Obesity remains one of the leading risk factors for the development of heart failure (HF), which is largely attributable to alterations in cardiac structure and function and a reduced cardiorespiratory fitness (CRF).1 However, once HF is diagnosed, patients with obesity, particularly those with class I obesity [body mass index (BMI) ≥30 and <35 kg/m2], present a more favourable survival, despite a greater risk for HF-related hospitalizations.2 This phenomenon has been termed the ‘obesity paradox’ in both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF).3 Weight loss has also been associated with worse survival in HF in observational studies; however, although long-term adherence and weight regain remain a major concern, smaller single-centre randomized trials support the use of intentional weight loss using caloric restriction to improve CRF and patient-reported quality of life (QoL) in patients with obesity and concomitant HF.4 The obesity paradox ‘heavily’ challenges the concept that obesity should be even treated with weight loss in patients with established HF, to the extent that current recommendations do not universally recommend weight loss, unless patients have class II obesity or greater.
In an analysis of the DAPA-HF study,5 which had previously shown that dapagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, improves clinical outcomes in patients with HFrEF, with and without type 2 diabetes mellitus (T2DM),6 Adamson and colleagues investigated (i) the effects of BMI categories and short-term BMI reduction on survival and the risk for hospitalization in the overall population; (ii) the potential interaction of BMI categories for the effects of dapagliflozin; (iii) the effect of dapagliflozin on body weight and cardiometabolic risk factors; and (iv) the safety of dapagliflozin across BMI categories.
Patients with obesity enrolled in DAPA-HF were younger, more likely to be white and female, with lower N-terminal pro-B-type natriuretic peptide (NT-proBNP) level, higher prevalence of T2DM, atrial fibrillation and a dramatically worse patient-reported QoL compared to those in the lower BMI categories. Patients with obesity were more likely to receive angiotensin receptor-neprilysin inhibitor, beta-blockers, statins, diuretics, and implantable cardioverter defibrillators. Patients with higher BMI were more likely to receive biguanides and insulin, and less likely to receive dipeptidyl peptidase-4 inhibitors.
In the whole study population, the authors found a non-linear relationship between BMI and the incidence of the primary composite endpoint of cardiovascular death or a worsening HF event, with patients with class I obesity presenting the most favourable prognosis compared to those with higher BMI, but also compared to those with lower BMI, including the underweight/normal weight group, confirming the previously reported obesity paradox. Consistently, also a BMI reduction of 2% in the overall population at 8 months was further associated with an increased risk for the composite cardiovascular death, HF hospitalizations and all-cause mortality. Of note, the number of individuals who were underweight was low; in fact, this group was analysed together with those who had a normal body weight, which might have affected the results of the study.
Consistent with the effects of most SGLT2 inhibitors in prior studies, dapagliflozin reduced systolic blood pressure and NT-proBNR and increased haematocrit. Dapagliflozin was also associated with a greater reduction in glycated haemoglobin in those with greater BMI and T2DM and a lower increase in creatinine in those with obesity. There was no significant interaction for BMI with the beneficial effects of dapagliflozin on clinical outcomes and QoL, substantiating that these favourable effects may be independent of BMI. In addition, the number of adverse events was not increased in patients with obesity treated with dapagliflozin, which considering the growing epidemic of obesity in the United States and worldwide, including in patients with established HFrEF, is reassuring.
Dapagliflozin has been also associated with favourable outcomes in primary prevention of cardiovascular disease (CVD) in patients with T2DM,7 proposing it as a powerful therapeutic agent available to clinicians across the board of different patient populations to reduce CVD risk. Despite the benefits of SGLT2 inhibitors, the mechanisms through which these agents improve clinical outcomes, especially HF-related outcomes, remain unclear.
One of the potential mechanisms through which SGLT2 inhibitors may improve outcomes is through improvement in CRF (Figure 1), which is a strong and modifiable risk factor for clinical outcomes in patients with HF, and SGLT2 inhibitors have been associated with improved CRF in this population.8,9 The determinants of CRF (central cardiac vs. peripheral) have been only partially investigated in patients treated with SGLT2 inhibitors, with reported beneficial effects on cardiac structure and function.8–10 Of note, the study by Adamson and colleagues did not provide data on physical activity or on measured nor estimated CRF. In fact, the obesity paradox has not been evident in patients with preserved CRF11 or greater levels of physical activity.12 Importantly, we want to point out that despite the fact that the obesity paradox exists in patients with established HF and many other CVD, we obviously should not encourage normal-weight individuals to accumulate adiposity; in fact, if obesity was prevented, many likely would have not developed HF in the first place.
Figure 1.
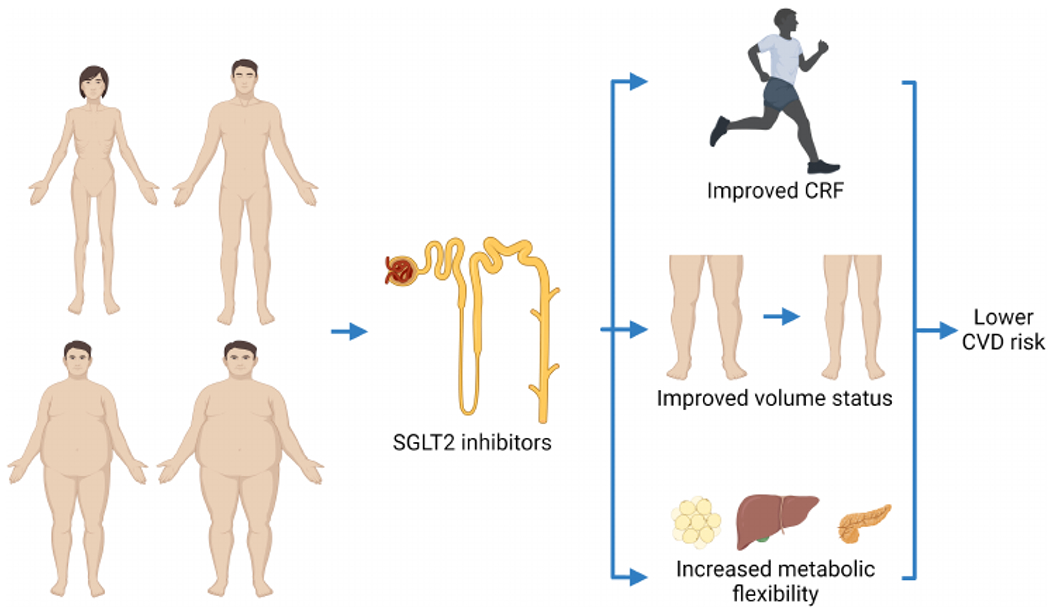
The potential beneficial effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors, such as dapagliflozin, on heart failure-related outcomes and patient-reported quality of life, independent of body mass index. SGLT2 inhibitors may exert their beneficial effects by improving fitness, volume status (without activating the nenin-angiotensin-aldosterone system), and metabolic flexibility, in addition to other factors. CRF, cardiorespiratory fitness; CVD, cardiovascular disease.
Another potential mechanism through which SGLT2 inhibitors might exert their beneficial effects is their ability to act as ‘non-traditional diuretics’. To the contrary of what is typically seen in patients treated with diuretics (i.e. loop diuretics), those concomitantly treated with SGLT2 inhibitors do not experience an increase in the activation of the renin-angiotensin-aldosterone system, which is detrimental in HF. SGLT2 inhibitors improve volume status, but without causing the negative effects characteristically resulting from volume contraction typically seen with other diuretics. In fact, these agents have been associated with either neutral, or even a reduction, in renin-angiotensin-aldosterone system activation.10 Of note, a recent analysis of the EMPEROR-Reduced trial with empagliflozin in HFrEF does not suggest that the benefits of SGLT2 inhibitors can be fully explained by its diuretic effects13; however, we cannot exclude the partial contribution from diuresis.
Improvements in metabolic flexibility, defined as the ability to switch from carbohydrates to lipid oxidation as energetic substrate of the body measured as a drop in the respiratory exchange ratio (or respiratory quotient when referring to direct cellular respiration; i.e. the ratio between carbon dioxide/oxygen production), have also been proposed as a potential beneficial mechanism of SGLT2 inhibitors to improve clinical HF-related outcomes. In patients with insulin resistance, characteristic of overweight and obesity, but also of other chronic diseases, such as HF, metabolic inflexibility is frequent, with the inability of the body, including the heart, to utilize fatty acids as an energetic substrate. In an elegant recent cross-over randomized controlled trial of patients with T2DM, but without HF, treatment with dapagliflozin for 5 weeks reduced 24 h respiratory exchange ratio measured using a whole-room indirect calorimeter, and increased fatty acid oxidation resulting in a negative fat balance.14 Taken together, the results of the study suggest that dapagliflozin improves metabolic flexibility resulting in a greater capability of the individual to utilize lipids as energetic substrate as opposed to carbohydrates.
The improvements in metabolic flexibility might also potentially explain how the SGLT2 inhibitor empagliflozin reduced, for the first time in the history of CVD outcome trials in HFpEF, the composite primary endpoint of hospitalization and cardiovascular death in this population.15 In fact, most patients with HFpEF present with overweight and obesity, resulting in metabolic abnormalities, such as metabolic inflexibility and insulin resistance, and this metabolic approach had not been previously targeted by other agents for CVD. Importantly, also sotagliflozin, a dual SGLT1/SGLT2 inhibitor, has recently been associated with favourable outcomes in HFpEF in a sub-analysis of a larger study including both HFrEF and HFpEF.16
When evaluating the effects of dapagliflozin on body weight in the study by Adamson and colleagues, dapagliflozin induced a weight loss of 0.9 kg, without heterogeneity across BMI categories compared to placebo.5 This is of utmost importance considering that weight loss in observational studies has been associated with worse prognosis in HFrEF, and also in this study, when patients were analysed as a whole without considering the effects of dapagliflozin, BMI reduction at 8 months was associated with a poor prognosis.5 However, when dapagliflozin was analysed separately, the benefits remained despite the weight reduction. This supports that weight loss achieved with SGLT2 inhibitors, due to increased glycosuria of approximately 50–100 g glucose per day, and therefore causing a daily caloric deficit, is safe. SGLT2 inhibitors have been proposed to mimic the molecular beneficial effects of caloric restriction.17 Some of the beneficial effects of caloric restriction are mediated by a nicotinamide adenine dinucleotide-dependent deacetylase, sirtuin 1 (Sirtl).17 Activation of Sirtl, through resver-atrol, a polyphenol and well-known clinically available activator of Sirtl,17 has been associated with cardiovascular benefits in patients without HF, but also in pre-clinical models of HF.17 By inducing a mild yet continuous caloric deficit, SGLT2 inhibitors may mimic its downstream effects, ultimately increasing Sirtl and resulting in CVD protection.
Although understanding the exact mechanisms of action of SGLT2 inhibitors responsible for their evident cardiovascular and metabolic benefits in both HFrEF and HFpEF remains important, more importantly is concern that their implementation in clinical practice remains low. This is problematic considering their use is currently recommended by major cardiovascular-and T2DM-related guidelines, proposing that clinicians should make a greater effort to prescribe SGLT2 inhibitors.
In conclusion, we congratulate Adamson and colleagues5 for confirming the powerful beneficial effects of SGLT2 inhibitors in HFrEF in both patients with and without T2DM and with and without obesity, on major clinical cardiovascular outcomes and patient-reported QoL, an effect which is evidently independent of their glucose-lowering effects. Moreover, dapagliflozin was proven safe also in patients with obesity. We also believe that understanding the mechanisms driving the benefits of SGLT2 inhibitors and developing strategies to promote their use in clinical practice are urgently needed.
Conflict of interest:
S.C. is supported by a Career Development Award 19CDA34660318 from the American Heart Association and by a Clinical and Translational Science Award UL1TR002649 from the National Institutes of Health to Virginia Commonwealth University. C.J.L. has served as a consultant and promotional speaker for AstraZeneca for Farxiga in the US. A.S.A. has nothing to disclose.
Footnotes
This article refers to ‘Efficacy of dapagliflozin in heart failure with reduced ejection fraction according to body mass index’ by C. Adamson et al., published in this issue on pages 1662-1672.
References
- 1.Kokkinos P, Faselis C, Franklin B, Lavie CJ, Sidossis L, Moore H, Karasik P, Myers J. Cardiorespiratory fitness, body mass index and heart failure incidence. Eur J Heart Fail 2019;21:436–444. [DOI] [PubMed] [Google Scholar]
- 2.Carbone S, Lavie CJ. Disparate effects of obesity on survival and hospitalizations in heart failure with preserved ejection fraction. Int J Obes (Lond) 2020;44:1543–1545. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428–1434. [DOI] [PubMed] [Google Scholar]
- 4.Billingsley HE, Hummel SL, Carbone S. The role of diet and nutrition in heart failure: a state-of-the-art narrative review. Prog Cardiovasc Dis 2020;63:538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamson C, Jhund PS, Docherty KF, Bělohlávek J, Chiang CE, Diez M, Drożdż J, Dukát A, Howlett J, Ljungman CEA, Petrie MC, Schou M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Langkilde AM, Lindholm D, Sjöstrand M, McMurray JJV. Efficacy of dapagliflozin in heart failure with reduced ejection fraction according to body mass index. Eur J Heart Fail 2021. July 16. 10.1002/ejhf.2308 ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 8.Carbone S, Billingsley HE, Canada JM, Bressi E, Rotelli B, Kadariya D, Dixon DL, Markley R, Trankle CR, Cooke R, Rao K, B Shah K, Medina de Chazal H, Chiabrando JG, Vecchié A, Dell M, L Mihalick V, Bogaev R, Hart L, Van Tassell BW, Arena R, Celi FS, Abbate A. The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: the CANA-HF study. Diabetes Metab Res Rev 2020;36:e3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 2019;73:1931–1944. [DOI] [PubMed] [Google Scholar]
- 10.Carbone S, Canada JM, Billingsley HE, Kadariya D, Dixon DL, Trankle CR, Buckley LF, Markley R, Vo C, Medina de Chazal H, Christopher S, Buzzetti R, Van Tassell BW, Abbate A. Effects of empagliflozin on cardiorespiratory fitness and significant interaction of loop diuretics. Diabetes Obes Metab 2018;20:2014–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, Arena R. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc 2013;88:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moholdt T, Lavie CJ, Nauman J. Sustained physical activity, not weight loss, associated with improved survival in coronary heart disease. J Am Coll Cardiol 2018;71:1094–1101. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Brueckmann M, Jamal W, Cotton D, Iwata T, Zannad F; EMPEROR-Reduced Trial Committees and Investigators. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-Reduced trial. J Am Coll Cardiol 2021;77:1381–1392. [DOI] [PubMed] [Google Scholar]
- 14.Op den Kamp YJM, de Ligt M, Dautzenberg B, Kornips E, Esterline R, Hesselink MKC, Hoeks J, Schrauwen-Hinderling VB, Havekes B, Oscarsson J, Phielix E, Schrauwen. Effects of the SGLT2 inhibitor dapagliflozin on energy metabolism in patients with type 2 diabetes: a randomized, double-blind crossover trial. Diabetes Care 2021;44:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl j Med 2021. https://doi-org.proxy.library.vcu.edu/10.1nejmoa2107038 [Epub ahead of print]. [Google Scholar]
- 16.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 17.Packer M Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail 2021;9:535–549. [DOI] [PubMed] [Google Scholar]


