Abstract
BACKGROUND:
Neonatal tracheal intubation (TI) is a high-risk procedure associated with adverse safety events. In our newborn and infant ICU, we measure adverse tracheal intubation–associated events (TIAEs) as part of our participation in National Emergency Airway Registry for Neonates, a neonatal airway registry. We aimed to decrease overall TIAEs by 10% in 12 months.
METHODS:
A quality improvement team developed an individualized approach to intubation using an Airway Bundle (AB) for patients at risk for TI. Plan-do-study-act cycles included AB creation, simulation, unit roll out, interprofessional education, team competitions, and adjusting AB location. Outcome measure was monthly rate of TIAEs (overall and severe). Process measures were AB initiation, AB use at intubation, video laryngoscope (VL) use, and paralytic use. Balancing measure was inadvertent administration of TI premedication. We used statistical process control charts.
RESULTS:
Data collection from November 2016 to August 2020 included 1182 intubations. Monthly intubations ranged from 12 to 41. Initial overall TIAE rate was 0.093 per intubation encounter, increased to 0.172, and then decreased to 0.089. System stability improved over time. Severe TIAE rate decreased from 0.047 to 0.016 in June 2019. AB initiation improved from 70% to 90%, and AB use at intubation improved from 18% to 55%. VL use improved from 86% to 97%. Paralytic use was 83% and did not change. The balancing measure of inadvertent TI medication administration occurred once.
CONCLUSIONS:
We demonstrated a significant decrease in the rate of severe TIAEs through the implementation of an AB. Next steps include increasing use of AB at intubation.
Neonatal tracheal intubation (TI) is a life-saving and high-risk procedure. Factors associated with neonatal TI success and safety include airway provider experience, premedication, and video laryngoscope (VL).1-5 Despite identification of these factors, neonatal TI success and safety outcomes are suboptimal. Reported first attempt success rates range from 30% to 57%,6-10 and adverse neonatal TI event rates range between 9% and 50%.3,6,9,11
The National Emergency Airway Registry for Neonates (NEAR4NEOS) was developed in 2014 to understand the process and safety of neonatal TI. NEAR4NEOS is an international multicenter neonatal airway registry modeled after National Emergency Airway Registry and National Emergency Airway Registry for Children.3 As a safety metric, NEAR4NEOS reports tracheal intubation–associated events (TIAEs).
At the Children’s Hospital of Philadelphia (CHOP) newborn and infant ICU (N/IICU), we measure TIAEs through participation in NEAR4NEOS. Through NEAR4NEOS, we became aware of the need for improved TI safety as we measured direct patient harm during TI. In response, we developed a quality improvement (QI) initiative in the CHOP N/IICU to address TI safety. Our rate of TIAEs was 0.093 per TI encounter at the start of this project, and the most frequent TIAEs were dysrhythmia (including heart rate <60 beats per minute without chest compressions) and esophageal intubation. The project goal was to reduce TIAEs through evidence-based interventions and improved teamwork.
METHODS
Specific Aim
Our SMART (Specific, Measurable, Applicable, Realistic, and Timely) aim was to reduce the rate of TIAEs by 10% in 12 months from 0.093 to 0.084.
Context
The CHOP N/IICU is a 100-bed level IV referral unit with >1200 admissions per year, including 500 deliveries per year in the special delivery unit for patients with known congenital anomalies. This project only included N/IICU TIs. The N/IICU is staffed by >600 providers from >15 health care professions. The unit is composed of 6 teams: a surgical team, a chronic lung team, and 4 medical teams. Patients range from extremely preterm to 1 year old, and diagnoses include complications of prematurity, surgical congenital anomalies, and chronic lung disease. Approximately 25 TIs per month occur within the N/IICU. Fellows and front line clinicians (nurse practitioners, physician assistants, hospitalists, and residents) routinely perform TIs. Each provider is limited to 1 to 2 attempts and use of VL (C-MAC; Karl Storz SE & Co, Tuttlingen, Germany) and premedication, including paralytic, are strongly encouraged. Attending physicians supervise the majority of intubations. An airway response team, composed of anesthesia and otolaryngology, is available for airway emergencies.
Each NEAR4NEOS site collects data for all TIs through self-report. Data include patient, provider, and TI characteristics, including success and safety outcomes. NEAR4NEOS classifies TIAEs as severe and nonsevere (Table 1).3 Within the CHOP N/IICU, respiratory therapists (RTs) complete the NEAR4NEOS form immediately after TI with team input.
TABLE 1.
Types of Adverse TIAEs
| Severe TIAE | Nonsevere TIAE |
|---|---|
| Cardiac arrest, patient died | Dysrhythmiaa |
| Cardiac arrest, patient survived | Emesis without aspiration |
| Chest compressions <1 min | Epistaxis |
| Delayed recognition esophageal intubation | Immediate recognition esophageal intubation |
| Direct airway injury | Hypertension requiring intervention |
| Emesis with aspiration | Lip trauma |
| Gum or dental trauma | Mainstem intubation |
| Hypotension requiring intervention | Medication error |
| Laryngospasm | Pain or agitation requiring additional medication causing delay |
| Malignant hyperthermia | — |
| Pneumothorax and/or pneumomediastinum | — |
Information from Foglia et al, 2019.3 —, not applicable.
Includes heart rate <60 beats per minute without chest compressions.
Planning and Development of the Airway Bundle
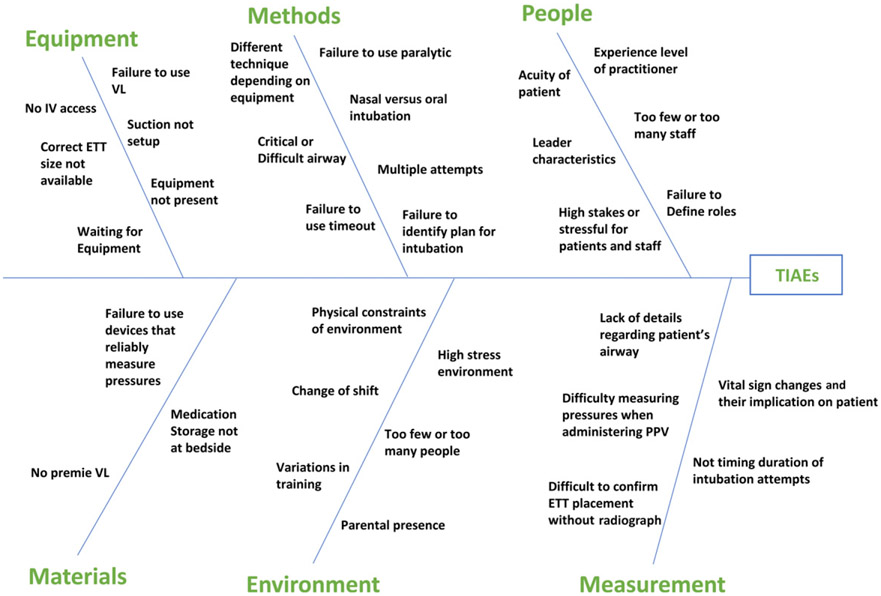
We created an interprofessional working group including attending physicians, fellows, front line clinicians, nurses, RTs, and QI experts. After creating a TIAE fishbone diagram (Fig 1) and reviewing available literature that demonstrated improved safety and success with use of premedication and VL, we developed a driver diagram identifying 4 primary drivers for reducing TIAEs: (1) risk assessment, (2) team preparation, (3) premedication, and (4) team communication (Fig 2).1-5
FIGURE 1.
Fishbone diagram. ETT, endotracheal tube; IV, intravenous; PPV, positive pressure ventilation.
FIGURE 2.
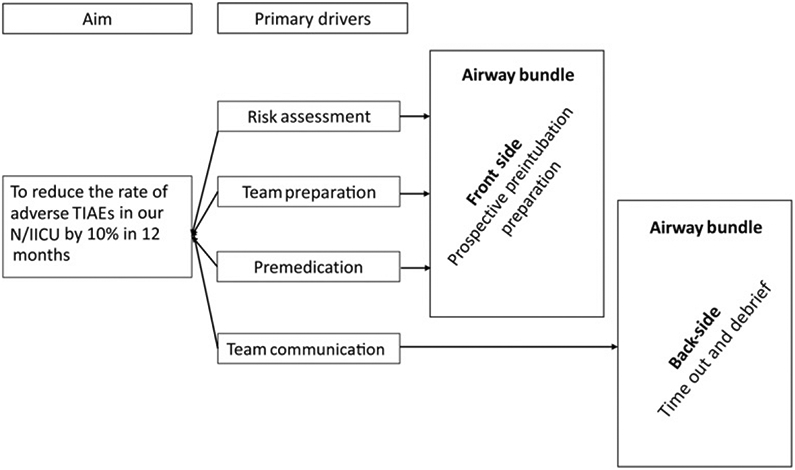
Driver diagram.
To address these drivers, we developed an Airway Bundle (AB; Supplemental Fig 9), modeled after the CHOP PICU AB.12,13 Because a substantial proportion of our N/IICU population is admitted for nonrespiratory diagnoses, we took a selective approach to identify at-risk patients. We used the presence of an AB at the bedside as a method to prospectively identify at-risk patients, addressing our first primary driver. At-risk patients were defined as intubated patients, patients with a planned intubation, recently extubated patients, patients with increasing respiratory support, and any patient the team deemed at risk. The AB further stratified patient risk through assessing risk factors for a difficult intubation or difficult bag mask ventilation.
The second section of the AB addressed team preparation: identification of an intubation threshold for nonintubated patients, need for endotracheal tube replacement after an unplanned extubation, equipment for intubation (emphasizing use of VL), and whether the patient needed a specialized airway provider (anesthesia or otolaryngology). This section also addressed premedication.
As part of the AB, we created an intubation premedication order set, which included atropine (0.02 mg/kg), fentanyl (2 μg/kg), and vecuronium (0.1 mg/kg). The team was expected to order premedication for all patients with an AB as “ON CALL PRN” to avoid medication delays at time of intubation. The medication section asked providers to identify which medications to use, confirm premedications were ordered, and identify contraindications to premedication.
The back side of the AB was designed for use at intubation and addressed the final primary driver, team communication. This primarily focused on performing a standardized time out before intubation. We created a time out card that was separate from the AB (Supplemental Fig 10). The final section of the AB encouraged postintubation debriefing reviewing primary drivers: (1) Was the AB filled out before intubation? (2) Was VL used? (3) Was paralysis used? and (4) Was the time out card used?
The AB and time out card were hung in a prominent orange folder at the bedside of any at-risk patient to aid with both preintubation preparation and use at intubation.
Interventions and Plan-Do-Study-Act Cycles
Designing the AB was our initial plan-do-study-act (PDSA) cycle. We tested and refined the AB through multiple rounds of simulation. After simulation, we trialed the AB in a 25-bed section of our unit. After 3 months of weekly rounding, including nursing and medical team education, we expanded the AB to an additional section of the unit and then the entire unit 2 months later. With the unit-wide roll out, we added AB-specific questions to the clinical care questions asked on rounds targeting QI efforts and harm reduction. We continued weekly rounding and education as we expanded to the entire unit.
After noticing minimal change in both initiation of the AB and use of the AB at time of intubation, we increased interprofessional education including in-person and e-mail education for all stakeholders. We instituted a unit-wide competition, rewarding the medical team and nursing unit with the highest AB initiation rate for at-risk patients with donuts. We also moved the time out cards to a more prominent location on the VL cart. We performed another round of interprofessional education when we asked teams to review and update the AB on a weekly basis, documenting the review date. On the basis of staff concerns that the AB location on the bedside cart was not in the line of sight of clinicians, we trialed moving the AB to the vitals monitor to hang with the code medication sheet. This was quickly abandoned because this created unforeseen workflow issues for RTs. Because RTs often work in multiple units, this location was different from the PICU and lead to confusion. Additionally, the monitor was less accessible to RTs, especially during an emergency. This test of change led to renewed interest and increased buy-in by the clinical RT team. As a result, AB posters were placed in each RT workroom, and RTs were empowered to encourage time out card use and completion of the backside of the AB after intubation. Last, RTs were reminded to collect the completed AB and to ask the team to initiate a new AB after intubation.
When clinical care questions were integrated into the electronic medical record (EMR), we transitioned from tracking AB initiation during weekly in-person rounding to provider self-report measured through weekly AB clinical care questions.14
The AB underwent 13 rounds of modifications (Table 2). Modifications included removing time out instructions and creating a separate time out card and adding difficult bag mask ventilation assessment, need for reintubation after unplanned extubation, intramuscular premedication dosing for difficult intravenous (IV) access, premedication dosing for opioid tolerant patients, and revision date to encourage weekly premedication dose and equipment updates as neonates grew.
TABLE 2.
Time line of PDSA Cycles and Interventions
| Date | PDSA or Intervention | AB Version |
|---|---|---|
| September 2017 | AB Creation | 1 |
| October 2017 | AB Simulations | — |
| November 2017 | Piloted AB in 25-bed section of unit | 2 |
| February 2018 | Expanded AB to second section of unit | 3–4 |
| April 2018 | Expanded AB to entire unit; AB clinical care question created | 5–6 |
| November 2018 | Interprofessional education; donut competition; time out card moved to VL | 7–9 |
| February 2019 | Interprofessional education; AB changed to include date reviewed by team | 10 |
| February 2019 | Piloted location change of AB to vitals monitor in 25-bed section of unit | — |
| March 2019 | AB location moved to vitals monitor for entire unit | — |
| April 2019 | AB moved back to bedside cart; increased RT involvement | — |
| November 2019 | Clinical care questions moved to EMR; AB initiation data collected from EMR | 11–12 |
| January 2020 | Unplanned extubation QR code added to back of AB | 13 |
QR, quick response. —, not applicable.
Measures
Data were collected from NEAR4NEOS forms, in-person rounding, self-report, and the EMR.
The outcome measure was rate of TIAEs per intubation encounter reported monthly. An intubation encounter is an episode of airway management including all intubation attempts.3 TIAEs were defined according to NEAR4NEOS and reported as rates of overall and severe TIAEs (Table 1).3 We reported all TIAEs during an encounter, thus 1 encounter could have multiple TIAEs. To compare our results with those reported in the literature, we performed a post hoc analysis reporting monthly percentage of TI encounters with a TIAE as a binary outcome of yes or no, regardless of number of TIAEs in an encounter.
We monitored 4 process measures based on the driver diagram: initiation of AB for at-risk patients, AB use at intubation, VL use, and paralytic use. AB initiation was defined as completion of the front side of the AB, detailing a prospective individualized TI plan. Goals for AB initiation and use at intubation were 80%. Initiation of AB was audited on a weekly basis, initially through in-person rounding, and was reported by the number of patients with an AB divided by the number of at-risk patients as previously defined. We changed auditing mechanisms in November 2019 when clinical care questions were integrated into the EMR. We thus relied on provider self-report of whether at-risk patients had an AB. AB initiation was calculated by the number of reported ABs divided by the number of reported ABs plus the number of intubated patients who did not have a reported AB (either the question was not completed or answered as no). Use of the AB and time out card at intubation was measured through completion of the back side of the AB. This form was submitted with the NEAR4NEOS form and reported monthly. Use of VL and paralytic were extracted from NEAR4NEOS data monthly and were reported per course. A course is defined as 1 method for intubation, including device, approach, and premedication. If device, approach, or premedication were changed during an encounter, this constituted a new course within the same encounter.3
Given intubation premedication was ordered “ON CALL PRN,” a balancing metric was inadvertent administration of premedication. We audited this through in-person rounding and monitoring the safety event reporting system.
Analysis
Data were analyzed by using QI Macros 2019 (KnowWare International, Inc, Denver, CO) and interpreted initially by using run charts followed by statistical process control charts (SPCCs) once enough data points were available. SPCCs were annotated with PDSA cycles, interventions, and goal lines. Shewhart chart methodology was used to interpret and analyze SPCCs. We adjusted centerlines according to special cause rules for variation.15 We performed a post hoc analysis of patient characteristics who underwent TI by 12 month epochs to explore changes in patient characteristics over time. The article was written following the Standards for Quality Improvement Reporting Excellence 2.0 guidelines.16
Ethics
This project was deemed exempt by CHOP Institutional Review Board.
RESULTS
Data collection occurred from November 2016 through August 2020 for TIAE rate and use of paralytic and VL by using NEAR4NEOs data. Data collection for AB initiation, use at intubation, and inadvertent administration of premedication started November 2017 when the AB was piloted. The initial AB was designed in September of 2017 with subsequent PDSA cycles implemented over time (Table 2).
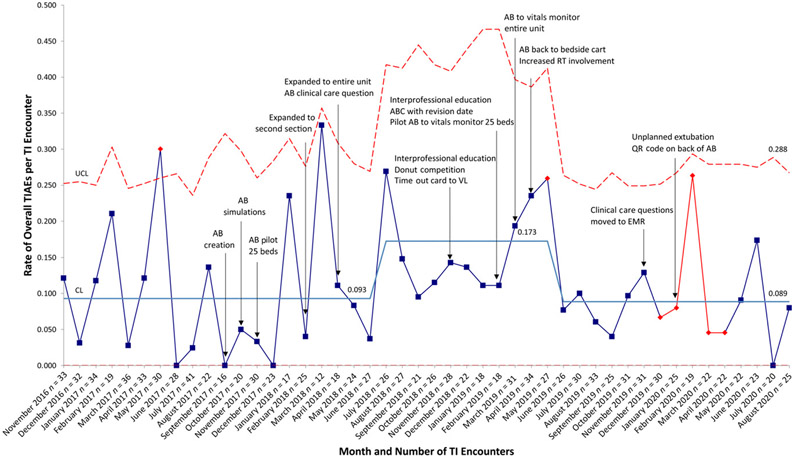
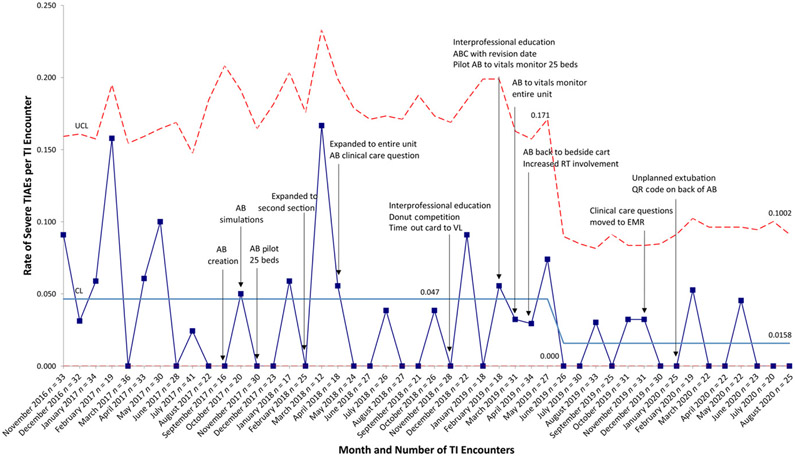
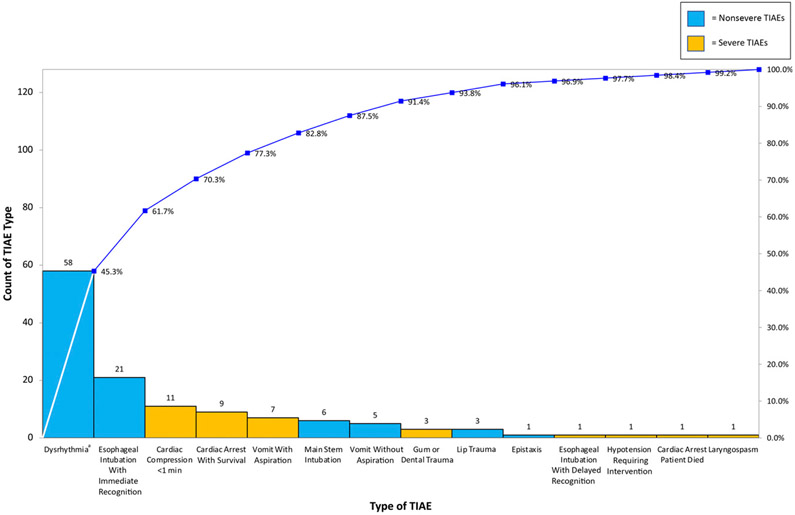
Monthly number of intubations ranged from 12 to 41. Mean monthly TIAE rate increased from 0.093 to 0.172 in July 2018 and then decreased to 0.089 in June 2019 (Fig 3). System stability improved after AB expansion to the entire unit in April 2018. When reporting TIAEs as a binary outcome of yes or no, regardless of number of TIAEs per encounter, mean monthly percentage of TI encounters with a TIAE was 7.6% and did not change. Mean severe TIAE rate decreased significantly in June 2019 from 0.047 to 0.016, corresponding to a 66% reduction (Fig 4). A pareto chart demonstrating the types TIAEs is presented in Fig 5. Types of TIAEs did not vary over time.
FIGURE 3.
Rate of overall TIAEs per TI encounter, u chart. CL, center line; QR, quick response; UCL, upper control limit.
FIGURE 4.
Rate of severe TIAEs per TI encounter, u chart. CL, center line; QR, quick response; UCL, upper control limit.
FIGURE 5.
Types of TIAEs pareto chart. aDysrhythmia includes heart rate <60 beats per minute without chest compressions.
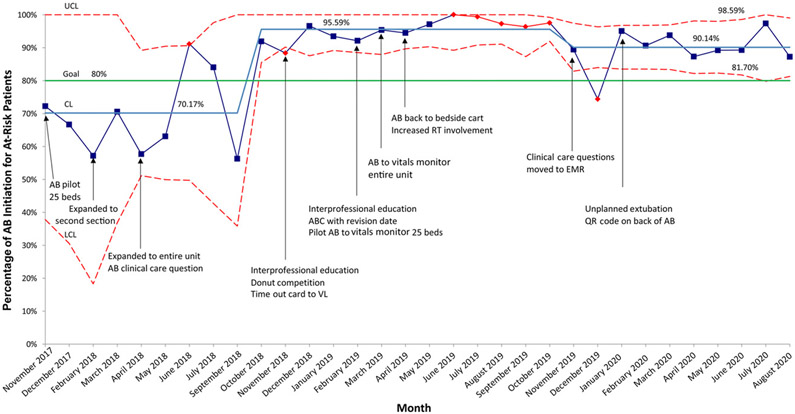
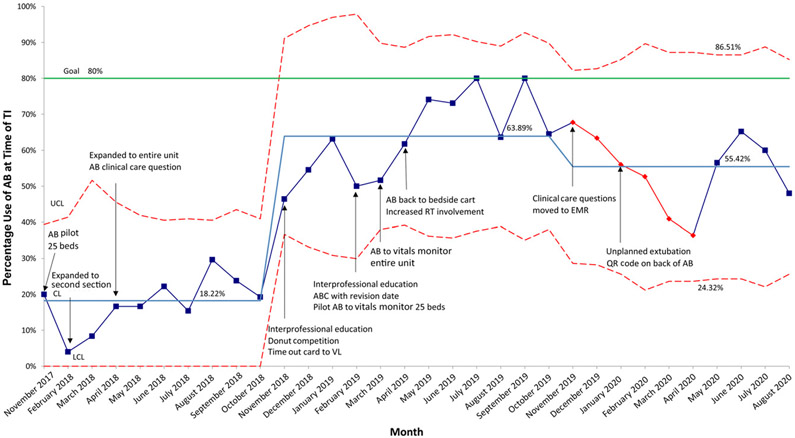
AB initiation for at-risk patients improved significantly from a baseline mean of 70.2% to 95.6% in October 2019. AB initiation decreased to 90.1% in November 2019 but remained above the 80% goal (Fig 6). AB use at intubation increased from a baseline mean of 18.2% to 63.9% in November 2018 but decreased to 55.4% in November 2019 (Fig 7).
FIGURE 6.
AB Initiation for at-risk patients, p chart. At-risk patients are defined as intubated patients, patients with a recent extubation, patients with a planned intubation, patients with escalating respiratory support, and any other patient the team feels is at risk. CL, center line; LCL, lower control limit; QR, quick response; UCL, upper control limit.
FIGURE 7.
AB use at time of TI, p chart. CL, center line; LCL, lower control limit; QR, quick response; UCL, upper control limit.
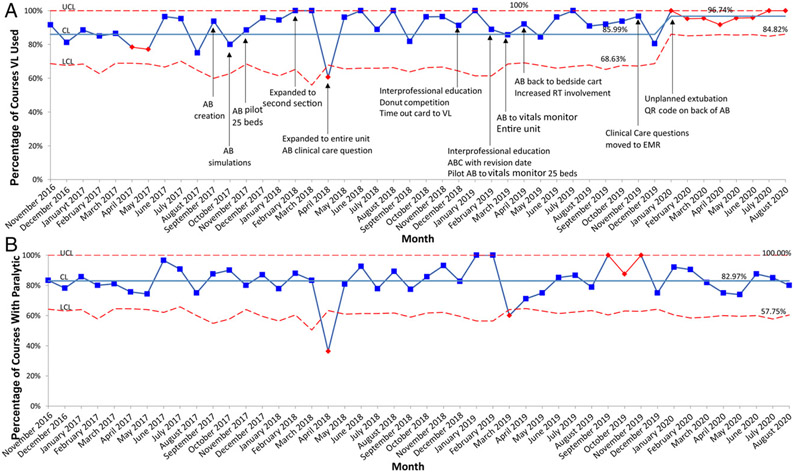
VL use improved from 86.0% to 96.7% in January 2020 (Fig 8A). Paralytic use remained consistent at 83.0% (Fig 8B). There was 1 event where an intubated patient received an “ON CALL PRN for intubation” dose of vecuronium instead of the routine PRN dose of vecuronium. Doses were identical and there was no harm to the patient. No other inadvertent administrations were reported. The majority of patient characteristics did not change significantly over time (Supplemental Table 3).
FIGURE 8.
A, Percentage of courses with VL, p chart. B, Percentage of courses with paralytic, p chart. A course is defined as 1 method for intubation, including device, approach, and premedication. If device, approach, or premedication were changed during an encounter, this would be a new course within the same encounter. CL, center line; LCL, lower control limit; QR, quick response; UCL, upper control limit.
DISCUSSION
Although we did not achieve our goal of 10% reduction in overall TIAEs, the introduction of an AB resulted in a significantly decreased rate of severe TIAEs in our N/IICU. Through multiple PDSA cycles, we increased VL use, AB initiation for at-risk patients, and AB use at intubation. Paralytic use remained persistently high throughout the project.
There are limited data in the neonatal literature with which to compare our results. In a survey conducted in the United Kingdom, 42% of responding NICUs had a preintubation checklist, but the survey did not elaborate on components or use of the checklist.17 Hatch et al11 showed a reduction of adverse events during neonatal TI from 46% to 36% with introduction of a standardized preintubation checklist. Hatch et al11 focused on standardizing actions immediately before and during intubation, whereas our intervention emphasized a proactive, individualized approach before the actual intubation. The AB focused on prospectively identifying neonates at risk for intubation and difficult airway management, developing an individualized airway plan, and ordering medications for intubation as PRN.
Baseline data revealed lack of system control that improved for overall and severe TIAEs in April 2018 when the AB was expanded to the entire unit. Although the mean rate of overall TIAEs increased in July 2018, the system became more stable over time, and the mean decreased to 0.089 in June 2019.
We hypothesize we were unable to achieve 10% reduction in overall TIAEs for 2 reasons. First, our TIAE rate are low at 0.089 to 0.172, or 7.6% if reported as a binary outcome. This is below the TIAE rate reported at other individual NEAR4NEOS sites (9%–50%) and those reported by Hatch et al3,9,11 Second, use of VL and paralytic, both shown to decrease TIAEs, were already well established before the project began.1-5
Despite not decreasing overall TIAEs by 10%, we saw significant decrease in the rate of severe TIAEs. Although we believe each PDSA cycle contributed to this reduction, the decrease came after 2 interprofessional education interventions and directly after increased RT engagement. As previously stated, the increased RT involvement resulted from a failed trial of moving the AB to the vitals monitor. This test of change demonstrated our lack of understanding of RT workflow. Although RTs were an important part of the initial stakeholder analysis, increased involvement of clinical and operational leaders highlighted important aspects of their work. This may have been more clearly understood if we initially developed a swim lane process map by roles. A swim lane diagram is a process map that delineates process steps and tasks by provider role. Ultimately, this failed test of change highlighted RT importance in TI safety.
The impact of the AB on reduction of severe TIAEs is supported by improvement in AB initiation for at-risk patients and improvement in VL use. Identifying at-risk patients created situational awareness and a shared mental model for all caregivers. AB initiation improved significantly from 70% to 96% as we increased interprofessional education including a team competition. The response to team competition was positive, promoting team comradery and increasing AB knowledge. The decrease in AB initiation in November 2019 to 90% corresponds to the reporting mechanism change for AB initiation from in-person auditing to automated reporting using EMR clinical care questions. Although automated data acquisition saved significant time, this measure now depended on clinical care question completion, which was often <100% and thus could underestimate AB initiation rate.14 Despite this decrease, reported AB initiation remains above our 80% goal.
To further support the impact of the AB on severe TIAE reduction, post hoc analysis revealed that most patient characteristics did not change over time. Of the few characteristics that changed significantly, only corrected gestational age and history of difficult airway have a known associations with TIAEs.18 Median corrected gestational age was higher in epoch 1 versus all other epochs, and history of difficult airway was less frequent in epoch 1 versus all other epochs. These changes would suggest higher risks of TIAEs in later epochs and thus do not explain the decrease in severe TIAEs. Given other characteristics associated with TIAEs such as weight and difficult airway features at time of TI did not change over time, we do not believe patient characteristics impacted rates of TIAEs.
AB use at intubation remained below our 80% goal. This is likely because PDSA cycles primarily were focused on education surrounding AB initiation rather than use at intubation. Additionally, the manner in which we measure AB use may underreport use of the time out because it depended on team completion of the backside of the AB after intubation and RT collection and submission of the AB.
Next steps include understanding barriers to AB use during intubation and focusing on interventions to address bradycardia, which mediates 3 of the top 4 most common TIAEs (dysrhythmia, cardiac arrest with survival, and chest compressions <1 minute) accounting for >60% of TIAEs. Proposed interventions include confirming use of atropine and video recording intubations to identify inefficiencies that increase nonventilatory time.
We acknowledge limitations. First, NEAR4NEOS data are self-reported. Sites undergo extensive training before initiating data collection, but reporting bias remains a possibility. Second, these results may not generalize to other settings because this was a single-center QI project at a large level IV referral N/IICU with a unique patient population. However, our primary drivers, risk assessment, team preparation, premedication, and team communication are applicable to all units performing TIs. The AB could be more impactful in units with higher TIAE rates and in units that do not routinely use premedication or VL.
CONCLUSIONS
We demonstrated a significant decrease in the rate of severe TIAEs through implementation of an AB. An AB is an important strategy to consider for neonatal airway safety improvement.
Supplementary Material
FUNDING:
Supported by the National Institutes of Child Health and Human Development (NICHD; R21HD089151-01A1). Ms Napolitano and Dr Nishisaki are supported by the NICHD (R21HD089151-01A1). Ms Napolitano has research and consulting relationships with Dräger, Smiths Medical, Philips/Respironics, Aerogen, and VERO-Biotech. Dr Foglia is supported by NICHD Career Development Award (K23HD084727). Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- AB
Airway Bundle
- CHOP
Children’s Hospital of Philadelphia
- EMR
electronic medical record
- IV
intravenous
- NEAR4NEOS
National Emergency Airway Registry for Neonates
- N/IICU
newborn and infant ICU
- PDSA
plan-do-study-act
- RT
respiratory therapist
- SPCC
statistical process control charts
- TI
tracheal intubation
- TIAE
tracheal intubation–associated event
- VL
video laryngoscope
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.O’Shea JE, Thio M, Kamlin CO, et al. Video-laryngoscopy to teach neonatal intubation: a randomized trial. Pediatrics. 2015;136(5):912–919 [DOI] [PubMed] [Google Scholar]
- 2.Moussa A, Luangxay Y, Tremblay S, et al. Videolaryngoscope for teaching neonatal endotracheal intubation: a randomized controlled trial. Pediatrics. 2016;137(3):e20152156. [DOI] [PubMed] [Google Scholar]
- 3.Foglia EE, Ades A, Sawyer T, et al. ; NEAR4NEOS Investigators. Neonatal intubation practice and outcomes: an international registry study. Pediatrics. 2019;143(1):e20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foglia EE, Ades A, Napolitano N, Leffelman J, Nadkarni V, Nishisaki A. Factors associated with adverse events during tracheal intubation in the NICU. Neonatology. 2015;108(1):23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouppirt NR, Nassar R, Napolitano N, et al. Association between video laryngoscopy and adverse tracheal intubation-associated events in the neonatal intensive care unit. J Pediatr. 2018;201:281–284.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrick HM, Glass KM, Johnston LC, et al. ; for the NEAR4NEOS Investigators. Comparison of neonatal intubation practice and outcomes between the neonatal intensive care unit and delivery room. Neonatology. 2020;117(1):65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane B, Finer N, Rich W. Duration of intubation attempts during neonatal resuscitation. J Pediatr. 2004;145(1): 67–70 [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh V, Ponnusamy V, Anandaraj J, et al. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur J Pediatr. 2011;170(2):223–227 [DOI] [PubMed] [Google Scholar]
- 9.Hatch LD, Grubb PH, Lea AS, et al. Endotracheal intubation in neonates: a prospective study of adverse safety events in 162 infants. J Pediatr. 2016;168:62–66.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haubner LY, Barry JS, Johnston LC, et al. Neonatal intubation performance: room for improvement in tertiary neonatal intensive care units. Resuscitation. 2013;84(10): 1359–1364 [DOI] [PubMed] [Google Scholar]
- 11.Hatch LD, Grubb PH, Lea AS, et al. Interventions to improve patient safety during intubation in the neonatal intensive care unit. Pediatrics. 2016;138(4): e20160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Rehder KJ, Giuliano JS Jr, et al. ; National Emergency Airway Registry for Children (NEAR4KIDS) Investigators; Pediatric Acute Lung Injury and Sepsis Investigator PALISI Network Investigators. Development of a quality improvement bundle to reduce tracheal intubation-associated events in pediatric ICUs. Am J Med Qual. 2016;31(1):47–55 [DOI] [PubMed] [Google Scholar]
- 13.Nishisaki A, Lee A, Li S, et al. Sustained improvement in tracheal intubation safety across a 15-center quality-improvement collaborative: an interventional study from the National Emergency Airway Registry for Children Investigators. Crit Care Med. 2021;49(2):250–260 [DOI] [PubMed] [Google Scholar]
- 14.Carr L, Padula M, Chuo J, et al. Improving compliance with a rounding checklist through low- and high-technology interventions: a quality improvement initiative. Pediatr Qual Saf, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provost LP, Murry SK. The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey-Bass;2011 [Google Scholar]
- 16.Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25 (12):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foy KE, Mew E, Cook TM, et al. Paediatric intensive care and neonatal intensive care airway management in the United Kingdom: the PIC-NIC survey. Anaesthesia. 2018;73(11): 1337–1344 [DOI] [PubMed] [Google Scholar]
- 18.Sawyer T, Foglia EE, Ades A, et al. ; National Emergency Airway Registry for Neonates (NEAR4NEOS) investigators. Incidence, impact and indicators of difficult intubations in the neonatal intensive care unit: a report from the National Emergency Airway Registry for Neonates. Arch Dis Child Fetal Neonatal Ed. 2019;104(5):F461–F466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.