Abstract
Radiation therapy treatments are typically planned based on a single image set, assuming that the patient’s anatomy and its position relative to the delivery system remains constant during the course of treatment. Similarly, the prescription dose assumes constant biological dose-response over the treatment course. However, variations can and do occur on multiple time scales. For treatment sites with significant intra-fractional motion, geometric changes happen over seconds or minutes, while biological considerations change over days or weeks. At an intermediate timescale, geometric changes occur between daily treatment fractions. Adaptive radiation therapy is applied to consider changes in patient anatomy during the course of fractionated treatment delivery. While traditionally adaptation has been done off-line with replanning based on new CT images, online treatment adaptation based on on-board imaging has gained momentum in recent years due to advanced imaging techniques combined with treatment delivery systems. Adaptation is particularly important in proton therapy where small changes in patient anatomy can lead to significant dose perturbations due to the dose conformality and finite range of proton beams. This review summarizes the current state-of-the-art of on-line adaptive proton therapy and identifies areas requiring further research.
1. Introduction
There are various sources of geometrical changes in the patient that may occur in between fractions. Most random uncertainties, with the exception of significant tumor volume changes, are approached by applying appropriate margins around the target volume. There are changes, however, that are not considered in the planning target volume either by design, because they are not easy to predict, such as unforeseeable changes in the patient’s anatomy, i.e., anatomical changes. Some of these are treatment-related such as tumor shrinkage or change in patient’s weight or shape. Others might be unrelated to radiation therapy such as mucosal filling of the sinuses. In general, geometry changes might dosimetrically impact organs at risk more than the target as they might have been anticipated when designing the planning target volume (Duma et al., 2012).
The goal of adaptive radiation therapy is to ensure the delivery of the prescribed dose while maintaining healthy tissue constraints even if the patient geometry changes during the course of a fractionated treatment. Thus, the treatment plan and consequently the delivery parameters are tailored to the “geometry of the day” (Wu et al., 2009; Yan et al., 1997). As stated in the seminal paper by Yan et al., “Adaptive radiation therapy is a closed-loop radiation treatment process where the treatment plan can be modified using a systematic feedback of measurements. Adaptive radiation therapy intends to improve radiation treatment by systematically monitoring treatment variations and incorporating them to re-optimize the treatment plan early on during the course of treatment” (Yan et al., 1997). Consequently, adaptive therapy strategies do allow a reduction of margins (van Kranen et al., 2016). Furthermore, while classical plan adaptation uses the initial treatment plan with its description and constraints as reference, adaptation can potentially alter those initial plan parameters during the course of fractionated treatment delivery. Heukelom and Fuller (Heukelom and Fuller, 2019) define adaptive radiation therapy (ART) paradigms that depend on its intent:
ARTex-aequo: ART to maintain original plan coverage and constraints
ARTOAR: ART to reduce the dose to organs at risk during the course of fractionated treatment
ARTamplio: ART aiming at tumor dose escalation
ARTreduco: ART based on a reduction of CTV volume
ARTtotale: ART aiming at dose escalation plus CTV volume reduction during the course of treatment
A major obstacle for the implementation of plan adaptation is the clinical workflow and available clinical resources. Adaptation requires imaging, contour definition, plan evaluation, plan adaptation and, finally, plan verification. If done on-line, these steps in turn rely on fast image reconstruction, a fast and reliable method for contour definition, and fast and accurate dose calculation. It is often unclear what the appropriate frequency of adaptation should be and whether plan adaptation is even needed for most patients both from a pure dosimetric as well as from a tumor control or normal tissue complication point of view (Chen et al., 2014). Figure 1 shows the prominent adaptive workflows. Adaptation is often done at fixed intervals (e.g., halfway through the fractionated treatment) in cases where anatomical changes are expected (e.g., tumor shrinkage). With the advent of in-room imaging it is now often triggered by observations on a given treatment day, e.g., reacting to an observed feature change in the setup and/or in the patient resulting in a negative effect on the plan quality. If resources permit, adaptation can also be done frequently (e.g., daily) potentially in combination with a trigger when a certain dosimetric or anatomical threshold is reached. In each of these scenarios, the initial treatment plan is typically used as a reference. However, in case of iterative adaptation, the reference is the image taken on the previous day as well as the plan of the previous day. This iterative approach is most accurate also in the context of adapting based on the cumulative dose.
Figure 1:
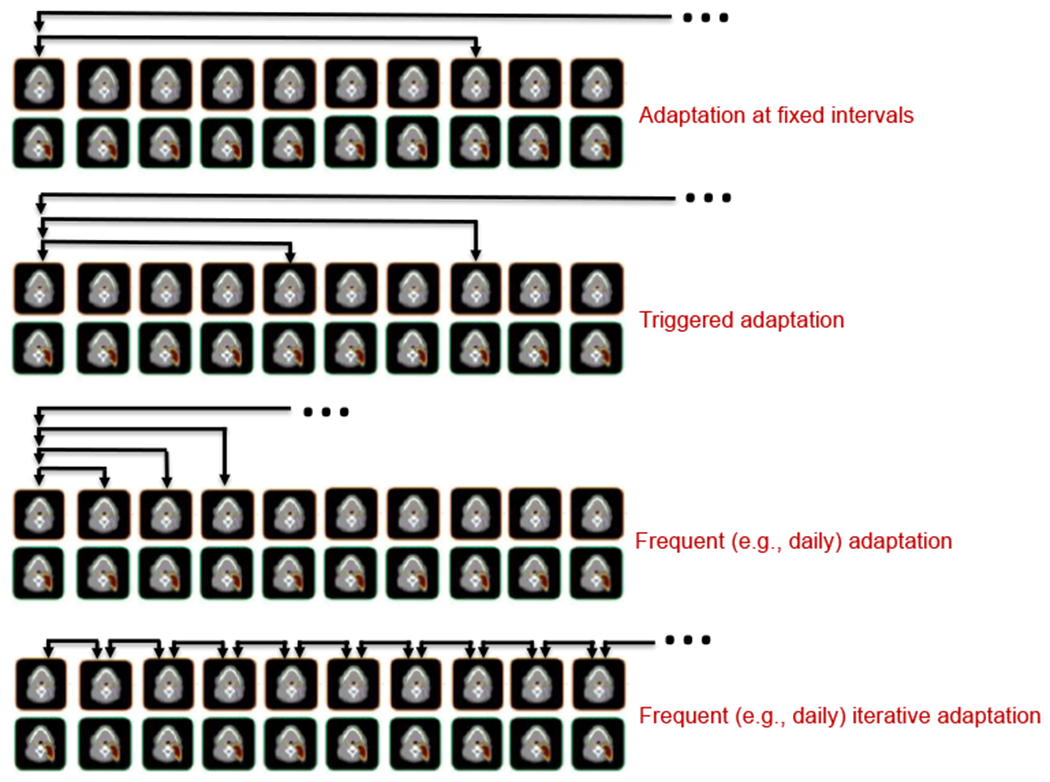
Adaptation workflow: A: Adaptation at constant intervals using the initial plan assessed on the new image; B: Adaptation triggered by on-line imaging based on the initial plan; C: Daily adaptation using the initial plan assessed; D: Frequent (e.g., daily) adaptation using the accumulated dose and previous day image and adapted plan as reference. Figure adapted from Heukelom and Fuller (Heukelom and Fuller, 2019).
The impact of adaptive therapy depends on the frequency of imaging (e.g., daily versus weekly) but also on the treatment schedule because anatomical changes might be more pronounced during early fractions (Bhide et al., 2010; Sanguineti et al., 2013). Furthermore, the quality of imaging plays a role. Infrequent adaptation can be accomplished without in-room imaging whereas daily adaptation generally does require in-room (on-line) imaging with potential compromises on image quality.
In general, compared to standard (un-adapted) radiation therapy, plan adaptation can follow many different strategies (Figure 2). In off-line adaptation, images taken during fractionated radiation therapy can be used for complete re-planning, a technique particularly common for tumors known to regress during treatment. Systematic errors have been addressed off-line by using an average anatomy model for off-line correction (van Kranen et al., 2013). There are numerous off-line adaptation workflows depending on the imaging being used, the re-optimization process, and the adaptive correction based on previous fractions (Yan et al., 2000; Lof et al., 1998). Off-line adaptation can also be used in combination with in-room imaging. A common approach is to use daily on-board imaging or serial CT to analyze geometrical variations and then re-optimize the treatment plan based on new target delineations on the registered original planning CT (Birkner et al., 2003; Wu et al., 2002; Woodford et al., 2007). In any case, off-line adaptive therapy generally largely follows the standard clinical workflow as far as delivery is concerned. Off-line adaptation is by definition a correction method for geometrical changes and its dosimetric impact is delayed for subsequent fractions. It is thus desirable to do on-line adaptation with images taken immediately before treatment to correct before the potentially flawed dose distribution is administered (Lim-Reinders et al., 2017).
Figure 2:

Frameworks for adaptive radiation therapy (RT). Off-line imaging is used for standard radiation therapy as well as off-line adaptive therapy for a selected number of fractions (left). Using image registration, on-line imaging also allows off-line adaptive therapy as well as image-guided therapy and in-line adaptation (middle) On-line adaptive therapy, the focus of this review, is shown on the right (dark colors) and aims at complete re-planning or plan adjustment.
As with off-line techniques, there are various ways to perform on-line adaptation used in combination with in-room imaging (Wu et al., 2011). The simplest form of on-line adaptive therapy is the re-positioning of the patient using in room imaging. For instance, a couch shift might correct for setup errors or anatomical changes. Adaptive radiation therapy is thus closely related to image guided therapy and treatment verification (Wu et al., 2009; Xing et al., 2007; Lim-Reinders et al., 2017). Image guided radiation therapy not only allows for more accurate patient setup and positioning, it also opens the door for daily treatment adaptation as no separate CT scan might be needed and imaging can be done in the same position as treatment (Dawson and Sharpe, 2006). Methods have been developed to do on-line adaptation using multi-leaf collimator position adjustments based on daily CT imaging assuming that there is no change within one CT slice (Court et al., 2005; Fu et al., 2009). Online positioning after offline re-optimization was proposed by Lei et al (Lei and Wu, 2010) while subsequently others have discussed the feasibility of doing both on-line (Li et al., 2011; Li et al., 2010; Mohan et al., 2005; Feng et al., 2006; Wu et al., 2008).
The ultimate goal is to adjust or even re-optimize the treatment plan based on daily imaging (Botas et al., 2018). Rapid on-line adaptation in a clinical workflow has to be completed within minutes, thus posing computational challenges to image registration, optimization and dose calculation alike (Men et al., 2010; Xing et al., 2007). Tumor shrinkage is a significant obstacle for on-line adaptation due to contour changes and deformations of healthy tissue caused by tumor volume changes that may require human intervention (Hamming-Vrieze et al., 2017). Furthermore, on-line adaptation is challenging because of limitations in imaging and computational efficiency. Wu et al. described a workflow capable of on-board daily imaging and re-optimization within 2 minutes (Wu et al., 2008). Due to the impact on computational and treatment planning efficiency, automated planning using machine learning has drawn attention particularly in adaptive radiation therapy (Moore, 2019). There are methods to reduce the required optimization steps as the plan-of-the-day is presumably based on a geometry very similar to the one used for the initial plan. Some of these methods re-optimize solely based on virtual couch shifts method (Bol et al., 2013) or limited aperture segmentation (Li et al., 2013).
In principle the delivery system can also react to changes during delivery, e.g., to account for respiratory motion. Potential variations in patient’s breathing pattern add an additional complexity that can be addressed by in-line (real-time) treatment adaptation based on on-board imaging (Keall et al., 2019). This review is limited to adaptation due to changes in between fractions and does not address changes that occur within a treatment session.
Because plan adaptation, both on-line as well as off-line, does alter clinical workflow, criteria for plan adaptation need to be defined. There needs to be a compromise between expected impact on treatment outcome and extra efforts for planning and delivery. The criteria will likely be different between off-line and on-line adaptation due to the different time frames. Thresholds for any type of plan adaptation are typically based on dosimetric criteria equivalent to prescription and constraints used in the initial planning (Heukelom et al., 2013; Dial et al., 2016; Lim et al., 2014; Green et al., 2019).
Anatomical changes in tumor and non-tumor anatomy clearly suggest a benefit of dosimetric treatment adaptation (Ahunbay et al., 2008; Mohan et al., 2005; Fu et al., 2009; Burridge et al., 2006; Barker et al., 2004; Hansen et al., 2006). Frequent adaptation using various imaging devices has been studied specifically in photon therapy for head and neck cancers (Hansen et al., 2006; Barker et al., 2004; Wu et al., 2009; O’Daniel et al., 2007; Schwartz et al., 2012; Wu et al., 2002; Castadot et al., 2010; Chen et al., 2014; Heukelom and Fuller, 2019). Furthermore, virtual clinical trials have been conducted to assess the benefit of adaptive therapy in photon treatments (Schwartz et al., 2012; Schwartz et al., 2013). The impact of PTV margin reduction on tumor coverage for oropharynx tumors in an adaptive setting with VMAT revealed problems with target coverage as well as a significant reduction in lymph node and parotid volumes due to volume reduction and patient weight (van Kranen et al., 2016). On-line adaptive therapy for nasopharynx patients has been suggested to improve outcome in photon therapy and reduce toxicities (Han et al., 2008; Wang et al., 2009).
The requirement for adaptation due to internal geometry change depends highly on the treatment site (Sonke et al., 2019; Lim-Reinders et al., 2017) and has been studied for many sites such as for head and neck cancers (Heukelom et al., 2013; Schwartz et al., 2013; Schwartz et al., 2012) and prostate carcinoma (Posiewnik and Piotrowski, 2019; Deutschmann et al., 2012), in addition to adaptation including respiratory motion in lung (Sonke and Belderbos, 2010; Kavanaugh et al., 2019). Brouwer et al. (Brouwer et al., 2015) as well as Castelli et al. (Castelli et al., 2018) have reviewed numerous published studies on anatomic changes during radiation therapy for head and neck cancer. An estimated 20% of head and neck cancer cases treated with IMRT require re-planning in the first 3 weeks of fractionated radiation therapy (Brouwer et al., 2015). The dosimetric impact of geometry changes can be substantial. For instance, Cheng et al. found an increase in mean dose to the parotid glands of 10.4 Gy during intensity-modulated photon radiotherapy for nasopharyngeal carcinoma patients (Cheng et al., 2012). Consequently, a significant improvement of patient’s quality of life was found with re-planning during fractionated therapy of these patients (Zhao et al., 2011; Yang et al., 2013).
Adaptive strategies ideally also include dose verification or daily dose reconstruction based on delivery information such as delivery log files and/or on-line imaging (Lee et al., 2008a; Lee et al., 2008b; van Elmpt et al., 2006). Consequently, it is feasible to perform dosimetric corrections in subsequent fractions based on the previously delivered dose (Yan et al., 1999; Wu et al., 2006; Jaffray et al., 2010; de la Zerda et al., 2007; Xing et al., 2007). Furthermore, plan adaptation typically aims at preserving the goals of the initial treatment plan. However, adaptation can be used in the context of adapted dose escalation and local boosts (Ciarmatori et al., 2019; Hafeez et al., 2016; Weiss et al., 2013).
While much can be learned from online adaptation in photon therapy, the dosimetric characteristics of proton therapy plans might require different adaptive strategies.
2. The rationale for plan adaptation in proton therapy
2.1. Proton therapy
As a result of electromagnetic interactions, nuclear interactions, and Coulomb scattering, the kinetic energy of a proton deposited in a medium can be described by the Bethe Bloch formula and the shape of the energy deposition curve is commonly termed the Bragg curve with the Bragg peak near the end of range (Paganetti, 2019). The initial energy of the protons (E0) determines the range (R0) of the proton beam and can be fit with an exponential, assuming a water medium, Ro = 0.0022 E01.77 (Bortfeld, 1997). Due to the peaked energy deposition, proton therapy can delivery therapeutic doses to cancer tissues with less dose deposited into surrounding healthy tissues, particularly in the distal region. Photon therapy, with recent technological developments, can deliver conformal therapeutic doses through intensity modulated deliveries from multiple entrance paths but the volume of tissues receiving low doses (integral dose) in surrounding tissue is always greater than in proton therapy (Barten et al., 2015; Lomax et al., 1999; van de Water et al., 2011; Zhang et al., 2010).
While proton therapy was formerly limited to passively scattered beam delivery using absorbers to shape the dose distribution, most modern proton therapy centers use scanned beam delivery systems. A scanning system uses magnets to scan the proton beam laterally to cover the intended treatment volume while energy modulation covers the target depth. Beam scanning allows the delivery of intensity-modulated proton therapy (IMPT), where beamlet position, energy and fluence are considered and optimized independently. This can be used to create multi-field dose distributions, summed to create a prescribed dose distribution, of higher complexity than plans where each field has uniform dose. The increased flexibility of scanned beam systems allow for rapid changes in the treatment delivery to accommodate online adaptive therapy workflows.
2.2. Range Uncertainties
While the physical dose deposition is predominantly localized in the Bragg Peak, the precision of the peak dose position is subject to uncertainties (Lomax, 2008a, b; Knopf and Lomax, 2013). The physical range uncertainties can be divided into CT imaging (relative stopping power calibration), dose calculation (nuclear interactions and Coulomb scattering), treatment delivery (proton energy variations), and patient anatomy (rigid or deformable patient changes) (Paganetti, 2012). Systematic range uncertainties from CT imaging can be reduced by the use of stoichiometric calibrations (Meijers et al., 2020; Yang et al., 2012) and dual energy CT imaging (Lee et al., 2019). Furthermore, research is ongoing towards the use of proton CT (Dedes et al., 2019; Rit et al., 2013; Deffet et al., 2020; Dickmann et al., 2021), which would be attractive particularly in an adaptive setting as it allows direct assessment of relative stopping power. In addition to imaging, random variations in patient anatomy or setup position can result in changes in proton range (Kim et al., 2017; Liebl et al., 2014; Trofimov et al., 2011; Stutzer et al., 2017).
The effects of the range uncertainties due to patient anatomy and setup changes can vary for treatment sites and treatment delivery techniques. While proton therapy can potentially be a more precise dose delivery technique, the daily variations of the patient setup or anatomy can impact the range and decrease the dose to the target or increase the dose to the surrounding organs (Ashida et al., 2020; Hamming-Vrieze et al., 2019; Liebl et al., 2014; Lomax, 2008b; Perumal et al., 2018; van de Water et al., 2011). Setup uncertainties and immobilization for various patient populations have been detailed in several publications (Engelsman et al., 2005; Winey et al., 2012). For different sites, the setup uncertainties can vary from less than 1mm (cranial) to several mm even without accounting for breathing or other anatomic motions. More recent studies have analyzed the effects of setup uncertainties on range uncertainties, combined with patient changes in head and neck patient populations since this treatment site is subject to both setup and anatomic variations during the course of treatment (Kim et al., 2017; Liebl et al., 2014; Malyapa et al., 2016). Kim et. al. demonstrated that patient weight loss and setup uncertainties in head and neck patient populations can have equally large effects on the range uncertainties.
Other studies have looked at prostate (Moteabbed et al., 2017; Sejpal et al., 2009; Trofimov et al., 2011; Wang et al., 2011). The prostate can move within the patient depending upon the bladder filling, rectal motion, and patient setup. Given the motion of the prostate over different time periods, the resulting range uncertainties depend upon the surrounding tissues, particularly the femoral heads when using lateral treatment beams. Range uncertainties in proton therapy for lung treatments have been reviewed in prior studies (Engelsman et al., 2006; Park et al., 2013; Szeto et al., 2016). Motion of the target tissues can vary depending upon the location and size of the target. The magnitude of the motion and the treatment beam angles impact the magnitude of range uncertainties. Since the target tissues are generally denser than the surrounding lung tissues, the physical range uncertainties can be dramatically larger than other cases of targets surrounded by more water equivalent tissues.
2.3. Margins
Given the dose uncertainties of the proton dose delivery, margins have been implemented to decrease the risk of missing the treatment volume due to set up and range uncertainties (Knopf et al., 2013; Taasti et al., 2019; Thomas, 2006). The margins rely upon the sum of the range uncertainties for the specific beam angle and can involve local tissue density variations. While margins can be applied uniformly, proton therapy plans typically apply the margins in a beam-by-beam design or by conversion of range margins into beam specific PTV (Lin et al., 2015; Park et al., 2012; Perumal et al., 2018). Schuemann et al evaluated the site specific range uncertainties when considering tissue homogeneity (Schuemann et al., 2014). In addition to margin recipes or recommendations, several studies have reviewed beam angle optimization as an additional metric to reduce the range uncertainties and the margins required for target coverage (Gu et al., 2018; Kim et al., 2020; Taasti et al., 2020).
As daily or repeat imaging is increasingly available, the sufficiency of traditional planning workflows based upon a single CT with margins applied to the CTV can be evaluated. For example, GTVs with larger volumes can have different, regional deformations or daily changes due to motion, setup uncertainties, weight loss, or lesion shrinkage. In the example of head and neck patients, there are different treatment volumes with different kinds, magnitudes, and directions of changes. The neck nodal treatment volume can be affected by weight loss and shrinkage, the oral pharyngeal treatment volume by sinus filling, and the lower neck nodes by the daily shoulder positions (Kim et. al., 2017), thus a single range margin will not capture the local variation of the patient specific uncertainties. Similarly, prostate positioning and deformation can vary between the primary gland and the seminal vesicles, in additional to potential rotational changes (Wang et al., 2011). Some studies have proposed patient specific uncertainty models for photon treatments for “plan of the day” applications (Murthy et al., 2011; Reijtenbagh et al., 2020; Sharfo et al., 2016) but have not been demonstrated in the case of proton therapy.
2.4. Robust Optimization
While margins have been the simplest response to range, setup, and anatomic uncertainties, robust optimization has also been suggested as a possible solution to balance target coverage and OAR avoidance in the presence of physical or biological dose uncertainties when using intensity modulated proton therapy (Barten et al., 2015; Cubillos-Mesias et al., 2019; Liu et al., 2014; Liu et al., 2013; Malyapa et al., 2016; Unkelbach et al., 2009; Unkelbach et al., 2007; Perumal et al., 2018; van de Water et al., 2018; Yock et al., 2019). Robust planning considers uncertainty models during plan optimization such that the resulting plan performs well under most scenarios within the uncertainty boundaries of the considered effects (Unkelbach and Paganetti, 2018; Unkelbach et al., 2018). Uncertainties considered in robust optimization include dose calculation, imaging, biological effect (Unkelbach and Paganetti, 2018; An et al., 2017), patient setup (Tang et al., 2017), tumor intra-fractional movement (Engwall et al., 2018; Buti et al., 2019; Cummings et al., 2018), and beam angle selection (Gu et al., 2019).
In general, robust plans result in less sharp dose gradients and regions of high dose around the target to increase the probability of achieving the prescribed doses to the target or OARs (Jagt et al., 2020; van de Water et al., 2016). The primary advantage of robust planning is the determination of the optimal tradeoff of target dose coverage and OAR sparing when accounting for physical and biological dose uncertainties. The assumption of robust optimization is twofold: the model of uncertainties accounts for future combinations of uncertainties and the reference imaging set is sufficiently representative of the uncertainty distributions. Prior studies have analyzed the sufficiency of robust plans for treatment delivery (Kirk et al., 2015; Wang et al., 2011; Yang et al., 2019).
More recently, Cubillos-Mesias et al (Cubillos-Mesias et al., 2019) and van de Water et al (van de Water et al., 2018) proposed geometrical and anatomical robust optimizations to account for setup variations with repeat CT and sinus filling with synthetic CTs, respectively. In both cases, the additional CT information increased the variables in the optimization space and the additional CT anatomical information increased the optimizer robustness to patient specific anatomical variations compared to a simple margin based robust optimization.
Anatomical variations during the course of a treatment are sometimes difficult to consider in robust planning due to the complexity of potential scenarios, i.e. gas bubbles in the GI tract, bladder filling, weight loss, or uterus position. Even when applied retrospectively through multiple-CT optimization, the robust plan may not be able to account for the anatomical changes completely (Cubillos-Mesias el al., 2019; Lalonde et al., 2021; Yang et al., 2020). Failure to maintain plan robustness may lead to fractions that require adaptation (Li et al., 2015; van de Water et al., 2018). The need for adaptation, however, can be reduced by including more scenarios in the uncertainty distributions used for robust planning, although there is a potential increase in OAR doses with wider uncertainty distributions or inclusion of anatomic variation scenarios to increase tumor control probability (TCP) using softer dose gradients around the target. This trade-off between OAR dose and TCP is known as the “price of robustness” (van de Water et al., 2016). Creating plan libraries with individual plans for each uncertainty scenario instead of a single robust plan for all scenarios may provide a solution to this trade-off by removing the need of softer dose gradients (Jagt et al., 2019).
Given the patient specific range, setup, and anatomic uncertainties observed in prior studies, robust optimization might be insufficient or require large uncertainty models that increase the dose to OARs. In these cases, a more optimal course of action would be daily or weekly adaptive proton therapy.
2.5. Adaptation
Since both margins and robust optimization increase the dose to surrounding organs to achieve a high confidence of tumor control probability (TCP) for a given patient anatomy, there is a clinical need for daily dose analysis and access to daily optimized plans particularly when the dose delivery exceeds normal tissue complication probability (NTCP) limits. Additionally, the use of daily adaptive plans could reduce the margins or uncertainty width for robust optimization to the intra-fractional variation of patient anatomy. It has been shown that using a simple daily spot intensity re-optimization can achieve superior target coverage and lower dose to OARs than robust optimization methods (Lalonde et al., 2021).
With current technology, inter-fractional variations are the most straightforward variations to address with adaptive proton therapy workflows. Weight loss is one factor that contributes to slowly changing, inter-fractional range variations, both by a potential decrease in the tissue in the entrance region of the proton treatment beam and additional challenges in patient setup positioning when the immobilization devices are not as conformal to the patient. Tumor shrinkage during the course of treatment can cause additional inter-fractional range variations, particularly in the lung but also in other sites such as head and neck. The benefit of daily adaptive proton therapy increases when the impact on NTCP is more pronounced where the critical OARs are nearby the tumor.
Margin reductions and planning adaptation have been shown to be most impactful for proton therapy of sites that are subject to inter-fractional variations such as prostate (Kupelian and Meyer, 2011; Trofimov et al., 2011; Wang et al., 2011), head and neck (Kim et al., 2017; Placidi et al., 2017; Hague et al., 2020; Wu et al., 2017), and NSCLC (den Otter et al., 2020). Simone et al demonstrated that adaptive IMPT can dramatically spare OARs in squamous cell carcinoma head and neck patients compared to IMRT and adaptive IMRT (Simone et al., 2011). Combining setup uncertainties and anatomic variations with the complexities of proton therapy for lung cancer, Hoffmann et al found that proton therapy for NSCLC requires adaptive techniques to restore the dose to the target while photon therapy is more robust to the setup and anatomic variations (Hoffmann et al., 2017).
More recent MR-Linac studies, however, have demonstrated significant inter-fractional and intra-fractional target position variations of the pancreas, prostate, and esophagus (Lagerwaard et al., 2018; Padgett et al., 2020; Boekhoff et al., 2020), with more treatment sites being explored. Currently, proton therapy is limited to targets that are visualized with in-room CT, CBCT, surrogate fiducials, or surrogate bony landmarks. To achieve adaptive proton therapy for additional treatment sites, the current imaging options must be validated, or additional imaging will be needed to supplement.
Aside from cases with large inter-fractional variations, daily adaptive proton therapy may have reduced benefits for sites with little changes over the course of treatment and have precise, rigid immobilization such as brain targets. Additionally, sites that are subject to large motion during the beam delivery (lung, liver, pancreas) may have less benefit from adaptive proton workflows unless motion management or online imaging tools are also employed (Veiga et al., 2016).
Finally, in addition to the benefits of more conformal dose distributions or reduced margins, daily adaptive proton therapy would provide the clinician with greater degrees of freedom for dose prescriptions. The traditional static course dose prescription for the target or OAR limits will be more flexible with daily adaptation and re-optimization. Figure 1 shows the variety of adaptation possibilities but, in general, the clinician will be presented with opportunities to alter the daily tumor or OAR doses. For example, the tumor/OAR geometry may have limited the tumor prescription due to increased OAR NTCP, but a daily image may provide a more favorable tumor/OAR geometry allowing for increased dose to the tumor. Similar dose variations could be possible for OARs. If plans are optimized on a daily image, the clinician may consider daily dose constraints instead of constraints based upon the entire course.
3. Imaging
On-line plan adaptation requires a current image of the patient geometry suitable for treatment planning. This section introduces recent progress in using in-room imaging and other measurements to enable adaptive proton therapy using CT, CBCT, MRI, and in-vivo range verification. Each of these approaches offer distinct advantages and challenges.
3.1. Computed Tomography (CT)
In-room imaging can be used to perform plan adaptation using either a plan-of-the-day approach, or by replanning (Zou et al., 2018). In current practice, proton and ion therapy centers with volumetric imaging systems have either CT or CBCT. Because of their size and space requirements at the treatment nozzle, CT systems usually do not offer isocentric imaging. One of the first systems was developed at PSI that uses a patient docking table that can be moved between the CT and treatment couch using a trolley (Pedroni et al., 1995). Another early system developed at NIRS used a horizontal CT that is lowered around a seated patient (Kamada et al., 1999). This device could be used in treatment position, albeit having a larger air gap. In current practice, CT are generally placed within the treatment room, and the patient is imaged on the treatment bed using CT-on-rails with a robotic patient positioner (Landry and Hua, 2018). In-room CT has many practical advantages over CBCT for adaptive therapy: better HU accuracy, better image contrast, and 4D scanning capabilities. The disadvantages of in-room CT are that it requires a larger treatment room and the patient must be moved between imaging and treatment positions. The gap between imaging and beam on time increases the risk of the patient moving prior to treatment (Ma and Paskalev, 2006) although the CT imaging could be supplemented with x-ray radiography or surface imaging at the isocenter.
3.2. Cone-beam CT (CBCT)
CBCT systems for proton therapy can be gantry mounted, nozzle mounted, room mounted, or couch mounted (Landry and Hua, 2018). Imaging at treatment position is easiest to accomplish for the gantry and nozzle mounted options. The main characteristics which affect their utility for plan adaptation are the field of view and HU number accuracy. Because of the popularity of CBCT imaging, considerable effort has been made to allow its use for dose calculation. For photon adaptive therapy, CBCT-based dose calculation is generally considered to be of acceptable accuracy (Jarema and Aland, 2019), and is commercially available. However, dose calculation on CBCT in proton therapy is still considered a research topic, and the best method for correcting HU in CBCT is under debate. Broadly speaking, there are two classes of algorithms: projection-space methods and image-space methods. Projection-space methods attempt to remove scatter from the x-ray projection images prior to volumetric reconstruction, and image-space methods attempt to improve the HU number of voxels after reconstruction.
Projection-space methods model a projection image as a combination of primary image and scatter image components, and attempt to estimate either the scatter component or primary component (Ruhrnschopf and Klingenbeck, 2011). It is known that the uniform scatter estimate (Boellaard et al., 1997) is not accurate enough for patient proton dose calculations, and more advanced methods are needed (Park et al., 2015). A conceptually simple method for estimating scatter is to measure it using a beam block between the x-ray tube and the patient. The areas of the image where the beam is blocked contain no primary signal, and therefore contains only scatter. Using a similar principle, you can modulate the beam intensity without completely blocking the beam. Various forms of static (Shi et al., 2017; Zhu et al., 2006; Liu et al., 2006) and moving (Wang et al., 2010) beam blockers have been proposed. We are not aware of any study that evaluates this technique for proton dose calculation, but RMS accuracy of +/− 15 HU has been reported (Chen et al., 2017).
Alternatively, the scatter or primary component can be estimated algorithmically from an unblocked image. Niu et al. introduced a method that uses a previously acquired CT image of the patient (Niu et al., 2010). The prior CT is deformably registered with the reconstructed CBCT, and then estimates of the primary image for each projection are created. The primary estimates are subtracted from the projection image, with a low pass filter used to remove effects of misregistration or anatomical change. This results in a scatter estimate that can be subtracted from the projection. This method was found to reduce range errors in proton planning to between 1.8 and 3.6 % (Park et al., 2015). The reconstructed CBCT can be used instead of a registered CT to estimate primary attenuation using ray tracing (Zhao et al., 2016). However, this approach requires an image-based correction for incorrect HU prior to ray tracing. This approach has been independently validated and was found to result in range errors of less than 5 mm in a phantom (Andersen et al., 2020).
The third major category of projection-based correction methods are model-based, which includes physics-based or deep-learning collections. The most accurate physics-based corrections use Monte Carlo to estimate and remove scatter (Jarry et al., 2006; Mainegra-Hing and Kawrakow, 2010). The CBCT image is reconstructed and then Monte Carlo simulation is used to correct the projections. This process can be performed iteratively if reconstruction speed is not an issue. Alternatively, analytic approximations can be used to estimate scatter using 2D or 3D scatter kernels. In 3D, a single-scatter model can be used in conjunction with ray tracing and pre-calculated kernels (Rinkel et al., 2007; Yao and Leszczynski, 2009). The 2D methods avoid the computational expense of ray tracing, a spatially varying scatter kernel is inferred from the projection image intensities (Sun and Star-Lack, 2010). More recently, deep learning scatter correction has become popular (Landry et al., 2019; Maier et al., 2019) (Figure 3). A network is trained to map CT volumes into the scatter component of a projection image using Monte Carlo to create training data. Treatment plans calculated with this method have been shown to achieve an average 2%/2mm gamma pass rate of over 98% when compared with scatter free ground truth (Lalonde et al., 2020).
Figure 3:
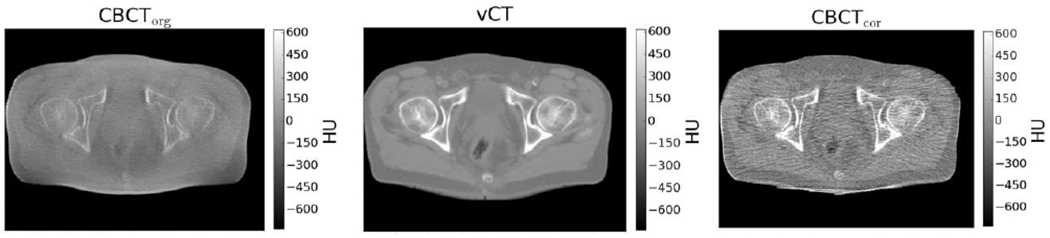
Uncorrected CBCT (left) is unsuitable for proton dose calculation because of large HU errors. This may be corrected by deforming the planning CT to match the CBCT (center), or removing scatter from CBCT projection images prior to reconstruction (right). From (Landry et al., 2019), with permission.
In contrast to projection-space methods, image-space methods attempt to correct HU from a reconstructed image. One popular approach is to use deformable image registration (DIR) to map a previously acquired diagnostic quality CT of the patient onto the daily CBCT and use the warped CT directly (often called either virtual CT, vCT, or synthetic CT, sCT) (Veiga et al., 2015; Veiga et al., 2014; Landry et al., 2015; Moteabbed et al., 2015). This method was shown to be slightly inferior to the prior CT scatter correction method, possibly due to registration accuracy or anatomical change unrelated to tissue deformation (Kurz et al., 2019; Kurz et al., 2016a). Another approach is to use tissue segmentation to classify voxels to locate fat and muscle. By assigning these regions and expected HU for the tissue type, the low frequency scatter-induced intensity variations can be removed (Shi et al., 2019). Machine learning is also proposed for performing image-space corrections. In a traditional architecture, paired CT and CBCT images are needed. This can be done using image registration to create a deformed CT, or by training the network using images corrected with another method (Landry et al., 2019; Kida et al., 2018). An alternative is to use a Cycle-GAN architecture, which eliminates the need for registration (Harms et al., 2020; Liang et al., 2019).
3.3. Magnetic resonance imaging (MRI)
In photon therapy, MRI has become a popular imaging modality for on-line adaptation. The integration of MRI with proton therapy has many challenges: perturbation of dose due to the magnetic field of the MR scanner, effects of beamline and scanning system magnets on image quality, dose calculation on MRI, and system integration. The dose effects due to the magnetic field are considerable but can be well-modeled by Monte Carlo. Lateral deflection of a pencil beam increases with both beam energy and field strength, with a deflection of 1.2 mm for a 90 MeV beam in a 0.5 T magnet, and 28.0 mm for a 200 MeV beam in a 1.5 T magnet (Moteabbed et al., 2014). An accurate model of this effect requires knowledge of the magnetic field in both the imaging field and the fringe field (Oborn, 2019). The effect on secondary electrons is low, due to their low energy (Raaymakers et al., 2008). Dose calculation on MR is much more challenging for proton beams than it is for photon beams due to uncertainties in the synthetic CT construction. Nevertheless, there is some indication that dose differences can be as low as a few percent for some treatment sites (Rank et al., 2013; Koivula et al., 2016). Equipment design remains a highly challenging problem (Hoffmann et al., 2020). Neither closed bore magnets nor robotic patient positioners are possible, and extensive magnetic shielding of the gantry and scanning system is likely needed. A preliminary investigation of an MRI-guided proton beamline has been demonstrated recently at OncoRay (Schellhammer et al., 2018). One can expect that developments towards proton-MR machines will accelerate in the near future with significant impact for proton adaptive therapy (Paganetti et al., 2020).
3.4. In vivo range imaging
In addition to anatomic imaging for dose calculation, range verification is an essential component in producing a high-quality adaptive plan. There is a broad category of methods both proposed and in use (Knopf and Lomax, 2013; Parodi, 2020; Parodi and Polf, 2018), with considerable interest in proton radiography and tomography, positron imaging, and prompt-gamma imaging. Proton radiography is a transmission imaging technique that uses a high energy proton beam to measure the energy loss when protons traverse an object (Poludniowski et al., 2015). It can be used for either 2D imaging technique, or for tomographic reconstruction. Proton beam imaging was first proposed by Cormack in 1963 (Cormack, 1963), and was first used to image an animal patient in 2004 (Schneider et al., 2004). To date, there have not yet been human studies, and there remain challenges. Most proton radiography designs use a very low dose rate which allows individual protons to be tracked. At patient exit, the position and residual energy of the proton can be measured using a variety of detectors, such as scintillators, GEM chambers, or solid state devices (Parodi and Polf, 2018). The energy is usually measured by a range telescope, which is a stack of detectors sandwiched between energy degraders. The path of each proton can be more accurately tracked by adding an additional detector between the beam and the patient. An alternative approach uses an energy modulated broad beam (Testa et al., 2013). This approach has the advantage that a range telescope is not required, but it requires higher patient dose. Multiple coulomb scattering within the patient causes the protons to diverge from a straight path, which causes loss of spatial resolution. This effect can be mitigated to some degree using deconvolution or most-likely path approaches (Rit et al., 2013; Deffet et al., 2020).
Positron imaging measures radioactive positron decay from short-lived radioactive isotopes produced in the body during therapy (Figure 4). The first use of positron imaging in humans was performed in 1997, where GSI used in-line PET to monitor patients during hadron therapy (Enghardt et al., 2004). The isotopes of greatest interest to proton therapy are O-15 and C-11, having half-lives of 2 mins and 20 mins respectively. These isotopes decay by positron emission, resulting in a pair of 511 keV annihilation photons. The relatively long half-lives of these isotopes allows PET imaging to be deployed for either on-line or off-line operation. On-line operation is preferred due to better registration with pre-treatment imaging and rapid capture of short-lived isotopes but is technically challenging due to space constraints (Shakirin et al., 2011; Min et al., 2013). There are several challenges in using PET for treatment adaptation. First, the image signal does not correlate directly with dose because the isotopes are created from nuclear interactions which have a different cross-section than the electromagnetic interactions that produce a large fraction of dose. Thus, it is more useful as a tool for treatment verification than dose reconstruction. Further, PET resolution is generally limited to 3-4 mm due to positron travel, photon non-collinearity, depth of interaction within the crystal, and other effects (Moses, 2011). Another challenge is biological washout, whereby isotopes travel within blood vessels before they decay (Grogg et al., 2015). Nevertheless, PET remains a feasible approach for detecting large deviations from planned treatment.
Figure 4:
Indirect imaging of proton dose using PET (left) and proton range verification using prompt gamma (right). From (Min et al., 2013) and (Richter et al., 2016), with permission.
Prompt gamma rays are emitted from nuclei excited during inelastic scattering events. The emission is immediate, within a few nanoseconds, and like PET is correlated with both dose and range. Several high energy photons are released for each event, with energies that depend on the nuclear species. Because it offers no coincidence photons as in PET, prompt gamma measurement cannot localize the exact location of an event but it does provide useful information on the proton range. The first patient measurements of prompt gamma emission were performed in 2015 at OncoRay in Dresden using a slit camera (Richter et al., 2016). The slit camera design places a pixelated scintillator at 90 degrees from the beam, collimated with a 1D slit collimator. The resulting measurement allows assessment of the beam range from a histogram of counts along a 1D line profile (Perali et al., 2014). Alternative strategies include time-energy resolved prompt gamma spectroscopy, prompt gamma timing measurements, and Compton cameras (Hueso-Gonzalez et al., 2015; Hueso-Gonzalez et al., 2018; Xie et al., 2017). In most cases, the expected emission pattern is computed using Monte Carlo, and compared with measurement. Although 3D information is not usually possible using prompt gamma, sub-millimeter spatial accuracy of depth information is feasible.
4. On-line adaptation strategies in proton therapy
4.1. Imaging, contour definition and registration approaches
Imaging is required in order to evaluate the original plan on the new setup and anatomy. The selection of the imaging technique has implications on the uncertainties involving the adaptation and on the workflow. Currently, CT or CBCT have been used in proton therapy related publications on adaptive therapy workflow, although it is safe to assume that MRI imaging could be leveraged to provide higher contrast for soft tissues. Procedures to translate MRI images to substitute CTs that allow for photon dose calculation have been developed (Johnstone et al., 2018; Edmund and Nyholm, 2017). The published proton therapy adaptation workflows have mainly focused on the plan adaptation itself, using either CT or CBCT as daily imaging, but without tailoring the workflow to any imaging technique. This approach of treating the imaging technique as a place holder assumes that imaging is sufficiently fast, presents sufficient accuracy for the adaptation and the consequences of any bias introduced by the imaging technique is small.
As discussed in the imaging section, registration between planning CT and CBCT poses challenges when defining the geometry for dose calculation. A recent review focuses entirely on dose calculation methods and their accuracy when using CBCT in adaptive therapy using photons or protons (Giacometti et al., 2020). Kurz et al compared dose calculation accuracy for different imaging strategies in adaptive proton therapy for head and neck and prostate tumor patients (Kurz et al., 2016a). Deformable image registration of the planning CT to the daily CBCT image yielded a vCT and dose calculations on vCT and scatter corrected CBCT were compared. Both methods resulted in acceptable results in terms of dose and proton range for head and neck but uncertainties due to anatomic variations of the bladder were observed for prostate treatments which were overcome in the scatter corrected CBCT by using the vCT only as prior for scatter correction of the CBCT projections. Thummerer et al. compared three methods to create synthetic CTs from CBCTs in the context of adaptive proton therapy (Thummerer et al., 2020b). This included a deep convolution neural network (DCNN), DIR, and an analytical image-based correction method using pixel histogram matching. The DCNN approach resulted in superior image quality. Only DCNN and DIR resulted in images capable of accurate proton dose calculation. The same authors also assessed the use of MR-based synthetic CT for proton dose calculation and reported on similar performance (Thummerer et al., 2020a).
In general, the Hounsfield Unit distribution and calibration will differ between planning CT and CBCT. Clinically significant dosimetric discrepancies have been reported leading to the introduction of a histogram matching algorithm to normalize the distributions for proton dose calculation in head and neck cancer patients (Arai et al., 2017).
One important aspect connecting imaging and plan adaptation is contour propagation. The correct definition of contours is a vital step in the initial treatment planning. It is generally challenging to define contours accurately on the reference planning image, mainly due to the unknown normal tissue infiltration, and the low contrast at some treatment sites, particularly for the target volume. Organs at risk typically provide better tissue contrast (with the exception of some treatment sites in the abdomen). Adapting contours is arguably the most challenging step in online adaptation workflows because contouring typically requires human intervention or approval and the target volumes might not have high contrast. Depending on the specific adaptive workflow, automated image registration may be required to properly define the contours (Cardenas et al., 2019; Rigaud et al., 2019). Specifically for adaptive proton therapy, there have been two approaches using either rigid registration between the planning CT and the daily image (Bernatowicz et al., 2018; Jagt et al., 2017) or deformable registration (Kurz et al., 2016b; Botas et al., 2018; Bobic et al., 2021; Nenoff et al., 2021). Deep learning approaches have been investigated for contour propagation for on-line proton therapy in prostate cancer (Elmahdy et al., 2019).
There is a higher complexity in the analysis of the effects of uncertainties and errors in contour definition, compared to uncertainties and errors in the imaging technique itself. Imaging uncertainties result in deviations that, accumulated over the beamlets path, build Gaussian uncertainty profiles (Paganetti, 2012; Knopf and Lomax, 2013; Lomax, 2020). This Gaussian distribution is a result of the beamlet paths traversing many CT voxels. By contrast, the contours defined in a patient image are a single sample from the uncertainty distribution of contouring (Shusharina et al., 2018; Vinod et al., 2016; Riegel et al., 2016). Because plans are evaluated based on contours, they are consistent with the errors committed during contouring. Approaches to solve or alleviate this through probabilistic contour definitions have been published (Shusharina et al., 2018; Unkelbach et al., 2020; Bortfeld et al., 2021).
4.2. Dose calculation approaches
Dose calculations are required to (1) evaluate the original plan quality, (2) calculate dose distributions to optimize the plan to the daily anatomy and setup, and (3) provide quality assurance. For on-line adaptation, the time constraint is especially pressing on item 2 due to the general need of calculating dose on a per proton beamlet basis in order to optimize IMPT plans. Two types of dose calculation algorithms have been applied in studies of daily IMPT adaptation, showing a trade-off between time and accuracy. Monte Carlo (MC) dose calculations are considered the gold standard in accuracy (Paganetti, 2012; Schuemann et al., 2015) but require more computational time, while analytical dose calculation (ADC) algorithms can be faster but less accurate.
Because analytical dose calculations algorithms provide high efficiency, they have been applied in adaptation pipelines (Kurz et al., 2016b; Jagt et al., 2017; Jagt et al., 2019; Jagt et al., 2018; Bernatowicz et al., 2018). Additional efficiency can be gained by implementing ADC algorithms on Graphical Processing Units (GPU) architectures. These GPU algorithms show high calculational efficiency because they are inherently multi-threaded. Several implementations of ADC algorithms have been published (da Silva et al., 2015; Matter et al., 2019), with Matter et al. introducing a fully functional IMPT plan generation package capable of generating an IMPT plan in ~10 seconds with the same accuracy as an ADC algorithm used for routine IMPT adaptation, which is well suitable for daily online plan adaptation.
MC codes have been implemented in GPU architectures and validated in patients (Kohno et al., 2011; Yepes et al., 2010; Jia et al., 2012; Qin et al., 2016; Maneval et al., 2019; Schiavi et al., 2017; Wan Chan Tseung et al., 2015). The algorithm presented by Qin et al. has been tested within an automated online adaptation workflow, potentially performing original plan evaluation, IMPT plan adaptation and verification in time scales of 2-5 minutes (Botas et al., 2018). In order to further increase the efficiency of the dose calculation, methodologies where the emphasis lies on beamlets that carry the highest proton fluence have been developed (Li et al., 2017).
Hybrid dose calculation algorithms have been applied in photon-based radiotherapy (Siebers et al., 2007; Siebers et al., 2002) and show promise in the adaptation regime also in proton therapy (Barragan Montero et al., 2018).
Nenoff et al. (Nenoff et al., 2020) studied the trade-off between the uncertainty in dose calculation and patient anatomy in an adaptive proton therapy workflow. For the patients and calculation algorithms analyzed, the study found that the advantage of daily adaptation outweighs a compromise on dose calculation accuracy when performing lower quality but much faster dose calculation using ADC. The study includes 5 non-small-cell lung cancer patients with 9 repeat CTs and 5 paranasal patients with 10 simulated CTs with sinus fillings. The authors report an average CTV V95 drop of ADC-calculated adaptations with respect to MC-calculated adaptations of 2 %, while not adapting implies a drop of up to 34 %. This study indicates that ADC and MC calculations could be combined in hybrid workflows in scenarios where MC calculations are not feasible due to time constraints. Accurate MC-based offline dose calculation of the dose distribution delivered with ADC-based adaptation and consider cumulative dose deviations from the nominal dose fractionation in the next fractions would minimize the effect of dose calculation inaccuracies.
4.3. Dose accumulation
For proper dose accumulation, the total dose delivered to each anatomical position needs to be mapped to the same anatomical position in all fractions, which in principle can be determined via DIR. However, dose accumulation with DIR poses an intrinsic problem: its accuracy can be difficult or even impossible to verify. Tissues within the body change through the course of treatment with some tissues shrinking or disappearing and others swelling or appearing. Under these scenarios, accumulating dose between CT scans taken weeks apart may or may not be appropriate. For a point/counterpoint discussion on this fundamental issue please see Schultheiss et al. (Schultheiss et al., 2012).
Additionally, to the perspective problem, the specific DIR algorithm used also can have important implications. Chetty et al. reviewed dose accumulation for photon therapy, discussing the center of mass, interpolation, direct voxel tracking and energy transfer DIR methods (Chetty and Rosu-Bubulac, 2019). An obstacle to be solved, independently of the deformable vector field calculation, is the fact that the equivalent region of a voxel from the original image is not necessarily rectangular or of the same volume or mass as in the reference image. Given the definition of dose, this change in mass of a voxel might produce unphysical dose accumulation with implications that depend on the specific case, such as for example the type of tumor regression (Chetty and Rosu-Bubulac, 2019). This could be impactful particularly in conformal dose delivery such as proton therapy (Amstutz et al., 2021).
Dose accumulation can be included within the adaptation workflow in different ways. As described in Heukelom et al. (Heukelom and Fuller, 2019), dose can be either accumulated on the new patient CT scan or back-projected into the initial planning CT. These two frames of reference produce different results due to the patient anatomical evolution. This is termed as “the perspective problem” in Heukelom et al. (see Figure 1) Re-calculation can be done on daily delivery log files (Winterhalter et al., 2019a; Meijers et al., 2019). If doses are analyzed daily, corrections could be made based on dosimetric indices and incorporated into an adaptive workflow, i.e., daily adaptation based on the accumulated dose from prior fractions.
4.4. Online plan adaptation strategies
Several proton adaptation workflows have been published (Kurz et al., 2016b; Jagt et al., 2017; Jagt et al., 2018; Jagt et al., 2019; Bernatowicz et al., 2018; Botas et al., 2018; Bobic et al., 2021; Moriya et al., 2018; Nenoff et al., 2019; Matter et al., 2019), with only the publication by Moriya et al focusing on passive scattering proton therapy. They can be divided in two groups, depending on the amount of information inherited from the original plan. The first group (“re-planning”) is based on optimizing the initial plan again, either inheriting the same objectives and constraints or providing new ones to adapt to the new geometry. Re-planning entails execution of the traditional planning pipeline. This requires very fast dose calculation and optimization as well as proper QA and plan approval. This workflow has been studied by Matter et al. (Matter et al., 2019) and, focusing on paranasal cancer patients, by Nenoff et al. (Nenoff et al., 2019). The second group (“re-optimization and dose restoration”) focuses on changes to the original plan to restore either the original plan quality or the original dose distribution. Depending on the changes required, this method may converge to full re-planning. In the following discussion we focus on publications that present adaptation approaches that include beamlet energy adaptation and optimization in proton beam scanning, i.e., on the second group.
Jagt et al. (Jagt et al., 2017) presented a method in which a reference plan is adapted to a new CT image with contours rigidly aligned from the planning CT. The method involved two steps: restoration of Bragg peak positions by energy corrections and re-optimization of beamlet weights to recover the reference plan. All beamlets were included in the re-optimization, for which the dose-influence matrix must be recomputed (voxels outside the regions of interest were not considered). Several prioritization strategies to balance target and OAR dose were presented. Ten prostate cancer patients were planned with two laterally opposing beams impinging on three PTVs for high, intermediate and low dose. It is not reported if the design of the PTVs was altered to test the method. It is reported, however, that the plans were created with a lower degree of robustness in order to challenge the adaptation algorithm. With the presented methods, 96.3% of the fractions resulted in V95% >= 98% in the prostate CTV, while V107% <= 2% was fulfilled in up to 92.5% of the fractions in the same contour.
Bernatowicz et al. (Bernatowicz et al., 2018) presented a workflow that considered rigidly translated contours from the planning CT to the daily anatomy on the example of 3 patients: a nasopharyngeal case with varying nasal fillings, an oropharyngeal and a 4D-robustly optimized lung case. Their approach aimed to restore the original dose distribution by creating new plans. In order to reach this goal, they investigated 3 ways of minimizing the difference between the original and the new plan, focusing on: DVH points, voxel-wise dose comparisons and isodose contours. All adaptation methods recovered V95% >= 95% and V107% <= 2% for the CTV on all repeat CTs, with the isodose approach yielding the best dose distribution as compared to the one originally planned. The dose restoration for the isodose approach time ranged from ~25s to ~200s, including dose calculation and optimization.
Jagt et al. (Jagt et al., 2018) expanded their previous methodology and tested it on daily manual contours, removing the limitation to rigid registration. The focus remained on prostate cancer. They also restricted the number of proton energy layers, implemented a reference point method (RPM) optimization and allowed up to two iterations of 2500 beamlets in addition to the original plan. In this method, once the energies of the beamlets are corrected and the random beamlets are added, the plan is optimized, which may remove unused beamlets and energy layers. Beamlet addition and optimization may be repeated for a second iteration. The contours were manually defined for the sake of testing the new approach and additional margins of 2 and 3.5 mm to the high- and low-dose CTV were added to account for the delineation and auto-segmentation uncertainties. In this case, 100% of the 88 scans produced V95% >= 98% in the true prostate CTV, while V107% <= 2% was fulfilled in 94.3% of the fractions in the same contour. This method required 2.9 to 4.6 min, depending on the number of iterations.
Botas et al. (Botas et al., 2018) studied different adaptation strategies involving the use of range shifters and isocentre shifts with and without beamlet weight optimization for head and neck cancer patients. The combination of isocentre shifts with beamlet weight optimization was shown to be the superior strategy. The first step in this approach consisted of adapting the position of the original Bragg peaks for each beamlet by applying the deformation field from the reference CT to the daily patient anatomy. Afterwards, the beamlet energy was modified without constraining to discrete energy layers. The fluence map produced was then evaluated. In case the result was not satisfactory, the set of beamlets with the highest number of protons was selected and their weights optimized, by fixing the dose provided by the beamlets with lower proton fluence. Using the deformation field this way, its inherent uncertainty may impact the position of the Bragg peaks, but not necessarily the dose distribution. Ten head and neck patients were studied, each having 6 scatter-corrected CBCTs and contours were translated using deformable image registration. The plans were optimized on the CTV directly without adding margins in order to test the efficacy of the adaptation algorithm. The authors reported an average V95 in the CTV across all patients and fractions of 99.0+−1.0 % a V107% = 12.8+−5.8% and V110%=2.1+−2.4%. Dose-volume histograms for OARs show the dependency on patient and fraction. The average adaptation time per patient was 322.7s. The approach was not evaluated imposing machine-dependent deliverability constraints on the minimum fluence per beamlet and total number of energy layers following approaches similar to van de Water 2015 (van de Water et al., 2015).
In 2019, Jagt et al. (Jagt et al., 2019) published another step of their framework by applying the previously developed techniques to a library of prior plans accounting for altered target geometries. At each fraction, the best library plan was adapted using energy adaptation. Next, the spot addition and RPM optimization presented in their previous publication was applied (Jagt et al., 2018). The framework was demonstrated with 6 locally advanced cervical cancer patients, with a total of 23 repeat-CTs. Using a 2-plan library and applying two iterations of beamlet addition-RPM optimization recovered V95% >= 95% and V107% <= 2% in all CTVs.
Bobic et al. (Bobic et al., 2021) built on the framework developed in Botas et al., comparing daily and weekly adaptation on a cohort of 10 head and neck patients with a total of 320 CBCT image sets. The authors demonstrated that, although daily adaptation yielded better results, weekly adaptation was typically successful in maintaining high plan quality throughout the plan delivery while reducing the clinical workload (see Figure 5). Specifically, random errors caused by daily positioning and anatomy variations are only effectively accounted for by daily adaptation. Furthermore, they showed that removing the energy and position adaptation initially implemented in the framework (Botas et al., 2018), maintained the deliverability of the initial plans so that adaptation only required beamlet weight adjustments. Thus, this method might be considered a delivery correction, instead of a plan re-optimization, a distinction with potential impact on clinical workflow guidelines.
Figure 5.
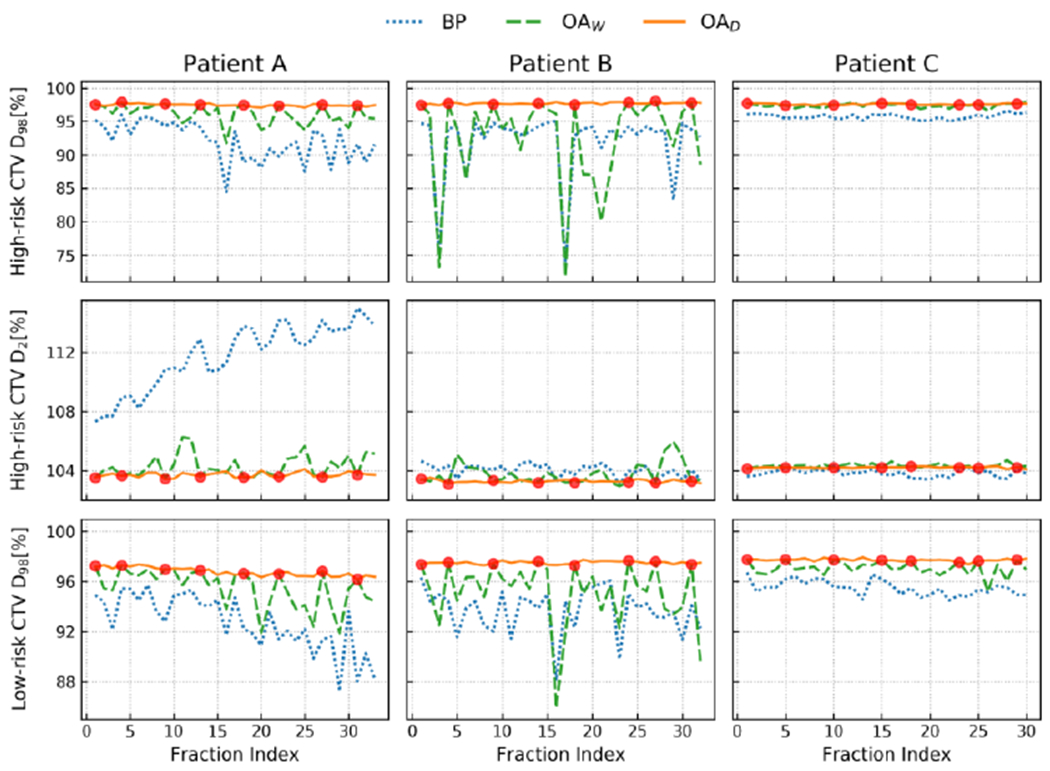
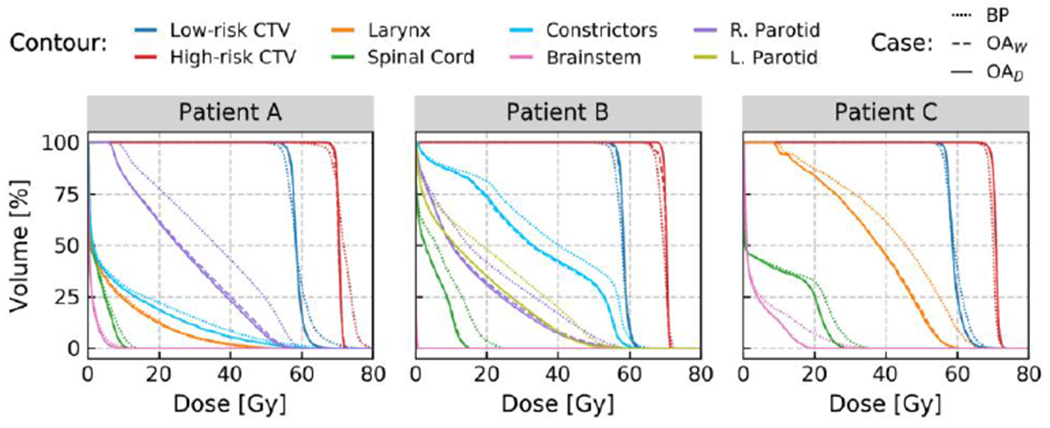
a: Evolution of the target conformity (high-risk CTV and low-risk CTV) over the full course of treatment for a three head and neck cancer patients. The target coverage and overdose are evaluated using the D98 and D2, respectively (both in % of the prescription dose). The red dots represent which fractions were adapted in a weekly adaptation scenario (OAW). Overall, daily adaptation (OAD) achieved the best target coverage, while both weekly and daily outperform treatment based on the base plan (BP). ((Bobic et al., 2021); with permission).
b: DVHs for the three patients shown in figure 5a, comparing the accumulated doses over the course of treatment, delivered by BP (dotted line), OAW (dashed line), and OAD (solid line). OAW yields remarkably similar performances to OAD for patients A and C, both in terms of target coverage and OAR sparing. For patient B, a clear difference regarding the high-risk CTV coverage can be observed between OAW and OAD. In terms of target coverage, patient B exhibits the worst result for OAW, which barely reached the clinical objective for the high-risk CTV. (Bobic et al., 2021); with permission).
4.5. Treatment verification
Patient specific plan verification with water phantom-based measurements are not viable in an online adaptation workflow. To carry out plan verification, it has been proposed to utilize independent MC-based simulations on the patient anatomy (Meier et al., 2015; Zhu et al., 2015; Matter et al., 2018; Johnson et al., 2019; Mackin et al., 2013). In order to also test the information pipelines feeding the plan parameters to the delivery system, it has been proposed to perform QA using the machine delivery files as inputs for the MC calculation. This approach has been shown to have higher sensitivity compared to patient specific verification measurements (Matter et al., 2018). The downside as compared to performing QA based on the delivery files is that it can only be performed after the patient is treated (Matter et al., 2018; Meijers et al., 2019; Toscano et al., 2019; Winterhalter et al., 2019a; Belosi et al., 2017). Corrections can only be applied in subsequent fractions.
4.6. Robust planning vs adaptation
Uncertainties other than the patient anatomy, such as imaging, patient setup, contouring, dose calculation or beam delivery are not explicitly considered in the plan adaptation approaches reviewed in this work. Performing robust plan adaptation online during plan adaptation would guarantee a higher degree of robustness of the online-generated plan. Direct comparisons of online adaptation vs robust planning were performed in prostate and head and neck cohorts. Jagt et al. (Jagt et al., 2020) compared robust planning of 11 prostate cancer cases with plan adaptation on a total of 88 repeat CT scans. Plan adaptations improved median OAR doses up to 16%. Lalonde et al. (Lalonde et al., 2021) compared online adaptation against classical robust optimization and anatomical robust optimization in a cohort of 10 head and neck patients with a median of 33 CBCT images each (range: 31 - 35). Online adaptation was shown to fulfill clinical objectives for all cases, while anatomical and classical robust optimization maintained them only in 8 and 4 cases, respectively. The studies are indicative of the advantage adaptation has on robust planning, although precise conclusions about the extent of these advantages should be considered site and case specific.
5. Biological aspects of plan adaptation in proton therapy
Plan adaptation as described in the previous sections is based on imaging and dosimetric indices. The main focus is on matching the treatment plan for each fraction. While the goal is to meet the prescription dose to the target and meet dose constraints for all organs at risk, there will be deviations from the original plan. For the tumor, adaptation may lead to dose hot spots, which may not necessarily of concern, except for when trying to analyze outcome differences in a clinical trial. For OARs, plan adaptation could lead to higher or lower doses than initially shown in the treatment plan, albeit according to adaptation guidelines, still below the defined constraints. Variations in dose distributions and higher dose heterogeneity cause deviations from the intended uniform fractionation scheme and might have implications on the total equivalent biological dose. Treatment outcome depends on the delivered biological dose, which has a complex dependency on the physical dose delivered at each anatomical scenario at each point in time (Boman et al., 2017). Consequently, for retrospective outcome analysis, the accumulated delivered doses instead of the planned doses should be used.
Treatment adaptation can be applied to address biological considerations particularly in the context of fractionation (Yang and Xing, 2005). A variation of the dose between fractions always leads to a larger biologic effect than does the same total dose delivered with standard uniform fractionation. It has been shown that the effect is negligible if the standard deviation of the dose is less than ~10% of the dose per fraction (Bortfeld and Paganetti, 2006). In adaptive dose-correction schemes, the dose correction should be applied uniformly over the remaining fractions, to minimize the variance (Bortfeld and Paganetti, 2006).
An alternative to maintaining the planned dose distribution (based on DVH constraints) in the adaptive workflow would be to adapt daily delivery towards maintaining estimated TCP or NTCP for organs at risk. Currently, treatment planning is mainly done based on dosimetric indices and not directly considering TCP and NTCP because of inherent uncertainties in TCP and NTCP models. Daily adaptation, as a relative concept, could potentially be based on TCP and NTCP. Adapting radiotherapy to temporal and spatial variations in tumor oxygenation has been suggested as well but is currently far from clinical reality (Sovik et al., 2007). Furthermore, plan adaptation can include biological indices deduced from image features (Matuszak et al., 2019).
In proton therapy, there is an additional effect due to variations in RBE (relative biological effectiveness). Despite patient variability and uncertainties in RBE, biological treatment planning can be incorporated via the linear energy transfer (LET) aiming at reducing LET in OARs (Unkelbach et al., 2016; Unkelbach and Paganetti, 2018). This can be partially achieved through robust optimization (Hirayama et al., 2020; Unkelbach and Paganetti, 2018). Maintaining LET considerations in the adaptive workflow would require full re-planning. It is thus likely that LET considerations cannot be fully maintained during the course of adaptive therapy. Re-calculating not only the dose but also the LET based on daily log files might therefore be necessary to confirm biologically equivalent doses. In theory, the impact of the biological uncertainty could be reduced by explicitly influencing LET in the adaptive workflow.
6. Summary and Outlook
On-line adaptive therapy faces many challenges as discussed in this review. The impact on the treatment delivery workflow depends on the chosen adaptation strategy and the tools applied for imaging, dose calculation, and dose assessment. There is ongoing research into the technical realization of on-line adaptive therapy. Equally important are efforts towards understanding the impact on treatment outcome, which will decide whether the disruption of the current clinical workflow by implementing adaptive strategies is generally warranted.
Treatment adaptation is expected to be clinically more significant in highly conformal treatment techniques such as proton therapy. Furthermore, range variations due to geometrical changes, including both anatomic and setup variations, result in dose perturbations that can be more severe. At the same time, there are unique challenges in proton therapy due to typically more inhomogeneous dose distributions in organs at risk compared to photon therapies. On the other hand, the complexity of intensity-modulated treatment fields in proton therapy offers great potential for plan adaptation since not only the fluence but also the energy (Bragg peak position) can be utilized in an adaptive workflow.
Radiation therapy is moving towards hypo-fractionated treatments for many disease sites particularly when using proton therapy with its low integral dose to normal tissue. This will impact also strategies for plan adaptation. First, changes in patient geometry might be smaller with a decrease in the overall time of the course of treatment. On the other hand, fewer fractions leave less room for retroactive plan corrections.
Table 1:
Published adaptive proton therapy strategies.
| Target results | Cases | Contour definition | Method | Dose calc. | Optimization | Timing | |
|---|---|---|---|---|---|---|---|
| Jagt 2017 (Jagt et al., 2017) | 96.3% of true prostate CTV’s V95%>= 98% and 92.5% of V107% <= 2% | 10 prostate cases. 80 repeat scans. Single plan. | Rigid registration | IMPT, beamlet energy, beamlet weight re-optimization | Analytical (Astroid) | Minimization of voxel-wise quadratic difference to reference plan | Dose 5-10 min, optimization 10s |
| Bernatowicz 2018 (Bernatowicz et al., 2018) | All CTV V95% >= 95% and V107% <= 2% | 3 cases: nasopharyngeal, oropharyngeal and lung. Single plan. | Rigid registration | IMPT, energy and beamlets positions | Analytical (Raystation 6) | Three approaches: DVH-based, voxel-wise and isodose-based objectives. | 25 to 200s |
| Jagt 2018 (Jagt et al., 2018) | 100% true prostate CTVs: V95% >= 98% and 94.3% of V107% <= 2% | 11 prostate cases. 88 repeat scans. Single plan. | Manual contouring | IMPT, beamlet energy, beamlet weight re-optimization | Analytical (Astroid) | Reference point method to original objectives, guided by plan | Dose 1.1 min plus 1.1 per spot addition iteration. Opt. 37s |
| Botas 2018 (Botas et al., 2018) | Mean CTV V95% = 99.0+−1.0%, V107% = 12.8+−5.8% and V110%=2.1+−2.4%. | 10 head and neck. 60 repeat scans. Single plan. | Deformable registration | IMPT, beamlet energy and beamlets positions | GPU MC (Qin et al., 2016) | Opt4D (Trofimov et al., 2005) with original objectives on subset of spots | Energy-position adaptation 11.7-26.6 s. MC dose 115.6-419.2 s Opt. 12.0-198.0 |
| Jagt 2019 (Jagt et al., 2019) | 100% primal & nodal CTVs: V95% >= 95%, V107% <= 2% | 6 cervix. 23 repeat scans. Plan library (max 2 plans). | Manual contouring | IMPT, beamlet energy, beamlet addition, plan library | Jagt et al 2018 | Jagt et al 2018 | 318-504 s |
| Matter 2019 (Matter et al., 2019) | Paraspinal: V95plan-adapt ~ −2% Brain: V95plan-adapt ~ 0% Cranio-spinal axis: V95plan-adapt ~ 0% Paranasal: V95plan-adapt ~ 1% |
4 patients: paraspinal, brain, cranio-spinal axis and paranasal | Manual contouring | IMPT, full replanning | Same publication (Matter et al., 2019) | Same publication (Matter et al., 2019) | 5-10 s. Up to 25 s for cranio-spinal axis. |
| Nenoff 2019 (Nenoff et al., 2019) | V95plan-adapt < 1% for all cases | 5 paranasal patients. 1845 setup errors and nasal fillings scenarios simulated | Same as original | IMPT, new plan generation | Analytical (in-house) | In-house ((Winterhalter et al., 2019b)) | Seconds |
| Bobic 2021 (Bobic et al., 2021), daily adaptation | High risk CTV: Median D98% = 98.07%, (97.15%-99.73%) | 10 head and neck patients. 320 image sets. | Deformable registration | IMPT, beamlet weight re-optimization | GPU MC (Qin et al., 2016 | Opt4D (Trofimov et al., 2005 | Median of 12 min (8 – 22 min) |
Acknowledgement
This work was funded by the National Cancer Institute (NCI R01CA229178, Fast Individualized Delivery Adaptation in Proton Therapy).
References
- Ahunbay EE, Peng C, Chen GP, Narayanan S, Yu C, Lawton C and Li XA 2008. An on-line replanning scheme for interfractional variations Med Phys 35 3607–15 [DOI] [PubMed] [Google Scholar]
- Amstutz F, Nenoff L, Albertini F, Ribeiro CO, Knopf AC, Unkelbach J, Weber DC, Lomax AJ and Zhang Y 2021. An approach for estimating dosimetric uncertainties in deformable dose accumulation in pencil beam scanning proton therapy for lung cancer Phys Med Biol 66. [DOI] [PubMed] [Google Scholar]
- An Y, Shan J, Patel SH, Wong W, Schild SE, Ding X, Bues M and Liu W 2017. Robust intensity-modulated proton therapy to reduce high linear energy transfer in organs at risk Med Phys 44 6138–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AG, Park YK, Elstrom UV, Petersen JBB, Sharp GC, Winey B, Dong L and Muren LP 2020. Evaluation of an a priori scatter correction algorithm for cone-beam computed tomography based range and dose calculations in proton therapy Phys Imaging Radiat Oncol 16 89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Kadoya N, Kato T, Endo H, Komori S, Abe Y, Nakamura T, Wada H, Kikuchi Y, Takai Y and Jingu K 2017. Feasibility of CBCT-based proton dose calculation using a histogram-matching algorithm in proton beam therapy Phys Med 33 68–76 [DOI] [PubMed] [Google Scholar]
- Ashida R, Nakamura M, Yoshimura M and Mizowaki T 2020. Impact of interfractional anatomical variation and setup correction methods on interfractional dose variation in IMPT and VMAT plans for pancreatic cancer patients: A planning study J Appl Clin Med Phys [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JL Jr., Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, Morrison WH, Rosenthal DI, Chao KS, Tucker SL, Mohan R and Dong L 2004. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system Int J Radiat Oncol Biol Phys 59 960–70 [DOI] [PubMed] [Google Scholar]
- Barragan Montero AM, Souris K, Sanchez-Parcerisa D, Sterpin E and Lee JA 2018. Performance of a hybrid Monte Carlo-Pencil Beam dose algorithm for proton therapy inverse planning Med Phys 45 846–62 [DOI] [PubMed] [Google Scholar]
- Barten DL, Tol JP, Dahele M, Slotman BJ and Verbakel WF 2015. Comparison of organ-at-risk sparing and plan robustness for spot-scanning proton therapy and volumetric modulated arc photon therapy in head-and-neck cancer Med Phys 42 6589–98 [DOI] [PubMed] [Google Scholar]
- Belosi MF, van der Meer R, Garcia de Acilu Laa P, Bolsi A, Weber DC and Lomax AJ 2017. Treatment log files as a tool to identify treatment plan sensitivity to inaccuracies in scanned proton beam delivery Radiother Oncol 125 514–9 [DOI] [PubMed] [Google Scholar]
- Bernatowicz K, Geets X, Barragan A, Janssens G, Souris K and Sterpin E 2018. Feasibility of online IMPT adaptation using fast, automatic and robust dose restoration Phys Med Biol 63 085018. [DOI] [PubMed] [Google Scholar]
- Bhide SA, Davies M, Burke K, McNair HA, Hansen V, Barbachano Y, El-Hariry IA, Newbold K, Harrington KJ and Nutting CM 2010. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study Int J Radiat Oncol Biol Phys 76 1360–8 [DOI] [PubMed] [Google Scholar]
- Birkner M, Yan D, Alber M, Liang J and Nusslin F 2003. Adapting inverse planning to patient and organ geometrical variation: algorithm and implementation Med Phys 30 2822–31 [DOI] [PubMed] [Google Scholar]
- Bobic M, Lalonde A, Sharp GC, Grassberger C, Verburg JM, Winey BA, Lomax AJ and Paganetti H 2021. Comparison of weekly and daily online adaptation for head and neck intensity-modulated proton therapy Phys Med Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff MR, Defize IL, Borggreve AS, Takahashi N, van Lier A, Ruurda JP, van Hillegersberg R, Lagendijk JJW, Mook S and Meijer GJ 2020. 3-Dimensional target coverage assessment for MRI guided esophageal cancer radiotherapy Radiother Oncol 147 1–7 [DOI] [PubMed] [Google Scholar]
- Boellaard R, van Herk M, Uiterwaal H and Mijnheer B 1997. Two-dimensional exit dosimetry using a liquid-filled electronic portal imaging device and a convolution model Radiother Oncol 44 149–57 [DOI] [PubMed] [Google Scholar]
- Bol GH, Lagendijk JJ and Raaymakers BW 2013. Virtual couch shift (VCS): accounting for patient translation and rotation by online IMRT re-optimization Phys Med Biol 58 2989–3000 [DOI] [PubMed] [Google Scholar]
- Boman E, Kapanen M, Pickup L and Lahtela SL 2017. Importance of deformable image registration and biological dose summation in planning of radiotherapy retreatments Med Dosim 42 296–303 [DOI] [PubMed] [Google Scholar]
- Bortfeld T 1997. An analytical approximation of the Bragg curve for therapeutic proton beams Medical Physics 24 2024–33 [DOI] [PubMed] [Google Scholar]
- Bortfeld T and Paganetti H 2006. The biologic relevance of daily dose variations in adaptive treatment planning Int J Radiat Oncol Biol Phys 65 899–906 [DOI] [PubMed] [Google Scholar]
- Bortfeld T, Shusharina N and Craft D 2021. Probabilistic definition of the clinical target volume-implications for tumor control probability modeling and optimization Phys Med Biol 66 01NT. [DOI] [PubMed] [Google Scholar]
- Botas P, Kim J, Winey B and Paganetti H 2018. Online adaption approaches for intensity modulated proton therapy for head and neck patients based on cone beam CTs and Monte Carlo simulations Phys Med Biol 64 015004. [DOI] [PubMed] [Google Scholar]
- Brouwer CL, Steenbakkers RJ, Langendijk JA and Sijtsema NM 2015. Identifying patients who may benefit from adaptive radiotherapy: Does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol 115 285–94 [DOI] [PubMed] [Google Scholar]
- Burridge N, Amer A, Marchant T, Sykes J, Stratford J, Henry A, McBain C, Price P and Moore C 2006. Online adaptive radiotherapy of the bladder: small bowel irradiated-volume reduction Int J Radiat Oncol Biol Phys 66 892–7 [DOI] [PubMed] [Google Scholar]
- Buti G, Souris K, Montero AMB, Lee JA and Sterpin E 2019. Towards fast and robust 4D optimization for moving tumors with scanned proton therapy Med Phys 46 5434–43 [DOI] [PubMed] [Google Scholar]
- Cardenas CE, Yang J, Anderson BM, Court LE and Brock KB 2019. Advances in Auto-Segmentation Semin Radiat Oncol 29 185–97 [DOI] [PubMed] [Google Scholar]
- Castadot P, Lee JA, Geets X and Gregoire V 2010. Adaptive radiotherapy of head and neck cancer Semin Radiat Oncol 20 84–93 [DOI] [PubMed] [Google Scholar]
- Castelli J, Simon A, Lafond C, Perichon N, Rigaud B, Chajon E, De Bari B, Ozsahin M, Bourhis J and de Crevoisier R 2018. Adaptive radiotherapy for head and neck cancer Acta Oncol 57 1284–92 [DOI] [PubMed] [Google Scholar]
- Chen AM, Daly ME, Cui J, Mathai M, Benedict S and Purdy JA 2014. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning Head & neck 36 1541–6 [DOI] [PubMed] [Google Scholar]
- Chen X, Ouyang L, Yan H, Jia X, Li B, Lyu Q, Zhang Y and Wang J 2017. Optimization of the geometry and speed of a moving blocker system for cone-beam computed tomography scatter correction Med Phys 44 e215–e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Wu VW, Ngan RK, Tang KW, Chan CC, Wong KH, Au SK and Kwong DL 2012. A prospective study on volumetric and dosimetric changes during intensity-modulated radiotherapy for nasopharyngeal carcinoma patients Radiother Oncol 104 317–23 [DOI] [PubMed] [Google Scholar]
- Chetty IJ and Rosu-Bubulac M 2019. Deformable Registration for Dose Accumulation Semin Radiat Oncol 29 198–208 [DOI] [PubMed] [Google Scholar]
- Ciarmatori A, Maffei N, Mistretta GM, Ceroni P, Bernabei A, Meduri B, D’Angelo E, Bruni A, Giacobazzi P, Lohr F and Guidi G 2019. Evaluation of the effectiveness of novel single-intervention adaptive radiotherapy strategies based on daily dose accumulation Med Dosim 44 379–84 [DOI] [PubMed] [Google Scholar]
- Cormack AM. Representation of a Function by Its Line Integrals, with Some Radiological Applications. Journal of Applied Physics. 1963;34:2722. [Google Scholar]
- Court LE, Dong L, Lee AK, Cheung R, Bonnen MD, O’Daniel J, Wang H, Mohan R and Kuban D 2005. An automatic CT-guided adaptive radiation therapy technique by online modification of multileaf collimator leaf positions for prostate cancer Int J Radiat Oncol Biol Phys 62 154–63 [DOI] [PubMed] [Google Scholar]
- Cubillos-Mesias M, Troost EGC, Lohaus F, Agolli L, Rehm M, Richter C and Stutzer K 2019. Including anatomical variations in robust optimization for head and neck proton therapy can reduce the need of adaptation Radiother Oncol 131 127–34 [DOI] [PubMed] [Google Scholar]
- Cummings D, Tang S, Ichter W, Wang P, Sturgeon JD, Lee AK and Chang C 2018. Four-dimensional Plan Optimization for the Treatment of Lung Tumors Using Pencil-beam Scanning Proton Radiotherapy Cureus 10 e3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J, Ansorge R and Jena R 2015. Sub-second pencil beam dose calculation on GPU for adaptive proton therapy Phys Med Biol 60 4777–95 [DOI] [PubMed] [Google Scholar]
- Dawson LA and Sharpe MB 2006. Image-guided radiotherapy: rationale, benefits, and limitations Lancet Oncol 7 848–58 [DOI] [PubMed] [Google Scholar]
- de la Zerda A, Armbruster B and Xing L 2007. Formulating adaptive radiation therapy (ART) treatment planning into a closed-loop control framework Phys Med Biol 52 4137–53 [DOI] [PubMed] [Google Scholar]
- Dedes G, Dickmann J, Niepel K, Wesp P, Johnson RP, Pankuch M, Bashkirov V, Rit S, Volz L, Schulte RW, Landry G and Parodi K 2019. Experimental comparison of proton CT and dual energy x-ray CT for relative stopping power estimation in proton therapy Phys Med Biol 64 165002. [DOI] [PubMed] [Google Scholar]
- Deffet S, Farace P and Macq B 2020. Sparse deconvolution of proton radiography data to estimate water equivalent thickness maps Med Phys 47 509–17 [DOI] [PubMed] [Google Scholar]
- den Otter LA, Anakotta RM, Weessies M, Roos CTG, Sijtsema NM, Muijs CT, Dieters M, Wijsman R, Troost EGC, Richter C, Meijers A, Langendijk JA, Both S and Knopf AC 2020. Investigation of inter-fraction target motion variations in the context of pencil beam scanned proton therapy in non-small cell lung cancer patients Med Phys 47 3835–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschmann H, Kametriser G, Steininger P, Scherer P, Scholler H, Gaisberger C, Mooslechner M, Mitterlechner B, Weichenberger H, Fastner G, Wurstbauer K, Jeschke S, Forstner R and Sedlmayer F 2012. First clinical release of an online, adaptive, aperture-based image-guided radiotherapy strategy in intensity-modulated radiotherapy to correct for inter- and intrafractional rotations of the prostate Int J Radiat Oncol Biol Phys 83 1624–32 [DOI] [PubMed] [Google Scholar]
- Dial C, Weiss E, Siebers JV and Hugo GD 2016. Benefits of adaptive radiation therapy in lung cancer as a function of replanning frequency Med Phys 43 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann J, Kamp F, Hillbrand M, Corradini S, Belka C, Schulte RW, Parodi K, Dedes G and Landry G 2021. Fluence-modulated proton CT optimized with patient-specific dose and variance objectives for proton dose calculation Phys Med Biol 66 064001. [DOI] [PubMed] [Google Scholar]
- Duma MN, Kampfer S, Schuster T, Winkler C and Geinitz H 2012. Adaptive radiotherapy for soft tissue changes during helical tomotherapy for head and neck cancer Strahlenther Onkol 188 243–7 [DOI] [PubMed] [Google Scholar]
- Edmund JM and Nyholm T 2017. A review of substitute CT generation for MRI-only radiation therapy Radiat Oncol 12 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmahdy MS, Jagt T, Zinkstok RT, Qiao Y, Shahzad R, Sokooti H, Yousefi S, Incrocci L, Marijnen CAM, Hoogeman M and Staring M 2019. Robust contour propagation using deep learning and image registration for online adaptive proton therapy of prostate cancer Med Phys 46 3329–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsman M, Rietzel E and Kooy HM 2006. Four-dimensional proton treatment planning for lung tumors. International Journal of Radiation Oncology, Biology, Physics 64 1589–95 [DOI] [PubMed] [Google Scholar]
- Engelsman M, Rosenthal SJ, Michaud SL, Adams JA, Schneider RJ, Bradley SG, Flanz JB and Kooy HM 2005. Intra- and interfractional patient motion for a variety of immobilization devices Med Phys 32 3468–74 [DOI] [PubMed] [Google Scholar]
- Enghardt W, Crespo P, Fiedler F, Hinz R, Parodi K, Pawelke J and Ponisch F 2004. Charged hadron tumour therapy monitoring by means of PET Nuclear Instruments and Methods in Physics Reseach A 525 284–8 [Google Scholar]
- Engwall E, Fredriksson A and Glimelius L 2018. 4D robust optimization including uncertainties in time structures can reduce the interplay effect in proton pencil beam scanning radiation therapy Med Phys [DOI] [PubMed] [Google Scholar]
- Feng Y, Castro-Pareja C, Shekhar R and Yu C 2006. Direct aperture deformation: an interfraction image guidance strategy Med Phys 33 4490–8 [DOI] [PubMed] [Google Scholar]
- Fu W, Yang Y, Yue NJ, Heron DE and Huq MS 2009. A cone beam CT-guided online plan modification technique to correct interfractional anatomic changes for prostate cancer IMRT treatment Phys Med Biol 54 1691–703 [DOI] [PubMed] [Google Scholar]
- Giacometti V, Hounsell AR and McGarry CK 2020. A review of dose calculation approaches with cone beam CT in photon and proton therapy Phys Med 76 243–76 [DOI] [PubMed] [Google Scholar]
- Green OL, Henke LE and Hugo GD 2019. Practical Clinical Workflows for Online and Offline Adaptive Radiation Therapy Semin Radiat Oncol 29 219–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogg K, Alpert NM, Zhu X, Min CH, Testa M, Winey B, Normandin MD, Shih HA, Paganetti H, Bortfeld T and El Fakhri G 2015. Mapping (15)O production rate for proton therapy verification Int J Radiat Oncol Biol Phys 92 453–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Neph R, Ruan D, Zou W, Dong L and Sheng K 2019. Robust beam orientation optimization for intensity-modulated proton therapy Med Phys 46 3356–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, O’Connor D, Nguyen D, Yu VY, Ruan D, Dong L and Sheng K 2018. Integrated beam orientation and scanning-spot optimization in intensity-modulated proton therapy for brain and unilateral head and neck tumors Med Phys 45 1338–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez S, Warren-Oseni K, McNair HA, Hansen VN, Jones K, Tan M, Khan A, Harris V, McDonald F, Lalondrelle S, Mohammed K, Thomas K, Thompson A, Kumar P, Dearnaley D, Horwich A and Huddart R 2016. Prospective Study Delivering Simultaneous Integrated High-dose Tumor Boost (</=70 Gy) With Image Guided Adaptive Radiation Therapy for Radical Treatment of Localized Muscle-Invasive Bladder Cancer Int J Radiat Oncol Biol Phys 94 1022–30 [DOI] [PubMed] [Google Scholar]
- Hague C, Aznar M, Dong L, Fotouhi-Ghiam A, Lee LW, Li T, Lin A, Lowe M, Lukens JN, McPartlin A, O’Reilly S, Slevin N, Swisher-Mcclure S, Thomson D, Van Herk M, West C, Zou W and Teo BK 2020. Inter-fraction robustness of intensity-modulated proton therapy in the post-operative treatment of oropharyngeal and oral cavity squamous cell carcinomas Br J Radiol 93 20190638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming-Vrieze O, Depauw N, Craft DL, Chan AW, Rasch CRN, Verheij M, Sonke JJ and Kooy HM 2019. Impact of setup and range uncertainties on TCP and NTCP following VMAT or IMPT of oropharyngeal cancer patients Phys Med Biol 64 095001. [DOI] [PubMed] [Google Scholar]
- Hamming-Vrieze O, van Kranen SR, Heemsbergen WD, Lange CAH, van den Brekel MWM, Verheij M, Rasch CRN and Sonke JJ 2017. Analysis of GTV reduction during radiotherapy for oropharyngeal cancer: Implications for adaptive radiotherapy Radiother Oncol 122 224–8 [DOI] [PubMed] [Google Scholar]
- Han C, Chen YJ, Liu A, Schultheiss TE and Wong JY 2008. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy Int J Radiat Oncol Biol Phys 70 1256–62 [DOI] [PubMed] [Google Scholar]
- Hansen EK, Bucci MK, Quivey JM, Weinberg V and Xia P 2006. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer Int J Radiat Oncol Biol Phys 64 355–62 [DOI] [PubMed] [Google Scholar]
- Harms J, Lei Y, Wang T, McDonald M, Ghavidel B, Stokes W, Curran WJ, Zhou J, Liu T and Yang X 2020. Cone-beam CT-derived relative stopping power map generation via deep learning for proton radiotherapy Med Phys 47 4416–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukelom J and Fuller CD 2019. Head and Neck Cancer Adaptive Radiation Therapy (ART): Conceptual Considerations for the Informed Clinician Semin Radiat Oncol 29 258–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukelom J, Hamming O, Bartelink H, Hoebers F, Giralt J, Herlestam T, Verheij M, van den Brekel M, Vogel W, Slevin N, Deutsch E, Sonke JJ, Lambin P and Rasch C 2013. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer BMC cancer 13 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama S, Matsuura T, Yasuda K, Takao S, Fujii T, Miyamoto N, Umegaki K and Shimizu S 2020. Difference in LET-based biological doses between IMPT optimization techniques: Robust and PTV-based optimizations J Appl Clin Med Phys 21 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Oborn B, Moteabbed M, Yan S, Bortfeld T, Knopf A, Fuchs H, Georg D, Seco J, Spadea MF, Jakel O, Kurz C and Parodi K 2020. MR-guided proton therapy: a review and a preview Radiat Oncol 15 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L, Alber M, Jensen MF, Holt MI and Moller DS 2017. Adaptation is mandatory for intensity modulated proton therapy of advanced lung cancer to ensure target coverage Radiother Oncol 122 400–5 [DOI] [PubMed] [Google Scholar]
- Hueso-Gonzalez F, Enghardt W, Fiedler F, Golnik C, Janssens G, Petzoldt J, Prieels D, Priegnitz M, Romer KE, Smeets J, Vander Stappen F, Wagner A and Pausch G 2015. First test of the prompt gamma ray timing method with heterogeneous targets at a clinical proton therapy facility Phys Med Biol 60 6247–72 [DOI] [PubMed] [Google Scholar]
- Hueso-Gonzalez F, Rabe M, Ruggieri TA, Bortfeld T and Verburg JM 2018. A full-scale clinical prototype for proton range verification using prompt gamma-ray spectroscopy Phys Med Biol 63 185019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffray DA, Lindsay PE, Brock KK, Deasy JO and Tome WA 2010. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue Int J Radiat Oncol Biol Phys 76 S135–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagt T, Breedveld S, van de Water S, Heijmen B and Hoogeman M 2017. Near real-time automated dose restoration in IMPT to compensate for daily tissue density variations in prostate cancer Phys Med Biol 62 4254–72 [DOI] [PubMed] [Google Scholar]
- Jagt T, Breedveld S, van Haveren R, Heijmen B and Hoogeman M 2018. An automated planning strategy for near real-time adaptive proton therapy in prostate cancer Phys Med Biol 63 135017. [DOI] [PubMed] [Google Scholar]
- Jagt TZ, Breedveld S, van Haveren R, Heijmen BJM and Hoogeman MS 2020. Online-adaptive versus robust IMPT for prostate cancer: How much can we gain? Radiother Oncol 151 228–33 [DOI] [PubMed] [Google Scholar]
- Jagt TZ, Breedveld S, van Haveren R, Nout RA, Astreinidou E, Heijmen BJM and Hoogeman MS 2019. Plan-library supported automated replanning for online-adaptive intensity-modulated proton therapy of cervical cancer Acta Oncol 58 1440–5 [DOI] [PubMed] [Google Scholar]
- Jarema T and Aland T 2019. Using the iterative kV CBCT reconstruction on the Varian Halcyon linear accelerator for radiation therapy planning for pelvis patients Phys Med 68 112–6 [DOI] [PubMed] [Google Scholar]
- Jarry G, Graham SA, Moseley DJ, Jaffray DJ, Siewerdsen JH and Verhaegen F 2006. Characterization of scattered radiation in kV CBCT images using Monte Carlo simulations Med Phys 33 4320–9 [DOI] [PubMed] [Google Scholar]
- Jia X, Schuemann J, Paganetti H and Jiang S 2012. GPU-based fast Monte Carlo dose calculation for proton therapy Physics in Medicine and Biology 57 7783–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Beltran C, Wan Chan Tseung H, Mundy DW, Kruse JJ, Whitaker TJ, Herman MG and Furutani KM 2019. Highly efficient and sensitive patient-specific quality assurance for spot-scanned proton therapy PloS one 14 e0212412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E, Wyatt JJ, Henry AM, Short SC, Sebag-Montefiore D, Murray L, Kelly CG, McCallum HM and Speight R 2018. Systematic Review of Synthetic Computed Tomography Generation Methodologies for Use in Magnetic Resonance Imaging-Only Radiation Therapy Int J Radiat Oncol Biol Phys 100 199–217 [DOI] [PubMed] [Google Scholar]
- Kamada T, Tsujii H, Mizoe JE, Matsuoka Y, Tsuji H, Osaka Y, Minohara S, Miyahara N, Endo M and Kanai T 1999. A horizontal CT system dedicated to heavy-ion beam treatment Radiother Oncol 50 235–7 [DOI] [PubMed] [Google Scholar]
- Kavanaugh J, Hugo G, Robinson CG and Roach MC 2019. Anatomical Adaptation-Early Clinical Evidence of Benefit and Future Needs in Lung Cancer Semin Radiat Oncol 29 274–83 [DOI] [PubMed] [Google Scholar]
- Keall P, Poulsen P and Booth JT 2019. See, Think, and Act: Real-Time Adaptive Radiotherapy Semin Radiat Oncol 29 228–35 [DOI] [PubMed] [Google Scholar]
- Kida S, Nakamoto T, Nakano M, Nawa K, Haga A, Kotoku J, Yamashita H and Nakagawa K 2018. Cone Beam Computed Tomography Image Quality Improvement Using a Deep Convolutional Neural Network Cureus 10 e2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park YK, Sharp G, Busse P and Winey B 2017. Water equivalent path length calculations using scatter-corrected head and neck CBCT images to evaluate patients for adaptive proton therapy Phys Med Biol 62 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park YK, Sharp G, Busse P and Winey B 2020. Beam angle optimization using angular dependency of range variation assessed via water equivalent path length (WEPL) calculation for head and neck proton therapy Phys Med 69 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk ML, Tang S, Zhai H, Vapiwala N, Deville C, James P, Bekelman JE, Christodouleas JP, Tochner Z and Both S 2015. Comparison of prostate proton treatment planning technique, interfraction robustness, and analysis of single-field treatment feasibility Pract Radiat Oncol 5 99–105 [DOI] [PubMed] [Google Scholar]
- Knopf AC, Boye D, Lomax A and Mori S 2013. Adequate margin definition for scanned particle therapy in the incidence of intrafractional motion Phys Med Biol 58 6079–94 [DOI] [PubMed] [Google Scholar]
- Knopf AC and Lomax A 2013. In vivo proton range verification: a review Physics in Medicine and Biology 58 R131–R60 [DOI] [PubMed] [Google Scholar]
- Kohno R, Hotta K, Nishioka S, Matsubara K, Tansho R and Suzuki T 2011. Clinical implementation of a GPU-based simplified Monte Carlo method for a treatment planning system of proton beam therapy Phys Med Biol 56 N287–94 [DOI] [PubMed] [Google Scholar]
- Koivula L, Wee L and Korhonen J 2016. Feasibility of MRI-only treatment planning for proton therapy in brain and prostate cancers: Dose calculation accuracy in substitute CT images Med Phys 43 4634. [DOI] [PubMed] [Google Scholar]
- Kupelian P and Meyer JL 2011. Image-guided, adaptive radiotherapy of prostate cancer: toward new standards of radiotherapy practice Front Radiat Ther Oncol 43 344–68 [DOI] [PubMed] [Google Scholar]
- Kurz C, Kamp F, Park YK, Zollner C, Rit S, Hansen D, Podesta M, Sharp GC, Li M, Reiner M, Hofmaier J, Neppl S, Thieke C, Nijhuis R, Ganswindt U, Belka C, Winey BA, Parodi K and Landry G 2016a. Investigating deformable image registration and scatter correction for CBCT-based dose calculation in adaptive IMPT Med Phys 43 5635. [DOI] [PubMed] [Google Scholar]
- Kurz C, Maspero M, Savenije MHF, Landry G, Kamp F, Pinto M, Li M, Parodi K, Belka C and van den Berg CAT 2019. CBCT correction using a cycle-consistent generative adversarial network and unpaired training to enable photon and proton dose calculation Phys Med Biol 64 225004. [DOI] [PubMed] [Google Scholar]
- Kurz C, Nijhuis R, Reiner M, Ganswindt U, Thieke C, Belka C, Parodi K and Landry G 2016b. Feasibility of automated proton therapy plan adaptation for head and neck tumors using cone beam CT images Radiat Oncol 11 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerwaard F, Bohoudi O, Tetar S, Admiraal MA, Rosario TS and Bruynzeel A 2018. Combined Inter- and Intrafractional Plan Adaptation Using Fraction Partitioning in Magnetic Resonance-guided Radiotherapy Delivery Cureus 10 e2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde A, Bobic M, Winey B, Verburg J, Sharp GC and Paganetti H 2021. Anatomic changes in head and neck intensity-modulated proton therapy: Comparison between robust optimization and online adaptation Radiother Oncol 159 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde A, Winey BA, Verburg JM, Paganetti H and Sharp GC 2020. Evaluation of CBCT scatter correction using deep convolutional neural networks for head and neck adaptive proton therapy Phys Med Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry G, Hansen D, Kamp F, Li M, Hoyle B, Weller J, Parodi K, Belka C and Kurz C 2019. Comparing Unet training with three different datasets to correct CBCT images for prostate radiotherapy dose calculations Phys Med Biol 64 035011 [DOI] [PubMed] [Google Scholar]
- Landry G and Hua CH 2018. Current state and future applications of radiological image guidance for particle therapy Med Phys 45 e1086–e95 [DOI] [PubMed] [Google Scholar]
- Landry G, Nijhuis R, Dedes G, Handrack J, Thieke C, Janssens G, Orban de Xivry J, Reiner M, Kamp F, Wilkens JJ, Paganelli C, Riboldi M, Baroni G, Ganswindt U, Belka C and Parodi K 2015. Investigating CT to CBCT image registration for head and neck proton therapy as a tool for daily dose recalculation Med Phys 42 1354–66 [DOI] [PubMed] [Google Scholar]
- Lee HHC, Li B, Duan X, Zhou L, Jia X and Yang M 2019. Systematic analysis of the impact of imaging noise on dual-energy CT-based proton stopping power ratio estimation Med Phys 46 2251–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Le QT and Xing L 2008a. Retrospective IMRT dose reconstruction based on cone-beam CT and MLC log-file Int J Radiat Oncol Biol Phys 70 634–44 [DOI] [PubMed] [Google Scholar]
- Lee L, Mao W and Xing L 2008b. The use of EPID-measured leaf sequence files for IMRT dose reconstruction in adaptive radiation therapy Med Phys 35 5019–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y and Wu Q 2010. A hybrid strategy of offline adaptive planning and online image guidance for prostate cancer radiotherapy Phys Med Biol 55 2221–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang X, Park P, Liu W, Chang J, Liao Z, Frank S, Li Y, Poenisch F, Mohan R, Gillin M and Zhu R 2015. Robust optimization in intensity-modulated proton therapy to account for anatomy changes in lung cancer patients Radiother Oncol 114 367–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Thongphiew D, Zhu X, Lee WR, Vujaskovic Z, Yin FF and Wu QJ 2011. Adaptive prostate IGRT combining online re-optimization and re-positioning: a feasibility study Phys Med Biol 56 1243–58 [DOI] [PubMed] [Google Scholar]
- Li T, Wu Q, Zhang Y, Vergalasova I, Lee WR, Yin FF and Wu QJ 2013. Strategies for automatic online treatment plan reoptimization using clinical treatment planning system: a planning parameters study Med Phys 40 111711. [DOI] [PubMed] [Google Scholar]
- Li T, Zhu X, Thongphiew D, Lee WR, Vujaskovic Z, Wu Q, Yin FF and Wu QJ 2010. On-line adaptive radiation therapy: feasibility and clinical study J Oncol 2010 407236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tian Z, Song T, Wu Z, Liu Y, Jiang S and Jia X 2017. A new approach to integrate GPU-based Monte Carlo simulation into inverse treatment plan optimization for proton therapy Phys Med Biol 62 289–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Chen L, Nguyen D, Zhou Z, Gu X, Yang M, Wang J and Jiang S 2019. Generating synthesized computed tomography (CT) from cone-beam computed tomography (CBCT) using CycleGAN for adaptive radiation therapy Phys Med Biol 64 125002. [DOI] [PubMed] [Google Scholar]
- Liebl J, Paganetti H, Zhu M and Winey BA 2014. The influence of patient positioning uncertainties in proton radiotherapy on proton range and dose distributions Med Phys 41 091711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, Stewart J, Kelly V, Xie J, Brock KK, Moseley J, Cho YB, Fyles A, Lundin A, Rehbinder H, Lof J, Jaffray DA and Milosevic M 2014. Dosimetrically triggered adaptive intensity modulated radiation therapy for cervical cancer Int J Radiat Oncol Biol Phys 90 147–54 [DOI] [PubMed] [Google Scholar]
- Lim-Reinders S, Keller BM, Al-Ward S, Sahgal A and Kim A 2017. Online Adaptive Radiation Therapy Int J Radiat Oncol Biol Phys 99 994–1003 [DOI] [PubMed] [Google Scholar]
- Lin L, Kang M, Huang S, Mayer R, Thomas A, Solberg TD, McDonough JE and Simone CB 2nd 2015. Beam-specific planning target volumes incorporating 4D CT for pencil beam scanning proton therapy of thoracic tumors J Appl Clin Med Phys 16 5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Frank SJ, Li X, Li Y, Zhu RX and Mohan R 2013. PTV-based IMPT optimization incorporating planning risk volumes vs robust optimization Med Phys 40 021709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Mohan R, Park P, Liu Z, Li H, Li X, Li Y, Wu R, Sahoo N, Dong L, Zhu XR and Grosshans DR 2014. Dosimetric benefits of robust treatment planning for intensity modulated proton therapy for base-of-skull cancers Pract Radiat Oncol 4 384–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shaw CC, Wang T, Chen L, Altunbas MC and Kappadath SC 2006. An Accurate Scatter Measurement and Correction Technique for Cone Beam Breast CT Imaging Using Scanning Sampled Measurement (SSM) Technique Proc SPIE Int Soc Opt Eng 6142 6142341–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof J, Lind BK and Brahme A 1998. An adaptive control algorithm for optimization of intensity modulated radiotherapy considering uncertainties in beam profiles, patient set-up and internal organ motion Phys Med Biol 43 1605–28 [DOI] [PubMed] [Google Scholar]
- Lomax AJ 2008a. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties Phys Med Biol 53 1027–42 [DOI] [PubMed] [Google Scholar]
- Lomax AJ 2008b. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions Phys Med Biol 53 1043–56 [DOI] [PubMed] [Google Scholar]
- Lomax AJ. Myths and realities of range uncertainty. Br J Radiol. 2020;93 doi: 10.1259/bjr.20190582. 20190582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AJ, Bortfeld T, Goitein G, Debus J, Dykstra C, Tercier P-A, Coucke PA and Mirimanoff RO 1999. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy Radiotherapy and Oncology 51 257–71 [DOI] [PubMed] [Google Scholar]
- Ma CM and Paskalev K 2006. In-room CT techniques for image-guided radiation therapy Med Dosim 31 30–9 [DOI] [PubMed] [Google Scholar]
- Mackin D, Li Y, Taylor MB, Kerr M, Holmes C, Sahoo N, Poenisch F, Li H, Lii J, Amos R, Wu R, Suzuki K, Gillin MT, Zhu XR and Zhang X 2013. Improving spot-scanning proton therapy patient specific quality assurance with HPlusQA, a second-check dose calculation engine Med Phys 40 121708. [DOI] [PubMed] [Google Scholar]
- Maier J, Eulig E, Voth T, Knaup M, Kuntz J, Sawall S and Kachelriess M 2019. Real-time scatter estimation for medical CT using the deep scatter estimation: Method and robustness analysis with respect to different anatomies, dose levels, tube voltages, and data truncation Med Phys 46 238–49 [DOI] [PubMed] [Google Scholar]
- Mainegra-Hing E and Kawrakow I 2010. Variance reduction techniques for fast Monte Carlo CBCT scatter correction calculations Phys Med Biol 55 4495–507 [DOI] [PubMed] [Google Scholar]
- Malyapa R, Lowe M, Bolsi A, Lomax AJ, Weber DC and Albertini F 2016. Evaluation of Robustness to Setup and Range Uncertainties for Head and Neck Patients Treated With Pencil Beam Scanning Proton Therapy Int J Radiat Oncol Biol Phys 95 154–62 [DOI] [PubMed] [Google Scholar]
- Maneval D, Ozell B and Despres P 2019. pGPUMCD: an efficient GPU-based Monte Carlo code for accurate proton dose calculations Phys Med Biol 64 085018. [DOI] [PubMed] [Google Scholar]
- Matter M, Nenoff L, Meier G, Weber DC, Lomax AJ and Albertini F 2018. Alternatives to patient specific verification measurements in proton therapy: a comparative experimental study with intentional errors Phys Med Biol 63 205014. [DOI] [PubMed] [Google Scholar]
- Matter M, Nenoff L, Meier G, Weber DC, Lomax AJ and Albertini F 2019. Intensity modulated proton therapy plan generation in under ten seconds Acta Oncol 58 1435–9 [DOI] [PubMed] [Google Scholar]
- Matuszak MM, Kashani R, Green M, Lee C, Cao Y, Owen D, Jolly S and Mierzwa M 2019. Functional Adaptation in Radiation Therapy Semin Radiat Oncol 29 236–44 [DOI] [PubMed] [Google Scholar]
- Meier G, Besson R, Nanz A, Safai S and Lomax AJ 2015. Independent dose calculations for commissioning, quality assurance and dose reconstruction of PBS proton therapy Phys Med Biol 60 2819–36 [DOI] [PubMed] [Google Scholar]
- Meijers A, Free J, Wagenaar D, Deffet S, Knopf AC, Langendijk JA and Both S 2020. Validation of the proton range accuracy and optimization of CT calibration curves utilizing range probing Phys Med Biol 65 03NT2. [DOI] [PubMed] [Google Scholar]
- Meijers A, Jakobi A, Stutzer K, Guterres Marmitt G, Both S, Langendijk JA, Richter C and Knopf A 2019. Log file-based dose reconstruction and accumulation for 4D adaptive pencil beam scanned proton therapy in a clinical treatment planning system: Implementation and proof-of-concept Med Phys 46 1140–9 [DOI] [PubMed] [Google Scholar]
- Men C, Jia X and Jiang SB 2010. GPU-based ultra-fast direct aperture optimization for online adaptive radiation therapy Phys Med Biol 55 4309–19 [DOI] [PubMed] [Google Scholar]
- Min CH, Zhu X, Winey BA, Grogg K, Testa M, El Fakhri G, Bortfeld TR, Paganetti H and Shih HA 2013. Clinical application of in-room positron emission tomography for in vivo treatment monitoring in proton radiation therapy Int J Radiat Oncol Biol Phys 86 183–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Zhang X, Wang H, Kang Y, Wang X, Liu H, Ang KK, Kuban D and Dong L 2005. Use of deformed intensity distributions for on-line modification of image-guided IMRT to account for interfractional anatomic changes Int J Radiat Oncol Biol Phys 61 1258–66 [DOI] [PubMed] [Google Scholar]
- Moore KL 2019. Automated Radiotherapy Treatment Planning Semin Radiat Oncol 29 209–18 [DOI] [PubMed] [Google Scholar]
- Moriya S, Tachibana H, Hotta K, Nakamura N, Sakae T and Akimoto T 2018. Range optimization for target and organs at risk in dynamic adaptive passive scattering proton beam therapy - A proof of concept Phys Med 56 66–73 [DOI] [PubMed] [Google Scholar]
- Moses WW 2011. Fundamental Limits of Spatial Resolution in PET Nucl Instrum Methods Phys Res A 648 Supplement 1 S236–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moteabbed M, Schuemann J and Paganetti H 2014. Dosimetric feasibility of real-time MRI-guided proton therapy Med Phys 41 111713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moteabbed M, Sharp GC, Wang Y, Trofimov A, Efstathiou JA and Lu HM 2015. Validation of a deformable image registration technique for cone beam CT-based dose verification Med Phys 42 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moteabbed M, Trofimov A, Sharp GC, Wang Y, Zietman AL, Efstathiou JA and Lu HM 2017. Proton therapy of prostate cancer by anterior-oblique beams: implications of setup and anatomy variations Phys Med Biol 62 1644–60 [DOI] [PubMed] [Google Scholar]
- Murthy V, Master Z, Adurkar P, Mallick I, Mahantshetty U, Bakshi G, Tongaonkar H and Shrivastava S 2011. ‘Plan of the day’ adaptive radiotherapy for bladder cancer using helical tomotherapy Radiother Oncol 99 55–60 [DOI] [PubMed] [Google Scholar]
- Nenoff L, Matter M, Amaya EJ, Josipovic M, Knopf AC, Lomax AJ, Persson GF, Ribeiro CO, Visser S, Walser M, Weber DC, Zhang Y and Albertini F 2021. Dosimetric influence of deformable image registration uncertainties on propagated structures for online daily adaptive proton therapy of lung cancer patients Radiother Oncol 159 136–43 [DOI] [PubMed] [Google Scholar]
- Nenoff L, Matter M, Hedlund Lindmar J, Weber DC, Lomax AJ and Albertini F 2019. Daily adaptive proton therapy - the key to innovative planning approaches for paranasal cancer treatments Acta Oncol 58 1423–8 [DOI] [PubMed] [Google Scholar]
- Nenoff L, Matter M, Jarhall AG, Winterhalter C, Gorgisyan J, Josipovic M, Persson GF, Munck Af Rosenschold P, Weber DC, Lomax AJ and Albertini F 2020. Daily Adaptive Proton Therapy: Is it Appropriate to Use Analytical Dose Calculations for Plan Adaption? Int J Radiat Oncol Biol Phys 107 747–55 [DOI] [PubMed] [Google Scholar]
- Niu T, Sun M, Star-Lack J, Gao H, Fan Q and Zhu L 2010. Shading correction for on-board cone-beam CT in radiation therapy using planning MDCT images Med Phys 37 5395–406 [DOI] [PubMed] [Google Scholar]
- O’Daniel JC, Garden AS, Schwartz DL, Wang H, Ang KK, Ahamad A, Rosenthal DI, Morrison WH, Asper JA, Zhang L, Tung SM, Mohan R and Dong L 2007. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys 69 1290–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oborn BM 2019. Real-Time MRI-Guided Particle Therapy MRI for Radiotherapy In: Liney G, van der Heide U. (eds) MRI for Radiotherapy. Springer, Cham. [Google Scholar]
- Padgett KR, Simpson G, Asher D, Portelance L, Bossart E and Dogan N 2020. Assessment of online adaptive MR-guided stereotactic body radiotherapy of liver cancers Phys Med 77 54–63 [DOI] [PubMed] [Google Scholar]
- Paganetti H 2012. Range uncertainties in proton therapy and the role of Monte Carlo simulations Phys Med Biol 57 R99–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganetti H, Beltran CJ, Both S, Dong L, Flanz JB, Furutani KM, Grassberger C, Grosshans DR, Knopf AC, Langendijk JA, Nystrom H, Parodi K, Raaymakers BW, Richter C, Sawakuchi GO, Schippers JM, Shaitelman SF, Teo K, Unkelbach J, Wohlfahrt P and Lomax AJ 2020. Roadmap: proton therapy physics and biology Phys Med Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganetti HE 2019. Proton Therapy Physics CRC Press; / Taylor & Francis; 2nd Edition [Google Scholar]
- Park PC, Cheung JP, Zhu XR, Lee AK, Sahoo N, Tucker SL, Liu W, Li H, Mohan R, Court LE and Dong L 2013. Statistical assessment of proton treatment plans under setup and range uncertainties Int J Radiat Oncol Biol Phys 86 1007–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PC, Zhu XR, Lee AK, Sahoo N, Melancon AD, Zhang L and Dong L 2012. A beam-specific planning target volume (PTV) design for proton therapy to account for setup and range uncertainties Int J Radiat Oncol Biol Phys 82 e329–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YK, Sharp GC, Phillips J and Winey BA 2015. Proton dose calculation on scatter-corrected CBCT image: Feasibility study for adaptive proton therapy Med Phys 42 4449–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi K. Latest developments in in-vivo imaging for proton therapy. Br J Radiol. 2020;93 doi: 10.1259/bjr.20190787. 20190787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi K and Polf JC 2018. In vivo range verification in particle therapy Med Phys 45 e1036–e50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroni E, Bacher R, Blattmann H, Boehringer T, Coray A, Lomax A, Lin S, Munkel G, Scheib S, Schneider U and Tourovsky A 1995. The 200-MeV proton therapy project at the Paul Scherrer Institute: Conceptual design and practical realization Medical Physics 22 37–53 [DOI] [PubMed] [Google Scholar]
- Perali I, Celani A, Bombelli L, Fiorini C, Camera F, Clementel E, Henrotin S, Janssens G, Prieels D, Roellinghoff F, Smeets J, Stichelbaut F and Vander Stappen F 2014. Prompt gamma imaging of proton pencil beams at clinical dose rate Phys Med Biol 59 5849–71 [DOI] [PubMed] [Google Scholar]
- Perumal B, Sundaresan HE and Vaitheeswaran R 2018. A Pilot Study on the Comparison between Planning Target Volume-based Intensity-Modulated Proton Therapy Plans and Robustly Optimized Intensity-Modulated Proton Therapy Plans Journal of medical physics / Association of Medical Physicists of India 43 179–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placidi L, Bolsi A, Lomax AJ, Schneider RA, Malyapa R, Weber DC and Albertini F 2017. Effect of Anatomic Changes on Pencil Beam Scanned Proton Dose Distributions for Cranial and Extracranial Tumors Int J Radiat Oncol Biol Phys 97 616–23 [DOI] [PubMed] [Google Scholar]
- Poludniowski G, Allinson NM and Evans PM 2015. Proton radiography and tomography with application to proton therapy Br J Radiol 88 20150134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posiewnik M and Piotrowski T 2019. A review of cone-beam CT applications for adaptive radiotherapy of prostate cancer Phys Med 59 13–21 [DOI] [PubMed] [Google Scholar]
- Qin N, Botas P, Giantsoudi D, Schuemann J, Tian Z, Jiang SB, Paganetti H and Jia X 2016. Recent developments and comprehensive evaluations of a GPU-based Monte Carlo package for proton therapy Phys Med Biol 61 7347–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaymakers BW, Raaijmakers AJ and Lagendijk JJ 2008. Feasibility of MRI guided proton therapy: magnetic field dose effects Phys Med Biol 53 5615–22 [DOI] [PubMed] [Google Scholar]
- Rank CM, Hunemohr N, Nagel AM, Rothke MC, Jakel O and Greilich S 2013. MRI-based simulation of treatment plans for ion radiotherapy in the brain region Radiother Oncol 109 414–8 [DOI] [PubMed] [Google Scholar]
- Reijtenbagh DMW, Godart J, Mens JM, Heijkoop ST, Heemsbergen WD and Hoogeman MS 2020. Patient-reported acute GI symptoms in locally advanced cervical cancer patients correlate with rectal dose Radiother Oncol 148 38–43 [DOI] [PubMed] [Google Scholar]
- Richter C, Pausch G, Barczyk S, Priegnitz M, Keitz I, Thiele J, Smeets J, Stappen FV, Bombelli L, Fiorini C, Hotoiu L, Perali I, Prieels D, Enghardt W and Baumann M 2016. First clinical application of a prompt gamma based in vivo proton range verification system Radiother Oncol 118 232–7 [DOI] [PubMed] [Google Scholar]
- Riegel AC, Antone JG, Zhang H, Jain P, Raince J, Rea A, Bergamo AM, Kapur A and Potters L 2016. Deformable image registration and interobserver variation in contour propagation for radiation therapy planning J Appl Clin Med Phys 17 347–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud B, Simon A, Castelli J, Lafond C, Acosta O, Haigron P, Cazoulat G and de Crevoisier R 2019. Deformable image registration for radiation therapy: principle, methods, applications and evaluation Acta Oncol 58 1225–37 [DOI] [PubMed] [Google Scholar]
- Rinkel J, Gerfault L, Esteve F and Dinten JM 2007. A new method for x-ray scatter correction: first assessment on a cone-beam CT experimental setup Phys Med Biol 52 4633–52 [DOI] [PubMed] [Google Scholar]
- Rit S, Dedes G, Freud N, Sarrut D and Letang JM 2013. Filtered backprojection proton CT reconstruction along most likely paths Med Phys 40 031103 [DOI] [PubMed] [Google Scholar]
- Ruhrnschopf EP and Klingenbeck K 2011. A general framework and review of scatter correction methods in x-ray cone-beam computerized tomography. Part 1: Scatter compensation approaches Med Phys 38 4296–311 [DOI] [PubMed] [Google Scholar]
- Sanguineti G, Ricchetti F, Thomas O, Wu B and McNutt T 2013. Pattern and predictors of volumetric change of parotid glands during intensity modulated radiotherapy Br J Radiol 86 20130363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhammer SM, Gantz S, Luhr A, Oborn BM, Bussmann M and Hoffmann AL 2018. Technical Note: Experimental verification of magnetic field-induced beam deflection and Bragg peak displacement for MR-integrated proton therapy Med Phys 45 3429–34 [DOI] [PubMed] [Google Scholar]
- Schiavi A, Senzacqua M, Pioli S, Mairani A, Magro G, Molinelli S, Ciocca M, Battistoni G and Patera V 2017. Fred: a GPU-accelerated fast-Monte Carlo code for rapid treatment plan recalculation in ion beam therapy Phys Med Biol 62 7482–504 [DOI] [PubMed] [Google Scholar]
- Schneider U, Besserer J, Pemler P, Dellert M, Moosburger M, Pedroni E and Kaser-Hotz B 2004. First proton radiography of an animal patient Med Phys 31 1046–51 [DOI] [PubMed] [Google Scholar]
- Schuemann J, Dowdell S, Grassberger C, Min CH and Paganetti H 2014. Site-specific range uncertainties caused by dose calculation algorithms for proton therapy. Physics in Medicine and Biology 59 4007–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuemann J, Giantsoudi D, Grassberger C, Moteabbed M, Min CH and Paganetti H 2015. Assessing the Clinical Impact of Approximations in Analytical Dose Calculations for Proton Therapy Int J Radiat Oncol Biol Phys 92 1157–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TE, Tome WA and Orton CG 2012. Point/counterpoint: it is not appropriate to “deform” dose along with deformable image registration in adaptive radiotherapy Med Phys 39 6531–3 [DOI] [PubMed] [Google Scholar]
- Schwartz DL, Garden AS, Shah SJ, Chronowski G, Sejpal S, Rosenthal DI, Chen Y, Zhang Y, Zhang L, Wong PF, Garcia JA, Kian Ang K and Dong L 2013. Adaptive radiotherapy for head and neck cancer--dosimetric results from a prospective clinical trial Radiother Oncol 106 80–4 [DOI] [PubMed] [Google Scholar]
- Schwartz DL, Garden AS, Thomas J, Chen Y, Zhang Y, Lewin J, Chambers MS and Dong L 2012. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial Int J Radiat Oncol Biol Phys 83 986–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejpal SV, Amos RA, Bluett JB, Levy LB, Kudchadker RJ, Johnson J, Choi S and Lee AK 2009. Dosimetric changes resulting from patient rotational setup errors in proton therapy prostate plans Int J Radiat Oncol Biol Phys 75 40–8 [DOI] [PubMed] [Google Scholar]
- Shakirin G, Braess H, Fiedler F, Kunath D, Laube K, Parodi K, Priegnitz M and Enghardt W 2011. Implementation and workflow for PET monitoring of therapeutic ion irradiation: a comparison of in-beam, in-room, and off-line techniques Phys Med Biol 56 1281–98 [DOI] [PubMed] [Google Scholar]
- Sharfo AW, Breedveld S, Voet PW, Heijkoop ST, Mens JM, Hoogeman MS and Heijmen BJ 2016. Validation of Fully Automated VMAT Plan Generation for Library-Based Plan-of-the-Day Cervical Cancer Radiotherapy PloS one 11 e0169202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Vedantham S, Karellas A and Zhu L 2017. X-ray scatter correction for dedicated cone beam breast CT using a forward-projection model Med Phys 44 2312–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wang A, Wei J and Zhu L 2019. Fast shading correction for cone-beam CT via partitioned tissue classification Phys Med Biol 64 065015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusharina N, Craft D, Chen YL, Shih H and Bortfeld T 2018. The clinical target distribution: a probabilistic alternative to the clinical target volume Phys Med Biol 63 155001. [DOI] [PubMed] [Google Scholar]
- Siebers JV, Kawrakow I and Ramakrishnan V 2007. Performance of a hybrid MC dose algorithm for IMRT optimization dose evaluation Med Phys 34 2853–63 [DOI] [PubMed] [Google Scholar]
- Siebers JV, Lauterbach M, Tong S, Wu Q and Mohan R 2002. Reducing dose calculation time for accurate iterative IMRT planning Med Phys 29 231–7 [DOI] [PubMed] [Google Scholar]
- Simone CB 2nd, Ly D, Dan TD, Ondos J, Ning H, Belard A, O’Connell J, Miller RW and Simone NL 2011. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer Radiother Oncol 101 376–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonke JJ, Aznar M and Rasch C 2019. Adaptive Radiotherapy for Anatomical Changes Semin Radiat Oncol 29 245–57 [DOI] [PubMed] [Google Scholar]
- Sonke JJ and Belderbos J 2010. Adaptive radiotherapy for lung cancer Semin Radiat Oncol 20 94–106 [DOI] [PubMed] [Google Scholar]
- Sovik A, Malinen E, Skogmo HK, Bentzen SM, Bruland OS and Olsen DR 2007. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia Int J Radiat Oncol Biol Phys 68 1496–504 [DOI] [PubMed] [Google Scholar]
- Stutzer K, Jakobi A, Bandurska-Luque A, Barczyk S, Arnsmeyer C, Lock S and Richter C 2017. Potential proton and photon dose degradation in advanced head and neck cancer patients by intratherapy changes J Appl Clin Med Phys 18 104–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M and Star-Lack JM 2010. Improved scatter correction using adaptive scatter kernel superposition Phys Med Biol 55 6695–720 [DOI] [PubMed] [Google Scholar]
- Szeto YZ, Witte MG, van Kranen SR, Sonke JJ, Belderbos J and van Herk M 2016. Effects of anatomical changes on pencil beam scanning proton plans in locally advanced NSCLC patients Radiother Oncol 120 286–92 [DOI] [PubMed] [Google Scholar]
- Taasti VT, Hong L, Deasy JO and Zarepisheh M 2020. Automated proton treatment planning with robust optimization using constrained hierarchical optimization Med Phys [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taasti VT, Jeong J, Jackson A and Deasy JO 2019. A theoretical investigation of adequate range uncertainty margins in proton treatment planning to preserve tumor control probability Acta Oncol 58 1446–50 [DOI] [PubMed] [Google Scholar]
- Tang S, Song L, Sturgeon JD and Chang C 2017. Robust Planning for a Patient Treated in Decubitus Position with Proton Pencil Beam Scanning Radiotherapy Cureus 9 e1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Verburg JM, Rose M, Min CH, Tang S, Bentefour el H, Paganetti H and Lu HM 2013. Proton radiography and proton computed tomography based on time-resolved dose measurements Phys Med Biol 58 8215–33 [DOI] [PubMed] [Google Scholar]
- Thomas SJ 2006. Margins for treatment planning of proton therapy Phys Med Biol 51 1491–501 [DOI] [PubMed] [Google Scholar]
- Thummerer A, de Jong BA, Zaffino P, Meijers A, Marmitt GG, Seco J, Steenbakkers R, Langendijk JA, Both S, Spadea MF and Knopf AC 2020a. Comparison of the suitability of CBCT- and MR-based synthetic CTs for daily adaptive proton therapy in head and neck patients Phys Med Biol 65 235036. [DOI] [PubMed] [Google Scholar]
- Thummerer A, Zaffino P, Meijers A, Marmitt GG, Seco J, Steenbakkers R, Langendijk JA, Both S, Spadea MF and Knopf AC 2020b. Comparison of CBCT based synthetic CT methods suitable for proton dose calculations in adaptive proton therapy Phys Med Biol 65 095002. [DOI] [PubMed] [Google Scholar]
- Toscano S, Souris K, Goma C, Barragan-Montero A, Puydupin S, Stappen FV, Janssens G, Matic A, Geets X and Sterpin E 2019. Impact of machine log-files uncertainties on the quality assurance of proton pencil beam scanning treatment delivery Phys Med Biol 64 095021. [DOI] [PubMed] [Google Scholar]
- Trofimov A, Nguyen PL, Efstathiou JA, Wang Y, Lu HM, Engelsman M, Merrick S, Cheng CW, Wong JR and Zietman AL 2011. Interfractional variations in the setup of pelvic bony anatomy and soft tissue, and their implications on the delivery of proton therapy for localized prostate cancer Int J Radiat Oncol Biol Phys 80 928–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimov A, Rietzel E, Lu HM, Martin B, Jiang S, Chen GT and Bortfeld T 2005. Temporo-spatial IMRT optimization: concepts, implementation and initial results Phys Med Biol 50 2779–98 [DOI] [PubMed] [Google Scholar]
- Unkelbach J, Alber M, Bangert M, Bokrantz R, Chan TCY, Deasy JO, Fredriksson A, Gorissen BL, van Herk M, Liu W, Mahmoudzadeh H, Nohadani O, Siebers JV, Witte M and Xu H 2018. Robust radiotherapy planning Phys Med Biol 63 22TR02. [DOI] [PubMed] [Google Scholar]
- Unkelbach J, Bortfeld T, Cardenas CE, Gregoire V, Hager W, Heijmen B, Jeraj R, Korreman SS, Ludwig R, Pouymayou B, Shusharina N, Soderberg J, Toma-Dasu I, Troost EGC and Vasquez Osorio E 2020. The role of computational methods for automating and improving clinical target volume definition Radiother Oncol 153 15–25 [DOI] [PubMed] [Google Scholar]
- Unkelbach J, Bortfeld T, Martin BC and Soukup M 2009. Reducing the sensitivity of IMPT treatment plans to setup errors and range uncertainties via probabilistic treatment planning Med Phys 36 149–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkelbach J, Botas P, Giantsoudi D, Gorissen BL and Paganetti H 2016. Reoptimization of Intensity Modulated Proton Therapy Plans Based on Linear Energy Transfer Int J Radiat Oncol Biol Phys 96 1097–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkelbach J, Chan TC and Bortfeld T 2007. Accounting for range uncertainties in the optimization of intensity modulated proton therapy Phys Med Biol 52 2755–73 [DOI] [PubMed] [Google Scholar]
- Unkelbach J and Paganetti H 2018. Robust Proton Treatment Planning: Physical and Biological Optimization Seminars in Radiation Oncology 28 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water S, Albertini F, Weber DC, Heijmen BJM, Hoogeman MS and Lomax AJ 2018. Anatomical robust optimization to account for nasal cavity filling variation during intensity-modulated proton therapy: a comparison with conventional and adaptive planning strategies Phys Med Biol 63 025020. [DOI] [PubMed] [Google Scholar]
- van de Water S, Kooy HM, Heijmen BJ and Hoogeman MS 2015. Shortening delivery times of intensity modulated proton therapy by reducing proton energy layers during treatment plan optimization Int J Radiat Oncol Biol Phys 92 460–8 [DOI] [PubMed] [Google Scholar]
- van de Water S, van Dam I, Schaart DR, Al-Mamgani A, Heijmen BJ and Hoogeman MS 2016. The price of robustness; impact of worst-case optimization on organ-at-risk dose and complication probability in intensity-modulated proton therapy for oropharyngeal cancer patients Radiother Oncol 120 56–62 [DOI] [PubMed] [Google Scholar]
- van de Water TA, Bijl HP, Schilstra C, Pijls-Johannesma M and Langendijk JA 2011. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature Oncologist 16 366–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elmpt WJ, Nijsten SM, Schiffeleers RF, Dekker AL, Mijnheer BJ, Lambin P and Minken AW 2006. A Monte Carlo based three-dimensional dose reconstruction method derived from portal dose images Med Phys 33 2426–34 [DOI] [PubMed] [Google Scholar]
- van Kranen S, Hamming-Vrieze O, Wolf A, Damen E, van Herk M and Sonke JJ 2016. Head and Neck Margin Reduction With Adaptive Radiation Therapy: Robustness of Treatment Plans Against Anatomy Changes Int J Radiat Oncol Biol Phys 96 653–60 [DOI] [PubMed] [Google Scholar]
- van Kranen S, Mencarelli A, van Beek S, Rasch C, van Herk M and Sonke JJ 2013. Adaptive radiotherapy with an average anatomy model: evaluation and quantification of residual deformations in head and neck cancer patients Radiother Oncol 109 463–8 [DOI] [PubMed] [Google Scholar]
- Veiga C, Janssens G, Teng CL, Baudier T, Hotoiu L, McClelland JR, Royle G, Lin L, Yin L, Metz J, Solberg TD, Tochner Z, Simone CB 2nd, McDonough J and Kevin Teo BK 2016. First Clinical Investigation of Cone Beam Computed Tomography and Deformable Registration for Adaptive Proton Therapy for Lung Cancer Int J Radiat Oncol Biol Phys 95 549–59 [DOI] [PubMed] [Google Scholar]
- Veiga C, Lourenco AM, Mouinuddin S, van Herk M, Modat M, Ourselin S, Royle G and McClelland JR 2015. Toward adaptive radiotherapy for head and neck patients: Uncertainties in dose warping due to the choice of deformable registration algorithm Med Phys 42 760–9 [DOI] [PubMed] [Google Scholar]
- Veiga C, McClelland J, Moinuddin S, Lourenco A, Ricketts K, Annkah J, Modat M, Ourselin S, D’Souza D and Royle G 2014. Toward adaptive radiotherapy for head and neck patients: Feasibility study on using CT-to-CBCT deformable registration for “dose of the day” calculations Med Phys 41 031703. [DOI] [PubMed] [Google Scholar]
- Vinod SK, Jameson MG, Min M and Holloway LC 2016. Uncertainties in volume delineation in radiation oncology: A systematic review and recommendations for future studies Radiother Oncol 121 169–79 [DOI] [PubMed] [Google Scholar]
- Wan Chan Tseung H, Ma J and Beltran C 2015. A fast GPU-based Monte Carlo simulation of proton transport with detailed modeling of nonelastic interactions Med Phys 42 2967–78 [DOI] [PubMed] [Google Scholar]
- Wang J, Bai S, Chen N, Xu F, Jiang X, Li Y, Xu Q, Shen Y, Zhang H, Gong Y, Zhong R and Jiang Q 2009. The clinical feasibility and effect of online cone beam computer tomography-guided intensity-modulated radiotherapy for nasopharyngeal cancer Radiother Oncol 90 221–7 [DOI] [PubMed] [Google Scholar]
- Wang J, Mao W and Solberg T 2010. Scatter correction for cone-beam computed tomography using moving blocker strips: a preliminary study Med Phys 37 5792–800 [DOI] [PubMed] [Google Scholar]
- Wang Y, Efstathiou JA, Sharp GC, Lu HM, Ciernik IF and Trofimov AV 2011. Evaluation of the dosimetric impact of interfractional anatomical variations on prostate proton therapy using daily in-room CT images Med Phys 38 4623–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Fatyga M, Wu Y, Dogan N, Balik S, Sleeman W t and Hugo G 2013. Dose escalation for locally advanced lung cancer using adaptive radiation therapy with simultaneous integrated volume-adapted boost Int J Radiat Oncol Biol Phys 86 414–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey B, Daartz J, Dankers F and Bussiere M 2012. Immobilization precision of a modified GTC frame J Appl Clin Med Phys 13 3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalter C, Meier G, Oxley D, Weber DC, Lomax AJ and Safai S 2019a. Log file based Monte Carlo calculations for proton pencil beam scanning therapy Phys Med Biol 64 035014. [DOI] [PubMed] [Google Scholar]
- Winterhalter C, Zepter S, Shim S, Meier G, Bolsi A, Fredh A, Hrbacek J, Oxley D, Zhang Y, Weber DC, Lomax A and Safai S 2019b. Evaluation of the ray-casting analytical algorithm for pencil beam scanning proton therapy Phys Med Biol 64 065021. [DOI] [PubMed] [Google Scholar]
- Woodford C, Yartsev S, Dar AR, Bauman G and Van Dyk J 2007. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images Int J Radiat Oncol Biol Phys 69 1316–22 [DOI] [PubMed] [Google Scholar]
- Wu C, Jeraj R, Olivera GH and Mackie TR 2002. Re-optimization in adaptive radiotherapy Phys Med Biol 47 3181–95 [DOI] [PubMed] [Google Scholar]
- Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D and Martinez A 2009. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT Int J Radiat Oncol Biol Phys 75 924–32 [DOI] [PubMed] [Google Scholar]
- Wu Q, Liang J and Yan D 2006. Application of dose compensation in image-guided radiotherapy of prostate cancer Phys Med Biol 51 1405–19 [DOI] [PubMed] [Google Scholar]
- Wu QJ, Li T, Wu Q and Yin FF 2011. Adaptive radiation therapy: technical components and clinical applications Cancer J 17 182–9 [DOI] [PubMed] [Google Scholar]
- Wu QJ, Thongphiew D, Wang Z, Mathayomchan B, Chankong V, Yoo S, Lee WR and Yin FF 2008. On-line re-optimization of prostate IMRT plans for adaptive radiation therapy Phys Med Biol 53 673–91 [DOI] [PubMed] [Google Scholar]
- Wu RY, Liu AY, Sio TT, Blanchard P, Wages C, Amin MV, Gunn GB, Titt U, Ye R, Suzuki K, Gillin MT, Zhu XR, Mohan R and Frank SJ 2017. Intensity-Modulated Proton Therapy Adaptive Planning for Patients with Oropharyngeal Cancer Int J Part Ther 4 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Bentefour EH, Janssens G, Smeets J, Vander Stappen F, Hotoiu L, Yin L, Dolney D, Avery S, O’Grady F, Prieels D, McDonough J, Solberg TD, Lustig RA, Lin A and Teo BK 2017. Prompt Gamma Imaging for In Vivo Range Verification of Pencil Beam Scanning Proton Therapy Int J Radiat Oncol Biol Phys 99 210–8 [DOI] [PubMed] [Google Scholar]
- Xing L, Siebers J and Keall P 2007. Computational challenges for image-guided radiation therapy: framework and current research Semin Radiat Oncol 17 245–57 [DOI] [PubMed] [Google Scholar]
- Yan D, Jaffray DA and Wong JW 1999. A model to accumulate fractionated dose in a deforming organ International Journal of Radiation Oncology, Biology, Physics 44 665–75 [DOI] [PubMed] [Google Scholar]
- Yan D, Lockman D, Brabbins D, Tyburski L and Martinez A 2000. An off-line strategy for constructing a patient-specific planning target volume in adaptive treatment process for prostate cancer Int J Radiat Oncol Biol Phys 48 289–302 [DOI] [PubMed] [Google Scholar]
- Yan D, Vicini F, Wong JW and Martinez A 1997. Adaptive radiation therapy. Physics in Medicine and Biology 42 123–32 [DOI] [PubMed] [Google Scholar]
- Yang H, Hu W, Wang W, Chen P, Ding W and Luo W 2013. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma Int J Radiat Oncol Biol Phys 85 e47–54 [DOI] [PubMed] [Google Scholar]
- Yang M, Zhu XR, Park PC, Titt U, Mohan R, Virshup G, Clayton JE and Dong L 2012. Comprehensive analysis of proton range uncertainties related to patient stopping-power-ratio estimation using the stoichiometric calibration Phys Med Biol 57 4095–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y and Xing L 2005. Optimization of radiotherapy dose-time fractionation with consideration of tumor specific biology Med Phys 32 3666–77 [DOI] [PubMed] [Google Scholar]
- Yang Z, Chang Y, Brock KK, Cazoulat G, Koay EJ, Koong AC, Herman JM, Park PC, Poenisch F, Li Q, Yang K, Wu G, Anderson B, Ohrt AN, Li Y, Zhu XR, Zhang X and Li H 2019. Effect of setup and inter-fraction anatomical changes on the accumulated dose in CT-guided breath-hold intensity modulated proton therapy of liver malignancies Radiother Oncol 134 101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang X, Wang X, Zhu XR, Gunn B, Frank SJ, Chang Y, Li Q, Yang K, Wu G, Liao L, Li Y, Chen M and Li H 2020. Multiple-CT optimization: An adaptive optimization method to account for anatomical changes in intensity-modulated proton therapy for head and neck cancers Radiother Oncol 142 124–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W and Leszczynski KW 2009. An analytical approach to estimating the first order x-ray scatter in heterogeneous medium Med Phys 36 3145–56 [DOI] [PubMed] [Google Scholar]
- Yepes PP, Mirkovic D and Taddei PJ 2010. A GPU implementation of a track-repeating algorithm for proton radiotherapy dose calculations Phys Med Biol 55 7107–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yock AD, Mohan R, Flampouri S, Bosch W, Taylor PA, Gladstone D, Kim S, Sohn J, Wallace R, Xiao Y and Buchsbaum J 2019. Robustness Analysis for External Beam Radiation Therapy Treatment Plans: Describing Uncertainty Scenarios and Reporting Their Dosimetric Consequences Pract Radiat Oncol 9 200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li Y, Pan X, Xiaoqiang L, Mohan R, Komaki R, Cox JD and Chang JY 2010. Intensity-Modulated Proton Therapy Reduces the Dose to Normal Tissue Compared with Intensity-Modulated Radiation Therapy or Passive Scattering Proton Therapy and Enables Individualized Radical Radiotherapy for Extensive Stage IIIB Non-Small-Cell Lung Cancer: A Virtual Clinical Study Int J Radiat Oncol Biol Phys 77 357–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wan Q, Zhou Y, Deng X, Xie C and Wu S 2011. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma Radiother Oncol 98 23–7 [DOI] [PubMed] [Google Scholar]
- Zhao W, Vernekohl D, Zhu J, Wang L and Xing L 2016. A model-based scatter artifacts correction for cone beam CT Med Phys 43 1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bennett NR and Fahrig R 2006. Scatter correction method for X-ray CT using primary modulation: theory and preliminary results IEEE Trans Med Imaging 25 1573–87 [DOI] [PubMed] [Google Scholar]
- Zhu XR, Li Y, Mackin D, Li H, Poenisch F, Lee AK, Mahajan A, Frank SJ, Gillin MT, Sahoo N and Zhang X 2015. Towards effective and efficient patient-specific quality assurance for spot scanning proton therapy Cancers 7 631–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Dong L and Kevin Teo BK 2018. Current State of Image Guidance in Radiation Oncology: Implications for PTV Margin Expansion and Adaptive Therapy Semin Radiat Oncol 28 238–47 [DOI] [PubMed] [Google Scholar]


