Abstract
This document summarizes suggestions of the central sleep apnea (CSA) Technical Expert Panel working group. This paper shares our vision for bringing the right device to the right patient at the right time. For patients with CSA, current coverage criteria do not align with guideline treatment recommendations. For example, CPAP and oxygen therapy are recommended but not covered for CSA. On the other hand, bilevel positive airway pressure (BPAP) without a backup rate may be a covered therapy for OSA, but it may worsen CSA. Narrow coverage criteria that require near elimination of obstructive breathing events on CPAP or BPAP in the spontaneous mode, even if at poorly tolerated pressure levels, may preclude therapy with BPAP with backup rate or adaptive servoventilation, even when those devices provide demonstrably better therapy. CSA is a dynamic disorder that may require different treatments over time, sometimes switching from one device to another; an example is switching from BPAP with backup rate to an adaptive servoventilation with automatic end-expiratory pressure adjustments, which may not be covered. To address these challenges, we suggest several changes to the coverage determinations, including: (1) a single simplified initial and continuing coverage definition of CSA that aligns with OSA; (2) removal of hypoventilation terminology from coverage criteria for CSA; (3) all effective therapies for CSA should be covered, including oxygen and all PAP devices with or without backup rates or servo-mechanisms; and (4) patients shown to have a suboptimal response to one PAP device should be allowed to add oxygen or change to another PAP device with different capabilities if shown to be effective with testing.
Key Words: central sleep apnea, CPAP, noninvasive ventilation, oxygen
Abbreviations: AHI, apnea-hypopnea index; ASV, adaptive servoventilation; BPAP, bilevel positive airway pressure; CAHI, central apnea-hypopnea index; CSA, central sleep apnea; HFrEF, heart failure with reduced ejection fraction; OAHI, obstructive apnea-hypopnea index; ONMAP, Optimal NIV Medicare Access Promotion; PSG, polysomnogram; Spo2, oxygen saturation by pulse oximetry; TECSA, treatment-emergent central sleep apnea
Note to the Reader: The current document is one of a series produced by a Technical Expert Panel (TEP), whose purpose was to propose changes to Centers for Medicare & Medicaid Services national coverage determinations for the use of noninvasive ventilation and home mechanical ventilation, which were formulated in 1998. Specifically, the TEP proposed changes to national coverage determinations for thoracic restrictive disorders (neuromuscular disease), COPD, hypoventilation syndromes, central sleep apnea, and OSA. The background, makeup of the TEP, and key recommendations are highlighted in an Executive Summary. CHEST, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society formed the “Optimal NIV Medicare Access Promotion (ONMAP)” to provide processes to obtain the “right device for the right patient at the right time.” More details and rationale for the proposed changes are available in the companion documents.
Central sleep apnea (CSA) is characterized by repetitive transient instability of respiratory drive, resulting in repetitive lessening in ventilatory effort during sleep, in turn leading to apneas, hypopneas, and hyperpneas.1 Central apneas are identified by diagnostic devices that record absence of airflow while there is little or no movement of respiratory muscles; by contrast, during obstructive apneas, the respiratory muscles are active. Alternating patterns of apneas or hypopneas followed by hyperpnea occurs in about one-third of patients with heart failure (called CSA with Cheyne-Stokes breathing), during excursion to high altitude (CSA due to high-altitude periodic breathing), and rarely without any accompanying disease (idiopathic or primary CSA). Medical disorders such as brainstem lesions may directly impair ventilatory control neurons and lead to CSA. CSA also occurs acutely in 5% to 15% of patients with OSA when treatment restores pharyngeal patency (treatment-emergent CSA [TECSA], also called complex sleep apnea).2 TECSA may be more common in patients living at high altitudes.3 CSA can occur in patients using opioids and may follow either a periodic or more ataxic pattern (CSA due to a medication or substance).4 It may also occur in the presence of other respiratory or sleep-related breathing disorders such as congenital central hypoventilation syndrome and in patients with myopathies. Finally, it is worth noting that many patients have features of both OSA and CSA during a single night or within individual respiratory events, and it may be challenging to differentiate the underlying mechanism.5 CSA causes poor sleep quality and adverse effects on cardiovascular health. CSA is associated with symptoms including but not limited to insomnia, frequent awakenings, snoring, witnessed apneas, nonrestorative sleep, hypersomnia, and nocturnal dyspnea.1
Moderate to severe CSA is associated with increased mortality in patients with heart failure with reduced ejection fraction (HFrEF). In a prospective study evaluating 963 patients with chronic stable HFrEF, those with moderate to severe CSA had almost 20% higher mortality rates than those without sleep-disordered breathing following adjustments for multiple clinical variables.6 In another prospective study of 88 patients with HFrEF, the median survival of patients with CSA was 45 months compared with 90 months for those without CSA (hazard ratio, 2.14; P = .02).7 Other studies generally suggest a worse prognosis associated with CSA in heart failure.8
The morbidity and mortality associated with CSA may vary greatly depending on the underlying cause. CSA has also been associated with higher disability or mortality in patients with stroke and transient ischemic attacks.9 CSA at high altitude impairs sleep quality but generally resolves with acclimatization or descent. In a population-based study of primary CSA, 24% of the patients died during a median population follow-up duration of 4.4 years.10 This high mortality rate may reflect unrecognized cardiac or neurologic comorbidities not known at the time of diagnosis. The prognostic implications of CSA on patients chronically using opioids are not known.
Background
Concerns With Current Coverage Criteria
Although serving many patients with CSA, the current policies (Table 1) may be a barrier to effective treatment in several circumstances. As exemplars, coverage is not possible for these kinds of patients:
-
•
A 67-year-old patient presents with symptoms of frequent awakenings at night and spousal observations of frequent apneas. Polysomnogram (PSG) reveals CSA with a central apnea-hypopnea index (CAHI) 11 events/h, obstructive AHI (OAHI) 7 events/h, and frequent awakenings. On CPAP 8 cm H2O, the CAHI is 6 events/h, the OAHI is 1 event/h, and sleep continuity is much improved. Idiopathic CSA is diagnosed. The current policies do not allow CPAP coverage for this patient.
-
•
An 84-year-old patient with severe heart failure and an ejection fraction of 35% has significant symptoms, including disrupted sleep. PSG reveals CSA with Cheyne-Stokes breathing with an AHI of 30 events/h. Mean oxygen saturation by pulse oximetry (Spo2) is 93%, and the minimum is 87%. Spo2 is ≤ 88% for 3.5 min, and it is sporadic. The patient is diagnosed with CSA. The breathing does not improve on CPAP, and PAP is poorly tolerated. On oxygen 3 L/min via nasal cannula, without a PAP device, there are no apneas, and the arousal index is decreased by 75%. The current policies do not allow oxygen therapy for this patient.
-
•
A 76-year-old patient with a history of stroke now reports severe sleepiness. During PSG the CAHI is 20 events/h, and the OAHI is 30 events/h. As the CPAP pressure is raised, the CAHI rises, and the OAHI falls. The best setting on CPAP or BPAP yields a CAHI of 30 events/h and an OAHI of 9 events/h. Using BPAP with backup rate during a separate PSG, at best pressure the CAHI is 4 events/h, and the OAHI is 6 events/h. Despite significant objective and subjective improvement on BPAP with backup rate, the residual AHI of 6 events/h precludes the diagnosis of complex sleep apnea or coverage for appropriate therapy, as the national coverage determination definition of "CompSA" (complex sleep apnea) requires that central apnea be present “when titrated to the point where obstructive events have been effectively treated (obstructive AHI less than 5 per hour).”11
-
•
A 69-year-old man has severe TECSA and congestive heart failure with an ejection fraction of 65%. He has been using BPAP with backup rate for 13 months, but he still has a high AHI and persistent sleepiness and insomnia. Using adaptive servoventilation (ASV) during a repeat attended PSG, the AHI is improved, and the patient has improved sleep quality. The current policies do not allow for ASV therapy for 5 years because another E0471 (bilevel pressure capacity with backup rate) has been previously covered.
Table 1.
Current National Coverage Determination Criteria for Treatment of CSA and Complex Sleep Apnea
| 2021 NCD Criteria for Treating Central/Complex Sleep Apnea | 2021 Proposed NCD Criteria for Treating Central Sleep Apnea |
| CPAP or Oxygen: No coverage for CSA BPAP (E0470 or E0471) Requires both criteria A & B.
|
|
| Current Definitions | 2021 Proposed Definition |
CSA requires all:
|
CSAa requires all:
|
AHI = apnea-hypopnea index; BPAP = bilevel positive airway pressure; CAHI = central apnea-hypopnea index; CompSA = complex sleep apnea (more modern term is “treatment-emergent central sleep apnea”); CSA = central sleep apnea; PSG = polysomnogram.
Definition applies whether on or off PAP.
Current Evidence and Guidelines
There are relatively few studies of outcomes after CSA therapy, but clinical experience and early data support CSA therapy to improve symptoms and perhaps to improve outcomes.1 CANPAP (Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial), a randomized trial of CPAP therapy for patients with HFrEF and Cheyne-Stokes breathing, found no major benefit for CPAP therapy compared with medical treatment, but some post hoc analyses suggested better outcomes in patients who respond to CPAP intervention.12,13 Some studies showed improvement in ejection fraction with ASV therapy in congestive heart failure, and clinical practice repeatedly shows that some patients with CSA report markedly improved insomnia, fatigue, and other symptoms while on ASV therapy.14 However, the SERVE-HF (Treatment of Sleep-disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure) study reported potential for harm when patients with HFrEF and Cheyne-Stokes breathing were treated with an ASV therapy.15,16 Ongoing trials using different ASV treatment algorithms are designed to further examine the role for this therapy in patients with heart failure receiving optimized medical therapy.
Opioid-induced CSA is more frequent and more severe at higher narcotic doses, and clinical experience has shown that ASV treatment for CSA may improve breathing patterns, sleep continuity, and pain and might contribute to opioid weaning.4
Most TECSA resolves spontaneously, but randomized trials have shown that treatment of the CSA component is associated with improved PAP adherence for OSA therapy.17 Switching from CPAP to ASV may be associated with abrupt improvements in residual apnea and in adherence at the time of switching.18 Data in this area are evolving, but it is already clear that ASV is an important therapy for patients with persistent TECSA. Sleep transition CSA usually resolves without intervention. Idiopathic CSA is often treated with CPAP, BPAP with backup rate, or ASV, without a strong evidence base.19
Oxygen treatment may be effective in CSA from any of the aforementioned causes.20 High altitude periodic breathing seems to respond better to supplemental oxygen than to ASV therapy.21 Clinically, some patients intolerant of PAP therapy respond well to oxygen therapy.1
We expect ongoing studies to clarify the best CSA treatments for long-term outcomes. Until then, clinicians individualize therapy with CPAP, BPAP with backup rate, ASV, and supplemental oxygen to achieve PSG evidence of stabilized breathing patterns, improved blood oxygenation, and better sleep continuity, as well as to achieve symptomatic relief. Coverage determinations should allow therapy based on best current practices.
Optimal NIV Medicare Access Proposal (ONMAP): Revised National Coverage Determination and Related Policies for CSA
The current policies were developed over the last 20 years, during which time the understanding of CSA pathophysiology, responses to treatment, and device technology have changed. The aforementioned cases exemplify situations in which modifications to the current policies must allow better medical management of these patients. We consider the main areas requiring modification to be as follows: (1) the definition of CSA, to bring it into harmony with current clinical definitions; (2) precisely state the sleep testing required to diagnose CSA; (3) create coverage for positive airway pressure devices and/or oxygen therapy that provide clinical benefit; and (4) harmonize ongoing coverage criteria with those of OSA rather than with diseases with hypoventilation.
Specific Suggestions
Definition of CSA
As previously stated, current policies separately define “central sleep apnea” and “complex sleep apnea.” Treatment options may be similar for each of these disorders, and the distinct definitions do not always add clarity or clinical value. One particular issue is that the policies require that a patient with CSA must have OAHI < 5 events/h, which is not consistent with current CSA or complex sleep apnea/TECSA definitions. Some patients with CSA continue to have OAHI > 5 events/h at optimal tolerated treatment pressures. As treatment pressure rises, central events may predominate, and at times finding an ideal pressure that eliminates obstructive events worsens central events.
We suggest that revised policies adopt a single definition of CSA that aligns with accepted society definitions, with CSA predominance without necessitating resolution of obstructive events. In addition, we suggest that qualifying symptoms for CSA therapy should parallel the symptoms that qualify a patient for OSA therapy and be generalized to prevent frivolous rejection of prescriptions on the basis of unlisted specific symptoms.
Criteria for All Types of CSA (Patients Must Meet Both A and B)
-
A.AHI criteria: A PSG or a sleep study that measures airflow and respiratory muscle movement or that is otherwise validated as a diagnostic test for CSA, must show:
-
1.A CAHI is ≥ 5 events/h AND
-
2.The sum of central apneas and hypopneas is ≥ 50% of the sum of all apneas and hypopneas AND
-
1.
-
B.Symptom criteria:
-
1.Patients with an AHI ≥ 5 events/h and < 15 events/h should show any impairment in sleep-related quality of life that in the judgment of the treating physician may be expected to benefit from therapy or have co-morbid cardiovascular conditions (eg, hypertension, ischemic heart disease, congestive heart failure, stroke), OR
-
2.Patients have an AHI ≥ 15 events/h
-
1.
The current definition of CSA requires that, “There is no evidence of daytime or nocturnal hypoventilation.”11 Later, however, the policy requires, “Significant improvement of the sleep-associated hypoventilation.” Presumably, hypoventilation is thus used in two ways in the current policy, one referring to sustained retention of CO2, and the other referring to hypopneas. This does not add clarity or value.
The revised policies should not refer to hypoventilation in the section on CSA.
Coverage Criteria for Positive Airway Pressure Devices and/or Oxygen Therapy That Provide Clinical Benefit
In a minority of patients, CPAP and/or oxygen provide significant improvement in CSA,19 and these treatments are recommended in clinical guidelines. Unfortunately, the current national coverage determinations do not cover CPAP (E0601) or oxygen therapy for patients with CSA.
We suggest that the revised policies allow CPAP as therapy for CSA when it is shown to be effective.
We suggest that the revised policies allow oxygen therapy for CSA when it is shown to be effective for an individual patient during sleep testing in the following situations:
-
1.
Altitude-related periodic breathing that is shown to reduce the AHI and result in clinical improvement with oxygen therapy, OR
-
2.
CSA with cumulatively ≥ 5 min Spo2 ≤ 88%, OR
-
3.
CSA persistent on CPAP or E0471 device without sustained hypoxia but oxygen (either in addition to PAP or alone) is shown to lead to clinical improvement, such as improving sleep quality or reduce the CAHI < 10 events/h, OR
-
4.
CPAP or E0471 is not tolerated or is contraindicated, and oxygen alone is shown to lead to clinical improvement, such as improving polysomnographic sleep quality, bring the Spo2 ≥ 88% or CAHI < 10 events/h
E0471 devices operate with proprietary algorithms. As a result, some patients respond better to one servoventilation or BPAP with backup rate device than to another.
We suggest that the revised policies should cover treatment with any effective E0471, even if a patient is previously prescribed and using a less effective BPAP device with a backup rate.
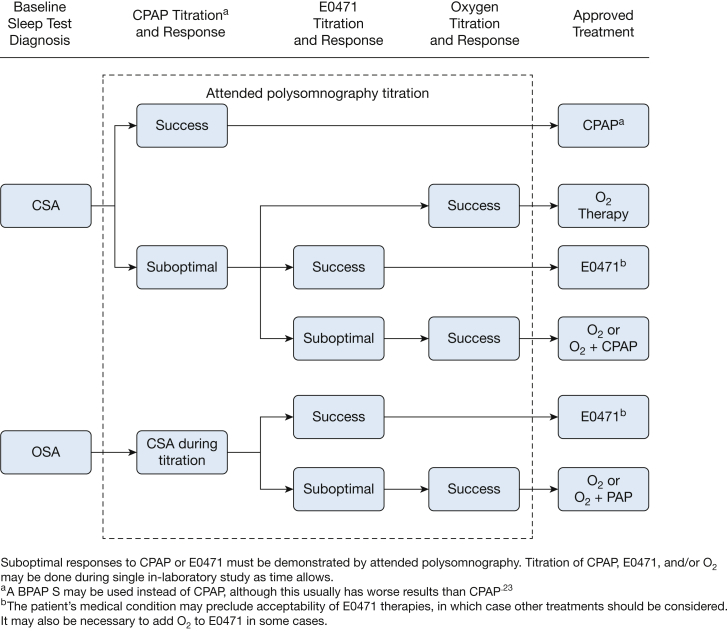
Clinical suboptimal responses or improvement with PAP devices or oxygen should be shown during PSG. As is common practice, split-night or trials of several different treatment modalities during one night of PSG may be appropriate, for example, when one modality is clearly ineffective following only a short exposure.22 The current policies require demonstration of “significant improvement” with the device at the setting that is prescribed for initial home use. Because AHI does not always resolve in CSA even when oxygen saturations, arousals, and symptoms improve, significant improvement should include measures of improvement other than AHI based on the discretion of the treating physician. A suggested evaluation pathway is shown in Figure 1.23
Figure 1.
Suggested evaluation pathway for determining treatment of CSA syndrome. E0471 is a bilevel device with a backup rate. BPAP = bilevel positive airway pressure; CSA = central sleep apnea; O2 = oxygen; PAP = positive airway pressure; S = spontaneous.
Patients with CSA requiring E0471 devices are currently required to have a face-to-face follow-up in 61 to 90 days following the start of therapy even if documentation of adherence and benefit can be made earlier. There is no clinical reason why delayed evaluation is necessary for this population.
We suggest that the policy providing continuing coverage for CSA should include the same criteria as patients with OSA and clinical documentation of benefit can be shown in the same 31 to 90 days as CPAP.
However, consistent with the other technical expert panel category suggestions, those still engaged with their noninvasive ventilation treating physician, and not yet meeting adherence criteria at day 90, should be allowed coverage for another 90-day period prior to consideration of alternative therapy.
Summary of New Suggestions
A summary of suggested changes in current coverage determinations, which will improve care for patients with CSA, is presented in Table 2.23
Table 2.
Suggested Changes to the Current Coverage Policies to Improve Access to the Right Treatments in a Timely Fashion
|
|
|
|
|
|
BPAP = bilevel positive airway pressure; CMS = Centers for Medicare & Medicaid Services; CSA = central sleep apnea; PAP = positive airway pressure; S/T = spontaneous/timed.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: T. I. M. has served as a consultant for Withings, Respicardia, Inc, and has received research funding from Withings and AR Medical Technologies Inc. A. M. is funded by National Institutes of Health (NIH). He reports income from Equillium, Corvus and Livanova related to medical education. ResMed provided a philanthropic donation to UC San Diego. R. B. B. serves on scientific advisory board of Cerebra. M. R. is a partial owner of Greater Washington Sleep Disorders Centers. None declared (K. G. J.).
∗ONMAP Technical Expert Panel members: Overall Chairs: Peter C. Gay, MD, FCCP, and Robert L. Owens, MD. Thoracic Restrictive Disorders: Chair/Co-Chair—Lisa F. Wolfe, MD, FCCP, and Joshua O. Benditt, MD, FCCP. Thoracic Restrictive Disorders Committee Members—Loutfi S. Aboussouan, MD, FCCP, John M. Coleman III, MD, and Dean R. Hess, PhD, RRT. COPD: Chair/Co-chair—Nicholas S. Hill, MD, FCCP, and Gerard J. Criner, MD, FCCP. COPD Committee Members—Richard D. Branson, MSc, RRT, Bartolome R. Celli, MD, FCCP, Neil R. MacIntyre, MD, FCCP, and Amen Sergew, MD. Central Sleep Apnea: Chair/Co-Chair—Timothy I. Morgenthaler, MD, and Atul Malhotra, MD, FCCP. Central Sleep Apnea Committee Members—Richard B. Berry, MD, Karin G. Johnson, MD, and Marc I. Raphaelson, MD. Hypoventilation: Chair/Co-chair—Babak Mokhlesi, MD, FCCP, and Christine H. Won, MD. Hypoventilation Committee Members—Bernardo J. Selim, MD, FCCP, Barry J. Make, MD, FCCP, and Bernie Y. Sunwoo, MBBS. OSA: Chair/Co-chair—Nancy A. Collop, MD, Master FCCP, and Susheel P. Patil, MD, PhD. OSA Committee Members—Alejandro D. Chediak, MD, FCCP, Eric J. Olson, MD, and Kunwar Praveen Vohra, MD, FCCP.
Contributor Information
Timothy I. Morgenthaler, Email: tmorgenthaler@mayo.edu.
ONMAP Technical Expert Panel:
Peter C. Gay, Robert L. Owens, Lisa F. Wolfe, Joshua O. Benditt, Loutfi S. Aboussouan, John M. Coleman, III, Dean R. Hess, Nicholas S. Hill, Gerard J. Criner, Richard D. Branson, Bartolome R. Celli, Neil R. MacIntyre, Amen Sergew, Timothy I. Morgenthaler, Atul Malhotra, Richard B. Berry, Karin G. Johnson, Marc I. Raphaelson, Babak Mokhlesi, Christine H. Won, Bernardo J. Selim, Barry J. Make, Bernie Y. Sunwoo, Nancy A. Collop, Susheel P. Patil, Alejandro D. Chediak, Eric J. Olson, and Kunwar Praveen Vohra
References
- 1.Aurora R.N., Chowdhuri S., Ramar K., et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgenthaler T.I., Kagramanov V., Hanak V., Decker P.A. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29(9):1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 3.Pagel J.F., Kwiatkowski C., Parnes B. The effects of altitude associated central apnea on the diagnosis and treatment of obstructive sleep apnea: comparative data from three different altitude locations in the mountain west. J Clin Sleep Med. 2011;7(6):610–615A. doi: 10.5664/jcsm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javaheri S., Malik A., Smith J., Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008;4(4):305–310. [PMC free article] [PubMed] [Google Scholar]
- 5.Javaheri S., McKane S.W., Cameron N., Germany R.E., Malhotra A. In patients with heart failure the burden of central sleep apnea increases in the late sleep hours. Sleep. 2019;42(1) doi: 10.1093/sleep/zsy195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldenburg O., Wellmann B., Buchholz A., et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2015;37(21):1695–1703. doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
- 7.Javaheri S., Shukla R., Zeigler H., Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49(20):2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 8.Lyons O.D., Bradley T.D. Heart failure and sleep apnea. Can J Cardiol. 2015;31(7):898–908. doi: 10.1016/j.cjca.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Parra O., Arboix A., Montserrat J.M., Quinto L., Bechich S., Garcia-Eroles L. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24(2):267–272. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- 10.Kouri I., Kolla B.P., Morgenthaler T.I., Mansukhani M.P. Frequency and outcomes of primary central sleep apnea in a population-based study. Sleep Med. 2020;68:177–183. doi: 10.1016/j.sleep.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Centers for Medicare & Medicaid Services. Local Coverage Determination Respiratory Assist Devices (L33800). Updated 08/08/2021. Accessed September 16, 2021.
- 12.Arzt M., Floras J.S., Logan A.G., et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115(25):3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 13.Bradley T.D., Logan A.G., Kimoff R.J., et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 14.Sharma B.K., Bakker J.P., McSharry D.G., Desai A.S., Javaheri S., Malhotra A. Adaptive servo-ventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis. Chest. 2012;142(5):1211–1221. doi: 10.1378/chest.12-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowie M.R., Woehrle H., Wegscheider K., et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javaheri S., Brown L.K., Randerath W., Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900–904. doi: 10.1016/j.chest.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Morgenthaler T., Kuzniar T.J., Wolfe L.F., Willes L., McLain W.C., Goldberg R. The Complex Sleep Apnea Resolution Study: a prospective randomized controlled trial of continuous positive airway pressure vs. adaptive servoventilation therapy. Sleep. 2014;37(5):927–934. doi: 10.5665/sleep.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pépin J.-L.D., Woehrle H., Liu D., et al. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med. 2018;14(1):57–63. doi: 10.5664/jcsm.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issa F.G., Sullivan C.E. Reversal of central sleep apnea using nasal CPAP. Chest. 1986;90(2):165–171. doi: 10.1378/chest.90.2.165. [DOI] [PubMed] [Google Scholar]
- 20.Sands S.A., Edwards B.A., Terrill P.I., et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J. 2018;52(3) doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr J.E., Heinrich E.C., Djokic M., et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178–184. doi: 10.1089/ham.2017.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzniar T.J., Golbin J.M., Morgenthaler T.I. Moving beyond empiric continuous positive airway pressure (CPAP) trials for central sleep apnea: a multi-modality titration study. Sleep Breathing. 2007;11(4):259–266. doi: 10.1007/s11325-007-0118-x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson K.G., Johnson D.C. Bilevel positive airway pressure worsens central apneas during sleep. Chest. 2005;128(4):2141–2150. doi: 10.1378/chest.128.4.2141. [DOI] [PubMed] [Google Scholar]