Abstract
Background
Although mucus plugging is a well-reported feature of asthma, whether asthma and type 2 inflammation affect mucociliary clearance (MCC) is unknown.
Research Question
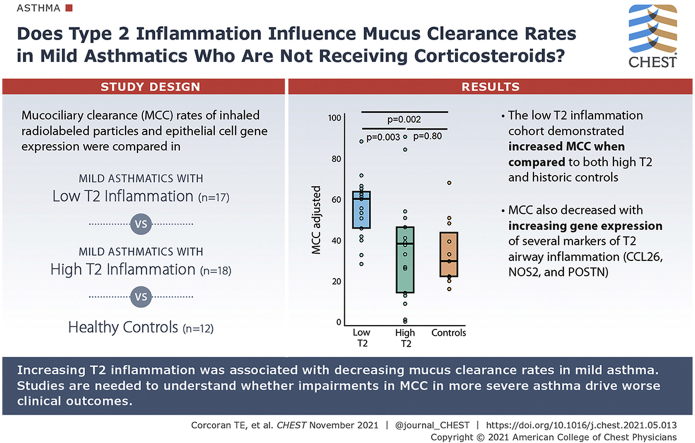
Does type 2 inflammation influence mucus clearance rates in patients with mild asthma who are not receiving corticosteroids?
Study Design and Methods
The clearance rates of inhaled radiolabeled particles were compared between patients with mild asthma with low (n = 17) and high (n = 18) levels of T2 inflammation. Fraction exhaled nitric oxide (Feno) was used to prospectively segregate subjects into T2 Lo (Feno < 25 ppb) and T2 Hi (Feno > 35 ppb) cohorts. Bronchial brush samples were collected with fiber-optic bronchoscopy, and quantitative polymerase chain reaction was performed to measure expression of genes associated with T2 asthma. MCC rate comparisons were also made with a historical group of healthy control subjects (HCs, n = 12).
Results
The T2 Lo cohort demonstrated increased MCC when compared with both T2 Hi and historic HCs. MCC within the T2 Hi group varied significantly, with some subjects having low or zero clearance. MCC decreased with increasing expression of several markers of T2 airway inflammation (CCL26, NOS2, and POSTN) and with Feno. MUC5AC and FOXJ1 expression was similar between the T2Lo and T2Hi cohorts.
Interpretation
Increasing T2 inflammation was associated with decreasing MCC. High rates of MCC in T2 Lo subjects may indicate a compensatory mechanism present in mild disease but lost with high levels of inflammation. Future studies are required to better understand mechanisms and whether impairments in MCC in more severe asthma drive worse clinical outcomes.
Key Words: asthma, cilia, lung imaging, mucociliary clearance, mucus, scintigraphy, type 2 inflammation
Abbreviations: ACT, Asthma Control Test; ALOX15, arachidonate 15-lipoxygenase; CCL26, chemokine ligand 26; Cen, percentage of aerosol deposition in the central lung zone; DTPA, diethylenetriamine pentaacetate; FEF25-75%, forced expiratory flowrate with 25% to 75% of FVC; FEF25-75%p, Forced expiratory flowrate with 25% to 75% of forced vital capacity, percent of predicted; Feno, fraction exhaled nitric oxide; FOXJ1, forkhead box protein J1; HCs, healthy control subjects; MCC, mucociliary clearance; MU5AC, mucin 5, subtypes A and C; NOS2, nitric oxide synthase 2; SLC26A4, solute carrier family 26 member 4; POSTN, periostin; T2Hi, group including subjects with Feno > 35 ppb; T2Lo, group including subjects with Feno < 25 ppb
Graphical Abstract
Mucus plugging is a common but poorly understood feature of airway inflammation and asthma. Extensive obstruction of the conducting airways with tenacious plugs is often observed in fatal asthma, and mucus plugging likely promotes airway obstruction and contributes to excessive morbidity as well. The type 2 cytokines, IL-4 and IL-13, bind to a heterodimeric IL-4/IL-13 receptor that activates signaling pathways that induce goblet cell differentiation and metaplasia, mucus hypersecretion, and, perhaps indirectly, airway hyperresponsiveness.1, 2, 3 Numerous studies have reported that IL-13 exposure increases mucus expression, secretion, and viscosity in vitro,4 which is at least partially controlled by 15-lipoxygenase/5(S)-hydroxyeicosatetraenoic acid.5,6 IL-13 was also reported to decrease mucus clearance in cultured primary human airways cells in association with expression of airway mucin 5, subtypes A and C (mucin 5AC).7
In addition to in vitro and mouse models, mucociliary clearance (MCC) and cough clearance can be assessed in human participants noninvasively by imaging the clearance of inhaled radiolabeled particles. Previous imaging studies of MCC in stable asthma inconsistently demonstrated both depression8,9 and enhancement of MCC rates compared with healthy control subjects (HCs).10,11 However, none of these studies included any specific phenotyping or compared outcomes with type 2 inflammation measurements. Another study demonstrated acute depression of MCC during asthma exacerbations, which recovered once the exacerbations resolved.12 Finally, and more recently, quantitative CT imaging of mucus plugs in asthma demonstrated increased mucus plugging in more severe disease, particularly in relation to biomarkers of type 2 inflammation.13
We hypothesized that mild corticosteroid-naïve asthma patients with higher levels of type 2 inflammation would demonstrate lower MCC when compared with similar mild patients without evidence of type 2 inflammation. Here we compared MCC between a “type 2 high” (T2Hi) group with fraction exhaled nitric oxide (Feno) >35ppb and a “type 2 low” (T2Lo) group with Feno < 25 ppb. These Feno limits were defined prospectively ahead of recruitment. Comparisons are also made with a historical group of HCs. We also performed research bronchoscopy with bronchial brushings to compare gene expression associated with mucus production, type 2 inflammation, and fibrosis with MCC.
Methods
Study Subjects
In this cross-sectional study, patients with asthma, ages 18 to 65 years, were included if they had an FEV1 of >70%, predicted with ≥12% reversibility (or a positive methacholine challenge in the previous 2 years), a BMI ≤ 38 and a ≤10 pack-year smoking history and no smoking for the past year. Patients were excluded if they were on inhaled or oral corticosteroids in the previous 30 days, had a respiratory infection, or used antibiotics in the last 30 days, were taking anticoagulants, were nursing or pregnant, or had an Feno between limits for the T2Lo and T2Hi groups (25-35 ppm).
Study Methods
All patients answered the Asthma Control Test (ACT) and performed pulmonary function testing (pre- and post-bronchodilator) and Feno measurements. We attempted to collect sputum for assessment of eosinophils via induction (Fahy method14), but very few of these mild asthma patients produced adequate samples. Thus, the patients were identified as T2Hi vs T2Lo asthma based on their Feno. Patients considered T2Hi had a Feno >35 ppb, whereas those considered T2Lo have a Feno of <25 ppb. As noted, patients with levels between 25 and 35 ppb were excluded. The lower limit was based on American Thoracic Society guidance for “low Feno.”15 We believed that the number of patients with mild asthma meeting the American Thoracic Society defined “high Feno” level (50 ppb) might be limited, but we still wanted to establish a margin between the groups, and thus settled on use of Feno measurements >35 ppb for our T2Hi group.
On separate study days, patients underwent MCC measurements and research fiber-optic bronchoscopy with airway brushings, using previously described procedures.16 Twenty-eight subjects successfully completed the bronchoscopy, and seven subjects were lost to follow-up or were otherwise unable to complete the bronchoscopy.
RNA was isolated from the airway epithelial cells collected from bronchial brushings, and quantitative real-time polymerase chain reaction was performed with TaqMan, using the following probes: MUC5AC (HS01365616_m1), Forkhead box protein J1 (FOXJ1; Hs00230964_m1), as well as the T2-associated genes, chemokine ligand 26 (CCL26; Hs00171146_m1), nitric oxide synthase 2 (NOS2; Hs00167248_m1), Solute Carrier Family 26 Member 4 (SLC26A4; Hs00166504_m1), arachidonate 15-lipoxygenase (ALOX15; Hs00609608_m1), and periostin (POSTN; Hs01566750_m1). Relative expression was determined using the ΔCt method with glucuronidase beta (Hs99999908_m1) as an internal control. Data are presented as the natural log of expression levels.
MCC measurements were performed by having the subjects inhale a liquid aerosol containing Technetium 99m sulfur colloid (a non-absorbable particle probe with a median size of 300 nm,17 296 MBq). A second absorbable, small-molecule radiopharmaceutical, indium 111- diethylenetriamine pentaacetate (DTPA) (In-DTPA, 56 MBq) was added to allow for measurement of absorptive clearance. Details of the clearance measurement technique are included in the supplement and in our previous reports.18 No bronchodilators were allowed on the day of the MCC studies. The method for measuring MCC in this study is identical to that used for a historical HC group.
Aerosol distribution pattern in the lungs is known to confound measures of MCC. High rates of central airway aerosol deposition are associated with increased MCC rates.19 Here we use that relationship to estimate an “adjusted” MCC rate. This method is explained in e-Appendix 1.
Analysis
Group comparisons were made using the Wilcoxon rank-sum test, except for distribution of sex, which was compared using χ2 testing. Spearman rank correlation coefficient was used to evaluate correlation between individual variables. Multivariable regression was used to estimate the relative effect of T2-associated gene expression and variables describing clinical condition on MCC in a model that includes a variable describing aerosol distribution—a known confounder of the MCC measurement. The P value for an individual variable within a multivariable model indicates the extent to which it contributes independently to the predictive value of the model. Model P and R2 values indicate the extent to which the multivariable model predicts the behavior of MCC. P values <.05 were considered significant. Stata/IC 14.0 (College Station, TX) was used for all calculations.
This study was approved by the University of Pittsburgh Institutional Review Board and the Human Use Subcommittee on Radiation Safety.
Results
Demographics and Clinical Profile
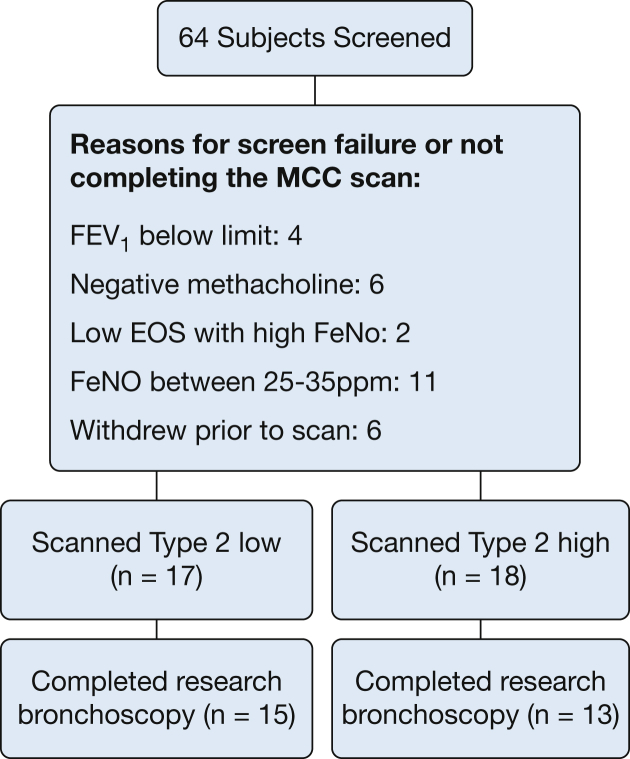
We screened a total of 64 subjects with asthma for this study. Of those, 35 patients enrolled and completed the MCC measurement, and 28 patients completed both research bronchoscopy and the MCC scan. Figure 1 shows the study screening, enrollment, and reasons for discontinuation, and Table 1 describes patient characteristics by group: T2Lo and T2Hi. ACT scores indicate worse asthma control in the T2Hi group. Pulmonary function is similar between the groups, and FEV1 is well preserved. Table 1 also includes details on the historical HC group.
Figure 1.
Breakdown of study screening, group assignment, and reasons for exclusion or not completing the MCC scan. Patients were assigned to the “type 2 low” (T2Lo) group if they had exhaled nitric oxide (Feno) <25 ppm and to the “type 2 high” (T2Hi) group (n = 18) with Feno > 35 ppm. Subjects in the range between groups were excluded.
Table 1.
Group Characteristics and Mucociliary Clearance (MCC) Outcomes
| T2 low (n = 17) | T2 high (n = 18) | T2 low vs T2 high P |
Historical control- subjects (n = 12) | |
|---|---|---|---|---|
| Age, years± | 22 ± 3± | 27 ± 9 | .14± | 22 ± 3 |
| Sex, M/F | 6/11 | 9/9 | .38 | 7/5 |
| ACT total± | 22 ± 4± | 19 ± 3 | .001 | … |
| ACT Question 1± | 4.8 ± 0.5± | 4.3 ± 0.6 | .006 | … |
| ACT Question 2± | 4.1 ± 1.0± | 3.6 ± 0.7 | .02 | … |
| ACT Question 3± | 4.5 ± 1.0± | 3.8 ± 0.9 | .003 | … |
| ACT Question 4± | 4.1 ± 0.9± | 3.5 ± 1.0 | .09 | … |
| ACT Question 5± | 4.2 ± 0.7± | 3.8 ± 0.7 | .09 | … |
| Feno± | 17 ± 5± | 80 ± 47 | <.0001 | … |
| FEV1% p (pre-bronchodilator)± | 89 ± 11± | 92 ± 11 | .53± | 102 ± 3 |
| FEF25-75% p± | 70 ± 21± | 70 ± 22 | .89 | …. |
| Reversibility, %± | 9 ± 7± | 12 ± 5 | .11 | … |
| MCC, % cleared/80 min± | 59 ± 19± | 32 ± 25 | .002± | 29 ± 20 |
| Central deposition, % (Cen)± | 56 ± 8± | 50 ± 12 | .09± | 49 ± 5 |
| Adjusted MCC, % cleared/80 min± | 55 ± 15± | 35 ± 25 | .003± | 33 ± 16 |
All means ± SD. All P values by Wilcoxon rank-sum except for sex, which was χ2. ACT = asthma control test; FEF25-75%p = forced expiratory flowrate with 25-75% of vital capacity, percent of predicted; Feno = fractional exhaled nitric oxide.
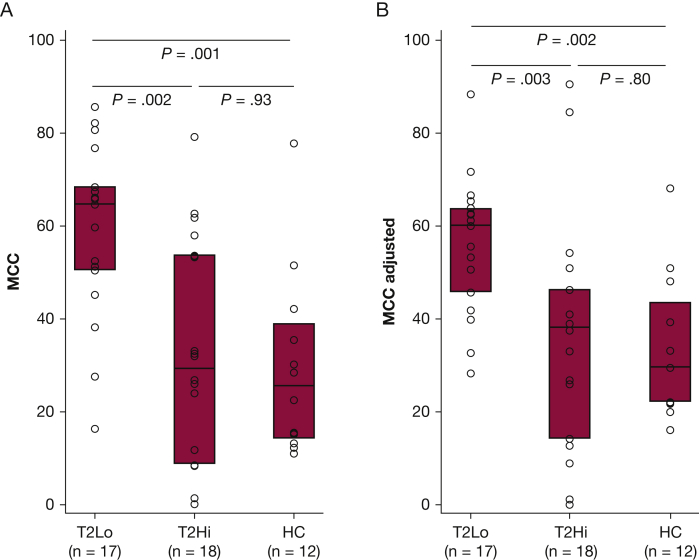
MCC Is Increased in T2Lo vs T2Hi and Historical HCs
MCC and MCCadjusted were significantly increased in the T2Lo group compared with T2Hi and historical HC groups (Fig 2). MCC rates higher than HCs have been demonstrated in some previous studies of asthma.10,11 MCC was highly variable in the T2Hi group (range, 0%-79% clearance/80 minutes). More than 25% of this group had clearance rates lower than any subject in the T2Lo or HC group. Two of T2Hi subjects had zero clearance. The following values were used to calculate MCCadjusted: Cenaverage = 53% and slope 1.07 (% cleared/% central). Calculation methods are included in e-Appendix 1. One calculated value for MCCadjusted was negative, and a zero value was substituted.
Figure 2.
A, Mucociliary clearance (MCC) in mild asthma subjects grouped as type 2 low (T2Lo) and type 2 high (T2Hi) based on exhaled nitric oxide (Feno) as compared with a historical group of healthy control subjects (HC). B, MCC-adjusted estimates and corrects for the effects of differences in aerosol distribution amongst the subjects based on their central aerosol deposition %. Methodology is included in e-Appendix 1.
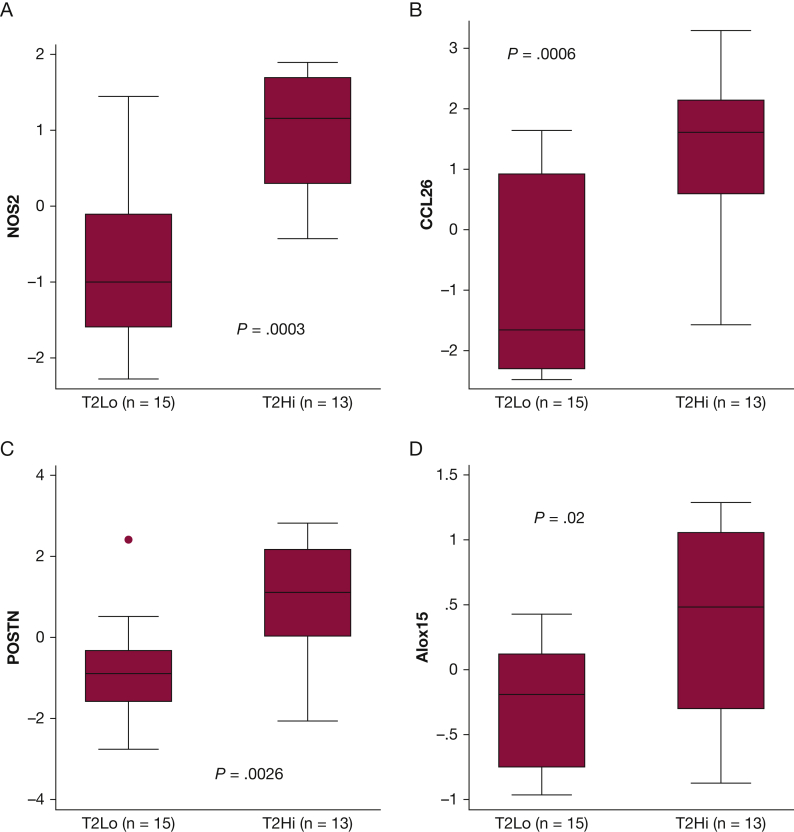
Type-2-Associated Epithelial Cell Gene Expression Correlates With MCC
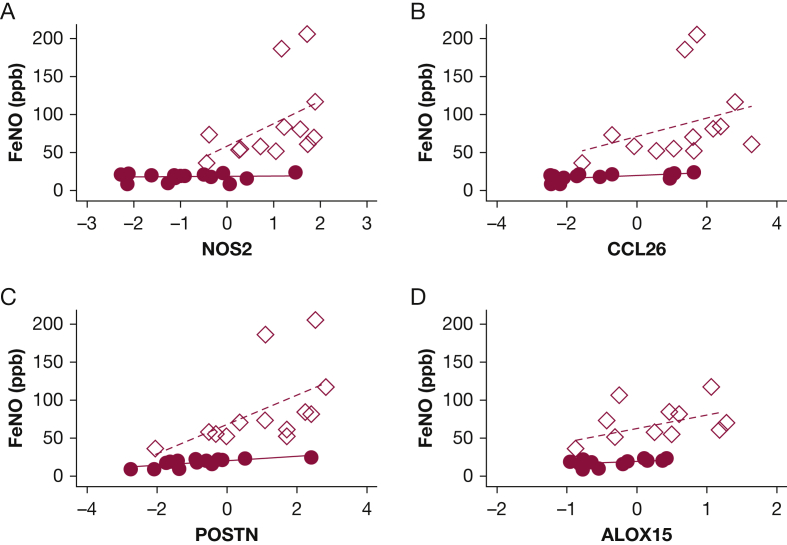
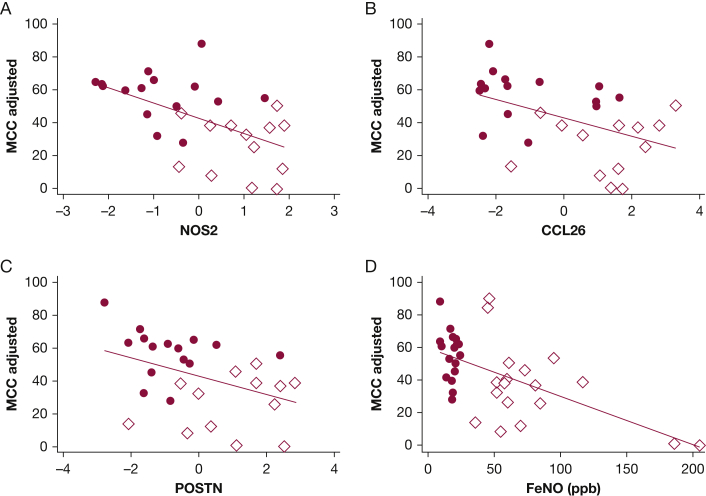
T2Hi subjects demonstrated increased mRNA expression of four genes linked to epithelial type 2 signatures, NOS2, CCL26, POSTN, and ALOX15 when compared with T2Lo (Fig 3). Feno measurements increased with the expression of these T2 signature genes,20 as shown in Figure 4. The relationship between Feno and gene expression appeared to vary by T2 grouping. Determining whether this is a simple consequence of the study design or whether expression independent factors may influence Feno is difficult. Feno generation has been previously shown to be primarily driven by NOS2 expression.21 MCC decreased with increasing expression of NOS2, CCL26, and POSTN, when considered through direct correlation with MCCadjusted (Fig 5A-C). MUC5AC, r = −0.13, P = .51; ALOX15, r = −0.28, P = .19, and SLC26A4, r = −0.25, P = .20, did not correlate with MCCadjusted. Unadjusted MCC demonstrated similar significance correlation: NOS2, r = −0.56, P = .0016; CCL26, r = −0.51, P = .006; POSTN, r = −0.42, P = .03; MUC5AC, r = −0.09, P = .64; ALOX15 r = −0.26, P = .22; SLC26A4r = −0.21, P = .28. Table 2 shows the results from multivariate regression models of MCC that include central deposition % (Cen) and measures of gene expression. Cen was included to help mitigate the known confounding factor associated with differences in aerosol distribution when measuring MCC. MCC increases with Cen because aerosol deposited in large central airways is more rapidly cleared. In a univariate model, 16% of MCC variation was explained by Cen alone (R2; e-Figs 1 and 2). In multivariate models, the addition of T2 gene expression levels increased the percentage of explained variation (48% when NOS2 expression was included). MCC decreased with increased T2 expression. FOXJ1 expression was similar in T2Hi and T2Lo groups and did not correlate with MCC, suggesting that MCC rate differences were not attributable to a loss of ciliated cells. SLC26A4 (pendrin) expression was similar between groups and did not correlate with MCC. ALOX15, whose expression may interfere with beta-adrenergic control of ciliary beat and MUC5AC expression, was increased in T2Hi.5,22
Figure 3.
Comparing gene expression levels between T2Hi and T2Lo groups based on samples obtained via bronchial brushings. Gene (protein): A, NOS2 (iNOS); B, CCL26 (eotaxin-3); C, POSTN (periostin); D, ALOX15 (arachidonate 15-lipoxygenase). All expression levels presented as the natural log of measured levels. Not depicted: MUC5AC P = .56, SLC26A4 (pendrin) P = .84, FOXJ1(Forkhead box protein J1) P = .88.
Figure 4.
Correlations between T2 gene expression patterns and Feno. All expression levels presented as the natural log of measured levels. Correlations by Spearman: gene (protein): A, NOS2 (iNOS) all r = 0.72, P < .0001; T2Hi r = 0.61, P = .03; T2Lo r = 0.06, P = .84. B, CCL26 (eotaxin-3) all r = 0.79, P < .0001; T2Hi r = 0.52, P = .07; T2Lo r = 0.59, P = .02. C, POSTN (periostin) all r = 0.81, P < .0001; T2Hi r = 0.72, P = .005; T2Lo r = 0.75, P = .001. D, ALOX15 all r = 0.66, P = .001; T2Hi r = 0.47, P = .17; T2Lo r = 0.57, P = .04. ● = T2Lo, ♢ = T2Hi. Dashed line = T2Hi linear fit. Solid line = T2Lo linear fit.
Figure 5.
Correlations between T2 gene expression patterns, Feno and MCCadjusted. All expression levels presented as the natural log of measured levels. Correlations by Spearman: gene (protein): A, NOS2 (iNOS) all r = -0.58, P = .001; T2Hi r = -0.06, P = .84; T2Lo r = -0.27, P = .33. B, CCL26 (eotaxin-3) all and r = -0.49, P = .008; T2Hi r = 0.19, P = .54; T2Lo r = -0.23, P = .40. C, POSTN (periostin) all r = -0.43, P = .02; T2Hi r = 0.12, P = .69; T2Lo r = -0.39, P = .14. D, Feno , all r = -0.54, P = .001 (if Feno > 150 ppb removed, r = -0.45, P = .008); T2Hi r = -0.32, P = .19; T2Lo r = -0.10, P = .70. ● = T2Lo, ♢= T2Hi. Solid line is whole group linear fit.
Table 2.
Multivariable Linear Regression Models of MCC Based on Gene Expression -Where MCC = B0 + B1 (Expression) + B2 (Central Deposition)
| Gene | Gene Expression |
Central Deposition |
Model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | SE | P | B2 | SE | P | B0 | SE | R2 | P | |
| MUC5AC | -7.89 | 4.38 | .08 | 1.74 | 0.43 | <.001 | -48 | 23 | 0.38 | .001 |
| CCL26 | -5.40 | 2.19 | .02 | 1.48 | 0.41 | .001 | -35 | 22 | 0.43 | .0003 |
| NOS2 | -9.07 | 3.04 | .006 | 1.31 | 0.40 | .003 | -26 | 22 | 0.48 | .001 |
| POSTN | -5.62 | 2.53 | .04 | 1.54 | 0.41 | .001 | -39 | 22 | 0.41 | .005 |
| ALOX15 | -8.92 | 5.32 | .10 | 2.26 | 0.41 | <.001 | -71 | 21 | 0.59 | .001 |
| FOXJ1 | -10.4 | 10.76 | .34 | 2.02 | 0.40 | <.001 | -62 | 21 | 0.49 | .001 |
| SLC26A4 | -3.27 | 3.09 | .30 | 1.63 | 0.44 | .001 | -43 | 24 | 0.33 | .003 |
Each row is a model including the named gene and central deposition % (Cen). Cen provides a measure of aerosol distribution, which is known to confound measures of MCC. Gene (associated protein): MUC5AC (Mucin 5AC), CCL26 (eotaxin-3), NOS2 (iNOS), and POSTN (periostin), ALOX15 (Arachidonate 15-lipoxygenase), FOXJ1(Forkhead box protein J1), and SLC26A4 (pendrin).
Biologic and Clinical Factors Related to MCC
The relationship between Feno and MCCadjusted is shown in Figure 5D. The results of multivariate regressions comparing MCC with clinical variables are included in Table 3. Cen is included to help mitigate the known confounding factor associated with differences in aerosol distribution when measuring MCC. This multivariate model indicates that Feno is an independent predictor of decreased MCC (P < .001). FEV1, age, and sex were not independent predictors of MCC. A multivariate model that included Cen and Feno explained 47% of the variation in MCC. This is similar to a model including Cen and NOS2 expression.
Table 3.
Multivariable Linear Regression Model of MCC Based on Individual Clinical Variables Where MCC = B0 + B1 (Clinical Variable) + B2 (Central Deposition)
| Variable | Clinical Variable |
Central Deposition |
Model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | SE | P | B2 | SE | P | B0 | SE | R2 | P | |
| Age, years | -0.83 | 0.56 | .15 | 0.95 | 0.39 | .02 | 15 | 27 | 0.20 | .01 |
| Sex, female | 0.57 | 8.48 | .95 | 1.08 | 0.41 | .01 | -12 | 24 | 0.14 | .04 |
| ACT score | 2.12 | 1.33 | .12 | 0.77 | 0.43 | .08 | -39 | 27 | 0.20 | .01 |
| Feno , ppb | -0.31 | 0.07 | <.001 | 0.99 | 0.31 | .003 | 8 | 17 | 0.47 | <.001 |
| FEV1%p | -0.21 | 0.39 | .59 | 1.01 | 0.42 | .02 | 10 | 46 | 0.14 | .03 |
| FEF25-75%p | -0.32 | 0.21 | .14 | 0.78 | 0.43 | .08 | 27 | 32 | 0.20 | .01 |
| Reversibility, % | -16.1 | 64.7 | .81 | 1.10 | 0.40 | .01 | -11 | 21 | 0.14 | .04 |
Each row describes a model including the named variable and central deposition % (Cen).
Cen provides a measure of aerosol distribution, which is known to confound measures of MCC. ACT = asthma control test; FEF25-75%p = forced expiratory flowrate with 25-75% of vital capacity, percent of predicted; Feno = fractional exhaled nitric oxide.
Forced Expiratory Flow at 25% to 75% of FVC Predicts Aerosol Penetration Into the Lung
Cen is highly and inversely correlated with forced expiratory flow at 25% to 75% of FVC (FEF25-75%; r = −0.44; P = .007), indicating that aerosol penetration is predicted by this measure commonly associated with small airway pathophysiology. FEV1 is not similarly predictive (r = −0.24, P = .12). This may indicate that small airway obstruction is associated with poor aerosol penetration into the deep lung and increased aerosol deposition in large central airways. There was no relationship between FEF25-75% and Feno (r = 0.08, P = .62).
Absorption Measurements
Absorptive clearance was significantly confounded by the high rates of MCC exhibited in the T2Lo group. This measurement becomes invalid if the small-molecule radiopharmaceutical used to measure absorption is cleared too rapidly by mucociliary clearance.23
Discussion
Although fatal asthma is often characterized by mucus occlusion of the conducting airways, little is known about mucus clearance in less severe asthma. Previous studies of MCC in asthma have yielded inconsistent results, likely because of the heterogenous nature of the disease. The presence or absence of type 2 inflammation provides one means to categorize patients with asthma and has previously been shown to predict therapeutic response and outcomes.24 Here we studied patients with very mild corticosteroid-naïve asthma and grouped them into T2Lo and T2Hi groups based on Feno measurements. Differences in T2-associated gene expression patterns were demonstrated between the groups, and Feno was correlated to expression levels of several T2-associated genes. The relationship between Feno and expression did appear to vary by T2 grouping, indicating that factors beyond expression may influence Feno levels.
Surprisingly, MCC rates in the T2Lo group were significantly higher than both the T2Hi group and a historic group of HCs. This effect was present even after correction for differences in aerosol distribution in the lungs. MCC rates within the T2Hi group varied widely, with some subjects having high rates and others having very low or no clearance. On average, they were similar to HCs, but with much more variability. Increased rates of MCC have been demonstrated in some other studies of asthma that were not phenotyped.10,11 We speculate that the high rates of MCC in the T2Lo group may illustrate a compensatory mechanism. Several lines of evidence suggest that the airway surface hydration and MCC may be increased in asthma. T2 inflammation increases the expression of transmembrane member 16A, a secretary chloride channel that is activated by extracellular purinergic signaling.25 While mechano-sensing, airway epithelia secrete adenosine triphosphate in response to compression, shear stress, and increased luminal mucus content.26 In addition to promoting airway hydration though transmembrane member 16A activation, adenosine triphosphate inhibits airway absorption though the epithelial sodium channel27 and stimulates ciliary beating.28 These secretory mechanisms may increase MCC in mild asthma in a manner similar to what is observed in patients with systemic pseudohypoaldosteronism, who have loss of function mutations to absorptive pathways and thereby have increased airway surface hydration and high rates of MCC.29
We did not observe a difference in MUC5AC expression between T2Lo and T2Hi subjects; however, it is entirely possible that alterations or increases in mucus production related to T2 inflammation contributed the decreasing rates of MCC that were noted with increasing T2 gene expression and Feno. Neither mucus production nor mucus properties were assessed in this study. High luminal mucus concentration creates an osmotic force that favors periciliary shrinkage or collapse, thereby inhibiting MCC.26 Additionally, T2Hi asthma is associated with increased oxidative stress that increases mucus viscosity by promoting mucin crosslinking.30 This oxidative stress may be augmented by the increased abundance of eosinophil peroxidase, which would be expected given the increased expression of CCL26, an eosinophilic chemokine.31 This is supported by the relationship between CCL26 expression and MCC (Table 2). (We are not aware of any way in which changes in mucus production or mucus properties would adversely affect the accuracy of the MCC measurement.) Activated eosinophils, perhaps through direct cytotoxic effects, have also been shown to depress ciliary beat, which could limit clearance.32 Potentially these effects may have offset the compensatory increases in MCC noted in the T2Lo subjects and contributed to the high levels of MCC variability within the T2Hi group.
IL-13 has also been shown to both decrease ciliated cell numbers and lower ciliary beat, both of which could impact MCC.33,34 No differences in FOXJ1 expression were observed between the T2Lo and T2Hi groups, suggesting that the MCC differences are not attributable to a loss of ciliated cells in these patients with mild asthma. However, given our lack of HCs undergoing bronchoscopy, it is impossible to know whether genes regulating cilia or their function could be increased in T2Lo asthma. Beta agonists have been shown to increase MCC35 and perhaps could have influenced clearance; however, subjects did not use bronchodilators on the MCC measurement day, and there was a trend toward more beta-agonist use in the T2Hi group based on ACT question 4.
Even in this cohort of patients with mild asthma, increased markers of T2 inflammation were associated with increased asthma symptom burden. This is consistent with the hypothesis that an overzealous inflammation response may disable appropriate and beneficial compensatory MCC effects. Treatment of patients with asthma with antibodies to the IL-4 receptor has been shown to lower Feno, improve FEV1, and markedly decrease exacerbations.36, 37, 38 In this mild T2 Hi group, MCC rates ranged widely, with some subjects demonstrating higher than normal MCC rates while others demonstrated low or zero clearance. Because these were patients with mild asthma with normal lung function, whether MCC is more severely depressed in more severe forms of asthma is unclear. Whether those with very low clearance would be at risk for long-term decline in lung function or increasing exacerbations is also not yet clear, but the strong relationship between MCC with inflammatory gene expression suggests that worsening MCC corresponds to worsening T2 inflammation.
Interpretation
In summary, our results demonstrate that MCC in a subset of patients with mild asthma appears to be dependent on background levels of T2 inflammation. Patients with mild asthma with low T2 inflammation appear to have activated mechanisms to enhance MCC, but increasing levels of T2 inflammation caused proportional decreases in MCC, limiting the effects of this potentially important compensatory mechanism. Additional studies are required to understand the enhanced MCC demonstrated in subjects with limited inflammation and to determine whether worsening or uncontrolled T2 inflammation more completely reduces MCC and drives severity of disease.
Take-home Points.
Study Question: Does type 2 inflammation influence mucus clearance rates in patients with mild asthma who are not receiving corticosteroids?
Results: MCC decreased with increasing expression of several markers of T2 airway inflammation (CCL26, NOS2, and POSTN) and with Feno.
Interpretation: Increasing T2 inflammation was associated with decreasing mucus clearance rates in mild asthma.
Acknowledgments
Author contributions: S. W. and M. M. M. completed the initial study design, participated in data collection and analysis, and reviewed and edited the manuscript. T. E. C. participated in the design of study analysis methods, performed data analysis, and wrote the first draft of the manuscript. A. H. and L. W. L. contributed to the design of study analysis methods and performed data analysis. L. W. and A. M. contributed to imaging study design and participated in data collection. S. L. H. and E. M. H. participated in data collection and analysis. T. E. C. takes responsibility for the integrity of this work from inception to publication.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. W. has received grant funding or personal fees from AstraZeneca, Sanofi-Genzyme, Pieris, Knopp, GSK, Teva, Regeneron, and Boehringer Ingelheim. T. E. C. has received research funding from Sanofi-Genzyme and Regeneron and received NIH funding to perform this work. M. M. M. received NIH funding to perform this work. None declared (A. S. H., S. L. H., L. W. L., L. W., A. M., and E. M. H.).
Additional information: The e-Appendix and e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by National Heart, Lung, and Blood Institute (NHLBI) [1R01 HL112863-01].
Supplementary Data
References
- 1.Grunig G., Warnock M., Wakil A.E., et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282(5397):2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wills-Karp M., Luyimbazi J., Xu X., et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z., Homer R.J., Wang Z., et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennox A.T., Coburn S.L., Leech J.A., et al. ATP12A promotes mucus dysfunction during type 2 airway inflammation. Sci Rep. 2018;8(1):2109. doi: 10.1038/s41598-018-20444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J., Maskrey B., Balzar S., et al. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179(9):782–790. doi: 10.1164/rccm.200811-1744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Minami Y., Etling E., et al. Preferential generation of 15-HETE-PE induced by IL-13 regulates goblet cell differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 2017;57(6):692–701. doi: 10.1165/rcmb.2017-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonser L.R., Zlock L., Finkbeiner W., Erle D.J. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126(6):2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman J.R., Pavia D., Sheahan N.F., Agnew J.E., Clarke S.W. Impaired tracheobronchial clearance in patients with mild stable asthma. Thorax. 1983;38(6):463–467. doi: 10.1136/thx.38.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilowite J.S., Bennett W.D., Sheetz M.S., Groth M.L., Nierman D.M. Permeability of the bronchial mucosa to 99mTc-DTPA in asthma. Am Rev Respir Dis. 1989;139(5):1139–1143. doi: 10.1164/ajrccm/139.5.1139. [DOI] [PubMed] [Google Scholar]
- 10.O’Riordan T.G., Zwang J., Smaldone G.C. Mucociliary clearance in adult asthma. Am Rev Respir Dis. 1992;146(3):598–603. doi: 10.1164/ajrccm/146.3.598. [DOI] [PubMed] [Google Scholar]
- 11.Lay J.C., Alexis N.E., Zeman K.L., Peden D.B., Bennett W.D. In vivo uptake of inhaled particles by airway phagocytes is enhanced in patients with mild asthma compared with normal volunteers. Thorax. 2009;64(4):313–320. doi: 10.1136/thx.2008.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messina M.S., O’Riordan T.G., Smaldone G.C. Changes in mucociliary clearance during acute exacerbations of asthma. Am Rev Respir Dis. 1991;143(5 Pt 1):993–997. doi: 10.1164/ajrccm/143.5_Pt_1.993. [DOI] [PubMed] [Google Scholar]
- 13.Svenningsen S., Haider E., Boylan C., et al. CT and functional MRI to evaluate airway mucus in severe asthma. Chest. 2019;155(6):1178–1189. doi: 10.1016/j.chest.2019.02.403. [DOI] [PubMed] [Google Scholar]
- 14.Gershman N.H., Wong H.H., Liu J.T., Mahlmeister M.J., Fahy J.V. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9(12):2448–2453. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 15.Dweik R.A., Boggs P.B., Erzurum S.C., et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (Feno) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore W.C., Evans M.D., Bleecker E.R., et al. Safety of investigative bronchoscopy in the Severe Asthma Research Program. J Allergy Clin Immunol. 2011;128(2):328–336. doi: 10.1016/j.jaci.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha GB. Fundamentals of Nuclear Pharmacy, 5th Edition. Springer.
- 18.Locke L.W., Myerburg M.M., Markovetz M.R., et al. Quantitative imaging of airway liquid absorption in cystic fibrosis. Eur Respir J. 2014;44(3):675–684. doi: 10.1183/09031936.00220513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke L.W., Myerburg M.M., Weiner D.J., et al. Pseudomonas infection and mucociliary and absorptive clearance in the cystic fibrosis lung. Eur Respir J. 2016;47(5):1392–1401. doi: 10.1183/13993003.01880-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff P.G., Modrek B., Choy D.F., et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chibana K., Trudeau J.B., Mustovich A.T., et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38(6):936–946. doi: 10.1111/j.1365-2222.2008.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albano G.D., Zhao J., Etling E.B., et al. IL-13 desensitizes beta2-adrenergic receptors in human airway epithelial cells through a 15-lipoxygenase/G protein receptor kinase 2 mechanism. J Allergy Clin Immunol. 2015;135(5):1144–1153. doi: 10.1016/j.jaci.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corcoran T.E., Huber A.S., Myerburg M.M., et al. Multiprobe nuclear imaging of the cystic fibrosis lung as a biomarker of therapeutic effect. J Aerosol Med Pulm Drug Deliv. 2019;32(4):242–249. doi: 10.1089/jamp.2018.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray A., Camiolo M., Fitzpatrick A., Gauthier M., Wenzel S.E. Are we meeting the promise of endotypes and precision medicine in asthma? Physiol Rev. 2020;100(3):983–1017. doi: 10.1152/physrev.00023.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caputo A., Caci E., Ferrera L., et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 26.Button B., Okada S.F., Frederick C.B., Thelin W.R., Boucher R.C. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal. 2013;6(279):ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danahay H., Atherton H., Jones G., Bridges R.J., Poll C.T. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282(2):L226–L236. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- 28.Schmid A., Salathe M. Ciliary beat co-ordination by calcium. Biol Cell. 2011;103(4):159–169. doi: 10.1042/BC20100120. [DOI] [PubMed] [Google Scholar]
- 29.Kerem E., Bistritzer T., Hanukoglu A., et al. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341(3):156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- 30.Yuan S., Hollinger M., Lachowicz-Scroggins M.E., et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015;7(276) doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman J.M., Naik C., Holguin F., et al. Epithelial eotaxin-2 and eotaxin-3 expression: relation to asthma severity, luminal eosinophilia and age at onset. Thorax. 2012;67(12):1061–1066. doi: 10.1136/thoraxjnl-2012-201634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devalia J.L., Sapsford R.J., Rusznak C., Davies R.J. The effect of human eosinophils on cultured human nasal epithelial cell activity and the influence of nedocromil sodium in vitro. Am J Respir Cell Mol Biol. 1992;7(3):270–277. doi: 10.1165/ajrcmb/7.3.270. [DOI] [PubMed] [Google Scholar]
- 33.Laoukili J., Perret E., Willems T., et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108(12):1817–1824. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomperts B.N., Kim L.J., Flaherty S.A., Hackett B.P. IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am J Respir Cell Mol Biol. 2007;37(3):339–346. doi: 10.1165/rcmb.2006-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazio F., Lafortuna C. Effect of inhaled salbutamol on mucociliary clearance in patients with chronic bronchitis. Chest. 1981;80(6 Suppl):827–830. doi: 10.1378/chest.80.6.827. [DOI] [PubMed] [Google Scholar]
- 36.Wenzel S., Ford L., Pearlman D., et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 37.Wenzel S., Castro M., Corren J., et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 38.Castro M., Corren J., Pavord I.D., et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.