Abstract
Metabolic encephalopathy is a critical condition that can be challenging to diagnose. Imaging provides early clues to confirm clinical suspicions and plays an important role in the diagnosis, assessment of the response to therapy, and prognosis prediction. Diffusion-weighted imaging is a sensitive technique used to evaluate metabolic encephalopathy at an early stage. Metabolic encephalopathies often involve the deep regions of the gray matter because they have high energy requirements and are susceptible to metabolic disturbances. Understanding the imaging patterns of various metabolic encephalopathies can help narrow the differential diagnosis and improve the prognosis of patients by initiating proper treatment regimen early.
Keywords: Metabolic brain disease, Excitotoxic edema, Encephalopathy, Diffusion weighted imaging, Reversibility
INTRODUCTION
The brain is vulnerable to damage from abnormal metabolites and toxins. Encephalopathy has been described as a clinical situation associated with diffuse or multifocal brain dysfunction [1,2,3]. Diffusion-weighted imaging (DWI) is useful for detecting early pathological alterations in membranes and membrane-related water transporting proteins [2,3,4,5]. Therefore, radiologists should be familiar with the magnetic resonance imaging (MRI) features for some relatively common acquired metabolic encephalopathies.
Here, we aim to review the DWI features of several well-recognized acute metabolic encephalopathies in adults.
Cerebral Edema
Cerebral edema is an excess accumulation of fluid in the intracellular or extracellular space of the brain and found in various brain diseases. This can be divided into three types depending on the mechanism: 1) vasogenic edema results from the breakdown of the blood-brain barrier (BBB) and accumulation of fluid in the extracellular space via leakage from the capillaries. MR shows hyper signal intensity on T2-weighted imaging (T2WI) and fluid-attenuated inversion recovery (FLAIR) images but not diffusion restriction, 2) cytotoxic edema results from the accumulation of intracellular fluid due to dysfunction of the Na+/K+ pump. Unlike vasogenic edema, the BBB is intact. In T2WI and FLAIR images, even though it usually shows hyper signal intensity, it is sometimes difficult to detect abnormal hypersignal intensity. DWI plays an important role in the diagnosis of cytotoxic edema, and 3) the last one is excitotoxic edema, which results from transsynaptic injury caused by excitotoxic amines (especially glutamate). Excessive glutamate allows for the entry of Ca2+ into the postsynaptic and can cause cytotoxic edema. Excitotoxic edema is due to less energy failure, so this type occasionally appears transiently and disappears on follow-up imaging. Brain injury in metabolic encephalopathy usually results from excitotoxic edema. MRI findings of excitotoxic edema are similar to cytotoxic edema. However, the diffusion restriction in excitotoxic edema is reversible, unlike in cytotoxic edema [1,2,3,6,7].
Disturbances in Glucose Metabolism
Glucose is an essential substrate for brain metabolism, and a constant supply is required for optimal function [1,2,6]. Therefore, the maintenance of stable plasma glucose levels is important for survival [1]. Significant alterations in plasma glucose levels, such as hypoglycemia or hyperglycemia, may mimic acute infarction symptoms [2,6,7].
Hypoglycemic Encephalopathy
Hypoglycemic encephalopathy refers to a brain injury resulting from a lack of glucose availability to brain cells [1]. Many conditions, including the overuse of insulin or oral hypoglycemic agents, insulinoma, and medical diseases, lead to hypoglycemia. Transient hypoglycemia is common in patients with diabetes and is usually not life-threatening, but severe persistent hypoglycemia may have serious clinical consequences [7,8,9,10,11]. Clinical symptoms can be divided into two types. The first type includes autonomic symptoms such as sweating, trembling, and palpitation. The second type includes neuroglycopenic symptoms such as weakness, confusion, change in personality, and seizures. Autonomic symptoms usually precede neuroglycopenic symptoms [1,7].
The exact pathophysiology is unclear. Some mechanisms have been proposed, and many authors believe that a loss of cellular homeostasis due to energy failure is a key factor. The depletion of the glucose supply results in severe energy failure and leads to excitotoxic edema in neuronal and glial cells [12,13,14].
On conventional MRI, abnormal signal intensity is seen in the posterior limb of the internal capsule, cortex (especially the parieto-occipital lobe and insular area), hippocampus, basal ganglia, and cerebral white matter. These lesions are usually seen bilaterally. Unlike hypoxic brain damage, the cerebellum, brain stem, and thalami are unaffected. These characteristics are significantly different between hypoglycemic encephalopathy and hypoxic brain damage [7,13,14,15]. Abnormal signal intensity changes are seen in DWI sequences in the early stages of the disease. DWI is more sensitive than conventional MRI in detecting abnormalities associated with hypoglycemic encephalopathy.
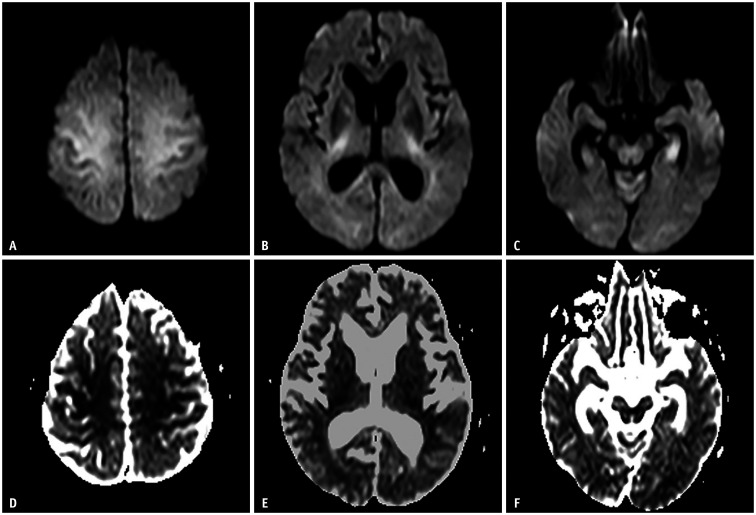
The DWI findings can be categorized into three patterns: 1) gray and white matter involvement (Fig. 1), 2) selective gray matter involvement (Fig. 2), and 3) selective white matter involvement (Fig. 3). However, these DWI patterns are not directly correlated with blood glucose levels [14]. Although the exact prevalence is unknown, previous studies have reported that pattern 1 is the most frequent, followed by patterns 2 and 3 with similar prevalence. In addition to these three patterns, patients can also show diffuse extensive cortical or white matter involvement patterns, but these are relatively uncommon [7,14]. Most DWI high signal lesions in hypoglycemic encephalopathy are reversible if the glucose supply is promptly reestablished. There are two major differences between hypoglycemic encephalopathy and ischemic stroke. One is that the distribution of lesions is not related to specific vascular territories, and the other is reversibility [1].
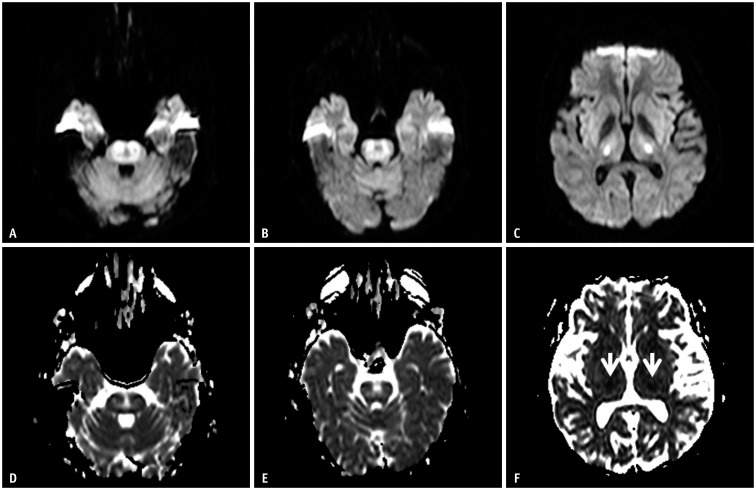
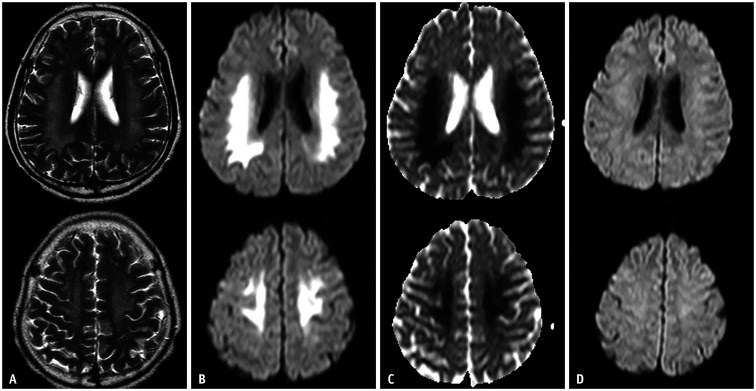
Fig. 1. A 63-year-old female was found unconscious (blood sugar level: 30 mg/dL).
A-C. Diffusion-weighted imaging shows high signal intensity along the centrum semiovale, posterior limb of the internal capsule, and hippocampus. D-F. These lesions are symmetrical, bilateral, and have low apparent diffusion coefficient values. These images show a hypoglycemic encephalopathy type one pattern (gray and white matter involvement).
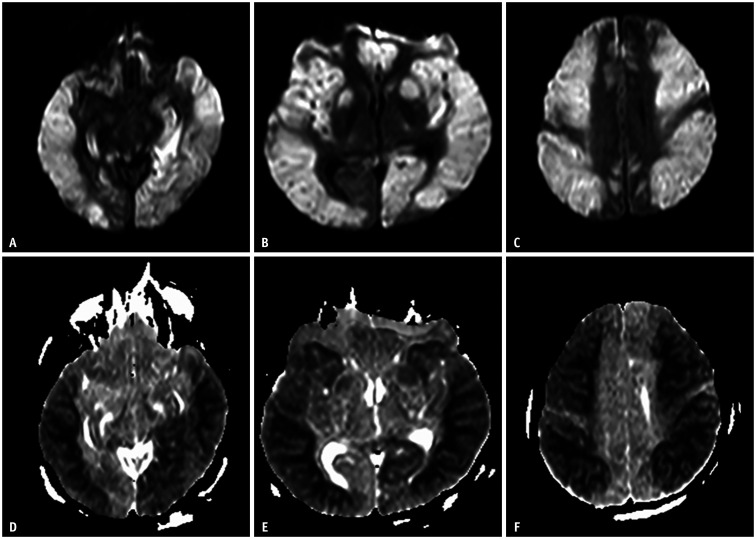
Fig. 2. A 53-year-old male who tried to commit suicide by injecting insulin.
A-F. Diffusion-weighted imaging shows diffuse restricted diffusion along the bilateral cerebral cortices, deep gray matter, and hippocampi. The lesion shows bilateral and symmetrical distribution. This case shows a hypoglycemic encephalopathy type two pattern (selective gray matter involvement).
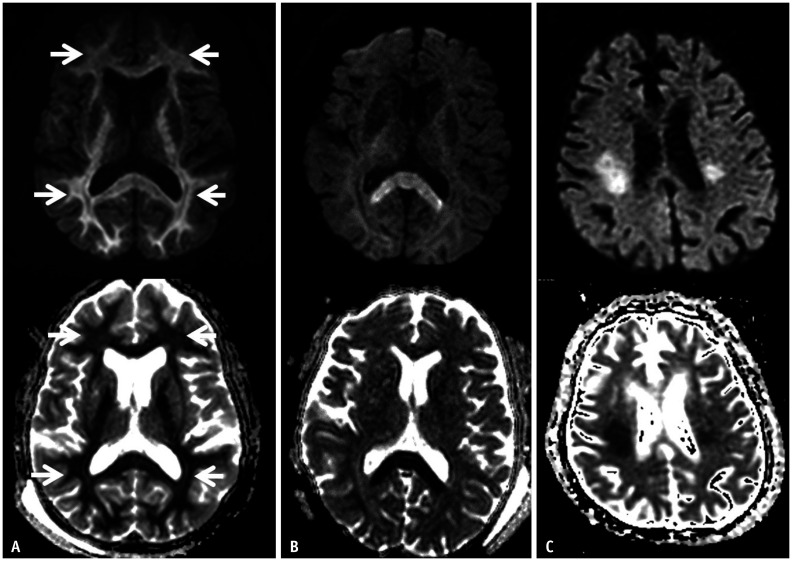
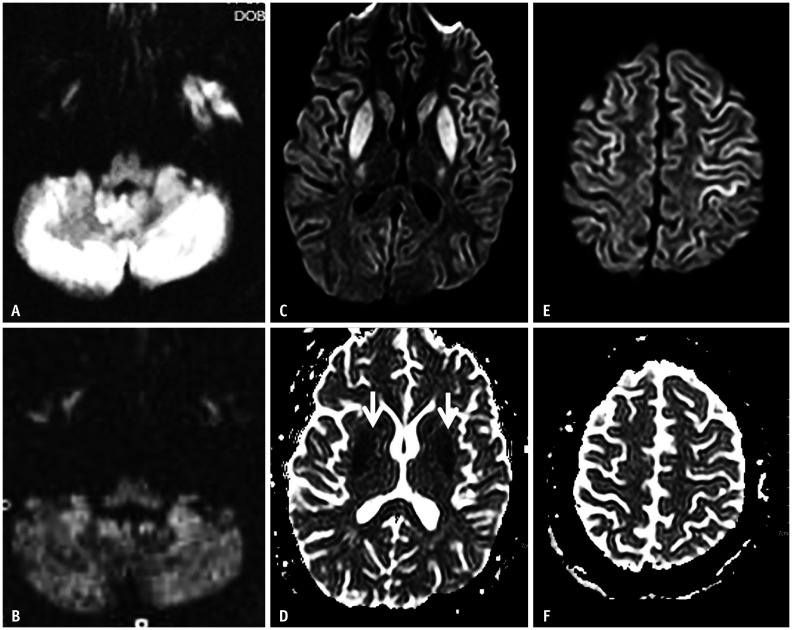
Fig. 3. A 75-year-old female presented with a drowsy mental status (blood glucose level: 10 mg/dL).
A-C. Diffusion-weighted imaging and the apparent diffusion coefficient map show a hypoglycemic encephalopathy type three pattern (selective white matter involvement). The lesions involve the subcortical white matter (A, arrows), corpus callosum (B), and corona radiata (C).
Many studies have shown that the severity and duration of hypoglycemia are related to the clinical prognosis, but the relationship between the extent or DWI pattern and clinical prognosis has not been reported. When prompt and appropriate treatment is provided, most patients with hypoglycemic encephalopathy show a favorable prognosis [8,14,16,17]. Previous studies failed to show an association between imaging patterns and prognosis. However, recent studies have found that diffuse lesions on DWI (Fig. 2) are more strongly correlated with a poor prognosis than specifically distributed patterns [7,8,13,14]. For short-term prognoses, a lack of lesions or the presence of focal lesions in the internal capsule on DWI indicates complete recovery within one day after treatment. In contrast, diffuse hemispheric white matter lesions indicate a poor one-week outcome, and patients tend to gradually recover over several weeks [13].
Hyperglycemic Encephalopathy
Hyperglycemic encephalopathy generally refers to hyperosmolar nonketotic hyperglycemia (HONK). Unlike diabetic ketoacidosis, HONK presents with hyperglycemia as the major feature, and acidosis is usually mild. The incidence of diabetic ketoacidosis is more common, but mortality is greater in patients with HONK [18]. Several complications result in HONK, such as cardiovascular collapse, stroke, cerebral edema, brain injury, and venous thrombosis [19]. Of these complications, cerebral edema and brain injury have characteristic imaging findings and neurologic symptoms, unlike the other symptoms of acute metabolic encephalopathy. Most patients with HONK usually show continuous, random, and involuntary movements of the unilateral, proximal, and distal muscle groups. This phenomenon is called hemichorea-hemiballism [20,21,22,23,24]. This is more common in elderly patients with uncontrolled diabetes mellitus and females because of estrogen [21,23,24,25,26]. HONK also has relatively benign and reversible symptoms [26].
There are many potential explanations about pathophysiology, but the most relevant hypothesis involves hyperviscosity. Hyperglycemia induces hyperviscosity and an increase in brain water content. This results in a reduction in cerebral blood flow and intracellular acidosis. This leads to the depletion of γ-aminobutyrate (GABA) and results in increased neuronal excitation through the thalamocortical pathway. This is why the lesions are located in the striatum [20,21,26,27].
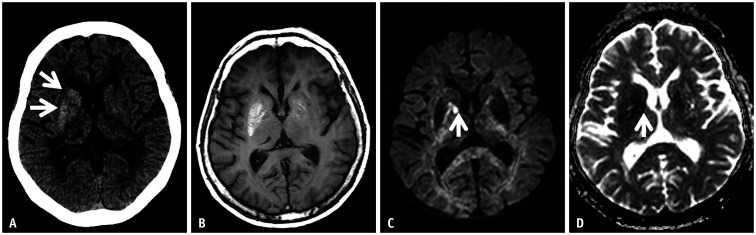
The imaging findings showed uni/bilateral basal ganglia hyperattenuation on computed tomography (CT) (Fig. 4A) and high signal intensity on T1-weighted images due to a T1 shortening effect (Fig. 4B). Several hypotheses have been proposed to explain the pathogenesis of these imaging findings, including reactive astrocytosis, mineral deposition, and petechial hemorrhage. On pathological analyses, the presence of reactive astrocytes was documented in five out of six available pathologic reports. Reactive astrocytes with rich protein contents, called gemistocytes usually appear in acute injuries. In addition, the rich protein content of these cells leads to the restricted motion of water molecules, which is expressed as high signal intensity on DWI with a low apparent diffusion coefficient (ADC) value (Fig. 4C, D) [20,21,22,28,29,30]. The abnormal MRI findings were reversible. In most cases, abnormal movement resolves within a few weeks or several days after hyperglycemia treatment, but complete resolution of the abnormal imaging findings requires more time (several months) [22]. Some poor prognosis indicators include cardiovascular disease, old age, hypotension, acidosis, high urea, and low ADC value. Overall, the prognosis is excellent, and there are few permanent neurologic sequelae [18,19,20].
Fig. 4. A 60-year-old female presented with athetosis on the left arm.
She was found to have nonketotic hyperglycemia (blood sugar level: 337 mg/dL).
A. Non-enhanced CT axial image shows hyperdense lesions in the caudate nucleus and lentiform nucleus without associated edema (arrows). B. T1-weighted axial images also demonstrate abnormal unilateral high signal intensity at the basal ganglia. C, D. On diffusion-weighted imaging, a small diffusion-restricted area is shown in the globus pallidus (arrows). The imaging findings represent hyperglycemic encephalopathy.
Sodium Regulation Disorder
Hyponatremic Encephalopathy
Hyponatremia is characterized by a low sodium concentration in the blood and generally refers to a serum sodium level < 135 mmoL/L, while severe hyponatremia with a serum sodium level < 125 mmoL/L can be classified into three types: 1) hypovolemic, involving a deficit in total body sodium and water (sodium deficit > water deficit), 2) euvolemic, is characterized by normal total body sodium but an increased water level. This state is the most common electrolyte disturbance in hospitalized patients who have a glucocorticoid deficiency, hypothyroidism, acute psychosis, post-surgery state, and 3) hypervolemic, increased total body sodium with water increased even more than sodium. The cause of this state is heart, liver, or kidney failure and nephritic syndrome [31,32,33,34].
The symptoms depend on the degree of hyponatremia and the rate of progression. When the sodium concentration is more than 125 mmoL/L, the patient is usually asymptomatic or suffers from predominant gastrointestinal symptoms. However, when the serum sodium concentrations fall below 125 mmoL/L, neurological symptoms develop, and immediate treatment is required because untreated severe symptomatic hyponatremia is fatal [31,32,33]. Hyponatremic brain injury is divided into two main groups: acute cerebral edema and osmotic demyelination syndrome. In the former, hyponatremia develops within 48 hours and has a greater risk of permanent neurological sequelae if not treated, whereas, in the latter, osmotic demyelination occurs due to excessive or rapid correction of serum sodium in patients with chronic hyponatremia [31].
Most reports describing the MR imaging findings of hyponatremic osmotic demyelination syndrome include the MR findings of central pontine myelinolysis (CPM) or extra pontine myelinolysis (EPM). The pathophysiology of demyelination involves disruption of the BBB after rapid correction of the sodium concentration, and histological examination shows an oligodendrocyte loss and reactive astrocytosis, while neurons and axons are usually preserved [12]. On MRI, the areas of demyelination show high signal intensity on T2WI but no mass effect. The location of the lesion is parallel to the distribution of the oligodendrocytes. In CPM, the pons (spared pontine tegmentum) is involved. A trident-shaped lesion in the central pons is a characteristic finding on T2WI and FLAIR, whereas, in EPM, the cerebellum, basal ganglia (putamen, caudate nucleus), thalamus, external capsule, lateral geniculate body, hippocampus, and cerebral cortex are characteristic [2,4,5,35,36]. Early diagnosis with conventional MRI is limited because demyelination lesions are not visible within the first two weeks. DWI showed bilateral, symmetrical high signal intensities in the regions mentioned above (Figs. 5, 6). ADC values are variable, and increased ADC values in the acute lesions may be due to inflammatory vasogenic edema and myelin destruction with axonal preservation. A decreased ADC value may be due to intramyelinic cytotoxic edema [4,35,36]. The severity of the demyelination is shown by the extent of the MRI signal abnormality [5]. The prognosis is good in cases with unremarkable DWI or cases involving rapid normalization of the diffusion high signal intensity. However, there is no correlation between the degree of demyelinating lesions on DWI and the severity of the clinical symptoms or outcomes [3,35,37,38]. A few reports showed diffuse cortical and lateral medullary lesions on DWI with low ADC values [39,40].
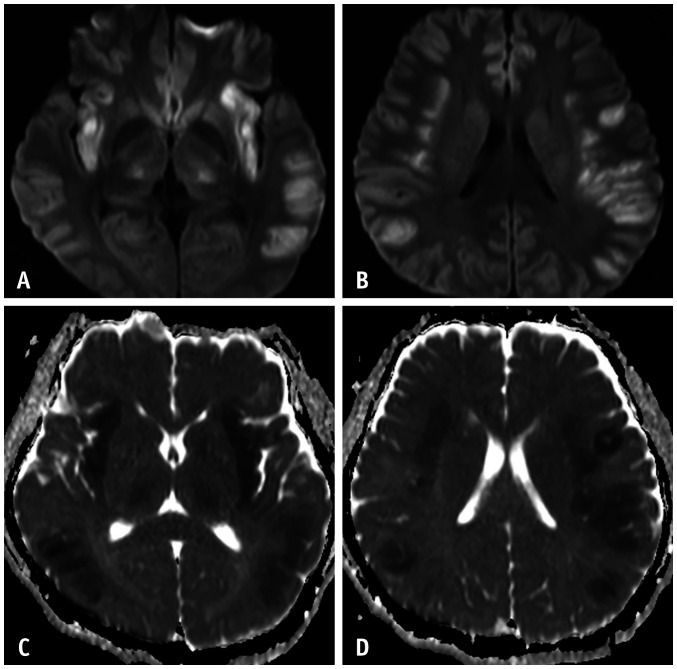
Fig. 5. A 39-year-old male who had general weakness and dysarthria.
A-F. Diffusion-weighted imaging at the level of the pons shows a high signal intensity in the central pons, which is sparing the pontine tegmentum (A, B). Bilateral symmetrical high signal intensities are noted in the bilateral thalami (C). On the ADC map, pontine lesions show high ADC values (D, E), but the thalamic lesions show restricted diffusion (F, arrows). This patient represents a central pontine case (the level of sodium at the time of admission was 128 mEq/L). ADC = apparent diffusion coefficient
Fig. 6. Two patients' imaging findings of extra pontine myelinolysis.
A-F. The first patient (71-year-old female) presented with a confused mentality, ataxia, and hyponatremia (A, B). This patient showed vasogenic edema in the bilateral cerebellar hemispheres. The second patient (a 16-year-old male) presented with a confused mental status, dysarthria, and hyponatremia. Diffusion-weighted imaging (C, E) shows bilateral symmetrical high signal intensities in the bilateral basal ganglia, thalami, and cerebral cortices. These lesions show variable ADC values (D, F), a high ADC value due to inflammatory vasogenic edema in the thalami and cortices, and low ADC values in the basal ganglia due to intramyelinic cytotoxic edema (D, arrows). ADC = apparent diffusion coefficient
In patients with asymptomatic hyponatremia, an aggressive therapeutic regimen is not necessary. Symptomatic hyponatremia is a medical emergency that requires constant monitoring in an intensive care unit. Treatment is initiated with intravenous hypertonic sodium chloride using an infusion pump, and the rate of the increase in plasma sodium is about 1 mmoL/L/hour. Furthermore, the sodium concentration should not increase to more than 25 mmoL/L during the initial 48 hours of treatment [31,32,33].
Hypernatremic Encephalopathy
Hypernatremia is defined as a serum sodium concentration of > 145 mmoL/L [31,41]. The total body sodium concentration is influenced by the extracellular fluid (ECF) volume. Hypernatremia should be considered according to the status of ECF volume (hypovolemia, euvolemia, and hypervolemia). Hypovolemic hypernatremia results from water loss from the kidneys (diuretics, glycerol, mannitol, hyperglycemia in diabetes) or the gastrointestinal tract. Euvolemic hypernatremia results from the restriction of water intake, excessive sweating, and diabetes insipidus. Hypervolemic hypernatremia is rare but can result from the excessive administration of sodium bicarbonate to treat acidosis. Among these, hypovolemic hypernatremia is more common [31]. Hypernatremia, similar to hyponatremia, is a potentially fatal condition that can cause encephalopathy, rhabdomyolysis, and osmotic demyelination [42]. Osmotic demyelination is related to rapid correction of hyponatremia, but it rarely occurs in acute severe hypernatremia.
Hypernatremic osmotic brain injury is relatively poorly described, and few studies have been reported that compared it to hyponatremic osmotic brain injury [43]. The exact incidence is not estimated, but hypernatremia is far less common than hyponatremia. The incidence is approximately 1% of hospitalized patients [31,42].
Patients can display various neurologic symptoms, and the most common symptoms include an altered mental status and motor deficits [31,43]. These symptoms are not related to age, the presence of comorbid conditions, initial sodium concentration, serum osmolality, and sodium correction rate [43].
Hypernatremia is a spectrum of osmotic demyelination syndrome. Therefore, the MRI findings in hypernatremia are similar to hyponatremia. The difference is that, unlike in hyponatremia, abnormal MR findings are usually present before the initiation of the treatment because osmotic myelination in hypernatremia is not a result of rapid correction but a result of the hypernatremia itself [44]. The MRI findings can be classified into three patterns: osmotic demyelination (CPM and EPM), vascular change (infarction, subdural hemorrhage, venous thrombosis), and cerebral and CSF volume changes (brain swelling, shrinkage, hydrocephalus, and subdural effusion). Among these patterns, most patients show an osmotic demyelination pattern, and mixed CPM and EPM are more common than CPM or EPM alone on MRI. In hypernatremic EPM, the white matter and corpus callosum are the most commonly involved sites (Fig. 7), but in hyponatremic EPM, the cerebellum and basal ganglia are more predominant. Unlike hyponatremia-related CPM, the lesion's location in hypernatremic CPM includes either the central or dorsolateral aspects of the pons, and an isolated CPM pattern is uncommon. Age is an important factor in topographic vulnerability to hyperosmolality. At a young age, the lesions are located in the white matter, corpus callosum, and basal ganglia but are not combined with the CPM or EPM sites or the vascular patterns. However, older age is associated with volume change patterns. In addition, a higher initial sodium level indicates a more frequent involvement of the hippocampus, which means that the hippocampus is vulnerable to the neurotoxic effects of osmotic derangement. There is no relationship between the clinical symptoms on presentation and the imaging patterns [43].
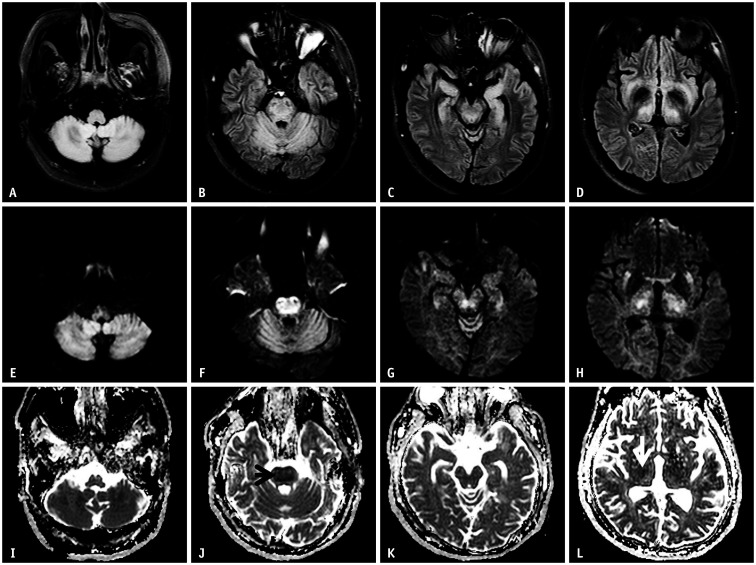
Fig. 7. A 33-year-old male presented to hospital with a drowsy mentality.
A-L. His initial serum sodium at the emergency department was 285 mEq/L, which corrected to 138 mEq/L after 72 hours. Fluid-attenuated inversion recovery shows abnormal high signal intensities at the cerebellum, central pons, hippocampus, external capsule, and thalamus (A-D). These lesions show high signal intensities on the diffusion-weighted imaging (E-H). On the apparent diffusion coefficient map (I-L), most lesions have high values (no diffusion restriction), except for the pons (J, arrow) and thalamus (L, arrow). MR images show that central pontine and extra pontine myelinolysis coexist. This case represents hypernatremic encephalopathy.
The clinical outcomes of acute hypernatremia are variable, but there is no relationship between the initial sodium levels and the outcomes. Generally, pediatric patients have better outcomes, and the MR abnormalities are more reversible because younger brains have a greater capacity to withstand osmotic disturbances. In adults, older age is associated with better recovery than young adults, but the presence of EPM lesions is associated with poor outcomes. Vascular complications and cerebral volume changes are not associated with the severity of the outcomes [43].
Uremic Encephalopathy
Uremia occurs when renal failure results in the systemic retention of nitrogenous and other waste products. These metabolic substances can result in tissue dysfunction, including of the central and peripheral nervous systems. Uremic encephalopathy is an acquired metabolic derangement associated with acute or chronic renal failure and occurs when the glomerular filtration rate decreases to less than 10% of the normal values [45,46].
The pathophysiology is complex, and many factors, including toxins and parathyroid hormone, may contribute to uremic encephalopathy [46,47,48,49,50,51]. A few theories have been proposed regarding the mechanisms of increased BBB permeability due to hyperglycemia in patients with diabetes. For example, increased nitrogenous waste products may inflict toxic and metabolic injuries on the brain tissue, especially the basal ganglia, and decreased oxygen consumption may affect the focal cellular metabolism and result in cellular edema [46,47,48,49,51].
The symptoms range from minor (e.g., lethargy, fatigue, mild sensory clouding) to severe (delirium, coma) and are sometimes associated with myoclonus, asterixis, and seizures. The former are features of depressed neuronal activity, and the latter involves neuronal excitation [47,48,49]. The degree of severity and progression depend on the rate of renal function deterioration.
The symptoms usually disappear after dialysis, renal transplantation, or recovery of renal function. The first treatment is dialysis. Most of the symptoms rapidly and completely improve following dialysis [52].
The MRI findings are classified into three patterns: 1) the cortical type, this type is the most common and is a part of the posterior reversible encephalopathy syndrome (PRES), 2) The basal ganglia type, which usually develops in diabetic uremic patients (Fig. 8), and 3) the diffuse white matter type, which is the rarest and may develop in non-diabetic uremic patients (Fig. 9). The cortical type can develop in uremic patients, and the MRI findings indicate vasogenic edema. The MRI findings of the basal ganglia type include mixed vasogenic and cytotoxic edema. This type usually has a worse prognosis than the other types, and many patients die or require prolonged intensive care due to complications related to uremic encephalopathy [46]. Diffuse white matter type shows cytotoxic edema on DWI. Patients suffering from this type often show facial palsy. This symptom may be a clue to differentiate this type from other types of uremic encephalopathy or other acute metabolic encephalopathies [46,49]. MRI abnormalities are usually reversible after dialysis or after the normalization of renal function. Therefore, the prognosis of uremic encephalopathy is good. However, neurologic and radiologic sequelae can remain when the ADC values are very low, especially in the medial portion of the basal ganglia. Early recognition of encephalopathy in patients with acute or chronic renal failure is important to prevent morbidity or mortality [53].
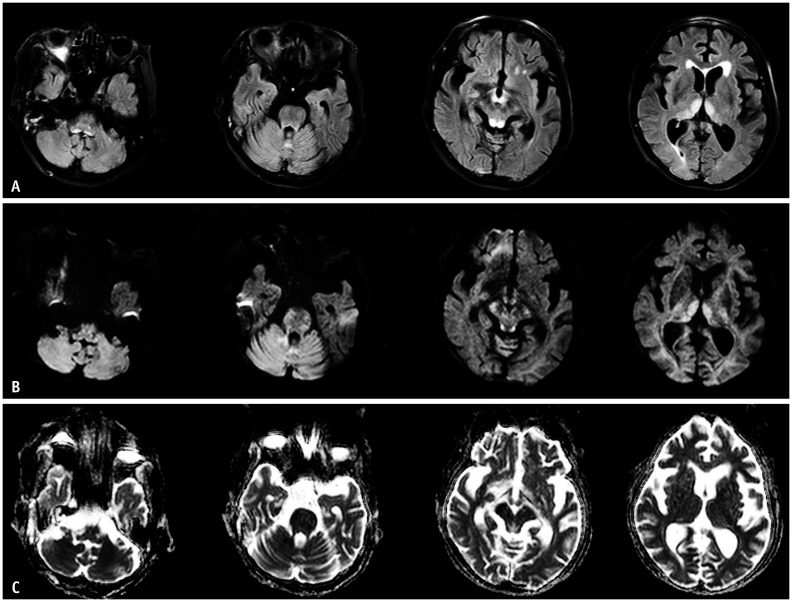
Fig. 8. A 67-year-old male patient with diabetes mellitus presented with gait imbalance to hospital.
Initial BUN/Cr was 80/16 mg/dL. A. T2-weighted imaging shows high signal intensity at the basal ganglia. B. The susceptibility-weighted image shows a few microbleeds in this lesion. C, D. The diffusion-weighted imaging (C) and apparent diffusion coefficient map (D) show diffuse vasogenic edema with focal cytotoxic edema (arrows). This is the basal ganglia type of uremic encephalopathy in a diabetic uremic patient.
Fig. 9. A 36-year-old male presented with right facial palsy and motor weakness.
A-D. His initial BUN/Cr was 58.7/6.06 mg/dL. T2-weighted imaging shows confluent high signal intensities of the bilateral cerebral white matter (A). These lesions show diffusion restriction on diffusion-weighted imaging (B) and the apparent diffusion coefficient map (C). On follow-up diffusion-weighted imaging, the previous abnormal signal intensities are completely resolved (D). This is the diffuse white matter type of uremic encephalopathy in a non-diabetic uremic patient.
Encephalopathy Related to Impaired Liver Function
Hyperammonemic Encephalopathy
Ammonia is a substance that contains nitrogen and results from protein digestion and bacterial metabolism. It is an essential substance for the synthesis of cellular compounds but is also a very toxic substance when too high a concentration is present in the blood [54]. The entry of ammonia to the brain is a cause of neurological disorders, and the condition is known as hyperammonemic encephalopathy [54,55,56]. The causes of hyperammonemia vary from inborn errors to acquired, such as liver failure, drugs, renal diseases, parenteral nutrition, and organ transplantation [54,56]. The most common cause of hyperammonemia in adults is acute liver dysfunction [54,55]. Therefore, it is believed that hyperammonemic encephalopathy may be similar to acute hepatic encephalopathy (HE). However, this hypothesis is controversial [57].
Excessive ammonia is converted to large amounts of glutamate and glutamine in astrocytes. This change leads to intracellular hyperosmolality and results in astrocyte swelling, brain edema, intracranial hypertension, and cerebral hypoperfusion [58,59,60,61].
Hyperammonemic encephalopathy appears to show specific imaging characteristics, with all patients in the literature showing bilateral symmetrical high signal intensities in the insular and cingulate cortices. Involvement of the dorsomedial thalamus was observed in more than 80% of patients (Fig. 10). The MRI findings did not differ between pediatric and adult patients. In addition, regardless of the etiologies, the MRI findings were similar in hyperammonemic encephalopathy [54,55,56,61,62]. DWI is very sensitive, and restricted diffusion can occur at a very early stage. However, abnormal signal intensities were observed after at least two days, especially cortical lesions on FLAIR. However, thalamic lesions appear at an early stage on both DWI and FLAIR. Pseudonormalization of ADC, the persistence of DWI, and high signal intensities on FLAIR were demonstrated over eight days [63]. Similar to acute hyperammonemic encephalopathy, hypoxic-ischemic encephalopathy diffusely involves the cerebral cortex and bilateral thalami. Distinctions between these two diseases are as follows: 1) involvement of the insular and cingulate cortex is more predominant in hyperammonemic encephalopathy than in hypoxic-ischemic encephalopathy (Fig. 10A, B). In contrast, the involvement of the basal ganglia and the hippocampus is more predominant in hypoxic-ischemic encephalopathy, and 2) cortical abnormal signal intensities are reversible in hyperammonemic encephalopathy but irreversible in hypoxic-ischemic encephalopathy. Above all, measurement of the ammonia level in the blood or verification of the presence of a hypoxic insult are major clues for distinguishing these two conditions [55,61].
Fig. 10. A 47-year-old male patient presented with confused mental status.
A-D. He had a history of heavy alcoholism (one bottle of soju/day for seven years). His plasma ammonium level was 63 µmoL/L. The diffusion-weighted imaging shows bilateral, asymmetrical high signal intensity involving the insular cortex, cingulate gyri, temporal, and parietal cortex (A, B). Most of these lesions show low values on the apparent diffusion coefficient map (C, D). This case represents hyperammonemic encephalopathy.
In general, regardless of the etiology, the prognosis of hyperammonemic encephalopathy is not good. However, if the degree of hyperammonemia is mild, and it is recognized at an early stage, the MRI changes recover completely without any neurologic sequelae after prompt treatment. The degree of hyperammonemia is related to the extent of injury, and the extent of injury is dependent on the severity and duration of hyperammonemia [54,55,56,61]. Radiologists should consider the possibility of acute hyperammonemic encephalopathy when DWI shows bilateral symmetrical cortical lesions involving the insular and cingulate cortex and the dorsomedial parts of the thalami in patients who do not have a history of hypoxic insult.
Hepatic Encephalopathy
HE is defined as a spectrum of neuropsychiatric disorders in patients with severe acute or chronic liver dysfunction [64,65]. Minimal HE is observed in a considerable number of patients with cirrhosis (approximately 70%). Overt HE develops in approximately one-third of patients with cirrhosis and patients who receive portosystemic shunt surgery. HE accompanying the acute onset of severe hepatic failure is a sign of acute liver failure [64,65,66].
Several pathophysiologic mechanisms of HE have been proposed, and most of these are common to encephalopathy associated with cirrhosis and acute liver failure. Among these, ammonia is known as the main contributor, but other mechanisms, including inflammation, oxidative stress, neurosteroids, and manganese accumulation, also contribute. The major difference between chronic and acute hepatic encephalopathies is that brain edema is much more prominent in acute liver failure than in cirrhosis [64]. In encephalopathy with acute liver failure, the mechanisms of brain edema and astrocytic changes are similar to those in hyperammonemic encephalopathy. However, brain edema is less in encephalopathy with cirrhosis because astrocytes initiate compensatory mechanisms, and these astrocytes show morphologic changes, a phenomenon called Alzheimer type 2 astrocytosis [64,65,67,68].
The known MRI findings of chronic liver failure include bilateral basal ganglia T1 high signal intensity, especially in the globus pallidus resulting from manganese deposition (Fig. 11A, B). FLAIR and DWI images show high signal intensity along with the hemispheric white matter or around the corticospinal tract (thalami, posterior limb of the internal capsule, and periventricular white matter). These findings reflect chronic astrocytic swelling, interstitial edema, or chronic demyelination. In acute HE, low ADC values in the affected areas reflect cytotoxic edema (Fig. 11C-F).
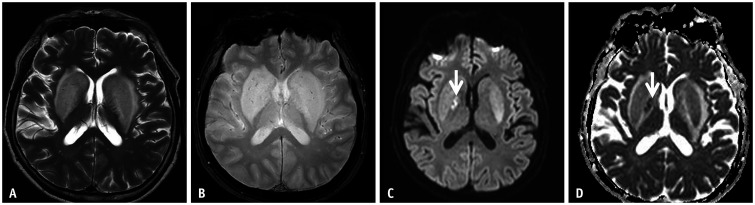
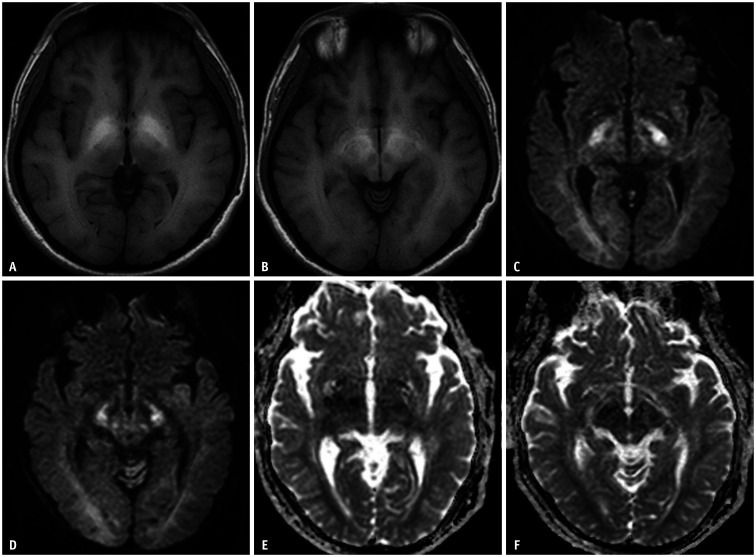
Fig. 11. A 44-year-old male patient was admitted due to liver failure.
A, B. T1-weighted images show bilateral symmetric high signal intensity involving the globus pallidus and anterior portion of the midbrain. These findings represent chronic hepatic encephalopathy, which is considered due to excessive manganese accumulation. C-F. Diffusion-weighted imaging high signal intensities (C, D) with subtle low apparent diffusion coefficient values (E, F) are noted in the bilateral thalami, cerebral peduncles, and cerebral white matters. This case represents chronic hepatic encephalopathy.
Acute HE can be fatal. On the contrary, chronic HE is more indolent. Studies show that 80% of patients can recover from HE if the exact etiology is identified and proper treatment received. Diffuse cortical involvement is more fatal but can be reversible [69,70,71].
Vitamin Deficiency-Related Encephalopathy
Wernicke's Encephalopathy (Vitamin B1; Thiamine)
Wernicke's encephalopathy was first described in 1881 by Carl Wernicke and indicates an uncommon condition characterized by severe neurological dysfunction resulting from thiamine (vitamin B1) deficiency. This condition has some distinct features and is clinically characterized by an acute confused mental status, ataxia, and ophthalmoplegia. Pathologically, it is characterized by punctate hemorrhage around the third and fourth ventricles and aqueduct [72]. The most common cause is chronic alcoholism, and in non-alcoholic conditions, long periods of starvation, hyperemesis gravidarum, gastrointestinal surgery, and chemical therapy can lead to thiamine deficiency. Wrong baby formula can cause this condition in healthy infants [73,74,75,76].
The prevalence typically ranges from 0.4% to 2.8% in Western countries and is much higher in alcoholics than in non-alcoholics. Females are more susceptible to developing Wernicke's encephalopathy than males [73,77].
Thiamine plays an important role in maintaining membrane integrity and osmotic gradients and acts as an essential cofactor for several enzymes. Thiamine deficiency lasting more than three weeks leads to cerebral lactic acidosis, failure to maintain cellular electrolyte homeostasis, and impairment of glial cells and neurons due to excessive glutamate. However, if thiamine is promptly supplied before cellular death occurs, these alterations are restored [78,79].
Damage often occurs in the structures surrounding the third ventricles (medial thalamus, tegmentum, periaqueductal area, mamillary bodies, and tectal plate of the midbrain) [72,75,80,81]. The cerebellum, cranial nerve nuclei in the pons, the corpus callosum, caudate nucleus, and cerebral cortex are usually less involved. The former is considered a “typical site” (surrounding the third ventricles) and is seen more frequently in alcoholic patients, but the latter, the “less common site,” frequently shows in non-alcoholic patients [74]. The symptoms related to the lesion sites include mental status or memory/cognitive impairments resulting from the involvement of the thalamus or mammillary bodies, ocular symptoms resulting from involvement of the pontine tegmentum, and ataxia resulting from involvement of the periaqueductal gray matter (cerebellar vermis) [73].
Although laboratory and neuroimaging studies can be useful for diagnosis, Wernicke's encephalopathy is primarily a clinical diagnosis. However, its diagnosis may be challenging in non-alcoholic patients without the triad of clinical symptoms. Therefore, early thiamine replacement is sometimes preceded, and the response to thiamine supplementation may be a diagnostic sign [73,74,75].
MRI shows bilateral and symmetrical T2 or FLAIR high signal intensity in the typical sites (Fig. 12). The sensitivity of DWI for detecting these lesions is superior to that of T2WI or FLAIR. However, high signal intensity on DWI and low ADC values do not always correspond to irreversible tissue damage. Since the signal abnormalities are usually normalized within two weeks when adequate thiamine is supplied, this indicates reversible cytotoxic edema before the cells enter a necrotic state [73,80,81]. In most cases, a follow-up MRI after an adequate supply of thiamine results in the complete disappearance of the signal abnormalities. Differential diagnoses include thalamic infarction, cytomegalovirus encephalitis, primary cerebral lymphoma, and mitochondrial disorders [73,80]. In general, the clinical symptoms also regress, but there is a time lag. Ophthalmic symptoms tend to improve within a few hours, and ataxia requires a longer time to improve. The prognosis is good with proper treatment. However, if untreated or inappropriately treated, it can lead to permanent brain damage that can cause death in about 20% of patients or Korsakoff syndrome in 85% of survivors [73,74].
Fig. 12. A 52-year-old female presented to the emergency department with malnutrition.
A-C. Her blood thiamine level was 1.6 µg/dL, and she was a chronic alcoholic. Fluid-attenuated inversion recovery imaging shows bilateral symmetrical high signal intensity at the thalamus, periventricular region of the third/fourth ventricle, periaqueductal area, and mamillary bodies (A). These lesions show abnormal high signal intensity on diffusion-weighted imaging (B) without low apparent diffusion coefficient values (C). This case displays the typical findings of Wernicke's encephalopathy.
CONCLUSION
Acute metabolic encephalopathies have the following similarities in imaging. First, they usually show bilaterally symmetrical imaging abnormalities. Second, they show the presence of vasogenic and cytotoxic edema, both of which can be reversible.
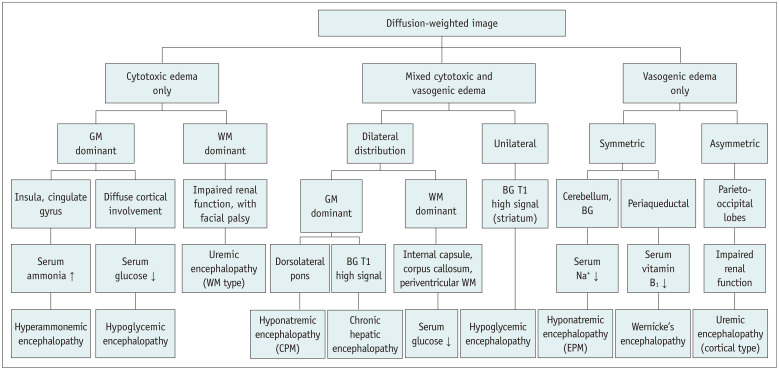
In some types of metabolic encephalopathies, the frequently involved structures on MRI may provide clues for diagnosis (Table 1). In particular, DWI can help detect metabolic encephalopathy even at an early stage, help evaluate the disease extent, and predict patient outcomes (Fig. 13).
Table 1. Summary of the Clinical and MRI Findings for Metabolic Encephalopathy.
| Type of Encephalopathy | Epidemiology | Symptoms | Laboratory Findings | MRI Findings | Px (With Proper Treatment) | |||
|---|---|---|---|---|---|---|---|---|
| Typically Affected Sites | Less Commonly Affected Sites | Other Findings | Edema Type (DWI/ADC) | |||||
| Hypoglycemic | Cause: insulin overuse or oral hyperglycemic agent, insulinoma | Autonomic-sweating, palpitation Neuroglycopenic weakness, confusion, seizure |
Serum glucose < 70 mg/dL | Bilateral, symmetric: BG Cerebral cortex |
Cerebral white matter Corpus callosum (splenium) | Cerebellum, brain stem, thalami are usually spared | Excitotoxic edema | Mostly good Diffuse, confluent cortex involve: poorer Px than other site involvement |
| Hyperglycemic | Usually nonketotic hyperglycemic patients | Hemichorea-hemiballism | Ketoacidosis > acidosis Serum osmolality > 320 mOsm/kg urine/serum ketone + | Uni- or Bilateral: BG (putamen) | *T1WI: high SI | Excitotoxic edema (complete resolution of abnormal SI: takes longer time, several months) | Mostly good Poor Px factors: cardiovascular Ds, old age, hypotension, hyponatremia, acidosis, high urea |
|
| Hyponatremic | Hyponatremic brain edema (rare): hyponatremia developing within 48 hours Osmotic demyelination (most common): rapid correction of hyponatremia |
Serum sodium < 125 mmoL/L (excess, too rapid correction) | Bilateral, symmetric - Osmotic demyelination: CPM (central pons, “trident” appearance) or EPM (BG, cerebral white matter, thalamus, cerebellum) lesion more than mixed lesion | - Hyponatremic brain edema (uncommon): diffuse cerebral cortex/lateral medulla | In hyponatremic CPM: central pons involvement In hyponatremic EPM: cerebellum is common involved site |
Excitotoxic edema and vasogenic edema (mixed) | Good in case that an unremarkable diffusion restriction or rapid normalization of diffusion high SI | |
| Hypernatremic | Hypovolemic hypernatremia is more common (water loss from kidneys or GI tract) Hypernatremia is far less common than hyponatremia |
Most common: altered mental status | Serum sodium > 145 mmoL/L | One spectrum of osmotic demyelination syndrome: CPM + EPM > only CPM or EPM finding | In hypernatremic CPM: central and dorsolateral pons In hypernatremic EPM: cerebral white matter and corpus callosum involvement are more common |
Excitotoxic edema and vasogenic edema (mixed) | Variable (no relation between initial sodium level) - Pediatric patients have better outcome and more reversible - In adults: old age is associated with better outcome - The presence of EPM lesions: related poor outcome | |
| Uremic | Acute/chronic renal failure GFR decrease less than 10% of normal (eGFR < 15 mL/min) |
Various (lethargy, fatigue, delirium, coma) | Cortical type: usually vasogenic edema BG type: mixed (cytotoxic and vasogenic edema) Diffuse white matter type: excitotoxic edema |
Good | ||||
| Hyperammonemic | Cause: inborn or acquired liver failure, drugs, renal Ds, parenteral nutrition, transplantation | Increase serum ammonia level | Other part of the cerebral cortex | Excitotoxic edema | Not good (the degree of hyperammonemia is related to extent of injury) | |||
| Hepatic | Acute and chronic liver cirrhosis with portosystemic shunt | Bilateral - BG - Around the corticospinal tract (thalamus, posterior limb of internal capsule) | Substantia nigra, subthalamic nuclei, cerebral white matter |
*TIWI: high SI in BG FLAIR, DWI: high SI along the cerebral white matter, and around the corticospinal tract |
Usually vasogenic edema: BG high SI on T1WI -persist: FLAIR, DWI high SI reversible | Good (> 80% of patients: can recover) | ||
| Wernicke’s | Cause - Chronic alcoholics - Non-alcoholics: starvation, GI surgery, chemotherapy Incidence: Alcoholic > non-alcoholic |
Confuse mental status, ataxia, opthalmoplegia | Low serum thiamine (vitamin B1) | Bilateral and symmetric: medial thalamus, mammillary body, periaqueductal region | Contrast enhancement can show on T1WI with contrast agent | Usually vasogenic edema | Good (if untreated or inappropriately treated, the mortality is about 20%) | |
*Characteristic finding. ADC = apparent diffusion coefficient, BG = basal ganglia, CPM = central pontine myelinolysis, Ds = disease, DWI = diffusion-weighted imaging, eGFR = estimated GFR, EPM = extra pontine myelinolysis, FLAIR = fluid-attenuated inversion recovery, GFR = glomerular filtration rate, GI = gastrointestine, PRES = posterior reversible encephalopathy syndrome, Px = prognosis, SI = signal intensity, T1WI = T1-weighted image
Fig. 13. Diagnostic algorithm of acute acquired metabolic encephalopathy.
BG = basal ganglia, CPM = central pontine myelinolysis, EPM = extra-pontine myelinolysis, GM = gray matter, WM = white matter
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: In Kyu Yu.
- Data curation: Se Jeong Jeon, See Sung Choi, Ha Yon Kim.
- Formal analysis: Se Jeong Jeon.
- Investigation: Se Jeong Jeon, In Kyu Yu.
- Methodology: Se Jeong Jeon, In Kyu Yu.
- Project administration: Se Jeong Jeon.
- Resources: Se Jeong Jeon.
- Software: Se Jeong Jeon, See Sung Choi, Ha Yon Kim.
- Supervision: In Kyu Yu, See Sung Choi.
- Visualization: Se Jeong Jeon.
- Writing—original draft: Se Jeong Jeon.
- Writing—review & editing: In Kyu Yu, See Sung Choi, Se Jeong Jeon.
References
- 1.Bathla G, Policeni B, Agarwal A. Neuroimaging in patients with abnormal blood glucose levels. AJNR Am J Neuroradiol. 2014;35:833–840. doi: 10.3174/ajnr.A3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Eesa M, Scott JN. Toxic and acquired metabolic encephalopathies: MRI appearance. AJR Am J Roentgenol. 2009;193:879–886. doi: 10.2214/AJR.08.2257. [DOI] [PubMed] [Google Scholar]
- 3.Sutter R, Kaplan PW. Neuroimaging correlates of acute encephalopathy. J Clin Neurophysiol. 2013;30:517–525. doi: 10.1097/WNP.0b013e3182a73be3. [DOI] [PubMed] [Google Scholar]
- 4.Karaarslan E, Arslan A. Diffusion weighted MR imaging in non-infarct lesions of the brain. Eur J Radiol. 2008;65:402–416. doi: 10.1016/j.ejrad.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Sutter R, Kaplan PW. What to see when you are looking at confusion: a review of the neuroimaging of acute encephalopathy. J Neurol Neurosurg Psychiatry. 2015;86:446–459. doi: 10.1136/jnnp-2014-308216. [DOI] [PubMed] [Google Scholar]
- 6.Koh E, Tsai LK, Hong CT. Serum calcium concentration affects signal changes on diffusion-weighted imaging in hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2012;33:297–300. doi: 10.3174/ajnr.A2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang EG, Jeon SJ, Choi SS, Song CJ, Yu IK. Diffusion MR imaging of hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2010;31:559–564. doi: 10.3174/ajnr.A1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witsch J, Neugebauer H, Flechsenhar J, Jüttler E. Hypoglycemic encephalopathy: a case series and literature review on outcome determination. J Neurol. 2012;259:2172–2181. doi: 10.1007/s00415-012-6480-z. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda T, Takahashi T, Sato A, Tanaka H, Igarashi S, Fujita N, et al. Predictors of outcome in hypoglycemic encephalopathy. Diabetes Res Clin Pract. 2013;101:159–163. doi: 10.1016/j.diabres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Berkovic SF, Bladin PF, Darby DG. Metabolic disorders presenting as stroke. Med J Aust. 1984;140:421–424. doi: 10.5694/j.1326-5377.1984.tb108105.x. [DOI] [PubMed] [Google Scholar]
- 11.Cordonnier C, Oppenheim C, Lamy C, Meder JF, Mas JL. Serial diffusion and perfusion-weighted MR in transient hypoglycemia. Neurology. 2005;65:175. doi: 10.1212/01.wnl.0000167128.14769.7b. [DOI] [PubMed] [Google Scholar]
- 12.Auer RN. Hypoglycemic brain damage. Forensic Sci Int. 2004;146:105–110. doi: 10.1016/j.forsciint.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Johkura K, Nakae Y, Kudo Y, Yoshida TN, Kuroiwa Y. Early diffusion MR imaging findings and short-term outcome in comatose patients with hypoglycemia. AJNR Am J Neuroradiol. 2012;33:904–909. doi: 10.3174/ajnr.A2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma JH, Kim YJ, Yoo WJ, Ihn YK, Kim JY, Song HH, et al. MR imaging of hypoglycemic encephalopathy: lesion distribution and prognosis prediction by diffusion-weighted imaging. Neuroradiology. 2009;51:641–649. doi: 10.1007/s00234-009-0544-5. [DOI] [PubMed] [Google Scholar]
- 15.Bathla G, Hegde AN. MRI and CT appearances in metabolic encephalopathies due to systemic diseases in adults. Clin Radiol. 2013;68:545–554. doi: 10.1016/j.crad.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt P, Böttcher J, Ragoschke-Schumm A, Mentzel HJ, Wolf G, Müller UA, et al. Diffusion-weighted imaging of hyperacute cerebral hypoglycemia. AJNR Am J Neuroradiol. 2011;32:1321–1327. doi: 10.3174/ajnr.A2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan R, Erbay S, Oljeski S, Thaler D, Bhadelia R. Case report: hypoglycemia and diffusion-weighted imaging. J Comput Assist Tomogr. 2003;27:420–423. doi: 10.1097/00004728-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739–2748. doi: 10.2337/dc06-9916. [DOI] [PubMed] [Google Scholar]
- 19.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherian A, Thomas B, Baheti NN, Chemmanam T, Kesavadas C. Concepts and controversies in nonketotic hyperglycemia-induced hemichorea: further evidence from susceptibility-weighted MR imaging. J Magn Reson Imaging. 2009;29:699–703. doi: 10.1002/jmri.21672. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Choi JY, Lee SH, Song SY, Lee YS. Hemichorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26:905–911. doi: 10.1097/00004728-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Atay M, Yetis H, Kurtcan S, Aralasmak A, Alkan A. Susceptibility weighted imaging features of nonketotic hyperglycemia: unusual cause of hemichorea-hemiballismus. J Neuroimaging. 2015;25:319–324. doi: 10.1111/jon.12084. [DOI] [PubMed] [Google Scholar]
- 23.Bizet J, Cooper CJ, Quansah R, Rodriguez E, Teleb M, Hernandez GT. Chorea, Hyperglycemia, Basal Ganglia Syndrome (C-H-BG) in an uncontrolled diabetic patient with normal glucose levels on presentation. Am J Case Rep. 2014;15:143–146. doi: 10.12659/AJCR.890179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BC, Hwang SH, Chang GY. Hemiballismus-hemichorea in older diabetic women: a clinical syndrome with MRI correlation. Neurology. 1999;52:646–648. doi: 10.1212/wnl.52.3.646. [DOI] [PubMed] [Google Scholar]
- 25.Nagai C, Kato T, Katagiri T, Sasaki H. Hyperintense putamen on T1-weighted MR images in a case of chorea with hyperglycemia. AJNR Am J Neuroradiol. 1995;16:1243–1246. [PMC free article] [PubMed] [Google Scholar]
- 26.Kandiah N, Tan K, Lim CC, Venketasubramanian N. Hyperglycemic choreoathetosis: role of the putamen in pathogenesis. Mov Disord. 2009;24:915–919. doi: 10.1002/mds.22277. [DOI] [PubMed] [Google Scholar]
- 27.Dewey RB, Jr, Jankovic J. Hemiballism-hemichorea. Clinical and pharmacologic findings in 21 patients. Arch Neurol. 1989;46:862–886. doi: 10.1001/archneur.1989.00520440044020. [DOI] [PubMed] [Google Scholar]
- 28.Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dillon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol. 2004;25:975–976. [PMC free article] [PubMed] [Google Scholar]
- 29.Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol. 2002;59:448–452. doi: 10.1001/archneur.59.3.448. [DOI] [PubMed] [Google Scholar]
- 30.Chua CB, Sun CK, Hsu CW, Tai YC, Liang CY, Tsai IT. “Diabetic striatopathy”: clinical presentations, controversy, pathogenesis, treatments, and outcomes. Sci Rep. 2020;10:1594. doi: 10.1038/s41598-020-58555-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Berl T. Sodium. Lancet. 1998;352:220–228. doi: 10.1016/S0140-6736(97)12169-9. [DOI] [PubMed] [Google Scholar]
- 32.Córdoba J, García-Martinez R, Simón-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab Brain Dis. 2010;25:73–80. doi: 10.1007/s11011-010-9172-3. [DOI] [PubMed] [Google Scholar]
- 33.Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102:67–77. doi: 10.1016/s0002-9343(96)00274-4. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds RM, Padfield PL, Seckl JR. Disorders of sodium balance. BMJ. 2006;332:702–705. doi: 10.1136/bmj.332.7543.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Förster A, Nölte I, Wenz H, Al-Zghloul M, Kerl HU, Brockmann C, et al. Value of diffusion-weighted imaging in central pontine and extrapontine myelinolysis. Neuroradiology. 2013;55:49–56. doi: 10.1007/s00234-012-1083-z. [DOI] [PubMed] [Google Scholar]
- 36.Chu K, Kang DW, Ko SB, Kim M. Diffusion-weighted MR findings of central pontine and extrapontine myelinolysis. Acta Neurol Scand. 2001;104:385–388. doi: 10.1034/j.1600-0404.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 37.Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44) J Neurol. 1999;246:700–705. doi: 10.1007/s004150050435. [DOI] [PubMed] [Google Scholar]
- 38.Graff-Radford J, Fugate JE, Kaufmann TJ, Mandrekar JN, Rabinstein AA. Clinical and radiologic correlations of central pontine myelinolysis syndrome. Mayo Clin Proc. 2011;86:1063–1067. doi: 10.4065/mcp.2011.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JJ, Chang HF, Chen DL. Recurrent episodic vertigo secondary to hyponatremic encephalopathy from water intoxication. Neurosciences (Riyadh) 2014;19:328–330. [PMC free article] [PubMed] [Google Scholar]
- 40.Yalcin-Cakmakli G, Karli Oguz K, Shorbagi A, Funda Bas D, Ergan-Arsava B, Kunt M, et al. Hyponatremic encephalopathy after excessive water ingestion prior to pelvic ultrasound: neuroimaging findings. Intern Med. 2010;49:1807–1811. doi: 10.2169/internalmedicine.49.3395. [DOI] [PubMed] [Google Scholar]
- 41.Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 42.Bhatia S, Kapoor AK, Sharma A, Gupta R, Kataria S. Cerebral encephalopathy with extrapontine myelinolysis in a case of postpartum hypernatremia. Indian J Radiol Imaging. 2014;24:57–60. doi: 10.4103/0971-3026.130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismail FY, Szóllics A, Szólics M, Nagelkerke N, Ljubisavljevic M. Clinical semiology and neuroradiologic correlates of acute hypernatremic osmotic challenge in adults: a literature review. AJNR Am J Neuroradiol. 2013;34:2225–2232. doi: 10.3174/ajnr.A3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saroja AO, Naik KR, Mali RV, Kunam SR. ‘Wine Glass’ sign in recurrent postpartum hypernatremic osmotic cerebral demyelination. Ann Indian Acad Neurol. 2013;16:106–110. doi: 10.4103/0972-2327.107719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004;107:1–16. doi: 10.1016/j.clineuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Kang E, Jeon SJ, Choi SS. Uremic encephalopathy with atypical magnetic resonance features on diffusion-weighted images. Korean J Radiol. 2012;13:808–811. doi: 10.3348/kjr.2012.13.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HC, Cheng SJ. The syndrome of acute bilateral basal ganglia lesions in diabetic uremic patients. J Neurol. 2003;250:948–955. doi: 10.1007/s00415-003-1122-0. [DOI] [PubMed] [Google Scholar]
- 48.Wang HC, Brown P, Lees AJ. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov Disord. 1998;13:952–957. doi: 10.1002/mds.870130615. [DOI] [PubMed] [Google Scholar]
- 49.Kim TK, Seo SI, Kim JH, Lee NJ, Seol HY. Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov Disord. 2006;21:1267–1270. doi: 10.1002/mds.20932. [DOI] [PubMed] [Google Scholar]
- 50.Moe SM, Sprague SM. Uremic encephalopathy. Clin Nephrol. 1994;42:251–256. [PubMed] [Google Scholar]
- 51.Okada J, Yoshikawa K, Matsuo H, Kanno K, Oouchi M. Reversible MRI and CT findings in uremic encephalopathy. Neuroradiology. 1991;33:524–526. doi: 10.1007/BF00588046. [DOI] [PubMed] [Google Scholar]
- 52.Biasioli S, D'Andrea G, Feriani M, Chiaramonte S, Fabris A, Ronco C, et al. Uremic encephalopathy: an updating. Clin Nephrol. 1986;25:57–63. [PubMed] [Google Scholar]
- 53.Prüss H, Siebert E, Masuhr F. Reversible cytotoxic brain edema and facial weakness in uremic encephalopathy. J Neurol. 2009;256:1372–1373. doi: 10.1007/s00415-009-5125-3. [DOI] [PubMed] [Google Scholar]
- 54.U-King-Im JM, Yu E, Bartlett E, Soobrah R, Kucharczyk W. Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR Am J Neuroradiol. 2011;32:413–418. doi: 10.3174/ajnr.A2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JY, Yu IK. Acute hyperammonemic encephalopathy with features on diffusion-weighted images: report of two cases. J Korean Soc Radiol. 2015;72:131–135. [Google Scholar]
- 56.Rosario M, McMahon K, Finelli PF. Diffusion-weighted imaging in acute hyperammonemic encephalopathy. Neurohospitalist. 2013;3:125–130. doi: 10.1177/1941874412467806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKinney AM, Sarikaya B, Spanbauer J, Lohman BD, Uhlmann E. Acute hepatic (or hyperammonemic) encephalopathy: diffuse cortical injury and the significance of ammonia. AJNR Am J Neuroradiol. 2011;32:E142. doi: 10.3174/ajnr.A2622. author reply E143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott TR, Kronsten VT, Hughes RD, Shawcross DL. Pathophysiology of cerebral oedema in acute liver failure. World J Gastroenterol. 2013;19:9240–9255. doi: 10.3748/wjg.v19.i48.9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodrigo R, Cauli O, Boix J, ElMlili N, Agusti A, Felipo V. Role of NMDA receptors in acute liver failure and ammonia toxicity: therapeutical implications. Neurochem Int. 2009;55:113–118. doi: 10.1016/j.neuint.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Lichter-Konecki U, Mangin JM, Gordish-Dressman H, Hoffman EP, Gallo V. Gene expression profiling of astrocytes from hyperammonemic mice reveals altered pathways for water and potassium homeostasis in vivo. Glia. 2008;56:365–377. doi: 10.1002/glia.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bindu PS, Sinha S, Taly AB, Christopher R, Kovoor JM. Cranial MRI in acute hyperammonemic encephalopathy. Pediatr Neurol. 2009;41:139–142. doi: 10.1016/j.pediatrneurol.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Takanashi J, Barkovich AJ, Cheng SF, Kostiner D, Baker JC, Packman S. Brain MR imaging in acute hyperammonemic encephalopathy arising from late-onset ornithine transcarbamylase deficiency. AJNR Am J Neuroradiol. 2003;24:390–393. [PMC free article] [PubMed] [Google Scholar]
- 63.Capizzano AA, Sanchez A, Moritani T, Yager J. Hyperammonemic encephalopathy: time course of MRI diffusion changes. Neurology. 2012;78:600–601. doi: 10.1212/WNL.0b013e318247ccd1. [DOI] [PubMed] [Google Scholar]
- 64.Atluri DK, Prakash R, Mullen KD. Pathogenesis, diagnosis, and treatment of hepatic encephalopathy. J Clin Exp Hepatol. 2011;1:77–86. doi: 10.1016/S0973-6883(11)60126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008;29:1612–1621. doi: 10.3174/ajnr.A1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 67.Thumburu KK, Taneja S, Vasishta RK, Dhiman RK. Neuropathology of acute liver failure. Neurochem Int. 2012;60:672–675. doi: 10.1016/j.neuint.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 68.Desjardins P, Du T, Jiang W, Peng L, Butterworth RF. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int. 2012;60:690–696. doi: 10.1016/j.neuint.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 69.McKinney AM, Lohman BD, Sarikaya B, Uhlmann E, Spanbauer J, Singewald T, et al. Acute hepatic encephalopathy: diffusion-weighted and fluid-attenuated inversion recovery findings, and correlation with plasma ammonia level and clinical outcome. AJNR Am J Neuroradiol. 2010;31:1471–1479. doi: 10.3174/ajnr.A2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang LJ, Zhong J, Lu GM. Multimodality MR imaging findings of low-grade brain edema in hepatic encephalopathy. AJNR Am J Neuroradiol. 2013;34:707–715. doi: 10.3174/ajnr.A2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bleibel W, Al-Osaimi AM. Hepatic encephalopathy. Saudi J Gastroenterol. 2012;18:301–309. doi: 10.4103/1319-3767.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernicke C. In: Lehrbuch der Gehirnkrankheiten für Ärzte und Studierende, Vol. 1-3. Wernicke C, editor. Kassel: Fisher Verlag; 1881. Die akute hamorrhagische Polioencephalitis superior; pp. 229–242. [Google Scholar]
- 73.Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke's encephalopathy: a review. Biomed Res Int. 2014;2014:503596. doi: 10.1155/2014/503596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cerase A, Rubenni E, Rufa A, Vallone I, Galluzzi P, Coratti G, et al. CT and MRI of Wernicke's encephalopathy. Radiol Med. 2011;116:319–333. doi: 10.1007/s11547-011-0618-x. [DOI] [PubMed] [Google Scholar]
- 75.Halavaara J, Brander A, Lyytinen J, Setälä K, Kallela M. Wernicke's encephalopathy: is diffusion-weighted MRI useful? Neuroradiology. 2003;45:519–523. doi: 10.1007/s00234-003-1043-8. [DOI] [PubMed] [Google Scholar]
- 76.Fattal-Valevski A, Kesler A, Sela BA, Nitzan-Kaluski D, Rotstein M, Mesterman R, et al. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics. 2005;115:e233–e238. doi: 10.1542/peds.2004-1255. [DOI] [PubMed] [Google Scholar]
- 77.Harper C. The incidence of Wernicke's encephalopathy in Australia--a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46:593–598. doi: 10.1136/jnnp.46.7.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hazell AS, Todd KG, Butterworth RF. Mechanisms of neuronal cell death in Wernicke's encephalopathy. Metab Brain Dis. 1998;13:97–122. doi: 10.1023/a:1020657129593. [DOI] [PubMed] [Google Scholar]
- 79.Moritani T, Smoker WR, Sato Y, Numaguchi Y, Westesson PL. Diffusion-weighted imaging of acute excitotoxic brain injury. AJNR Am J Neuroradiol. 2005;26:216–228. [PMC free article] [PubMed] [Google Scholar]
- 80.Weidauer S, Rösler A, Zanella FE, Lanfermann H. Diffusion-weighted imaging in Wernicke encephalopathy associated with stomach cancer: case report and review of the literature. Eur Neurol. 2004;51:55–57. doi: 10.1159/000075093. [DOI] [PubMed] [Google Scholar]
- 81.Chu K, Kang DW, Kim HJ, Lee YS, Park SH. Diffusion-weighted imaging abnormalities in wernicke encephalopathy: reversible cytotoxic edema? Arch Neurol. 2002;59:123–127. doi: 10.1001/archneur.59.1.123. [DOI] [PubMed] [Google Scholar]