Abstract
Knockout of C/EBPα causes a severe loss of liver function and, subsequently, neonatal lethality in mice. By using a gene replacement approach, we generated a new C/EBPα-null mouse strain in which C/EBPβ, in addition to its own expression, substituted for C/EBPα expression in tissues. The homozygous mutant mice C/ebpαβ/β are viable and fertile and show none of the overt liver abnormalities found in the previous C/EBPα-null mouse line. Levels of hepatic PEPCK mRNA are not different between C/ebpαβ/β and wild-type mice. However, despite their normal growth rate, C/ebpαβ/β mice have markedly reduced fat storage in their white adipose tissue (WAT). Expression of two adipocyte-specific factors, adipsin and leptin, is significantly reduced in the WAT of C/ebpαβ/β mice. In addition, expression of the non-adipocyte-specific genes for transferrin and cysteine dioxygenase is reduced in WAT but not in liver. Our study demonstrates that when expressed from the C/ebpα gene locus, C/EBPβ can act for C/EBPα to maintain liver functions during development. Moreover, our studies with the C/ebpαβ/β mice provide new insights into the nonredundant functions of C/EBPα and C/EBPβ on gene regulation in WAT.
CCAAT/enhancer-binding proteins (C/EBPs) are transcriptional regulators of the basic leucine zipper family. Members of the C/EBP family (C/EBPα, C/EBPβ, Ig/EBP, C/EBPδ, and C/EBPɛ) recognize a common DNA-binding sequence and display similar dimerization specificities (4, 9, 24). Although tissue expression patterns of these members often overlap, recent studies using gene knockout analysis of C/EBPs in mice revealed that C/EBPs differ significantly in their physiological functions and in their downstream target genes. For example, mice lacking C/EBPα die shortly after birth due to severe hypoglycemia and the absence of glycogen storage in liver (23), whereas knockout of C/EBPβ causes defects in female reproduction (18).
Members of the C/EBP family also work in conjunction with each other or in sequence to support development of a tissue and to maintain its functions. For example, C/EBPα, -β, and -δ are expressed at defined times during adipogenesis, and each has a crucial role in adipocyte development (4, 19, 25). The expression of C/EBPβ and -δ precedes C/EBPα and peroxisome proliferator activated receptor γ (PPARγ) in adipogenesis, so it is believed that C/EBPβ and -δ are important in early adipocyte differentiation as well as in activating expression of C/EBPα and PPARγ. In contrast, C/EBPα seems to play a critical role in the later stage of adipogenesis by maintaining a differentiated adipocyte phenotype, such as fat storage (5, 6).
In this study, we used a gene replacement strategy to generate a viable and fertile C/EBPα-null mouse line in which C/EBPβ substitutes for C/EBPα in tissues during development. We show that C/EBPβ functions for C/EBPα in liver to maintain normal blood glucose levels but does not act for C/EBPα to regulate fat storage in white adipose tissue (WAT). We also show that in WAT, C/EBPβ does not substitute for C/EBPα to stimulate expression of several factors, including adipsin, leptin, and transferrin. These findings provide new insights into nonredundant functions of C/EBPα and -β isoforms.
MATERIALS AND METHODS
Generation of C/ebpαβ/β mice.
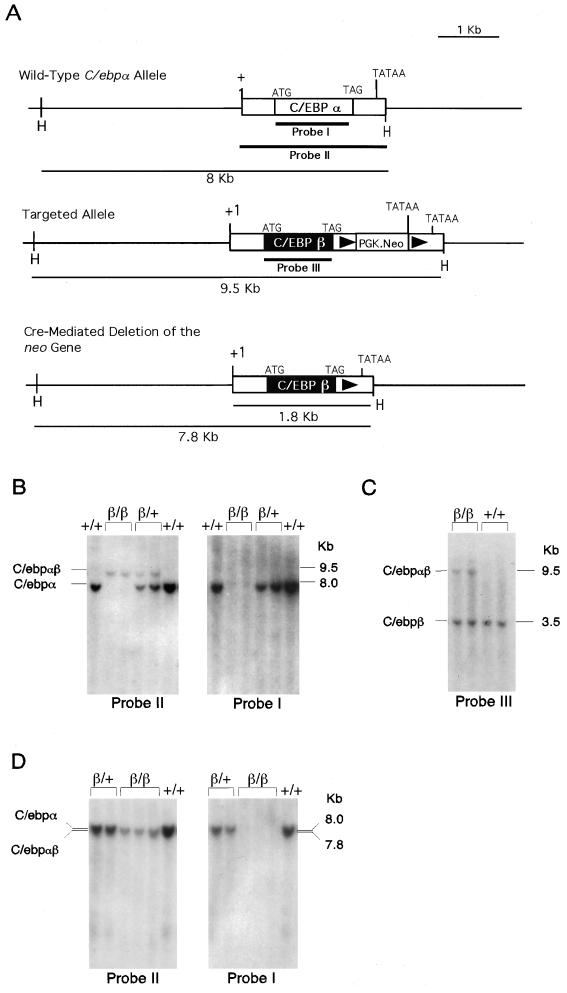
The C/ebpα genomic fragment used for preparing the gene-targeting construct was described earlier (12). In this targeting construct, the entire protein-coding region of C/ebpα (1,188 bp, from the start to stop codons) was deleted and replaced with a 831-bp DNA fragment containing the mouse C/EBPβ protein-coding region (Fig. 1). The loxP-PGK.neo-loxP expression cassette was then inserted into an SpeI site 159 bp downstream of the stop codon TAG. The targeting vector contained 4.1 kb of homologous DNA upstream of the C/EBPα start codon and 3.8 kb of homologous DNA downstream of the loxP-PGK.neo-loxP cassette.
FIG. 1.
Targeted modification of the C/ebpα gene locus. (A) C/ebpα gene (top), targeted allele (middle), and the expected Cre-loxP-mediated removal of the PGK.neo transgene (bottom). (B and C) Southern blot analysis of representative F2 (before crossing with the EIIa-cre mice) mouse tail biopsies. Tail DNA was digested with HindIII (B) and with HindIII and BamHI (C) and probed with the probes shown in panel A. (D) Southern blot analysis of representative F2 mouse tail biopsies after crossing with the EIIa-cre mice. DNA was digested with HindIII and probed with the probes shown in panel A. +, wild-type allele; β, targeted C/ebpα allele.
Embryonic stem (ES) cells (RW4; Genome Systems) were electroporated with the linearized targeting vector DNA, and G418-resistant clones were selected, expanded, and analyzed by Southern blotting with a 5′ probe and a coding region probe to identify specific homologous recombinants. The correctly targeted ES cells were injected into C57BL/6J blastocysts to generate chimeric founder mice as previously described (8). Chimeric male founders with close to 90 to 100% agouti coat color were bred with C57BL/6J females. Offspring mice having germ line transmission of the mutant allele were bred with homozygous EIIa-cre mice (11) to remove the targeting marker PGK.neo gene from the mouse genome as previously described (12). F1 mice carrying one mutant C/ebpα allele, termed C/ebpαβ/+ (+, wild-type allele; β, mutant allele), were interbred to generate homozygous C/ebpαβ/β mice.
Diet experiments.
All mice used in this study were F3 siblings derived from interbreeding the F2 C/ebpαβ/+ mice. Mice were kept in a sterile microisolator and were observed closely throughout the experiment. For measuring the food intake, three mice of each sex and of each genotype were placed separately in microisolators 2.5 weeks after birth. Fresh food comprising a regular diet for mice (LabDiet, Richmond, Ind.) was provided daily and the amount of food consumed was recorded daily. For the fasting experiment, 5-week-old mice were kept in a sterile microisolator and were provided with only sterile water for 24 h.
Southern blot analysis.
Isolated ES cell DNA or mouse tail DNA was digested with the appropriate restriction enzyme, electrophoresed in a 0.5% agarose gel, transferred to a nylon membrane (GeneScreen Plus; Dupont), and hybridized with probes derived from the C/ebpα or C/ebpβ gene as indicated in Fig. 1A.
Histological analysis.
Fat pads and livers were fixed in 4% phosphate-buffered saline-buffered paraformaldehyde solution for embedding in paraffin or were embedded immediately in tissue-freezing medium (OCT; Tissue-Trek, Miles, Inc.) and frozen in liquid nitrogen. Sections of 5 μm (for paraffin-embedded tissues) or 10 μm (for frozen tissues) were cut and mounted on silanized slides. The frozen tissue sections were stained with oil red O and counter-stained with hematoxylin as previously described (22). The paraffin tissue sections were stained with hematoxylin and eosin solutions (H&E staining).
Analyses of serum chemistries and levels of leptin and insulin.
Mouse sera taken from various developmental stages were analyzed in an autodry chemical analyzer (SP4410; Spotchem) to monitor levels of serum glucose and other blood chemistries. Serum leptin and insulin levels were assayed with the respective kits (Crystal Chem, Inc., Chicago, Ill.) based on enzyme-linked immunosorbent assay according to the manufacturer's instructions.
Hemolytic assay.
Serum factor D activity was monitored by hemolytic assay using rabbit erythrocytes and factor D-depleted human serum (1). In a total assay volume of 150 μl, 25 μl of mouse serum and 25 μl of factor D-depleted human serum were added to rabbit erythrocytes (107 cells in 100 μl of 5 mM Veronal-buffered saline–12 mM MgCl2, pH 7.4). After incubation at 37°C for 1 h, the hemolytic activity was determined, and the activity was expressed as a percentage of the activity in a lysed control (1).
LPS treatment.
Four-week-old C/ebpαβ/β mice were injected intraperitoneally with 5 mg of lipopolysaccharide (LPS) per kg of body weight to induce a generalized inflammatory response. Twelve hours after the injection, livers were removed and snap-frozen in liquid nitrogen for later RNA extraction.
RNA extraction and Northern blot analysis.
Frozen mouse tissues were homogenized in TRIzol RNA reagent (GIBCO-BRL), and total RNAs were isolated according to the manufacturer's protocol. Total RNA (10 μg) was denatured, electrophoresed, transferred to a nylon membrane, and probed with cDNA probes using standard protocols.
Western blot analysis.
Tissues were homogenized in a 10× volume of 10 mM Tris-HCl (pH 7.0)–1% Triton X-100 containing the protease inhibitors phenylmethylsulfonyl fluoride and pepain. Fifty micrograms of total protein was electrophoresed in a 10% denaturing polyacrylamide-bis gel and transferred to a nitrocellulose membrane. The membrane was blocked in phosphate-buffered saline containing 5% (wt/vol) nonfat milk and 0.2% Tween 20, incubated with the primary rabbit immunoglobulin G against mouse C/EBPβ (Santa Cruz, Santa Cruz, Calif.) and developed by using peroxidase-labeled goat anti-rabbit immunoglobulin G (Santa Cruz).
RESULTS
Generation of C/EBPβ knockin homozygous C/ebpαβ/β mice.
The C/EBPβ knockin targeting vector was constructed to replace the entire protein-coding region of C/ebpα with that of C/ebpβ in the C/ebpα gene locus (Fig. 1A). A loxP-PGK.neo-loxP cassette was inserted downstream of the stop codon to serve as the targeting selection marker. After transfection of the linearized targeting vector DNA into ES cells, 1% of the G418-resistant ES clones were found to carry a mutant C/ebpα allele in which the protein coding region of C/ebpα was deleted and replaced with that of C/ebpβ (Fig. 1A). Mice having germ line transmission of the mutant allele C/ebpαβ/+ were produced. The C/ebpαβ/+ mice were interbred to generate homozygous C/ebpαβ/β mice. Deletion of the C/EBPα protein-coding region in the manipulated C/ebpα gene locus was confirmed by Southern analysis with a 1.2-kb DNA probe (probe I) containing the entire protein-coding region and a 2-kb probe (probe II) covering the full-length C/EBPα transcript (Fig. 1B). In addition, the presence of the C/EBPβ protein-coding region in the C/ebpα gene locus was confirmed by probing the C/ebpαβ/β mouse genome with a C/EBPβ cDNA probe (probe III) (Fig. 1C). Furthermore, to avoid the potential interference of the PGK.neo marker gene on the expression of C/EBPαβ mRNA (the transcript encoded by the mutant allele), C/ebpαβ/β mice were then bred with a cre transgenic mouse line to remove the PGK.neo gene from the targeted C/ebpα locus (11). The resulting F1 mice heterozygous for the C/ebpαβ allele and lacking the marker gene were selected for interbreeding to yield F2 C/ebpαβ/β mice (Fig. 1D). The absence of the C/EBPα mRNA and the presence of C/EBPαβ mRNA (1.8 kb) in C/ebpαβ/β mice were further confirmed by probing total RNAs from various tissues with the respective cDNA probes (Fig. 2 and 3).
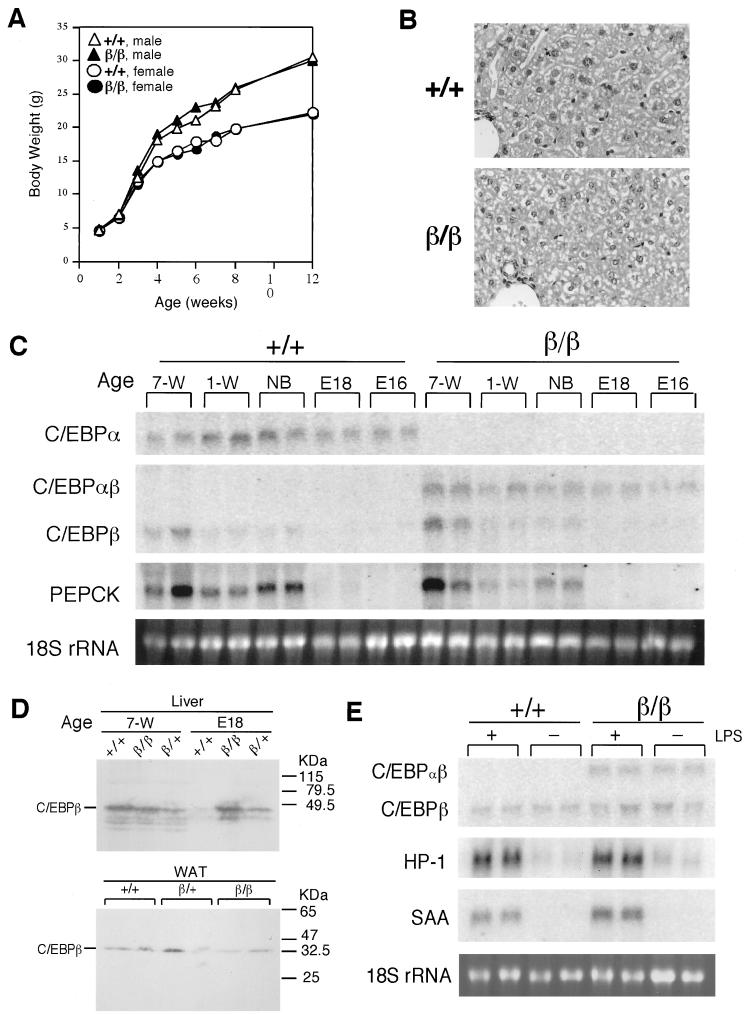
FIG. 2.
Normal growth and liver functions of C/ebpαβ/β mice. (A) Growth curves for C/ebpαβ/β mice on a standard diet. The value for each point is the average body weight of five mice of the same genotype. The standard deviation for each group is within 10% of the respective mean value and is not shown. (B) Histological sections and H&E staining of livers of 9-week-old C/ebpαβ/β mice. Magnification, ×360 (original magnification, ×400). (C) Northern blotting analysis of representative liver biopsies of C/ebpαβ/β mice. Liver total RNAs (20 μg) from C/ebpαβ/β mice of different ages were denatured and electrophoresed on a formaldehyde-containing 1% agarose gel, blotted to a nylon membrane, and probed with the indicated cDNA probes. Each lane contains RNA from an individual animal. E, embryonic stage; NB, newborn; W, week. (D) Western analysis of C/EBPβ protein levels of representative liver and WAT biopsies of C/ebpαβ/β mice. Total cellular protein (50 μg) from livers from mice of different ages and from WAT of 7-week-old mice were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, blotted to a nitrocellulose membrane, and probed with an antibody against mouse C/EBPβ. Each lane contains protein from an individual animal. E, embryonic stage; W, week. (E) Northern blotting analysis of representative liver biopsies of C/ebpαβ/β mice after the LPS treatment. Liver total RNA (20 μg) from 4-week-old C/ebpαβ/β mice injected with LPS were denatured and electrophoresed on a formaldehyde-containing 1% agarose gel, blotted to a nylon membrane, and probed with the indicated oligonucleotide probes. Each lane contains RNA from an individual animal. +, wild-type allele; β, targeted C/ebpα allele.
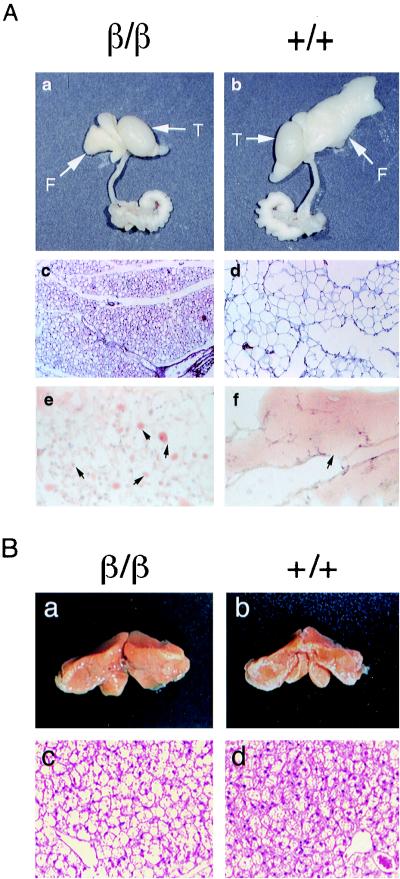
FIG. 3.
Inhibited adipocyte hypertrophy and gene expression in WAT of C/ebpαβ/β mice. (A) Appearance of epididymal fat pads (panels a and b), histological sections, and H&E staining of epididymal fat pads (panels c and d) (magnification, ×86; original magnification, ×100) and histological sections and oil red O staining of epididymal fats (panels e and f) of 4-week-old C/ebpαβ/β mice. (B) Appearance (panels a and b) and histological sections and H&E staining (panels c and d) (magnification, ×344; original magnification, ×400) of brown fat pads of 4-week-old C/ebpαβ/β mice. (C through E) Northern blotting analysis of representative epididymal fat (C and E) and brown fat (D) biopsies of 4-week-old C/ebpαβ/β mice. Total RNA (8 μg) from 4-week-old C/ebpαβ/β mice was denatured and electrophoresed on a formaldehyde-containing 1% agarose gel, blotted to a nylon membrane, and probed with the indicated cDNA probes. Each lane contains RNA from an individual animal. F, epididymal fat pads; T, testes; +, wild-type allele; β, targeted C/ebpα allele.
Normal growth and hepatic functions in C/ebpαβ/β mice.
C/ebpαβ/β mice were viable and grossly normal. C/ebpαβ/β mice did not differ from their wild-type littermates in their growth rate as measured by body weight gain (Fig. 1A). Both sexes of C/ebpαβ+/+ mice are fertile. Liver dysfunction and other reported abnormalities associated with the previous knockout of C/EBPα (3, 12, 23), such as severe hypoglycemia at birth, were not found in C/ebpαβ/β mice. C/ebpαβ/β mice had normal blood glucose levels and other blood chemistry, such as albumin (Table 1), indicating that liver functions in C/ebpαβ/β mice are not defective. In addition, histological analysis of livers from C/ebpαβ/β mice did not suggest abnormality (Fig. 2B).
TABLE 1.
Serum chemistry of C/ebpαβ/β micea
| Serum component | Concn in mouse group
|
|
|---|---|---|
| C/ebpα+/+ | C/ebpαβ/β | |
| Glucose (mg/dl) | 164 ± 25 | 183 ± 6 |
| Triacylglyceride (mg/dl) | 46 ± 9 | 49 ± 9 |
| Albumin (g/dl) | 2.3 ± 0.2 | 2.4 ± 0.1 |
| Calcium (mg/dl) | 9.3 ± 0.2 | 9.4 ± 0.2 |
| Total cholesterol (mg/dl) | 83 ± 14 | 67 ± 6 |
| Total bilirubin (mg/dl) | ≤0.1 | ≤0.1 |
| Total protein (g/dl) | 4.5 ± 0.1 | 4.5 ± 0.4 |
| Glucose oxidase (IU/l) | 53 ± 8 | 66 ± 27 |
| Glutamic pyruvic transaminase (IU/l) | 5 ± 1 | 5 ± 1 |
| Glucose (mg/dl)b | ||
| Before fasting | 157 ± 13 | 147 ± 9 |
| After fasting | 94 ± 13 | 91 ± 5 |
Sera were collected from groups of mice of each genotype at 4 weeks old and subjected to analysis for glucose and other serum components. Values presented are the means ± standard errors of at least five serum samples.
Sera were collected from mice of each genotype at 5 weeks old before and after fasting for 24 h. Values presented are the means ± standard errors of 7 serum samples.
At the gene expression level, C/ebpαβ/β mice lack C/EBPα but have a concomitant gain of C/EBPβ expressed from the C/ebpα gene locus (shown as C/EBPαβ) (Fig. 2C). The expression pattern of C/EBPαβ in C/ebpαβ/β mice did not differ from that of C/EBPα in wild-type littermates, indicating that the expression activity of the C/ebpα gene locus was not affected by our gene replacement approach. Similarly, the mRNA levels of C/EBPβ expressed from its own gene locus were not altered by the presence of C/EBPαβ (Fig. 2C). Furthermore, the levels of hepatic C/EBPβ protein were correlated with the mRNA levels (C/EBPβ or C/EBPαβ) in each genotype at the embryo stage (day 18), but at the age of 7 weeks, the levels of C/EBPβ protein in liver or WAT were not different between genotypes (Fig. 2D).
The previously described C/EBPα-null mice died shortly after birth, possibly due to severe hypoglycemia and lack of glycogen storage in liver (12, 23). The molecular mechanism that caused neonatal death might have involved a defect in gene expression of phosphoenolpyruvate carboxykinase (PEPCK) (23), a key enzyme in gluconeogenesis. We therefore examined the PEPCK mRNA levels in livers of C/ebpαβ/β mice during development. As expected, PEPCK mRNA was present in the livers of C/ebpαβ/β mice, and the timing and levels of its expression were similar to those of wild-type mice (Fig. 2C). This indicates that the PEPCK gene expression is not affected in the livers of C/ebpαβ/β mice. To further support this observation, C/ebpαβ/β mice were subjected to fasting. After fasting for 24 h, C/ebpαβ/β mice did not appear different from their wild-type littermates in viability and mobility, and both kinds of mice developed a similar degree of hypoglycemia (Table 1).
We next examined the acute-phase response in livers of C/ebpαβ/β mice. C/EBPα was previously found to be absolutely required for the acute-phase response in neonatal mice (3). However, in response to LPS treatment, C/ebpαβ/β mice did not differ from wild-type mice in the up-regulated expressions of hepatic acute-phase responsive genes, such as haptoglobin and serum amyloid A (Fig. 2E), suggesting that the acute-phase response is not affected in livers of C/ebpαβ/β mice.
Reduced fat storage in WAT of C/ebpαβ/β mice.
Although C/ebpαβ/β and wild-type mice had similar body weights, the size and appearance of their WAT were strikingly different (Fig. 3A). WAT in C/ebpαβ/β mice appeared small and yellowish, whereas WAT in C/ebpαβ/+ and wild-type littermates was significantly enlarged and appeared whitish (Fig. 3A, panels a and b). Nuclear DNA contents of the epididymal fat pads were not different between C/ebpαβ/β mice and their wild-type littermates (data not shown). This suggests that the reduced fat mass in C/ebpαβ/β mice is not due to a reduction in fat cell numbers. On the other hand, histological analysis showed that the size of adipocytes and the degree of lipid accumulation were markedly reduced in WAT of C/ebpαβ/β mice (Fig. 3A, panels c through f), indicating that the lipid accumulation in WAT of C/ebpαβ/β mice is inhibited. By contrast, C/ebpαβ/β mice had 60% more brown adipose tissue (BAT) than their wild-type littermates (Fig. 3B, panels a and b), and the adipocytes in their BAT appeared more vacuolated, presumably containing more lipid droplets (Fig. 3B, panels c and d).
The reduction of lipid store in WAT of C/ebpαβ/β mice is not due to a decrease of lipid supply in circulation. As shown in Table 1, the levels of serum triacylglyceride were not different between C/ebpαβ/β and wild-type mice. In addition, despite their lipodystrophic status, C/ebpαβ/β mice did not develop fatty liver as analyzed by oil red staining of frozen liver sections (data not shown).
Reduced mRNA levels of leptin and adipsin in WAT of C/ebpαβ/β mice.
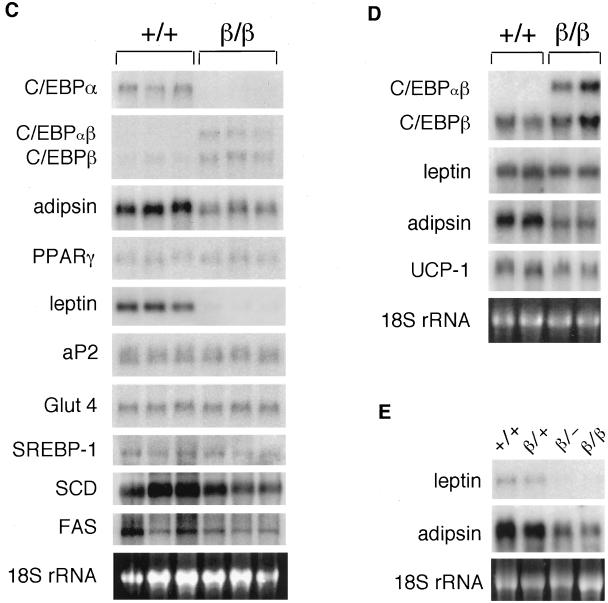
To understand the molecular mechanism underlying the resistance of fat accumulation in WAT of C/ebpαβ/β mice, we first examined the expression of numerous genes whose functions are related to adipocyte differentiation and maturation. The mRNA levels of C/EBPβ expressed from its endogenous gene locus (as a 1.5-kb transcript) appeared to be up-regulated in WAT of C/ebpαβ+/+ mice (Fig. 3C). The levels of C/EBPβ expressed from the C/ebpα gene locus (as a 1.8-kb transcript) were similar to those from the C/ebpβ gene locus. mRNA levels of other transcription regulators involved in adipocyte development, such as PPARγ and adipocyte determination and differentiation-dependent factor (ADD1, also called SREBP-1), were not different in WAT of C/ebpαβ/β and wild-type mice. Similarly, aP2 and glucose transporter IV that are expressed in mature adipocytes were not affected in WAT of C/ebpαβ/β mice. Expression of enzymes involved in fatty acid synthesis, such as fatty acid synthetase and stearyl-coenzyme A-desaturase, were not significantly different between wild-type and C/ebpαβ/β mice. These results indicate that the expression of the above factors important in adipogenesis is not inhibited in WAT of C/ebpαβ/β mice. However, mRNA levels encoding two factors expressed specifically in mature adipocytes, adipsin (factor D) and leptin (product of the ob gene), were significantly reduced in WAT of C/ebpαβ/β mice (Fig. 3C). Interestingly, unlike expression of adipsin, whose mRNA level was markedly reduced in both WAT and BAT, leptin expression was not significantly affected in BAT of C/ebpαβ/β mice (Fig. 3D). The uncoupling protein 1 mRNA levels were not affected in BAT of C/ebpαβ/β mice (Fig. 3D).
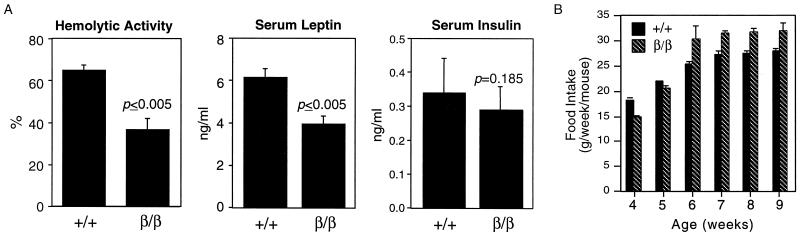
Adipsin and leptin are both produced in WAT as well as in BAT, are released into circulation, and are involved in maintaining energy homeostasis (5, 7, 13, 17). To determine whether the adipsin levels in the blood of C/ebpαβ/β mice were reduced to reflect their mRNA levels in WAT, a hemolysis assay was carried out to determine the adipsin activity in sera of C/ebpαβ/β mice. Indeed, hemolytic activity in sera of C/ebpαβ/β mice was reduced to 50% of that in sera of wild-type mice (Fig. 4A). Similarly, based on an enzyme-linked immunosorbent assay, serum leptin levels in C/ebpαβ/β mice were reduced to 60% of the normal level (Fig. 4A). On the other hand, serum insulin levels in C/ebpαβ/β mice were not different from those in wild-type littermates (Fig. 4A). Despite their normal body weight, C/ebpαβ/β mice consumed more food (on average, 9% more) than wild-type mice, as revealed by measuring their food intake for 6 consecutive weeks (Fig. 4B). Since leptin has an inhibitory effect on food intake, this moderately higher food consumption by C/ebpαβ/β mice might be associated with the decreased leptin levels in circulation.
FIG. 4.
Reduced serum adipsin and leptin levels and increased food intake in C/ebpαβ/β mice. (A) Sera were collected from groups of mice of each genotype at the age of 4 weeks for analysis of hemolytic activity and insulin and leptin levels. Values presented are the means ± standard errors for at least five serum samples. The P value indicates the level of significance for differences between C/ebpαβ/β and wild-type mice. (B) Weekly food intake during a 6-week diet experiment. Values presented are the means ± standard errors for six mice. +, wild-type allele; β, targeted C/ebpα allele.
To examine whether the down-regulated expression of adipsin and leptin genes in WAT of C/ebpαβ/β mice is associated with the lack of C/EBPα or with the gain of C/EBPβ, C/ebpαβ/β mice were bred with the C/EBPα knockout heterozygotes generated by the Cre-loxP system (12). Mice carrying one allele of C/ebpαβ and one allele of the Cre-loxP-mediated C/EBPα deletion (designated C/ebpαβ/− mice) were selected and compared to the C/ebpαβ/+ mice, in which a wild-type C/ebpα allele remained. The morphology and histology of WAT in C/ebpαβ/− mice were similar to those in C/ebpαβ/β mice, while the WAT of C/ebpαβ/+ mice was not different from that of wild-type mice (data not shown). Similarly, the levels of adipsin and leptin mRNA were reduced significantly in WAT of C/ebpαβ/− mice but not in WAT of C/ebpαβ/+ mice (Fig. 3E). These results indicate that C/EBPα is required to maintain normal expression of adipsin and leptin genes and that C/EBPβ, regardless of its level of expression, cannot substitute for C/EBPα for this function in WAT.
WAT-specific reduction of CDO and transferrin gene expression.
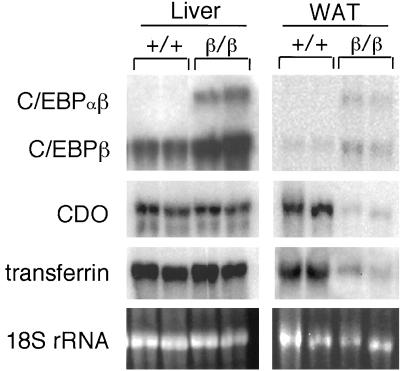
Adipsin and leptin are adipocyte-specific factors (5). To further elucidate the nonredundant function of C/EBPα in adipose tissues, we examined the expression of other genes whose expression is not restricted to adipocytes and whose expression is regulated by C/EBP proteins. Transferrin and cysteine dioxygenase (CDO) are expressed at a high level in liver, and both are expressed at a low level in other tissues, including adipose tissues (10, 20). In addition, C/EBP proteins play an important role in regulating their expression (16, 20, 26). As shown in Fig. 5, mRNA levels for both CDO and transferrin were not reduced in liver but were significantly decreased in WAT. Again, this result indicates that C/EBPβ can substitute for C/EBPα in regulating CDO and transferrin gene expression in liver but not in WAT.
FIG. 5.
Decreased expressions of transferrin and CDO genes in WAT, but not in liver, of C/ebpαβ/β mice. Northern blotting analysis of representative tissue biopsies of C/ebpαβ/β mice. Total RNA isolated from tissues of C/ebpαβ/β mice were denatured and electrophoresed on a formaldehyde-containing 1% agarose gel, blotted to a nylon membrane, and probed with the indicated cDNA probes. Each lane contains RNA from an individual animal. +, wild-type allele; β, targeted C/ebpα allele.
DISCUSSION
Our approach of knocking C/ebpβ into the C/ebpα gene locus allows C/EBPβ to be expressed from the endogenous C/ebpα gene locus to replace C/EBPα in tissues during development. The resulting homozygous mutant mice, which are C/ebpαβ/β, lack C/EBPα but have a concomitant gain of C/EBPβ in tissues. C/ebpαβ/β mice are viable and fertile. The neonatal lethality and other adverse physiological effects that were associated with the knockout of C/EBPα are overcome in C/ebpαβ/β mice. However, C/ebpαβ/β mice have a significant reduction in their fat accumulation and gene expression in WAT, a defect that is not overcome by our gene replacement approach, thus providing a unique system for studying in detail the regulatory role of C/EBPα in maintaining fat cell functions.
In C/ebpαβ/β mice, during embryonic and early postnatal developments the levels of hepatic C/EBPβ mRNA expressed from the C/ebpα allele appear to be significantly higher than those of C/EBPβ mRNA expressed from its endogenous allele. In the earlier C/EBPα knockout studies (12, 23), the normal expression of C/EBPβ was not able to compensate for C/EBPα deficiency and thus overcome those adverse physiological effects in liver. It is therefore possible that C/EBPβ, during the early developmental stage, is not expressed at a level sufficient to compensate for C/EBPα deficiency in the livers of the knockout mice. In contrast, in C/ebpαβ/β mice, the C/EBPβ insufficiency is overcome due to the higher expression from the C/ebpα allele. Nevertheless, the mechanism of how C/EBPβ functions for C/EBPα in the livers of C/ebpαβ/β mice awaits further study.
C/EBPα is required for adipogenesis of both WAT and BAT (4, 25). However, its regulation in maintaining mature fat cells and fat cell function is not yet fully understood, especially in adipose tissues in animals. Adipsin is a serine protease involved in generating the active complement C3a, also known as acylation-stimulating protein (15). Acylation-stimulating protein stimulates triglyceride synthesis in adipocytes (2). Therefore, the decrease of adipsin production might be responsible in part for the defect of lipid accumulation in WAT of C/ebpαβ/β mice. However, the molecular mechanism underlying the prevention of fat accumulation in WAT C/ebpαβ/β mice awaits further elucidation.
Previously, leptin expression was found to be modulated by C/EBPα in humans (14), whereas the regulatory role of C/EBPα on adipsin gene expression is not yet clear. The reduced expression of both adipsin and leptin genes in the WAT of C/ebpαβ/− mice but not in the WAT of C/ebpαβ/+ mice indicates that C/EBPα is the major trans-activator for both genes in mice. Although C/EBPβ appears to function for C/EBPα in maintaining the expression of hepatic PEPCK, it does not substitute for C/EBPα to activate adipsin and leptin gene expressions in WAT. On the other hand, the expression of other C/EBPα-regulated genes, such as those for SREBP and aP2 (5, 13), whose functions are also related to the adipocyte maturation, were not affected in the WAT of C/ebpαβ/β mice. This indicates a gene-dependent mechanism that determines whether C/EBPβ can substitute for C/EBPα in regulating transcription of a gene. Similarly, the finding that the expression of the transferrin and CDO genes was affected in WAT but not in liver suggests a tissue-specific mechanism that determines whether C/EBPβ can substitute for C/EBPα in regulating expression of certain genes in a tissue. Taken together, our studies with the C/ebpαβ/β mice indicate that the gene regulation by C/EBP proteins likely involves a complex mechanism that is tissue and target gene dependent. Further studies with C/ebpαβ/β mice should provide new insights into the mechanism of the functional specificity of C/EBPα on regulating gene expression.
Adipose tissues play an important role in regulating energy balance in mammals (17). Obesity is a disorder of energy balance and is a significant risk factor for many serious illnesses, such as heart disease, arthritis, and diabetes. C/EBPα plays a critical role in lipid accumulation in adipose tissues. Therefore, understanding the C/EBPα regulatory mechanism of fat storage in adipose tissues might ultimately lead to development of therapeutic approaches to preventing the onset of obesity and its associated pathologies. The lean C/ebpαβ/β mice provide an excellent system to further uncover the mechanism and to explore therapeutic strategies.
REFERENCES
- 1.Baker B C, Campbell C J, Grinham C J, Turcatti G. Purification and partial characterization of rat factor D. Biochem J. 1991;279:775–779. doi: 10.1042/bj2790775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldo A, Sniderman A D, St.-Luce S, Avramoglu R K, Maslowska M, Hoang B, Monge J C, Bell A, Mulay S, Cianflone K. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J Clin Investig. 1993;92:1543–1547. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess-Beusse B L, Darlington G J. C/EBPα is critical for the neonatal acute-phase response to inflammation. Mol Cell Biol. 1998;18:7269–7277. doi: 10.1128/mcb.18.12.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Z, Umek R M, Mcknight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 5.Cowherd R M, Lyle R E, McGehee R E., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 6.Fajas L, Fruchart J-C, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998;10:165–173. doi: 10.1016/s0955-0674(98)80138-5. [DOI] [PubMed] [Google Scholar]
- 7.Gregoire F M, Smas C M, Sul H S. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 8.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 9.Johnson J F, Williams S C. CCAAT/enhancer binding (C/EBP) proteins. In: Yaniv M, Tronche F, editors. Liver gene expression. R. G. Austin, Tex: Landes Company; 1994. pp. 231–258. [Google Scholar]
- 10.Kahn A, Levin M J, Zakin M M, Bloch B. The transferrin gene. In: Guroff G, editor. Oncogenes, genes, and growth factors. New York, N.Y: Wiley-Interscience; 1987. pp. 277–309. [Google Scholar]
- 11.Lasko M, Pichel P B, Gorman R, Sauer B, Okamoto Y, Lee E, Alt F W, Westphal H. Efficient in vivo manipulation of mouse genomic sequence at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y-H, Sauer B, Johnson P F, Gonzalez F J. Disruption of the c/ebpα gene in adult mouse liver. Mol Cell Biol. 1997;17:6014–6022. doi: 10.1128/mcb.17.10.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loftus T M, Lane M D. Modulating the transcriptional control of adipogenesis. Curr Opin Genet Dev. 1997;7:603–608. doi: 10.1016/s0959-437x(97)80006-8. [DOI] [PubMed] [Google Scholar]
- 14.Miller S G, De Vos P, Guerre-Millo M, Wong K, Hermann T, Staels B, Briggs M R, Auwerx J. The adipocyte specific transcription factor C/EBPα modulates human ob gene expression. Proc Natl Acad Sci USA. 1996;93:5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen B S, Cook K S, Yaglom J, Groves D L, Volanakis J E, Damm D, White T, Speigelman B M. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483–1486. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer E, Guilou F, Part D, Zakin M M. A different combination of transcription factors modulates the expression of the human transferrin promoter in liver and Sertoli cells. J Biol Chem. 1993;268:23399–23408. [PubMed] [Google Scholar]
- 17.Spiegelman B M, Flier J S. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 18.Sterneck E, Tessarollo L, Johnson P F. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q Q, Lane M D. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuboyama N, Hosokawa Y, Totani M, Oka J, Matsumoto A, Koide T, Kodama H. Structural organization and tissue-specific expression of the gene encoding rat cysteine dioxygenase. Gene. 1996;181:161–165. doi: 10.1016/s0378-1119(96)00496-9. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboyama-Kasaoka N, Hosokawa Y, Kodama H, Matsumoto A, Oka J, Totani M. Human cysteine dioxygenase gene: structural organization, tissue-specific expression and downregulation by phorbol 12-myristate 13-acetate. Biosci Biotechnol Biochem. 1999;63:1017–1024. doi: 10.1271/bbb.63.1017. [DOI] [PubMed] [Google Scholar]
- 22.van Goor H, Gerrits P O, Grond J. The application of lipid-soluble stains in plastic-embedded sections. Histochemistry. 1986;85:251–253. doi: 10.1007/BF00494811. [DOI] [PubMed] [Google Scholar]
- 23.Wang N-D, Finegold M J, Bradley A, Ou C N, Abdelsayed S V, Wilde M D, Taylor R, Wilson D R, Darlington G J. Impaired energy homeostasis in C/EBPα knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 24.Williams S C, Cantwell C A, Johnson P F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- 25.Yeh W C, Cao Z, Classon M, McKnight S L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 26.Zakin M M. Regulation of transferrin gene expression. FASEB J. 1992;6:3253–3258. doi: 10.1096/fasebj.6.14.1426763. [DOI] [PubMed] [Google Scholar]