Abstract
Introduction
Emerging adulthood is a phase in life that is associated with an increased risk to develop a variety of mental health disorders including anxiety and depression. However, less than 25% of university students receive professional help for their mental health reports. Internet-based cognitive behavioural therapy (iCBT) may entail useful interventions in a format that is attractive for university students. The aim of this study protocol is to test the effectiveness of a therapist-guided versus a computer-guided transdiagnostic iCBT programme with a main focus on anxiety and depression.
Methods and analysis
University students with anxiety and/or depressive symptoms will be randomised to a (1) 7-week iCBT programme (excluding booster session) with therapist feedback, (2) the identical iCBT programme with computer feedback only or (3) care as usual. Participants in the care as usual condition are informed and referred to conventional care services and encouraged to seek the help they need. Primary outcome variables are self-reported levels of anxiety as measured with the General Anxiety Disorder-7 and self-reported levels of depression as measured with the Patient Health Questionnaire-9. Secondary outcomes include treatment adherence, client satisfaction, medical service use, substance use, quality of life and academic achievement. Assessments will take place at baseline (t1), midtreatment (t2), post-treatment (t3), at 6 months (t4) and 12 months (t5) postbaseline. Social anxiety and perfectionism are included as potentially important predictors of treatment outcome. Power calculations are based on a 3 (group) × 3 (measurement: pretreatment, midtreatment and post-treatment) interaction, resulting in an aimed sample of 276 participants. Data will be analysed based on intention-to-treat and per protocol samples using mixed linear models.
Ethics and dissemination
The current study was approved by the Medical Ethics Review Committee (METC) of the Academic Medical Centre, Amsterdam, The Netherlands (number: NL64929.018.18). Results of this trial will be published in peer-reviewed journals.
Trial registration number
NL7328.
Keywords: adult psychiatry, anxiety disorders, depression & mood disorders
Strength and limitations of this study.
The current study protocol describes a randomised controlled trial (RCT) focused on university students with anxiety and/or depressive symptoms.
A transdiagnostic internet-based cognitive behavioural therapy programme, with either therapist guidance or computerised guidance, is compared with care as usual (three-arm RCT).
The procedure of the trial is developed to make it easily accessible and anonymous, and the full procedure is online.
The therapists are not fully blinded to the treatment condition.
The post-treatment outcomes rely on self-reports of the participants.
Background
Emerging adulthood is a crucial stage in life that includes the transition from the teenage years to full-fledged adulthood (18–25 years). It is a phase during which many young adults attend university to receive education and training to obtain a degree that will be the basis for their adult working life.1 It is also the phase that is associated with an increased risk of developing a variety of mental health disorders such as anxiety and depression,2–4 whereby comorbidity is the rule rather than the exception.5 Recent studies found that between 16% and 46% of all students suffer from mental health disorders.2 6 Moreover, these problems have been associated with poorer academic performance, academic dropout, decreased labour market functioning and poorer social and health outcomes.6–10 Early detection and effective intervention are, thus, crucial with regard to the long-term prognosis of mental disorders, especially as untreated mental disorders have a mean duration between 4 and 23 years.11
Online treatment programmes may be useful as an intervention in a format that is particularly attractive for university students, given the fact that less than 25% of students with mental health problems use available face-to-face services.12 University students might avoid help due to several reasons, including fear of stigmatisation, lack of perceived urgency, preference to deal with it themselves, threat to identity formation and goal setting and/or scepticism regarding treatment effectiveness.13 14 This group may be more likely to seek help if an effective, more flexible, easily accessible and anonymous intervention strategy is provided. A scoping review by Boydell et al15 concluded that internet therapies have (untapped) potential to provide better information about mental health and may improve the cost efficacy of mental health services. Moreover, their study indicated that young people, in general, prefer eHealth treatments relative to face-to-face services.
Several studies demonstrated that the effectiveness of guided internet-based cognitive behavioural therapy (iCBT) programmes for anxiety and depression is comparable to conventional (face-to-face) CBT (for meta-analyses, see Carlbring et al and Cuijpers et al16 17). There is also some evidence for the effectiveness of internet interventions for young adults and university student populations with various mental health problems, including depression, anxiety, eating disorders, stress, alcohol and sleep problems18–22. For example, a recent meta-analysis20 focused on university students with mental problems, who self-selected a psychological online intervention (guided/unguided). They found small intervention effects for depression (g=0.18, 95% CI (0.08 to 0.27) and anxiety (g=0.27, 95% CI (0.13 to 0.40)) in university students. It should be noted, however, that the effects on anxiety were not significant anymore after adjusting for publication bias. Harrer and colleagues20 concluded that clearly more research is needed to explore ways to increase treatment effectiveness for university students and to study predictors of treatment outcome.
The current study includes a group of university students and focuses on two of the most common mental disorders in this group, namely, anxiety and depression.2 As comorbidity is high, we will use a transdiagnostic iCBT intervention, which has the advantage to target both disorders at the same time and thereby might increase treatment effectiveness (for a systemic review and meta-analysis, see Newby et al 23). Moreover, iCBT programmes can be provided with or without additional personal guidance. Guidance usually implies written or verbal feedback by the therapist to the patient’s homework assignments and other forms of (safe) communication (eg, chat, telephone) within an online environment with varying degrees of interactive features.24 Both approaches (with and without personal guidance) have shown to be efficacious, although programmes with (therapist) guidance generally report a higher effect size (for meta-analyses, see Cuijpers et al and Richards and Richardson 17 25), even though it should be noted that a recent meta-analysis20 found no difference between personal or no personal guidance. To date, little is known about the effectiveness for therapist-guided versus a computer-guided version of the same intervention tested within the same study when applied to a university student sample with mental health concerns20 26 27. This could also have important health economic implications considering the additional cost of human support. Therefore, the current study tests this transdiagnostic iCBT for anxiety and depression in three groups; a therapist-guided online self-help version, a computer-guided version of the same programme and care as usual (CAU). Primary outcomes were anxiety and depression; secondary measures were included to evaluate treatment adherence and satisfaction, academic achievement and medical service use.
Internet-based programmes, either therapist-guided with personal feedback or computer-guided with automated feedback, might suit some individuals better than others. It is important to focus on possible treatment predictors (eg, including baseline symptoms severity, gender, personality factors and comorbidity), as this may help us understand more about the heterogeneity of treatment response.20 28 Specially, a recent meta-analysis20 found limited effectiveness on anxiety in university students in their meta-analysis. The current study will focus on the possible role of social anxiety and perfectionism, as there is some evidence that adults and children with social anxiety might benefit less from general CBT programmes (for meta-analyses, see Hudson et al and Norton and Price29 30). Likewise, self-critical perfectionism is a transdiagnostic personality factor that is generally associated with worse treatment outcome, including CBT.31 Importantly, individuals with self-critical perfectionism have poorer social relationships, which in turn negatively impacts therapeutic progress.32
The aim of this study is to test the effectiveness of a therapist-guided versus a computer-guided transdiagnostic iCBT programme with a focus on anxiety and depression. Based on previous studies in the general population,17 we hypothesise that the therapist-guided iCBT and computer-guided iCBT conditions are both significantly more effective than CAU. In addition, we expect that the therapist-guided iCBT condition is significantly more effective than the computer-guided iCBT condition; an effect we expect to be mediated by higher treatment adherence.
Methods
Study design
The current study includes a randomised controlled trial (RCT) design with three arms, consisting of (1) a therapist-guided transdiagnostic iCBT programme with personalised feedback, (2) a computer-guided version of the same CBT programme with automated feedback and (3) CAU. We used the Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines for this study33; see online supplemental appendix A.
bmjopen-2021-049554supp001.pdf (83.1KB, pdf)
Eligibility criteria
All students scoring 16 or higher on the Center for Epidemiological Studies Depression Scale (CES-D)34 and/or a score of 5 or higher on the General Anxiety Disorder-7 (GAD-7)35 were included in the study. There is no higher end cut-off. Participants are excluded from the study if they (1) are younger than 16 years of age, (2) currently receive psychological treatment (as described by the American Psychological Association (APA) dictionary) for anxiety and/or depression, (3) do not have access to a stable internet connection for the next 2 months, (4) if they meet the diagnostic criteria for (recent or current) psychotic disorder according to the Mini International Neuropsychiatric Interview (MINI),36 (5) if they are at active high risk for suicide based on the MINI and (6) if they do not give informed consent. Participants meeting exclusion criteria 4 and/or 5 are referred to local healthcare facilities.
Recruitment
All participants were recruited at the University of Amsterdam (UvA), The Netherlands. The UvA is a public research university located in Amsterdam. It is one of the largest research universities in Europe with 31 186 students enrolled in 2018 and includes seven faculties: Humanities, Social and Behavioural Sciences, Economics and Business, Science, Law, Medicine and Dentistry. Recruitment for the study is done in two phases: a first broad screening phase for all university students studying at the UvA and a second more specific screening of eligible students who showed interest to participate. In the first broad screening phase, all students enrolled at the UvA receive an invitation via email that are sent centrally from the study platform. In addition, study advisors and study counsellors of the UvA are informed about the study and are asked to refer students who are interested in participating in the study to the research team, so that they can be invited by email.
This first broad-screening invitation email contains general information about the study and a unique link to fill out the screening questionnaire. This screening questionnaire takes approximately 20 min to complete and also includes other questionnaires related to the mental health of university students. This screening questionnaire was developed in collaboration with other universities to collect multicentre data. Participants who click on the invitation link are referred to the project platform. Here, they find an information letter and are asked to give online informed consent to the research team. This information letter explicitly states that participation is voluntary and that participants can withdraw at any time without consequences. Next, participants are asked to create an account after which they are directed to the screening questionnaire. Following the original invitation, two reminder emails are sent 1 and 2 weeks after the first invitation. As the study runs for three academic years (2019–2021), participants are asked at the beginning of the screening if they consent to being invited again later. Moreover, participants can also indicate that they do not want to receive any emails again.
In the second more specific screening phase, university students who score above the cut-off on either anxiety (GAD-7) and/or depressive symptoms (CES-D) receive an email with an online information brochure and informed consent document34 35; see online supplemental appendix B. After informed consent is given by the participant, they are called by a trained clinical research assistant to check all of the inclusion and exclusion criteria. A telephonic diagnostic interview (MINI) 36 is administered to establish possible diagnoses with respect to mood and anxiety disorders, bipolar disorder, psychosis and suicidal ideation (not fulfilling the criteria for an anxiety and/or mood disorder is not an exclusion criterion).
bmjopen-2021-049554supp002.pdf (135.5KB, pdf)
Randomisation, blinding and treatment allocation
Participants are randomly assigned to either the therapist-guided online intervention, the computer-guided online intervention or CAU (control) condition (1:1:1 allocation ratio) directly following the baseline measurement. Randomisation is stratified by gender and anxiety and/or depressive symptoms (based on the cut-off scores on the screening questionnaire). Sequence generation is based on computer-generated random numbers and are allocated by an automatic system.
Due to the nature of the intervention, participants and researchers cannot be completely blinded to the assigned treatment condition, though note that the measurements in the intervention are blinded. Researchers are aware of the participants’ condition because they provide participants with feedback in the therapist-guided condition. In the computer-guided condition, feedback is automatically generated by the system, but therapists do send reminders to this group as part of the intervention.
Intervention
The online transdiagnostic intervention that is used in this study, ‘ICare Prevent’, was originally developed by Weisel et al37 for the general German-speaking population and translated and adapted by Bolinski et al38 and Karyotaki et al39 for a Dutch undergraduate student population into Dutch and English. The intervention was based on other effective protocols40–43 and can be seen as a variant of an iCBT protocol for which several studies have proven its effectiveness. The intervention is provided to the participants through the eHealth platform Minddistrict. This platform enables researchers and healthcare providers to provide digital therapy to their participants and patients. On this platform, e-coaches create a personal account for each participant directly following the baseline assessment and randomisation. Once the personal account has been created, the participant receives an email with information how to activate the account. Once the account is activated, the participant can start with the intervention. The platform monitors the progress of the participants, e-coaches provide support for the participants in the therapist-guided treatment condition and respond to questions from the participants with the messaging function. Data processing and storage are in accordance with the ISO 27000 and NEN 7510 norms. A data processing agreement that complies with the European General Data Protection Regulation (GDPR) has been signed between eHealth provider Minddistrict and the UvA.
The intervention is based on basic cognitive behavioural therapy principles for anxiety and depression with components of psychoeducation, including online exercises and home assignments. The intervention consists of seven regular sessions (45–60 min per session) and one booster session (4 weeks after the completion of the last session). From the second session onwards, participants are able to follow eight additional optional modules based on their personal needs, including sleep, perfectionism, alcohol use, reduce rumination, self-worth, acceptance, appreciations and rest and relaxation. They can decide to choose one additional module per session, and they are free to repeat this module or choose other modules in later sessions. In sessions 5 and 6, users decide to follow either content directed at changing negative cognitions or exposure to feared situations. Participants are free to decide when they would like to start a treatment session and if they want to do an additional module, but it is advised to do at least one and no more than two treatment sessions per week. For a full description of the intervention, see Karyotaki et al.39
Therapist-guided intervention versus computer-guided intervention
During the intervention, participants in both conditions receive online support in the form of messages or notifications in the messaging function (e-mails). In both conditions, participants receive up to 3 weekly reminders via the messaging function when they are inactive. Moreover, participants in both conditions can use the messaging function to ask questions. All participants can ask technical or usability related questions. There is one key difference between the two conditions: Participants in the computer-guided iCBT condition receive automatically generated feedback messages (see online supplemental appendix C for an example). In contrast, in the therapist-guided condition, the e-coaches (trained psychologists on minimal Bachelor (BA) level) provide detailed feedback based on participants’ output of the sessions, and this feedback is displayed at the bottom of the session they completed (see Appendix C for an example). The coach spends approximately 30 min on providing feedback per session and intends to give feedback within five working days after the session is completed by the participant. During the trial, training and weekly supervision for the e-coaches who are guiding the participants are provided by a licensed mental healthcare psychologist.
bmjopen-2021-049554supp003.pdf (98.2KB, pdf)
Care as usual
Participants in the CAU condition are informed and referred to conventional care services and encouraged to seek the help they need. Medical services used by the participants during the RCT are monitored through self-report questions at t3, t4 and t5. Participants in the CAU condition are assessed at the same time points as the two intervention conditions including the weekly questionnaires during the first 7 weeks.
Suicide risk monitoring
All participants receive an information brochure with all relevant contact information as part of the information package they receive prior to the study. In addition, the brief version of the PHQ-9, the PHQ-444 is administered before each treatment session or weekly via email (CAU). Suicide risk is monitored with the help of a suicide risk protocol that was created specifically for this study (Klein et al Suicide protocol). Suicide risk is monitored using item 3 (‘feeling down, depressed or hopeless’) of the PHQ-4 and item 9 (‘thought that you would be better off dead, or of hurting yourself’) of the Beck Depression Inventory scale.45 If deemed necessary, participants are called and referred to appropriate services, including their General Practitioner (GP) or 113zelfmoordpreventie (online platform for suicide prevention). In addition, the participant also receives a pop-up message on the questionnaire page with relevant contact information right after filling in the questionnaire (ie, where they can find help if needed with relevant contact information).
Assessments
The RCT includes five assessment points: (1) a baseline assessment including a diagnostic interview and questionnaires, (2) a midtreatment questionnaire, 5 weeks after baseline, (3) a post-treatment questionnaire, 8 weeks after baseline and (4) follow-up measurements, 6 months and (5) 12 months after baseline (figure 1). In addition, prior to each treatment session, or weekly in the CAU condition, participants fill out a short online questionnaire. This questionnaire briefly assesses symptom severity and monitors suicidal ideation.
Figure 1.
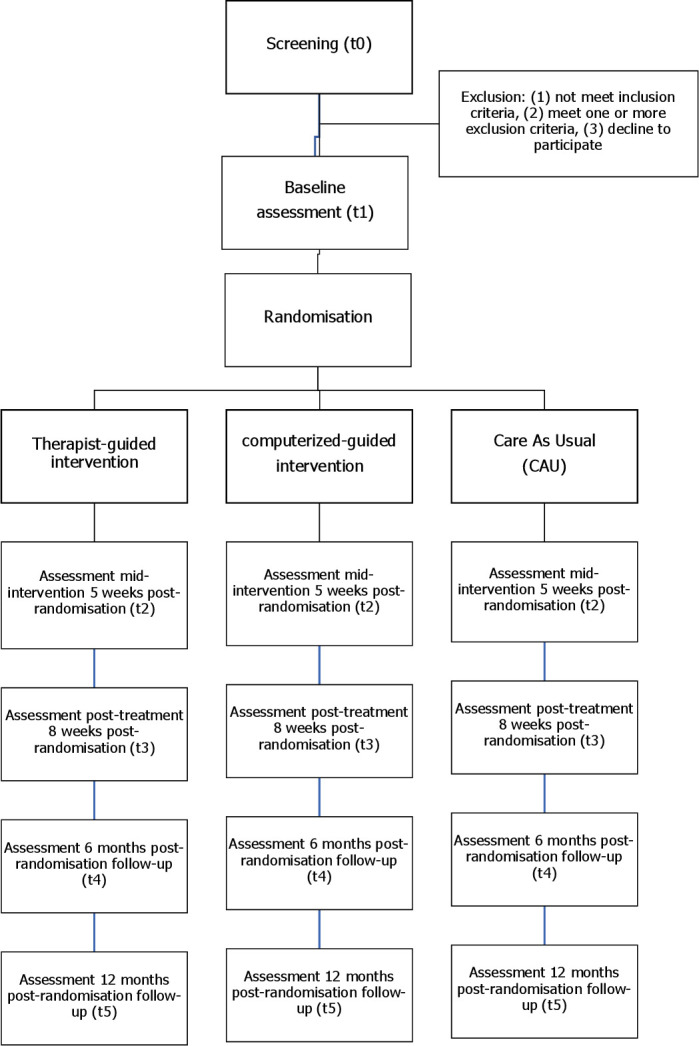
Flow of the study.
Primary outcomes
The Generalised Anxiety disorder-7 (GAD-7)35 and the Patient Health Questionnaire-9 (PHQ-9)46 are the primary outcomes in the study and assess respectively anxiety and depressive symptoms on a continuous scale. The 7-item GAD is a self-report questionnaire measuring anxiety symptoms. All items are scored on a scale from 0 (‘not at all’) to 3 (‘nearly every day’), with total scores ranging from 0 to 21. A higher score on the questionnaire is associated with a more severe experience of anxiety symptoms. The GAD-7 questionnaire has good psychometric properties including a good test retest reliability (ICC=0.83)35 and a good internal consistency (o.79<α<0.91).47 The PHQ-9 questionnaire is used to screen for self-reported symptoms of depression. The nine items are scored on a 0–3 scale, resulting in a total score between 0 and 27. A higher score on the scale indicates a more severe experience of depressive symptoms. The PHQ-9 is a valid and reliable tool with good sensitivity (0.88) and high specificity (0.94)48 49 (see table 1 for an overview of all questionnaires on all time points).
Table 1.
Overview of the measures separately for each assessment point
| Questionnaire | Assessment points | ||||||
| Baseline (t1) | Presessions | Midtreatment (t2) | Post-treatment (t3) | 6 months follow-up (t4) | 12 months follow-up (t5) | ||
| Anxiety | GAD-7 | X | X* | X | X | X | X |
| Depression | PHQ-9 | X | X* | X | X | X | X |
| Social anxiety | SIAS-6 | X | X | X | X | X | |
| Social anxiety | Mini-SPIN | X | X | X | X | X | X |
| Alcohol use | AUDIT-C | X | X | X | X | X | |
| Drug use | DAST-10 | X | X | X | X | X | |
| Quality of life | EQ-5D | X | X | X | X | ||
| Objective academic achievement | † | X | X | X | X | ||
| Subjective academic achievement | PSS-WIS | X | X | X | X | ||
| Perfectionism | DEQ-SCP (short) | X | |||||
| Suicide risk | BDI-II (item 9) PHQ-4 (item 3) |
X | |||||
| Medical service use | TiC-P | X | X | X | |||
| Satisfaction with intervention | CSQ-8 | X | |||||
*PHQ-4, brief anxiety and depression measure to monitor symptoms.
†Self-developed questionnaire.
AUDIT-C, Alcohol Use Disorders Identification Test-Concise; BDI-II, Beck Depression Inventory scale –II; CSQ-8, Client Satisfaction Questionnaire—eight items; DAST-10, Drug Abuse Screening Test—ten items; DEQ-SCP, Depressive Experiences Questionnaire - Self-Critical Perfectionism; ECS-R-SF, Revised Experiences in Close Relationships—Short Form; EQ-5D-5L, EuroQol-5D-5L; GAD-7, General Anxiety Disorder-seven items; Mini-SPIN, Mini-Social Phobia Inventory; PHQ-4, Patient Health Questionnaire—four items; PHQ-9, Patient Health Questionnaire-nine items; PSS-WIS, Presenteeism Scale for Students—Work Impairment Scale; SIAS-6, Social Interaction Anxiety Scale—six items; TiC-P, Treatment Inventory of Costs in Patients.
Secondary outcomes
Alcohol and drug use are measured with respectively the Alcohol Use Disorders Identification Test-Concise (AUDIT-C)50 51 and the Drug Abuse Screening Test – 10 items (DAST-10).52 The AUDIT-C is a brief screening tool for identifying early and/or heavy alcohol use. The scale consists of 3 items, all scored on a 0–4 scale, resulting in a total score ranging from 0 to 12. The DAST-10 consists of 10 items. Item responses are ‘yes’ or ‘no’. Higher scores indicate a more severe level of drug abuse problems. The DAST-10 reports high validity (α=0.94) and reliability in a broad range of settings and populations.53
Quality of life is measured with the EuroQol-5D-5L scale (EQ-5D-5L).54 The EQ-5D-5L assesses health-related well-being on five dimensions: mobility, self-care, ordinary activities, discomfort and mood state. The sixth item of the EQ-5D-5L is a VAS scale, ranging from 0 to 100, asking the participant to rate their health perception at that moment. The EQ-5D-5L has high levels of acceptability and sensitivity.55 56
Academic achievement is measured with objective and subjective measures. Students are asked to report their number of European Credit Transfer System as an objective measure of their academic performance. Additionally, the work impairment subscale of the Presenteeism Scale for Students (PSS-WIS)57 is administered to assess subjective academic achievement on a 5-point Likert scale ranging from ‘always’ (0) to ‘never’ (5). The PSS-WIS is a valid (ICC=0.88 (95% CI p<0.001)) and reliable (test–retest reliability r=0.80, p<0.001) self-report measure to screen for impaired work performance due to (mental) health problems.57
Medical service use is measured with two items from the Treatment Inventory of Costs in Patients with mental disorders.58 The first item includes the frequency of contact with conventional care services (eg, general practitioner, study advisor, psychologist, medical specialist). The second item includes the use of medication during the length of the treatment period.
Client satisfaction with treatment is measured with the Client Satisfaction Questionnaire—eight items (CSQ-8).59 60 The CSQ-8 consists of 8 items scored on a 1 (‘quite dissatisfied’) to 4 (‘very satisfied’) scale, with total scores ranging between 8 and 32. The CSQ-8 is a standardised satisfaction measure reporting very good internal consistency (α=0.83–0.93) and high validity.61
Finally, following previous studies (for a systematic review), 62 treatment adherence is measured by tracking the activities in Minddistrict. We collect the total number of modules completed, time spent per module and the number of times the participants log into the Minddistrict platform.
Predictors of treatment outcome
Social anxiety is measured with the Social Interaction Anxiety Scale-6 item (SIAS-6)63 and the brief Mini-Social Phobia Inventory (Mini-SPIN).64 The brief Mini-SPIN64 is included to screen for generalised social anxiety disorder. The scale shows strong sensitivity (93.8%) and good internal consistency (α=0.85).65 66 Besides the administration on t1–t5, the Mini-SPIN is administered before each treatment session, or weekly via email (CAU), to monitor the social anxiety status of the participant.
Perfectionism is measured with the short version of the Self-Critical Perfectionism subscale of the Depressive Experiences Questionnaire (DEQ-SCP).67 The subscale consists of 12 items. The DEQ-SCP scale is a simple, valid scale demonstrating good psychometric properties, in both clinical and student samples.68
Besides the inclusion of social anxiety and perfectionism, we include several variables to study possible predictors of treatment outcome including demographic variables (ie, age, gender, study direction, faculty, national/international student), baseline severity, personality factors, number of sessions completed/adherence rate and comorbidity for explorative analyses.
Statistical analyses
Sample size calculation
The power analysis is based on the mixed factors 3×3 interaction difference between the three treatment arms and three time points (premeasurement, midmeasurement and postmeasurement). We anticipate a small interaction effect (Cohen’s f=0.1) at post-treatment based on a recent meta-analysis that focused on the effectiveness of therapist-guided versus computer-guided psychotherapy in treating depression and anxiety.17 We determined a statistical power (1-ß) of.8 and corrected alpha of α=0.05. With a two-tailed hypothesis, we need 204 participants per intervention group to be able to detect a statistically significant result. Previous literature has shown that web-based interventions have a relatively high reported range of drop-out in university students, varying between approximately 30% and 40% posttreatment.20 Calculating with a dropout rate of 35%, the minimum required total sample for the RCT is Ntotal=276 participants (with a ratio of 1:1:1), with 92 participants in each group.
Clinical analyses
Mixed Linear Model analyses will be conducted with SPSS and R.
To get a more realistic picture of the true efficacy of treatment, completer analyses will also be performed for those participants who adhere to the protocol.
Predictor analyses
Predictors of treatment outcome will be investigated on an exploratory basis with predefined predictors: social anxiety and perfectionism, baseline symptoms severity, gender, personality factors and comorbidity. Analyses will be conducted using regression analyses, with interaction effects and structural equation modelling.
Patient and public involvement
A focus group of university students helped with the design of the study. This group also helped with writing the consent letters, the website texts and checked the clearness of all instructions on the online platform. All university students of the UvA will receive an email with the (anonymised) results at the group level when the project is finished.
Discussion
Even though many university students face mental problems, only relatively few students use available services.12 iCBT programmes are easily accessible, flexible and relatively anonymous.24 Hence, they might be a good alternative for reaching out to this group14. The current study aims to compare the effectiveness of online interventions for elevated levels of anxiety and/or depression in university students enrolled at the UvA: a therapist-guided transdiagnostic iCBT programme, a computer-guided version of the same programme and CAU. Several moderators are included that may potentially amplify or attenuate treatment response and so may provide new knowledge on effectiveness for different subgroups.
Strengths of this study are the use of a transdiagnostic iCBT programme, its large sample size, and the generalisability of the findings since exclusion criteria are minimal (eg, there are almost no exclusion criteria concerning comorbid disorders; we created a Dutch and English version, so that both national and international student can participate). Moreover, the current iCBT programme is flexible, because participants can complete sessions at their own pace and it includes separate modules focusing on related problems (eg, stress, sleep, alcohol use). This flexibility has both strengths and limitations. On the one hand, participants will not feel pressured, they can work on a session whenever they have time do to so, and they have freedom in choosing additional modules that fit their situation. On the other hand, this might result in a different therapeutic process for each participant, which makes it more difficult to compare the different groups. Also, this flexibility makes it more difficult to investigate the effective components of the programme. One way to investigate this is to follow the course of the symptoms by analysing the presession questionnaire. Some participants might find it difficult to stay motivated or decide between the different treatment module options. Therefore, one of the limitations of this study is a potentially high degree of drop out, especially in the computer-guided programme and in the CAU condition.25 To minimise drop out, participants receive up to 3 weekly reminders via the messaging function when they are inactive. In addition, we provide all participants with technical support and suicide risk monitoring. Also, there may be a potential confound of ‘feedback length’, as the feedback in the therapist-guided condition is textually longer than the automated feedback in the computer-guided condition. Due to the large sample size but limited human resources, and our priority to maintain the privacy of participants, all measurements are self-reported and may, therefore, be more subjective than clinician-rated reports. Moreover, participants may not report certain information, for example, whether they receive face-to-face treatment or counselling for their complaints during the current study. Finally, our automated study design allows for blinded assessments throughout the intervention, which on the downside may lead to some assessments not being aligned with the intervention phase (eg, ‘midtreatment’ questionnaires being completed before session 4 has been reached.)
In sum, the current trial is unique as it compares two versions of the same transdiagnostic iCBT intervention; a therapist-guided versus a computer-guided version in a large sample that includes a 1-year follow-up. This study will, therefore, improve our understanding of the need of individualised feedback for computer-based interventions. The findings will significantly add to the knowledge on the treatment of depression and anxiety in university students and will give insight into treatment predictors/moderators that may determine why some students benefit and others do not.
Ethics and dissemination
The current study was approved by the Medical Ethics Review Committee (METC) of the Academic Medical Centre, Amsterdam, The Netherlands (number: NL64929.018.18) and follows all Dutch ethical legislations. Any unintended effects will be reported to the METC committee. All procedures are in accordance with the latest version of the Helsinki declaration and comply to the GDPR rules (2018). All protocol changes will be reported to the ethical committees, and trial participants per email. Active informed consent from participants is asked for the entire procedure. Results of this trial will be published in peer-reviewed journals.
Supplementary Material
Acknowledgments
We would like to thank the student psychologists, the student deans, colleagues of academic affairs, the colleagues from the Behavioural Science Lab (BSL) the student advisory boards who were involved in the focus group from the University of Amsterdam and M. van der Hoff and R. Atteveld for their help with the design of the project.
Footnotes
Twitter: @KaryotakiEirini
Contributors: RWW (PI), PV (PI), AK (Co-PI) and CvdH (Co-PI) received funding for the project. AK, RWW, PV, CvdH, NEW, EJMB, JK, SSMR, JJvB, TP, ES, FB, HR, EK, PC, SS and RMR designed the study. AK drafted the main part of the manuscript, NW and LdK assisted in writing parts of the manuscript under supervision of AK. EJMB, JL, LdK, SSMR, JJvB, CvdH, ES, FB, HR, EK, PC, SS, RMR, PV and RWW provided critical feedback for important intellectual content on earlier versions of the manuscript. AK, SSMR, NEW, JK, EJMB and JJvB coordinated the data recruitment and data collection. All authors approved the final version of the manuscript for submission and all authors agreed to be accountable for all aspects of the work.
Funding: This work was supported by a grant from the University of Amsterdam (Spui 21, 1012GC,+31205251400) awarded to AK (Co-PI), PV (PI; p.vonk@uva.nl; Oude Turfmarkt 151, 1012LA, Amsterdam, University of Amsterdam), CvdH (Co-PI) and RWW (PI; r.w.h.j.wiers@uva.nl; Nieuwe achtergracht 129, 1001NK, Amsterdam, University of Amsterdam). Award/grant number non-applicable. A steering group from the University of Amsterdam monitors the processes of the entire trial and approves mid-term evaluation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000;55:469–80. [PubMed] [Google Scholar]
- 2.Auerbach RP, Mortier P, Bruffaerts R, et al. Who world mental health surveys international college student project: prevalence and distribution of mental disorders. J Abnorm Psychol 2018;127:623–38. 10.1037/abn0000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Girolamo G, Dagani J, Purcell R, et al. Age of onset of mental disorders and use of mental health services: needs, opportunities and obstacles. Epidemiol Psychiatr Sci 2012;21:47–57. 10.1017/s2045796011000746 [DOI] [PubMed] [Google Scholar]
- 4.Zivin K, Eisenberg D, Gollust SE, et al. Persistence of mental health problems and needs in a college student population. J Affect Disord 2009;117:180–5. 10.1016/j.jad.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 5.Auerbach RP, Mortier P, Bruffaerts R, et al. Mental disorder comorbidity and suicidal thoughts and behaviors in the world Health organization world mental health surveys international college student initiative. Int J Methods Psychiatr Res 2019;28:e1752. 10.1002/mpr.1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auerbach RP, Alonso J, Axinn WG, et al. Mental disorders among college students in the world Health organization world mental health surveys. Psychol Med 2016;46:2955–70. 10.1017/S0033291716001665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso J, Mortier P, Auerbach RP, et al. Severe role impairment associated with mental disorders: results of the who world mental health surveys international college student project. Depress Anxiety 2018;35:802–14. 10.1002/da.22778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolinski F, Boumparis N, Kleiboer A, et al. The effect of e-mental health interventions on academic performance in university and college students: a meta-analysis of randomized controlled trials. Internet Interv 2020;20:100321. 10.1016/j.invent.2020.100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGorry PD, Purcell R, Goldstone S, et al. Age of onset and timing of treatment for mental and substance use disorders: implications for preventive intervention strategies and models of care. Curr Opin Psychiatry 2011;24:301–6. 10.1097/YCO.0b013e3283477a09 [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund PA, Bruce ML, et al. The prevalence and correlates of untreated serious mental illness. Health Serv Res 2001;36:987–1007. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang PS, Berglund P, Olfson M, et al. Failure and delay in initial treatment contact after first onset of mental disorders in the National comorbidity survey replication. Arch Gen Psychiatry 2005;62:603–13. 10.1001/archpsyc.62.6.603 [DOI] [PubMed] [Google Scholar]
- 12.Buchanan JL. Prevention of depression in the College student population: a review of the literature. Arch Psychiatr Nurs 2012;26:21–42. 10.1016/j.apnu.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis 2011;199:301–8. 10.1097/NMD.0b013e3182175123 [DOI] [PubMed] [Google Scholar]
- 14.Van der Heijde CM, Vonk P, Meijman FJ. Self-Regulation for the promotion of student health. traffic lights: the development of a tailored web-based instrument providing immediate personalized feedback. Health Psychol Behav Med 2015;3:169–89. 10.1080/21642850.2015.1049950 [DOI] [Google Scholar]
- 15.Boydell KM, Hodgins M, Pignatiello A, et al. Using technology to deliver mental health services to children and youth: a scoping review. J Can Acad Child Adolesc Psychiatry 2014;23:87–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Carlbring P, Andersson G, Cuijpers P, et al. Internet-Based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther 2018;47:1–8. 10.1080/16506073.2017.1401115 [DOI] [PubMed] [Google Scholar]
- 17.Cuijpers P, Noma H, Karyotaki E. Individual, group, telephone, self-help and Internet-based cognitive behavior therapy for adult depression: a network meta-analysis of delivery methods. JAMA Psychiatry 2019;76:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies EB, Morriss R, Glazebrook C. Computer-delivered and web-based interventions to improve depression, anxiety, and psychological well-being of university students: a systematic review and meta-analysis. J Med Internet Res 2014;16:e130. 10.2196/jmir.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebert DD, Zarski A-C, Christensen H, et al. Internet and computer-based cognitive behavioral therapy for anxiety and depression in youth: a meta-analysis of randomized controlled outcome trials. PLoS One 2015;10:e0119895. 10.1371/journal.pone.0119895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrer M, Adam SH, Baumeister H, et al. Internet interventions for mental health in university students: a systematic review and meta-analysis. Int J Methods Psychiatr Res 2019;28:e1759. 10.1002/mpr.1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethi S, Campbell AJ, Ellis LA. The use of computerized self-help packages to treat adolescent depression and anxiety. J Technol Hum Serv 2010;28:144–60. 10.1080/15228835.2010.508317 [DOI] [Google Scholar]
- 22.Riper H, van Straten A, Keuken M, et al. Curbing problem drinking with personalized-feedback interventions: a meta-analysis. Am J Prev Med 2009;36:247–55. 10.1016/j.amepre.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 23.Newby JM, McKinnon A, Kuyken W, et al. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clin Psychol Rev 2015;40:91–110. 10.1016/j.cpr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Andersson G, Cuijpers P, Carlbring P, et al. Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry 2014;13:288–95. 10.1002/wps.20151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards D, Richardson T. Computer-Based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev 2012;32:329–42. 10.1016/j.cpr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Berger T, Hämmerli K, Gubser N, et al. Internet-Based treatment of depression: a randomized controlled trial comparing guided with unguided self-help. Cogn Behav Ther 2011;40:251–66. 10.1080/16506073.2011.616531 [DOI] [PubMed] [Google Scholar]
- 27.Renfrew ME, Morton DP, Morton JK, et al. The influence of three modes of human support on attrition and adherence to a Web- and mobile App-Based mental health promotion intervention in a nonclinical cohort: randomized comparative study. J Med Internet Res 2020;22:e19945. 10.2196/19945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessler RC, van Loo HM, Wardenaar KJ, et al. Using patient self-reports to study heterogeneity of treatment effects in major depressive disorder. Epidemiol Psychiatr Sci 2017;26:22–36. 10.1017/S2045796016000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson JL, Rapee RM, Lyneham HJ, et al. Comparing outcomes for children with different anxiety disorders following cognitive behavioural therapy. Behav Res Ther 2015;72:30–7. 10.1016/j.brat.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 30.Norton PJ, Price EC. A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. J Nerv Ment Dis 2007;195:521–31. 10.1097/01.nmd.0000253843.70149.9a [DOI] [PubMed] [Google Scholar]
- 31.Löw CA, Schauenburg H, Dinger U. Self-criticism and psychotherapy outcome: a systematic review and meta-analysis. Clin Psychol Rev 2020;75:101808. 10.1016/j.cpr.2019.101808 [DOI] [PubMed] [Google Scholar]
- 32.Shahar G, Blatt SJ, Zuroff DC, et al. Perfectionism impedes social relations and response to brief treatment for depression. J Soc Clin Psychol 2004;23:140–54. 10.1521/jscp.23.2.140.31017 [DOI] [Google Scholar]
- 33.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beekman AT, Deeg DJ, Van Limbeek J, et al. Criterion validity of the center for epidemiologic studies depression scale (CES-D): results from a community-based sample of older subjects in the Netherlands. Psychol Med 1997;27:231–5. 10.1017/s0033291796003510 [DOI] [PubMed] [Google Scholar]
- 35.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 36.Lecrubier Y, Sheehan DV, Weiller E, et al. The mini international neuropsychiatric interview (mini). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 1997;12:224–31. 10.1016/S0924-9338(97)83296-8 [DOI] [Google Scholar]
- 37.Weisel KK, Zarski A-C, Berger T, et al. Transdiagnostic tailored Internet- and Mobile-Based guided treatment for major depressive disorder and comorbid anxiety: study protocol of a randomized controlled trial. Front Psychiatry 2018;9:274. 10.3389/fpsyt.2018.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolinski F, Kleiboer A, Karyotaki E, et al. Effectiveness of a transdiagnostic individually tailored Internet-based and mobile-supported intervention for the indicated prevention of depression and anxiety (ICARE prevent) in Dutch college students: study protocol for a randomised controlled trial. Trials 2018;19:118–30. 10.1186/s13063-018-2477-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karyotaki E, Klein AM, Riper H, et al. Examining the effectiveness of a web-based intervention for symptoms of depression and anxiety in college students: study protocol of a randomised controlled trial. BMJ Open 2019;9:e028739. 10.1136/bmjopen-2018-028739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buntrock C, Ebert DD, Lehr D, et al. Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA 2016;315:1854–63. 10.1001/jama.2016.4326 [DOI] [PubMed] [Google Scholar]
- 41.Ebert DD, Buntrock C, Lehr D, et al. Effectiveness of Web- and Mobile-Based treatment of subthreshold depression with Adherence-Focused guidance: a single-blind randomized controlled trial. Behav Ther 2018;49:71–83. 10.1016/j.beth.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 42.Ebert DD, Nobis S, Lehr D, et al. The 6-month effectiveness of Internet-based guided self-help for depression in adults with type 1 and 2 diabetes mellitus. Diabet Med 2017;34:99–107. 10.1111/dme.13173 [DOI] [PubMed] [Google Scholar]
- 43.Etzelmueller A, Vis C, Karyotaki E, et al. Effects of Internet-based cognitive behavioral therapy in routine care for adults in treatment for depression and anxiety: systematic review and meta-analysis. J Med Internet Res 2020;22:e18100. 10.2196/18100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JBW, et al. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613–21. 10.1176/appi.psy.50.6.613 [DOI] [PubMed] [Google Scholar]
- 45.Beck AT, Steer RA, Ball R, et al. Comparison of Beck depression inventories -Ia and -II in psychiatric outpatients. J Pers Assess 1996;67:588–97. 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- 46.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:509–15. 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 47.Dear BF, Titov N, Sunderland M, et al. Psychometric comparison of the generalized anxiety disorder scale-7 and the Penn state worry questionnaire for measuring response during treatment of generalised anxiety disorder. Cogn Behav Ther 2011;40:216–27. 10.1080/16506073.2011.582138 [DOI] [PubMed] [Google Scholar]
- 48.Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the patient health questionnaire: a systematic review. Gen Hosp Psychiatry 2007;29:388–95. 10.1016/j.genhosppsych.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 49.Levis B, Benedetti A, Thombs BD. Accuracy of patient health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ 2019;64:365:l1476. 10.1136/bmj.l1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush K, Kivlahan DR, McDonell MB. The audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 51.Verhoog S, Dopmeijer JM, de Jonge JM, et al. The Use of the Alcohol Use Disorders Identification Test - Consumption as an Indicator of Hazardous Alcohol Use among University Students. Eur Addict Res 2020;26:1–9. 10.1159/000503342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skinner HA. The drug abuse screening test. Addict Behav 1982;7:363–71. 10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
- 53.Maisto SA, Carey MP, Carey KB, et al. Use of the audit and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2000;12:186–92. 10.1037//1040-3590.12.2.186 [DOI] [PubMed] [Google Scholar]
- 54.EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 55.Heslin M, Chua K-C, Trevillion K, et al. Psychometric properties of the five-level EuroQoL-5 dimension and short Form-6 dimension measures of health-related quality of life in a population of pregnant women with depression. BJPsych Open 2019;5:388. 10.1192/bjo.2019.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.König H-H, Heider D, Lehnert T, et al. Health status of the advanced elderly in six European countries: results from a representative survey using EQ-5D and SF-12. Health Qual Life Outcomes 2010;8:143–53. 10.1186/1477-7525-8-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsushita M, Adachi H, Arakida M, et al. Presenteeism in college students: reliability and validity of the Presenteeism scale for students. Qual Life Res 2011;20:439–46. 10.1007/s11136-010-9763-9 [DOI] [PubMed] [Google Scholar]
- 58.Bouwmans C, De Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res 2013;13:217–25. 10.1186/1472-6963-13-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen DL, Attkisson CC, Hargreaves WA, et al. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197–207. 10.1016/0149-7189(79)90094-6 [DOI] [PubMed] [Google Scholar]
- 60.de Brey H. A cross-national validation of the client satisfaction questionnaire: the Dutch experience. Eval Program Plann 1983;6:395–400. 10.1016/0149-7189(83)90018-6 [DOI] [PubMed] [Google Scholar]
- 61.Attkisson CC, Greenfield TK. Client Satisfaction Questionnaire-8 and Service Satisfaction Scale-30. In: Maruish ME, ed. The use of psychological testing for treatment planning and outcome assessment. Lawrence Erlbaum Associates, Inc: 402–20. [Google Scholar]
- 62.Christensen H, Griffiths KM, Farrer L. Adherence in Internet interventions for anxiety and depression. J Med Internet Res 2009;11:e13. 10.2196/jmir.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters L, Sunderland M, Andrews G, et al. Development of a short form social interaction anxiety (SIAS) and social phobia scale (SPS) using nonparametric item response theory: the SIAS-6 and the SPS-6. Psychol Assess 2012;24:66–76. 10.1037/a0024544 [DOI] [PubMed] [Google Scholar]
- 64.Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety 2001;14:137–40. 10.1002/da.1055 [DOI] [PubMed] [Google Scholar]
- 65.Weeks JW, Spokas ME, Heimberg RG. Psychometric evaluation of the mini-social phobia inventory (Mini-SPIN) in a treatment-seeking sample. Depress Anxiety 2007;24:382–91. 10.1002/da.20250 [DOI] [PubMed] [Google Scholar]
- 66.Seeley-Wait E, Abbott MJ, Rapee RM. Psychometric properties of the mini-social phobia inventory. Prim Care Companion J Clin Psychiatry 2009;11:231–6. 10.4088/PCC.07m00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blatt SJ, D'Afflitti JP, Quinlan DM. Experiences of depression in normal young adults. J Abnorm Psychol 1976;85:383. 10.1037//0021-843x.85.4.383 [DOI] [PubMed] [Google Scholar]
- 68.Desmet M, Vanheule S, Groenvynck H, et al. The depressive experiences questionnaire. Eur J Psychol Assess 2007;23:89–98. 10.1027/1015-5759.23.2.89 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049554supp001.pdf (83.1KB, pdf)
bmjopen-2021-049554supp002.pdf (135.5KB, pdf)
bmjopen-2021-049554supp003.pdf (98.2KB, pdf)


