Abstract
Background
Pseudomonas aeruginosa (PA) is a common cause of respiratory infection and morbidity. Pseudomonas elastase is an important virulence factor regulated by the lasR gene. Whether PA elastase activity is associated with worse clinical outcomes in ICU patients is unknown.
Research Question
Is there an association between PA elastase activity and worse host outcomes in a cohort of ICU patients?
Methods
PA respiratory isolates from 238 unique ICU patients from two tertiary-care centers within the University of Pittsburgh Medical Center health system were prospectively collected and screened for total protease and elastase activity, biofilm production, antimicrobial resistance, and polymicrobial status. The association between pathogen characteristics and 30-day and 90-day mortality was calculated using logistic regression. For subgroup analysis, two patterns of early (≤72 h) and late sample (>72 h) collection from the index ICU admission were distinguished using a finite mixture model. Lung inflammation and injury was evaluated in a mouse model using a PA high elastase vs low elastase producer.
Results
PA elastase activity was common in ICU respiratory isolates representing 75% of samples and was associated with increased 30-day mortality (adjusted OR [95% CI]: 1.39 [1.05-1.83]). Subgroup analysis demonstrated that elastase activity was a risk factor for 30- and 90-day mortality in the early sample group, whereas antimicrobial resistance was a risk factor for 90-day mortality in the late sample group. Whole genome sequencing of high and low elastase producers showed that predicted loss-of-function lasR genotypes were less common among high elastase producers. Mice infected with a high elastase producer showed increased lung bacterial burden and inflammatory profile compared with mice infected with a low elastase producer.
Interpretation
Elastase activity is associated with 30-day ICU mortality. A high elastase producing clinical isolate confers increased lung tissue inflammation compared with a low elastase producer in vivo.
Key Words: critical illness, pneumonia, Pseudomonas aeruginosa, pseudomonas elastase activity
Abbreviations: MDR, multidrug resistant; mSOFA, modified sequential organ failure assessment score; PA, Pseudomonas aeruginosa; T2SS, Type 2 Secretion System; XDR, extensively drug resistant
Take-home Points.
Research Question: Is there an association between Pseudomonas aeruginosa elastase activity and worse clinical outcomes in a cohort of ICU patients?
Results:Pseudomonas aeruginosa elastase activity was identified in 75% of respiratory isolates and is associated with increased 30-day mortality. Mice infected with a high elastase producing clinical isolate demonstrate increased lung tissue damage in vivo.
Interpretation: Pseudomonas elastase activity may serve as a target for point of care biomarkers and novel adjuvant therapies.
Pseudomonas aeruginosa (PA) is an opportunistic, gram-negative bacterium and a major cause of acute lower respiratory tract infections in the ICU.1 PA infection has been previously associated with increased ICU length of stay and prolonged mechanical ventilation.2 Additionally, PA infection has been associated with increased ICU mortality and remains a global health care burden despite implementation of targeted antimicrobial interventions.3, 4, 5 PA uses multiple mechanisms to adapt to diverse environments, including the deployment of extracellular virulence factors, either secreted or injected exoproteins, that are capable of causing lung tissue damage and are regulated by two-component regulatory systems.6, 7, 8, 9, 10 In particular, the type 2 secretion system (T2SS) enables the secretion of exoproteins that require intracellular folding11,12 and include elastase (LasB), lipases, phospholipases, lipoxygenase A, and exotoxin A, among others.13
We previously showed that LasB, a T2SS exoprotease (also known as Pseudomonas elastase), is associated with increased neutrophilic inflammation and lung tissue damage during acute intrapulmonary infection in mice.14 This is consistent with other preclinical and clinical studies that indicate elastase activity is an important virulence factor.15, 16, 17 Although prior studies have focused primarily on characterizing PA phenotypes in chronic lung infections and in patients with underlying structural lung disease,18,19 little information exists regarding pathogen characteristics of PA clinical isolates, including secreted elastase and protease activity in a large cohort of ICU patients. Additionally, whether PA protease or elastase activity are associated with worse clinical outcomes in critically ill patients is not known. In this study, we characterized PA pathogen factors including elastase and protease activity, biofilm production, antimicrobial resistance, and polymicrobial culture status, in ICU patients, and we report patient-associated outcomes.
Methods
Sample Collection
PA respiratory isolates were prospectively collected from ICUs at two tertiary-care hospitals within the University of Pittsburgh Medical Center health care system between July 2016 and April 2018. The study was granted a waiver of informed consent and approved by the University of Pittsburgh Institutional Review Board (STUDY20010070). Additional methods for patient data extraction, in vitro analysis of pathogen characteristics, in vivo experiments, and statistical analysis are outlined in e-Appendix 1.
Results
Pathogen and Host Characteristics
PA isolates were identified from respiratory samples of 238 unique patients who were admitted to the ICU. The pathogen characteristics of the PA respiratory isolates were screened in vitro for secreted protease activity, elastase activity, and biofilm production. Multidrug or extensively drug resistant (MDR/XDR) status and polymicrobial culture results were obtained from the clinical microbiology data. PA elastase and protease activity were detectable and common in ICU respiratory isolates, representing 75% and 71% of isolates, respectively (Table 1). Compared with the reference PA14 research strain, the median (IQR) for percent elastase activity was 25.9 (0-106.5), and 12.0 (0-65.3) for percent protease activity. Forty-four percent of isolates showed high elastase activity, and 32% showed high protease activity. The discrepancy in percent elastase and percent protease activity lies in the fact that the activity assays used different substrates for cleavage (elastin and casein, respectively). As expected, PA protease and elastase activity were highly correlated (r = 0.82, e-Fig 1) but not identical. MDR/XDR was seen in 32% of isolates, and biofilm formation was seen in 61% of isolates (Table 1). The antimicrobial resistance patterns for the PA ICU respiratory isolates are outlined in e-Table 1. Protease and elastase activity were not associated with antimicrobial resistance, polymicrobial culture results, or biofilm production (e-Table 2).
Table 1.
Pseudomonas aeruginosa Isolates With Protease and Elastase Activity Are Common in the ICU
| PA Pathogen Characteristics | No. Samples (%) |
|---|---|
| Elastase activitya | |
| Any (>0%) | 178 (74.8) |
| Low (0%-50%) | 134 (56.3) |
| High (>50%) | 104 (43.7) |
| Protease activitya | |
| Any (>0%) | 170 (71.4) |
| Low (0%-50%) | 161 (67.7) |
| High (>50%) | 77 (32.3) |
| Biofilm productionb | 143 (60.6) |
| MDR/XDRc | 74 (31.6) |
| Polymicrobiald | 88 (37.5) |
P aeruginosa (PA) respiratory isolates were obtained from unique ICU patients admitted to the ICU at two academic medical referral centers. MDR = multidrug-resistant; XDR = extensively drug resistant.
Elastase and protease activity were normalized to a research PA reference strain (PA14) and reported as % elastase or protease activity: Any (>0%), Low (0%-50%), High (>50%). The elastase activity used fluorogenic elastin as a substrate, and protease activity used fluorogenic casein as the substrate. N = 238 for elastase activity. N = 238 for protease activity.
Biofilm production was measured using a crystal violet staining assay as described in the Methods section. n = 236 for biofilm production.
Multidrug or extensively drug resistant (MDR/XDR) was defined by resistance to ≥3 or ≥6 antibiotic classes, respectively, and were combined for the analysis. n = 234 for MDR/XDR status
Polymicrobial was defined as the presence of other pathogens from the same respiratory sample, n = 235 for polymicrobial.
Baseline characteristics of the 238 unique ICU patients are outlined in Table 2. The median age was 63 years, and 58% were male. Seventy-nine percent of patients were White, reflecting the demographics of the surrounding region. The median modified sequential organ failure assessment (mSOFA) score was 6 (IQR, 4-8), and 74% showed a Charlson Co-morbidity Index (CCI) of ≥2. The median number of days from the index ICU admission to sample collection was 2 days (IQR, 1-6). One hundred eighty-four patients (77%) were mechanically ventilated. The median ICU length of stay was 18 days (IQR, 8-34) with 30- and 90-day mortality of 34% and 47%, respectively.
Table 2.
Clinical Characteristics of ICU Patients With Low vs High Elastase Producers
| ICU Patient Characteristics | Total N = 238 |
Low Elastase n = 134 |
High Elastase n = 104 |
P |
|---|---|---|---|---|
| Age, median (IQR) | 63 (55-72) | 61 (48-70) | 66 (56-73) | .02 |
| Male, No. (%) | 138 (58.0) | 77 (57.5) | 61 (58.7) | .90 |
| Race, No. (%)a | .02 | |||
| White | 189 (79.4) | 106 (79.1) | 83 (79.8) | |
| Black | 32 (13.5) | 12 (15.7) | 11 (10.6) | |
| Asian | 5 (2.1) | 0 | 5 (4.8) | |
| Unknown | 12 (5.0) | 7 (5.2) | 5 (4.8) | |
| mSOFA score,b median (IQR) | 6 (4-8) | 6 (4-8) | 6 (4-9) | .08 |
| CCI, median (IQR) | 3 (1-5) | 2 (1-5) | 3 (1-4) | .79 |
| CCI score, No. (%) | .61 | |||
| 0 | 28 (11.8) | 14 (10.5) | 14 (13.5) | |
| 1 | 35 (14.7) | 22 (16.4) | 13 (12.5) | |
| ≥2 | 175 (73.5) | 98 (73.1) | 77 (74.0) | |
| Sample collection day,c median (IQR) | 2 (1-6) | 2 (0-6) | 2 (1-6) | .34 |
| ICU LOS,c median (IQR), d | 18 (8-34) | 19 (9-37) | 14 (7-31) | .03 |
| Mechanical ventilation, No. (%) | 184 (77.3) | 101 (75.4) | 83 (79.8) | .44 |
| HHFNC, No. (%) | 11 (4.6) | 3 (2.2) | 8 (7.8) | .06 |
| Bloodstream infection, No. (%) | 32 (13.5) | 14 (10.5) | 18 (17.3) | .13 |
| 30-d mortality,c No. (%) | 81 (34.0) | 38 (28.4) | 43 (41.4) | .04 |
| 90-d mortality,c No. (%) | 112 (47.1) | 59 (44.0) | 53 (51.0) | .30 |
238 ICU patients demonstrated a respiratory culture growing P aeruginosa. Elastase and protease activity were normalized to a research PA reference strain (PA14) and reported as % elastase activity. Low (0-50%); High (>50%). P values ≤ .05 are significant and are shown in bold. IQR = interquartile range; mSOFA = modified sequential organ failure assessment score; CCI = Charlson Co-morbidity Index; HHFNC = heated high-flow nasal canula oxygen
Race P value analysis excludes unknown race category.
Modified SOFA score calculated with neurologic score equal to 0; therefore, maximum score is 20.
Sample collection date, ICU length of stay (LOS), and 30-d and 90-d mortality were calculated from the index ICU admission date.
In stratifying the entire cohort (N = 238) into low vs high elastase producers, subjects with high elastase producing PA strains were older (P = .02). There was a significant difference in race demographics between groups, likely attributable to an increase in the Asian population in the high elastase producer group. This finding may be due to the small sample size of Asian subjects in the cohort (n = 0 vs n = 5). The frequency of mechanical ventilation in the low elastase producer group was 75% vs 80% in the high elastase producer group (P = .44). Frequency of heated high-flow nasal cannula oxygen use in the low producer group was 2% vs 8% in the high elastase producer group (P = .06). The high elastase producer group was associated with higher 30-day mortality (P = .04) but inversely associated with increased length of stay (P = .03). We speculate that the latter association is confounded by the competing risk of increased 30-day mortality in the high elastase producer group.
Elastase and Protease Activity From PA ICU Respiratory Isolates Were Associated With 30-Day ICU Mortality
In logistic regression analysis adjusted for age, sex, and mSOFA, elastase (OR [95% CI], 1.39 [1.05-1.83] for each SD increase in activity) and protease activity (OR [95% CI], 1.34 [1.02-1.76] for each SD increase in activity) were significantly associated with 30-day mortality (Table 3). No association was found between elastase or protease activity and 90-day mortality in the unadjusted or adjusted model. Additionally, there was no association between antimicrobial resistance or biofilm activity and 30-day or 90-day mortality. In the unadjusted analysis, there was a statistically significant association between polymicrobial infection and 30-day and 90-day mortality; however, this association did not remain significant in the adjusted models.
Table 3.
Association of PA Pathogen Characteristics and Mortality
| PA Pathogen Characteristics, N = 238 |
30-d Mortality |
90-d Mortality |
||
|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusteda OR (95% CI) |
Unadjusted OR (95% CI) |
Adjustedb OR (95% CI) |
|
| Elastase activity, per SD | 1.38 (1.05-1.82) | 1.39 (1.05-1.83) | 1.27 (0.97-1.66) | 1.26 (0.96-1.66) |
| Protease activity, per SD | 1.37 (1.05-1.78) | 1.34 (1.02-1.76) | 1.15 (0.89-1.48) | 1.07 (0.81-1.42) |
| Biofilm production,c per SD | 1.13 (0.87-1.47) | 1.11 (0.85-1.45) | 1.11 (0.85-1.44) | 1.03 (0.78-1.36) |
| MDR/XDRc | 1.09 (0.68-1.74) | 0.89 (0.48-1.65) | 1.26 (0.81-1.95) | 1.18 (0.64-2.15) |
| Polymicrobiald | 0.49 (0.30-0.78) | 0.60 (0.33-1.09) | 0.57 (0.37-0.87) | 0.69 (0.38-1.23) |
A logistic regression model was used to determine the association between PA pathogen factors and 30-d and 90-d mortality from time of index ICU admission. CCI = Charlson Co-morbidity Index; MDR = multidrug-resistant; mSOFA = modified sequential organ failure assessment score; XDR = extensively drug resistant
The 30-d mortality model was adjusted for age, sex and mSOFA.
The 90-d mortality model was adjusted for age, sex, mSOFA, and CCI. P < .05 are in bold.
n = 236 for Biofilm production, n = 234 for MDR/XDR.
n = 235 for polymicrobial.
To determine the distribution of PA respiratory isolate sample collection, we calculated the time in days from the date of the index ICU admission to the date of sample collection (e-Fig 2). The distribution of time in days to sample collection was 0 to 74 days. Using a finite mixture model, the distribution of sample collection dates clustered into two subgroups. The subgroups include patients who underwent early PA respiratory isolate collection, defined as ≤72 h from the date of index ICU admission (n = 156) and patients with late PA respiratory isolate sample collection, defined as >72 h from the date of the index ICU admission (n = 82). Subsequently, logistic regression models were used to determine the association between PA pathogen characteristics and ICU mortality at 30 and 90 days in the early and late collection subgroups (Table 4 and Table 5, respectively).
Table 4.
Association of PA Pathogen Characteristics and Mortality in Early Samples
| PA Pathogen Characteristics, n = 156 | 30-d Mortality |
90-d Mortality |
||
|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusteda OR (95% CI) |
Unadjusted OR (95% CI) |
Adjustedb OR (95% CI) |
|
| Elastase activity, per SD | 1.67 (1.17-2.38) | 1.56 (1.10-2.23) | 1.62 (1.12-2.34) | 1.55 (1.08-2.22) |
| Protease activity, per SD | 1.58 (1.14-2.20) | 1.46 (1.04-2.07) | 1.39 (1.01-1.92) | 1.30 (0.92-1.86) |
| Biofilm production,c per SD | 1.03 (0.77-1.38) | 1.01 (0.75-1.36) | 1.10 (0.82-1.47) | 1.05 (0.77-1.44) |
| MDR/XDRc | 0.98 (0.56-1.72) | 0.83 (0.39-1.78) | 0.86 (0.51-1.48 | 0.72 (0.34-1.51) |
| Polymicrobiald | 0.41 (0.23-0.73) | 0.57 (0.27-1.22) | 0.66 (0.39-1.12) | 0.91 (0.44-1.87) |
A logistic regression model was used to determine the association between PA pathogen factors and 30-d and 90-d mortality in early samples collected between 0 and 3 days from the index ICU admission date. The early and late subgroups were determined using a finite mixture model. CCI = Charlson Co-morbidity Index; MDR = multidrug-resistant; mSOFA = modified sequential organ failure assessment score; XDR = extensively drug resistant.
The 30-d mortality model was adjusted for age, sex, and mSOFA.
The 90-d mortality model was adjusted for age, sex, mSOFA, and CCI. P < .05 are in bold.
n = 154 for Biofilm production and n = 153 for MDR/XDR.
n = 154 for polymicrobial.
Table 5.
Association of PA Pathogen Characteristics and Mortality in Late Samples
| PA Pathogen Characteristics, n = 82 | 30-d Mortality |
90-d Mortality |
||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjustedb | |
| Elastase activity, per SD | 0.79 (0.42-1.50) | 0.80 (0.41-1.57) | 0.71 (0.41-1.22) | 0.67 (0.36-1.27) |
| Protease activity, per SD | 0.97 (0.57-1.64) | 0.98 (0.56-1.73) | 0.75 (0.47-1.22) | 0.69 (0.40-1.19) |
| Biofilm production, per SD | 1.51 (0.82-2.77) | 1.55 (0.82-2.94) | 1.14 (0.64-2.02) | 0.96 (0.52-1.77) |
| MDR/XDRc | 1.33 (0.55-3.22) | 1.10 (0.35-3.42) | 2.79 (1.26-6.15) | 3.41 (1.10-10.58) |
| Polymicrobialc | 0.77 (0.33-1.81) | 0.88 (0.31-2.54) | 0.42 (0.20-0.89) | 0.38 (0.14-1.09) |
A logistic regression model was used to determine the association between PA pathogen factors and 30-d and 90-d ICU mortality in late samples collected ≥4 d from the index ICU admission date. The early and late subgroups were determined using a finite mixture model. CCI = Charlson Co-morbidity Index; MDR = multidrug-resistant; mSOFA = modified sequential organ failure assessment score; XDR = extensively drug resistant.
The 30-d mortality model was adjusted for age, sex, and mSOFA.
The 90-d mortality model was adjusted for age, sex, mSOFA, and CCI. P < .05 are in bold.
n = 81 for MDR/XDR and polymicrobial.
PA Elastase Activity Was Associated With 30-Day and 90-Day ICU Mortality in Early Samples
In the early sample collection subgroup, PA elastase and protease activity were significantly associated with 30-day mortality in both the unadjusted and adjusted model (Table 4). Elastase activity but not protease activity was also associated with 90-day mortality in the adjusted model (OR [95% CI], 1.55 [1.08-2.22]). To address any potential immortal time bias, we performed an additional analysis of 30- and 90-day mortality from the time of specimen collection rather than from the index ICU admission date. By this approach, we defined a risk period time (time from ICU admission to PA isolate collection) that is excluded from the analysis. The new analysis shows the same directionality as the original analysis, in that elastase activity in the early sample collection is associated with both 30-day (OR, 1.47 [1.05-2.06]) and 90-day mortality (1.55 [1.08-2.22]). In the early sample subgroup, there was no association between biofilm formation or MDR/XDR status and 30- or 90-day mortality. Polymicrobial infection was significantly associated with decreased ICU mortality in the 30-day unadjusted model, but this association was no longer significant in the adjusted model.
PA Elastase Activity Was Not Associated With 30-Day and 90-Day Mortality in Late Samples
In the late sample collection group, there was no association between PA elastase or protease activity and 30 or 90-day mortality in the unadjusted or adjusted model (Table 5). However, in the late sample collection group, MXR/XDR status was significantly associated with higher 90-day mortality in the unadjusted and adjusted model (adjusted OR [95% CI], 3.41 [1.10-10.58]). No association was found between biofilm formation and 30- or 90-day mortality. Polymicrobial infection was associated with decreased 90-day mortality in the unadjusted model, but this relationship was no longer significant in the adjusted model.
No statistically significant differences were found between baseline characteristics in the early and late sample groups, including age, sex, and race (e-Table 3). No difference was seen in the length of hospital stay before ICU transfer between the two groups for those patients not directly admitted to the ICU, suggesting that our subgroups were not confounded by pre-ICU hospital stays. In the early sample collection group, there was a trend toward more co-morbidities by CCI score (P = .05), but no difference was seen when comorbidities were compared by individual diagnoses or organ system. Notably, the early sample group had an increased likelihood of ever having a prior PA infection documented in the Electronic Medical Records (P < .001) and were also more likely to have had a PA infection within the last year when compared with the late sample group (P = .03). There was no difference between PA infection within the prior month, although the sample size was very small.
PA Respiratory Isolates With Low Elastase Activity Were Associated With lasR Mutations When Analyzed by Whole Genome Sequencing
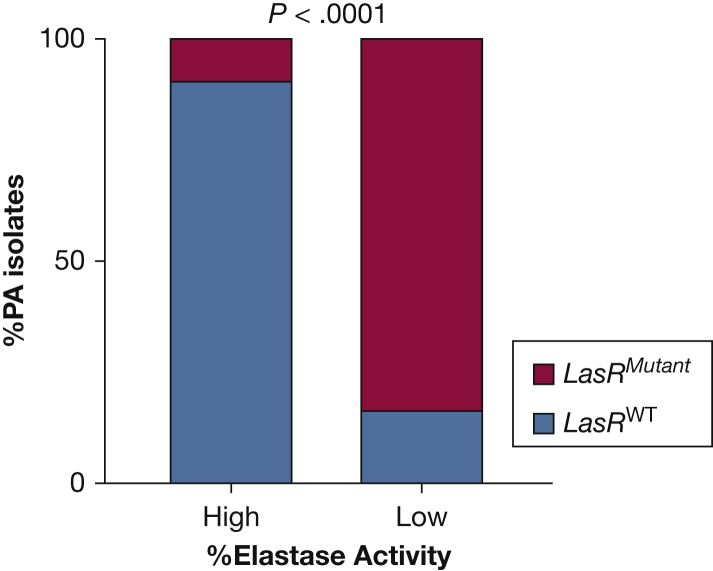
The lasR gene encodes the positive regulator of type II secretion gene expression, which controls LasB, and strains with mutations in lasR have low type II secretion activity.20, 21, 22 PA often adapts to the host environment and develops mutations in the lasR transcriptional regulator, such as in the case of chronic infections in people with cystic fibrosis.23 To determine whether lasR mutations could explain why some PA strains in the ICU cohort exhibited low elastase activity, the genomes of 23 PA isolates with high elastase activity and 24 with low elastase activity were sequenced, and sequencing reads were aligned to the PA14 reference genome (Fig 1). Among these isolates, mutant lasR genotypes were significantly more common among strains with low elastase production than strains with high elastase production (20/24 low elastase producers vs 2/23 high elastase producers, P < .0001). One of the 2/23 high elastase producers with detectable lasR mutations only had lasR mutations which were detected in other strains as well, suggesting that these mutations may be natural lasR variations that do not affect lasB gene expression (e-Fig 3). Finally, we also analyzed the rhlR gene sequences in every strain, because rhlR also can affect lasB transcription, though to a lower degree than lasR. Although some rhlR mutations were detected, these mutations were only detected either in strains that also had lasR mutations or in strains with high elastase activity (e-Fig 3). Therefore, it is unlikely that rhlR mutations are affecting lasB activity in these isolates.
Figure 1.
LasR mutation is associated with low elastase producing clinical isolates. The genomes of 23 high elastase producing and 24 no/low elastase producing PA ICU respiratory isolates were sequenced, and sequencing reads were aligned to the lasR and lasI reference gene sequences from PA reference strain PA14. Mutations were determined using Breseq. Statistical analysis was completed using Fisher exact test. Note that LasR regulates LasB, or Pseudomonas elastase.
Mice Infected With a High Elastase Producer Show Higher Lung Bacterial Burden, Increased Barrier Disruption, and Higher Inflammatory Cytokines In Vivo
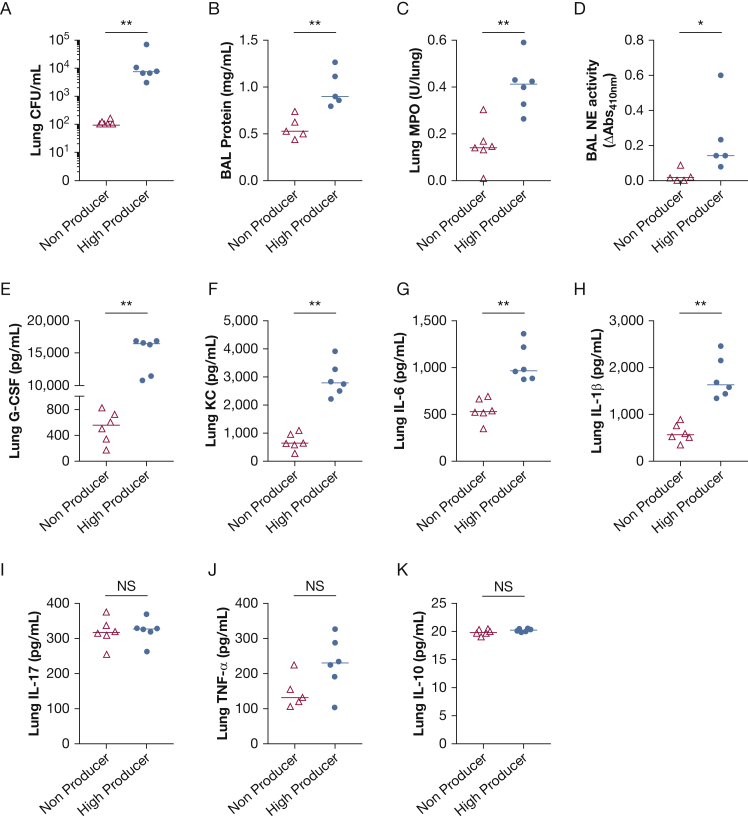
In our clinical data analysis, patients with high elastase producing PA respiratory isolates were associated with increased 30-day mortality. To evaluate a possible mechanism for our clinical findings, one high elastase producer and one low elastase producer were selected and evaluated for pathogenicity in an acute lung infection model. For a similar inoculum delivered, mice infected with a high elastase producer showed significantly higher lung bacterial burden (P = .002) (Fig 2A). Furthermore, infection with a high elastase producer showed increased total BAL protein, a marker of microvascular permeability, compared with mice infected with a low elastase producer (P = .008) (Fig 2B). The BAL total cell counts and neutrophil counts were not significantly different between mice undergoing acute intrapulmonary infection with the PA low elastase producer vs PA high elastase producer (e-Fig 4). However, mice infected with a high elastase producer showed increased lung tissue neutrophil content (myeloperoxidase content) and BAL free neutrophil elastase activity (P = .004 and P = .02, respectively) (Fig 2C-D). This suggests that neutrophils in the airspaces, although not different in total numbers in the airspace compartment, showed increased activation in the mice infected with a high elastase producer. Moreover, infection with a high elastase producer resulted in increased lung inflammatory cytokines including granulocyte colony-stimulating factor, keratinocyte-derived cytokine, IL-6, and IL-1β (Fig 2E-H), but no difference in IL-17, tumor necrosis factor alpha, or IL-10 levels in lung tissue (Fig 2I-K). This suggests that infection with a PA ICU respiratory clinical isolate exhibiting high elastase activity results in higher lung bacterial burden and increased microvascular permeability, and aggravates lung inflammation in vivo when compared with infection with a low elastase producing PA respiratory isolate.
Figure 2.
High elastase activity is associated with increased lung bacterial burden, microvascular permeability, and enhanced lung inflammatory cytokines in vivo. WT mice were infected with a PA ICU respiratory isolate from low elastase group (non-producer) or high elastase group (high producer). Mice underwent intratracheal inoculation with 1-2 × 106 CFU/mL and were killed at a predetermined time of 20 h after infection. A, Lung CFU/mL; B, BAL total protein concentrations (mg/mL); C, Lung myeloperoxidase content (units/lung); and D, BAL neutrophil elastase activity (ΔAbs410 nm) and lung tissue cytokines (pg/mL). E, G-CSF; F, KC; G, IL-6; H, IL-1ß; I, IL-17; J, TNF-α; and K, IL-10 were measured. Each data point represents an individual mouse. Lines indicate the median. ∗P < .05; ∗∗P < .01. Mann-Whitney test was used in the statistical analysis. CFU = colony-forming units; G-CSF = granulocyte colony-stimulating factor; MPO = myeloperoxidase; NE = neutrophil elastase; PA = Pseudomonas aeruginosa; TNF-α = tumor necrosis factor alpha; WT = wild-type.
Discussion
We examined the in vitro characteristics of PA pathogen factors, including elastase and protease activity, biofilm production, as well as MDR/XDR and polymicrobial status from 238 respiratory isolates collected from unique ICU patients. Although prior cohort studies examining PA virulence factors have largely focused on patients with chronic lung disease such as COPD and cystic fibrosis,18,24,25 this study represents the largest cohort to date of PA respiratory isolates collected from unique patients in the ICU. We demonstrate that PA elastase activity was common in respiratory isolates collected from critically ill patients, accounting for 75% of isolates studied. Moreover, PA elastase activity was associated with increased 30-day mortality. In contrast, MDR/XDR status, polymicrobial status, and biofilm production were not associated with increased 30-day mortality.
We identified two subgroups of patients based on the timing of the respiratory isolate collection from their index ICU admission date. In patients with early sample collection (0-72 h from index ICU admission), PA elastase activity was associated with increased 30-day and 90-day mortality even after adjusting for age, sex, and severity of acute illness. Conversely, in patients with late sample collection (>72 h from index ICU admission), there was no association between PA elastase or protease activity and mortality, suggesting that patients with early sample collection were driving the association between PA elastase activity and mortality seen in examination of the whole cohort. Finally, we demonstrate that mice infected with a high elastase producing clinical strain show higher bacterial burden, increased lung injury and inflammation compared with mice infected with a low elastase producing clinical strain. Taken together, these findings suggest that PA ICU respiratory clinical isolates exhibiting elastase activity are associated with increased acute mortality and enhanced lung inflammation in vivo.
One proposed mechanism for the association between elastase activity and acute mortality seen in this study is increased proteolytic lung tissue damage and inflammation. In vitro, LasB has been shown to degrade structural components of the lung, including elastin and collagen.26,27 LasB has also been found to disrupt intercellular junctions of the lung epithelia, leading to increased permeability.28,29 Furthermore, LasB is capable of degrading components of the innate and acquired immune system,30 including cytokines and chemokines,31 antimicrobial peptides, immunoglobulins, serum complement factors, and surfactant proteins.32 We previously showed that PA LasB activity and T2SS drive host neutrophil serine protease activity and lung tissue damage in mice susceptible to lung injury and that administration of LasB inhibitor, functional deletion of LasB, or genetic disabling of the T2SS improved host defense and neutrophilic injury in mice.14 We now show that mice infected with a high elastase producing clinical isolate show increased inflammation and tissue damage in vivo, suggesting a possible mechanism for pathogenicity and the association between elastase activity and 30-day mortality.
PA LasB, the source of PA elastase activity,14 is an important virulence factor regulated by quorum sensing and the transcriptional regulator lasR.21 We evaluated the association between elastase activity and genetic mutations in the lasR gene. Whole genome sequencing of the high and low elastase producers showed that low elastase producing strains were significantly more likely to have lasR mutations compared with those with high elastase production. These findings are consistent with prior studies that have shown that lasR mutations are associated with decreased elastase activity in laboratory and clinical PA strains.33 One surprising finding is that two thirds of our samples were obtained within the first 72 h of ICU admission, suggesting that most of the patients in our cohort are likely presenting to the ICU with PA infection rather than developing it during the ICU stay. Our statement is supported by the finding that the early sample collections are from patients with higher co-morbidities, cystic fibrosis, history of stroke, and prior PA infections. These are individuals with risk profiles compatible with PA infection from the community. Although PA often develops loss of function mutations in the lasR transcriptional regulator during chronic infections that shut off elastase activity,19,23 our findings invite the intriguing possibility that patients with a history of PA infections may still be at risk for high elastase producing isolates.
Our study has several strengths, including a large patient cohort, allowing study of both phenotypic and genotypic variations in PA respiratory isolates as well as a translational model of infection to study the mechanism of injury. However, data presented in this study were collected from two hospitals in one health care system, which may limit the generalizability of our findings.
One possible source of bias is that inclusion in our study required the patient to be alive in the ICU and have a respiratory culture positive for PA. We do not know the actual number of patients with PA infection that die in the ICU before a sample collection can be obtained. However, our estimation is that this would be a small number, because microbiology culture data are obtained in most patients admitted to the ICU with suspected infection. Because of the nature of data extraction methods and subgroup sample size, there are limitations in providing an intermediary phenotype that could explain the association between elastase activity and mortality; however, this study provides the initial framework for future hypothesis testing.
Interpretation
In conclusion, we have shown that elastase activity is a common pathogen characteristic of PA identified in the ICU and is associated with increased mortality. Mice infected with a high elastase producing clinical isolate demonstrate increased lung tissue damage in vivo. Further work is needed to determine mechanisms of lung injury caused by high elastase activity, because it may serve as a target for point-of-care biomarkers and novel adjuvant therapies.
Acknowledgments
Author contributions: J. Z. is the guarantor of the paper. J. Z. performed the experiments, designed, analyzed, and interpreted the data, and wrote the manuscript. H. P. performed the experiments, analyzed the data, and wrote the manuscript. M. H. and R. M. performed the experiments and analyzed the data. W. B., P. J., Y. D., J. B., and J. P. interpreted the data and revised the work for important intellectual content. M. N. provided critical statistical expertise, designed, analyzed, interpreted the data, and wrote the manuscript. J. S. L. conceived, designed, analyzed, interpreted the data, and wrote the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. S. L. discloses a paid consultantship with Janssen Pharmaceuticals, Inc. unrelated to this study. None declared (J. Z., H. F. P., W. B., M. H., R. M., P. J., Y. D., J. B., J. P., M. N.).
Role ofsponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers F32 HL152504 (J. Z.); R01 HL136143, R01 HL142084, K24 HL143285 (J. S. L.); Career Development Award Number IK2 BX004886 from the United States Department of Veterans Affairs Biomedical Laboratory R&D (BLRD) Service (W. B.); K22 AI127473 (P. J.), Cystic Fibrosis FoundationJORTH17F5 (P. J.); Cystic Fibrosis Foundation RDP BOMBER19RO (J. B.); The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (J. Z.); content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other sponsoring agency.
Supplementary Data
References
- 1.Hernández G., Rico P., Díaz E., Rello J. Nosocomial lung infections in adult intensive care units. Microbes Infect. 2004;6(11):1004–1014. doi: 10.1016/j.micinf.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Rello J., Jubert P., Vallés J., Artigas A., Rué M., Niederman M.S. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 1996;23(5):973–978. doi: 10.1093/clinids/23.5.973. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J.-L., Rello J., Marshall J., et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Nathwani D., Raman G., Sulham K., Gavaghan M., Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014;3(1):32. doi: 10.1186/2047-2994-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park D.R. The microbiology of ventilator-associated pneumonia. Respir Care. 2005;50(6):742–763. [PubMed] [Google Scholar]
- 6.Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: from bacterial pathogenesis to host response. J Intensive Care. 2014;2(1):10. doi: 10.1186/2052-0492-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharazmi A. Mechanisms involved in the evasion of the host defence by Pseudomonas aeruginosa. Immunol Lett. 1991;30(2):201–205. doi: 10.1016/0165-2478(91)90026-7. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez P.N., Koch G., Thompson J.A., Xavier K.B., Cool R.H., Quax W.J. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76(1):46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipnis E., Sawa T., Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect. 2006;36(2):78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Cadoret F., Ball G., Douzi B., Voulhoux R. Txc, a new type II secretion system of Pseudomonas aeruginosa strain PA7, is regulated by the TtsS/TtsR two-component system and directs specific secretion of the CbpE chitin-binding protein. J Bacteriol. 2014;196(13):2376–2386. doi: 10.1128/JB.01563-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirst T.R., Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci. 1987;84(21):7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douzi B., Filloux A., Voulhoux R. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci. 2012;367(1592):1059–1072. doi: 10.1098/rstb.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filloux A. Protein secretion systems in Pseudomonas aeruginosa: an essay on diversity, evolution, and function. Front Microbiol. 2011;2:155. doi: 10.3389/fmicb.2011.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu Y., Olonisakin T., Bain W., et al. Thrombospondin-1 protects against pathogen-induced lung injury by limiting extracellular matrix proteolysis. J Clin Invest Insight. 2018;3(3) doi: 10.1172/jci.insight.96914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H.B., DiMango E., Bryan R., et al. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64(1):37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackwood L.L., Stone R.M., Iglewski B.H., Pennington J.E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Berre R., Nguyen S., Nowak E., et al. Relative contribution of three main virulence factors in Pseudomonas aeruginosa pneumonia. Crit Care Med. 2011;39(9):2113–2120. doi: 10.1097/CCM.0b013e31821e899f. [DOI] [PubMed] [Google Scholar]
- 18.LaFayette S.L., Houle D., Beaudoin T., et al. Cystic fibrosis-adapted Pseudomonas aeruginosa quorum sensing lasR mutants cause hyperinflammatory responses. Sci Adv. 2015;1(6) doi: 10.1126/sciadv.1500199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith E.E., Buckley D.G., Wu Z., et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A. 2006;103(22):8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rust L., Pesci E.C., Iglewski B.H. Analysis of the Pseudomonas aeruginosa elastase (lasB) regulatory region. J Bacteriol. 1996;178(4):1134–1140. doi: 10.1128/jb.178.4.1134-1140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gambello M.J., Iglewski B.H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toder D.S., Ferrell S.J., Nezezon J.L., Rust L., Iglewski B.H. lasA and lasB genes of Pseudomonas aeruginosa: analysis of transcription and gene product activity. Infect Immun. 1994;62(4):1320–1327. doi: 10.1128/iai.62.4.1320-1327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Argenio D.A., Wu M., Hoffman L.R., et al. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol. 2007;64(2):512–533. doi: 10.1111/j.1365-2958.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marvig R.L., Sommer L.M., Molin S., Johansen H.K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet. 2015;47(1):57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo-Troyano A., Melo V., Marcos P.J., et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease patients with frequent hospitalized exacerbations: a prospective multicentre study. Respiration. 2018;96(5):417–424. doi: 10.1159/000490190. [DOI] [PubMed] [Google Scholar]
- 26.Hamdaoui A., Wund-Bisseret F., Bieth J.G. Fast solubilization of human lung elastin by Pseudomonas aeruginosa elastase. Am Rev Respir Dis. 1987;135(4):860–863. doi: 10.1164/arrd.1987.135.4.860. [DOI] [PubMed] [Google Scholar]
- 27.Heck L.W., Morihara K., McRae W.B., Miller E.J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun. 1986;51(1):115–118. doi: 10.1128/iai.51.1.115-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bain W., Olonisakin T., Yu M., et al. Platelets inhibit apoptotic lung epithelial cell death and protect mice against infection-induced lung injury. Blood Adv. 2019;3(3):432–445. doi: 10.1182/bloodadvances.2018026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azghani A.O. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am J Respir Cell Mol Biol. 1996;15(1):132–140. doi: 10.1165/ajrcmb.15.1.8679217. [DOI] [PubMed] [Google Scholar]
- 30.Theander T.G., Kharazmi A., Pedersen B.K., et al. Inhibition of human lymphocyte proliferation and cleavage of interleukin-2 by Pseudomonas aeruginosa proteases. Infect Immun. 1988;56(7):1673–1677. doi: 10.1128/iai.56.7.1673-1677.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W.W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990;58(9):3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alcorn J.F., Wright J.R. Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J Biol Chem. 2004;279(29):30871–30879. doi: 10.1074/jbc.M400796200. [DOI] [PubMed] [Google Scholar]
- 33.Woo T.E., Duong J., Jervis N.M., Rabin H.R., Parkins M.D., Storey D.G. Virulence adaptations of Pseudomonas aeruginosa isolated from patients with non-cystic fibrosis bronchiectasis. Microbiology (Reading) 2016;162(12):2126–2135. doi: 10.1099/mic.0.000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.