Axillary adenopathy has been rarely reported following the administration of Influenza and human papilloma vaccinations [1]. As reported by Dr. Lane et al. [2], during the Covid-19 pandemic in 2020 and since the beginning of the vaccination campaigns worldwide in 2021, the incidence of axillary lymphadenopathy has been recently reported by the Society of Breast Imaging (SBI) [3] after vaccination with the Moderna Covid-19 and Pfizer-BioNTech Covid-19 vaccines [4,5], such new observations have caused diagnostic dilemma particularly in oncology patients [6,7].
We have encountered Covid-19 vaccination associated axillary lymphadenopathy in our breast unit, such incidence was observed in patients undergoing breast examination such as breast ultrasonography (US) after vaccination for screening and diagnosis of breast cancer. This had led us to conduct a descriptive observational retrospective case series analysis in the UK. Our primary outcome was describing the US findings of lymphadenopathy among vaccinated candidates. Our secondary outcomes were to evaluate other associations, such as: breast cancer or disease, vaccine type, injection side, the need of biopsy and further follow-up imaging.
During our study period over 31 days, from 16 March 2021 to 16 April 2021, we have encountered three cases, all had received the AstraZeneca vaccine on the same side of the adenopathy. Two cases were after the first dose and one after both the first and second doses. One was found on screening mammography subsequently diagnosed with high-grade ductal carcinoma in situ (DCIS), one presented with an axillary lump, and another with axillary pain. Axillary US in two cases showed a uniform cortical thickening, and one showed an eccentrically thickened cortex required a biopsy.
Case Number 1
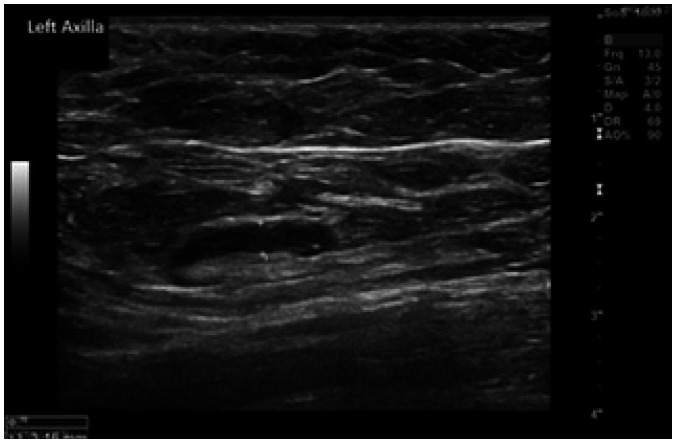
A 72-year-old female presented with a left sided breast and axillary pain 5 days after receiving the first dose of AstraZeneca vaccine. Axillary US showed a lymph node with an intact fatty hilum, but with a uniformly thickened nodal cortex measuring > 3 mm (Fig. 1). This was deemed indeterminate and therefore a follow-up US was performed 23 days later demonstrating that the cortical thickness of the lymph node had reduced and measured less than 3 mm and was considered to be normal, therefore no further follow-up was needed. Her mammogram was normal.
Fig. 1. Case no 1. A 72 years old female presented with a left sided breast and axillary pain 5 days after receiving the first dose of AstraZeneca vaccine.
Axillary ultrasonography showed a lymph node with an intact fatty hilum, but with a uniformly thickened nodal cortex measuring > 3 mm. This was deemed indeterminate in appearance and therefore a follow up ultrasound scan was performed.
Case Number 2
A 61-year-old female noticed a left axillary swelling, associated with left sided shoulder and arm pain before vaccination with AstraZeneca. Axillary US was performed at 27 days after vaccination demonstrated a single lymph node with an intact fatty hilum and a uniform cortex. The cortex measured less than 3 mm and considered normal. Her mammogram was normal. No follow-up was needed.
Case Number 3
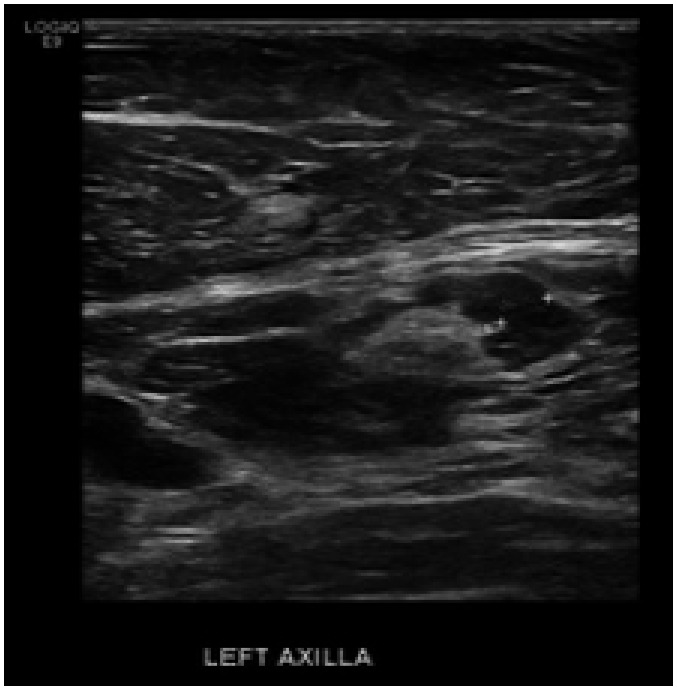
A 61-year-old female was found to have suspicious calcifications on screening mammography, subsequently diagnosed as high-grade DCIS. She had her first dose of AstraZeneca vaccine 79 days prior to screening and her second dose the day before. All on the left arm. US of the left axilla showed two lymph nodes that whilst the fatty hilum was intact the cortex was thickened and measured > 3 mm. One of the lymph nodes had an eccentrically thickened cortex that measured 4.9 mm (Fig. 2). The US was graded indeterminate, and in view of the extensive malignant appearing calcification, a biopsy was undertaken rather than follow-up US. Biopsy confirmed benign reactive changes and hence no follow-up imaging was requested.
Fig. 2. Case no. 3. A 61 years old female, who had her first and second dose of AstraZeneca vaccine, (79 days) and (one day) prior to examination.
Ultrasonography of the left axilla showed two mildly enlarged lymph nodes, one has an eccentrically thickened cortex that measured 4.9 mm. Biopsy confirmed benign reactive changes.
Conventional US is the imaging modality of choice for the assessment of the axillary lymph nodes, including in women with a previous breast cancer [8,9]. On US, a normal lymph node appears generally ovoid in shape, with a uniform, thin peripheral cortex and a fatty hyperechoic hilum, while in a malignant lymph node, the node may become rounded, the fatty hilum is replaced and the cortex may display either a cortical bulge or appear uniformly thickened. Such structural changes are named axillary adenopathy [10,11]. Axillary lymph nodes morphological changes can be related to benign or inflammatory conditions or malignancies. In case of radiological suspicion, US-guided core biopsy or fine needle aspiration biopsy are performed [8].
Since axillary adenopathy in women without a previous history of breast cancer is a rare, reported as 0.02%–0.04% in the literature [9] and malignant axillary adenopathy are more detected during the follow-up of breast cancer with an estimated prevalence from 0.8% up to 4% [12,13,14], finding of an axillary lymphadenopathy in vaccinated patients can cause uncertainty. On the light of this we recommend the following:
- Vaccination should be advised on the opposite side arm of the previous cancer.
- Short term imaging follow-up (e.g., in 6 weeks as per our unit’s guidelines or in 4–12 weeks after the second dose of vaccination as per SBI recommendations [3]) to ensure resolution and nodal sampling in case of no resolution.
- Accurate documentation at the time of breast examination such as mammography or US detailing vaccination date, type of vaccine and the side of injection.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
References
- 1.Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28:1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 2.Lane DL, Neelapu SS, Xu G, Weaver O. COVID-19 vaccine-related axillary and cervical lymphadenopathy in patients with current or prior breast cancer and other malignancies: cross-sectional imaging findings on MRI, CT, and PET-CT. Korean J Radiol. 2021;22:1938–1945. doi: 10.3348/kjr.2021.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm L, Destounis S, Dogan B, Nicholson B, Dontchos B, Sonnenblick E, et al. SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. Reston: Society of Breast Imaging; 1891. [Google Scholar]
- 4.Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine's local reactions, systemic reactions, adverse events, and serious adverse events. cdc.gov Web site. [Accessed January 16, 2021]. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html .
- 5.Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. cdc.gov Web site. [Accessed January 16, 2021]. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html .
- 6.Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300:E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, et al. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Royal College of Radiologists. Guidance on screening and symptomatic breast imaging, fourth edition. rcr.ac.uk Web site. [Accessed March 23, 2021]. https://www.rcr.ac.uk/publication/guidance-screening-and-symptomatic-breast-imaging-fourth-edition .
- 9.Lee SH, Yi A, Jang MJ, Chang JM, Cho N, Moon WK. Supplemental screening breast US in women with negative mammographic findings: effect of routine axillary scanning. Radiology. 2018;286:830–837. doi: 10.1148/radiol.2017171218. [DOI] [PubMed] [Google Scholar]
- 10.Lernevall A. Imaging of axillary lymph nodes. Acta Oncol. 2000;39:277–281. doi: 10.1080/028418600750013014. [DOI] [PubMed] [Google Scholar]
- 11.Dialani V, James DF, Slanetz PJ. A practical approach to imaging the axilla. Insights Imaging. 2015;6:217–229. doi: 10.1007/s13244-014-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogiya A, Kimura K, Nakashima E, Sakai T, Miyagi Y, Iijima K, et al. Long-term prognoses and outcomes of axillary lymph node recurrence in 2,578 sentinel lymph node-negative patients for whom axillary lymph node dissection was omitted: results from one Japanese hospital. Breast Cancer. 2016;23:318–322. doi: 10.1007/s12282-014-0576-5. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Anderson S, Fisher ER, Redmond C, Wickerham DL, Wolmark N, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet. 1991;338:327–331. doi: 10.1016/0140-6736(91)90475-5. [DOI] [PubMed] [Google Scholar]
- 14.Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE, Jr, Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]