CONSPECTUS
The familiar pungent taste of spicy food, the refreshing taste of mint, and many other physiological phenomena are mediated by transient receptor potential (TRP) ion channels. TRP channels are a superfamily of ion channels that are sensitive to diverse chemical and physical stimuli and play diverse roles in biology. In addition to chemical regulation, some family members also sense common physical stimuli, such as temperature or pressure. Since their discovery and cloning in the 1990s and 2000s, understanding the molecular mechanisms governing TRP channel function and polymodal regulation has been a consistent but challenging goal. Until recently, a general lack of high-resolution TRP channel structures had significantly limited a molecular understanding of their function.
In the past few years, a flood of TRP channel structures have been released, made possible primarily by advances in cryo-electron microscopy (cryo-EM). The boon of many structures has unleashed unparalleled insight into TRP channel architecture. Substantive comparative studies between TRP structures provide snapshots of distinct states such as ligand-free, stabilized by chemical agonists, or antagonists, partially illuminating how a given channel opens and closes. However, the now ~75 TRP channel structures have ushered in surprising outcomes, including a lack of an apparent general mechanism underlying the channel opening and closing among family members. Similarly, the structures reveal a surprising diversity in which chemical ligands bind TRP channels.
Several TRP channels are activated by temperature changes in addition to ligand binding. Unraveling mechanisms of thermosensation have proven an elusive challenge to the field. Although some studies point to thermosensitive domains in the transmembrane region of the channels, results have sometimes been contradictory and difficult to interpret; in some cases, a domain that proves essential for thermal sensitivity in one context can be entirely removed from the channel without affecting thermosensation in another context. These results are not amenable to simple interpretations and point to allosteric networks of regulation within the channel structure.
TRP channels have evolved to be fine-tuned for the needs of a species in its environmental niche, a fact that has been both a benefit and burden in unlocking their molecular features. Functional evolutionary divergence has presented challenges for studying TRP channels, as orthologs from different species can give conflicting experimental results. However, this diversity can also be examined comparatively to decipher the basis for functional differences. As with structural biology, untangling the similarities and differences resulting from evolutionary pressure between species has been a rich source of data guiding the field. This Account will contextualize the existing biochemical and functional data with an eye to evolutionary data and couple these insights with emerging structural biology to better understand the molecular mechanisms behind chemical and physical regulation of TRP channels.
Conspectus Figure
1. Introduction
Much of biology is determined by relative concentrations of ions across biological membranes. These concentration gradients are tightly regulated and used to transmit signals typically via ion channels. Ion channels are pore-forming proteins embedded in lipid bilayer membranes. Hundreds of different ion channels exist, with vast structural and functional diversity, but in principle, all work the same way: the channel pore has one or more "gates" that can open and close in response to specific stimuli such as chemical ligands, electrical potential across the membrane, temperature, or mechanical force. When open, ions freely flow through the pore down their concentration gradient forming cascades of information transmission.
Transient receptor potential (TRP) ion channels make up a diverse superfamily of membrane proteins that are widely expressed in higher organisms. Based on sequence homology this superfamily is divided into seven subfamilies, six of which are found in humans (Figure 1 and Tables S1 and S2).1 Canonically the best studied ion channels are “gated”, that is opened or closed, by either chemical ligands or changes in potential (i.e., voltage) across the membrane. A feature that appears common to many TRP channels is responsiveness to multiple types of stimuli, such as chemical, electrical, thermal, pH, and mechanical stimuli. Diverse classes of endogenous lipids also provide a variety of regulatory effects on TRP channels and can act as cofactors required for function or even as direct agonists and antagonists.2,3 Channel activity is further modulated by phosphorylation, which can directly affect channel gating or modify membrane trafficking.4-7 Presumably because of their role as polymodal sensors, TRP channels are expressed in a variety of tissues and function broadly in physiology. Because a given TRP channel integrates diverse physical and chemical stimuli, a thorough understanding of the molecular mechanisms, including the interplay between stimuli and the similarities and differences between TRP channels, has been complicated and often controversial.
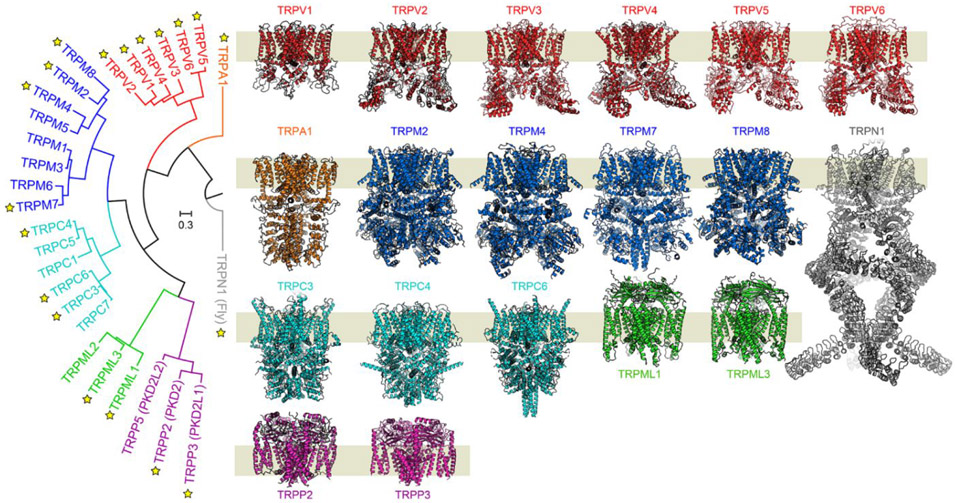
Figure 1. TRP channel evolutionary relationships and representative structures.
Left, a phylogenetic tree of human TRP channels, including an ancestral non-mammalian TRPN1 channel (Gray). Yellow stars indicate that a structure of either the human or an ortholog channel has been determined. To date, at least two structures have been determined from each human TRP subfamily, with exception of TRPA1, where there is a lone human subfamily member. Representative structures have been determined for the entire vanilloid (TRPV) subfamily. The structures reveal a conserved general transmembrane architecture with highly diverse extramembrane loops and N- and C-terminal domains. The collective structural information has shaped understanding of how TRP channels gate in response to chemical and physical stimuli. TRPA is for ankyrin, -V for vanilloid, -M for melastatin, -C for canonical, -ML for mucolipin, -P for polycystic.
Since the cryo-EM structures of TRPV1 reported in 2013, ~75 structures representing orthologs to about two-thirds of human TRP channels have been determined (Figure 1).8,9 This advancement has arisen primarily from developments in cryo-electron microscopy (cryo-EM). As a result, TRP channel structures with resolutions between 2.9–5 Å have provided unprecedented insight into the molecular architecture of channels from various TRP subfamilies and bound in different states to a variety to chemical ligands. These structures show that TRP channels have widely divergent termini and loop regions. The only conserved structural features across all TRP channels are two transmembrane structural domains and the amphipathic “TRP” helix (Figure 2). The conserved membrane regions include a four-helix bundle domain comprising the S1-S4 transmembrane helices that have been referred to nondescriptly as the S1-S4 domain or, because of homology to voltage-sensing domains found in voltage-gated channels, the voltage-sensing like domain (VSLD). The S5-S6 transmembrane helices tetramerize to form the pore domain (PD) where ion conduction occurs. Following the S6 transmembrane helix is the conserved amphipathic TRP helix that lies along the intracellular plane of the membrane. Besides these features shared across all TRP channels, other features are also shared between a few subfamilies. TRPA, TRPM, and TRPC channels have a C-terminal coiled-coil domain, thought to mediate and stabilize the tetrameric assembly. TRPA, TRPV, and TRPN channels have N-terminal ankyrin repeats that are capable of transducing conformational change to the pore. While TRP channels are typically considered homomeric, and indeed all current structures recapitulate this, there is evidence which suggests these channels can form heteromeric channel assemblies between TRP family members10 or complexes between two homomeric TRP channels.11 Alternative splicing isoforms of TRP channels further increase the complexity of regulation and diversity of function.12 TRP channel function is further fine-tuned by other protein interactions. For example, TRPV1, the canonical heat and capsaicin sensor, has been implicated in interactions with at least 94 non-TRP channel proteins.13
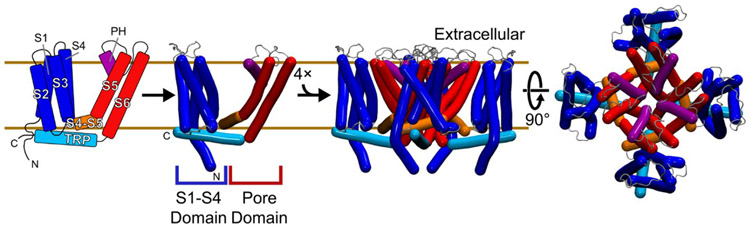
Figure 2. The conserved transmembrane architecture of TRP ion channels.
A TRP channel monomer (left panels) contains six transmembrane helices, including two conserved structural domains. The S1-S4 ligand sensing domain (blue) is a four-helix bundle of first four transmembrane helices, S1 to S4. The last two transmembrane helices, S5 and S6, form the pore domain (PD, red) where the tetramer of the PD forms the conductance pathway of the channel. The S1-S4 domain and the PD are linked by an S4-S5 linker (orange). Two additional conserved helices are the pore helix (PH, purple) and the amphipathic TRP helix (cyan). A functional channel is composed of a domain-swapped tetramer, with the PD helices interacting with adjacent subunits (right panels). Structural and functional studies suggest that allosteric networks between binding, temperature sensing, and other stimuli regulate these channels.
Numerous TRP channel structures have provided detailed information, such as domain architectures, domain packing, and key insights into chemical ligand-induced gating mechanisms.8,9 Despite significant successes in TRP channel structural biology and a well conserved transmembrane domain (TMD, helices S1-S6, Figure 2) architecture, surprisingly a general TRP channel gating mechanism has not emerged from the structural biology. Indeed, it is possible that the ability to detect a variety of stimuli and the diverse N- and C-terminal structural features preclude a single conserved gating mechanism across all the channels. Notwithstanding these challenges, and as outlined more thoroughly below, the conserved TMD seems to be central to function. Interactions between the TRP helix, the S1-S4 domain, and a linker helix between the S4 and S5 helices are crucial to transducing stimuli from peripheral domains to gate the PD. Apart from the TMD and TRP helix, there are no domains that are present in all TRP channels, indicating the importance of these regions. Coupled with the recent structural information, TRP channel function is enriched and complicated by species-specific diversity, with some channels activated by a particular chemical while the same chemical inhibits the equivalent (orthologous) channel in a different species.14 There are also reports characterizing a given thermosensitive TRP channel as a heat sensor in one species and a cold sensor in another.15 Speciation can also extend to partner proteins that modulate the channels, such as opposite effects of the PIRT protein on human and mouse orthologs of TRPM8.16 The emerging examples of species dependent function indicate that TRP channels are functionally plastic and that allosteric networks regulate channel function. In this Account, we highlight TRP channel structural and functional studies and leverage evolutionary and genetic data with the goal of identifying similarities and differences between family members. We also focus on the emerging role of allostery in functional regulation, with “function” defined as gating of the channel pore, and propose that TRP channels are a model system to probe and understand the fundamentals of allostery in biomolecular systems.
2. Biochemical insights from genetics and evolution
After the identification of the fly trp gene in 1989,17 hundreds of TRP channels have since been identified primarily from organisms in the phylogenetic kingdom of Animalia and spanning a wide variety of animals including fish, sea squirts, rodents, flies, and humans. Each species genome typically encodes about 15-30 TRP channels, with 27 found in humans. Beyond animals, a few examples of TRP channels have been identified in fungi and non-land plants.18 To date, no TRP channels have been identified in bacteria nor archaea.
Evolutionary-based studies of protein orthologs from distinct species with variable physiologies can be leveraged to help elucidate molecular mechanisms, either through direct comparative studies or by systematic analysis to identify different regions and their susceptibility to genetic variation. Evolutionary analysis of the menthol and cold-sensing receptor TRPM8 indicates that it is found only in vertebrates and emerged about 400 MYA during vertebrate evolution.19 Analysis of evolutionary conservation of TRPM8 orthologs indicates that diverse regions of TRPM8 have evolved with distinct selection pressures.20 Presumably channel regions that are most well conserved are central to core channel function. Not surprisingly, the most highly conserved TRPM8 regions are in the TMD and include the S3, S4, S5, and S6 transmembrane helices from the S1-S4 domain and the PD respectively. Two small membrane regions including the intracellular side of the S4 helix, S4-S5 loop, and intracellular S5 region that connect the S1-S4 domain to the PD appear to be completely conserved across the ~40 TRPM8 orthologs that were sequenced at the time of the study.19 These conserved regions in TRPM8 are reminiscent of the regions in the heat and capsaicin receptor TRPV1 vanilloid binding site.
Another direct result from expansive sequencing and evolutionary data is leveraged from statistical models of protein sequence evolution that can be used to identify residues that coevolve. This has been successfully employed and validated in structure prediction algorithms by identifying interacting residue pairs21 but also provides insight into conformational changes, critical functional residues, and protein-protein interaction information.22 Looking at the evolutionary couplings of TRPM8 and TRPV1 TMD regions, interesting patterns emerge (Figure 3A). While the technique is typically used to identify residue-residue contacts (i.e., residues that are close in space), the TRPM8 and TRPV1 coevolutionary analysis shows that there are many evolutionary couplings that are spatially far apart. This suggests that these channels are allosterically regulated, and the patterns of evolutionary couplings are consistent with allosteric communication between the S1-S4 domain and PD. Another feature to emerge from this analysis is that these coevolution patterns of allosteric networks between TRPV1 and TRPM8 are distinct. The differences indicate that there are likely some conserved activation and regulation mechanisms between TRPV1 and TRPM8, focused on coupling the S1-S4 domain to the PD via the S4-S5 linker. However, other distinct types of allostery and or mechanistic conformational changes might have emerged through evolution.
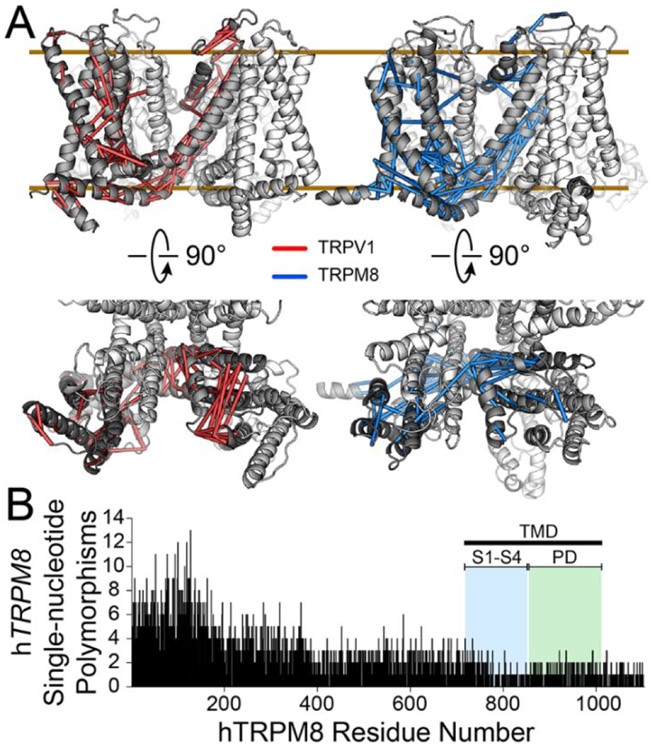
Figure 3. Insights from evolutionary studies.
A) TRPM8 and TRPV1 show distinct patterns of coevolution. Using GREMLIN software the 100 highest probability predicted coevolving residues were plotted on homology models of the human TRPV1 (red) and TRPM8 (blue) TMD regions (including helices S1-S6), with pseudo-bonds shown between coevolving pairs. The analysis identifies coevolution of the intracellular S1-S4 domain to the pore domain. However, patterning differences of the evolutionary constraints suggests there are distinct mechanisms and allosteric networks. B) The frequency of exonic human TRPM8 single-nucleotide polymorphisms (SNPs) as a function of residue number. A decreased SNPs frequency in the TMD indicates that this region is less tolerant of mutations. Data were aggregated from the Ensembl database searching exclusively deposited human genomes.
Significant advances in DNA sequencing technology and the ease of obtaining human genetic information can also help to elucidate regions that are most prone to mutations and therefore presumably least crucial to function. Our analysis of human genomes found in the Ensembl database identify 26,975 human TRPM8 variants including insertions, deletions, and single nucleotide polymorphisms. Analysis of the human TRPM8 variants reflects frequency and tolerance to divergence and shows that this variance is not random. Figure 3B plots the exonic single nucleotide polymorphisms (SNPs) of the human TRPM8 gene as a function of protein residue number. As expected from protein domain conservation across species and from TRP structural studies, the TRPM8 TMD shows fewer SNPs than other domains, suggesting its importance in TRPM8 function. This general trend is shown for additional TRP channels that are similarly analyzed in Figure S1.
Analysis of TRP channel genetic data provides important insights into function, identifying the TMD as crucial to function and suggesting the importance of allosteric mechanisms. Leveraging the similarities and differences between orthologous and paralogous TRP channels has also provided biochemical insight into how these channels are gated. Although many of these channels are polymodally regulated, significant inroads have been made in understanding how chemical ligands regulate TRP channels and similarly, a framework for temperature activation is beginning to emerge. One constant from these comparative studies is that distal regulation of function, or allostery, is key in understanding the biochemical mechanism.
3. Mechanisms of ligand gating
Many TRP channels are regulated by a variety of diverse chemical ligands, and structural studies are beginning to identify intriguing mechanistic commonalities and surprising differences between TRP channels. Among the best studied is TRPV1, which is activated by elevated temperatures and sensitive to a variety of ligands including the vanilloid compound capsaicin. Structural, functional, and computational studies of TRPV1 have delineated a clear vanilloid binding pocket and mechanism for TRPV1 ligand activation.23,24 Protein engineering studies of two non-vanilloid activated channels, TRPV225,26 and TRPV327, have shown that this binding site is likely conserved as are the mechanistic framework, at least in the TRPV family and potentially other TRP channels.28-32 One key feature that emerges from the TRPV3 vanilloid engineering study is that allostery plays a significant role in ligand activation.27
Highlighting the diversity of allosteric mechanisms of ligand activation, TRPA1 is uniquely activated via covalent modification by pungent electrophilic compounds such as allyl isothiocyanate and diallyl disulfide, found in wasabi, mustard oil, or garlic. These compounds covalently bond with cysteine residues in the N-terminal ankyrin repeat and pre-S1 regions. This induces conformational changes that propagate from the modification site to the pore.33 A final unique example is allosteric regulation of TRPM7 by a kinase domain, which mediates nucleotide inhibition of the channel.34 Human TRPM2, on the other hand has an evolutionary inactivated enzyme domain that still retains the ability to bind the historical substrate and this binding regulates channel activity allosterically.35 Allostery seems to emerge as a key contributor in TRP channel ligand activation as highlighted below.
Menthol ligand-gating in TRPM8
While experimental, computational, and structural data identify the vanilloid binding site in TRPV1, the TRPM8 menthol binding site is more controversial. Early mutagenesis data identified residues in the S1-S4 domain that selectively abrogate TRPM8 menthol sensitivity while leaving intact cold activation.36 From this and other studies, Y745 (S1) and Arg842 (S4) have emerged as potential TRPM8 residues involved in menthol binding. Later radioligand binding studies of 3H-labeled menthol showed that the mutation Y745H decreases TRPM8 affinity for the radioligand, suggesting that it may be directly involved in menthol binding.37 More recently an NMR and microscale thermophoresis (MST) binding study of an isolated human TRPM8 S1-S4 domain showed that menthol and WS-12, a menthol analog and more potent TRPM8 agonist, directly bind to this domain.38 The authors further tested the Y745H and R842H mutations in the isolated S1-S4 membrane domain, but unexpectedly neither impacted menthol affinity as monitored by NMR or MST. With the flood of TRPM cryo-EM structures, including TRPM2, TRPM4, TRPM7, and TRPM8, it is clear that the equivalent tyrosine and arginine residues for Y745 and R842 in TRPM8 are structurally conserved. These residue identities are also conserved across the human TRPM family. Given that TRPM8 is the only menthol-sensitive TRPM channel, it seems unlikely that either Y745 or R842 are key determinants for menthol selectivity. This idea is supported by the identification of a TRPM8 isoform (isoform 4) protein in epidermal cells that is missing parts of the N-terminus and a region of the TMD that includes helices S1 and S2.39 The epidermally expressed TRPM8 isoform 4 trafficks differently from the full-length protein but retains key functions such as cold sensitivity and activation by menthol and other cooling agents, namely WS-12 and icilin. Convoluting these data are recent agonist bound structures of TRPM8 identifying overlapping but distinct binding sites for WS-12 and icilin in an intracellular side cavity of the S1-S4 domain between helices S1-S4.40 Presumably the aggregate data suggest that Y745 modulates menthol sensitivity allosterically since mutating the residue influences TRPM8 menthol sensitivity and in the context of the full channel reduces affinity. However, TRPM8 isoform 4, which lacks the S1 helix that includes Y745, retains menthol sensitivity. It is clear from structural studies that TRPM8 and TRPV1 have distinct agonist binding sites.8,40 Nonetheless, there is experimental evidence of reciprocal ligand effects between TRPM8 and TRPV1. The TRPM8 agonist menthol inhibits TRPV1 capsaicin activation, and the TRPV1 agonist capsaicin antagonizes menthol evoked TRPM8 currents.41 Additionally, an early study published before the identification of TRPM8 used 3H-labeled menthol for radioligand binding studies in whole cell membranes, and among other things noted that labeled menthol is displaced by TRPV1 agonist capsaicin and antagonist capsazepine.42 Menthol has also been implicated in activating or inhibiting other TRP channels, like TRPV3 and TRPA1.43,44 While TRPM8 ligand binding is coming into focus, the allosteric relationships and mechanisms of activation are still relatively opaque but emerging evidence suggests that there is likely mechanistic overlap between TRP channels.
The convoluted case of 2-APB
While the canonical vanilloid binding site seems to be conserved in TRPVs, clear mechanistic relationships between ligand regulation of TRP channels is still unresolved. The compound 2-APB (2-aminoethoxydiphenyl borate) modulates the activity of channels from at least the TRPA, -M, -V, and -C families. Such a compound presumably should provide tremendous insight into what constitutes conserved mechanisms of chemical activation. 2-APB regulates five of six TRPV family members, activating TRPV1, V2, and V3, and inhibiting TRPV5, and V6.45-47 Structures from TRPV3 and TRPV6 have been determined in the presence of 2-APB which activates or inhibits the respective channel. The 2-APB bound TRPV6 structure identifies the inhibitor on the intracellular side in the S1-S4 domain that is distinct from the canonical vanilloid binding pocket.47 The proposed mechanism whereby 2-APB inhibits TRPV6 is allosteric in nature where conformational rearrangements in the S1-S4 domain, specifically motions in the S3 helix, pull on the S4-S5 linker shifting an activating lipid that binds at the interface between the S1-S4 domain and the PD, which ultimately results in channel inhibition.
In contrast, the TRPV3 structure identifies three different binding sites for 2-APB: one near, but distinct from the intracellular S1-S4 TRPV6 binding site, one below the intracellular TRP-helix, and one in the extracellular side of the S1-S4 domain (Figure 4A).48 According to this proposed activation mechanism of TRPV3, 2-APB binds the extracellular side of the S1-S4 domain, causing the S1-S2 loop to move; this results in the release of an extracellular leaflet lipid which couples 2-APB binding and allosteric gating across the membrane. The authors conclude that the extracellular binding site is key for TRPV3 activation since 2-APB binding in the other two intracellular sites were populated in closed state structures.49 We note that other TRPV structures have a lipid binding site near that in the TRPV3 structure, raising potential that this may be a non-specific binding site.
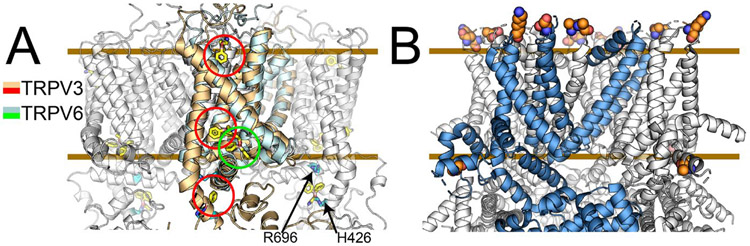
Figure 4. Allosteric coupling in ligand and temperature activation.
Panel A shows structures of 2-APB bound TRPV3 and TRPV6 in gold and cyan respectively. Between the two structural studies, four 2-APB binding sites have been identified in related TRPV channels. These coupled with functional studies suggest that in 2-APB ligand regulation of TRP channels allostery is key to modulating function. Panel B identifies residues essential to exchanging cold-sensing properties between TRPM8 channels from hibernating and non-hibernating rodents. The six residues are far away in space indicating that allosteric networks are key to deciphering these outcomes. Comparing these six mutations with human TRPM8 show that not only are allosteric networks important but also that there must be compensating pairs of residues that impact allostery.
The TRPV6 residues near the 2-APB binding site were also functionally studied, and a tyrosine residue in S4 (Y467A) was identified that increases 2-APB inhibitory potency of TRPV6.47 This tyrosine in the S4 helix is conserved across the TRPV family, and the same mutation made in mouse TRPV3 caused a 20-fold increase in 2-APB agonist potency.48 This complicates the interpretation of 2-APB in activating TRPV3, suggesting that either TRPV6 and TRPV3 share the 2-APB binding site, in contradiction of the structural data, or imply that multiple 2-APB binding sites contribute to TRPV3 activation but only one to TRPV6 inhibition. Another interpretation could be that regulation is achieved by allosteric coupling between the binding sites.
To complicate 2-APB modulation of TRPV channels further, a high-throughput mutagenesis functional screen of ~14,000 mutations in mouse TRPV3 identified two key residues (H426 and A696) that reside near the TRP-helix 2-APB binding site that was deemed non-activating in the TRPV3 structure studies.50 TRPV4 has not been reported to be modulated by 2-APB; however, by mutating the equivalent residues (N456 and W737) to histidine and arginine respectively, the mutated TRPV4 becomes sensitive to 2-APB activation. Taken together, these three studies suggest a complicated binding landscape for TRPV family modulation by 2-APB, where allostery and multiple binding sites in and/or near the S1-S4 domain couple to channel gating in the S5-S6 PD.
These studies clearly show that allosteric coupling between distinct binding sites in the S1-S4 domain and pore domain gating is a common mechanism to regulate TRP channel function. Figure 5 illustrates the relative structural rearrangements of the TRPV3 and TRPV6 S1-S4 domain induced by 2-APB that results in activation and inhibition, respectively. Other motions of S1-S4 domain in TRPV family gating are also shown, highlighting the apparently disparate structural mechanisms and diverse allosteric coupling TRPV channel gating.
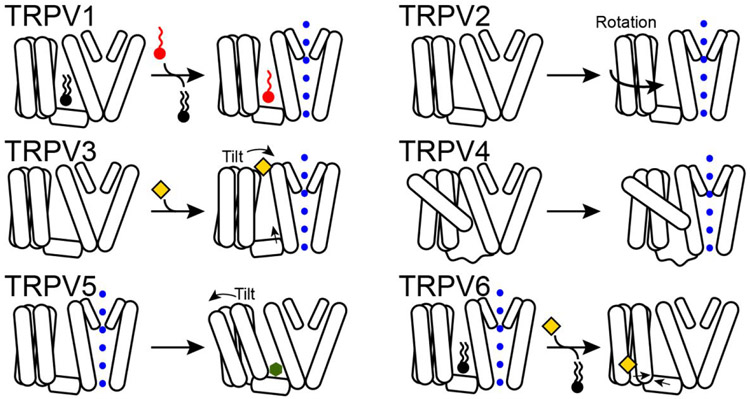
Figure 5. Diverse S1-S4 gating-coupled movements identified from TRPV structural studies.
In TRPV1, the S1-S4 domain remains immobile while a residing lipid (black rectangle) is replaced by a vanilloid ligand (red circle). In TRPV2, the S1-S4 domain, along with intracellular ankyrin repeat domains, rotates when the lower gate opens. In TRPV3, the S1-S4 domain tilts towards the PD when the agonist 2-APB (yellow diamond) binds to the S1-S4 domain. TRPV4 gating details are presently unclear, but a unique S1-S4 arrangement causes the S3 helix to contact S6 helix. In TRPV5, the S1-S4 domain tilts away from the PD when the antagonist econazole (dark green hexagon) enters the binding pocket, causing the lower gate to close. In TRPV6, the inhibitor 2-APB (yellow diamond) causes the S3 helix to move toward S4-S5 linker to close the lower gate.
4. Mechanisms of temperature gating
Eleven TRP channels from the TRPA, TRPV, TRPC, and TRPM families can integrate biological scale temperature changes into conformational change and eventually signal transduction. These channels, including TRPM8 and TRPV1, are inherently and acutely thermosensitive;51,52 however, the mechanisms and conformational changes associated with thermosensing are currently unknown. A number of studies implicate cross-talk between TRP channel thermal and ligand activation, in which small amounts of chemical agonist potentiate the channel to be more temperature sensitive.53 This is also recapitulated in the aforementioned TRPV3 vanilloid engineering studies in which a form of TRPV3 is RTx insensitive until it has been prestimulated with heat.27 While chemical and thermal activation are linked to some extent, there is also evidence of distinct mechanistic pathways; for example, TRPV1 channels maximally stimulated with high concentrations of capsaicin nevertheless show increased current when temperature is simultaneously elevated.54 Reciprocity between chemical and temperature activation has also been observed at the organismal level, for example, chemical-based antagonism of TRPM8 in rodents transiently decreases body temperature.55 Alternatively, chemical activation of TRPM8 triggers thermogenesis increasing metabolic output and has been suggested as a mechanism to chemically regulate obesity.56,57 Indeed, there is a correlation between a species body temperature set point and TRPM8 activation temperature, suggesting that the temperature sensitivity is relatively plastic between orthologs.20,58 The fact that a given thermosensitive TRP channel will respond to temperature in a species dependent manner indicates a potential direction for investigating thermosensing mechanisms.
The regions that are directly sensitive to temperature and mechanisms that underlie the structural rearrangements are not currently clear and diverse regions have been implicated in modulating thermosensing.14,59-61 Nonetheless, the thermodynamics that forms the basis of TRP channel temperature sensitivity likely mirror standard protein folding/unfolding frameworks. Suitably large temperature-induced thermodynamic driving forces can arise from entropic and enthalpic contributions to charged or hydrophobic residues sequestered to or released from the hydrophobic membrane bilayer or the aqueous environment (entropic) and balanced by local changes in secondary structure, such as an α-helix forming or unfurling (enthalpic) to give biologically accessible changes in free energy. One can also think about the conformational changes in terms of changes in heat capacity (ΔCp); however, ΔCp can be thought of in terms of how temperature impacts enthalpy, entropy, or free energy. Similarly, it has long been recognized that the hydrophobic effect is temperature dependent suggesting that changes in hydrophobic exposure as a function of temperature is likely key to the inherent TRP channel thermosensitivity. Experimental data supporting these thermodynamic principles are beginning to emerge. For example, engineering changes in hydrophobic accessibility of a temperature insensitive voltage-gated potassium ion channel endowed the channel to be temperature sensitive.62 A separate study performed a high-throughput functional screen of ~7,300 random mutants of rat TRPV1 for ligand and temperature sensitivity.63 This impressive feat did not explicitly identify the structural domains or mechanisms that give rise to thermosensitivity, but two crucial insights emerge from the data. First, TRPV1 mutations that decrease hydrophobicity are generally less tolerated for retention of temperature activation relative to chemical activation by capsaicin. Second, in general all TRPV1 regions and domains are functionally tolerant to incorporation of mutations, emphasizing that allosteric networks across the channel must be important. Still, there are hints that the core thermosensing regions are found in the TMD and that these structural domains are sufficient for temperature activation. For example, the engineered temperature-sensing voltage-gated potassium channel mentioned above, relied exclusively on mutations in the equivalent S1-S4 domain, indicating that a single domain can be sufficient to confer thermosensitivity.62 On the other hand, transferring the TRPV1 PD to a non-thermosensitive channel scaffold is also sufficient to confer thermosensitivity.64 Although these results implicate different regions in thermosensing, it is noteworthy that they are both in the transmembrane domain. Given that the TMD is the most conserved feature across TRP channels, it seems likely that this region is central to mechanisms of thermal activation.
While the key regions involved in thermosensing may be narrowed down to the TMD, studies attempting to identify more specific domains responsible for temperature activation have given seemingly contradictory results, as illustrated by the following examples of TRPV1, TRPM8, and TRPA1. This lack of an identified thermosensing domain suggests allosteric modulation is also present in thermosensing. The first high-resolution TRP channel structure was a cryo-EM structure of a truncated rat TRPV1.9 The rTRPV1 truncation removed 109 residues from the N-terminus, 23 residues from the pore turret loop, and, and 74 residues from the distal C-terminus. The final construct represents 632/838 residues or about 75% of the wild-type rat protein. Despite these deletions, the truncated channel behaves well structurally and recapitulates the general features of the biological channel including sensitivity to capsaicin and other vanilloids, pH, and elevated temperatures. In an unrelated study of mouse TRPV1 thermosensitivity, it was found that exchanging a 14 residue subset of the pore turret in mouse TRPV1 for an artificial polyglycine sequence abolishes temperature sensitivity while retaining vanilloid sensitivity.54 Strikingly in the minimal rat TRPV1 structural construct, the exact pore turret region is deleted with no effect on thermosensitivity. Presumably, this suggests that parts of the pore turret in the PD form an allosteric network that modulates thermosensitivity but is not crucial mechanistically.
Differences in thermosensing between TRPM8 orthologs have been used to gain a foothold in identifying domains or residues that are mechanistically important, but again the results have been difficult to interpret. A recent study leveraged the fact that mouse TRPM8 is more cold sensitive than chicken TRPM8. Swapping the pore loop was sufficient to recapitulate the relative differences suggesting that this region either directly or allosterically modulates TRPM8 cold sensitivity.65 This region overlaps with the pore loop in TRPV1 mentioned above that allosterically modulates thermosensitivity. Another recent study of TRPM8 orthologs showed that TRPM8 from hibernating rodents retained menthol chemical activation but was poorly activated by low temperatures.58 By swapping the TMD from rat TRPM8, which is potently activated by cold, that cold-sensitivity could be transferred to the less sensitive hibernating rodent TRPM8 channel. Of the 15 amino acids that vary between rat and a hibernating squirrel TRPM8, exchanging six amino acids between the rat and squirrel orthologs was sufficient to alter the cold sensitivity without affecting chemical sensitivity. All six residues were absolutely required to swap the cold sensitivity; swapping any five particular residues was not sufficient to transfer the phenotype between channels. Two key points emerge from these studies: first, allosteric coupling across the membrane likely contributes to the observed effects, because five of the six residues are on the extracellular side, one is on the intracellular side, and none reside in transmembrane helices (Figure 4B). Second, two of the six hibernating rodent residues are identical with the equivalent human TRPM8 residues; as human TRPM8 is robustly cold-sensitive, this suggests that not only is there allosteric coupling, but there must also be compensation between networks of residues.
TRPA1 provides a final example of thermosensation regulation by allosteric networks. TRPA1 can be either hot– or cold–sensing depending on the species.15 Mutating three amino acid residues that cluster to a discrete soluble ankyrin repeat domain in mouse TRPA1 can flip the cold sensing channel to warm sensing.15 However, TRPA1 truncations that completely remove this large N-terminal region result in channels that are still temperature sensitive; therefore, the fundamental origins of temperature sensitivity of TRPA1 seem to reside in the transmembrane region.66
5. Conclusions and Outlook
The intersection of structural and genetic revolutions has provided tremendous tools and insight into how TRP channels function. An emergent property of these channels is the identification of the fundamental role of allostery in function. We speculate the centrality of allosteric networks in TRP channel regulation arises primarily because of the evolution of these channels to function in thermosensing, where biologically accessible changes in temperature are poor thermodynamic driving forces. Current studies have identified the importance of both the S1-S4 and PD in sensing, binding, and coupling to gating, giving a platform to better understand the molecular mechanisms that underlie basic biological processes such as tasting spiciness or feeling cold. Despite the significant progress in recent years, there is a need to dissect these channels with spectroscopic tools such as EPR,67 fluorescence,68,69 and NMR.38 These tools should unravel the allosteric networks that seemingly make allosteric coupling so challenging to interpret from current structural and functional measurements. Understanding these basic mechanisms will provide access to the fundamental rules of mechanistic cross talk and will also reveal opportunities for pharmacological intervention and manipulation.
Supplementary Material
Funding Sources
This work was supported by the National Institutes of General Medical Sciences R01GM112077 to W.D.V.H.
Biography
Jacob Hilton is currently a Ph.D. candidate at Arizona State University, School of Molecular Sciences. His research interests focus on TRP channel mechanisms, function, and regulation. He received his B.A. in chemistry from Brigham Young University.
Minjoo Kim is a Ph.D. candidate at Arizona State University, School of Molecular Sciences, studying the activation mechanism of TRP channel using NMR. She received her B.S. in biochemistry and microbiology from Arizona State University.
Wade Van Horn is an Associate Professor of Molecular Sciences at Arizona State University and member of the Biodesign centers of Personalized Diagnostics and Mechanisms of Evolution. He received his Ph.D. from the University of Utah, Department of Chemistry and pursued postdoctoral studies at Vanderbilt University.
Footnotes
REFERENCES
- (1).Nilius B; Owsianik G The transient receptor potential family of ion channels. Genome Biol 2011, 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Rohacs T Phosphoinositide regulation of TRP channels. Handb Exp Pharmacol 2014, 223, 1143–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ciardo MG; Ferrer-Montiel A Lipids as central modulators of sensory TRP channels. Biochim Biophys Acta Biomembr 2017, 1859, 1615–1628. [DOI] [PubMed] [Google Scholar]
- (4).Numazaki M; Tominaga T; Toyooka H; Tominaga M Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 2002, 277, 13375–13378. [DOI] [PubMed] [Google Scholar]
- (5).Bhave G; Hu HJ; Glauner KS; Zhu W; Wang H; Brasier DJ; Oxford GS; Gereau R. W. t. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A 2003, 100, 12480–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Studer M; McNaughton PA Modulation of single-channel properties of TRPV1 by phosphorylation. J Physiol 2010, 588, 3743–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liu J; Du J; Yang Y; Wang Y Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface localization, leading to inflammatory thermal hyperalgesia. Exp Neurol 2015, 273, 253–262. [DOI] [PubMed] [Google Scholar]
- (8).Cao E; Liao M; Cheng Y; Julius D TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Liao M; Cao E; Julius D; Cheng Y Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hellwig N; Albrecht N; Harteneck C; Schultz G; Schaefer M Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 2005, 118, 917–928. [DOI] [PubMed] [Google Scholar]
- (11).Staruschenko A; Jeske NA; Akopian AN Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 2010, 285, 15167–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bidaux G; Beck B; Zholos A; Gordienko D; Lemonnier L; Flourakis M; Roudbaraki M; Borowiec AS; Fernandez J; Delcourt P; Lepage G; Shuba Y; Skryma R; Prevarskaya N Regulation of activity of transient receptor potential melastatin 8 (TRPM8) channel by its short isoforms. J Biol Chem 2012, 287, 2948–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shin YC; Shin SY; Chun JN; Cho HS; Lim JM; Kim HG; So I; Kwon D; Jeon JH TRIP database 2.0: a manually curated information hub for accessing TRP channel interaction network. PLoS One 2012, 7, e47165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hilton JK; Rath P; Helsell CV; Beckstein O; Van Horn WD Understanding thermosensitive transient receptor potential channels as versatile polymodal cellular sensors. Biochemistry 2015, 54, 2401–2413. [DOI] [PubMed] [Google Scholar]
- (15).Jabba S; Goyal R; Sosa-Pagan JO; Moldenhauer H; Wu J; Kalmeta B; Bandell M; Latorre R; Patapoutian A; Grandl J Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron 2014, 82, 1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hilton JK; Salehpour T; Sisco NJ; Rath P; Van Horn WD Phosphoinositide-interacting regulator of TRP (PIRT) has opposing effects on human and mouse TRPM8 ion channels. J Biol Chem 2018, 293, 9423–9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Montell C; Rubin GM Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [DOI] [PubMed] [Google Scholar]
- (18).Lange M; Weihmann F; Schliebner I; Horbach R; Deising HB; Wirsel SG; Peiter E The Transient Receptor Potential (TRP) Channel Family in Colletotrichum graminicola: A Molecular and Physiological Analysis. PLoS One 2016, 11, e0158561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Majhi RK; Saha S; Kumar A; Ghosh A; Swain N; Goswami L; Mohapatra P; Maity A; Kumar Sahoo V; Kumar A; Goswami C Expression of temperature-sensitive ion channel TRPM8 in sperm cells correlates with vertebrate evolution. PeerJ 2015, 3, e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Myers BR; Sigal YM; Julius D Evolution of thermal response properties in a cold-activated TRP channel. PLoS One 2009, 4, e5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ovchinnikov S; Kinch L; Park H; Liao Y; Pei J; Kim DE; Kamisetty H; Grishin NV; Baker D Large-scale determination of previously unsolved protein structures using evolutionary information. Elife 2015, 4, e09248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Nicoludis JM; Gaudet R Applications of sequence coevolution in membrane protein biochemistry. Biochim Biophys Acta Biomembr 2018, 1860, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yang F; Xiao X; Cheng W; Yang W; Yu P; Song Z; Yarov-Yarovoy V; Zheng J Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat Chem Biol 2015, 11, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yang F; Xiao X; Lee BH; Vu S; Yang W; Yarov-Yarovoy V; Zheng J The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel. Nat Commun 2018, 9, 2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yang F; Vu S; Yarov-Yarovoy V; Zheng J Rational design and validation of a vanilloid-sensitive TRPV2 ion channel. Proc Natl Acad Sci U S A 2016, 113, E3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang F; Hanson SM; Jara-Oseguera A; Krepkiy D; Bae C; Pearce LV; Blumberg PM; Newstead S; Swartz KJ Engineering vanilloid-sensitivity into the rat TRPV2 channel. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zhang F; Swartz KJ; Jara-Oseguera A Conserved allosteric pathways for activation of TRPV3 revealed through engineering vanilloid-sensitivity. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gao Y; Cao E; Julius D; Cheng Y TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 2016, 534, 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zubcevic L; Herzik MA Jr.; Chung BC; Liu Z; Lander GC; Lee SY Cryo-electron microscopy structure of the TRPV2 ion channel. Nat Struct Mol Biol 2016, 23, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hughes TET; Lodowski DT; Huynh KW; Yazici A; Del Rosario J; Kapoor A; Basak S; Samanta A; Han X; Chakrapani S; Zhou ZH; Filizola M; Rohacs T; Han S; Moiseenkova-Bell VY Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM. Nat Struct Mol Biol 2018, 25, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).McGoldrick LL; Singh AK; Saotome K; Yelshanskaya MV; Twomey EC; Grassucci RA; Sobolevsky AI Opening of the human epithelial calcium channel TRPV6. Nature 2018, 553, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Autzen HE; Myasnikov AG; Campbell MG; Asarnow D; Julius D; Cheng Y Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 2018, 359, 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Samanta A; Kiselar J; Pumroy RA; Han S; Moiseenkova-Bell VY Structural insights into the molecular mechanism of mouse TRPA1 activation and inhibition. J Gen Physiol 2018, 150, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Demeuse P; Penner R; Fleig A TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol 2006, 127, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Iordanov I; Mihalyi C; Toth B; Csanady L The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bandell M; Dubin AE; Petrus MJ; Orth A; Mathur J; Hwang SW; Patapoutian A High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 2006, 9, 493–500. [DOI] [PubMed] [Google Scholar]
- (37).Voets T; Owsianik G; Janssens A; Talavera K; Nilius B TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 2007, 3, 174–182. [DOI] [PubMed] [Google Scholar]
- (38).Rath P; Hilton JK; Sisco NJ; Van Horn WD Implications of Human Transient Receptor Potential Melastatin 8 (TRPM8) Channel Gating from Menthol Binding Studies of the Sensing Domain. Biochemistry 2016, 55, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bidaux G; Borowiec AS; Gordienko D; Beck B; Shapovalov GG; Lemonnier L; Flourakis M; Vandenberghe M; Slomianny C; Dewailly E; Delcourt P; Desruelles E; Ritaine A; Polakowska R; Lesage J; Chami M; Skryma R; Prevarskaya N Epidermal TRPM8 channel isoform controls the balance between keratinocyte proliferation and differentiation in a cold-dependent manner. Proc Natl Acad Sci U S A 2015, 112, E3345–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Yin Y; Le SC; Hsu AL; Borgnia MJ; Yang H; Lee SY Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Takaishi M; Uchida K; Suzuki Y; Matsui H; Shimada T; Fujita F; Tominaga M Reciprocal effects of capsaicin and menthol on thermosensation through regulated activities of TRPV1 and TRPM8. J Physiol Sci 2016, 66, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wright CE; Bowen WP; Grattan TJ; Morice AH Identification of the L-menthol binding site in guinea-pig lung membranes. Br J Pharmacol 1998, 123, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Macpherson LJ; Hwang SW; Miyamoto T; Dubin AE; Patapoutian A; Story GM More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 2006, 32, 335–343. [DOI] [PubMed] [Google Scholar]
- (44).Karashima Y; Damann N; Prenen J; Talavera K; Segal A; Voets T; Nilius B Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci 2007, 27, 9874–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Colton CK; Zhu MX 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb Exp Pharmacol 2007, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kovacs G; Montalbetti N; Simonin A; Danko T; Balazs B; Zsembery A; Hediger MA Inhibition of the human epithelial calcium channel TRPV6 by 2-aminoethoxydiphenyl borate (2-APB). Cell Calcium 2012, 52, 468–480. [DOI] [PubMed] [Google Scholar]
- (47).Singh AK; Saotome K; McGoldrick LL; Sobolevsky AI Structural bases of TRP channel TRPV6 allosteric modulation by 2-APB. Nat Commun 2018, 9, 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Singh AK; McGoldrick LL; Sobolevsky AI Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat Struct Mol Biol 2018, 25, 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Zubcevic L; Herzik MA Jr.; Wu M; Borschel WF; Hirschi M; Song AS; Lander GC; Lee SY Conformational ensemble of the human TRPV3 ion channel. Nat Commun 2018, 9, 4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hu H; Grandl J; Bandell M; Petrus M; Patapoutian A Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci U S A 2009, 106, 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Cao E; Cordero-Morales JF; Liu B; Qin F; Julius D TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013, 77, 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zakharian E; Thyagarajan B; French RJ; Pavlov E; Rohacs T Inorganic polyphosphate modulates TRPM8 channels. PLoS One 2009, 4, e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Voets T; Droogmans G; Wissenbach U; Janssens A; Flockerzi V; Nilius B The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [DOI] [PubMed] [Google Scholar]
- (54).Yang F; Cui Y; Wang K; Zheng J Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci U S A 2010, 107, 7083–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Gavva NR; Davis C; Lehto SG; Rao S; Wang W; Zhu DX Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain 2012, 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ma S; Yu H; Zhao Z; Luo Z; Chen J; Ni Y; Jin R; Ma L; Wang P; Zhu Z; Li L; Zhong J; Liu D; Nilius B; Zhu Z Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol 2012, 4, 88–96. [DOI] [PubMed] [Google Scholar]
- (57).Rossato M; Granzotto M; Macchi V; Porzionato A; Petrelli L; Calcagno A; Vencato J; De Stefani D; Silvestrin V; Rizzuto R; Bassetto F; De Caro R; Vettor R Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol Cell Endocrinol 2014, 383, 137–146. [DOI] [PubMed] [Google Scholar]
- (58).Matos-Cruz V; Schneider ER; Mastrotto M; Merriman DK; Bagriantsev SN; Gracheva EO Molecular Prerequisites for Diminished Cold Sensitivity in Ground Squirrels and Hamsters. Cell Rep 2017, 21, 3329–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Yao J; Liu B; Qin F Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A 2011, 108, 11109–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wen H; Zheng W Decrypting the Heat Activation Mechanism of TRPV1 Channel by Molecular Dynamics Simulation. Biophys J 2018, 114, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Zheng W; Wen H Heat activation mechanism of TRPV1: New insights from molecular dynamics simulation. Temperature 2019, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Chowdhury S; Jarecki BW; Chanda B A molecular framework for temperature-dependent gating of ion channels. Cell 2014, 158, 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Sosa-Pagan JO; Iversen ES; Grandl J TRPV1 temperature activation is specifically sensitive to strong decreases in amino acid hydrophobicity. Sci Rep 2017, 7, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Zhang F; Jara-Oseguera A; Chang TH; Bae C; Hanson SM; Swartz KJ Heat activation is intrinsic to the pore domain of TRPV1. Proc Natl Acad Sci U S A 2018, 115, E317–E324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Pertusa M; Rivera B; Gonzalez A; Ugarte G; Madrid R Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J Biol Chem 2018, 293, 12454–12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Moparthi L; Survery S; Kreir M; Simonsen C; Kjellbom P; Hogestatt ED; Johanson U; Zygmunt PM Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A 2014, 111, 16901–16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Velisetty P; Stein RA; Sierra-Valdez FJ; Vasquez V; Cordero-Morales JF Expression and Purification of the Pain Receptor TRPV1 for Spectroscopic Analysis. Sci Rep 2017, 7, 9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Zagotta WN; Gordon MT; Senning EN; Munari MA; Gordon SE Measuring distances between TRPV1 and the plasma membrane using a noncanonical amino acid and transition metal ion FRET. J Gen Physiol 2016, 147, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Steinberg X; Kasimova MA; Cabezas-Bratesco D; Galpin JD; Ladron-de-Guevara E; Villa F; Carnevale V; Islas L; Ahern CA; Brauchi SE Conformational dynamics in TRPV1 channels reported by an encoded coumarin amino acid. Elife 2017, 6, e28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.