Abstract
Breast radiologists are increasingly seeing patients with axillary adenopathy related to COVID-19 vaccination. Vaccination can cause levels I–III axillary as well as cervical lymphadenopathy. Appropriate management of vaccine-related adenopathy may vary depending on clinical context. In patients with current or past history of malignancy, vaccine-related adenopathy can be indistinguishable from nodal metastasis. This article presents imaging findings of oncology patients with adenopathy seen in the axilla or neck on cross-sectional imaging (breast MRI, CT, or PET-CT) after COVID-19 vaccination. Management approach and rationale is discussed, along with consideration on strategies to minimize false positives in vaccinated cancer patients. Time interval between vaccination and adenopathy seen on breast MRI, CT, or PET-CT is also reported.
Keywords: COVID-19, Vaccine, Lymphadenopathy, Breast cancer, Breast MRI, CT, PET-CT
INTRODUCTION
Since the first COVID-19 vaccine was issued emergency use authorization by the U.S. Food and Drug Administration [1] and COVID-19 vaccination in the United States began in December, 2020, COVID-19 vaccine-related axillary and cervical adenopathy has been reported on the side of inoculation within 2–4 days after vaccination [2,3]. After vaccination, migration of antigens from the injection site to the draining nodes can result in lymph node enlargement [4]. The median duration of adenopathy reported after Moderna vaccine (mRNA-1273) administration is 1–2 days and approximately 10 days duration after Pfizer vaccine (BNT162b2) administration [2,3].
COVID-19 vaccination-induced lymphadenopathy is increasingly seen on breast imaging; management can be confounded by current or past cancer history. Management of vaccine-related adenopathy detected on breast MRI or other cross-sectional imaging currently varies across radiology practices. The management algorithm for adenopathy seen after recent COVID-19 vaccination in a high risk or known breast cancer patient might differ from an average risk patient with adenopathy seen on an otherwise benign-appearing screening mammogram. As the population continues to be vaccinated in larger numbers, adenopathy caused by COVID-19 vaccination will be increasingly seen by breast radiologists and could result in screening callbacks, additional workups, and false positive biopsies. In this article, we describe six patients with current or prior cancer history who had adenopathy seen on breast MRI, CT, or PET-CT after COVID-19 vaccination; the management approach used for each patient is discussed.
Clinical Scenarios and Patient Management
Breast Cancer Staging
Accurate locoregional staging of breast cancer includes status of the axillary, internal mammary, and supraclavicular lymph nodes. Abnormal imaging appearance of the regional lymph nodes, particularly if ipsilateral to known breast cancer, can indicate metastatic nodal involvement. If COVID-19 vaccination is performed in the arm ipsilateral to known breast cancer, reactive vaccine-related adenopathy can be seen on imaging and be indistinguishable from metastasis. Management decisions for adenopathy seen in patients with concurrent cancer and regional adenopathy after vaccination should take into account the likelihood of nodal metastasis based on primary tumor histology and stage, expected lymphatic drainage patterns, laterality of adenopathy compared to malignancy, and time since vaccination [4]. A clinical need for timely definitive diagnosis might sway management toward biopsy [4]. Patients 1–3 illustrate our management of regional adenopathy in patients who have both current breast cancer and recent COVID-19 vaccination.
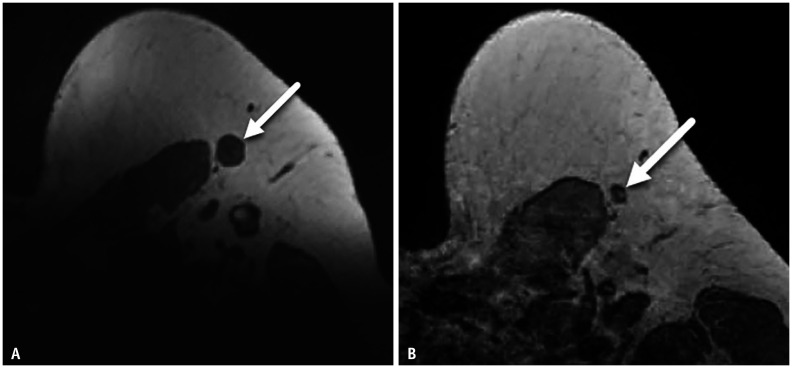
Patient 1 is a 44-year-old female with current left breast cancer (high grade solid and cribriform ductal carcinoma in situ with comedonecrosis). Staging breast MRI shows two ipsilateral nonpalpable enlarged left axillary nodes, asymmetric when compared to the right (Fig. 1). Patient received 1st dose Pfizer COVID-19 vaccination in left arm 4 days prior to MRI. So that appropriate oncologic treatment could be initiated, ultrasound-guided biopsy was performed. Biopsy yielded benign findings with no evidence of nodal metastasis.
Fig. 1. A-44-year-old female with left breast cancer.
Contrast-enhanced T1-weighted, fat-saturated axial MR image performed 4 days after COVID-19 vaccination shows two enlarged round nodes (arrows) with no visible hilum in the left axilla, asymmetric when compared to a normal-appearing right axillary node.
Patient 2 is a 47-year-old female who underwent elective bilateral breast reduction surgery. Invasive and in situ ductal right breast cancer was incidentally found at surgical pathology, along with benign left breast findings. Postoperative breast MRI to evaluate for residual disease 3 months post-surgery showed enlarged left axillary lymph node (Fig. 2A). No residual malignancy was detected by breast MRI. Patient provided history of 1st dose Moderna COVID-19 vaccination in left arm 9 days prior to MRI. The enlarged node had normal benign appearance on MRI performed 54 days earlier (Fig. 2B). Interval enlargement of the left axillary node was attributed to recent COVID-19 vaccination, particularly since it was contralateral to the known right breast cancer and right axillary sentinel node biopsy was negative. No biopsy or follow-up was performed. The patient underwent subsequent right breast mastectomy.
Fig. 2. A-47-year-old female with right breast cancer.
A, B. Axial T1-weighted MR image performed after recent COVID-19 vaccination shows enlarged left axillary node (arrow, A). The same node had a normal appearance prior to vaccine administration with visible fatty hilum on axial T1-weighted MR image obtained 54 days earlier (arrow, B).
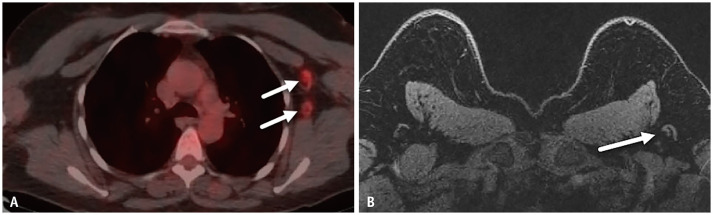
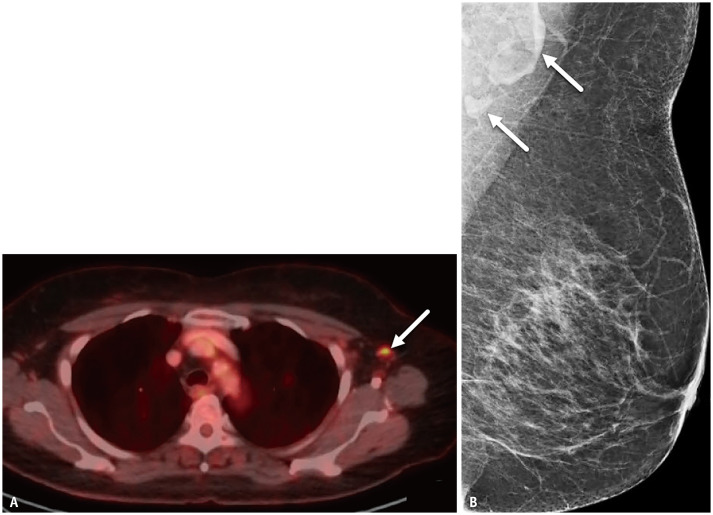
Patient 3 is a 50-year-old female with recent diagnosis of T3N0M0 right breast cancer. PET-CT showed contralateral left axillary nodes with increased fluorodeoxyglucose (FDG) uptake (Fig. 3A). Patient had received 2nd dose Pfizer COVID-19 vaccination 3 days prior to PET-CT and 1st dose 24 days prior to PET-CT. Left axillary nodes on breast MRI performed 13 days prior to 1st vaccination dose appeared normal (Fig. 3B). No palpable adenopathy was present. Given contralateral location to node-negative right breast malignancy, as well as congruent history of recent vaccination, the left axillary nodes seen on PET-CT were presumed reactive to COVID-19 vaccination and no further action was required.
Fig. 3. A-50-year-old female with newly-diagnosed T3N0M0 right breast cancer.
A. Axial fused PET-CT image performed 3 days after 2nd dose COVID-19 vaccination shows left axillary nodes (arrows) with increased fluorodeoxyglucose uptake. B. Breast MRI performed for breast cancer 13 days prior to 1st vaccination dose demonstrates normal-appearing left axillary nodes (arrow).
Imaging Surveillance in Patients with History of Cancer
In patients with remote history of cancer undergoing imaging surveillance, vaccine-related adenopathy can confound the clinical picture and raise concern for possible recurrence. Management of adenopathy in patients with a history of cancer should consider the probability of nodal metastasis, based on original cancer diagnosis (type and location of primary malignancy, expected recurrence patterns) and presence of other suspicious imaging findings or suspicious clinical findings [4,5]. Close correlation with vaccination details is required. Management might be guided in some cases by level of patient concern, particularly in a patient with prior cancer diagnosis and high level of anxiety regarding new adenopathy. If a patient is thought to be low risk for nodal disease, discussion with the patient to reassure them that adenopathy is an expected result after vaccination might be helpful. Appropriate management of a patient in cancer imaging surveillance may differ in symptomatic versus non-symptomatic patients, as illustrated by Patients 4–6.
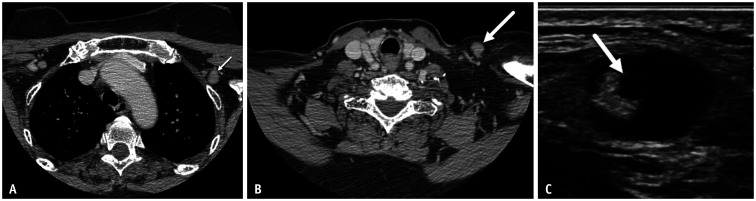
Patient 4 is a 70-year-old female with history of right breast stage IIIC, T2N3M0 invasive ductal cancer status post bilateral mastectomy. CT scan neck performed to evaluate left neck pain and palpable abnormality showed enlarged 1.3 cm left supraclavicular lymph node and enlarged left axillary nodes (Fig. 4), palpable on same day physical examination. The patient received Dose 1 and Dose 2, left arm mRNA COVID-19 vaccination 26 days and 5 days, respectively, prior to CT scan. Due to palpable nature of dominant left supraclavicular node, advanced stage at time of original diagnosis, and high level of patient concern, ultrasound-guided biopsy was performed yielding benign findings with no metastatic carcinoma.
Fig. 4. A-70-year-old female with history of right breast cancer status post bilateral mastectomy.
A, B. Axial post-contrast CT images performed 5 days after 2nd vaccine dose show adenopathy in the left level II axilla (arrow, A) and left supraclavicular region (arrow, B). C. Ultrasound shows the palpable left supraclavicular node with focal cortical bulge (arrow) and effacement of fatty hilum.
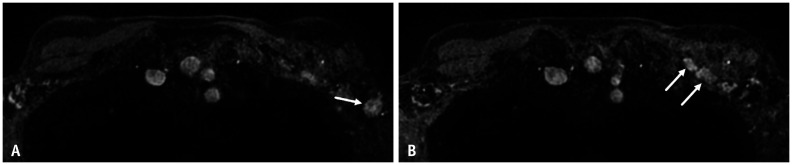
Patient 5 is a 55-year-old female with history of T1N0M0, grade 2 invasive left breast cancer treated with breast conservation. Surveillance breast MRI in this asymptomatic patient (Fig. 5) showed numerous enlarged nodes in the levels I-III left axilla, but no suspicious breast lesion. The patient had received 2nd dose Pfizer vaccination in the left arm 2 days prior to MRI. Adenopathy was not clinically palpable. Due to lack of associated suspicious imaging or clinical finding, small node-negative tumor at time of original diagnosis, and recent COVID-19 vaccination, benign etiology was favored and ultrasound follow-up was recommended in 8–12 weeks to document resolution of adenopathy.
Fig. 5. A-55-year-old female with history of left breast cancer treated with breast conservation.
A, B. Contrast-enhanced T1-weighted, fat-saturated axial MR images performed 2 days after 2nd dose COVID-19 vaccination show new left axillary adenopathy involving level I axilla (arrow, A) and extending to the level II–III axilla (arrows, B). No suspicious lesion was identified within the breast.
Patient 6 is a 76-year-old female with prior history of right axillary grade 1 follicular lymphoma in remission and no evidence of disease on routine surveillance PET-CT for past 12 years. Recent PET-CT showed new increased FDG uptake in normal sized left axillary nodes with max standardized uptake value 4.5 (Fig. 6). No other suspicious findings or FDG-avid adenopathy to indicate active lymphoma. Same day screening mammogram showed stable normal axillary nodes. Physical exam revealed no palpable adenopathy. The patient had received 1st dose Moderna vaccine in left arm 15 days prior to PET-CT. Considering no other suspicious imaging or clinical finding, lengthy time of remission, and coincidental recent COVID-19 vaccination, the left axillary nodes were presumed reactive to vaccination and the patient was scheduled to return in 1 year for annual surveillance imaging.
Fig. 6. A-76-year-old female with history of lymphoma.
A. Axial fused PET-CT image performed 15 days after 1st dose vaccine demonstrates normal sized left axillary node with increased fluorodeoxyglucose uptake (arrow). B. Left axillary nodes (arrows) appear normal in morphology and stable since prior mammography.
DISCUSSION
Given the prevalence of COVID-19 vaccine-related axillary adenopathy already encountered by breast radiologists, there is discussion within the breast imaging community regarding the potential for excessive callbacks or unnecessary biopsies. The Society of Breast Imaging guidelines issued in January, 2021, suggested that unilateral axillary adenopathy seen on screening mammogram be issued a Breast Imaging-Reporting and Data System (BI-RADS) 0 report to allow callback for further assessment of the ipsilateral breast and axilla [6]. More recent literature reveals that some centers consider recent COVID-19 vaccination a known inflammatory cause of unilateral axillary adenopathy and therefore recommend a benign assessment (BI-RADS category 2), if adenopathy is ipsilateral to the site of recent COVID-19 vaccination and not palpable [5]. Management recommendations vary by institution, although recommendations have been recently published that offer practical algorithms for management of adenopathy seen after COVID-19 vaccination [4,5].
Management can be confounded by a concurrent or past cancer diagnosis; vaccine-related adenopathy in such patients can be indistinguishable from nodal metastasis. It is critical to correlate with thorough clinical history and vaccination details. When patients with current or prior history of breast cancer receive COVID-19 vaccination, we recommend that injection be given in the arm contralateral to the malignancy; this helps avoid unnecessary biopsy of a benign vaccine-related reactive lymph node which may overlap in imaging appearance with a metastatic node if ipsilateral to the breast cancer. Although ideal to schedule imaging exams prior to the first injection as this would eliminate vaccine-related findings, this is not always feasible due to scheduling constraints. Delay of screening or surveillance until at least 6 weeks after the 2nd dose may help minimize false positive findings; however, breast screening has already been delayed due to the ongoing pandemic. During the pandemic, the utilization of screening mammography dropped 99% and the number of new diagnosed cancers decreased; delayed diagnosis of these cancers could have detrimental impact on their prognosis [7]. Moreover, patients with a breast complaint require timely diagnostic workup and cannot be safely deferred. Physical exam should evaluate for palpable adenopathy or other suspicious clinical findings. Lehman et al. [8] suggest an approach for management of vaccine-related adenopathy based on clinical presentation, which encourages breast imaging regardless of vaccination status and acknowledges that in appropriate clinical scenarios, unilateral axillary adenopathy can be safely recognized as a benign finding after COVID-19 vaccination. To mitigate false positive nodal findings after COVID-19 vaccination, history of COVID-19 vaccination, number of doses and dates, along with site and laterality of injection should be documented at patient intake.
Patients who have concurrent breast cancer and ipsilateral indeterminate node may require nodal biopsy for accurate staging and treatment plan; the need to initiate oncologic treatment does not allow time to assess resolution of possible vaccine-related adenopathy, so we recognize that some false positive biopsies will be inevitable in these vaccinated patients. Biopsy in Patient 1 who had asymptomatic round lymph nodes was required due to ipsilateral breast cancer; the need to initiate oncologic treatment did not allow time for imaging follow-up. Even though adenopathy in Patient 4 was contralateral to the patient’s original breast malignancy, biopsy was pursued due to palpable nature of adenopathy and history of stage IIIC breast cancer. Contralateral nodal breast cancer metastases have rarely been reported [9,10], and therefore if patients have history of more aggressive, higher stage, or locally advanced breast cancer, a lower threshold to biopsy an indeterminate node may be appropriate, even if contralateral to the original malignancy. In contrast, Patients 2 and 3 were considered low risk for nodal metastasis due to negative axillary lymph node status on the side ipsilateral to primary malignancy; the contralateral lymph nodes could therefore be safely assumed benign without biopsy.
Vaccination has been cited as a potential cause of false-positive results in FDG PET-CT imaging, previously reported after vaccination against influenza, H1N1, and human papillomavirus [11]. An early case report of FDG uptake in axillary nodes after COVID-19 vaccination described a patient with history of breast cancer and moderately increased FDG uptake in normal-sized axillary nodes 10 days after COVID-19 vaccination [12]. A second case report described a new cluster of subcentimeter axillary nodes with FDG uptake 2 days after COVID-19 vaccination [13]. Our Patient 6 demonstrates similar findings of increased FDG uptake in normal sized axillary nodes 15 days after ipsilateral injection of 1st dose Moderna vaccine. While there are now multiple studies describing COVID-19 vaccination-related adenopathy on FDG PET-CT [14,15], the duration of vaccine-related FDG-avid nodal uptake remains not clearly defined. FDG nodal uptake has been previously reported up to 7 days after influenza vaccination and 1–14 days after H1N1 vaccination [11,16,17]. However, abnormal nodal appearance may persist longer on FDG-PET after COVID-19 vaccination, even in nonenlarged nodes [4]. The current mRNA vaccines might require longer time for resolution of adenopathy compared to other vaccines [18]. One study reports that 29% mRNA vaccine recipients have persistent FDG uptake between 7 to 10 weeks after second dose [19].
COVID-19 vaccines may cause a higher incidence of axillary adenopathy on breast MRI than other vaccines. Although little data exists regarding duration of vaccine-related adenopathy on breast MRI, it has been suggested that the time course of adenopathy seen on breast MRI might mirror the duration of increased nodal uptake seen on FDG PET-CT [20]. A published study regarding unilateral axillary adenopathy caused by COVID-19 vaccination and seen on breast MRI [20] described two patients with axillary adenopathy 13 days and 16 days after vaccination, respectively. The authors suggest that COVID-19 vaccine-related adenopathy visible on breast MRI may occur as early as 1–2 days after injection [20]. Indeed, review of our patients with known or prior history of breast cancer agrees that early onset of adenopathy can be seen on breast MRI after COVID-19 vaccination. Patient 1 showed axillary adenopathy on breast MRI only 4 days after receiving the 1st dose of Pfizer vaccine. Patient 5 had adenopathy on MRI 2 days after 2nd vaccine dose. Consistent with previous reports [20,21], we have also observed that vaccine-related adenopathy seen on breast MRI can involve multiple levels of the axilla (seen in Patient 5) and can be more extensive than that seen on mammography, which only visualizes the level I axilla. COVID-19 vaccination can also cause cervical adenopathy on the side ipsilateral to injection, as demonstrated in Patient 4, who had adenopathy involving the level II axilla and supraclavicular region seen on neck CT.
In summary, as the population becomes increasingly vaccinated, more incidental axillary and cervical adenopathy is anticipated on imaging studies. Radiologist and referring clinician awareness is critical to avoid a large number of false positive biopsies. Axillary or cervical adenopathy after recent COVID-19 vaccination should be interpreted with knowledge of COVID-19 vaccination details and in the context of patient’s risk for nodal metastasis, potential limitations of the imaging modality, and impact on clinical treatment plans. In general, we favor a conservative management plan if possible, but recognize that false positive nodal biopsy may be inevitable in some breast cancer patients. Correlation with injection site and pathology of known malignancy may help minimize false positives. Further study regarding duration of COVID-19 related adenopathy is needed, and as it becomes available over the next months, timing guidelines for follow-up may be amended. Documentation of COVID-19 information will prove important for other radiology subspecialties as well, as they read cross-sectional imaging of studies that include the axilla and neck, where vaccine-related adenopathy may be seen.
Footnotes
Conflicts of Interest: Dr. Neelapu received grants and personal fees from Kite/Gilead, personal fees from Celgene/BMS, grants and personal fees from Adicet Bio, grants and personal fees from Precision Biosciences, grants and personal fees from Allogene, personal fees from Kuur Therapeutics, personal fees from Novartis, personal fees from Bluebird Bio, grants and personal fees from Merck, grants from Poseida, grants from Cellectis, grants from Karus, personal fees from Legend Biotech, personal fees from Incyte, personal fees from Calibr, however all of these have no concerning conflicts of interest with the submitted work. Other authors have no conflicts of interest to disclose.
- Conceptualization: all authors.
- Resources: all authors.
- Supervision: Deanna L Lane.
- Writing—original draft: Deanna L Lane.
- Writing—review & editing: all authors.
Funding Statement: None
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.US Food & Drug Adminstration. FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. FDA.gov Web site. [Accessed February 10, 2021]. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 .
- 2.Centers for Disease Control and Prevention. The moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. CDC.gov Web site. [Accessed February 10, 2021]. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html .
- 3.Centers for Disease Control and Prevention. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. CDC.gov Web site. [Accessed February 10, 2021]. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html .
- 4.Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KN, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;300:E323–E327. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman CD, D’Alessandro HA, Mendoza DP, Succi MD, Kambadakone A, Lamb LR. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol. 2021;18:843–852. doi: 10.1016/j.jacr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm L, Destounis S, Dogan B, Nicholson B, Dontchos B, Sonnenblick E, et al. SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. SBI-online.org Web site. [Accessed February 10, 2021]. https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf .
- 7.Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59:1–11. doi: 10.1016/j.rcl.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman CD, Lamb LR, D'Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am J Roentgenol. 2021;217:584–586. doi: 10.2214/AJR.21.25688. [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Martínez Y, Acevedo-Bañez I, De-Bonilla-Damiá Á, Fernández-Rodríguez P, Sousa JM, Jiménez-Hoyuela García JM. Contralateral axillary lymph node metastasis in a patient with relapsed breast cancer: locoregional event or distant metastasis disease? Oncol Res Treat. 2021;44:128–131. doi: 10.1159/000513661. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Zhang Y, Sun X. Therapeutic options for contralateral axillary lymph node metastasis in breast cancer. Curr Probl Cancer. 2021 Jan; doi: 10.1016/j.currproblcancer.2020.100706. [Epub] [DOI] [PubMed] [Google Scholar]
- 11.Katal S, Pouraryan A, Gholamrezanezhad A. COVID-19 vaccine is here: practical considerations for clinical imaging applications. Clin Imaging. 2021;76:38–41. doi: 10.1016/j.clinimag.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eifer M, Eshet Y. Imaging of COVID-19 vaccination at FDG PET/CT. Radiology. 2021;299:E248. doi: 10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G, Lu Y. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med. 2021;46:353–354. doi: 10.1097/RLU.0000000000003597. [DOI] [PubMed] [Google Scholar]
- 14.Orevi M, Chicheportiche A, Ben-Haim S. Lessons learned from post-COVID-19 vaccination PET/CT studies. J Nucl Med. 2021 Jul; doi: 10.2967/jnumed.121.262348. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter UJ, Maurer A, et al. [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2021 Jun; doi: 10.1007/s00330-021-08122-2. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26:248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 17.Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36:848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol. 2021;217:975–983. doi: 10.2214/AJR.21.25728. [DOI] [PubMed] [Google Scholar]
- 19.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;300:E345–E347. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021;217:831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 21.Plaza MJ, Wright J, Fernandez S. COVID-19 vaccine-related unilateral axillary lymphadenopathy: pattern on screening breast MRI allowing for a benign assessment. Clin Imaging. 2021;80:139–141. doi: 10.1016/j.clinimag.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.