Introduction
Leukocyte adhesion deficiency type 1 (LAD-1) is a genetically determined loss or defect of integrin β2 (CD18) on leukocytes,1,2 which leads to impaired leukocyte migration, particularly of neutrophils, and manifests as abnormal pus formation, poor wound healing, and persistent neutrophilia. Mortality rates among juveniles are high, as affected patients are more likely to experience recurrent and severe bacterial and fungal infections. The only curative approach is hematopoietic stem cell transplantation (HSCT), which, however, is limited by transplant-associated toxicity and graft-versus-host disease.1, 2, 3 In this case report, we describe the natural course of severe LAD-1 in a patient who reached adult age without HSCT and with a high quality of life.
Case report
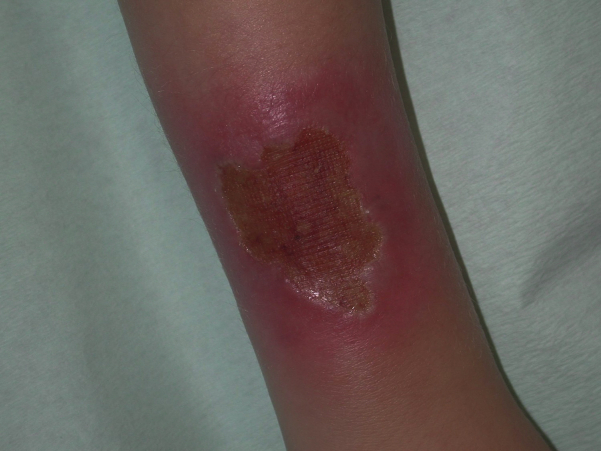
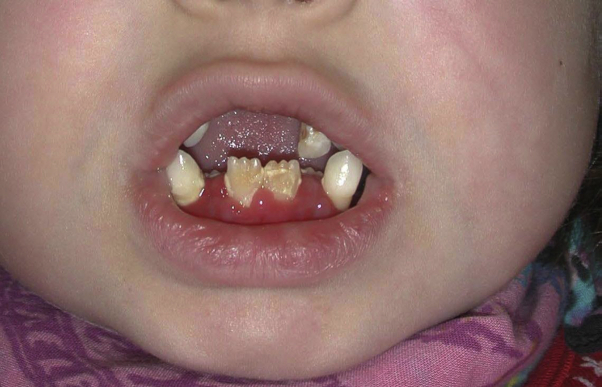
We report a case of a 24-year-old female patient with LAD-1 who has been monitored since 2002. At the age of 6, the patient first presented to our department with a wound on her right arm that did not heal despite intensive topical therapy for 4 weeks (Fig 1). Clinical examination revealed progressive periodontitis and gingivitis (Fig 2).
Fig 1.
Chronic wound on the right forearm of the 6-year-old patient.
Fig 2.
Gingivitis in the 6-year-old patient.
The patient's clinical history included one episode of severe facial soft tissue infection and consecutive surgical intervention at the age of 3. Laboratory testing revealed leukocytosis. Abdominal ultrasound and chest radiography did not result in any pathologic findings. Skin biopsy revealed nonspecific changes.
Impaired wound healing accompanied by advanced periodontitis and leukocytosis led us to suspect LAD-1; this was confirmed by flow cytometry, which showed low CD18 expression on leukocytes. No matching bone marrow donor was available; we thus proceeded without allogeneic HSCT and with frequent monitoring. The wound was treated with topical granulocyte-macrophage colony-stimulating factor, which induced healing over 8 weeks. Over the following years, the patient sustained recurrent wounds following minimal trauma, such as insect bites (Fig 3). Therapy included parenteral antibiotics (depending on skin cultures) and intravenous immunoglobulins (2 g/kg body weight), resulting in prompt wound healing. Apart from recurrent, manageable wounds, the patient leads a high-quality life as a college student.
Fig 3.
Inflamed ulcer on the left shoulder of the 9-year-old patient.
The surveillance schedule included visits to our dermatological and hematological outpatient clinic every 6 months, vaccinations against viral infections to prevent bacterial superinfections, genetic counseling, intensive skin disinfection before blood sampling, and administration of high doses of antibiotics (parenteral) and immunoglobulins in cases of severe infection.
Discussion
LAD-1 is a rare (incidence < 1:1 million), autosomal, recessive primary immunodeficiency disorder caused by a mutation in the integrin β2 (ITGB2) gene on chromosome 21q22.3, which encodes the CD18 leukocyte antigen (ITGB subunit).1,2 LAD-1 was first described in 1980, and since then, approximately 300 patients with LAD-1 have been reported worldwide. More than 80 mutations of the ITGB2 gene have been reported to lead to severe (<2% expression of β2 integrins) or moderate (2%-30% expression of β2 integrins) LAD-1 phenotypes.1 Mutations very rarely produce nonfunctional but normally expressed β2 integrins.2
Patients with LAD-1 have high mortality rates at a young age because of necrotizing enterocolitis, pneumonia, and respiratory or cardiac failure. HSCT is the only curative treatment in early childhood.3 Mortality is highest in patients with severe LAD-1, who did not receive HSCT (61%-75% at the age of 2 years).4
Our patient expressed <2% CD18 on her leukocytes, compared with healthy individuals. Molecular analysis revealed that she was a compound heterozygote with 2 novel mutations, which we had previously reported, and which were present in both of the patient's parents.5 There is no known correlation between these novel mutations and the severity of LAD-1.
As our patient with LAD-1 expressed <2% of CD18 molecules on her leukocytes and reached adulthood with a high quality of life without receiving HSCT, this raises the question of whether LAD-1 severity is influenced not only by mutations in the ITGB2 gene but also by other factors. For example, LAD-1 patients with a mild phenotype who survive infancy show severe progressive periodontitis with tooth loss. Recent studies showed that periodontitis in LAD-1 patients is dominated by the infiltration of T helper 17 cells and overproduction of interleukin (IL-) 23 and IL-17.6,7 In healthy individuals, IL-23 production by macrophages is regulated by neutrophils in tissues. In LAD-1 patients, neutrophils in tissues are lacking, which leads to overproduction of IL-23 and downstream cytokines. A recent study reported healing of chronic ulcers and improved periodontitis in a patient with LAD-1 after one year of therapy with ustekinumab. Beneficial effects were attributed to blocking of IL-23 and inhibition of IL-23-dependent production of IL-17.8 Thus, LAD-1 disease appears to change its character from life-threatening infections in childhood to chronic inflammatory disease in adulthood, mediated by IL-23 and IL-17. In addition to mutations in the ITGB2 gene, other genetic factors regulating IL-23 and IL-17 may also affect the severity of LAD-1. Inflammatory processes mediated by IL-23 and IL-17, such as periodontitis, which was strongly manifested in our patient during childhood, may have been a positive prognostic indicator in this patient reaching adulthood.
We observed high efficacy of parenteral immunoglobulins for wound healing in our patient with LAD-1, which is in line with previously published reports.9 Moreover, parenteral immunoglobulins accelerated wound healing in our patient, compared with treatment with parenteral antibiotics (days versus weeks, respectively). Topical granulocyte-macrophage colony-stimulating factor administration was also effective in our LAD-1 patient. It was suggested that topical granulocyte-macrophage colony-stimulating factor administration supports wound healing due to induction of cytokines (IL-1, tumor necrosis factor, and macrophage colony-stimulating factor) and stimulation of monocytes and macrophages.10
In summary, in children, chronic wounds accompanied by leukocytosis and periodontitis are “red flags” with respect to the possible diagnosis of LAD-1. Our patient with LAD-1expressed CD18 molecules on leukocytes at less than 2% and survived infancy with a good quality of life without HSCT. Periodontitis during childhood may have been a positive prognostic indicator in our patient. Early antibiotic therapy for wound infection and additional therapy with parenteral immunoglobulins for more severe wounds are crucial for LAD-1 management and patient survival. Further studies are needed to examine the role of IL-23 and IL-17 for LAD-1 severity.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Crowley C.A., Curnutte J.T., Rosin R.E., et al. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- 2.Hogg N., Stewart M.P., Scarth S.L., et al. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J Clin Invest. 1999;103(1):97–106. doi: 10.1172/JCI3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qasim W., Cavazzana-Calvo M., Davies E.G., et al. Allogeneic hematopoietic stem-cell transplantation for leukocyte adhesion deficiency. Pediatrics. 2009;123(3):836–840. doi: 10.1542/peds.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almarza Novoa E., Kasbekar S., Thrasher A.J., et al. Leukocyte adhesion deficiency-I: a comprehensive review of all published cases. J Allergy Clin Immunol Pract. 2018;6(4):1418–1420.e10. doi: 10.1016/j.jaip.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Bernard Cher T.H., Chan H.S., Klein G.F., et al. A novel 3′ splice-site mutation and a novel gross deletion in leukocyte adhesion deficiency (LAD)-1. Biochem Biophys Res Commun. 2011;404(4):1099–1104. doi: 10.1016/j.bbrc.2010.12.124. [DOI] [PubMed] [Google Scholar]
- 6.Hajishengallis G., Moutsopoulos N.M. Etiology of leukocyte adhesion deficiency-associated periodontitis revisited: not a raging infection but a raging inflammatory response. Expert Rev Clin Immunol. 2014;10(8):973–975. doi: 10.1586/1744666X.2014.929944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moutsopoulos N.M., Konkel J., Sarmadi M., et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 2014;6(229):229ra40. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moutsopoulos N.M., Zerbe C.S., Wild T., et al. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med. 2017;376(12):1141–1146. doi: 10.1056/NEJMoa1612197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki-Nakashimada M., Maravillas-Montero J.L., Berrón-Ruiz L., et al. Successful adjunctive immunoglobulin treatment in patients affected by leukocyte adhesion deficiency type 1 (LAD-1) Immunol Res. 2015;61(3):260–268. doi: 10.1007/s12026-014-8619-8. [DOI] [PubMed] [Google Scholar]
- 10.De Ugarte D.A., Roberts R.L., Lerdluedeeporn P., Stiehm E.R., Atkinson J.B. Treatment of chronic wounds by local delivery of granulocyte-macrophage colony-stimulating factor in patients with neutrophil dysfunction. Pediatr Surg Int. 2002;18(5-6):517–520. doi: 10.1007/s00383-002-0733-3. [DOI] [PubMed] [Google Scholar]