) during 1L and notes on changes in 1L (regimen or dose or schedule modifications), on the basis of the Common Terminology Criteria for Adverse Events criteria (version 5). AEs up to 90 days after the last 1L dose were captured. Serious adverse events (SAEs) were evaluated, that is, any AE that is life threatening or resulted in death, hospitalization, prolonging an existing hospitalization, persistent or marked disruption of a person’s ability to conduct normal life functions, birth defect, or interventions required to prevent one of the above-mentioned outcomes (e.g., 1L regimen or dose modifications, interruptions/withdrawal, other medications [steroids, hormone replacement] instituted), as defined in the regulations.25 Immune-related AE (irAE) was captured as described by the treating physicians or events deemed to be associated with 1L which required steroids to resolve.
Statistical Analysis
PFS (in mo) was calculated from the start of 1L to disease progression (i.e., as per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1 criteria or clinically by treating physician or death), whichever occurred earlier. OS was defined as the time in months from starting 1L to death from any cause. AEs during 1L were also evaluated. Patients were censored at the last follow-up or data cutoff date (up to May 21, 2021).
Clinical characteristics, treatment, safety, and survival outcomes were compared using the Fisher’s exact (descriptive analysis) and log-rank (Kaplan-Meier) tests. Multivariate Cox regression models were fit to investigate the predictive factors for survival outcomes. A p value of less than 0.05 was considered a priori to be statistically significant. Analysis was performed using SPSS Statistics for Windows (version 27.0; IBM SPSS, Armonk, NY).
Results
Patient and Treatment Characteristics
A total of 67 patients were identified, 34 in the atezolizumab and 33 in the comparator chemotherapy-only group. Baseline patient characteristics are summarized in Table 1. There was no marked difference in patients’ characteristics between the two groups.
Table 1.
Clinical Characteristics of Atezolizumab Plus Chemotherapy (Atezolizumab) Versus Platinum-Doublet Chemotherapy (Chemotherapy) Groups, n (%)
| Variables | Atezolizumab |
Chemotherapy |
p Value |
|---|---|---|---|
| n = 34 | n = 33 | ||
| Median age (range), y | 65 (54–78) | 65 (45–85) | |
| ≥65 y | 17 (50) | 17 (52) | 1.0 |
| ECOG performance status | 1.0 | ||
| 0 | 2 (6) | 1 (3) | |
| 1 | 7 (21) | 6 (18) | |
| 2 | 5 (15) | 4 (12) | |
| 3 | 3 (9) | 4 (12) | |
| 4 | 0 (0) | 1 (3) | |
| Unknown | 17 (50) | 17 (52) | |
| Male | 18 (53) | 15 (46) | 0.63 |
| Ever smokera | 30 (88) | 30 (91) | 1.0 |
| TNM (AJCC eighth) M-status | 0.65 | ||
| M0 | 2 (6) | 0 (0) | |
| M1a | 5 (15) | 4 (12) | |
| M1b | 9 (27) | 11 (33) | |
| M1c | 18 (53) | 18 (55) | |
| Had brain metastasis at diagnosis | 8 (24) | 5 (15) | 0.54 |
| Had any comorbidity at diagnosis | 29 (85) | 31 (94) | 0.43 |
| Treatment characteristic and outcomes | |||
| Had adverse events | 21 (62) | 16 (49) | 0.33 |
| Median chemotherapy cycle# (range) | 4 (1–6) | 4 (1–6) | |
| RT—any site | 22 (65) | 19 (58) | 0.62 |
| Thoracic RT (frontline) | 4 (12) | 10 (30) | 0.08 |
| Prophylactic cranial irradiation | 4 (12) | 2 (6) | 0.67 |
| Reason for 1L discontinuation | 0.02 | ||
| Adverse events | 11 (32) | 5 (15) | |
| Disease progression (as per RECIST criteria) | 15 (44) | 20 (61) | |
| Health decline or patient’s request | 2 (6) | 5 (15) | |
| Regimen completed and stable disease | 0 (0) | 1 (3) | |
| Unknown | 1 (3) | 3 (9) | |
| Received second line | 9 (27) | 5 (15) | 0.37 |
#, number; 1L, first-line; AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; RT, radiotherapy.
Includes current and previous smokers.
At median follow-up of 18 months, 13 patients (19%) were still alive: 12 (18%) in the atezolizumab and one (1%) in the chemotherapy group. Of the 34 patients within the atezolizumab group, 24% had Eastern Cooperative Oncology Group (ECOG) performance status greater than or equal to 2 at presentation and approximately 50% aged more than or equal to 65 years. Because of delays in obtaining access to atezolizumab through a special access program, 74% (n = 25) of the patients in the atezolizumab group had received at least one cycle of platinum-etoposide chemotherapy before initiation of atezolizumab. The median number (range) of chemotherapy cycles received before starting atezolizumab was 1 (one to three). The median time (range) from diagnosis to atezolizumab receipt was 37 (12–128) days. The median duration of atezolizumab treatment (range) was 136 (2–406) days (Table 1). At the time of analysis, 15% of the patients (n = 5) were still undergoing treatment with atezolizumab. Furthermore, 12% of the patients (n = 4) had tRT in addition to atezolizumab, all during maintenance atezolizumab treatment.
Treatment and Survival Outcomes
At the time of data cutoff, 91% of the atezolizumab and 97% of the chemotherapy group had developed progressive disease as defined by the RECIST criteria or clinically by treating physician or death (p = 0.61). The most common reason for 1L termination was RECIST criteria-defined progressive disease in both atezolizumab (44%) and chemotherapy (66%) groups (Table 1).
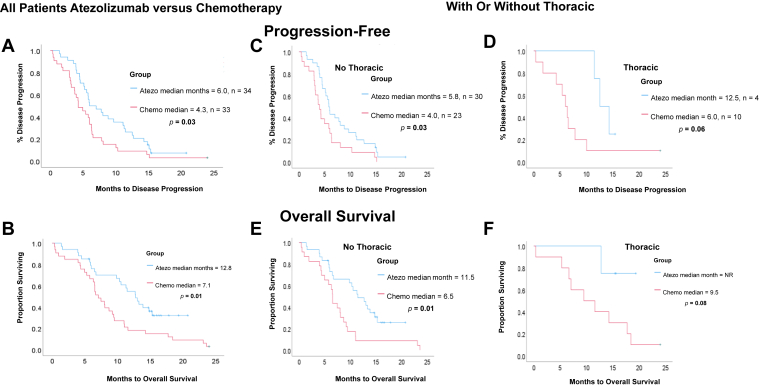
Median PFS (mPFS) was 6.0 months in the atezolizumab group and 4.3 months in the chemotherapy group. mOS was 12.8 months in the atezolizumab versus 7.1 in the chemotherapy cohort (Fig. 1A and B). Although the mOS among patients with ECOG performance status of 2 was shorter for the patients in the atezolizumab group in comparison to those with good ECOG at 0 to 1 ([ECOG 2 versus 0–1] = 5.9 months versus not reached, p = 0.07), there was no significant difference in survival between atezolizumab and chemotherapy groups with ECOG 2 (mOS = 5.9 versus 6.3 mo, p = 0.97).
Figure 1.
Survival outcomes in patients treated with atezo plus chemo (atezo) compared with platinum-doublet chemo (chemo) for all patients (A and B) and stratified by if treated with (D and F) or without thoracic radiotherapy (C and E). Atezo, atezolizumab; chemo, chemotherapy; NR, not reached.
Among the atezolizumab group (n = 34), seven patients did not receive maintenance atezolizumab owing to AEs in four patients and one patient each experienced declining health, progressive disease, or unknown reasons. The mOS was 13.3 months for those with and 6.0 months for those without maintenance atezolizumab (p = 0.01).
Although 74% of the patients in the atezolizumab group had received previous 1L cycle of platinum-etoposide treatment (21% being cisplatin-etoposide), the mOS was not statistically different in those who received cisplatin-etoposide versus carboplatin-etoposide chemotherapy (mOS = 9.8 versus 13.3 mo, p = 0.21).
Radiation Treatment
Among the atezolizumab group, survival outcomes were as follows in tRT versus no tRT subgroup, respectively: mPFS 12.5 versus 5.8 months (p = 0.095) and mOS not reached versus 11.5 months (p = 0.095). Within the chemotherapy group, mPFS is 6.0 versus 4.0 months (p = 0.22) and mOS is 9.5 versus 6.5 months (p = 0.08). Of the 14 patients who received tRT, those treated with atezolizumab had a longer PFS (p = 0.06) and OS (p = 0.08) compared with those treated with chemotherapy (Fig. 1C–F). At presentation, however, a lower proportion of patients treated with tRT had distant metastasis than those who did not receive tRT (distant metastasis = 64 versus 89%, p = 0.04, M1c = 29 versus 60%, p = 0.06) (Supplementary Table 1). The median number of chemotherapy plus or minus atezolizumab cycles (range) at tRT commencement was five (one to nine), median tRT dose (range) was 30 (20–40) Gy, and fraction (range) was 10 (5–16).
In multivariate analyses, favorable prognostic factors of OS include receipt of atezolizumab (hazard ratio = 0.42, 95% confidence interval: 0.20–0.88, p = 0.02) and tRT (hazard ratio = 0.33, 95% confidence interval: 0.13–0.88, p = 0.03) (Table 2).
Table 2.
Prognostic Factors of Survival in Atezolizumab Plus Chemotherapy Versus Platinum-Doublet Chemotherapy Groups
| Variable Categories | Reference Category | PFS Hazard Ratio (95% CI) | p Value | OS Hazard Ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| AEs | No AE | 0.72 (0.40–1.29) | 0.27 | 1.04 (0.53–2.01) | 0.91 |
| Atezolizumab plus chemotherapy | Chemotherapy | 0.53 (0.28–1.02) | 0.06 | 0.42 (0.20–0.88) | 0.02 |
| tRT | No tRT | 0.50 (0.22–1.15) | 0.11 | 0.33 (0.13–0.88) | 0.03 |
| PCI | No PCI | 0.39 (0.13–1.32) | 0.11 | 0.44 (0.13–1.46) | 0.18 |
| 2L systemic | No 2L | - | - | 0.57 (0.25–132) | 0.19 |
| ECOG status | 0 | ||||
| 1 | 1.89 (0.39–9.25) | 0.43 | 0.91 (0.16–5.10) | 0.92 | |
| 2 | 5.49 (1.0–30.08) | 0.05 | 7.72 (1.32–45.01) | 0.02 | |
| 3 | 3.32 (0.54–20.36) | 0.19 | 4.98 (0.74–33.63) | 0.10 | |
| 4 | 20.56 (1.42–297.48) | 0.03 | 26.15 (1.63–418.98) | 0.02 | |
| ≥65 y | <65 y | 0.53 (0.27–1.01) | 0.06 | 0.37 (0.17–0.81) | 0.01 |
| Male | Female | 0.99 (0.56–1.76) | 0.97 | 0.89 (0.48–1.67) | 0.72 |
| No distant metastasis | Distant metastasis | 1.37 (0.56–3.34) | 0.50 | 1.73 (0.66–4.55) | 0.27 |
| Brain metastasis | No brain metastasis | 1.26 (0.62–2.60) | 0.52 | 1.43 (0.64–3.19) | 0.39 |
2L, second-line; AE, adverse event; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PCI, prophylactic cranial irradiation; PFS, progression-free survival; tRT, thoracic radiation.
Adverse Events
There were 21 patients (62%) in the atezolizumab group who had an AE of any grade compared with 16 (49%) in the chemotherapy group (Table 1). The most common AE was hematology related in patients in both atezolizumab (35%) and chemotherapy (33%) groups. In addition, 11 (32%) and seven (21%) patients of atezolizumab versus chemotherapy, respectively, had more than one AEs, and median treatment cycle (range) at first AE was one (1–17) in atezolizumab group compared with two (one to four) in chemotherapy group.
There were 18 patients (53%) in the atezolizumab group compared with 13 patients (39%) in the chemotherapy group who had SAEs, mainly hematological in both (32% versus 24%). AEs led to treatment discontinuation in 32% (n = 11) and 15% (n = 5) of patients in the atezolizumab and chemotherapy groups, respectively, and this represents the second leading cause of treatment termination in both groups (Table 1). The most common AE leading to termination was hematology related in atezolizumab (n = 4) and death in chemotherapy (n = 3) groups.
Within the tRT subgroup, rate of AE-related 1L discontinuation was 25% versus 20% in the atezolizumab (n = 1, autoimmune colitis) versus chemotherapy (n = 2, one death and one neutropenia) group, respectively (Supplementary Table 1).
In the atezolizumab group, eight patients (24%) developed irAE, which were distributed as rash or skin disorder in five patients and hypothyroidism in three patients, whereas adrenal insufficiency and autoimmune colitis (diarrhea) were found in one patient each. In addition, two patients had more than one irAEs (hypothyroidism and rash or skin disorder). Half (n = 4) of the irAEs led to permanent treatment discontinuation. The median number of cycles (range) at 1L discontinuation was five (1–16) in the patients in the atezolizumab group. Supplementary Table 2 reveals the distribution of type and grade of AEs with atezolizumab use.
Discussion
This study evaluated the effectiveness and safety of adding atezolizumab to chemotherapy for ESCLC treatment in a real-world Canadian population and compared it with a cohort of chemotherapy-treated patients. Our findings revealed a longer PFS and OS outcomes with atezolizumab plus chemotherapy than chemotherapy alone, consistent with the foundational trial reports.3, 4, 5, 6 The major causes of treatment discontinuation in both groups were disease progression and AEs. Nonetheless, 18% of the atezolizumab group compared with 1% in the chemotherapy group were still alive at the time of this retrospective analysis (∼18 mo median follow-up).
Our findings are notable because of the heterogeneity found in our real-world patients which was absent in the clinical trials. Among the patients with ECOG 2 performance status, this study reveals no significant difference in survival between atezolizumab and chemotherapy groups (mOS = 5.9 versus 6.3 mo, respectively, p = 0.97). Although the addition of atezolizumab is feasible, its impact on survival seems lower than in patients with ECOG 0 and 1. Furthermore, although, a substantial proportion of patients in the atezolizumab group (74%) had received at least one cycle of platinum-etoposide treatment (21% being cisplatin-etoposide) before initiation of atezolizumab owing to the special access issues, the time to treatment for atezolizumab (median of 37 [range: 12–128] d) in most patients still falls within the standard 30 to 52 days, as previously described.26 We also found no marked difference in survival outcomes among the atezolizumab group whether the patients had received cisplatin or carboplatin-etoposide as part of front-line ESCLC management.
Despite the paucity of safety and efficacy evidence on using radiation treatment with ICI in lung cancers, approximately 12% of patients in the atezolizumab group had tRT for ESCLC in our real-world study. We found that receiving tRT was associated with improved OS in multivariate analysis (Table 2). Importantly, tRT did not seem to negatively affect the efficacy of atezolizumab treatment (Fig. 1). Moreover, the rate of AE-related treatment withdrawal was similar in patients in both atezolizumab and chemotherapy groups who received tRT (Supplementary Table 1), which is suggestive of acceptable AE risks associated with tRT in combination with immunotherapy, consistent with other reports.15, 16, 17,20,27,28 Nevertheless, evidence on efficacy of radiation and ICI for SCLC remains inconclusive, and more study is needed to determine benefit on survival outcomes in this patient population.15,20
In this study, all patients who received tRT in the atezolizumab group received it during the maintenance phase in the 1L setting. Because we also observed a trend toward improved outcomes among the patients in the chemotherapy group who had tRT and given evidence suggesting ICI can augment the antitumor effect of RT, it is possible that combined chemoimmunotherapy provides enhanced radiosensitization than when either chemotherapy or ICI is used alone.18,19 With the report that the most dominant pattern of SCLC progression was at initial thoracic sites of the disease, it is also likely that tRT provides treatment consolidation to residual tumor cells that might have escaped the antitumor effect of systemic therapy.3,29 Alternatively, improved OS with tRT may be accounted for by the fact that patients who received tRT had numerically lower proportion (but not statistically significant, p = 0.06) of M1c disease (American Joint Committee on Cancer TNM eighth edition M-status) than those who did not receive such treatment as found in our Supplementary Table 1.
Furthermore, our findings indicate a lower rate of AEs among real-world patients receiving atezolizumab than the IMpower133 study (62% versus 95%, respectively). Nevertheless, approximately half were SAEs (53% versus 29%, respectively) and were mainly hematological in nature. An important proportion of AEs resulted in atezolizumab termination (35% versus 11%) and was the leading cause for lack of maintenance atezolizumab in real world compared with patients with ESCLC in clinical trial in receipt of ICI.4,6,30 By virtue of the retrospective nature of our study, there could be a tendency toward less AE reporting and documentation in real-world clinical setting. The patient population in our study included approximately 25% with poor performance status (ECOG ≥ 2), approximately 50% with higher metastatic disease burden (M1c disease), and a quarter of patients with brain metastases at presentation. These factors might have affected the ability of real-world patients to tolerate AEs compared with the IMpower133 population and may account for differences in the rate of grade 5 AEs or that leading to treatment discontinuation. We found a lower survival associated with poor ECOG greater than or equal to 2 versus good ECOG of 0 in patients receiving atezolizumab (Table 2).
To the best of our knowledge, this is the first real-world study revealing the beneficial effect of adding atezolizumab to chemotherapy in the treatment of ESCLC. Future studies will need to confirm whether there is benefit in adding tRT to chemoimmunotherapy for ESCLC management and importantly the optimal sequencing for the combination treatment.
These findings should be interpreted with caution given the small sample size of our cohort and random selection of our control group as a potential source of bias and the fact that other confounding factors may have been missed in our retrospective data analyses. For example, ECOG performance status was either not documented or could not be estimated from chart reviews for a substantial proportion of patients. Noting the observed lower OS for our chemotherapy cohort compared with that reported in the control arm of the IMpower133 trial (7.1 versus 10.3 mo, respectively) with comparable OS in the atezolizumab group suggests that there could be additional differences between these groups, including performance status. Similarly, important AEs may have been missed. As these are data from a single province, our results may not be representative of populations with ESCLC elsewhere. Nevertheless, these are SCLC cases from a population of approximately 4.4 million people, with a single-payer health care system and where population demographics are similar to the rest of Canada.
In conclusion, this real-world study confirmed improved PFS and OS outcomes with atezolizumab plus chemotherapy relative to chemotherapy alone for ESCLC, consistent with recent clinical trials. Nevertheless, our study found higher rates of AEs resulting in treatment discontinuation with atezolizumab use. Our data suggest that tRT in patients with ESCLC receiving chemoimmunotherapy is feasible; however, the impact on survival outcomes warrants further investigation in larger prospective studies.
CRediT Authorship Contribution Statement
Anifat A. Elegbede, D. Gwyn Bebb, Aliyah Pabani: Conceptualization.
Anifat A. Elegbede, Michelle L. Dean: Data curation.
Anifat A. Elegbede: Formal analysis, Methodology, Project administration, Writing—original draft.
D. Gwyn Bebb: Funding acquisition.
Anifat A. Elegbede, Aliyah Pabani, Amanda J. Gibson: Investigation.
D. Gwyn Bebb, Aliyah Pabani: Supervision.
Anifat A. Elegbede, Aliyah Pabani, Amanda J. Gibson, Andrea S. Fung, Winson Y. Cheung, D. Gwyn Bebb: Writing—review and editing.
Acknowledgments
We acknowledge Dr. Ezeife D. for critically proofreading the article and Dr. Karen Kopciuk for providing statistical advice and support. We thank the Glans-Look Lung Cancer Research Initiative donors for their continuous financial support in maintaining the database. The authors acknowledged that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the JTO Clinical and Research Reports.
Footnotes
Disclosure: Dr. Bebb reports receiving advisory roles for Roche. The remaining authors declare no conflict of interest.
Cite this article as: Elegbede AA, Gibson AJ, Fung AS, et al. A real-world evaluation of atezolizumab plus platinum-etoposide chemotherapy in patients with extensive-stage SCLC in Canada. JTO Clin Res Rep. 2021;2:100249.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100249.
Supplementary Data
References
- 1.Bryan S., Masoud H., Weir H.K., et al. Cancer in Canada: stage at diagnosis. Health Rep. 2018;29:21–25. [PubMed] [Google Scholar]
- 2.American Cancer Society Lung cancer survival rates: 5-year survival rates for lung cancer. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html
- 3.Liu S.V., Reck M., Mansfield A.S., et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133) J Clin Oncol. 2021;39:619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 5.Goldman J.W., Dvorkin M., Chen Y., et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 6.Horn L., Mansfield A.S., Szczęsna A., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 7.Rudin C.M., Awad M.M., Navarro A., et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38:2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network National Comprehensive Cancer Network guideline version 3.2021: small cell lung cancer. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- 9.Pan-Canadian Oncology Drug Review pCODR expert review committee (pERC) final recommendation for atezolizumab (Tecentric) for small cell lung cancer. https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10156AtezolizumabSCLC_FnRec_ProceduralReview_approvedbyChair_Post_30Jan2020_final.pdf
- 10.Canadian Agency for Drugs and Technologies in Health CADTH reimbursement recommendation draft: durvalumab (Imfinzi) https://www.cadth.ca/sites/default/files/attachments/2021-06/PC0234%20Imfinzi%20-%20Draft%20CADTH%20Recommendation%20For%20posting%20June%2010%2C%202021.pdf [PubMed]
- 11.Simone C.B., Bogart J.A., Cabrera A.R., et al. Radiation therapy for small cell lung cancer: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2020;10:158–173. doi: 10.1016/j.prro.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingemans A.C., Früh M., Ardizzoni A., et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:839–853. doi: 10.1016/j.annonc.2021.03.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badiyan S.N., Roach M.C., Chuong M.D., et al. Combining immunotherapy with radiation therapy in thoracic oncology. J Thorac Dis. 2018;10(suppl 21):S2492–S2507. doi: 10.21037/jtd.2018.05.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S.H., Rimner A., Cohen R.B. Combining immunotherapy and radiation therapy for small cell lung cancer and thymic tumors. Transl Lung Cancer Res. 2017;6:186. doi: 10.21037/tlcr.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pakkala S., Higgins K., Chen Z., et al. Durvalumab and tremelimumab with or without stereotactic body radiation therapy in relapsed small cell lung cancer: a randomized phase II study. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theelen W.S.M.E., Peulen H.M.U., Lalezari F., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh J., Menon H., Chen D., et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson C.W., Sherer M.V., Zamarin D., et al. Immunotherapy and radiation therapy sequencing: state of the data on timing, efficacy, and safety. Cancer. 2021;127:1553–1567. doi: 10.1002/cncr.33424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C.T., Chen W.C., Chang Y.H., Lin W.Y., Chen M.F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci Rep. 2016;6:19740. doi: 10.1038/srep19740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid S., Mauti L.A., Friedlaender A., et al. Outcomes with immune checkpoint inhibitors for relapsed small-cell lung cancer in a Swiss cohort. Cancer Immunol Immunother. 2020;69:1605–1613. doi: 10.1007/s00262-020-02565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh J.W., Heymach J.V., Guo C., et al. Phase 1/2 trial of pembrolizumab and concurrent chemoradiation therapy for limited-stage SCLC. J Thorac Oncol. 2020;15:1919–1927. doi: 10.1016/j.jtho.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X., Wang S., Zhang Y., Wei H., Yu J. Efficacy and safety of immune checkpoint inhibitors (ICIs) in extensive-stage small cell lung cancer (SCLC) J Cancer Res Clin Oncol. 2021;147:593–606. doi: 10.1007/s00432-020-03362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Research randomizer. https://www.randomizer.org/
- 24.Brazauskas R., Logan B.R. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016;22:557–563. doi: 10.1016/j.bbmt.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration What is a serious adverse event? https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
- 26.Kasymjanova G., Small D., Cohen V., et al. Lung cancer care trajectory at a Canadian centre: an evaluation of how wait times affect clinical outcomes. Curr Oncol. 2017;24:302–309. doi: 10.3747/co.24.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bang A., Wilhite T.J., Pike L.R.G., et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98:344–351. doi: 10.1016/j.ijrobp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 28.von Reibnitz D., Chaft J.E., Wu A.J., et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv Radiat Oncol. 2018;3:391–398. doi: 10.1016/j.adro.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins K.A., Curran W.J., Jr., Liu S.V., et al. Patterns of disease progression after carboplatin/etoposide + atezolizumab in extensive-stage small-cell lung cancer (ES-SCLC) Int J Radiat Oncol Biol Phys. 2020;108:1398. doi: 10.1016/j.ijrobp.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield A.S., Każarnowicz A., Karaseva N., et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol. 2020;31:310–317. doi: 10.1016/j.annonc.2019.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.