Abstract
The existing coverage criteria for home noninvasive ventilation (NIV) do not recognize the diversity of hypoventilation syndromes and advances in technologies. This document summarizes the work of the hypoventilation syndromes Technical Expert Panel working group. The most pressing current coverage barriers identified were: (1) overreliance on arterial blood gases (particularly during sleep); (2) need to perform testing on prescribed oxygen; (3) requiring a sleep study to rule out OSA as the cause of sustained hypoxemia; (4) need for spirometry; (5) need to show bilevel positive airway pressure (BPAP) without a backup rate failure to qualify for BPAP spontaneous/timed; and (6) qualifying hospitalized patients for home NIV therapy at the time of discharge. Critical evidence support for changes to current policies includes randomized controlled trial evidence and clinical practice guidelines. To decrease morbidity and mortality by achieving timely access to NIV for patients with hypoventilation, particularly those with obesity hypoventilation syndrome, we make the following key suggestions: (1) given the significant technological advances, we advise acceptance of surrogate noninvasive end-tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases; (2) not requiring Pco2 measures while on prescribed oxygen; (3) not requiring a sleep study to avoid delays in care in patients being discharged from the hospital; (4) remove spirometry as a requirement; and (5) not requiring BPAP without a backup rate failure to approve BPAP spontaneous/timed. The overarching goal of the Technical Expert Panel is to establish pathways that improve clinicians’ management capability to provide Medicare beneficiaries access to appropriate home NIV therapy. Adoption of these proposed suggestions would result in the right device, for the right type of patient with hypoventilation syndromes, at the right time.
Key Words: Bilevel PAP, CPAP, home mechanical ventilator, noninvasive ventilation, obesity hypoventilation, volume assured pressure support
Abbreviations: AASM, American Academy of Sleep Medicine; ABG, arterial blood gas; ATS, American Thoracic Society; BPAP, bilevel positive airway pressure; DME, durable medical equipment; EPAP, expiratory positive airway pressure; EtPco2, end-tidal Pco2; HMV, home mechanical ventilator; NIV, noninvasive ventilation; OHS, obesity hypoventilation syndrome; ONMAP, Optimal NIV Medicare Access Promotion; PAP, positive airway pressure; PSG, polysomnogram; S, spontaneous; S/T, spontaneous/timed; TcPco2, Pco2 by transcutaneous CO2 tension; TEP, Technical Expert Panel; VAPS, volume-assured pressure support; VBG, venous blood gas
Note to the Reader: The current document is one of a series produced by a Technical Expert Panel (TEP) whose purpose was to propose changes to Centers for Medicare & Medicaid Services national coverage determinations for the use of noninvasive ventilation and home mechanical ventilation, which were formulated in 1998. Specifically, the TEP proposed changes to national coverage determinations for thoracic restrictive disorders (neuromuscular disease), COPD, hypoventilation syndromes, central sleep apnea, and OSA. The background, makeup of the TEP, and key recommendations are highlighted in an Executive Summary. CHEST, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society formed the “Optimal NIV Medicare Access Promotion (ONMAP)” to provide processes to obtain the “right device for the right patient at the right time.” More details and rationale for the proposed changes are available in the companion documents.
Hypoventilation syndromes are a heterogeneous group of disorders caused by loss of normal homeostasis and are characterized by hypercapnia, defined as a Paco2 ≥ 45 mm Hg at sea level. Noninvasive ventilation (NIV) includes a group of bilevel positive airway pressure (BPAP) devices that are effective in improving hypercapnia and are accepted as standard of care for treating various hypoventilation syndromes. Obesity is a leading cause of hypoventilation in the United States, and obesity hypoventilation syndrome (OHS) refers to the development of awake daytime hypercapnia in obese individuals (BMI ≥ 30 kg/m2) in the absence of other known causes of hypoventilation. OHS is associated with significant morbidity and higher risk of hospitalizations, ICU utilization, and death.1, 2, 3, 4, 5 Since a prior consensus conference in 1998, further clinical evidence, including the largest randomized trial to date, has reinforced the benefits of positive airway pressure (PAP) in treating OHS.6,7
Many hypoventilatory syndromes with hypercapnic respiratory failure are included in this category, such as those caused by obesity (eg, OHS), central respiratory drive depression associated with medication or substance use (eg, opioids), and decompensated hypercapnic respiratory failure other than COPD and neuromuscular disease (increased work of breathing due to increased respiratory system load [eg, end-stage interstitial lung disease]). The Technical Expert Panel (TEP) has predominately focused on reviewing evidence and consensus practice guidelines on OHS due to low strength of evidence of NIV therapy in chronic alveolar hypoventilation disorders other than neuromuscular disease and COPD (also reviewed separately by the TEP).
There is a pressing need to reevaluate the current coverage criteria for both durable medical equipment (DME) devices and respiratory support services to ensure the most appropriate care of patients with hypoventilation syndromes requiring NIV.
Background
Current Coverage Guidelines
The current coverage criteria for BPAP devices with a backup rate (BPAP spontaneous/timed [S/T]) used to provide NIV have resulted in overly restrictive regulatory barriers to the delivery of appropriate equipment to support patients with hypoventilation syndromes.8 Currently, initial coverage for a BPAP S/T to treat hypoventilation syndromes are specified in the National Coverage Determination criteria and related policies (https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33800) and is described in Table 1.
Table 1.
Current NCD Criteria for Initial Coverage of BPAP Devices for Hypoventilation Syndromes
| BPAP Device Without Backup Rate (E0470) Feature | BPAP Device With Backup Rate (E0471) Feature |
|---|---|
Requires both criteria A and B and either criterion C or D.
|
Requires both criteria A and B and either criterion C or D.
|
ABG = arterial blood gas; AHI = apnea-hypopnea index; BPAP = bilevel positive airway pressure; NCD = national coverage determination.
By failing to recognize the spectrum of disease severity and advances in technology, the current coverage criteria have led to inappropriate use of costly home mechanical ventilators (HMVs) when BPAP S/T devices may suffice.9 For example, the criteria to qualify appropriate patients with home NIV are particularly challenging and burdensome for hospitalized patients being discharged and may have led to both inappropriate use of HMVs and inappropriate/ineffective treatments such as supplemental oxygen alone. Many clinicians find it easier to prescribe a costlier HMV than a BPAP S/T. This is discussed further in the companion ONMAP for Thoracic Restrictive Disorders TEP article.
Perhaps as a result, the US Department of Health and Human Services, Office of Inspector General, concluded that Medicare paid 85 times more claims for E0464 home ventilators in 2015 than it did in 2009, with a dramatic shift in diagnoses on these claims from neuromuscular to respiratory conditions.9 However, timely NIV is clearly important for such patients, given that hospital discharge on NIV has been shown to reduce mortality at 3 months following acute-on-chronic hypercapnic respiratory failure in patients suspected of having OHS.10 In fact, the 2019 American Thoracic Society (ATS) Clinical Practice Guidelines for the management of OHS recommends such patients hospitalized with respiratory failure be discharged with NIV until they undergo outpatient diagnostic procedures.10,11
Extending DME coverage beyond 3 months currently requires that the patient be reevaluated by the treating physician at 61 to 90 days with confirmed adherence of ≥ 4 h per day over 21 of 30 days (70%). Initial acclimation and subsequent access to sleep physicians and sleep studies are problematic. Many patients discharged on NIV are cared for at intermediate-care facilities, including short-term rehabilitation, prior to returning home. The existing criteria for continued DME coverage are not forgiving to these challenges and have led to unintended consequences such as withdrawing therapies based on an arbitrary threshold of adherence and follow-up period, especially in these often-debilitated patients who may ultimately benefit from NIV.
Current Evidence/Clinical Consensus Practice Guidelines
Comorbidities and Mortality in OHS
Compared with hospitalized eucapnic obese patients, those with hypoventilation have higher rates of transfer to ICUs, mechanical ventilation, long-term care needs, and mortality.1,12,13 The in-patient mortality among subjects with OHS in need of NIV for acute-on-chronic hypercapnic respiratory failure ranges from 0% to 15%.1,3,5,14 Of those discharged, Meservey et al14 described a 30-day readmission rate of 23% in a mixed hypercapnic respiratory failure population, with 66% of the readmissions related to recurrent hypercapnic respiratory failure. In those with OHS identified on the general medicine wards, Nowbar et al1 reported an estimated mortality rate of 23% at 18 months’ postdischarge (hazard ratio, 4.0; 95% CI, 1.5-10.4) compared with those with simple obesity. In this same study, despite a high postdischarge mortality, BPAP therapy for hypoventilation was initiated in only 13% of the patients with OHS. In another observational study of 600 hospitalized patients with OHS, of whom 61% were initially admitted to the ICU, 15% died in the hospital and, at 3 years, all-cause mortality was 31%.5 Unfortunately, it is unclear what percentage of patients who survived hospitalization eventually received outpatient PAP therapy. Compared with patients with OHS discharged without PAP therapy, Mokhlesi et al10 reported a decreased 3- and 6-month mortality in patients with OHS initiated on BPAP S/T at time of discharge (3-month mortality rate, 2.3% treated vs 16.8% untreated [P < .0001]; 6-month mortality rate, 4.9% treated vs 22.7% untreated [P < .0001). In a retrospective observational cohort study, Berg et al14 found that in the 2 years following a diagnosis of OHS and the initiation of PAP treatment, there was a significant reduction in outpatient physician costs and days of hospitalization per year (5 years prior, 7.9 days per patient per year; 2 years following, 2.5 days per patient per year; P = .01). These data indicate that patients with untreated OHS are at a high risk of 30-day readmission and mortality due to recurrent hypercapnic respiratory failure.
PAP Therapy
The BPAP mode provides more ventilatory support to effectively unload accumulated CO2 during hypoventilation than CPAP. Adding the backup rate with the BPAP S/T provides additional mechanical breaths should the patient’s breath rate fall below the preset backup respiratory rate needed to keep a minimum minute ventilation.
Volume-assured pressure support (VAPS) devices are BPAP S/T modes that self-adjust inspiratory positive airway pressure to maintain either a consistent preset target expiratory tidal volume or minute ventilation depending on the device’s proprietary algorithm. Based on current technology, VAPS may also adjust the respiratory rate to treat hypoventilation and apply auto-expiratory positive airway pressure (EPAP) to stabilize an open upper airway in case of increased upper airway resistance (eg, OSA).
Benefits of BPAP or VAPS Over Lifestyle Modifications Alone
When compared with lifestyle counseling alone, BPAP has consistently proven to be more effective on short- and long-term outcomes. Several observational studies have shown that BPAP therapy produced an improvement in gas exchange, symptoms, and measures of health-related quality of life. Furthermore, observational studies of PAP therapy have been associated with long-term improvement in mortality as well as a reduction in hospitalization days compared with no therapy.15
In patients with OHS and concomitant severe OSA, randomized controlled trials have shown that BPAP S/T and VAPS therapy significantly decrease daytime Paco2, sleep-disordered breathing, and daytime sleepiness and improve health-related quality of life compared with lifestyle changes alone.6 Furthermore, VAPS therapy has resulted in significant improvements in pulmonary function and functional capacity (6-min walk distance).
In OHS without concomitant severe OSA, Masa et al16 found VAPS therapy to be more effective than lifestyle modification in improving blood gas parameters, a health-related quality of life measure (physical component of the 36-item Short Form Health Survey), and daytime sleepiness.7 VAPS therapy led to lower ED visits, and post hoc analysis of adherence subgroups showed that higher level of adherence to VAPS therapy was associated with reduced ED visits and mortality.
Comparison of BPAP S, BPAP S/T, or VAPS vs CPAP
Several randomized controlled trials have compared various modes of positive airway pressure (PAP) therapy in patients with OHS. Only one small randomized controlled trial (N = 36) compared BPAP in spontaneous (S) mode (ie, without a backup rate) vs CPAP.17 In this study, patients who failed CPAP titration were actually excluded. Most clinical trials have compared CPAP vs BPAP S/T,18 CPAP vs VAPS,19 or BPAP S/T vs VAPS.20,21 Importantly, all these clinical trials were performed in ambulatory patients with OHS and concomitant severe OSA. These randomized controlled trials have shown similar treatment effectiveness among different PAP modes. In the largest clinical trial, VAPS and CPAP therapy resulted in similar outcomes such as hospital resource utilization, BP, arterial blood gas (ABG) parameters, spirometry, quality of life, clinical symptoms, and need for supplemental oxygen therapy.19 Both VAPS and CPAP also similarly improved the pulmonary artery pressure and left ventricular diastolic dysfunction.22
In patients with OHS without co-existing severe OSA, the only randomized controlled study to date used the VAPS mode with a backup rate and compared it vs lifestyle modification, thereby supporting use of a backup rate until additional data emerge for the subset of OHS patients without concomitant severe OSA.16
The ATS Clinical Practice Guidelines identified 10 observational studies that included hospitalized patients with acute-on-chronic respiratory failure with OHS or suspected of having OHS.10,11 In seven studies, NIV was prescribed at hospital discharge. At 3 months, the ORs for mortality were significantly lower in the group discharged on PAP (adjusted OR, 0.16; 95% CI, 0.08-0.33; P < .0001) than those discharged without PAP. The vast majority of patients (92%) were discharged from the hospital on NIV, as opposed to CPAP, but different modes of NIV (including S, S/T, and VAPS) were used, and the ATS Clinical Practice Guidelines could not recommend empiric settings for NIV.
The Optimal NIV Medicare Access Promotion (ONMAP) Technical Expert Panel (TEP) recognizes that VAPS with the auto-EPAP feature is currently available only in HMV in the United States. As such, we suggest that hospitalized patients be discharged on BPAP S/T with a backup rate and empiric EPAP setting. VAPS with auto-EPAP may be considered when the auto-EPAP feature is readily available in respiratory assist devices, as it is currently in Europe. However, if there is concern for failure of empiric BPAP S/T, VAPS with auto-EPAP may be clinically indicated, and its prescription via HMV should be at the provider’s discretion while the patient is awaiting outpatient workup.
Effectiveness of VAPS Compared With Manually Titrated BPAP S/T
There is growing evidence that VAPS is as effective as manually titrated BPAP S/T for treating respiratory insufficiency or failure. In OHS, VAPS therapy has been shown to have similar treatment effectiveness in controlling sleep-disordered breathing and gas exchange compared with BPAP S/T.20 Auto-EPAP technology in VAPS modes may facilitate outpatient PAP set-up with a device achieving titration in the home environment with reduction in health care utilization of polysomnogram (PSG) studies. In a randomized crossover study, Orr et al23 showed that auto-EPAP was noninferior to manual EPAP in controlling upper airway obstruction while remaining effective at treating hypoventilation, without requiring titration by a sleep laboratory technician. In another clinical trial, 60 ambulatory patients with OHS were randomized to receive BPAP S/T or VAPS with auto-EPAP. Although there were no differences in sleep quality and gas exchange, the burden of NIV set-up was less in those randomized to VAPS with auto-EPAP.21 Although VAPS with the auto-EPAP feature is only available in HMVs, the TEP encourages that auto-EPAP technology be added to respiratory assist devices with the VAPS modality, currently only available in Europe, to avoid having to prescribe HMVs.
Transition From BPAP S/T or VAPS to CPAP in Stable Ambulatory OHS With Severe OSA
The current literature suggests that many patients with OHS and concomitant severe OSA initially treated with BPAP S/T or VAPS can be safely switched to CPAP following a 2- to 3-month period of nocturnal BPAP S/T therapy.24,25 This step-down intervention applied to OHS with coexistent severe OSA has the potential to deliver effective health care at a lower cost for much of the population.
Although CPAP could become a suitable therapy for the majority of patients with OHS and concomitant severe OSA, some cases complicated by weight gain, the need for oxygen supplementation following discharge, and CPAP failure may require continuation of BPAP S/T or VAPS therapy to remain eucapnic. Therefore, the effectiveness of CPAP therapy for OHS with severe OSA should be evaluated with in-laboratory titration PSG in 2 to 3 months of home NIV therapy following hospital discharge. Once switched to CPAP, patients will need follow-up to ensure adequate and maintained response to therapy. In contrast, for those patients with OHS but without severe OSA, BPAP S/T or VAPS is recommended as the long-term therapy of choice.
Noninvasive Assessment of Nocturnal Arterial CO2 Tension
Assessment of Paco2 is essential for evaluating the adequacy of ventilation in patients with NIV therapy. To date, repeated ABG sampling remains the gold standard test. However, in addition to discomfort, repeated awake ABG sampling does not reliably assess control of nocturnal hypoventilation as the patient needs to be awake to take the blood sample.26 While awake, a normal morning Paco2 does not actually reflect the abnormal time course of Paco2 during the night. In these cases, nocturnal noninvasive assessment of Paco2 by transcutaneous CO2 tension (TcPco2) is an acceptable alternative.
Despite potential technical limitations, continuous TcPco2 recordings have shown good agreement with arterial measurements. TcPco2 monitoring has also been shown to correlate well with ABG in adults requiring NIV for acute respiratory failure, even in those with obesity. Kelly et al27 found an average difference of 6.1 mm Hg between the TcPco2 and ABG when analyzing Paco2. This close correlation, however, was diminished with higher Paco2 levels, specifically those > 60 mm Hg. Janssens et al28 reported that during NIV, TcPco2 recordings could be performed continuously for 8 h without any local discomfort or significant signal drift.
The American Academy of Sleep Medicine (AASM) clinical practice guidelines for adjustment of NIV in stable chronic alveolar hypoventilation syndromes state that “during attended NIV titration, gas exchange can be monitored by pulse oximetry and the arterial Pco2 may be measured intermittently by ABG testing or continuously estimated by transcutaneous Pco2 or end-tidal Pco2 (EtPco2) monitoring to allow precise documentation of an adequate level of NIV support.”29 Therefore, we recommend monitoring TcPco2 as a noninvasive alternative to ABG to determine the presence of alveolar hypoventilation and its response to PAP therapy at the levels provided earlier in this document.
ONMAP Suggestions for New National Coverage Determinations for Hypoventilation Syndromes
The existing coverage criteria for BPAP devices do not recognize the diversity of disorders that constitute hypoventilation syndromes, the variability in acuity and severity of presentation of hypoventilation syndromes over time, and advances in technologies. This results in regulatory barriers to appropriately support patients with hypoventilation syndromes, and these limitations are discussed in the following sections.
The current coverage criteria rely on an ABG (criterion A) as the gold standard for assessing hypercapnia. An ABG helps to differentiate acute, chronic, and acute-on-chronic hypercapnia and can also provide valuable information as to the possible cause. Access to ABG is not always readily available, however, especially in the sleep/pulmonary clinic or sleep laboratory. There are practical limitations of ABG monitoring during sleep, including concern whether the pain or anxiety induced by an arterial puncture leads to transient hyperventilation, which in turn normalizes Paco2. Furthermore, in morbidly obese patients, obtaining an ABG can be technically difficult and, as such, the patients may decline repetitive ABGs.
The ONMAP TEP acknowledges that there is a higher level of agreement between ABG and venous blood gas (VBG) for assessing pH and bicarbonate level, as opposed to Pco2. However, it is also important to consider that the vast majority of the studies that have compared ABG with VBG have been performed in critically ill or postoperative patients, or in patients who present to the ED with acute respiratory exacerbation.30 Studies have not focused on ambulatory patients with OHS or neuromuscular diseases who are in stable respiratory condition. One prospective study compared simultaneously obtained ABG and peripheral VBG in stable ambulatory patients with OHS (n = 36) and neuromuscular disease (n = 46) while they were resting and breathing ambient air.31 In this study, there was a strong correlation between the arterial and venous Pco2 (r = 0.725; P < .001). In patients with a Paco2 > 45 mm Hg, the cutoff value was found to be ≥ 50 mm Hg for venous blood (sensitivity 87.5% and specificity 72.4%). Importantly, a normal venous Pco2 or a Pco2 < 50 mm Hg in venous blood effectively excludes hypercapnia on ABG.31,32 The TEP believes that the higher the Pco2 on a VBG, the higher the likelihood that hypercapnia is present on an ABG. However, it is important to point out that the finding of an elevated Pco2 in venous blood does not necessarily mean that the patient should be prescribed home NIV. The elevated Pco2 on VBG should be interpreted in the right clinical context.
For these reasons, interest has been growing in noninvasive measures to estimate the Paco2, especially when frequent or continuous monitoring is required. There have been significant technological advances in surrogate measures of Paco2, including end-tidal Pco2 and TcPco2 monitoring. The AASM recommends use of Paco2, TcPco2, or EtPco2 for detection of sleep hypoventilation. Based on data that normal individuals rarely have a Paco2 >55 mm Hg during sleep, the AASM revised its scoring of sleep hypoventilation in adults in 2012 to include two criteria: (1) an increase in Paco2 >55 mm Hg for >10 min; or (2) an increase in Paco2 >10 mm Hg during sleep compared with awake supine measurements to a value exceeding 50 mm Hg for > 10 min.33 By relying solely on ABG, the current policies fail to acknowledge the technological advances made since the 1998 consensus conference and do not allow use of the updated AASM scoring criteria for diagnosing sleep hypoventilation. These surrogate Pco2 measures are sufficient to identify the condition of hypoventilation appropriate for treatment in patients with OHS. This differs from the necessary more exact Paco2 threshold (≥ 52 mm Hg) to fulfill the conditions consistent with the current scientific evidence supporting treatment for patients with hypercapnic COPD with NIV, as outlined by the ONMAP for COPD TEP article.
Technological advances extend beyond CO2 monitoring, to include increasingly sophisticated adherence data from PAP devices and rapidly evolving home sleep testing and consumer sleep technologies, among others. Although research on the exact role of these individual technological developments in both the diagnosis and management of hypoventilation syndromes continues to evolve, coverage criteria need to acknowledge and keep pace with the rapidly developing technologies.
Another challenge to the current BPAP coverage criteria in treating hypoventilation syndromes is the requirement for spirometry (criterion B). This is the only diagnosis category requiring this procedure as mandatory testing rather than clinical judgment. Spirometry may not always be readily available or possible, and therefore delays in treatment can result.34 In addition, although some pulmonary measurements such as functional residual capacity and expiratory reserve volume decrease with increasing BMI, spirometric variables are rarely below the normal range.
Hypoventilation often co-exists with hypoxemia, but the administration of supplemental oxygen alone in hypoventilation syndromes has the potential to worsen hypercapnia by various mechanisms.35 Even 20 min of supplemental oxygen use has been associated with worsening hypercapnia in OHS.36,37 Supplemental oxygen is often needed in addition to PAP during the initial treatment period for OHS, but oxygen alone is not recommended in OHS treatment. In many patients with OHS, adequate adherence to PAP therapy can improve hypoventilation and hypoxemia, and therefore the need for daytime and nocturnal oxygen requirements should be revisited to discontinue oxygen supplementation when no longer necessary.19 The current BPAP coverage criteria inappropriately force use of oxygen therapy on patients with OHS due to the existing challenges in meeting current BPAP coverage criteria, thereby potentially perpetuating misdiagnosis, delaying appropriate treatment, and even worsening hypercapnia. As recommended elsewhere, there is a need to discontinue current requirements to perform testing on the patient’s prescribed supplemental oxygen.
Clinical Practice Guidelines
In 2019, the ATS published clinical guidelines recommending that in stable ambulatory patients diagnosed with OHS and concomitant severe OSA, CPAP therapy rather than NIV should be considered as first-line treatment (conditional recommendation, very low level of certainty in the evidence).11 The ATS panel concluded that NIV may preferentially be used in patients with OHS who have sleep hypoventilation without severe OSA. For hospitalized patients with respiratory failure suspected of having OHS, the ATS clinical practice guidelines recommended that patients be discharged on NIV therapy and remain on NIV therapy during sleep until they undergo outpatient workup and titration of PAP therapy in the sleep laboratory, ideally within the first 3 months following hospital discharge (conditional recommendation, very low level of certainty in the evidence).10
After considering all the evidence, the ONMAP TEP recommends that hospitalized patients be discharged on NIV while awaiting outpatient workup. In this situation, the options are to prescribe NIV in the form of BPAP S/T with empiric settings or autotitrating NIV such as VAPS with the auto-EPAP feature, which has the capability to automatically adjust the respiratory rate to treat hypoventilation; the auto-EPAP feature can ensure upper airway patency in case of increased resistance (eg, OSA).
Applying a narrow set of coverage criteria to a heterogeneous group of diseases presenting with variable acuity and severity even within the same patient has limitations. As an example, patients with OHS can present as decompensated acute-on-chronic hypercapnic respiratory failure requiring hospitalization and even ICU level of care. At the other end of the spectrum, patients with OHS can present routinely to the outpatient sleep clinic with compensated chronic daytime hypoventilation. Sleep hypoventilation typically precedes daytime hypoventilation in the hypoventilation syndromes. Therefore, even within the same disorder and patient, the degree of ventilatory support needed and the optimal DME required for providing this support can vary over time.
Current Medicare policy requires patients receiving BPAP S/T devices to have a face-to-face follow-up in 61 to 90 days following the start of therapy, even if documentation of adherence and benefit can be made earlier. There is no clinical reason why delayed evaluation is necessary for this population. Consistent with the other TEP category suggestions, adherence to NIV therapy should be considered acceptable with ≥ 2 h per night on 70% of nights during a consecutive 30-day period anytime during the first 3 months of initial usage. Moreover, those still engaged with their NIV treating physician and not yet meeting adherence criteria at day 90 should be allowed coverage for another 90-day period before coverage for NIV is discontinued.
Clinical Vignette of a Hospitalized Patient
A 68-year-old severely obese (BMI 65 kg/m2) woman, life-long nonsmoker (no COPD suspicion) is hospitalized with shortness of breath, and a VBG analysis shows a pH of 7.20 and Pco2 of 80 mm Hg. She is admitted to the ICU and after 24 h of NIV therapy, the patient improves and NIV is weaned off. At time of transfer to the general medicine ward, her ABG reveals a pH of 7.34, Paco2 of 60 mm Hg, and Pao2 of 80 mm Hg on 3 L/min of supplemental oxygen. To minimize the risk of rehospitalization and long-term mortality, the medical team would like to qualify this patient for a home BPAP S/T (E0471) at hospital discharge.
Barriers to qualify this patient for BPAP S/T (E0471) based on current criteria include the following:
-
1.
“Trial of BPAP device without a backup rate should fail to qualify for one with backup rate,” which may increase the length of hospitalization as inpatient NIV therapy is commonly needed with backup rate to prevent respiratory decompensation.
-
2.
“Spirometry is needed to rule out COPD,” which is not widely available as an inpatient test and arguably not necessary for low clinical suspicion for COPD.
-
3.
“Repeat ABG during or immediately after sleep is needed to demonstrate a higher Paco2 than the ABG used to qualify her for a BPAP without a backup rate,” but this measurement technique produces sleep fragmentation, pain, anxiety, and possible false results due to resultant hyperventilation.
-
4.
Without an ABG (point 3), she would need a “facility-based PSG or home sleep test to rule out OSA as cause of sustained oxygen desaturations.” However, many institutions do not have the capability of sleep studies in hospitalized patients, and results are often compromised by inpatient sleep patterns and medications, and waits for outpatient PSG testing can be 2 to 3 months.
Clinical Vignette Follow-Up
Due to these current qualification barriers, the patient was ultimately discharged without a BPAP S/T and because of transportation issues due to severe obesity, she missed the sleep clinic appointment. She was readmitted to the hospital with acute-on-chronic hypercapnic respiratory failure 4 months following discharge, and this time, she was discharged on a costlier HMV using an alternative coverage pathway.
Summary of New Suggested Changes
Hypoventilation
Item/Service Description
NIV is achieved by using a device capable of bilevel pressure delivery; that is, different pressures during inspiration and expiration. NIV devices may have the option of a backup rate, which assures a minimum respiratory rate.
Clinical Indications
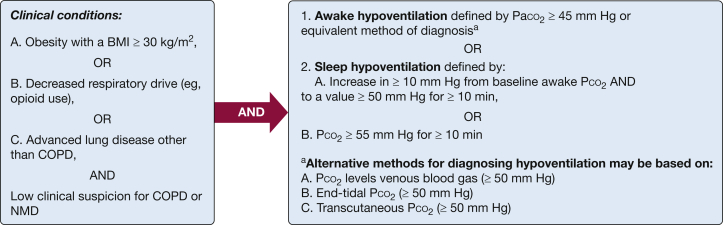
NIV is indicated for hypoventilation syndromes, as defined by an elevated Pco2 in arterial or venous blood, or elevated Pco2 measured by transcutaneous or end-tidal CO2 methods. This covers the following clinical conditions (Fig 1):
-
A.
Obesity-related hypoventilation (eg, E66.2).
-
B.
Hypoventilation due to central respiratory drive depression associated with medication, substance use, or other medical conditions (eg, opioids F19.982, G37.46; neurogenic R06.89; medical condition G47.36).
-
C.
Hypoventilation due to respiratory system disease other than COPD [eg, end-stage interstitial lung disease, J98.4, G47.36], neuromuscular diseases, or thoracic cage disorders, which are covered elsewhere.
Figure 1.
Definition of clinical conditions related to hypoventilation. NMD = neuromuscular disease.
Suggestions for Outpatient NIV Support for Hypoventilation Syndromes
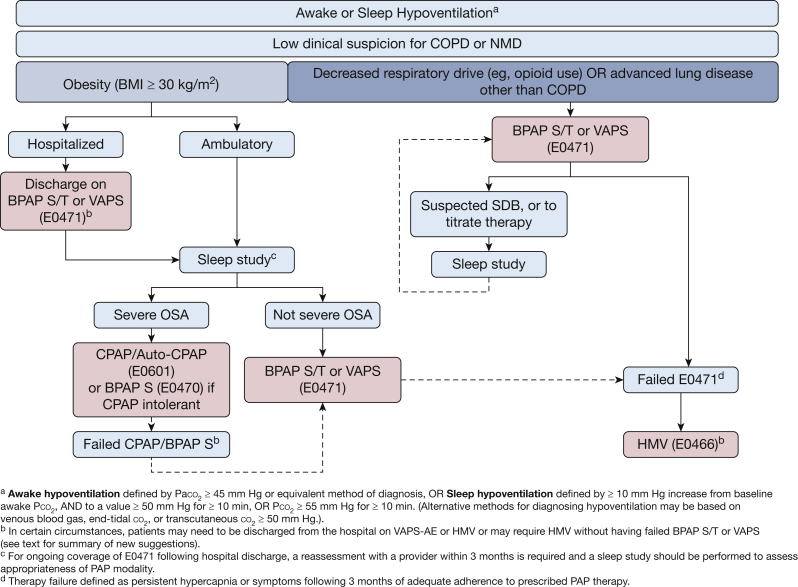
Figure 2 provides a flow diagram for suggested indications for outpatient NIV support for awake or sleep hypoventilation.
Figure 2.
Suggestions for outpatient NIV support for hypoventilation syndromes. AE = auto-EPAP; BPAP = bilevel positive airway pressure; HMV = home mechanical ventilation; NIV = noninvasive ventilation; NMD = neuromuscular disease; SDB = sleep-disordered breathing; S = spontaneous; S/T = spontaneous/timed; VAPS = volume-assured pressure support.
-
A.Obesity-related hypoventilation
-
•Hospitalized patients with persistent awake hypoventilation at the time of discharge following an episode of acute-on-chronic hypercapnic respiratory failure (J96.22: Acute and chronic respiratory failure with hypercapnia) should receive BPAP ventilation with backup rate (BPAP S/T or VAPS [E0471])
-
▪For ongoing coverage of equipment, a reassessment with a provider within 3 months is required and an attended PSG should be performed to assess appropriateness of PAP modality
-
▪Unattended type II/III/IV portable sleep apnea testing is not advised in patients with hypoventilation but is acceptable if attended PSG is not obtainable
-
▪
-
•Ambulatory obese patients with awake or sleep-related hypoventilation and without severe OSA (defined as an apnea-hypopnea index or respiratory disturbance index < 30 events/h), ideally based on an attended PSG, should be started on BPAP S/T or VAPS (E0471)
-
▪For ongoing coverage of equipment, a follow-up with a qualified practitioner within 3 months is required to assess response to therapy and assess appropriateness of PAP modality
-
▪A home sleep test is not advised in patients with hypoventilation but is acceptable if attended PSG is not obtainable
-
▪
-
▪
-
•Ambulatory obese patients with wake or sleep-related hypoventilation and with severe OSA (defined as an apnea-hypopnea index or respiratory disturbance index ≥ 30 events/h) based on attended PSG or home sleep test should be started on CPAP or auto-CPAP therapy (E0601)
-
▪In patients who are intolerant or proven ineffective with CPAP, providers may engage the protocol advised by the “CPAP and Bilevel PAP Therapy for OSA” TEP article.
-
▪If the patient with OHS remains hypercapnic (awake Paco2 ≥ 45 mm Hg, or Pco2 ≥ 50 mm Hg on VBG, EtPco2, or TcPco2 despite adequate adherence to CPAP (E0601) or BPAP S (E0470) after 3 months, a BPAP S/T (E0471) may be considered without need for repeat sleep testing
-
▪
-
•
-
B.Ambulatory patients with hypoventilation due to central respiratory drive depression associated with medication, substance use, or other medical conditions (eg, opioids) may be considered for BPAP S/T or VAPS (E0471) without the need of sleep testing
-
•Sleep testing may be considered if concomitant sleep apnea is suspected, or for titration of NIV
-
•
-
C.Hypoventilation due to respiratory system failure other than COPD or neuromuscular disease/thoracic cage disorder (eg, end-stage or advanced interstitial lung disease) should be considered for: BPAP S/T or VAPS without the need of sleep testing
-
•Sleep testing may be considered if concomitant sleep apnea is suspected, or for titration of NIV
-
•
NIV via an HMV (E0466) is advised for patients with hypoventilation who need:
-
•
Higher pressures than those deliverable by E0471
-
•
Fio2 > 40%, which is greater than can be supplied to an E0471
-
•
Need for bilevel modes with autoadjusting EPAP capability (presently not available in nonventilator devices such as VAPS E0471; VAPS with auto-EPAP is currently available in Europe in E0471 devices) in patients with hypoventilation syndromes when the presence and/or severity of OSA is unknown at time of device prescription
-
•
Need for daytime ventilation or > 10 h per day
-
•Severe disease that requires a device with batteries or alarms, or requires a backup ventilator, or inability to apply or disengage mask without assistance
-
oSevere disease defined as a history of ≥ 2 hospitalizations for hypercapnic respiratory failure (J96.02), OR persistent hypercapnia as defined by Paco2 ≥ 45 mm Hg (or surrogate Pco2 measurements ≥ 50 mm Hg) despite adequate adherence to BPAP S/T therapy.
-
o
Supplemental oxygen therapy suggestions with NIV
-
•
Oxygen supplementation should be adequate to achieve oxygen saturation by pulse oximetry 88% to 92% in all causes of chronic hypercapnic respiratory failure following achievement of optimization targets of NIV settings as determined by the treating physician.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
∗ONMAP Technical Expert Panel members: Overall Chairs: Peter C. Gay, MD, FCCP, and Robert L. Owens, MD. Thoracic Restrictive Disorders: Chair/Co-Chair—Lisa F. Wolfe, MD, FCCP, and Joshua O. Benditt, MD, FCCP. Thoracic Restrictive Disorders Committee Members—Loutfi S. Aboussouan, MD, FCCP, John M. Coleman III, MD, and Dean R. Hess, PhD, RRT. COPD: Chair/Co-chair—Nicholas S. Hill, MD, FCCP, and Gerard J. Criner, MD, FCCP. COPD Committee Members—Richard D. Branson, MSc, RRT, Bartolome R. Celli, MD, FCCP, Neil R. MacIntyre, MD, FCCP, and Amen Sergew, MD. Central Sleep Apnea: Chair/Co-Chair—Timothy I. Morgenthaler, MD, and Atul Malhotra, MD, FCCP. Central Sleep Apnea Committee Members—Richard B. Berry, MD, Karin G. Johnson, MD, and Marc I. Raphaelson, MD. Hypoventilation: Chair/Co-chair—Babak Mokhlesi, MD, FCCP, and Christine H. Won, MD. Hypoventilation Committee Members—Bernardo J. Selim, MD, FCCP, Barry J. Make, MD, FCCP, and Bernie Y. Sunwoo, MBBS. OSA: Chair/Co-chair—Nancy A. Collop, MD, Master FCCP, and Susheel P. Patil, MD, PhD. OSA Committee Members—Alejandro D. Chediak, MD, FCCP, Eric J. Olson, MD, and Kunwar Praveen Vohra, MD, FCCP.
Contributor Information
Babak Mokhlesi, Email: Babak_Mokhlesi@rush.edu.
ONMAP Technical Expert Panel:
Peter C. Gay, Robert L. Owens, Lisa F. Wolfe, Joshua O. Benditt, Loutfi S. Aboussouan, John M. Coleman, III, Dean R. Hess, Nicholas S. Hill, Gerard J. Criner, Richard D. Branson, Bartolome R. Celli, Neil R. MacIntyre, Amen Sergew, Timothy I. Morgenthaler, Atul Malhotra, Richard B. Berry, Karin G. Johnson, Marc I. Raphaelson, Babak Mokhlesi, Christine H. Won, Bernardo J. Selim, Barry J. Make, Bernie Y. Sunwoo, Nancy A. Collop, Susheel P. Patil, Alejandro D. Chediak, Eric J. Olson, and Kunwar Praveen Vohra
References
- 1.Nowbar S., Burkart K.M., Gonzales R., et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116(1):1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Budweiser S., Riedl S.G., Jorres R.A., Heinemann F., Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261(4):375–383. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo A., Ferrer M., Gonzalez-Diaz G., et al. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(12):1279–1285. doi: 10.1164/rccm.201206-1101OC. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Anon O., Perez de Llano L.A., De la Fuente Sanchez S., et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marik P.E., Chen C. The clinical characteristics and hospital and post-hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obes Sci Pract. 2016;2(1):40–47. doi: 10.1002/osp4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masa J.F., Corral J., Alonso M.L., et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick Study. Am J Respir Crit Care Med. 2015;192(1):86–95. doi: 10.1164/rccm.201410-1900OC. [DOI] [PubMed] [Google Scholar]
- 7.Masa J.F., Corral J., Caballero C., et al. Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax. 2016;71(10):899–906. doi: 10.1136/thoraxjnl-2016-208501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest. 1999;116(2):521–534. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 9.Office of Inspector General. Escalating Medicare Billing for Ventilators Raises Concerns. https://www.oversight.gov/sites/default/files/oig-reports/oei-12-15-00370.pdf. 2016. Accessed May 11, 2021.
- 10.Mokhlesi B., Masa J.F., Afshar M., et al. The effect of hospital discharge with empiric noninvasive ventilation on mortality in hospitalized patients with obesity hypoventilation syndrome. An individual patient data meta-analysis. Ann Am Thorac Soc. 2020;17(5):627–637. doi: 10.1513/AnnalsATS.201912-887OC. [DOI] [PubMed] [Google Scholar]
- 11.Mokhlesi B., Masa J.F., Brozek J.L., et al. Evaluation and management of obesity hypoventilation syndrome. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;200(3):e6–e24. doi: 10.1164/rccm.201905-1071ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marik P.E., Desai H. Characteristics of patients with the "malignant obesity hypoventilation syndrome" admitted to an ICU. J Intensive Care Med. 2013;28(2):124–130. doi: 10.1177/0885066612444261. [DOI] [PubMed] [Google Scholar]
- 13.Berg G., Delaive K., Manfreda J., Walld R., Kryger M.H. The use of health-care resources in obesity-hypoventilation syndrome. Chest. 2001;120(2):377–383. doi: 10.1378/chest.120.2.377. [DOI] [PubMed] [Google Scholar]
- 14.Meservey A.J., Burton M.C., Priest J., Teneback C.C., Dixon A.E. Risk of readmission and mortality following hospitalization with hypercapnic respiratory failure. Lung. 2020;198(1):121–134. doi: 10.1007/s00408-019-00300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afshar M., Brozek J.L., Soghier I., et al. The role of positive airway pressure therapy in adults with obesity hypoventilation syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2020;17(3):344–360. doi: 10.1513/AnnalsATS.201907-528OC. [DOI] [PubMed] [Google Scholar]
- 16.Masa J.F., Benitez I., Sanchez-Quiroga M.A., et al. Long-term noninvasive ventilation in obesity hypoventilation syndrome without severe OSA: the Pickwick Randomized Controlled Trial. Chest. 2020;158(3):1176–1186. doi: 10.1016/j.chest.2020.03.068. [DOI] [PubMed] [Google Scholar]
- 17.Piper A.J., Wang D., Yee B.J., Barnes D.J., Grunstein R.R. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63(5):395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 18.Howard M.E., Piper A.J., Stevens B., et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax. 2017;72(5):437–444. doi: 10.1136/thoraxjnl-2016-208559. [DOI] [PubMed] [Google Scholar]
- 19.Masa J.F., Mokhlesi B., Benitez I., et al. Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet. 2019;393(10182):1721–1732. doi: 10.1016/S0140-6736(18)32978-7. [DOI] [PubMed] [Google Scholar]
- 20.Murphy P.B., Davidson C., Hind M.D., et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012;67(8):727–734. doi: 10.1136/thoraxjnl-2011-201081. [DOI] [PubMed] [Google Scholar]
- 21.Patout M., Gagnadoux F., Rabec C., et al. AVAPS-AE versus ST mode: a randomized controlled trial in patients with obesity hypoventilation syndrome. Respirology. 2020;25(10):1073–1081. doi: 10.1111/resp.13784. [DOI] [PubMed] [Google Scholar]
- 22.Masa J.F., Mokhlesi B., Benitez I., et al. Echocardiographic changes with positive airway pressure therapy in obesity hypoventilation syndrome. Long-term Pickwick randomized controlled clinical trial. Am J Respir Crit Care Med. 2020;201(5):586–597. doi: 10.1164/rccm.201906-1122OC. [DOI] [PubMed] [Google Scholar]
- 23.Orr J.E., Coleman J., Criner G.J., et al. Automatic EPAP intelligent volume-assured pressure support is effective in patients with chronic respiratory failure: a randomized trial. Respirology. 2019;24(12):1204–1211. doi: 10.1111/resp.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arellano-Maric M.P., Hamm C., Duiverman M.L., et al. Obesity hypoventilation syndrome treated with non-invasive ventilation: is a switch to CPAP therapy feasible? Respirology. 2020;25(4):435–442. doi: 10.1111/resp.13704. [DOI] [PubMed] [Google Scholar]
- 25.de Llano L.P., Castro-Anon O., Castro-Cabana L., Mendez Marote L., Golpe R. Long-term effectiveness of CPAP in patients with severe obesity-hypoventilation syndrome. Sleep Breath. 2021;25(2):947–950. doi: 10.1007/s11325-020-02177-z. [DOI] [PubMed] [Google Scholar]
- 26.Georges M., Nguyen-Baranoff D., Griffon L., et al. Usefulness of transcutaneous PCO2 to assess nocturnal hypoventilation in restrictive lung disorders. Respirology. 2016;21(7):1300–1306. doi: 10.1111/resp.12812. [DOI] [PubMed] [Google Scholar]
- 27.Kelly A.M., Klim S. Agreement between arterial and transcutaneous PCO2 in patients undergoing non-invasive ventilation. Respir Med. 2011;105(2):226–229. doi: 10.1016/j.rmed.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Janssens J.P., Perrin E., Bennani I., de Muralt B., Titelion V., Picaud C. Is continuous transcutaneous monitoring of PCO2 (TcPCO2) over 8 h reliable in adults? Respir Med. 2021;95(5):331–335. doi: 10.1053/rmed.2001.1045. [DOI] [PubMed] [Google Scholar]
- 29.Berry R.B., Chediak A., Brown L.K., et al. NPPV Titration Task Force of the American Academy of Sleep Medicine: best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010;6(5):491–509. [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne A.L., Bennett M., Chatterji R., Symons R., Pace N.L., Thomas P.S. Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis. Respirology. 2014;19(2):168–175. doi: 10.1111/resp.12225. [DOI] [PubMed] [Google Scholar]
- 31.Orucova H., Cagatay T., Bingol Z., Cagatay P., Okumus G., Kiyan E. Comparison of arterial and venous blood gases in patients with obesity hypoventilation syndrome and neuromuscular disease. Ann Thorac Med. 2019;14(3):192–197. doi: 10.4103/atm.ATM_29_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloom B.M., Grundlingh J., Bestwick J.P., Harris T. The role of venous blood gas in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2014;21(2):81–88. doi: 10.1097/MEJ.0b013e32836437cf. [DOI] [PubMed] [Google Scholar]
- 33.Berry R.B., Budhiraja R., Gottlieb D.J., et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ip A., Asamoah-Barnieh R., Bischak D.P., Davidson W.J., Flemons W.W., Pendharkar S.R. Using operational analysis to improve access to pulmonary function testing. Can Respir J. 2016;2016:5269374. doi: 10.1155/2016/5269374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson T.D., Freiberg D.B., Regnis J.A., Young I.H. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1524–1529. doi: 10.1164/ajrccm.161.5.9904119. [DOI] [PubMed] [Google Scholar]
- 36.Hollier C.A., Harmer A.R., Maxwell L.J., et al. Moderate concentrations of supplemental oxygen worsen hypercapnia in obesity hypoventilation syndrome: a randomised crossover study. Thorax. 2014;69(4):346–353. doi: 10.1136/thoraxjnl-2013-204389. [DOI] [PubMed] [Google Scholar]
- 37.Wijesinghe M., Williams M., Perrin K., Weatherall M., Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011;139(5):1018–1024. doi: 10.1378/chest.10-1280. [DOI] [PubMed] [Google Scholar]