Abstract
Objective
We have reported previously that the IRAK4 inhibitor PF06426779 given to ubiquitin-binding-defective ABIN1[D485N] mice at 6 weeks of age prevents the major facets of lupus that develop 10 weeks later. The present study was undertaken to investigate whether PF06426779 could reverse the lupus phenotype when administered to 13-week-old ABIN1[D485N] mice that had already developed symptoms of lupus.
Methods
Splenomegaly, the number of splenic neutrophils, TFH and Germinal Centre B (GCB) cells, serum levels of immunoglobulins, the extent of kidney, liver and lung pathology, and glomerular IgA and IgM were measured after feeding 13-week-old ABIN1[D485N] and wild-type mice for another 10 weeks with R&M3 diet with and without PF06426779 (4 g/kg).
Results
Following drug treatment, spleen size and weight, splenic neutrophil numbers, and serum IgA and glomerular IgA levels of ABIN1[D485N] mice returned to those seen in wild-type mice. The rise in splenic TFH and GCB numbers, the increase in kidney and liver pathology, and the concentrations of serum IgG1, IgG2A and IgE between 13 and 23 weeks were suppressed. There was no reduction in the level of anti-self double-stranded DNA, anti-self nuclear antigens or IgM during the drug treatment.
Conclusions
The results demonstrate the therapeutic potential of IRAK4 inhibitors for the treatment of lupus and raise the possibility of monitoring efficacy by measuring decreases in the serum levels of IgA. Our results support the view that there may be a closer connection between lupus and IgA nephropathy than realised previously.
Keywords: lupus nephritis, autoantibodies, therapeutics, anti-inflammatory agents, non-steroidal
Key messages.
What is already known about this subject?
Polymorphisms in TNIP1, the gene encoding encoding ABIN1 (A20-binding inhibitor of NF-κB 1), predispose to lupus in many human populations.
Ubiquitin-binding-deficient ABIN1[D485N] knock-in mice spontaneously develop a lupus-like disease, which is driven by the TLR7-MyD88-IRAK4 pathway.
What does this study add?
The IRAK4 inhibitor PF06426779 reverses splenomegaly, neutrophil numbers and the rise in serum and glomerular IgA levels in ABIN1[D485N] mice that have already developed symptoms of lupus, and prevents further increases in splenic TFH and Germinal Centre B cell numbers, serum IgG1 and IgE concentrations, and kidney and liver pathology.
How might this impact on clinical practice or future developments?
The study suggests that IRAK4 inhibitors may have therapeutic potential for the treatment of lupus.
It also indicates that monitoring the serum levels of IgA, as well as patrolling monocyte numbers in the blood, may be a simple non-invasive way to examine drug efficacy, and may reveal whether IgA nephropathy and SLE are the same disease.
Introduction
ABIN1 (A20-binding inhibitor of NF-κB 1) is a polyubiquitin-binding protein that restricts the production of inflammatory mediators by the innate immune system. Polymorphisms in TNIP1, the gene encoding ABIN1, predispose to lupus in many human populations (reviewed,1), and mice deficient in ABIN12 or expressing a polyubiquitin-binding-deficient mutant of ABIN1 (ABIN1[D485N])3 develop a disease resembling type III/IV human lupus nephritis.4 All the major facets of lupus in ABIN1[D485N] mice can be prevented by crossing ABIN1[D485N] mice to mice expressing the kinase-inactive IRAK4[D329A] mutant5 or by oral administration of the small molecule IRAK4 inhibitor PF 06426779 prior to the appearance of the hallmark features of lupus, such as glomerulonephritis, liver and lung inflammation, and increased levels of autoantibodies.6 A different IRAK4 inhibitor (BMS-986126) was also shown to prevent kidney pathology in the lupus-prone MRL/lpr and NZB/W mouse lines.7
Lupus is usually diagnosed when humans have already developed the disease. To evaluate the potential of the IRAK4 inhibitor for the treatment of lupus, it was therefore critical to investigate whether it prevented the further rise, or even reversed, the hallmark features of lupus after they had already developed. Here, we present the results of such a study in ABIN1[D485N] mice, which has revealed some interesting and unexpected findings.
Methods
ABIN1[D485N] mice aged 13 weeks that had already developed splenomegaly, autoimmunity and organ inflammation, or control wild-type littermates, were fed for 10 weeks on chow containing the IRAK4 inhibitor PF06426779 or control chow. The sources of other reagents and the methods used are described elsewhere.6
Statistical analysis was carried out using GraphPad Prism 9 software. The distribution was determined using the Shapiro-Wilk normality test. Multiple comparisons of data with normal distribution were performed using one-way ANOVA followed by Tukey’s post hoc test. Multiple comparison of non-parametric data was done using the Kruskal-Wallis test, followed by the Mann-Whitney U test. Data in percentages were logit-transformed. Figures were drawn using GraphPad Prism and Adobe Illustrator.
Immunohistochemistry
Kidney sections were stained with haematoxylin and either anti-IgA (Abcam, ab97231) or anti-IgM (Abcam, ab97226) primary antibodies at 1:1600 dilution. The signal was detected using ImmPRESS[R] HRP Horse Anti-Goat IgG Polymer Detection Kit (Vector Laboratories). Slides for microscopy were scanned using Motic EasyScan Infinity at ×40 magnification and analysed using QuPath software. Forty glomeruli per mouse were gated and those staining positively with 3,3′-diaminobenzidine were quantified.
Results and discussion
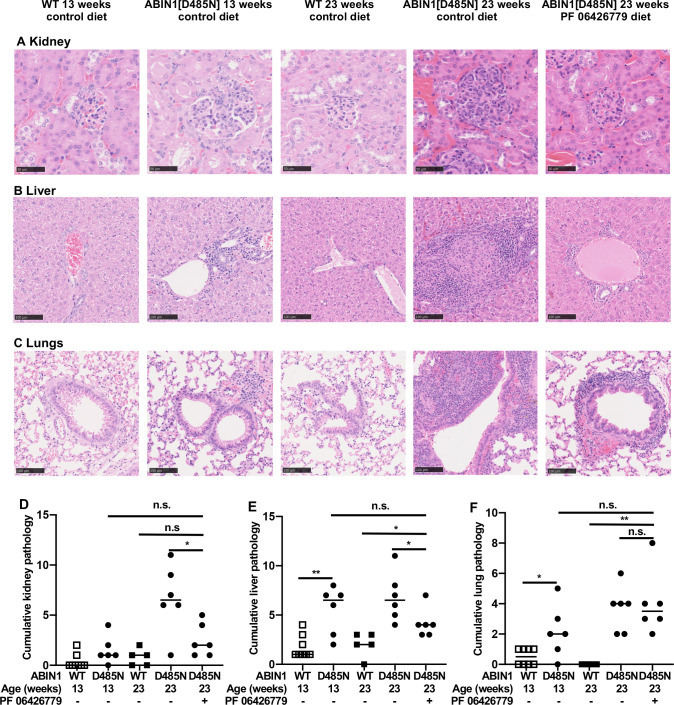
The IRAK4 inhibitor PF06426277 was given to ABIN1[D485N] mice in their food (4 g/kg) from 13 weeks. At this age, splenomegaly, autoimmunity and organ inflammation have already started to develop. Strikingly, 10 weeks after treatment with PF06426779 had commenced, the spleen size had almost returned to that seen in wild-type mice (figure 1A) and neutrophil numbers had also normalised (figure 1B). Splenic TFH and Germinal Centre B (GCB) cell numbers continued to increase over the 10-week period in ABIN1[D485N] mice not given the drug, but this was prevented by the IRAK4 inhibitor (figure 1C, D). Importantly, the further rise in kidney and liver pathology from 13 to 23 weeks was suppressed, but not lung pathology (figure 2A–F).
Figure 1.
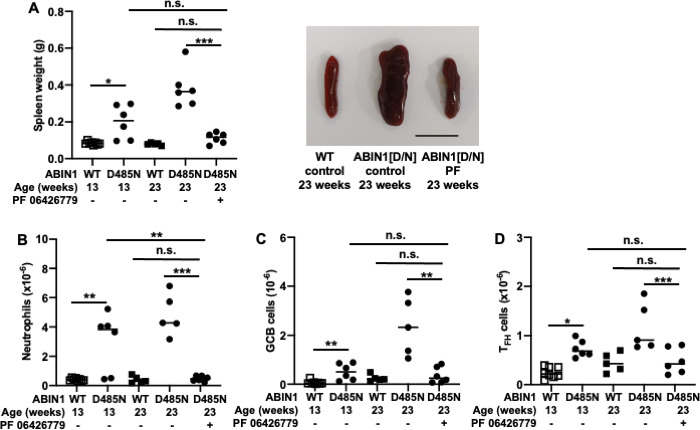
Reversal of splenic phenotypes in ABIN1[D485N] knock-in mice by an IRAK4 inhibitor. 13-week-old ABIN1[D485N] mice (n=6) and age-matched wild-type (WT) (n=5) mice were fed for 10 weeks on R&M3 diet containing or not containing PF 06426779 (4 g/kg). Mice were culled at the age of 23 weeks. An additional group of 8 WT and 6 ABIN1[D485N] knock-in mice were culled when they were 13 weeks old as a control. (A) Spleen weight and a representative image showing relative spleen size. Bar equals 1 cm. (B) Neutrophil numbers in the spleen. (C) Germinal Centre B cell numbers in the spleen. (D) Follicular T helper cell numbers in the spleen. Each symbol shows the result from a single individual mouse. Statistical significance was calculated using one-way ANOVA and Tukey’s post hoc test (A, B and D) or the Kruskal-Wallis and the Mann-Whitney U tests (C); * denotes p<0.05, ** denotes p<0.01 and *** denotes p<0.001. The methodology used is detailed in Nanda et al.6
Figure 2.
Effect of the IRAK4 inhibitor PF 06426277 on organ inflammation. (A–C) Representative histological images showing H&E staining of the glomerulus (A), liver (B) and lungs (C) of 13-week-old ABIN1[D485N] knock-in mice, 23-week-old WT and ABIN1[D485N] mice fed on R&M3 control diet for 10 weeks, and 23-week-old ABIN1[D485N] mice fed on R&M3 diet containing PF 06426779 (4 g/kg). (D–F) As in A–C, except that the graphs show the pathology scores of the kidney (D), liver (E) and lungs (F). The horizontal bars equal 50 µm in (A) and 100 µm in (B) and (C). Each symbol shows the result from a single individual mouse. Statistical significance was calculated using the Kruskal-Wallis and the Mann-Whitney U tests; * denotes p<0.05 and **p<0.01. In D–F, an ordinal grading scale from 0 to 4 was used to assess the pathology observed semi-quantitatively, where 0=absent, 1=mild, 2=moderate, 3=marked and 4=severe. In the images of the kidney sections, glomerulonephropathy, tubulointerstitial fibrosis, inflammatory cell infiltrates, basophilic tubules and protein cast were all scored separately. The five scores were then added together to produce a cumulative pathology score shown on the ordinate (D). Similarly, for the liver sections, the presence of perivascular, periportal and parenchymal inflammatory cell infiltrates, increased cellularity and oval cell hyperplasia were scored separately and the values combined (E), and for the lung sections, the peribronchiolar and perivascular inflammatory cell infiltrates, subpleural lymphoid focus and bronchus-associated lymphoid tissue were scored separately and the values combined (F). The pathologist (CS) carried out the scoring blind without knowledge of the identity of each sample.
ABIN1[D485N] mice display elevated levels of patrolling monocytes in their blood at only 4 weeks, which increases progressively up to 16 weeks. PF06426779 administered from 6 weeks prevented the subsequent rise in pMo seen in the absence of PF06426779.6 Here, we found that PF06426779 administered from 13 weeks reduced the number of pMo after 18 or 23 weeks, but not to the level seen in control WT mice (online supplemental figure S1).
lupus-2021-000573supp001.pdf (374.8KB, pdf)
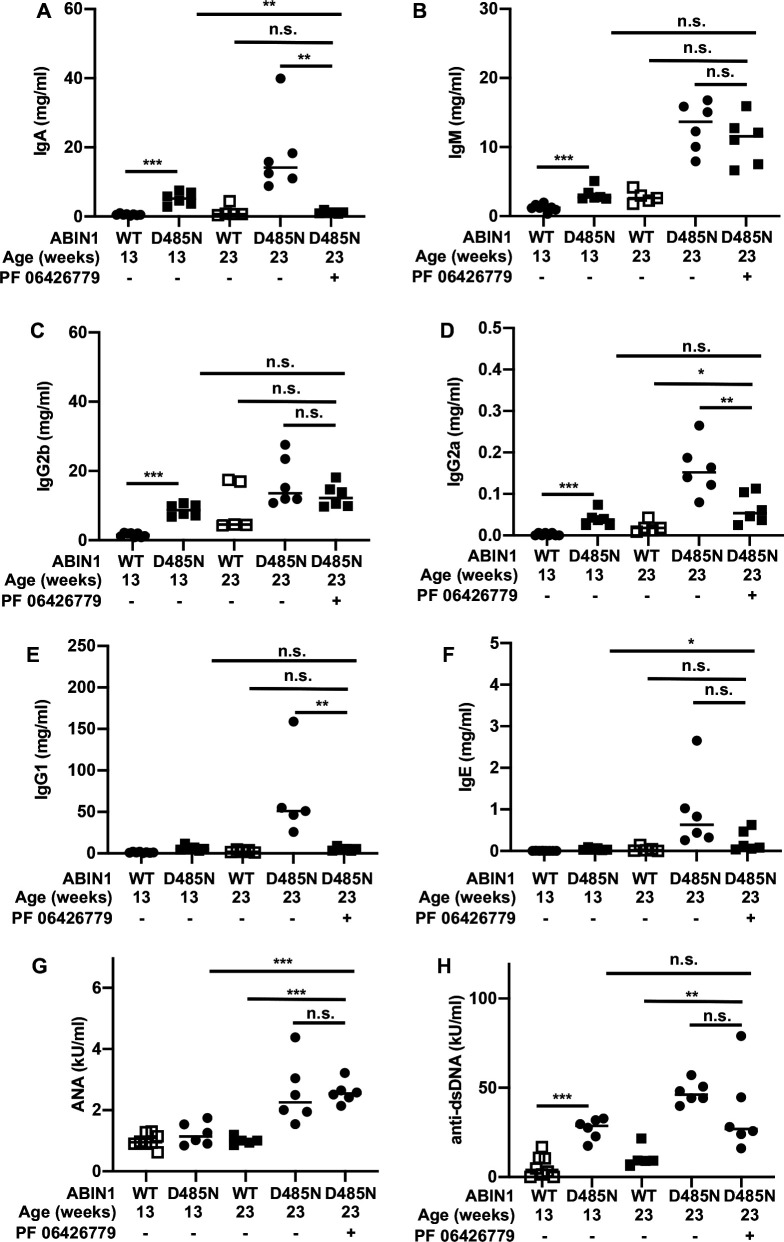
The kidneys of ABIN1[D485N] mice contain deposits of complement factors and immunoglobulins (IgA, IgM and IgG) at 5–6 months of age.4 We therefore measured the levels of these and other immunoglobulins in the serum of ABIN1[D485N] mice (figure 3). At 13 weeks, IgA, IgM, IgG2a and IgG2b levels were elevated (figure 3A–C), but IgG1 and IgE were not (figure 3E, F). Treatment of ABIN1[D485N] mice for 10 weeks with the IRAK4 inhibitor reversed the rise in serum IgA seen at 13 weeks of age, the levels returning to those measured in WT mice (figure 3A). In contrast, serum levels of IgM and IgG2b measured at 13 weeks in ABIN1[D485N] mice were not reduced after 10 weeks of IRAK4 inhibitor treatment (figure 3B, C). The serum levels of IgM continued to increase between 13 and 23 weeks in ABIN1[D485N] mice whether or not the mice were treated with the IRAK4 inhibitor. In contrast, the further rise in serum IgG2a observed between 13 and 23 weeks in ABIN1[D485N] was prevented by the drug (figure 3D).
Figure 3.
Effect of the IRAK4 inhibitor PF 06426779 on serum levels of immunoglobulins and autoantibodies in ABIN1[D485N] knock-in mice. (A) IgA; (B) IgM; (C) IgG2b; (D) IgG2a; (E) IgG1; (F) IgE; (G) ANA; (H) anti-double stranded DNA (anti-dsDNA). The measurements were made in wild-type and ABIN1[D485N] knock-in mice at the ages indicated. Where indicated (+) the R&M3 diet included PF 06426779 (4 g/kg) from the age of 13 weeks until the mice were culled at the age of 23 weeks. Different mouse cohorts were used for the studies at 13 and 23 weeks. Each symbol shows the result from a single individual mouse. Statistical significance was calculated using the Kruskal-Wallis and the Mann-Whitney U tests; * denotes p<0.05, ** denotes p<0.01 and *** denotes p<0.001. The methodology used is detailed in Nanda et al.6
Serum IgG1 and IgE levels were not elevated in 13-week-old ABIN1[D485N] mice but were greatly elevated after 23 weeks (figure 3E, F). Similar to IgG2a, this rise between 13 and 23 weeks was prevented by administration of the IRAK4 inhibitor (figure 3E, F).
The lack of effect of the drug on serum IgM and IgG2b levels does not appear to be explained by much longer half lives of these immunoglobulins in the blood. In normal adult mice, all the immunoglobulins studied have half lives of days and not weeks or months. The half lives of IgM and IgG2b are 2 and 4–6 days, respectively, shorter than IgG1 (6–8 days),8 but longer than IgA and IgE (1 day8 and 0.5 day,9 respectively).
Interestingly, the 10-week treatment with the IRAK4 inhibitor did not affect serum levels of ANA significantly (figure 3G) or anti-dsDNA (figure 3H) observed after 23 weeks. The reversal of splenomegaly and splenic neutrophil levels, the prevention of the further rise in splenic TFH and GCB cell numbers, and the improved kidney and liver pathology over this period following treatment with the IRAK4 inhibitor therefore occurred without any significant change in the serum levels of the autoantibodies examined.
Of all the antibodies studied, the effect of the IRAK4 inhibitor on serum IgA levels showed the strongest correlation with the appearance and disappearance of splenomegaly and splenic neutrophil numbers between 13 and 23 weeks of age. Consistent with these findings, glomerular IgA was also reduced by feeding 13-week-old ABIN1[D485N] mice for 10 weeks with diet containing 06426779, but IgM was not (online supplemental figure S2). Interestingly, the deposition of IgA in the kidney, termed IgA nephropathy (IgAN), is the most frequent primary glomerulopathy in the world. Although the co-association of SLE and IgAN had been reported in several human patients, the possibility that IgA deposition might be a subtype or even a primary trigger of kidney pathology in human SLE had not been considered until a patient diagnosed with SLE was found to contain glomerular IgA deposits.10 The ABIN1[D485N] mice also accumulate glomerular IgA deposits.4 Since IgAN and SLE are both relatively common conditions, it is possible that their co-existence is underdiagnosed.
lupus-2021-000573supp002.pdf (1.7MB, pdf)
Four IRAK4 inhibitors have entered clinical trials for the treatment of rheumatoid arthritis, psoriasis and lymphoma with little evidence of toxicity.11 The present study suggests that IRAK4 inhibitors may also have therapeutic potential for the treatment of some types of lupus and that it may be possible to monitor their efficacy rapidly and non-invasively by measuring the serum levels of IgA and/or other immunoglobulins, such as IgG1 or IgE or by a decrease in patrolling monocyte numbers. The routine monitoring of serum IgA levels in patients with lupus patients may also reveal whether IgAN and SLE are really the same disease.
Acknowledgments
We thank Clara Figueras Vadillo and Gail Gilmour for genotyping mouse lines; Lynn Oxford, Lynn Stevenson and Frazer Bell (Veterinary Diagnostic Services, University of Glasgow) for tissue processing for histological analysis; and Graeme Ball and Alan Prescott for microscopy analysis.
Footnotes
Contributors: TP and SKN performed the experiments and analysed the data. CS performed histological analysis. SWW and VR provided the IRAK4 inhibitor PF06426779. VR and PC acquired funding for the study. PC and VR conceived the study and PC, TP, SKN and VR wrote the paper.
Funding: We acknowledge UK Medical Research Council Programme grant MR/R021406/1 (to PC) and Wellcome Trust Investigator Award 209380/Z/17/Z (to PC). VR and SWW are funded by Pfizer Inc.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Experiments on mice were approved by the University of Dundee Ethical Review Committee under a UK Home Office project license.
References
- 1.Brady MP, Korte EA, Caster DJ, et al. TNIP1/ABIN1 and lupus nephritis: review. Lupus Sci Med 2020;7:e000437. 10.1136/lupus-2020-000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuriakose J, Redecke V, Guy C, et al. Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J Clin Invest 2019;129:2251–65. 10.1172/JCI125116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda SK, Venigalla RKC, Ordureau A, et al. Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J Exp Med 2011;208:1215–28. 10.1084/jem.20102177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caster DJ, Korte EA, Nanda SK, et al. ABIN1 dysfunction as a genetic basis for lupus nephritis. J Am Soc Nephrol 2013;24:1743–54. 10.1681/ASN.2013020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanda SK, Lopez-Pelaez M, Arthur JSC, et al. Suppression of IRAK1 or IRAK4 catalytic activity, but not type 1 IFN signaling, prevents lupus nephritis in mice expressing a ubiquitin binding-defective mutant of ABIN1. J Immunol 2016;197:4266–73. 10.4049/jimmunol.1600788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanda SK, Petrova T, Marchesi F, et al. Distinct signals and immune cells drive liver pathology and glomerulonephritis in ABIN1[D485N] mice. Life Sci Alliance 2019;2:e201900533. 10.26508/lsa.201900533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudhgaonkar S, Ranade S, Nagar J, et al. Selective IRAK4 inhibition attenuates disease in murine lupus models and demonstrates steroid sparing activity. J Immunol 2017;198:1308–19. 10.4049/jimmunol.1600583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 1988;18:313–6. 10.1002/eji.1830180221 [DOI] [PubMed] [Google Scholar]
- 9.Hirano T, Hom C, Ovary Z. Half-life of murine IgE antibodies in the mouse. Int Arch Allergy Appl Immunol 1983;71:182–4. 10.1159/000233385 [DOI] [PubMed] [Google Scholar]
- 10.da Silva LS, Almeida BLF, de Melo AKG, et al. IgA nephropathy in systemic lupus erythematosus patients: case report and literature review. Rev Bras Reumatol Engl Ed 2016;56:270–3. 10.1016/j.rbre.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Wiese MD, Manning-Bennett AT, Abuhelwa AY. Investigational IRAK-4 inhibitors for the treatment of rheumatoid arthritis. Expert Opin Investig Drugs 2020;29:475–82. 10.1080/13543784.2020.1752660 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2021-000573supp001.pdf (374.8KB, pdf)
lupus-2021-000573supp002.pdf (1.7MB, pdf)