Abstract
Objective:
This pilot study tested counselor access to participants’ digital self-monitoring (SM) data as a means of improving long-term lifestyle modification (LM) outcomes.
Methods:
After 12 weeks of weight loss treatment, participants (N = 77) were randomized to LM or LM+SHARE for weeks 13 to 52. All participants received monthly phone calls and weekly text messages from weeks 13 to 52 and were instructed to engage in daily, digital SM of weight, eating, and exercise. In LM+SHARE, but not LM, counselors had access to SM device data. Assessments were conducted as weeks 0, 13, 26, and 52.
Results:
Retention, engagement, and treatment satisfaction were excellent. LM+SHARE participants, compared to LM, had more frequent SM of weight and eating. Weight loss continued at a similar rate in both conditions from weeks 13 to 26. From weeks 26 to 52, those in LM regained approximately 2 kg, while those in LM+SHARE maintained weight loss, a significant difference. Nonetheless, total weight loss did not significantly differ by condition. Engagement in dietary SM mediated the effect of condition on weight.
Conclusions:
Counselor access to SM data is feasible and acceptable. Additional research is warranted to determine if it can meaningfully improve outcomes.
Keywords: obesity treatment, weight loss, counseling, lifestyle modifications, social support
Introduction
Maintenance of behavior change is a notoriously difficult aspect of lifestyle modification for adults with overweight or obesity (1). Many behavioral weight loss programs continue some form of contact with participants after the intensive phase of face-to-face treatment ends, but it is unclear how to make this continued contact most efficacious (2). One promising approach may be for counselors to closely monitor participant performance remotely, such that they regularly examine changes in weight, dietary intake, and physical activity and check how frequently self-monitoring in each of those domains is occurring. Digital tools that capture information about eating, exercise, and weight have previously been used primarily for self-monitoring purposes, but device data also can be accessed by counselors, creating new opportunities for performance monitoring.
Mohr and colleagues (3) have developed a theoretical model of supportive accountability to explain how performance monitoring by a trustworthy counselor can enhance engagement with eHealth interventions; the theory also may be applicable to remote interventions more broadly. When participants know that counselors are checking on progress towards goals, this may promote behavioral adherence because individuals strive to maintain outward appearances of intention-action consistency (4, 5). The presence of an “observing other” also can enhance adherence by prompting self-evaluation, and positive feedback from an observer serves to reinforce desirable behavior (6–8).
In addition, it is possible that the richness of the data available from digital devices may improve the quality of counseling, apart from any effect on supportive accountability. Device data access allows counselors to assess objectively, rather than subjectively, how frequently participants are self-monitoring weight, eating, and physical activity; how weight has changed; and daily pattern of physical activity. Counselors also can view self-report data regarding what and when food and drink has been consumed, along with daily calorie intake. If devices are regularly synced (and food intake recorded throughout the day), there can be minimal time lag in counselor surveillance of participant behavior or weight change. As a result, counselor feedback, goal setting, or problem-solving with participants may be more personalized, accurate, and timely, compared to when all information about goal progress is self-reported and available to counselors only at the time of an intervention session.
A number of interventions have been evaluated in which participants shared information from digital self-monitoring tools with counselors or peers (9–13). However, the present study is the first to our knowledge to hold constant the use of self-monitoring tools, behavioral skills training, and clinical contact time and vary whether or not counselors have access to device data and can integrate data observations into their counseling approach. This study was designed as a pilot RCT in order to inform future research. Primary aim 1 of the study was to establish the feasibility and acceptability of the digital data sharing approach in the remote treatment phase of a behavioral weight loss program. Primary aim 2 was to test the hypotheses that weight loss and physical activity would be improved for participants randomized to have digital device data shared with their counselor, versus those in a comparison condition. The secondary aims were to test the hypothesis that self-monitoring engagement would be greater in the experimental versus standard condition, and examine self-monitoring engagment and perceived accountability as mediators of the effect of condition on weight.
Methods
Setting and Population
This was a single-site, pilot randomized controlled clinical trial. Participants were recruited from the community from October 2017 to March 2018. Follow-up assessments were completed in May 2019. Inclusion criteria included age 18 to 70 years; BMI 25–45 kg/m2; regular access to a smartphone and internet connection; ability to begin a program of physical activity; and completion of all enrollment procedures. Participants were excluded if they had a medical condition or psychiatric condition (e.g., active substance abuse, eating disorder) that could pose a risk or limit ability to comply with program recommendations. Participants were also excluded if they were pregnant or planning to become pregnant or move out of the area during the data collection period, were using a pacemaker, had a history of bariatric surgery, had recently begun or changed the dose of a medication that could cause a significant change in weight, or if they had a weight loss of ≥ 10% in the previous 3 months. The most common reasons for exclusion were out-of-range BMI (n = 34) and lack of availability at group meeting times (n = 30). All participants provided informed consent, and all study procedures were approved by the Institutional Review Board at Drexel University, where the study was conducted.
Phase I: Uniform, Initial Weight Loss Treatment
All participants received identical treatment during Phase I, the initial, face-to-face phase of treatment. Participants attended 12, weekly behavioral weight loss group sessions. The treatment protocol was adapted from Look AHEAD and the Diabetes Prevention Program (14, 15). Treatment included problem solving skills, strategies for stimulus control, goal setting, and social support skills. Participants were given prescriptions for reducing calorie intake and were told to gradually increase their moderate-to-vigorous physical activity (MVPA) to 250 bouted minutes per week (i.e., a minimum of 10 minutes of exercise at a time).
All participants were provided with three self-monitoring tools: a Yunmai Smart Scale for measuring body weight, a Fitbit Flex for monitoring physical activity, and the Fitbit app for recording food intake and viewing data from all three devices. Participants were told to weight themselves weekly in weeks 1–10 and daily thereafter; to wear the physical activity sensor daily, paying particular attention to “active minutes” when reviewing device data; and to record food intake daily. During Phase I, device data were not available to counselors. Participants were instructed not to share device data with friends, family, or other participants at any point in the program.
Phase II: Remote Maintenance Treatment
At the conclusion of Phase I, participants were randomly assigned (matching for initial weight loss) to one of two treatments for Phase II (weeks 13–52): standard lifestyle modification (LM) or lifestyle modification plus device data sharing (LM+SHARE). Phase II treatment was delivered remotely. There were no further group meetings; participants received monthly, individual, 15-minute phone calls and weekly, one-way text messages. Participants in both conditions were instructed to continue using digital devices daily to self-monitor weight, physical activity, and food intake.
In LM+SHARE, counselors had access to a secure online portal that uploaded data from participants’ self-monitoring devices. Counselors were instructed to attend to changes in weight, adherence to dietary intake and physical activity goals, and self-monitoring frequency when viewing data. In phone calls and text messages with participants, counselors used their data observations to cultivate a sense of supportive accountability, conveying to participants that they were regularly monitoring adherence to program goals in order to sustain participant success. Counselors set goals with participants in monthly phone calls, subsequently viewed data relevant to the goals to determine if they were being met, and in the text messages and phone calls that followed provided praise to reinforce positive behaviors or expressed concern if adherence was poor. For example, a text message might say: “I’m so impressed that you started recording your food intake again! I saw you recorded for 5 days this week. Reflect on how you accomplished that, and how it was helpful, so that you can carry this success forward. Keep up this fantastic work!” As another example, in a phone call a counselor might say: “You set a goal last month of 5 bouts of exercise per week, and I see that you only averaged 2 per week. Tell me more about what happened.” Counselors also used phone calls and text messages to review core behavioral skills or strategies and encourage their use.
In the LM condition, counselors did not have access to device data. Counselors used participant self-report in monthly phone calls (prompted by questions such as, “How successful have you been in meeting your physical activity goals since our last call?”) to assess progress and adherence. As in LM+SHARE, LM counselors reviewed one or more core behavioral skills or strategies in each phone call. All participants in the LM condition received standardized, weekly text messages that highlighted a core behavioral skill or strategy; messages in this condition were not personalized.
The seven counselors who provided treatment had degrees in psychology or a related field (1 = PhD, 4 = MA/MS, 2 = BA/BS). Counselors had previous training in behavioral weight loss and also completed a training workshop and weekly supervision for this study. All counselors provided both forms of treatment.
Measures
Assessments were completed at weeks 0 (baseline), 13 (i.e., baseline of Phase II treatment), week 26, and week 52 (end of treatment).
Demographics.
Age, gender, education, race, and ethnicity were self-reported at baseline.
Height and weight.
At each assessment, research staff weighed participants using a Tanita model WB-3000 digital scale. Participants were weighed in light street clothes without shoes. Height was measured at baseline using a stadiometer. Measurements were taken twice and averaged.
Physical Activity.
Participants wore ActiGraph GT3X tri-axial, solid state accelerometers to measure MVPA at weeks 13 and 52. (To minimize “device burden,” given that participants were asked upon enrollment to begin using three other self-monitoring devices, Actigraph measurements were not done at week 0.) Participants were told to wear the accelerometer for seven consecutive days during all waking hours, and data were considered valid when available for a minimum of four days for at least 10 hours per day. ActiLife software was used to calculate MVPA using established cut-points (16). Consistent with the intervention focus on bouted exercise, MVPA totals included only episodes of MVPA detected by the accelerometer to be ≥ 10 minutes.
Self-monitoring engagement.
Device data were collected on a secure online portal, which allowed for calculation of the percent of days during which weight was recorded on the Yunmai Smart Scale; ≥ five foods were logged in the Fitbit app; and the Fitbit counted ≥ 500 steps.
Treatment engagement.
Engagement with treatment phone calls was calculated as the number of calls the participant completed during Phase II, out of 8 possible calls. The number of text messages sent by the counselor also was calculated.
Treatment satisfaction.
Treatment satisfaction was measured using the Treatment Acceptability Questionnaire (17), with some items modified to reflect specific treatment components of the current study. This measure has good internal consistency and concurrent validity.
Perceived accountability.
The “Perceptions of Accountability” subscale of the Supportive Accountability Scale (18) was administered to measure the extent to which the participants feel accountable to others to achieve their weight control goals. Reliability and validity for this scale has been established (18).
Data Analysis
Retention was compared between the two conditions using Fisher’s exact test. Permutation test was conducted to examine the difference between conditions in the number of phone calls and text messages (which could vary if a participant requested that contact be discontinued or temporarily suspended) and treatment acceptability ratings. Permutation test, a type of nonparametric test that uses sampling without replacement to test hypotheses, was chosen due to the violation of t-test assumptions in the data set (19). We conducted permutation based ANCOVA to compare self-monitoring engagement in Phase II between conditions controlling for Phase I self-monitoring engagement. This analytic method was implemented in the “permuco” package in R and has been shown to be robust to data with non-normal distribution (20). ANCOVA was used to test the difference in weight change between conditions, controlling for Phase I weight loss. Change in bouted MVPA during Phase II was compared between LM and LM+SHARE controlling for Fitbit-measured changes in Phase I MVPA using permutation based ANCOVA. One outlier for bouted MVPA at 13 weeks in the LM condition was removed from the analyses (MVPA was more than 3 SDs above others). Repeated measures ANCOVA was used to compare perceived accountability controlling for Phase I change. Analyses examined whether the effect of condition on weight loss during Phase II was mediated by change in monthly adherence to self-monitoring during the first 6 months of Phase II, or change in perceived accountability from weeks 13 to 26. If weight and MVPA outcome data were missing, the most recent data from the participant’s digital scale or Fitbit, respectively, were used for imputation; if those data were not available, baseline data were carried forward. Strong associations were found between weight measured in clinic and via home digital scale (r= .995, p < .001 at 52 weeks), and between Actigraph and Fitbit measurements of MVPA (r = .60, p < .001 at 52 weeks), validating this missing data approach. The study was designed as a pilot randomized trial, with a focus on having a large enough sample for estimates of feasibility, acceptability, and effect sizes to be reliable. Data were analyzed in SPSS version 25 and R version 4.0.0.
Results
Phase I (Pre-Randomization)
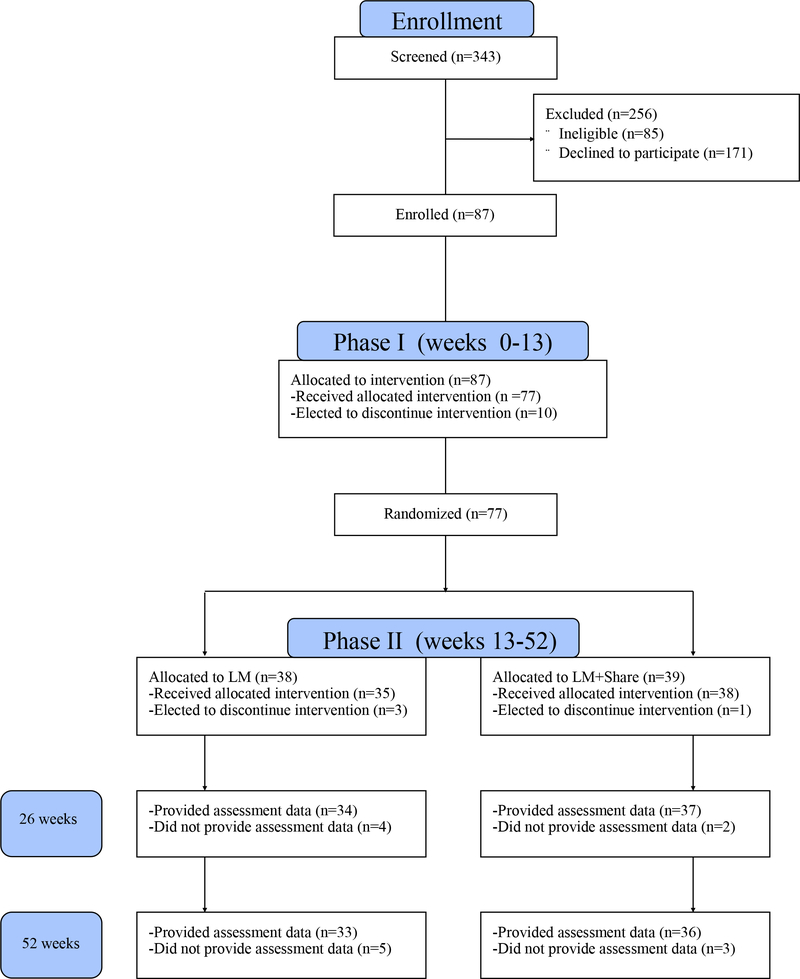
As shown in Figure 1, of the 87 participants who were enrolled in the study, 77 completed the initial weight loss treatment (Phase I) and were randomized to LM or LM+SHARE. Demographic information for these participants is shown in Table 1. At the time of randomization, weight loss averaged 5.78 kg (SD = 4.79) in LM participants and 5.80 kg (SD = 4.01) in LM+SHARE participants, and MVPA averaged 173.62 min/week (SD = 137.16) in LM participants and 125.86 min/week (SD = 134.16) in LM+SHARE participants. Phase I weight loss and MVPA at 13 weeks did not differ by condition (F(1,75) < 0.01, p = 0.98 and F(1,74) = 2.36, p = 0.12, respectively.) Additional sample information is shown in Table 1.
Figure 1.
Consort Diagram. LM, lifestyle modification.
Table 1.
Baseline and Phase I descriptive statistics by condition
| LM | LM+SHARE | |
|---|---|---|
|
| ||
| Age (years), M (SD) | 51.71 (13.33) | 49.84 (13.56) |
| Female, n (%) | 30 (78.9%) | 32 (82.1%) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 2 (5.3%) | 1 (2.6%) |
| Non-Hispanic | 36 (94.7%) | 38 (97.4%) |
| Race, n (%) | ||
| American Indian/Alaskan Native | 1 (2.6%) | 0 (0%) |
| Asian | 2 (5.3%) | 0 (0%) |
| Native Hawaiian or other Pacific Islander | 0 (0%) | 0 (0%) |
| Black or African American | 13 (34.2%) | 15 (38.5%) |
| White | 19 (50.0%) | 22 (56.4%) |
| Other or more than once race | 3 (7.9%) | 2 (5.1%) |
| Education, n % | ||
| High school or lower | 2 (5.3%) | 3 (7.7%) |
| Associate degree | 9 (23.7%) | 5 (12.8%) |
| Bachelor’s degree | 8 (21.1%) | 14 (35.9%) |
| Graduate or professional degree | 19 (50.0%) | 17 (43.6%) |
| Baseline BMI, M (SD) | 34.80 (4.8) | 34.9 (4.7) |
| Baseline weight (kg), M (SD) | 93.24 (15.3) | 96.25 (13.4) |
| Phase I outcomes, M (SD) | ||
| Weight loss (kg) | 5.78 (4.8) | 5.80 (4.01) |
| Min/week increase in MVPA (Fitbit measured) | 84.68 (116.4) | 57.61 (107.1) |
| Eating SM % days of adherence | 86.77 (13.4) | 82.94 (21.5) |
| Weight SM % days of adherence | 86.47 (16.4) | 84.66 (20.8) |
| Physical activity SM % days adherence | 96.65 (6.2) | 90.71 (18.2) |
| Increase in perceived accountability score | 9.49 (14.8) | 4.43 (13.9) |
Note. SD, standard deviation; BMI, body mass index; kg, kilograms; MVPA, moderate-to-vigorous physical activity; min/week, minutes per week; SM, self-monitoring.
Feasibility and Acceptability
During Phase II, high rates of completion of treatment contacts were observed in both LM and LM+SHARE. LM and LM+SHARE did not significantly differ in the number of phone calls (LM: M = 7.37, SD = 1.55, LM+SHARE: M = 7.20, SD = 1.79, p = 0.64) or text messages delivered (LM: M = 30.50, SD = 3.85, LM+SHARE: M =29.67, SD = 5.94, p = 0.499). Mean post-treatment scores on the Treatment Acceptability Questionnaire were 65.62 (SD = 8.43) in LM and 65.95 (SD = 7.76) in LM+SHARE (p = 0.86). Retention of randomized participants (defined as percent of participants returning to research clinic for outcomes assessment) did not differ at week 26 (LM = 89.5%, LM+SHARE = 94.9%, p = .43) or week 52 (LM = 86.8%, LM+SHARE = 92.3%, p = .48).
Self-Monitoring Engagement
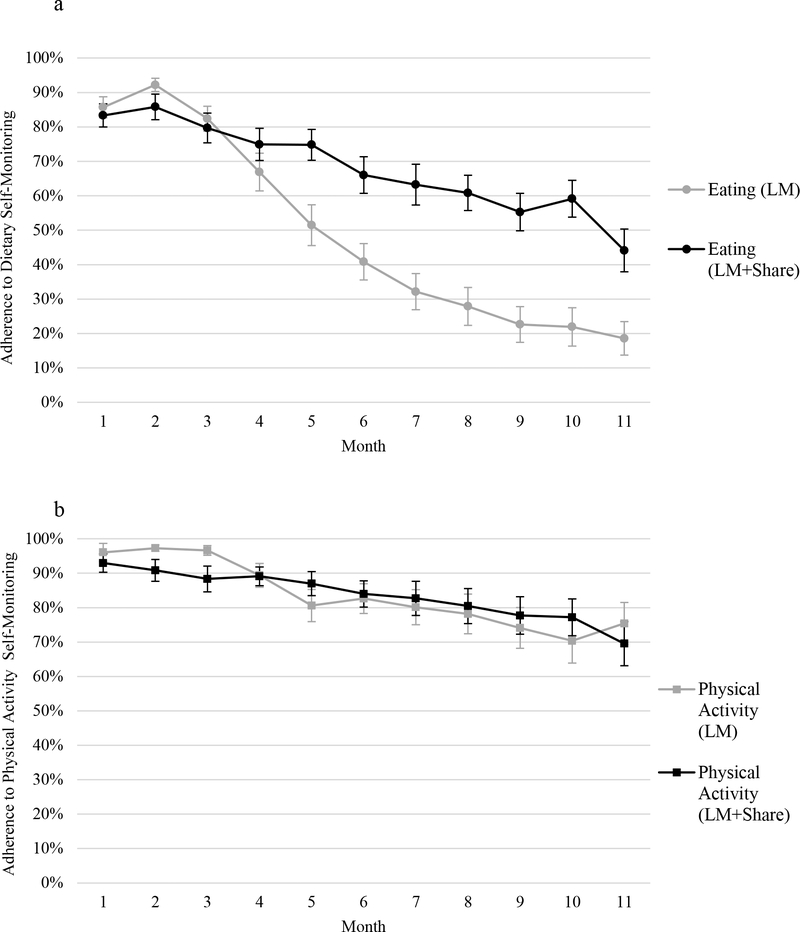
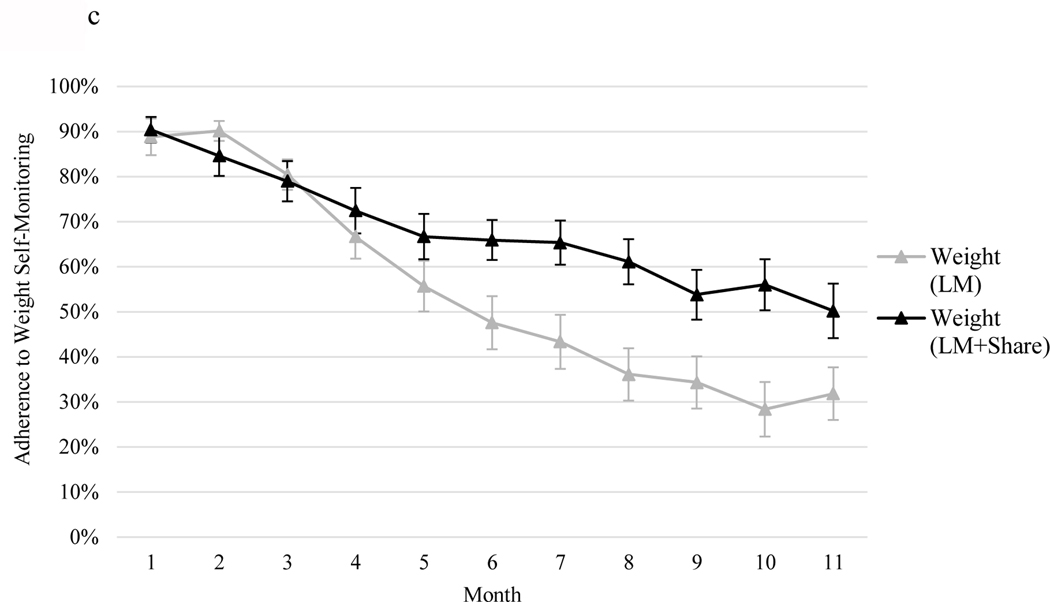
During Phase II, participants in LM+SHARE, compared to LM, engaged in more frequent self-monitoring of weight (58% vs. 43% of days, d = 0.52, F(1, 73) = 9.59, p = 0.002) and self-monitoring of eating (67% vs. 41% of days, d = 0.94, F(1, 73) = 32.84, p < .001). Self-monitoring of physical activity was high in both conditions and did not differ (80% vs. 79% of days, d = 0.04, F(1, 73) = 1.38, p = 0.25). Figure 2 illustrates self-monitoring engagement patterns over time by condition.
Figure 2.
a, 2b, and 2c. Percent adherence to self-monitoring by month and condition separated by self-monitoring type: 2a = adherence to dietary self-monitoring, 2b = adherence to physical activity self-monitoring, 2c = adherence to weight self-monitoring. LM, lifestyle modification. Error bars represent standard errors.
Weight Change
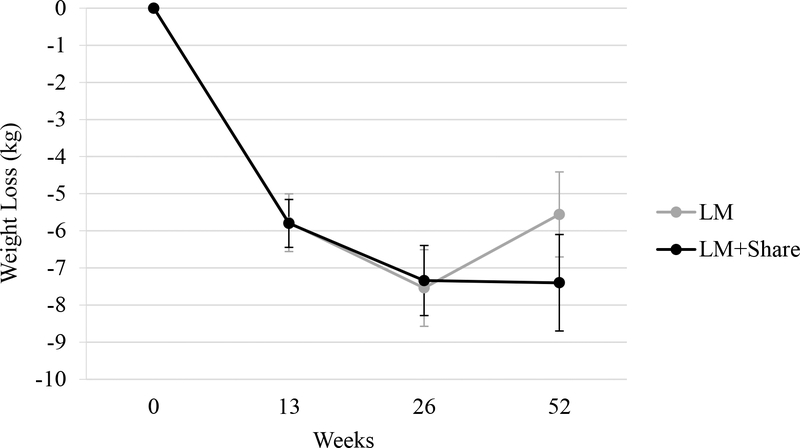
As shown in Figure 3, significant additional weight loss occurred in weeks 13 to 26 in LM (M = −1.76 kg, SD= 2.81, p < .001) and LM+SHARE (M = −1.54 kg, SD = 2.83, p = .002). This pattern did not significantly different between conditions (d = −0.08, p = 0.75). From weeks 26 to 52, significant weight regain occurred in LM (M = +1.98 kg, SD = 3.22, p = .001), while weight change in LM+SHARE was not significant (M = +0.06 kg, SD = 4.16, p = .93). Change in weight from 26 to 52 weeks significantly differed by condition (d = 0.55, p = 0.02), though significance decreased when only completers were included (d = 0.44, p = 0.08). Change in weight during the entirety of the Phase II period did not significantly differ by condition (LM: M = + 0.22 kg, SD = 4.02, LM+SHARE: M = −1.60 kg, SD = 5.47; d = 0.38, p = 0.099).
Figure 3.
Mean weight loss (kg) over time by condition. LM, lifestyle modification; kg, kilograms. Error bars represent standard errors.
Physical Activity
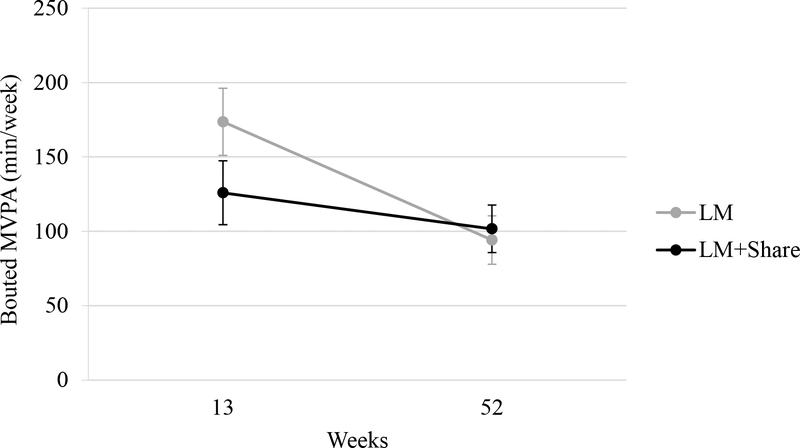
Change in MVPA in Phase II did not significantly differ by condition (d = 0.37, p = 0.16). Findings were similar with completers only (d = 0.58, p = 0.07). Change over time by condition is shown in Figure 4.
Figure 4.
Moderate-to-vigorous physical activity (mins/week) over time by condition. LM, lifestyle modification; min/week, minutes per week; MVPA, moderate-to-vigorous physical activity. Error bars represent standard errors.
Perceived Accountability
Perceived accountability declined significantly during Phase II in both LM (M = −8.28, SD = 15.25, p = .004) and LM+SHARE (M = −6.88, SD = 16.05, p = 0.02). However, these reductions were greater in LM, compared to LM+SHARE (F(1, 203) = 8.19, p = 0.005, ηp2 = 0.04). During Phase II, changes in perceived accountability were not associated with frequency of self-monitoring of weight (Kendall’s tau = 0.01, p = 0.88), eating (Kendall’s tau = 0.07, p = 0.40), or physical activity (Kendall’s tau = 0.02, p = 0.80).
Mediation
Frequency of dietary self-monitoring significantly mediated the effect of condition on weight loss during Phase II, after controlling for baseline weight (b = .63, 95% C.I. [.03, 2.05]). Frequency of weight self-monitoring (b = 0.36, 95% CI [−.13, 1.21]), physical activity self-monitoring (b = −.009, 95% CI [−.52, .42]) and early change in perceived accountability (b = −.11, 95% CI [−.86, .13]) were not significant mediators of the effect of condition on weight loss during Phase II.
Discussion
Adults who attempt lifestyle modification typically find that maintenance of behavior change is challenging. This pilot study was the first to our knowledge to experimentally test counselor access to and use of digital self-monitoring device data during the maintenance phase of lifestyle modification, while controlling for other intervention components. This experimental intervention approach was feasible and acceptable, and improvements in self-monitoring engagement were observed.
One hypothetical concern about digital device data sharing is that if participants become ashamed of or disappointed in their progress, they will drop out of the study, refuse attempts at clinical contact, or avoid self-monitoring. This study, consistent with related research, found no evidence to suggest that a negative response to data sharing occurred (9, 12, 13, 21). In fact, self-monitoring of weight and eating occurred more frequently in LM+SHARE, compared to LM (with medium to large effect sizes). These results are very encouraging, given the evidence that self-monitoring of weight and eating may ultimately promote long-term weight control (22–24). Across conditions, participants had a high level of adherence to physical activity self-monitoring, which may be viewed as especially low-effort, rewarding, or valuable (25), possibly creating a ceiling effect for further bolstering device use.
During the first three months of remote treatment, participants in both LM and LM+SHARE achieved additional weight loss. However, in the following six months, intent-to-treat analyses indicated that the LM+SHARE condition produced superior results. Specifically, participants in LM regained weight, on average, while participants in LM+SHARE maintained their weight losses. Thus, the benefit of device data sharing may be greatest when participants would otherwise begin to find weight loss maintenance most difficult. The difference in weight trajectory observed in weeks 26 to 52, as well as the effect size observed for total difference in Phase II weight loss, is encouraging, given how challenging it is to improve weight loss maintenance outcomes (26). However, the clinical significance of this difference in weight trajectory is not known, the pattern became non-significant when only completers were included, and the total difference in weight by condition did not reach significance.
Conditions did not statistically differ in change in MVPA during Phase II. The clinical significance of the pattern of results is difficult to interpret because MVPA at the time of Phase II randomization was approximately 50 min/week higher in LM versus LM+SHARE. Additional research with a larger sample size and randomization blocking for MVPA is needed to determine if counselor surveillance improves maintenance of physical activity.
This study can provide only limited information about mechanisms of action. Perceived accountability was maintained better in LM+SHARE, versus LM. However, significant reductions in perceived accountability were nonetheless observed in both conditions, suggesting that regardless of data sharing, participants found that remote treatment provided less accountability than weekly, face-to-face group sessions. Early change in perceived accountability did not significantly mediate the effect of condition on weight, nor was perceived accountability associated with self-monitoring frequency. Future research might use a larger sample and measure perceived accountability more frequently or with alternate tools. Future research also could measure the quality of counseling to determine if this mediates the effect of treatment condition, in that provider access to device data may make counseling processes such as goal setting and problem solving more effective.
Change in self-monitoring of eating mediated the effect of condition on weight loss. This finding is consistent with the aforementioned literature showing that individuals who engage in more consistent dietary self-monitoring have superior weight loss outcomes (12, 27–29). The failure of self-monitoring of physical activity to mediate the effect of condition on weight loss parallels prior work showing that frequency of physical activity self-monitoring is not associated with weight loss outcomes (11, 12, 28). On the other hand, it was surprising that weight self-monitoring was not a mediator of weight outcomes, given other work that has found that self-weighing is a valuable tool for weight loss (12, 22, 23, 29, 30). Given the small sample size, these null mediator results should be interpreted with caution. However, this pattern raises the possibility that to bolster weight loss maintenance while minimizing burden, future efforts to use device data sharing could investigate the use of dietary self-monitoring data only.
Strengths of the study, including the experimental design, racial diversity and retention of participants, and objective measurement of weight, physical activity, and self-monitoring engagement, are offset by a number of limitations. The proportion of men enrolled was small and adults over age 70 were excluded from participation, so the generalizability of findings to those populations may be limited. Phone calls and text messages were not qualitatively analyzed nor rated for treatment fidelity, and the actual length of each telephone session was not measured. Participants were instructed not to share device data with friends, family, or other participants, but it is unknown to what extent this may have occurred and contaminated either condition. Power for statistical analyses was limited and mediators were not comprehensively assessed. The study was designed such that participants in LM+SHARE differed from those in LM in two ways: they knew that their counselors were monitoring their data, and counselor observations from those data were integrated into phone calls and text messages. It is not known which of these components was most powerful.
As digital self-monitoring devices become more widely used by consumers, there is great potential for incorporating this approach into lifestyle modification programs. However, the cost and burden of device data surveillance is notable, so more work is required to determine if it provides a sufficient return on investment for large scale adoption. This study found no evidence to suggest that data surveillance was iatrogenic (e.g., that a sense of shame may cause treatment dropout), and longer-term research is warranted to determine if that remains the case even when more participants may begin to experience behavioral lapses or periods of weight regain.
Study Importance Questions.
What is already known about this subject?
Tools are needed to support maintenance of behavior change for adults attempting weight loss.
What are the new findings of this manuscript?
It was feasible and acceptable to participants for counselors to access their digital self-monitoring data (weight, exercise, and intake), and integrate observations from those data into counseling phone calls and text messages. This approach resulted in superior engagement in self-monitoring, compared to counseling without data surveillance.
Total weight change was similar whether counselors did or did not have access to participants’ data, although some separation between conditions in weight trajectory was detected later in the maintenance period.
How might your results change the direction of research or the focus of clinical practice?
Additional research designed to bolster the efficacy of this experimental approach and understand more about mechanism is warranted.
Acknowledgements
Data may be available in a deidentified format to researcher with the approval of the first author and the Institutional Review Board after all parent study analyses are completed. Information about the trial protocol can be obtained from the first author.
Funding: R21DK112741
Footnotes
Trial Registration: ClinicalTrials.gov identifier NCT03337139, https://www.clinicaltrials.gov
Disclosure: Dr. Forman is a member of the Tivity Health Scientific Advisory Board. The other authors declared no conflict of interest.
References
- 1.Wadden TA, Tronieri J, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75:235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asbjørnsen RA, Smedsrød ML, Nes LS, Wentzel J, Varsi C, Hjelmesæth J, et al. Persuasive system design principles and behavior change techniques to stimulate motivation and adherence in electronic health interventions to support weight loss maintenance: Scoping review. J Med Internet Res. 2019;21(6):e14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: A model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Festinger L. A theory of cognitive dissonance. Stanford: Stanford University Press; 1957. [Google Scholar]
- 5.Tedeschi JT. Impression management theory and social psychological research. Academic Press; 2013. [Google Scholar]
- 6.Cameron J, Pierce WD. Reinforcement, reward, and intrinsic motivation: A meta-analysis. Rev Educ Res. 1994;64(3):363–423. [Google Scholar]
- 7.Carver CS, Scheier MF. On the self-regulation of behavior. Cambridge University Press; 2001. [Google Scholar]
- 8.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. 2009;11(2):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godino JG, Merchant G, Norman GJ, et al. Using social and mobile tools for weight loss in overweight and obese young adults (Project SMART): A 2 year, parallel-group, randomised, controlled trial. Lancet Diabetes Endocrinol. 2016;4(9):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. JAMA. 2016;316(11):1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mameli C, Brunetti D, Colombo V, et al. Combined use of a wristband and a smartphone to reduce body weight in obese children: Randomized controlled trial. Pediatr Obes. 2018;13(2):81–7. [DOI] [PubMed] [Google Scholar]
- 12.Ross KM, Wing RR. Impact of newer self-monitoring technology and brief phone-based intervention on weight loss: A randomized pilot study. Obesity. 2016;24(8):1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JG, Bond DS, Raynor HA, Papandonatos GD, Wing RR. Comparison of smartphone-based behavioral obesity treatment with gold standard group treatment and control: a randomized trial. Obesity. 2019;27(4):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 17.Hunsley J. Development of the Treatment Acceptability Questionnaire. J Psychopathol and Behav Assess. 1992;14(1):55–64. [Google Scholar]
- 18.Chhabria K, Ross KM, Sacco S, Leahey TM. Development of a supportive accountability measure in adults seeking obesity treatment. JMIR Preprints. 2020(17967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good P. Permutation tests. New York: Springer; 2000. [Google Scholar]
- 20.Frossard J, Renaud O. Permutation tests for regression, ANOVA and comparison of signals: The permuco package. 2018.
- 21.Polzien KM, Jakicic JM, Tate DF, Otto AD. The efficacy of a technology-based system in a short-term behavioral weight loss intervention. Obesity. 2007;15(4):825–30. [DOI] [PubMed] [Google Scholar]
- 22.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005;30(3):210–6. [DOI] [PubMed] [Google Scholar]
- 23.Vanwormer JJ, French SA, Pereira MA, Welsh EM. The impact of regular self-weighing on weight management: A systematic literature review. Int J Behav Nutr Phys Act. 2008;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–5S. [DOI] [PubMed] [Google Scholar]
- 25.Butryn ML, Godfrey K, Martinelli MK, Roberts SR, Forman EM, Zhang F. Digital self-monitoring: Does adherence or association with outcomes differ by self-monitoring target? Obes Sci Pract. 2019;6(2):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchesson MJ, Tan CY, Morgan P, Callister R, Collins C. Enhancement of self-monitoring in a Web-based weight loss program by wxtra individualized feedback and reminders: Randomized trial. J Med Internet Res. 2016;18(4):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini CA, Verba SD, Otto AD, Helsel DL, Davis KK, Jakicic JM. The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity. 2012;20(2):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: The ENGAGED randomized clinical trial. Obesity. 2017;25(7):1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity. 2013;21(9):1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]