Take-home points
• The emergence of AI-based fully automated methods for the CT-based body composition analysis could revolutionize how this information is utilized.
• The true potential value of automated CT-based body composition analysis lies in its ability to identify patients at greatest risk for downstream adverse clinical events.
• Broad validation and widespread clinical implementation could add substantial value to existing patient care.
Cross-sectional body imaging can provide valuable objective data on internal tissues and organs. In particular, CT scans can quantify bone mineral density, visceral and subcutaneous fat, skeletal muscle, liver fat, and arterial vascular calcification, amongst other organ-based assessments [1,2]. When these body composition measures are incidental to the clinical indication for imaging, their consideration has been referred to as “opportunistic screening” [3]. To date, the labor-intensive nature of manual (or even semi-automated) body composition measurements has largely prevented their translation from the research realm to routine clinical practice or large-scale population health. However, the emergence of fully-automated artificial intelligence (AI)-based approaches has now paved the way for both population-based studies and efficient prospective clinical reporting [2,4,5]. The initial results to date for predicting downstream adverse clinical events based on automated body composition analysis are very encouraging [6,7,8,9]. The convergence of “explainable” AI solutions with robust predictive value along with the generally high CT utilization rates now holds great promise for improved pre-symptomatic detection of patients at unsuspected cardiometabolic risk.
A host of non-invasive approaches other than cross-sectional imaging exist for estimating body fat, including body mass index (BMI), hydrostatic densitometry, air displacement plethysmography, bioelectrical impedance analysis, and dual-energy X-ray absorptiometry (DXA) [10]. However, the importance of the relative distribution of adipose tissue, as well as ectopic fat in the liver (hepatic steatosis) and skeletal muscle (myosteatosis) elevate the status of cross-sectional imaging with CT or MR over these other techniques. To more fully extend the focus of body composition analysis beyond limited fat-based concerns and include vascular calcium load and bone mineral density assessment, CT becomes the clear comprehensive modality of choice [1,2].
Although abdominal CT is an ideal tool for objective non-invasive assessment of internal organs and tissues, in current practice nearly all of this valuable data is either completely ignored or only subjectively noted (eg, the presence of calcific aortic plaques). A number of manual measures utilizing regions-of-interest (ROI) to assess mean attenuation (in Hounsfield unit [HU]) have been around for many years, including L1-level trabecular bone for osteoporosis [3,11] and liver assessment for non-alcoholic fatty liver disease (NAFLD) [12,13]. More recently, it has been shown that liver HU at unenhanced CT correlates with the MR-based proton density fat fraction (PDFF) [14,15]. CT-based skeletal muscle assessment for sarcopenia includes both low density (myosteatosis) and low bulk (myopenia) measures [16]. In general, HU assessment for intra- and inter-muscular adipose tissue appears to be the more valuable measure, and is also easier to assess manually. Manual and semi-automated quantification of visceral and subcutaneous fat has also been in existence for many years (for both CT and MR) [17,18]. In particular, we have found the visceral-to-subcutaneous fat ratio to be a particularly useful singular measure [19]. Semi-automated quantification of abdominal aortic calcification using a coronary calcium scoring tool is feasible [20], but somewhat arduous and seldom used in clinical practice. As noted above, although analogous fat, liver, and muscle quantification is possible with MR, a more comprehensive cardiometabolic evaluation that considers osteoporosis and atherosclerosis favors the use of CT (Fig. 1). Furthermore, overall abdominal CT volumes continue to dwarf MR numbers [21].
Fig. 1. Fully automated CT-based body composition analysis in a 93-year-old female with history of both colon and breast cancer.
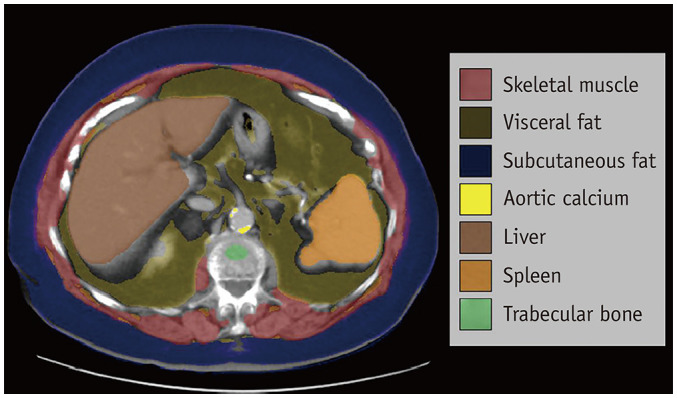
Post-contrast CT image at the L1 vertebral level demonstrates automated segmentation and display of skeletal muscle (red), visceral fat (amber), subcutaneous fat (blue), aortic calcium (bright yellow), liver (brown), spleen (orange), and trabecular bone (green). These all represent examples of “explainable artificial intelligence” that can be visually confirmed and compared against analogous manual measures, if desired.
The emergence of fully automated methods for the CT-based body composition analysis described above could revolutionize how this information is utilized [19,22,23,24,25]. Importantly, AI-based solutions that are “explainable” and analogous to previously validated manual and semi-automated approaches should be more acceptable to patients and the wider medical community (including payers) over more complex “black box” approaches that cannot be easily verified. For example, automated single-slice segmentation at the L1 or L3 level for bone, fat, and muscle analysis is more reproducible and can be readily confirmed for quality control purposes (Fig. 1). Appropriate multi-slice segmentation for aortic calcification or whole-organ analysis can also be visually verified by the radiologist. Examples of additional fully automated algorithms that further broaden the scope of CT-based analysis include abdominal organ volumetry (such as liver and spleen), detection of urolithiasis and volumetric stone burden, liver fibrosis staging, and focal lesion detection (such as fractures and tumors) [26,27]. Of course, robust computer-aided detection (CAD) of colorectal polyps at CT colonography has existed for many years [28].
The true potential value of automated CT-based body composition analysis lies in its ability to identify patients at greatest risk for downstream adverse clinical events. Our initial single-center investigations have demonstrated that CT-based measures can equal or outperform the current multivariable clinical reference standards for predicting future osteoporotic fractures, major cardiovascular events, and death [6,7]. More sophisticated models that also incorporate relevant demographic factors will further enhance CT-based performance, perhaps generating a “biological age” that may belie a patient's actual chronological age [29]. Opportunistic use of automated body composition analysis would leverage the unused data embedded in the many millions of body CT scans performed each year throughout the world. Ultimately, if the overall benefit related to these combined measures is great enough, a case could be made for “intended” CT screening, whereby adults undergo a virtual physical exam at a certain age. Such screening could also be combined with the already accepted CT screening indications for colorectal and lung cancer.
To address the enormous clinical potential of CT-based body composition analysis, as well as remaining issues prior to widespread implementation, we have gathered together a group of interested investigators to form the “Opportunistic Screening Consortium in Abdominal Radiology” or OSCAR (Fig. 2). This multi-center effort seeks to establish a generalizable, vendor-neutral automated body composition solution for clinical translation. A large retrospective CT trial will seek to fully characterize the normal distribution of automated bone, muscle, fat, liver, and aortic calcium measures according to patient age, sex, race/ethnicity, and socioeconomic status, as well as assess generalizability among different CT vendors and diverse scanning protocols. Future investigations may incorporate federated learning to improve generalizability across multi-national patient populations [30]. We will also further assess the ability of automated CT body composition analysis for predicting subsequent adverse clinical events and outcomes.
Fig. 2. The OSCAR.

OSCAR represents a group of investigators interested in image-based body composition analysis. In particular, a multi-center trial will seek to develop and validate a process for applying CT-based opportunistic cardiometabolic screening utilizing fully automated body composition tools. This large-scale effort aims to address variations related to patient demographics and different technical environments, as well as explore the prognostic value of the combined body composition measures for predicting future adverse events. The ultimate goal is to provide a generalizable, vendor-neutral CT solution that can translate to routine clinical use and add substantial value to patient care without the need for additional patient time or dose exposure. OSCAR = Opportunistic Screening Consortium in Abdominal Radiology
In summary, fully automated CT-based body composition analysis is now entering an exciting phase of investigation. Broad validation and widespread clinical implementation could add substantial value to existing patient care, without the need for additional patient time or dose exposure. Specifically, pre-symptomatic identification of patients at greatest cardiometabolic risk could translate into improved health care outcomes if appropriate interventions are implemented.
Footnotes
Conflicts of Interest: Dr. Pickhardt serves or has recently served as an advisor to Bracco, Zebra, and GE Healthcare; and is a shareholder in SHINE, Elucent, and Cellectar. Dr. Summers receives royalties from iCAD, PingAn, Philips and ScanMed and research support from PingAn and NVIDIA.
- Conceptualization: Perry J. Pickhardt.
- Writing—original draft: Perry J. Pickhardt.
- Writing—review & editing: all authors.
Funding Statement: None
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.Pickhardt PJ. Value-Added Opportunistic CT Screening: State of the Art. Radiology. (in press) [Google Scholar]
- 2.Pickhardt PJ, Graffy PM, Perez AA, Lubner MG, Elton DC, Summers RM. Opportunistic screening at abdominal CT: use of automated body composition biomarkers for added cardiometabolic value. Radiographics. 2021;41:524–542. doi: 10.1148/rg.2021200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magudia K, Bridge CP, Bay CP, Babic A, Fintelmann FJ, Troschel FM, et al. Population-scale CT-based body composition analysis of a large outpatient population using deep learning to derive age-, sex-, and race-specific reference curves. Radiology. 2021;298:319–329. doi: 10.1148/radiol.2020201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. 2019;290:669–679. doi: 10.1148/radiol.2018181432. [DOI] [PubMed] [Google Scholar]
- 6.Pickhardt PJ, Graffy PM, Zea R, Lee SJ, Liu J, Sandfort V, et al. Automated abdominal CT imaging biomarkers for opportunistic prediction of future major osteoporotic fractures in asymptomatic adults. Radiology. 2020;297:64–72. doi: 10.1148/radiol.2020200466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Graffy PM, Zea R, Lee SJ, Liu J, Sandfort V, et al. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: a retrospective cohort study. Lancet Digit Health. 2020;2:e192–e200. doi: 10.1016/S2589-7500(20)30025-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan N, Elnekave E, Barda N, Bregman-Amitai O, Bar A, Orlovsky M, et al. Automated opportunistic osteoporotic fracture risk assessment using computed tomography scans to aid in FRAX underutilization. Nat Med. 2020;26:77–82. doi: 10.1038/s41591-019-0720-z. [DOI] [PubMed] [Google Scholar]
- 9.Stemmer A, Shadmi R, Bregman-Amitai O, Chettrit D, Blagev D, Orlovsky M, et al. Using machine learning algorithms to review computed tomography scans and assess risk for cardiovascular disease: retrospective analysis from the National Lung Screening Trial (NLST) PLoS One. 2020;15:e0236021. doi: 10.1371/journal.pone.0236021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018;66:1–9. doi: 10.1136/jim-2018-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang S, Graffy PM, Ziemlewicz TJ, Lee SJ, Summers RM, Pickhardt PJ. Opportunistic osteoporosis screening at routine abdominal and thoracic CT: normative L1 trabecular attenuation values in more than 20000 adults. Radiology. 2019;291:360–367. doi: 10.1148/radiol.2019181648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol. 2010;194:623–628. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 13.Pickhardt PJ, Hahn L, Muñoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202:752–758. doi: 10.2214/AJR.13.11367. [DOI] [PubMed] [Google Scholar]
- 14.Guo Z, Blake GM, Li K, Liang W, Zhang W, Zhang Y, et al. Liver fat content measurement with quantitative CT validated against MRI proton density fat fraction: a prospective study of 400 healthy volunteers. Radiology. 2020;294:89–97. doi: 10.1148/radiol.2019190467. [DOI] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Graffy PM, Reeder SB, Hernando D, Li K. Quantification of liver fat content with unenhanced MDCT: phantom and clinical correlation with MRI proton density fat fraction. AJR Am J Roentgenol. 2018;211:W151–W157. doi: 10.2214/AJR.17.19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutin RD, Lenchik L. Value-added opportunistic CT: insights into osteoporosis and sarcopenia. AJR Am J Roentgenol. 2020;215:582–594. doi: 10.2214/AJR.20.22874. [DOI] [PubMed] [Google Scholar]
- 17.Pickhardt PJ, Jee Y, O'Connor SD, del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: association with the metabolic syndrome. AJR Am J Roentgenol. 2012;198:1100–1107. doi: 10.2214/AJR.11.7361. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Liu J, Yao J, Kanarek A, Summers RM, Pickhardt PJ. Fully automated segmentation and quantification of visceral and subcutaneous fat at abdominal CT: application to a longitudinal adult screening cohort. Br J Radiol. 2018;91:20170968. doi: 10.1259/bjr.20170968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the Framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology. 2019;290:108–115. doi: 10.1148/radiol.2018180562. [DOI] [PubMed] [Google Scholar]
- 21.Moreno CC, Hemingway J, Johnson AC, Hughes DR, Mittal PK, Duszak R., Jr Changing abdominal imaging utilization patterns: perspectives from Medicare beneficiaries over two decades. J Am Coll Radiol. 2016;13:894–903. doi: 10.1016/j.jacr.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Graffy PM, Liu J, O'Connor S, Summers RM, Pickhardt PJ. Automated segmentation and quantification of aortic calcification at abdominal CT: application of a deep learning-based algorithm to a longitudinal screening cohort. Abdom Radiol (NY) 2019;44:2921–2928. doi: 10.1007/s00261-019-02014-2. [DOI] [PubMed] [Google Scholar]
- 23.Graffy PM, Liu J, Pickhardt PJ, Burns JE, Yao J, Summers RM. Deep learning-based muscle segmentation and quantification at abdominal CT: application to a longitudinal adult screening cohort for sarcopenia assessment. Br J Radiol. 2019;92:20190327. doi: 10.1259/bjr.20190327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graffy PM, Sandfort V, Summers RM, Pickhardt PJ. Automated liver fat quantification at nonenhanced abdominal CT for population-based steatosis assessment. Radiology. 2019;293:334–342. doi: 10.1148/radiol.2019190512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Lee SJ, Liu J, Yao J, Lay N, Graffy PM, et al. Population-based opportunistic osteoporosis screening: validation of a fully automated CT tool for assessing longitudinal BMD changes. Br J Radiol. 2019;92:20180726. doi: 10.1259/bjr.20180726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns JE, Yao J, Summers RM. Vertebral body compression fractures and bone density: automated detection and classification on CT image. Radiology. 2017;284:788–797. doi: 10.1148/radiol.2017162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez AA, Noe-Kim V, Lubner MG, et al. Deep learning CT-based quantitative visualization tool for liver volume estimation: defining normal and hepatomegaly. Radiology. 2021 Oct; doi: 10.1148/radiol.2021210531. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summers RM, Yao J, Pickhardt PJ, Franaszek M, Bitter I, Brickman D, et al. Computed tomographic virtual colonoscopy computer-aided polyp detection in a screening population. Gastroenterology. 2005;129:1832–1844. doi: 10.1053/j.gastro.2005.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson BA, Bjornsdottir G, Thorgeirsson TE, Ellingsen LM, Walters GB, Gudbjartsson DF, et al. Brain age prediction using deep learning uncovers associated sequence variants. Nat Commun. 2019;10:5409. doi: 10.1038/s41467-019-13163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieke N, Hancox J, Li W, Milletarì F, Roth HR, Albarqouni S, et al. The future of digital health with federated learning. NPJ Digit Med. 2020;3:119. doi: 10.1038/s41746-020-00323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.


