Abstract
Vascular endothelial growth factor (VEGF) is an essential regulator of vascularization. It is expressed as several splice variants; the major forms contain 120 amino acids, 164 amino acids, and 188 amino acids. We utilized transformed cells nullizygous for VEGF to specifically express each of these isoforms in isolation, in order to determine the role of each in tumorigenic neo-vascularization. We found that only the intermediate isoform, VEGF164, could fully rescue tumor growth; VEGF120 partially rescued tumor growth, and VEGF188 failed completely to rescue tumor expansion. Surprisingly, the vascular density of VEGF188 isoform-expressing tumors is significantly greater than that of wild-type VEGF cells and the other isoform-specific tumors. The failure of the hypervascular VEGF188-expressing tumors to grow may be due to inadequate perfusion of the massive number of microvessels in these tumors; three-dimensional imaging of the tumorigenic vasculature indicated little or no recruitment of the peripheral vasculature. This demonstrates that the VEGF isoforms perform unique functions which together enable tumorigenic vascularization.
Vascular endothelial growth factor (VEGF) is an important regulator of both developmental and tumorigenic angiogenesis (4, 10, 14). It is an intensively studied molecule and one which has been shown to have significant potential as both a target for antiangiogenic therapies and for stimulated therapeutic angiogenesis (1, 3, 6). It was originally discovered as a mediator of both vascular permeability and endothelial cell proliferation (20, 34) and is widely expressed in development and in various pathological states, perhaps most notably tumorigenesis (21).
VEGF acts on endothelial cells as a chemotactic and mitogenic agent via two endothelial cell-specific receptor tyrosine kinases, flt-1–VEGFR1, and flk-1–kdr–VEGFR2 (13). Both can bind all of the VEGF isoforms; a third VEGF receptor, neuropilin-1, does not bind VEGF120, however (37). Neuropilin-1 also has a broader profile of expression and is found on neurons and endothelial cells; its expression on a number of tumor cell lines indicates that it may also have a role in tumor progression.
VEGF acts through its endothelial receptors to induce chemotaxis and increased permeability of existing blood vessels, as well as to stimulate endothelial cell mitosis and thus the proliferation of new vessels. The role of neuropilin-1 in VEGF signal transduction is less clear, although null mutations of the receptor have angiogenic defects, and ectopic expression of neuropilin-1 results in hemorrhage (19).
VEGF itself is regulated in a complex fashion, at least in part stimulated by lowered tissue oxygenation (16, 36). The hypoxia-stimulated induction of VEGF occurs bimodally, through increased stability of VEGF mRNA mediated by elements in its 3′ end (8, 16, 23, 35) and through transcriptional up-regulation caused by hypoxia inducible transcription factor 1 (9, 12, 17, 22, 25, 33). VEGF expression is also induced through the action of a number of oncogenes, including activated ras, and is found at increased levels in many solid tumors as well as cell lines derived from them (29, 32).
Each isoform of VEGF occurs via alternative splicing of the full-length mRNA. The variants encode 120-, 164-, and 188-amino-acid proteins (in mouse; the human proteins are one residue longer, and thus produce isoforms of 121, 165, and 189 amino acids, respectively) (11). These differ in their incorporation of exons 6 and 7 of the full-length gene. Each of these exons encodes a cationic domain which confers heparin-binding activity. In VEGF164, the possession of exon 7 results in increased heparin-binding affinity relative to VEGF120; in VEGF188, which contains both exons 6 and 7, there is a dramatic increase in charge and extracellular matrix (ECM) association relative to the other two isoforms. A number of studies have found that the close association of the highly charged forms of VEGF (VEGF188 and VEGF164) with the ECM can be abrogated by the action of proteases (15, 31). This proteolytic resolubilization thus allows them to be released from the ECM and become available ligands for VEGF receptors.
Although the regulation of VEGF expression is becoming well understood, its mode of action in a temporal-spatial context is not; particularly poorly understood is the regulation of expression and distribution of the three primary isoforms. Initial studies of the isoforms demonstrated that they are generally coexpressed in all tissues, with VEGF164 being the predominant form (28, 38). It was also found that there were significant tissue-specific differences in their expression, although without any clear functional pattern related to these differences (2).
The presence of a number of splice variants of VEGF which encode proteins of greatly differing charge and affinity for the ECM allows different models for their function to be developed and tested. Overexpression of each isoform in cell lines and tumors has been shown to result generally in increased angiogenesis, with at least one study demonstrating differential functions during tumorigenesis, i.e., that expression of the smaller isoforms resulted in hemorrhagic events but that expression of VEGF188 resulted in increased vessel density alone (7). The interpretation of this type of experiment is complicated by the continued presence in the experimental cells of the endogenous VEGF gene, which is normally regulated by hypoxia and other microenvironmental factors (36).
Another recent study of VEGF isoform function utilized “knock-in” mutations of the locus to force expression of only one isoform, VEGF120 (5). The phenotype of these animals is postnatal death due to cardiac ischemia; the vascularization of the animals is largely normal, however, up to the time of birth. In the VEGF120 animals created by Carmeliet and coworkers, there was considerable compensatory up-regulation of expression of the single remaining isoform, presumably through feedback via the still-intact endogenous VEGF promoter (5). This resulted in levels of expression three to four times over what is typically seen for VEGF120 as a component of a mixture of isoforms expressed by wild-type cells (5). Thus, for developmental vascularization, increased levels of VEGF120 can largely compensate for the absence of the other isoforms in setting up a vascular network and inducing formation of capillary beds and other VEGF-dependent vascular structures.
We have created H-ras-transformed cell lines which express each variant in isolation and behind a constitutively expressed viral promoter to determine how they separately induce the recruitment and capillary expansion of the systemic vasculature critical for tumorigenesis. We found that expression of each isoform at equivalent levels at the cellular level results in very different levels of local VEGF in tumors; we further show evidence that this dramatically affects tumor vascularization and growth. This supports a model for differential function of the VEGF isoforms during tumorigenic neo-vascularization, in which the more soluble isoform acts at more distal sites to promote vascular recruitment, and the more ECM-associated isoforms act to promote local expansion of capillary beds. Additionally, we provide evidence that both of these functions are critical for rapid expansion of ras-transformed solid tumors.
MATERIALS AND METHODS
Cloning of the VEGF splice variant cDNAs.
Reverse transcription-PCR was performed on total RNA derived from wild-type mouse embryonic fibroblasts according to the manufacturer's protocol (Stratagene). The nucleotide sequences of the primers were 5′-TGGATCCATGAACTTTCTGCT (5′ oligonucleotide) and 5′-GAATTCACCGCCTCGGCTTGTC (3′ oligonucleotide). The first ATG in the 5′ primer corresponds to nucleotide 13 to 15 from GenBank accession no. S37052. Following digestion with restriction endonucleases BamHI and EcoRI, the amplification products were purified and cloned and the resulting plasmid inserts were sequenced and determined to have no errors. The cDNA inserts were then subcloned into the pBABE retroviral DNA vector (18) and an adenoviral DNA vector[pAC-CMVpLpA(SR+)] (26) for use in expression studies.
Cell culture.
Generation of stable cell lines expressing VEGF isoforms was performed as follows: a VEGF-deficient embryonic fibroblast cell line was isolated and oncogenically transformed as previously described (14). Viable retrovirus expressing a VEGF isoform cDNA was obtained by transfection of the insert-containing retroviral vector into BOSC 23 cells, a retrovirus packaging cell line. VEGF-null, transformed fibroblasts were infected with the pBABE retrovirus containing one of the three VEGF isoform cDNA inserts. Infected cells were isolated by their resistance to hygromycin (400 μg/ml), conferred by the retroviral hygromycin resistance gene. Cell growth assays were performed as follows. Cells (2 × 104) were plated onto 37-mm-diameter tissue culture dishes in culture medium (high-glucose Dulbecco's modified Eagle medium [DMEM], 10% fetal bovine serum, glutamine, antibiotics). Cell counts were performed every 24 h in quadruplicate using a hemacytometer (Reichert). Chemotaxis assays were performed over 4 h with a Corning well insert (8-μm diameter), followed by counts of stained cells postfixation on the side opposite seeding. For adenovirus-expressing cell lines, VEGF-deficient cells were infected at a concentration of 100 virions per cell, 24 h before cells were harvested and injected. Persistent VEGF expression was detected at 10 days postinfection; tumors were harvested at 7 to 9 days.
Generation of fibrosarcomas.
A total of 107 cells in 100 μl of DMEM were injected subcutaneously intrascapularly into immunocompromised mice, from either stably or transiently virally infected cells.
Expression analysis and histology. (i) Northern hybridization for quantification of VEGF expression.
Total RNA was isolated from tissue culture cells using TRIzol Reagent (Gibco BRL) according to the manufacturer's protocol. RNA (15 μg) was loaded into each lane of a 1% agarose gel containing formaldehyde. The gel was transferred onto a nylon filter (MSI) and probed with an α-32P-labeled VEGF120 cDNA fragment. To control for loading variation, the filter was also probed with a fragment of GAPDH.
(ii) Metabolic labeling and immunoprecipitation of VEGF isoforms for quantification of VEGF expression.
Cells expressing VEGF isoforms or cells infected with vector alone were grown to confluency in 100-mm-diameter plates in the presence of complete medium. Cells were washed once in phosphate-buffered saline (PBS) and incubated in prewarmed, serum-free DMEM lacking cysteine and methionine for 45 min at 37°C. Cells were then incubated for 3 h in methionine- and cysteine-free DMEM containing 100 μCi of [35S]methionine and 100 μCi of [35S]cysteine (Translabel; ICN) per ml. Complete medium was added to the labeling reaction, and cells were incubated an additional 3 h in the presence of heparin (100 μg/ml; Sigma). An aliquot of conditioned medium from each labeling reaction was removed and cleared overnight at 4°C with 20 μl of protein A-agarose (Santa Cruz Biotechnology). After centrifugation, 10 μg of VEGF polyclonal Ab-3 (NeoMarkers) was added to the supernatant and incubated at 4°C for 3 h. Twenty microliters of protein A-agarose was then added to precipitate the antigen-antibody (Ab) complex for 1 h at 4°C. After centrifugation, the precipitate was washed three times with ice-cold radioimmunoprecipitation assay buffer and the pellet was resuspended in Laemmli sample buffer. Samples were heated to 95°C for 3 min prior to electrophoresis on a 5%–12% denaturing acrylamide gel. Gels were fixed in 5% acetic acid–10% methanol before being dried and exposed by autoradiography.
(iii) Tumor histology: CD31 staining.
Tumor cryosections were fixed in acetone for 10 min prior to staining and then rehydrated through consecutive washes in PBS. Nonspecific staining was blocked by a 30-min incubation with 5% normal rabbit serum. After several PBS rinses, sections were incubated for 2 h at room temperature with rat anti-mouse CD31 Ab (Mec13.3; PharMingen), diluted 1:100 in 3% bovine serum albumin (BSA)–PBS. After several PBS rinses, sections were incubated for 30 min with a biotinylated secondary Ab, rabbit anti-rat immunoglobulin G (Vector Laboratories, Inc.) diluted 1:400 in 3% BSA–PBS. After rinsing in PBS, an avidin-biotinylated horseradish peroxidase complex (Vectastain Elite ABC kit) was used to detect the Ab complex and color was developed with a DAB kit (both from Vector Laboratories, Inc.). Sections were counterstained with Mayer's hematoxylin and mounted.
(iv) Tumor histology: VEGF staining.
Several tumors of each type were harvested and fixed overnight in 4% paraformaldehyde. Tumor blocks were paraffin embedded and cut into 6-μm-thick sections. Slides were deparaffinized in xylene substitute (twice for 10 min) and then transferred to 100% ethanol (twice for 2 min). Sections were rehydrated through consecutively lower concentrations of ethanol washes before being rinsed in PBS. Heat-induced epitope retrieval in EDTA buffer was performed according to the manufacturer's protocol (NeoMarkers). Endogenous peroxidase activity was blocked by a 10-min incubation in 3% hydrogen peroxide, diluted in methanol. Serum blocking was performed for 30 min with 5% normal goat serum diluted in PBS. Sections were incubated for 1.5 h at room temperature with (VEGF) Ab-3 (JH121) (NeoMarkers) mouse monoclonal antibody, diluted 1:100 in 3% BSA–PBS. After several rinses in PBS, sections were incubated for 1 h with a fluorescein isothiocyanate-labeled goat anti-mouse secondary antibody (PharMingen), diluted 1:200 in 3% BSA–PBS. Sections were washed in PBS, counterstained for 20 min with 0.0005% Hoechst 33342, and rinsed several times in PBS before mounting with aqueous medium.
(v) Perfusion analysis.
Tumor-bearing animals were first perfused with a prewarmed heparin-PBS solution to ensure complete flushing of the visceral blood volume. Mice were then infused at physiological pressures with 5.0 ml of a curable yellow latex injection compound, Microfil MV-122 (Microfil). We allowed the curing to proceed overnight before we harvested the tumors along with surrounding skin. The tissue was cleared in increasing concentrations of glycerol, according to the manufacturer's protocol. The vascular architecture was visualized with a color video camera (Sony CCD-IRIS).
RESULTS
Only VEGF164 is able to fully restore tumorigenic capacity to VEGF-null transformed cells.
To determine isoform-specific capacity to restore tumor growth to VEGF-deficient transformed cells, we stably expressed each isoform through the use of retroviral vectors expressing each isoform behind the cytomegalovirus long terminal repeat (Fig. 1a). Transfection was done on clones derived from H-ras- and simian virus 40 large T antigen-transformed cells which contained a loxP-flanked allele of VEGF. These cells were made null for VEGF via cre recombinase expression (14) and then transfected with isoform-specific virus and selected for stable integration and pooled. Cell growth in culture, soft agar colony formation, cellular morphology, focus formation, and chemotaxis were all identical among the wild-type, VEGF-null, and isoform-expressing stably transfected cell lines (Fig. 1c shows cell growth curves; other assays data not shown), indicating that specific expression of each isoform does not alter transformed cell behavior. Due to the possibility that neuropilin-1 expression might alter the behavior of the individual clones, its expression was analyzed in each isoform-specific cell line (Fig. 1d). This indicates that expression was uniform in each cell line. To determine whether this expression altered motility, Boyden chamber assays were performed on VEGF wild-type and VEGF-null cell lines. No differences in motility were seen (data not shown), indicating that in this cell type the expression of VEGF and its action on the neuropilin-1 receptor do not affect motility in culture.
FIG. 1.
Expression levels in VEGF isoform-specific cell lines. (a) Northern blot analysis from retrovirally infected cell lines. Note the similar levels of isoform-specific RNA (5.0 to 5.2 kb). The cellular VEGF RNA (3.6 kb) is slightly smaller in the VEGF-null cell lines compared to that of wild type (WT) (3.7 kb) due to the absence of exon 2. The RNA filter was simultaneously probed with a fragment of GAPDH to control for gel loading differences. (b) Comparison of VEGF protein levels in conditioned medium from cell lines infected with isoform expressing adenoviruses. Cell lines were metabolically labeled and treated with heparin (100 μg/ml). (c) Comparison of cell growth rates of retrovirally infected, isoform-expressing cell lines and controls in tissue culture. (d) Northern blot analysis of neuropilin-1 (NP-1) in VEGF isoform-specific cell lines indicates similar expression levels of this receptor in each cell line used to generate tumors.
Tumor growth of the VEGF wild-type, null, and isoform-expressing stable cell lines was assayed in immunocompromised mice (27). As can be seen in Fig. 2, there was a highly significant reduction in tumor mass caused by lack of VEGF expression, as described previously (10, 14). When stably transfected lines expressing each of the specific isoforms were injected, each had a specific and significant effect on tumor growth. In the case of the VEGF120 isoform, tumor growth was partially but not completely restored to wild-type levels. Interestingly, the intermediate isoform, VEGF164, was able to fully rescue tumor mass, to within a range statistically identical to that seen in wild-type cells; this indicates that in this system this isoform is fully capable of rescuing the essential functions of the wild-type gene during tumor growth.
FIG. 2.
Tumorigenesis assay of VEGF isoform-specific cell lines. Shown is a comparison of the masses of fibrosarcomas generated from isoform-specific, stable cell lines and controls. A total of 107 cells in 100 μl of DMEM were injected subcutaneously, intracapularly into immunocompromised mice. Tumors were harvested and weighed 16 days postinjection (n = 9 animals per cell line). Error bars indicate 1 standard error.
Most strikingly, expression of the isoform with the greatest charge and highest affinity for heparin, VEGF188, was unable to rescue growth of the tumors: their mass was equivalent to that of tumors lacking VEGF expression. This inability of these tumors to grow beyond the rate seen in VEGF-null tumors indicates that they are deficient in some aspect of tumor vascularization induced by tumor cell VEGF expression and that it is a function supplied by the other isoforms of VEGF.
Vascular density induced by VEGF rescue construct expression does not correlate with tumor growth.
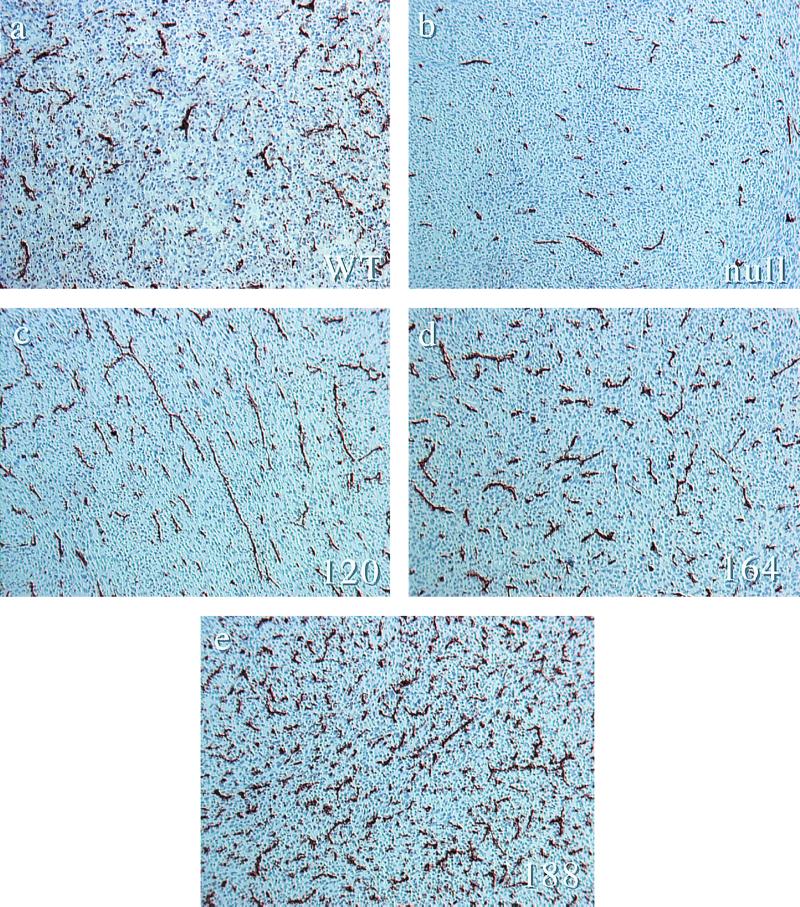
Examination of tumors expressing different VEGF isoforms revealed striking differences in vascularization. As can be seen in Fig. 3, histological examination of the differences between vessel densities shows that the degree of vascularization increases as a function of heparin-binding capacity and that a quantitative measure of this, Chalkley analysis (Fig. 4), shows expected differences between VEGF wild-type and null tumors. VEGF120 tumors demonstrate a significant increase in vascular density compared to null tumors; VEGF164-expressing tumors have an even greater increase in vascular density, one statistically identical to that of wild-type tumors; VEGF188 tumors, however, are hypervascular relative to wild-type tumors and to the other isoform-expressing fibrosarcomas.
FIG. 3.
Immunostaining of vascular endothelium from VEGF isoform-specific fibrosarcomas. (a) Representative anti-CD31 immunohistology of tumor cryosections from wild-type transformed fibroblasts. (b) The same cells following cre-mediated excision of the VEGF gene. (c) VEGF120-expressing tumors. (d) VEGF164-expressing tumors. (e) VEGF-188 expressing tumors. Magnification, ×100.
FIG. 4.
Microvessel density quantification by Chalkley analysis. For each tumor type, 10 representative fields from each of 3 individual tumors were scored for the maximum overlap of stained vessels with the random spot array on the Chalkley graticule. Error bars indicate 1 standard error.
Levels of tumor cell-associated VEGF are dependent on isoform identity.
To determine the role of heparin affinity and charge on cellular localization, we utilized adenoviral vectors to express significant and equivalent amounts of each isoform in VEGF-null cells; this resulted in equivalent amounts of VEGF protein in culture (Fig. 1b). As can be seen in Fig. 5, each isoform's expression results in very different localization in vivo. As expected, there is both vessel-associated VEGF and tumor cell membrane-bound VEGF in the wild-type section (Fig. 5a). In the photomicrograph of the VEGF-null tumor (Fig. 5b), it is apparent that only blood vessels show any expression of VEGF, possibly as an autocrine signal. In the VEGF120 tumor (Fig. 5c), there is striking labeling of the vasculature, but otherwise no local VEGF detectable. Since these cells express VEGF in culture at levels equivalent to those expressed by the other isoforms (Fig. 1), diffusion of the isoform is the most likely explanation for these lowered levels.
FIG. 5.
VEGF immunostaining from paraffin-embedded tumor sections demonstrates relative local concentration of VEGF isoforms. (a) Wild-type control; (b) VEGF-deficient control; (c) VEGF120; (d) VEGF164; (e) VEGF188. Fluorescein isothiocyanate-conjugated secondary Ab recognizes anti-VEGF Ab-3 (JH121). Nuclear counterstain is Hoechst 33342 (blue). Magnification, ×400. Note the high degree of cell-associated VEGF staining in VEGF188-expressing tumors compared to the strictly vessel-associated staining found in VEGF120-expressing tumors.
If this is the case, one would expect to see increased levels of VEGF as charge and heparin affinity increase. This is clearly the case, as demonstrated by the VEGF164 (Fig. 5d) tumors, which show significant labeling of both tumor cells and local vessels.
Most strikingly, the VEGF188 tumors demonstrate the highest local level of expression of any of the isoforms, with a concomitant high degree of tumor cell membrane association (Fig. 5e). This large increase in local concentration of VEGF is in keeping with the high degree of charge and heparin-binding affinity of this molecule and is again independent of expression levels at the cellular level in culture.
Altered concentrations act differentially on tumor vascularization and systemic vessel recruitment.
The expression profiles seen in Fig. 5 could act to alter bioavailability of isoform-specific VEGF to the systemic vasculature. These different local levels could also result in differing effects on vessel capture and endothelial cell proliferation. To determine whether this was in fact the case, we used a method for visualizing tumor vascularization in situ within the tissues of the animal following injection of either stably or transiently VEGF isoform-expressing cells (similar results were seen in both sets of experiments). This required an injection of latex as a perfusion into the left ventricle of anesthetized animals, followed by sacrifice and clearing and mounting of the tumors and surrounding tissues (24). Representative examples of such tissues are found in Fig. 6.
FIG. 6.
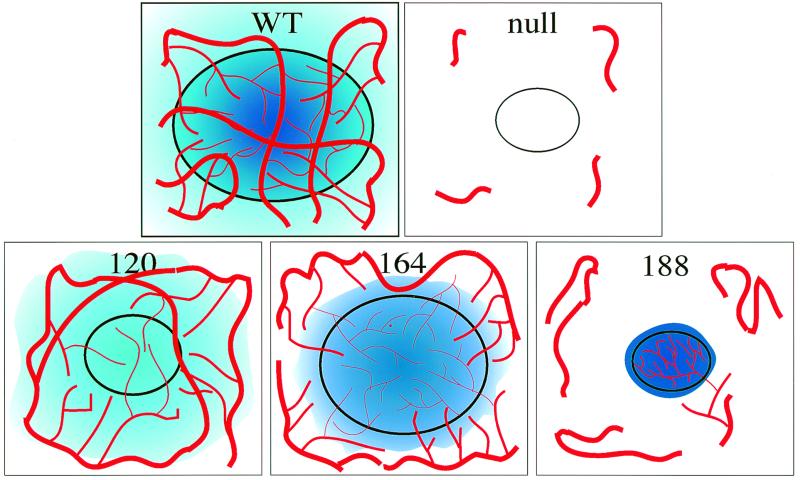
Macroscopic view of tumor vasculature and surrounding stroma. Perfusion of tumor-bearing mice with liquid latex (Microfil) compound results in a three-dimensional cast of major vessels and microvessels. (a and b) Wild-type control tumor at magnifications of ×7 and ×20. (c and d) VEGF-null tumor at similar magnifications. (e and f) VEGF120. Note the extreme degree of host vasculature localized to the periphery of the tumor (arrows) and poor infiltration, coincident with the low local concentration of VEGF in the tumor. (g and h) VEGF164. Note the high degree of systemic vasculature directed to the tumor as well as significant microvasculature. (i and j) VEGF188. Note the presence of fewer systemic vessels directed to the tumor yet many microvessels within the tumor itself.
As can be seen in Fig. 6a, and at higher magnification in Fig. 6b, tumors wild type for VEGF expression are well vascularized and possess both large vessels connecting them to the systemic vasculature and vessels of decreasing diameter connected to extensive internal capillary beds. Although many nonfunctional vessels are typically present in tumors, we assume that the vessels visualized here are at least patent, since visualization occurs via injection of latex into the systemic circulation at a remote site. They should thus be a good representation of the functional vasculature of each tumor.
VEGF-null tumors (Fig. 6c and d) have visibly smaller and less well developed connections to the local vasculature; in addition, there is evidence of reduced vessel branching in these tumors (Fig. 6d). This is as would be expected from the reduced size of these tumors as well as their reduced vessel density (Fig. 4).
Tumors expressing VEGF120 (Fig. 6e and f) effectively capture local vessels as predicted by the increased diffusion of this ligand; a ring of such vessels can be seen encircling the tumor. This highly effective recruitment of systemic vessels is typical of tumors expressing this isoform. However, the VEGF120 tumors do not possess extensive capillary beds and are relatively poorly vascularized internally, as shown by the absence of local capillary beds in the interior of these tumors. The small vessels that are present are largely unbranched (Fig. 6f), with morphologies similar to those seen in VEGF-null tumors.
VEGF164 is capable of inducing both external vessel capture and internal vascular expansion, and this can be seen in Fig. 6g and h, where VEGF164 is shown to phenocopy wild-type VEGF expression-induced vascularization. This result correlates with the intermediate nature of this isoform, enabling both local expression and a diffusible signal. As can be seen in Fig. 6h, there is also a stepped gradation in vessel size in these tumors, with larger vessels feeding into increasingly smaller ones. There are also evident a number of dense capillary beds within the tumor; these are absent from both VEGF-null and VEGF120-expressing tumors.
Tumors expressing VEGF188 (Fig. 6i and j) indicate how functional deficits in vascularization may be caused by high local concentrations of VEGF coupled to a lowered diffusibility of this isoform. The high local densities of VEGF188 present in these tumors (Fig. 6j) correlate with the very high density of vessels seen in CD31-labeled micrographs (Fig. 3) and quantified by Chalkley analysis. The vessels seen in the VEGF188-expressing tumors (Fig. 6j) also lack obvious gradations in vessel diameter seen in vasculature of VEGF wild-type or VEGF164-expressing tumors. Also absent is any obvious recruitment of vessels from neighboring tissues, and unlike the large vessels ringing the VEGF120- or VEGF164-expressing tumors, large vessels appear morphologically unaltered by the presence of VEGF188-expressing tumors (Fig. 6i). This observation combined with the large number of small vessels within the VEGF188 isoform-specific tumor lead us to conclude that the expression of VEGF188 was insufficient for tumor expansion, due in part to an inability to recruit vessels from the local systemic circulation.
Cooperation of VEGF isoforms during tumor expansion.
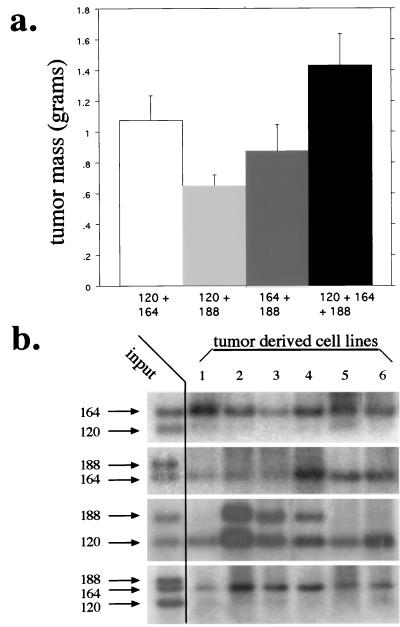
To test the hypothesis that the different isoforms act in concert to organize vessel recruitment and capillary bed expansion, we injected the stably transfected cell lines as mixtures into immunocompromised mice. None of the isoform-expressing lines showed differences in growth rates in culture (Fig. 1), and Southern blots were done to ensure that the mixtures were of equivalent amounts of each cell line with aliquots of cells taken immediately prior to each injection. As can be seen in Fig. 7a, the mixtures containing VEGF164 were the most rapidly expanding. Interestingly, the most successful mixture was the one containing all of the isoforms in combination, indicating that at a functional level, each isoform contributes to the overall success of tumorigenic vascularization.
FIG. 7.
Tumor microenvironment selects for a gradient of VEGF expression. (a) Tumor masses resulting from subcutaneous injection of cell lines composed of every combination of isoform-specific cell lines. Note that there is a slight growth advantage to tumors which express all isoforms. (b) Southern blot analysis of isoform-specific VEGF levels in cell lines harvested from representative tumors in panel a. Input lanes represent the equal contribution from each cell line prior to tumor formation. Note the competitive advantage of VEGF164-expressing cells and the decrease in selective pressure in tumors expressing only VEGF120 and VEGF188, indicating that the tumor requires both soluble and cell-associated VEGF.
As mentioned above, at the initial stages of tumor growth, each of the isoform-specific populations was equivalently represented. We also wished to determine the extent to which each isoform was present at the stage at which the tumors were harvested, in order to estimate the importance of continued expression of each isoform to an established tumor. As can be seen in Fig. 7b, in each case in which VEGF164-expressing cells were present, it was the predominantly represented isoform at the end stage of tumor growth. Since the other isoforms would not be detected below approximately 5% representation due to the limitations of the Southern blot detection levels, this indicates that VEGF164 represents >95% of the end stage tumor mass. This indicates that at this stage of the tumors development selective pressure favors cells expressing this intermediate isoform. This implies that the growth advantage for tumors containing all of the isoforms (seen in Fig. 7a) occurs at an earlier stage of the tumors expansion, perhaps during the initial establishment of its vascular structure.
The only case where there is clear evidence at the genetic level for collaboration or cooperation of the isoforms is in the mixture of cell lines expressing VEGF120 and VEGF188. Here half of the tumors are comprised of an equivalent number of cells expressing each isoform. This is very intriguing, as the evidence from our other experiments indicates that these two isoforms act in a nonoverlapping and possibly complementary manner. The evidence here lends support to the idea that each of these isoforms acts specifically to recruit systemic vasculature, in the case of VEGF120, and induce intratumoral capillary expansion, in the case of VEGF188. Further, since this is the only mixture in which there is evidence for a selective advantage of coexpression even at a late stage of tumor growth, this result implies that the presence of both activities of VEGF, distal and local, remains important throughout the lifespan of a solid tumor.
DISCUSSION
The specific role played by each isoform of VEGF in vivo is likely a very complex interplay of differential effects. In turn, these are presumably different in the wide variety of differing microenvironments that occur in human tumorigenesis. We have used a simple but powerful genetic system to dissect the specific roles of each of the isoforms of VEGF. We have shown in the present study that the specific expression of each isoform in this murine system results in very significant alterations in tumor growth and vascular density, although these two are surprisingly not completely correlated.
Many tumors and tumor cell lines have been shown to express the neuropilin-1 receptor. We have also found significant levels of expression of this receptor on the ras-transformed cells generated for this study (Fig. 1d). However, we did not find significant differences in cell growth, motility, or soft agar colony formation between VEGF wild-type and VEGF-null cells using serum-free media (J.G. and R.S.J., unpublished data [not shown]), indicating that if expression of this receptor has functional implications for transformed cell growth, they are not apparent in cell culture systems. That does not preclude them from being important factors in tumor growth, and experiments with transformed cells nullizygous for neuropilin-1 should help to elucidate this issue.
Interestingly, the VEGF164 isoform most completely phenocopies wild-type expression of VEGF in terms of tumor growth, local densities of VEGF, and vascular density and morphology. This is what would have been predicted from numerous studies of the isoforms and fits with its predominance in studies of isoform-specific expression patterns. There is some evidence in our data for a selective advantage in expression of all of the isoforms: when cells expressing all of the isoforms were mixed, it results in tumors larger than those produced from any single partial mixture of the isoforms. This indicates that expression of all of the isoforms has some advantage in solid tumor growth over expression of any one individually. Studies of human tumors have shown that it is indeed the case that variations in isoform expression occur from tumor type to tumor type and among clinically similar tumors (29). This may be an indication that variation in expression confers differential advantage on tumors as they expand in different sites, each possessing differing requirements for neovascularization.
Interestingly, our data indicate that VEGF188 expression in tumors results in a striking paradox: relatively retarded tumor growth coupled to relative hypervascularization. These same tumors have the highest local levels of VEGF. The tumors expressing VEGF188 show no evidence of successful recruitment of large vessels surrounding the body of the tumors themselves. Rather, they are virtually filled with small convoluted vessels distinctly different in morphology from those seen as a result of expression of the other isoforms. Our conclusion is that these vessels are likely to be functionally less important than vessels perfused by the larger number of connections seen in tumors expressing the other two isoforms.
It is important, however, to note that the high local VEGF levels and membrane association of the heparin-binding forms of VEGF have functional importance. Evidence for this is seen in the decreased internal vascular density of VEGF120-expressing tumors and in the evident cooperativity in VEGF120 and VEGF188 expression, demonstrated by their coselection in the mixing experiments shown in Fig. 7. These data would suggest a split in the roles that the VEGF isoforms generally play, where VEGF120 is used to recruit vessels to the site of expression and VEGF188 is used to induce locally elevated expression, in turn allowing elevated expansion of capillary beds.
Our findings demonstrate that the VEGF isoforms each contribute differentially to the process of tumor vascularization. A model which accounts for these observations is depicted in Fig. 8. Our model argues that at least in part, the greater diffusibility of the most soluble isoform, VEGF120, allows it to effectively recruit vessels at some remove from effector cells but also means that for a fixed amount of effector molecule, there will be lower local concentrations in the immediate vicinity of the secreting cell or tissue. The opposite is then true for the highly charged and ECM-associated VEGF188 isoform, which achieves high local concentrations without disseminating significantly towards the distal vasculature. This property can explain the inability of VEGF188 tumors to expand at the rates seen when other isoforms are used to rescue VEGF function: inability to coopt and recruit sufficient connections to the systemic circulation ultimately makes the high internal capillary densities found in these tumors less effective. These findings imply that signaling via VEGF acts in a bimodal fashion within the three-dimensional matrix of tumors, using association with the ECM to accomplish organization, arborization, and effective function during neovascularization.
FIG. 8.
Proposed gradient model of tumorigenic VEGF signaling. VEGF120 produces a diffuse signal (light blue) which recruits peripheral vessels but does little to vascularize the tumor itself. VEGF164 can both recruit vessels with a partially diffusible signal and vascularize the tumor with a partially cell-associated signal. VEGF188 fails to adequately recruit the host vasculature, but vascular endothelium which is captured forms a hypervascular capillary network due to the high local concentration of VEGF.
ACKNOWLEDGMENTS
We acknowledge Wayne McNulty for technical assistance and members of the Johnson laboratory for helpful discussions and comments.
REFERENCES
- 1.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacic M, Edwards N A, Merrill M J. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissues. Growth Factors. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]
- 3.Banga J D. Therapeutic angiogenesis through intramuscular injection of the gene for vascular endothelial growth factor (VEGF) Ned Tijdschr Geneeskd. 2000;144:113–116. . (In Dutch.) [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ng Y S, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar V V, Stalmans I, Mattot V, Perriard J C, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D'Amore P A, Shima D T. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 6.Chawla P S, Keelan M H, Kipshidze N. Angiogenesis for the treatment of vascular diseases. Int Angiol. 1999;18:185–192. [PubMed] [Google Scholar]
- 7.Cheng S Y, Nagane M, Huang H S, Cavenee W K. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci USA. 1997;94:12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claffey K P, Shih S C, Mullen A, Dziennis S, Cusick J L, Abrams K R, Lee S W, Detmar M. Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell. 1998;9:469–481. doi: 10.1091/mbc.9.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damert A, Machein M, Breier G, Fujita M Q, Hanahan D, Risau W, Plate K H. Up-regulation of vascular endothelial growth factor expression in a rat glioma is conferred by two distinct hypoxia-driven mechanisms. Cancer Res. 1997;57:3860–3864. [PubMed] [Google Scholar]
- 10.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Houck K A, Jakeman L B, Winer J, Leung D W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 12.Forsythe J A, Jiang B H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier E, Birnbaum D, Borg J P. Receptors for factors of the VEGF (vascular endothelial growth family) Bull Cancer. 1997;84:397–405. . (In French.) [PubMed] [Google Scholar]
- 14.Grunstein J, Roberts W G, Mathieu-Costello O, Hanahan D, Johnson R S. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999;59:1592–1598. [PubMed] [Google Scholar]
- 15.Houck K A, Leung D W, Rowland A M, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 16.Ikeda E, Achen M G, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- 17.Iyer N V, Kotch L E, Agani F, Leung S W, Laughner E, Wenger R H, Gassmann M, Gearhart J D, Lawler A M, Yu A Y, Semenza G L. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16:4504–4511. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 20.Keck P J, Hauser S D, Krivi G, Sanzo K, Warren T, Feder J, Connolly D T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 21.Kolch W, Martiny-Baron G, Kieser A, Marme D. Regulation of the expression of the VEGF/VPS and its receptors: role in tumor angiogenesis. Breast Cancer Res Treat. 1995;36:139–155. doi: 10.1007/BF00666036. [DOI] [PubMed] [Google Scholar]
- 22.Levy A P, Levy N S, Goldberg M A. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 23.Levy A P, Levy N S, Wegner S, Goldberg M A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 24.Lin G, Boijsen E, Hägerstrand I, Lunderquist A. Microvasculature architecture of small liver metastases in man. A correlation between microfil vascular preparations and histologic sections. Investig Radiol. 1984;19:296–302. doi: 10.1097/00004424-198407000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Cox S R, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 26.Lu C Y, Giordano F J, Rogers K C, Rothman A. Adenovirus-mediated increase of exogenous and inhibition of endogenous fosB gene expression in cultured pulmonary arterial smooth muscle cells. J Mol Cell Cardiol. 1996;28:1703–1713. doi: 10.1006/jmcc.1996.0160. [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 28.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 29.Okada F, Rak J W, Croix B S, Lieubeau B, Kaya M, Roncari L, Shirasawa S, Sasazuki T, Kerbel R S. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto K, Oshika Y, Fukushima Y, Ohnishi Y, Tokunaga T, Tomii Y, Kijima H, Yamazaki H, Ueyama Y, Tamaoki N, Nakumura M. Xenografts of human solid tumors frequently express cellular-associated isoform of vascular endothelial growth factor (VEGF) 189. Oncol Rep. 1999;6:1201–1204. doi: 10.3892/or.6.6.1201. [DOI] [PubMed] [Google Scholar]
- 31.Park J E, Keller G A, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel R S. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 33.Ryan H E, Lo J, Johnson R S. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senger D R, Galli S J, Dvorak A M, Perruzzi C A, Harvey V S, Dvorak H F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 35.Shima D T, Deutsch U, D'Amore P A. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- 36.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 37.Soker S, Takashima S, Miao H Q, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 38.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]