Abstract
Objective:
To evaluate the efficacy of a novel intervention aimed at enhancing self-advocacy in individuals living with traumatic brain injury (TBI).
Setting:
Community.
Participants:
Sixty-seven (35 allocated to treatment, 32 to control) community-dwelling adults 9 months or more post-TBI (mean of 8.9 years postinjury); previously discharged from inpatient or outpatient TBI rehabilitation; able to travel independently in the community, indicating a level of independence needed to engage in self-advocacy.
Design:
Longitudinal randomized 2-arm controlled trial (NCT no. 03385824). Computer-generated block randomization allocated participants to treatment/intervention or control/no-intervention. All outcome assessments completed by blinded study staff.
Intervention:
A manualized group intervention, Self-Advocacy for Independent Life (SAIL), addressing the self-efficacy beliefs, knowledge, and skills for self-advocacy following TBI.
Main Outcome Measures:
The Self-Advocacy Scale (SAS) (primary); General Self-Efficacy Scale (GSE); Personal Advocacy Activity Scale (PAAS); Satisfaction With Life Scale (SWLS).
Results:
The treatment group showed significantly greater improvement than controls from baseline to posttreatment on the primary measure (SAS) of self-efficacy specific to self-advocacy after TBI (effect size = 0.22). Similar improvements were found on secondary measures of general self-efficacy and satisfaction with life from baseline to posttreatment. However, significant between-groups gains for primary and secondary measures were not maintained over 6- and 12-week follow-up.
Conclusions:
Individuals living with chronic TBI sequelae can increase self-efficacy specific to self-advocacy, general self-efficacy, and satisfaction with life, through a TBI-specific intervention aimed at empowering individuals to advocate for their own needs and wishes. Sustaining gains over time may require ongoing community collaboration and support. This could involve community-based systems of self-advocacy education, resources, and peer support.
Keywords: chronic brain injury, group therapy, randomized controlled trial, self-advocacy, self-efficacy, self-management, satisfaction with life, traumatic brain injury
TRAUMATIC BRAIN INJURY (TBI) can lead to chronic changes in functioning, necessitating a lifelong need for resources not required prior to the injury such as behavioral health services, transportation, vocational supports, and financial assistance.1–6 Successfully accessing and making decisions about these resources can be difficult, requiring a clear understanding of one’s own needs, along with initiative, knowledge, and perseverance.3,7
Self-advocacy (SA), knowing and articulating oneʼs needs and wishes and making informed decisions to address them,8 is an important component of managing life after TBI and may involve efforts by both the individual and his or her family. This may occur within the community (accessing resources, finding employment, negotiating for services, resolving conflicts, etc) and at home (making wishes known to family, negotiating for independence, resolving family conflicts, etc). SA is one type of advocacy, along with systems advocacy (advocating for policy change) and peer advocacy (advocating for others with similar challenges). SA is conceptualized as a component of self-determination, making choices and decisions to direct the course of one’s own life.9,10 Self-management (managing aspects of one’s own chronic condition) and empowerment (helping someone gain control over their own life) are related concepts.11,12
This study reports on the trial of a psychoeducational intervention focused on SA after TBI. The Self-Advocacy for Independent Life (SAIL) program aims to empower individuals with the beliefs, knowledge, and skills of SA specific to TBI. The construct of SA is grounded in principles of self-efficacy.13–15 Self-efficacy, an individual’s belief in his or her ability to succeed at a task, is described as the basis for deciding what action to take and what effort to exert when facing challenges.16,17 Individuals with a high level of self-efficacy persevere through difficulties and have a sense of control over their own life. Those with low self-efficacy avoid challenges and are more susceptible to stress and depression.18,19 Self-efficacy is not only a key component of self-determination but also an important element of resilience, with high self-efficacy bolstering an individual’s ability to bounce back from challanges.18
Bandura17 describes 4 constructs for developing self-efficacy: performance accomplishments, vicarious experiences, verbal persuasion, and emotional arousal. These constructs are conducive to an interactive group treatment model, where participants exchange feedback and reinforcement, practice skills in a supportive environment, model and observe successful behaviors, and share support and encouragement. Group therapy theory relies on constructs of interpersonal learning and on the installation of hope and universality (realizing one is not alone in facing challenges).20
SA programs exist for individuals with various disabilities, for families of individuals with disabilities and for individuals with specific health conditions.14,21–27 These interventions focus on development of the self-efficacy, knowledge, and skills to advocate for oneself or one’s family.13–15,26,28 Research regarding SA after TBI has primarily focused on interventions targeting families advocating for an adult5 or child with TBI29,30 or for families and individuals with lived experience advocating together.31 There is a need to evaluate interventions focused on empowering the individual with TBI to advocate for himself or herself within the community and family. One challenge in applying SA to TBI is that successful SA requires self-awareness, learning new information, organization, problem solving, assertive communication, and tactful negotiation. These same cognitive, communicative, and regulatory abilities are commonly affected by TBI.32 Interventions addressing SA for individuals living with TBI must be adapted with these potential sequelae in mind.
The SAIL program addresses the beliefs, knowledge, and skills of SA specific to TBI. SAIL has undergone a history of development since its conception in the 1990s. Initially, interactive single-day workshops directed toward the family and the individual affected by TBI provided information, resources, and strategies for communication, problem solving, and accessing resources. The original SAIL Workbook (developed by a team of TBI professionals and individuals with lived experience, led by the primary author) is a resource guide outlining SA concepts and strategies, recommendations from experienced self-advocates, and information and resources.33 A manualized SAIL intervention was later developed by the primary author to more specifically address the cognitive needs of individuals living with the effects of TBI, adding emphasis on self-awareness/self-assessment, setting and attaining advocacy goals, compensatory strategies, and repeated practice of skills.34 A Group Member Guide outlines the intervention sessions, with homework assignments and session reviews to promote generalization.
PRELIMINARY DATA
Two preliminary SAIL studies addressed measurement and feasibility of efficacy research. Rasch analysis was employed to design TBI-specific outcome measures, resulting in the Self-Advocacy Scale (SAS) (assessing self-efficacy specific to SA) and the Personal Advocacy Activity Scale (PAAS) (assessing SA behaviors), with both instruments demonstrating strong psychometric characteristics.35 A 2-arm randomized feasibility study36 (n = 12) compared the SAIL intervention versus the workbook-only using outcome measures that included the SAS, PAAS, General Self-Efficacy Scale (GSE),37 and Satisfaction With Life Scale (SWLS).38 The study demonstrated feasibility for the methodology, showing positive trends on outcome measures, with the magnitude of change at posttreatment approaching significance in the SAS and the PAAS. These preliminary data were used to design the current randomized controlled trial.
Evaluation of the current trial was theoretically driven. Bandura17 suggested self-efficacy be investigated as a domain-specific construct. However, general self-efficacy, defined as an individual’s perception of his or her ability to perform tasks across various situations, has also been described.39 General self-efficacy is considered particularly useful when facing multiple demands related to a major event, such as an illness or injury.40 Both domain-specific self-efficacy and general self-efficacy are relevant when addressing SA after TBI.
Therefore, self-efficacy specific to advocating for oneself after TBI was selected as the primary outcome for this trial, measured by the SAS.35 General self-efficacy and SA behaviors were selected as secondary outcomes, along with measures of other constructs relevant to program outcomes, namely, satisfaction with life, flourishing, and participation. We hypothesized that, when compared with the control group, participants receiving the SAIL intervention would (1) demonstrate significantly greater improvements in self-efficacy specific to self-advocacy (SAS) from baseline to posttreatment; (2) maintain significantly greater improvements on SAS from baseline through 6- and 12-week follow-up; (3) have significantly greater improvements in SA behaviors, general self-efficacy, life satisfaction, flourishing, and societal participation from baseline to posttreatment and at 6- and 12-week follow-up.
METHODS
Design
This study was approved by HCA-HealthONE Institutional Review Board. A computer-generated block randomization sequence randomized “waves” of 16 participants to treatment or control. Block randomization was used to ensure that no more than 8 participants per wave were randomized to treatment (study treatment is designed for 6–8 participants at a time). There were 5 waves of participants enrolled, ranging in size from 12 to 16 participants.
Participants/location
Sixty-seven community-dwelling individuals enrolled. Inclusion criteria were as follows: TBI due to external mechanical force; more than 9 months post-TBI; discharged from inpatient or outpatient TBI rehabilitation; able to provide documentation of TBI; age 18 years or older; and able to travel independently in the community. Exclusion criteria were as follows: non–English-speaking; previously completed SAIL; participating in another clinical trial; and GSE score more than 32 (a high level of general self-efficacy). Two waves occurred in Englewood, Colorado, and 3 waves occurred in outlying geographic areas of Colorado including Grand Junction, Colorado Springs, and Westminster. Outlying locations were selected on the basis of potential availability of participants in areas not typically served by our institution’s research interventions. Four 3-hour interactive SAIL sessions took place in small meeting rooms within each community.
Procedure
Recruitment information was mailed to individuals who had been treated for TBI at our rehabilitation hospital, posted on our hospital’s website and social media sites, and provided to local organizations serving people with TBI. Individuals expressing interest completed a telephone screening to determine whether they met study inclusion/exclusion criteria.
Individuals meeting all study criteria completed informed consent with the study coordinator. Within 1 month before starting intervention, participants were randomized to treatment (n = 35) or control (n = 32). The study coordinator notified participants of concealed study allocation and instructed participants not to reveal this to staff completing study assessments. All assessments were administered over the phone by study personnel blind to randomization assignment.
Baseline assessment occurred 2 to 3 weeks before starting study intervention, and the study intervention lasted 10 weeks. All participants were reevaluated at posttreatment, 6-week follow-up, and 12-week follow-up (see Figure 1). Participants received $25 for each assessment, an additional $30 if they completed all assessments.
Figure 1.
SAIL Consort diagram. SAIL indicates Self-Advocacy for Independent Life.
Intervention
Treatment condition
Treatment participants underwent a 10-week SAIL intervention. The lead author, a licensed clinical social worker with extensive clinical TBI experience, provided the treatment (see Table 1). Sessions 1 to 3 took place on consecutive weeks, with the fourth session 3 weeks later, allowing time for practice toward goals. Each 3-hour session included scheduled breaks and refreshments.
TABLE 1.
Outline of SAIL intervention
| SAIL intervention |
|---|
|
|
| Session 1 (week 1) |
| • Introductions, sharing personal stories, importance of group process |
| • Defining SA After BI |
| Awareness of my strengths/challenges and needs |
| Knowledge of TBI and community resources |
| Strategies for self-care, organization and preparation, and effective communication |
| Practice in a supportive environment |
| • Self-assessment—Assessing my SA strengths and challenges; defining my SA team |
| • Goal-setting—Setting realistic SA goals based on my self-assessment |
| • Session review and home assignment |
| Session 2 (week 2) |
| • Review of session 1, goals, and home assignment |
| • Pillar 1: Take Care of Yourself |
| Emotionally, physically, cognitively, socially, and spiritually |
| • Pillar 2: Gather Information; Knowledge Empowers You |
| Understanding the brain and brain injury; community resources and how to access them |
| • Session review and home assignment |
| Session 3 (week 3) |
| • Review of session 2, goals, and home assignment |
| • Pillar 3: Become Organized and Prepared |
| Organizational strategies and tools; strategies for preparing for advocacy meetings/calls |
| • Pillar 4: Assertively Communicate and Negotiate |
| Assertive communication and active listening |
| • Session review and home assignment |
| Session 4 (week 6) |
| • Review of session 3, goals, and home assignment |
| • Review of 4 Pillars, Wellness Wheel, and Practice of Skills |
| • My SA Progress, New Goals, and My Advocacy Action Plan |
| Follow-up booster calls 1 (week 8) and 2 (week 10) |
| • Review progress on goals and Action Plan, problem solving |
Abbreviations: BI, brain injury; SA, self-advocacy; SAIL, Self-Advocacy for Independent Life; TBI, traumatic brain injury.
Participants received the SAIL Workbook as a resource, along with the session-by-session Group Member Guide. Sessions emphasized increasing self-efficacy and knowledge of TBI information/resources; group process to instill hope and universality; and learning/practicing strategies and skills in a supportive environment. Participants were encouraged to share knowledge, experiences, and feedback and to seek information/resources outside of the group. The Group Member Guide provides a road map for the group discussion, outlining SA concepts, information, and strategies, and includes worksheets for recording individual goals, plans, and problem-solving strategies. Discussion is elicited through Socratic questioning to allow participants a sense of ownership of information learned. Each session includes a review as well as a home assignment to be completed with a family member/support person to reinforce generalization. Participants developed individual self-advocacy goals as part of the intervention, based on self-assessment. Goal progress was monitored by the individual and the group to provide feedback and generalization. Individual booster phone calls at weeks 8 and 10 from the primary author offered additional feedback and problem solving to enhance maintenance and generalization.
After treatment, participants completed a brief, 5-item satisfaction survey to provide qualitative feedback on the SAIL intervention. Feedback revealed high satisfaction with the SAIL program (see Table, Supplemental Digital Content 1, available at: http://links.lww.com/JHTR/A447, for satisfaction survey questions and summary of responses).
Control condition
Control group participants did not receive the intervention but received the SAIL Workbook and Group Member Guide after all study assessments were completed.
MEASURES
Demographic, cognitive, and injury characteristics
Demographic information, cause of TBI, and severity of TBI as determined by the duration of loss of consciousness per the Ohio State University Traumatic Brain Injury Method (OSU TBI-ID)41 were collected at baseline. To characterize the symptomatic and cognitive status of participants, the following 2 assessments were administered at baseline:
The Neurobehavioral Symptom Inventory (NSI)42 is a 22-item self-report measure of physical, cognitive, affective, and sensory symptoms commonly experienced following TBI. Participants rate the extent to which each symptom has disturbed them in the last 2 weeks on a scale of 0 (none) to 4 (very severe). Total scores range from 0 to 88, with higher scores indicating greater TBI symptomatology.
The Brief Test of Adult Cognition by Telephone (BTACT)43 measures general cognitive function. Feasibility and utility of the BTACT in the TBI population have been established.44,45 Using methodology described by DiBlasio et al,46 a composite score was calculated from raw scores on the 6 BTACT subtests. Higher composite scores represent more favorable cognitive performance.
Outcome measures
The following measures were administered at all assessment points: baseline, posttreatment, 6-week follow-up, and 12-week follow-up.
Primary outcome measure
The SAS35 is an 8-item measure of domain-specific self-efficacy for SA after TBI. The SAS measures an individual’s current beliefs about his or her ability to produce desired self-advocacy outcomes. Examples of items include “I can get my questions answered during a meeting or call” and “I can keep track of important information that I need.” Items are scored on the basis of a response of 1 (not confident) to 4 (very confident). Total scores range from 8 to 32. Higher scores indicate that the respondent views himself or herself as having a high level of self-efficacy for SA. The SAS has been found to be reliable and valid.35
Secondary outcome measures
The GSE37 is a 10-item Likert scale assessing beliefs about one’s ability to cope with a variety of demands in life, a core component of SA (eg, “I can always manage to solve difficult problems if I try hard enough”). The GSE produces a single score ranging from 10 to 40, with higher scores indicating greater general self-efficacy. The GSE has been used in previous studies to assess general self-efficacy after TBI and stroke.47,48
The PAAS35 is a reliable and valid 12-item self-rating of personal advocacy behaviors. Participants indicate the frequency of various advocacy behaviors over the past 6 weeks by choosing 1 (not at all), 2 (1–4 times), or 3 (≥5 times). Total scores range from 12 to 36, with higher scores indicative of more personal advocacy behaviors.
The SWLS49 is a global measure of life satisfaction that has been validated in persons with TBI.38 Total scores on this 5-item Likert scale range from 5 to 35, with higher scores indicating higher global life satisfaction.
The Flourishing Scale (FS)50 is a global measure of self-perceived success in areas such as relationships, self-esteem, purpose, and optimism. Total scores on this 8-item Likert scale range from 8 to 56, with greater scores indicative of greater well-being.
The Participation Assessment with Recombined Tools–Objective (PART-O)51 is a 17-item objective measure of participation, representing functioning at the societal level, which may also improve as the result of greater self-advocacy skills. The PART-O has 3 subscales (Out and About, Productivity, and Social Relations) that contribute to a summary score ranging from 0 to 5, with higher scores indicating more participation.
Power and sample size calculations
Based on data from the preliminary pilot study,36 a sample size of 33 subjects per group had at least 80% power (α = .05) to detect a difference in the changes in the primary outcome measure (SAS) from baseline to posttreatment between the groups of at least 4.5 points, assuming the standard deviation (SD) at baseline was no larger than 6.4, and the correlation between the repeated measures was at least 0.5.
Statistical analysis
Statistical analyses were conducted using SAS version 9.4 (copyright 2016 SAS Institute Inc, Cary, North Carolina) assuming a significance level of α = .05, unless otherwise specified. Baseline demographic, cognitive, and injury characteristics were summarized by group using means and SDs for the continuous variables and frequency counts and percentages for categorical variables. Differences between groups at baseline were assessed using 2-samples t tests and chi-square tests for the continuous and categorical variables, respectively. Continuous variables that were not normally distributed were described using medians and interquartile ranges, and group differences were assessed using a Mann-Whitney U test. Each outcome was also summarized by treatment group using means and SDs across all time points.
Data were analyzed as intent to treat, using all available data from all participants. Each outcome was analyzed using a repeated-measures linear mixed-effects model. All models included fixed effects for the treatment group, assessment time, and the interaction between the treatment group and time. For each model, the omnibus test of the treatment by time interaction effect was first tested to determine whether the 2 treatment groups exhibited significantly different changes in the outcome variable over the duration of the study. If this interaction effect was significant (α = .05), then post hoc analyses were conducted to determine how the groups differed in their patterns of change from baseline to posttreatment. In particular, changes from baseline to posttreatment, 6-week follow-up, and 12-week follow-up were compared between groups using a Bonferroni adjustment (α < .05/3 = .0167) to control for multiple comparisons. Effect sizes (ESs) were estimated as the mean estimate (either the within-group change or the between-group difference in changes) divided by the square root of the model-based variance for each outcome at baseline.
All model results were tested for consistency and robustness using sensitivity analyses. For participants who had missing outcome data at a specific posttreatment week, a last observation carried forward imputation method was used and the respective models were run. Models were also run using only participants with complete data for each outcome and with 2 participants removed from the treatment group. These participants were randomized into the treatment group but could not attend any of the sessions. In addition, each respective outcome model was adjusted for by significant demographic, cognitive, and injury characteristics. Significance was determined through bivariate analyses with the outcome of interest.
RESULTS
The Consort diagram in Figure 1 summarizes the flow of participants in this study. Participants were 52 years of age on average, and the majority were male (55%), not employed (70%), had a greater than a high school diploma (90%), and had no military service (82%). All demographic, cognitive, and injury characteristics are summarized in Table 2 by the treatment group. The treatment groups did not differ significantly on any demographic or injury characteristics; however, the treatment group had significantly higher BTACT composite scores, indicating higher cognitive functioning.
TABLE 2.
Demographic and cognitive status at baseline, and injury characteristics
|
Control group (N = 32)
|
Treatment group (N = 35)
|
||||||
| n | Mean | SD | n | Mean | SD | P | |
|
| |||||||
| Age at injury | 32 | 51.1 | 12.6 | 35 | 52.8 | 16.7 | .6471 |
| Time since injury, median (IQR), y | 32 | 9.2 | 4.7–13.1 | 35 | 8.6 | 4.1–23.7 | .9851 |
| BTACT composite score | 32 | −0.8 | 0.9 | 35 | −0.2 | 1.1 | .0145 |
| NSI total | 31 | 35.7 | 16.8 | 35 | 36.2 | 15.0 | .9015 |
|
| |||||||
| n | % | n | % | ||||
|
| |||||||
| Gender | .9327 | ||||||
| Male | 17 | 53.1 | 20 | 57.1 | |||
| Female | 15 | 46.9 | 15 | 42.9 | |||
| Marital status | .8091 | ||||||
| Single (never married) | 8 | 25.0 | 11 | 31.4 | |||
| Married | 11 | 34.4 | 12 | 34.3 | |||
| No longer married | 13 | 40.6 | 12 | 34.3 | |||
| Education level | .6170 | ||||||
| High school diploma/GED or less | 3 | 9.4 | 4 | 11.4 | |||
| Some college | 11 | 34.4 | 14 | 40.0 | |||
| Bachelor’s degree | 11 | 34.4 | 7 | 20.0 | |||
| Graduate degree | 7 | 21.9 | 10 | 28.6 | |||
| Employment status | .1151 | ||||||
| Productive | 13 | 40.6 | 7 | 20.0 | |||
| Not productive | 19 | 59.4 | 28 | 80.0 | |||
| Living with | .9745 | ||||||
| Alone | 9 | 28.1 | 9 | 25.7 | |||
| Spouse/significant other | 14 | 43.8 | 16 | 45.7 | |||
| Others | 9 | 28.1 | 10 | 28.6 | |||
| Urbanicity | >.9999 | ||||||
| Suburban | 11 | 34.4 | 13 | 37.1 | |||
| Urban | 21 | 65.6 | 22 | 62.9 | |||
| Injury etiology | .3017 | ||||||
| Vehicular | 21 | 65.6 | 19 | 54.3 | |||
| Violence | 5 | 15.6 | 3 | 8.6 | |||
| Falls | 4 | 12.5 | 6 | 17.1 | |||
| Other | 2 | 6.3 | 7 | 20.0 | |||
| OSU TBI-ID (time of LOC) | .8561 | ||||||
| Mild (<30 min) | 4 | 12.5 | 5 | 14.3 | |||
| Moderate (30 min-1 d) | 5 | 15.6 | 7 | 20.0 | |||
| Severe (>1 d) | 23 | 71.9 | 23 | 65.7 | |||
| Military service | .1546 | ||||||
| No | 29 | 90.6 | 26 | 74.3 | |||
| Yes | 3 | 9.4 | 9 | 25.7 | |||
Abbreviations: BTACT, Brief Test of Adult Cognition by Telephone; GED, General Education Development; IQR, interquartile range; LOC, loss of consciousness; NSI, Neurobehavioral Symptom Inventory; OSU TBI-ID, Ohio State University Traumatic Brain Injury Method.
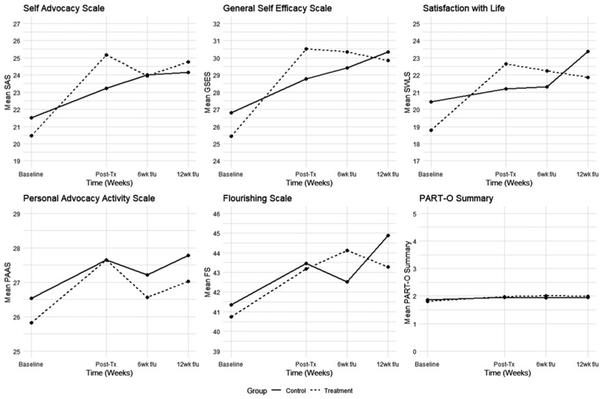
Table 3 displays the averages for each outcome across all time points for both the treatment and control groups. The model-based estimated changes from baseline to posttreatment and each follow-up within each group were calculated (see Table, Supplemental Digital Content 2, available at: http://links.lww.com/JHTR/A448, that shows adjusted changes from baseline), and the differences in changes between groups are summarized for each outcome in Table 4. The estimated means for each outcome across all endpoints from the repeated-measures models are plotted in Figure 2.
TABLE 3.
Average outcome scores across the time points
| Control (N = 32) |
Treatment (N = 35) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline, mean (SD) | Post-Tx, mean (SD) | 6-wk f/u, mean (SD) | 12-wk f/u, mean (SD) | Baseline, mean (SD) | Post-Tx, mean (SD) | 6-wk f/u, mean (SD) | 12-wk f/u, mean (SD) | |
|
| ||||||||
| SAS | 21.5 (3.5) | 23.2 (4.1) | 24.0 (4.6) | 24.2 (4.8) | 20.5 (3.9) | 25.2 (3.2) | 23.9 (3.8) | 24.8 (3.1) |
| GSE | 26.8 (3.0) | 28.8 (5.8) | 29.4 (5.9) | 30.3 (6.8) | 25.4 (4.2) | 30.5 (4.1) | 30.3 (4.2) | 29.9 (4.2) |
| SWLS | 20.5 (7.7) | 21.2 (9.7) | 21.3 (8.6) | 23.4 (8.1) | 18.8 (7.0) | 22.7 (7.5) | 22.3 (7.6) | 21.9 (7.8) |
| PAAS | 26.5 (4.0) | 27.7 (4.2) | 27.2 (4.3) | 27.8 (4.5) | 25.8 (4.1) | 27.7 (4.5) | 26.6 (3.4) | 27.0 (4.1) |
| FS | 41.3 (9.2) | 43.5 (8.8) | 42.5 (8.9) | 44.9 (8.0) | 40.7 (7.3) | 43.2 (10.8) | 44.1 (9.0) | 43.3 (9.1) |
| PART-O Summary | 1.9 (0.6) | 2.0 (0.6) | 1.9 (0.6) | 2.0 (0.7) | 1.8 (0.5) | 2.0 (0.7) | 2.0 (0.6) | 2.0 (0.6) |
Abbreviations: FS, Flourishing Scale; GSE, General Self-Efficacy Scale; PAAS, Personal Advocacy Activity Scale; PART-O, Participation Assessment with Recombined Tools–Objective; Post-Tx, posttreatment; SAS, Self-Advocacy Scale; SWLS, Satisfaction With Life Scale; wk f/u, week follow-up.
TABLE 4.
Adjusted changes from baseline on outcome measures
| Group | Endpoint | Estimate | SE | 95% CI | P a | Effect size |
|---|---|---|---|---|---|---|
|
| ||||||
| SAS | .0120 b | |||||
| Treatment-Control | Post-Tx | 3.00 | 0.97 | 1.06, 4.93 | .0029c | 0.22 |
| Treatment-Control | 6-wk f/u | 0.92 | 1.01 | −1.09, 2.94 | .3638 | 0.07 |
| Treatment-Control | 12-wk f/u | 1.56 | 1.04 | −0.51, 3.63 | .1375 | 0.11 |
| GSE | .0134 b | |||||
| Treatment-Control | Post-Tx | 3.05 | 1.21 | 0.64, 5.46 | .0139c | 0.23 |
| Treatment-Control | 6-wk f/u | 2.12 | 1.22 | −0.32, 4.55 | .0871 | 0.16 |
| Treatment-Control | 12-wk f/u | 0.70 | 1.31 | −1.91, 3.31 | .5923 | 0.05 |
| SWLS | .0086 b | |||||
| Treatment-Control | Post-Tx | 3.12 | 1.40 | 0.31, 5.92 | .0298b | 0.06 |
| Treatment-Control | 6-wk f/u | 2.07 | 1.41 | −0.75, 4.89 | .1480 | 0.04 |
| Treatment-Control | 12-wk f/u | −0.13 | 1.43 | −2.99, 2.73 | .9279 | 0.00 |
| PAAS | .8246 | |||||
| Treatment-Control | Post-Tx | 0.75 | 1.08 | −1.40, 2.91 | .4870 | 0.05 |
| Treatment-Control | 6-wk f/u | 0.06 | 0.84 | −1.61, 1.73 | .9434 | 0.00 |
| Treatment-Control | 12-wk f/u | −0.04 | 0.95 | −1.94, 1.85 | .9649 | 0.00 |
| FS | .1085 | |||||
| Treatment-Control | Post-Tx | 0.39 | 1.81 | −3.23, 4.01 | .8317 | 0.01 |
| Treatment-Control | 6-wk f/u | 1.20 | 1.82 | −2.44, 4.85 | .5119 | 0.02 |
| Treatment-Control | 12-wk f/u | −1.44 | 1.74 | −4.92, 2.04 | .4119 | 0.02 |
| PART-O Summary | .3754 | |||||
| Treatment-Control | Post-Tx | 0.10 | 0.08 | −0.07, 0.26 | .2488 | 0.28 |
| Treatment-Control | 6-wk f/u | 0.14 | 0.08 | −0.02, 0.30 | .0944 | 0.41 |
| Treatment-Control | 12-wk f/u | 0.13 | 0.09 | −0.05, 0.31 | .1588 | 0.38 |
Abbreviations: FS, Flourishing Scale; GSE, General Self-Efficacy Scale; PAAS, Personal Advocacy Activity Scale; PART-O, Participation Assessment with Recombined Tools–Objective; Post-Tx, posttreatment; SAS, Self-Advocacy Scale; SE, standard error; SWLS, Satisfaction With Life Scale; wk f/u, week follow-up.
P values in bold represent overall group by time interaction effects; P values not in bold represent pair-wise comparisons.
Significance level α = .05.
Bonferroni adjustment α = .0167 (.05/3).
Figure 2.
Outcome plots. Post-Tx indicates posttreatment; wk f/u, week follow-up.
Primary outcome
There were significant differences in changes in mean SAS change scores between groups over the study duration (treatment by time interaction P = .0120). Regardless of group, SAS scores increased from baseline to each endpoint. The within-group ESs ranged 0.12 to 0.19 for the controls and 0.25 to 0.34 for the treatment group. The treatment group showed significantly greater improvements from baseline to posttreatment than the control group by 3.0 units (P = .0029). The treatment group also showed greater improvements than the control group at 6-week follow-up (estimate = 0.92, P = .3638) and 12-week follow-up (estimate = 1.56, P = .1375); however, these differences were not significant. The between-group ESs ranged from 0.07 to 0.22.
Secondary outcomes
There were significant differences in changes in mean GSE scores (P = .0134) and mean SWLS scores (P = .0086) between groups over time. The treatment group showed significantly greater improvements from baseline to posttreatment by 3.05 points for the GSE (P = .0139) and by 3.12 points for the SWLS (P = .0298); however, difference in the SWLS did not remain statistically significant after adjusting for multiple comparisons. Between-group differences at 6-week and 12-week follow-up were not statistically significant for either outcome. There were not significant differences in the changes in the PAAS (P = .8246), FS (P = .1085), or PART-O (P = .3754) over time between groups.
Sensitivity analysis
All mixed-effects models were adjusted for relevant demographic, cognitive, and injury characteristics across each outcome. BTACT composite score was controlled for in all models with additional characteristics in the SAS (OSU TBI-ID), PAAS (Employment Status), FS (Employment Status and Urbanicity), SWLS (Military Service and Urbanicity), and PART-O Summary (Marital Status, Employment Status, and Living With) models. All mixed-effects model results were found to be robust after controlling for these relevant characteristics. All model conclusions also remained consistent when analyzing using last observation carried forward, subjects with complete data across all endpoints, and when removing subjects who did not receive the full treatment.
DISCUSSION
This study evaluated the efficacy of a manualized group SA intervention for individuals with TBI, emphasizing self-efficacy, self-assessment, goal-setting, assertive communication, and group support. Increased self-efficacy is an important outcome for individuals post-TBI who wish to manage and improve their lives. The treatment group demonstrated significantly greater improvement posttreatment than controls on the primary measure of self-efficacy specific to SA, supporting our primary hypothesis. Similar results were found for secondary measures of general self-efficacy and satisfaction with life. Participants also reported high levels of satisfaction with the program. These results offer insight into the building blocks of SA for those living with chronic TBI and related areas of self-determination and self-management.
The nonsignificant results at follow-up may be due to a variety of factors. Treatment gains may require ongoing support systems for this population of participants who experience challenges in retaining and organizing information, regulating emotions, and communicating needs. This finding is consistent with previous psychoeducational TBI trials.52 Treatment participants indicated (anecdotally and through feedback on the SAIL satisfaction survey) that they valued the group intervention and did not want it to end. Follow-up data were collected after the group ended, essentially taking away the supportive interaction the treatment participants valued. In contrast, control participants did not experience this loss of valued interaction, with their only study interaction (assessment calls from the data collector) continuing through follow-up.
It is unclear why the control group improved over time. Control participants did not receive treatment but did receive ongoing interaction from the data collector, which may have led to a desire to please the data collector. Social desirability (the tendency for study participants to present a favorable image of themselves) may have been a factor. In a review of studies using questionnaire-based research, almost half of those that assessed social desirability found that it influenced their results.53 Repeated exposure to assessment items provided control participants with an indication of the beliefs and behaviors targeted in the study, which may have been seen as the desired responses.54
Study limitations include use of a no-intervention control group. Future research of this intervention could include comparison with a control intervention. Another limitation is a sole reliance on self-report tools for outcome measures. Although the PAAS addresses SA activities, it is not clear whether the measures used fully capture behavior change, in addition to the noted changes in self-efficacy. In addition, the SAS, PAAS, and FS are still novel measures in the field, making it difficult to compare scores in the current sample with a broader TBI population.
CONCLUSION
Individuals living with the chronic effects of TBI, even those who are many years postinjury, can improve advocacy-specific self-efficacy, general self-efficacy, and satisfaction with life. SAIL offers a theoretically based program for empowering individuals living with TBI with self-efficacy and resources for SA. Continuation of gains postintervention may require an ongoing system of support. This could involve community networks providing SA peer support, feedback, skill practice, and up-to-date information and resources. Such networks could be under the umbrella of established TBI support groups or independent living centers. Peer-coaches could help facilitate this model, providing an opportunity for peer advocacy and self-efficacious activity for the peer-coaches. SAIL courses could be incorporated into community colleges as a preliminary course for those seeking further educational or vocational goals. SAIL groups specifically designed for family members advocating for a loved one would provide additional support by increasing family awareness, skills, and self-efficacy. Further research could address efficacy of a SAIL family intervention, as well as characteristics of optimal responders, efficacy of the intervention against a control intervention, and effectiveness of the intervention in various community settings. This study adds to a growing suite of evidence-based interventions to enhance community participation and self-determination after TBI in the chronic phase and reflects the importance of long-term supports.
Supplementary Material
Acknowledgments
The authors thank Richard Owens, consumer advisor, for his dedication and contributions to this study.
The contents of this article were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant no. 90DPT80007-01-00). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this article do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the federal government.
Footnotes
The authors have no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.headtraumarehab.com).
Contributor Information
Lenore Hawley, Research Department, Craig Hospital, Englewood, Colorado.
Clare Morey, Research Department, Craig Hospital, Englewood, Colorado.
Mitch Sevigny, Research Department, Craig Hospital, Englewood, Colorado.
Jessica Ketchum, Research Department, Craig Hospital, Englewood, Colorado.
Grahame Simpson, John Walsh Centre for Rehabilitation Research, Sydney School of Medicine, The University of Sydney, Sydney, New South Wales, Australia.
Cynthia Harrison-Felix, Research Department, Craig Hospital, Englewood, Colorado.
Candace Tefertiller, Research Department, Craig Hospital, Englewood, Colorado.
REFERENCES
- 1.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008; 23(6):394–400. doi: 10.1097/01.HTR.0000341435.52004.ac [DOI] [PubMed] [Google Scholar]
- 2.Heinemann AW, Sokol K, Garvin L, Bode RK. Measuring unmet needs and services among persons with traumatic brain injury. Arch Phys Med Rehabil. 2002;83(8):1052–1059. doi: 10.1053/apmr.2002.34283 [DOI] [PubMed] [Google Scholar]
- 3.Pickelsimer EE, Selassie AW, Sample PL, Heinemann AW, Gu JK, Veldheer LC. Unmet service needs of persons with traumatic brain injury. J Head Trauma Rehabil. 2007;22(1):1–13. doi: 10.1097/00001199-200701000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Degeneffe CE, Chang F, Dunlap L, Man D, Sung C. Development and validation of the Caregiver Empowerment Scale: a resource for working with family caregivers of persons with traumatic brain injury. Rehabil Psychol. 2011;56(3):243–250. doi: 10.1037/a0024465 [DOI] [PubMed] [Google Scholar]
- 5.Man DW. The empowering of Hong Kong Chinese families with a brain damaged member: its investigation and measurement. Brain Inj. 1998;12(3):245–254. doi: 10.1080/026990598122728 [DOI] [PubMed] [Google Scholar]
- 6.Tverdov AH, McClure KS, Brownsberger MG, Armstrong SL. Family needs at a post-acute rehabilitation setting and suggestions for supports. Brain Inj. 2016;30(3):324–333. doi: 10.3109/02699052.2015.1113566 [DOI] [PubMed] [Google Scholar]
- 7.Wilson L, Stewart W, Dams-OʼConnor K, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16(10):813–825. doi: 10.1016/S1474-4422(17)30279-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stodden RA, Conway MA, Chang KB. Findings from the study of transition, technology and postsecondary supports for youth with disabilities: implications for secondary school educators. J Spec Educ Technol. 2003;18(4):29–44. [Google Scholar]
- 9.Turnbull AP, Turnbull R. Self-determination: is a rose by any other name still a rose? Res Pract Persons Severe Disabil. 2006;31(1):83–88. [Google Scholar]
- 10.Wehmeyer ML, Abery BH. Self-determination and choice. Intellect Dev Disabil. 2013;51(5):399–411. doi: 10.1352/1934-9556-51.5.399 [DOI] [PubMed] [Google Scholar]
- 11.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1): 1–7. doi: 10.1207/S15324796ABM2601_01 [DOI] [PubMed] [Google Scholar]
- 12.“Empowerment.” Merriam-Webster.com. https://www.merriam-webster.com. Accessed October 6, 2020.
- 13.Wehmeyer M, Schwartz M. Self-determination and positive adult outcomes: a follow-up study of youth with mental retardation or learning disabilities. Except Child. 1997;63(2):245–255. [Google Scholar]
- 14.Test D, Fowler C, Wood W, Brewer D, Eddy S. A conceptual framework of self-advocacy for students with disabilities. Remedial Spec Educ. 2005;26(1):41–54. [Google Scholar]
- 15.Morton MV, Wehman P. Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Inj. 1995;9(1):81–92. doi: 10.3109/02699059509004574 [DOI] [PubMed] [Google Scholar]
- 16.Jones F Strategies to enhance chronic disease self-management: how can we apply this to stroke? Disabil Rehabil. 2006;28(13/14): 841–847. doi: 10.1080/09638280500534952 [DOI] [PubMed] [Google Scholar]
- 17.Bandura A Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191 [DOI] [PubMed] [Google Scholar]
- 18.Schwarzer R, Warner LM. Perceived self-efficacy and its relationship to resilience. In: Resilience in Children, Adolescents, and Adults. Springer; 2013:139–150. [Google Scholar]
- 19.Bandura A Exercise of personal and collective efficacy in changing societies. In: Bandura A, ed. Self-efficacy in Changing Societies. Cambridge University Press; 1995:1–45. [Google Scholar]
- 20.Yalom I, Leszcz M. The Theory and Practice of Group Psychotherapy. 5th ed. Basic Books; 2005. [Google Scholar]
- 21.Merchant D, Gajar A. A review of the literature on self-advocacy components in transition programs for students with learning disabilities. J Vocat Rehabil. 1997;8:223–231. [Google Scholar]
- 22.Roessler R, Brown P, Rumrill P. Self-advocacy training: preparing students with disabilities to request classroom accommodations. J Postsecondary Educ Disabil. 1998;13(3):20–31. [Google Scholar]
- 23.Curtin RB, Walters BA, Schatell D, Pennell P, Wise M, Klicko K. Self-efficacy and self-management behaviors in patients with chronic kidney disease. Adv Chronic Kidney Dis. 2008;15(2):191–205. doi: 10.1053/j.ackd.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Kramer JM. Identifying and evaluating the therapeutic strategies used during a manualized self-advocacy intervention for transition-age youth. OTJR (Thorofare N J). 2015;35(1):23–33. doi: 10.1177/1539449214564146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rusch D, Walden AL, DeCarlo Santiago C. A community-based organization model to promote Latinx immigrant mental health through advocacy skills and universal parenting supports. Am J Community Psychol. 2020;66(3/4):337–346. doi: 10.1002/ajcp.12458 [DOI] [PubMed] [Google Scholar]
- 26.The Arc of New Jersey. New Jersey Self-Advocacy Project. Accessed January 15, 2021. https://www.arcnj.org/programs/njsap/self_advocacy.html
- 27.Thomas TH, Donovan HS, Rosenzweig MQ, Bender CM, Schenker Y. A conceptual framework of self-advocacy in women with cancer. ANS Adv Nurs Sci. 2021;44(1):E1–E13. doi: 10.1097/ANS.0000000000000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noelle T Do you know the layers of self-advocacy. Published 2016. Accessed January 2021. https://www.nami.org/Blogs/NAMI-Blog/October-2016/Do-You-Know-the-Layers-of-Self-Advocacy
- 29.Glang A, McLaughlin K, Schroeder S. Using interactive multimedia to teach parent advocacy skills: an exploratory study. J Head Trauma Rehabil. 2007;22(3):198–205. doi: 10.1097/01.HTR.0000271121.42523.3a [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin KA, Glang A, Beaver SV, Gau JM, Keen S. Web-based training in family advocacy. J Head Trauma Rehabil. 2013; 28(5):341–348. doi: 10.1097/HTR.0b013e31824e1d43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AW, Moessner AM, Bergquist TF, Kendall KS, Diehl NN, Mandrekar J. A randomized practical behavioural trial of curriculum-based advocacy training for individuals with traumatic brain injury and their families. Brain Inj. 2015;29(13/14):1530–1538. doi: 10.3109/02699052.2015.1075173 [DOI] [PubMed] [Google Scholar]
- 32.Eslinger P, Zappala G, Chakara F, Barrett AM. Cognitive impairments after TBI. In: Zasler ND, Katz DI, Zafonte RD, eds. Brain Injury Medicine: Principles and Practice. Demos Medical Publishing; 2007:779–790. [Google Scholar]
- 33.Hawley L Self-advocacy after brain injury. In: SAIL—Self-Advocacy for Independent Life. Brain Injury Association of Colorado; 1992: 2008–2014. [Google Scholar]
- 34.Hawley L Self-advocacy for Independent Life, Group Member Guide. Self-Published, 2016. [Google Scholar]
- 35.Hawley L, Gerber D, Pretz C, Morey C, Whiteneck G. Initial validation of personal self-advocacy measures for individuals with acquired brain injury. Rehabil Psychol. 2016;61(3):308–316. doi: 10.1037/rep0000093 [DOI] [PubMed] [Google Scholar]
- 36.Hawley L, Gerber D, Morey C. Improving personal self-advocacy skills for individuals with brain injury: a randomized pilot feasibility study. Brain Inj. 2017;31(3):290–296. doi: 10.1080/02699052.2016.1250952 [DOI] [PubMed] [Google Scholar]
- 37.Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale. In: Weinman J, Wright S, Johnson M, eds. Measures in Health Psychology: A Userʼs Portfolio—Causal and Control Beliefs. NFER-NELSON; 1995:35–37. [Google Scholar]
- 38.Corrigan JD, Kolakowsky-Hayner S, Wright J, Bellon K, Carufel P. The Satisfaction With Life Scale. J Head Trauma Rehabil. 2013; 28(6):489–491. doi: 10.1097/HTR.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Gully SM, Eden D. Validation of a new General Self-Efficacy Scale. Organ Res Methods. 2001;4:62–83. [Google Scholar]
- 40.Luszczynska A, Scholz U, Schwarzer R. The General Self-Efficacy Scale: multicultural validation studies. J Psychol. 2005;139(5):439–457. doi: 10.3200/JRLP.139.5.439-457 [DOI] [PubMed] [Google Scholar]
- 41.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI identification method. J Head Trauma Rehabil. 2007;22(6):318–329. doi: 10.1097/01.HTR.0000300227.67748.77 [DOI] [PubMed] [Google Scholar]
- 42.Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. 1995;10(3):1–17. [Google Scholar]
- 43.Lachman ME, Agrigoroaei S, Tun PA, Weaver SL. Monitoring Cognitive Functioning: The Brief Test of Adult Cognition by Telephone (BTACT) Validation and Normative Data. Brandeis University; 2011. [Google Scholar]
- 44.Dams-OʼConnor K, Sy KTL, Landau A, et al. The feasibility of telephone-administered cognitive testing in individuals 1 and 2 years after inpatient rehabilitation for traumatic brain injury. J Neurotrauma. 2018;35(10):1138–1145. doi: 10.1089/neu.2017.5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson LD, Barber JK, Temkin NR, et al. Validity of the Brief Test of Adult Cognition by Telephone (BTACT) in level 1 trauma center patients 6 months posttraumatic brain injury: a TRACK-TBI study. J Neurotrauma. 2020. doi: 10.1089/neu.2020.7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiBlasio CA, Sima A, Kumar RG, Kennedy RE, et al. Performance of the brief test of adult cognition by telephone in a national sample. J Head Trauma Rehabil. Published online February 22, 2021. doi: 10.1097/HTR.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrence KA, Matthieu MM, Robertson-Blackmore E. Completion of a veteran-focused civic service program improves health and psychosocial outcomes in Iraq and Afghanistan veterans with a history of traumatic brain injury. Mil Med. 2017;182(7):e1763–e1770. doi: 10.7205/MILMED-D-16-00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones F, Mandy A, Partridge C. Changing self-efficacy in individuals following a first time stroke: preliminary study of a novel self-management intervention. Clin Rehabil. 2009;23(6):522–533. doi: 10.1177/0269215508101749 [DOI] [PubMed] [Google Scholar]
- 49.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 50.Diener E, Wirtz D, Tov W, New well-being measures: short scales to assess flourishing and positive and negative feelings. Soc Indicators Res. 2010;97:143–156. [Google Scholar]
- 51.Bogner JA, Whiteneck GG, Corrigan JD, Lai JS, Dijkers MP, Heinemann AW. Comparison of scoring methods for the Participation Assessment with Recombined Tools–Objective. Arch Phys Med Rehabil. 2011;92(4):552–563. doi: 10.1016/j.apmr.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 52.Whiting D, Deane F, McLeod H, Ciarrochi J, Simpson G. Can acceptance and commitment therapy facilitate psychological adjustment after a severe traumatic brain injury? A pilot randomized controlled trial. Neuropsychol Rehabil. 2020;30(7):1348–1371. doi: 10.1080/09602011.2019.1583582 [DOI] [PubMed] [Google Scholar]
- 53.Van de Mortel TF. Faking it: social desirability response bias in self-report research. Aust J Adv Nurs. 2008;25(4):40. [Google Scholar]
- 54.Polich G, Iaccarino MA, Kaptchuk TJ, Morales-Quezada L, Zafonte R. Placebo effects in traumatic brain injury. J Neurotrauma. 2018;35(11):1205–1212. doi: 10.1089/neu.2017.5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.