Abstract
Objectives:
Our objectives were to (1) quantify the frequency of wheezing episodes and asthma diagnosis in young children in a large pediatric primary care network and (2) assess the variability in practice-level asthma diagnosis, accounting for common asthma risk factors and comorbidities. We hypothesized that significant variability in practice-level asthma diagnosis rates would remain after adjusting for associated predictors.
Methods:
We generated a retrospective longitudinal birth cohort of children who visited one of 31 pediatric primary care practices within the first 6 months of life from 1/2005-12/2016. Children were observed for up to 8 years or until the end of the observation window. We used multivariable discrete time survival models to evaluate predictors of asthma diagnosis by 3-month age intervals. We compared unadjusted and adjusted proportions of children diagnosed with asthma by practice.
Results:
Of the 161,502 children in the cohort, 34,578 children (21%) received at least one asthma diagnosis. In multivariable modeling, male gender, minority race/ethnicity, gestational age <34 weeks, allergic rhinitis, food allergy, and prior wheezing episodes were associated with asthma diagnosis. After adjusting for variation in these predictors across practices, the cumulative incidence of asthma diagnosis by practice by age 6 years ranged from 11-47% (interquartile range (IQR): 24-29%).
Conclusions:
Across pediatric primary care practices, adjusted incidence of asthma diagnosis by age 6 years ranged widely, though variation gauged by the IQR was more modest. Potential sources of practice-level variation, such as differing diagnosis thresholds and labeling of different wheezing phenotypes as “asthma”, should be further investigated.
Keywords: childhood wheezing, asthma incidence, variation in diagnosis
Introduction
Asthma is the most common chronic physical illness in childhood with prevalence rates around 9% nationally.1 Diagnosing asthma in young children, however, is fraught with challenges. Asthma is a heterogeneous condition with a number of varying clinical phenotypes. There is no specific biomarker associated with the condition and no clear gold standard for diagnosis.2,3 The most common clinical presentation, recurrent wheezing, is non-specific4, and prior research suggests that up to 60% of young children who wheeze will no longer wheeze by age 6 years.5 Further, there are no agreed upon criteria for number or type of wheezing episodes that justify an asthma diagnosis. This uncertainty leaves substantial room for clinician interpretation and the potential for marked practice variation.
Variation in asthma diagnosis rates may reflect underlying differences in population-level risk (appropriate variation) or, alternatively, physicians employing differing clinical thresholds for diagnosis in patients with similar characteristics (inappropriate variation). An inappropriately low threshold for diagnosis would result in overdiagnosis and could lead to unnecessary treatment and misappropriation of clinical resources.6 An inappropriately high threshold would result in under-diagnosis, undertreatment, and potentially preventable morbidity. Although variation in asthma diagnosis has important implications for clinical care, quality reporting, and research, practice-level variation in asthma diagnosis in pediatric primary care has not been extensively studied.
The National Asthma Education and Prevention Program’s Expert Panel Report, the primary consensus guidelines on the care of asthma in the US, highlights “key indicators” for considering asthma diagnosis including wheezing and a history of cough or recurrent wheezing, shortness of breath, or difficulty breathing.2 Pediatric textbooks offer similar non-specific guidance, with one prominent text noting that “intermittent dry coughing and/or expiratory wheezing are the most common chronic symptoms of asthma” without offering explicit diagnostic criteria.7 More recently, experts have promoted a probability-based approach to diagnosis based on risk factors and the pattern of symptoms during and between viral respiratory illnesses.8 Risk factors include recurrent wheezing, childhood eczema, allergic rhinitis, parental asthma, and test results, such as eosinophilia and atopic sensitization.9-12
The extent to which this lack of a consensus on criteria for clinical diagnosis of asthma translates into variability in physicians’ diagnosis of asthma is unclear. Our specific objectives were to quantify the frequency of wheezing episodes and physician-diagnosed asthma in young children and to assess variability in diagnosed asthma between practices in a large pediatric primary care network, after adjusting for asthma risk factors and comorbities.
Methods
Study design
This is a retrospective longitudinal cohort study of a large academic pediatric healthcare system of 31 pediatric primary care practices in the Northeast that have been on the same electronic medical record (EMR) since 2005. This study was approved by the system’s institutional review board.
Study Population
All data from this study were obtained from the health system’s EMR. Children with an in-person visit to one of the health system’s primary care practices in the first six months of life from 1/2005 through 12/2016 were eligible for inclusion. Children were observed up to age 8 years (to allow observation into school age when viral-associated wheezing is less common) in sequential 18-month observation windows beginning at age 6 months (i.e. 6-23 months, 24-41 months, etc.). Eighteen-month windows were chosen to account for visits that may occur just beyond the recommended 12-month periodicity schedule for well-child visits for children older than three. Children’s data were included up to the end of the last 18-month interval for which they had an outpatient visit; subsequent intervals were not included (right-censored). Children who had no further in-person visits following age 6 months, or who had already received an asthma diagnosis by age 6 months were excluded.
Outcomes
Our primary outcome was physician-diagnosed asthma. When physicians record diagnoses in the EMR, they are stored as SNOMED CT codes, a comprehensive clinical terminology. We selected all 97 unique SNOMED asthma diagnostic codes to generate a comprehensive list of asthma diagnoses (Supplemental Table 1). The recording of any single instance of any of these codes was considered to be diagnosed asthma.
Covariates
The primary exposure was the number of wheezing episodes, which we derived from the COAST cohort definition as an office visits with an associated wheezing diagnosis or a beta-agonist medication prescriptions without a preceding or concurrent asthma diagnosis.13 There were 6 SNOMED wheezing diagnostic codes, including bronchiolitis (Supplemental Table 1); encounters with a “croup” diagnosis were excluded. Wheezing episodes occurring within 2 weeks of each other were considered one episode. We assessed additional asthma risk factors12 that were reliably documented in discrete fields in the EMR: gender, race/ethnicity, gestational age at delivery, and prior or concomitant diagnoses of cough, eczema, allergic rhinitis, and food allergy (Supplemental Table 1). Prior or concomitant diagnoses were considered time-varying (could have been diagnosed at any clinic encounter), whereas other covariates did not vary over time.
To assess variability in wheezing episodes and asthma diagnosis by practice, we included a variable for practice site, defined as the first practice that the child visited after birth. We consolidated two groups of three practices that shared clinical providers into two sites, reducing the number of practices from 31 to 27.
Statistical analysis
We first compared characteristics of children with diagnosed asthma to those who never received an asthma diagnosis. We then compared the percentage of children diagnosed with asthma by number of diagnosed wheezing episodes. We estimated the unadjusted frequency of wheezing and asthma diagnosis by practice and computed a Spearman’s rho coefficient to assess correlation in wheezing and asthma diagnosis across practices.
We used mixed effects logistic regression models to estimate the effects of age (operationalized as 3-month intervals, explained below), gender, race/ethnicity, gestational age <34 weeks, and clinical covariates present at the start of each age interval on odds of incident asthma diagnosis at a given age, accounting for random variation by practice site using random intercepts. Children were included in the analysis up until the end of their follow-up (the last 18-month interval in which they had an in-person, outpatient visit up to 8 years of age) or the first 3-month age interval in which they were diagnosed with asthma. We divided age into 3-month intervals (6-8 months, 9-12 months, etc.) to account for potentially rapid change in the epidemiology of wheezing and asthma diagnosis in early childhood. These models were used to estimate practice-specific probabilities of an incident asthma diagnosis in each 3-month age interval conditional on a given covariate profile (accounting for differences in these covariates – including age distributions – across clinics). These probabilities were then used to estimate the cumulative incidence of asthma diagnosis at a given age by aggregating probabilities across age-intervals to obtain the cumulative probability of an asthma diagnosis at or before that age interval. All covariates except for age were assumed to have non-time-varying effects.
Adjusted practice-specific asthma incidence estimates were generated from these models using marginal standardization, to standardize practice-level estimates to a common distribution of patient characteristics. We then compared the adjusted and unadjusted cumulative incidences at age 6 years, an age by which children are generally able to perform spirometry. To estimate the magnitude of variability explained by practice-site, we calculated the intra-class correlation coefficient (ICC) using a formula specific to the mixed effects logistic regression model: σ2/(σ2+π2/3) where σ2 is the estimated variance of the site-specific random effect and π is the numerical constant.14 ICC is a statistic that describes how strongly units in the same group resemble each other.
Sensitivity Analyses
Hypothesizing that children with only one asthma diagnosis may be unlikely to have an asthma phenotype, we ran a similar multivariable model but restricted the outcome definition to two or more asthma diagnoses.15 In this model, the time of asthma diagnosis was defined to be the time of the second diagnosis.
Second, anticipating children may have been diagnosed with asthma during an ED visit or hospitalization, we reran models including ED and hospital visits, but restricting the cohort to 4 practices whose patients are likely to be admitted to the health system’s ED and inpatient units, based on geographic location and referral patterns.16 We term this cohort the “proximity cohort.” We then repeated our initial multivariable model including all ED and inpatient visits, in addition to the primary care encounters for these 4 practices only.
Results
Cohort description
We identified 161,502 children who met our inclusion and exclusion criteria. (Figure 1) Of children in the cohort, 34,578 (21%) received at least one encounter-level asthma diagnosis. Median follow-up time was 87 months (IQR 63-93) for children with at least one asthma diagnosis and 61 months (IQR 28-88) for those without asthma. On average, the practice visited in the first 6 months of life was the location of 90% of all clinic visits for children with an asthma diagnosis and 95% of all clinic visits for children without an asthma diagnosis. With respect to wheezing, 64,687 (40%) children in the cohort had at least one diagnosed wheezing episode. Wheezing episodes occurred in 92% of children diagnosed with asthma and in 26% of children not diagnosed. (Table 1)
Figure 1.
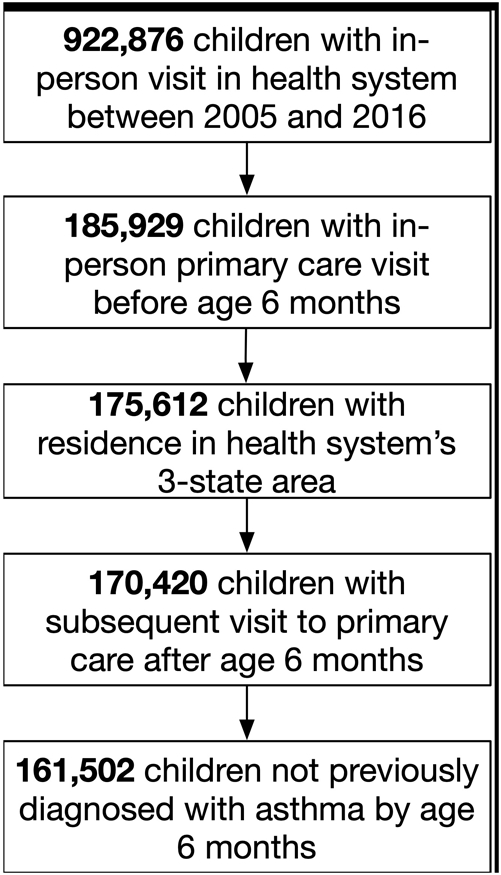
Consort diagram for cohort inclusion
Table 1.
Patient characteristics for (1) Overall cohort, (2) children with no asthma diagnosis and (3) children with at least one asthma diagnosis
| Overall | No Asthma Diagnosis |
Asthma Diagnosis |
||
|---|---|---|---|---|
| Total Population | 161,502 | 126,924 | 34,578 | |
| Sex | Female | 78,882 (48%) | 64,837 (51%) | 14,045 (41%) |
| Race/Eth combined, n (%) | Non-Hispanic Asian | 5,763 (4%) | 4,863 (4%) | 900 (3%) |
| Non-Hispanic Black | 43,706 (30%) | 31,378 (28%) | 12,328 (38%) | |
| Hispanic | 10,533 (7%) | 8,231 (7%) | 2,302 (7%) | |
| Missing | 15,806 (10%) | 13,295 (11%) | 2,511 (7%) | |
| Other | 2,587 (2%) | 2,105 (2%) | 482 (2%) | |
| Non-Hispanic White | 83,107 (57%) | 67,052 (59%) | 16,055 (50%) | |
| Mean gestational age (GA), in months (sd) | 38.6 (2.3) | 38.7 (2.1) | 38.1 (3.0) | |
| Missing GA, n (%) | 32,648 (20%) | 24,584 (19%) | 8,064 (23%) | |
| GA <34 weeks, n (%) | 4,915 (4%) | 3,026 (3%) | 1,889 (7%) | |
| Medications*, n (%) | Albuterol | 60,182 (37%) | 28,735 (23%) | 31,447 (91%) |
| Inhaled Steroid | 11,090 (7%) | 1,555 (1%) | 9,535 (28%) | |
| Other asthma medication† | 3,931 (2%) | 1,555 (1%) | 2,376 (7%) | |
| Systemic Steroid | 27,635 (17%) | 15,237 (12%) | 12,398 (36%) | |
| Comorbidities*, n (%) | Allergic Rhinitis | 29,485 (18%) | 21,889 (17%) | 7,596 (22%) |
| Eczema | 65,409 (41%) | 50,337 (40%) | 15,072 (44%) | |
| Food Allergy | 9,963 (6%) | 7,294 (6%) | 2,669 (8%) | |
| Wheezing episode* n (%) | 64,687 (40%) | 32,811 (26%) | 31,876 (92%) | |
| Bronchiolitis diagnosis*, n (%) | 13,665 (9%) | 6,771 (5%) | 6,894 (20%) | |
| Cough diagnosis*, n (%) | 51,549 (32%) | 37,633 (30%) | 13,916 (40%) | |
| Proportion of visits at initial practice, median (IQR) | 0.94 (0.82 - 1.00) | 0.95 (0.84 - 1.00) | 0.90 (0.77 – 0.98) | |
indicates number of events occurring over entire observation interval for “No Asthma Diagnosis” group and number of events before asthma diagnosis for "Asthma Diagnosis" group
includes montelukast, zafirlukast, theophylline, aminophylline, dyphylline, omalizumab, reslizumab, mepolizumab
Of the 34,578 children diagnosed with asthma, 21,236 (61%) had at least two prior wheezing episodes. In contrast, 13% of those who were not diagnosed with asthma had two or more wheezing episodes. (Table 2) Of the 37,860 children with at least 2 wheezing episodes, 21,236 (56%) were eventually diagnosed with asthma. Figure 2 shows the unadjusted frequency of physician-diagnosed wheezing episodes and asthma by practice site. The unadjusted range in practice-level diagnosed wheezing episodes was 30%-61% and for diagnosed asthma was 10%- 47%. Spearman’s rho between these per-practice percentages of wheezing and asthma was 0.75. One practice – site 27, which was a combination of three suburban, non-teaching practices in a neighboring state – was a clear outlier with 47% of children ever having an asthma diagnosis. Compared to the overall cohort described in Table 1, children from these clinics were more likely to be Non-Hispanic White (78% vs 57%), have a prescription for albuterol (57% vs 37%) or a systemic steroid (38% vs 17%), and be diagnosed with wheezing (61% vs 40%), and less likely to be diagnosed with cough (13% vs 32%).
Table 2.
Comparison of wheezing episodes between children with and without diagnosed asthma
| No Asthma Diagnosis |
Asthma Diagnosis |
|
|---|---|---|
| (n =126,924) | (n=34,578) | |
| Mean number of wheezing episodes (SD)* | 0.5 (1.2) | 2.2 (1.6) |
| Number of wheezing episodes (% of column) * | ||
| 0 | 94,113 (74.2%) | 2,702 (7.8%) |
| 1 | 16,187 (12.8%) | 10,640 (30.8%) |
| 2 | 7,968 (6.3%) | 9,484 (27.4%) |
| 3 | 4,281 (3.4%) | 5,870 (17.0%) |
| 4+ | 4,375 (3.5%) | 5,882 (17.0%) |
| Mean number of wheezing episodes/year (SD)* | 0.1 (0.4) | 1.1 (1.1) |
indicates number of events occurring over entire observation interval for “No Asthma Diagnosis” group and number of events before asthma diagnosis for "Asthma Diagnosis" group; SD=standard deviation
Figure 2.
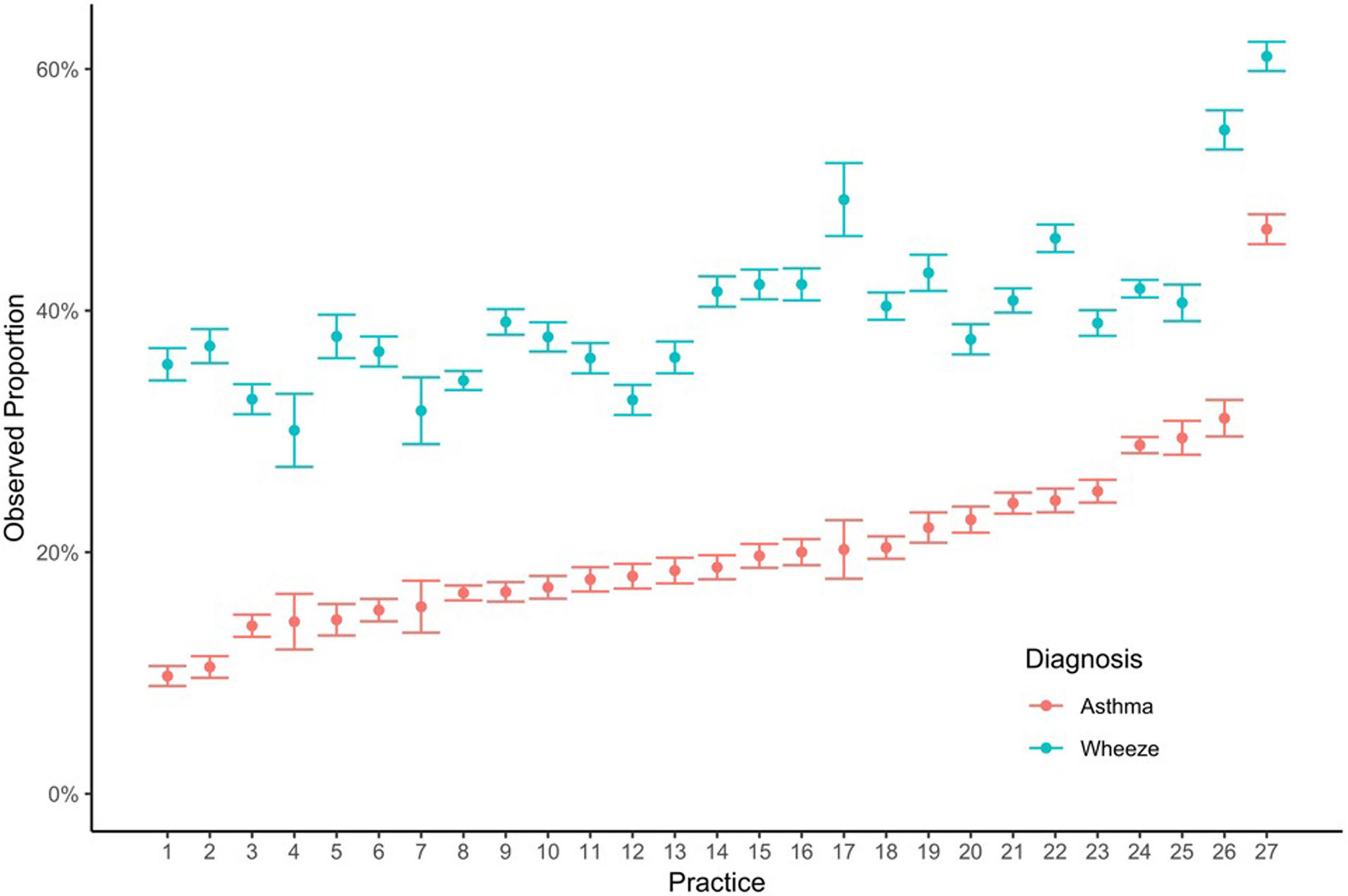
Observed proportion of children diagnosed with wheezing episodes and asthma across the 27 practice site locations (ordered by increasing proportion with asthma)
Main multivariable model results
In our main multivariable model, the covariate most strongly associated with asthma diagnosis was prior wheezing episode (OR 28.56, 95% CI 27.42-29.75). Among those with a prior wheezing episode, each additional wheezing episode was associated with an increase in odds of asthma diagnosis by a factor of 1.37 (95% CI 1.36-1.38), such that children with two prior wheezing episodes had a 53.60 (i.e., 28.56*1.372) higher odds of asthma diagnosis than a similarly aged child with no prior wheezing episodes. To confirm that this large OR was not an artifact of the selected model structure, we re-ran the model using categorical definitions for both prior wheezing and cough (0, 1, 2, 3, 4+), and demonstrated nearly identical results (results not shown). Age, Hispanic ethnicity, non-White race, male gender, and preterm birth, as well as prior or concomitant diagnosis of allergic rhinitis and food allergy were also associated with increased odds of asthma diagnosis. (Table 3)
Table 3.
Adjusted odds ratios (OR) for asthma risk factors and asthma diagnosis from main model and 2 sensitivity analyses**
| Main Analysis | Second Asthma Diagnosis | Proximity cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Race/ethnicity (ref non-Hispanic White) | |||||||||
| Non-Hispanic Asian | 1.13 | 1.05-1.21 | < 0.001 | 1.12 | 1.03-1.21 | 0.006 | 1.26 | 1.10-1.45 | 0.001 |
| Non-Hispanic Black | 1.67 | 1.62-1.73 | < 0.001 | 1.68 | 1.61-1.74 | < 0.001 | 1.77 | 1.63-1.92 | < 0.001 |
| Hispanic | 1.2 | 1.15-1.26 | < 0.001 | 1.3 | 1.23-1.37 | < 0.001 | 1.26 | 1.11-1.42 | < 0.001 |
| Missing | 0.95 | 0.91-0.99 | 0.026 | 0.99 | 0.94-1.04 | 0.651 | 1.12 | 0.97-1.30 | 0.128 |
| Other | 1.1 | 1.00-1.21 | 0.047 | 1.14 | 1.02-1.26 | 0.016 | 1.31 | 1.06-1.62 | 0.012 |
| Male gender | 1.18 | 1.15-1.21 | < 0.001 | 1.16 | 1.13-1.19 | < 0.001 | 1.09 | 1.05-1.14 | < 0.001 |
| Prematurity (<34 weeks) | 1.21 | 1.15-1.27 | < 0.001 | 1.17 | 1.11-1.24 | < 0.001 | 1.09 | 1.00-1.18 | 0.04 |
| Food allergy | 1.27 | 1.21-1.32 | < 0.001 | 1.27 | 1.22-1.33 | < 0.001 | 1.28 | 1.18-1.39 | < 0.001 |
| Allergic rhinitis | 2.01 | 1.96-2.08 | < 0.001 | 2.05 | 1.99-2.12 | < 0.001 | 1.83 | 1.72-1.95 | < 0.001 |
| Eczema | 1 | 0.97-1.02 | 0.747 | 1.05 | 1.02-1.08 | < 0.001 | 1.2 | 1.15-1.25 | < 0.001 |
| Any wheeze episode | 28.56 | 27.42-29.75 | < 0.001 | 48.92 | 45.69-52.38 | < 0.001 | 30.99 | 28.49-33.72 | < 0.001 |
| Additional wheeze episodes | 1.37 | 1.36-1.38 | < 0.001 | 1.47 | 1.46-1.48 | < 0.001 | 1.54 | 1.51-1.56 | < 0.001 |
| Any cough episodes | 1.01 | 0.97-1.03 | 0.95 | 1.01 | 0.98-1.04 | 0.635 | 0.92 | 0.88-0.97 | < 0.001 |
| Additional cough episodes | 1.06 | 1.04-1.07 | < 0.001 | 1.03 | 1.01-1.04 | < 0.001 | 1 | 0.98-1.03 | 0.79 |
| Proportion diagnosed with asthma | 21.41% | 17.14% | 27.72% | ||||||
| Variance from GLMM† | 0.1413 | 0.1621 | 0.0047 | ||||||
| ICC‡ | 0.0412 | 0.0470 | 0.0014 | ||||||
A11 analyses adjusted for age. See Supplemental Table 2 in Appendix for ORs for 3-month age intervals.
Generalized linear mixed model
Intraclass correlation coefficient
After adjusting for factors included in the main multivariable model, substantial variability in asthma diagnosis by clinic site remained. Figure 3 shows the cumulative incidence of asthma diagnosis by age 6 years before and after adjustment for the model covariates. At the practice-level, adjusted cumulative incidence of asthma diagnosis ranged from 11% to 47% (IQR 24-29%). The proportion of variance in cumulative incidence of asthma diagnosis incidence attributable to the variation among sites (ICC) was 0.0412.
Figure 3.
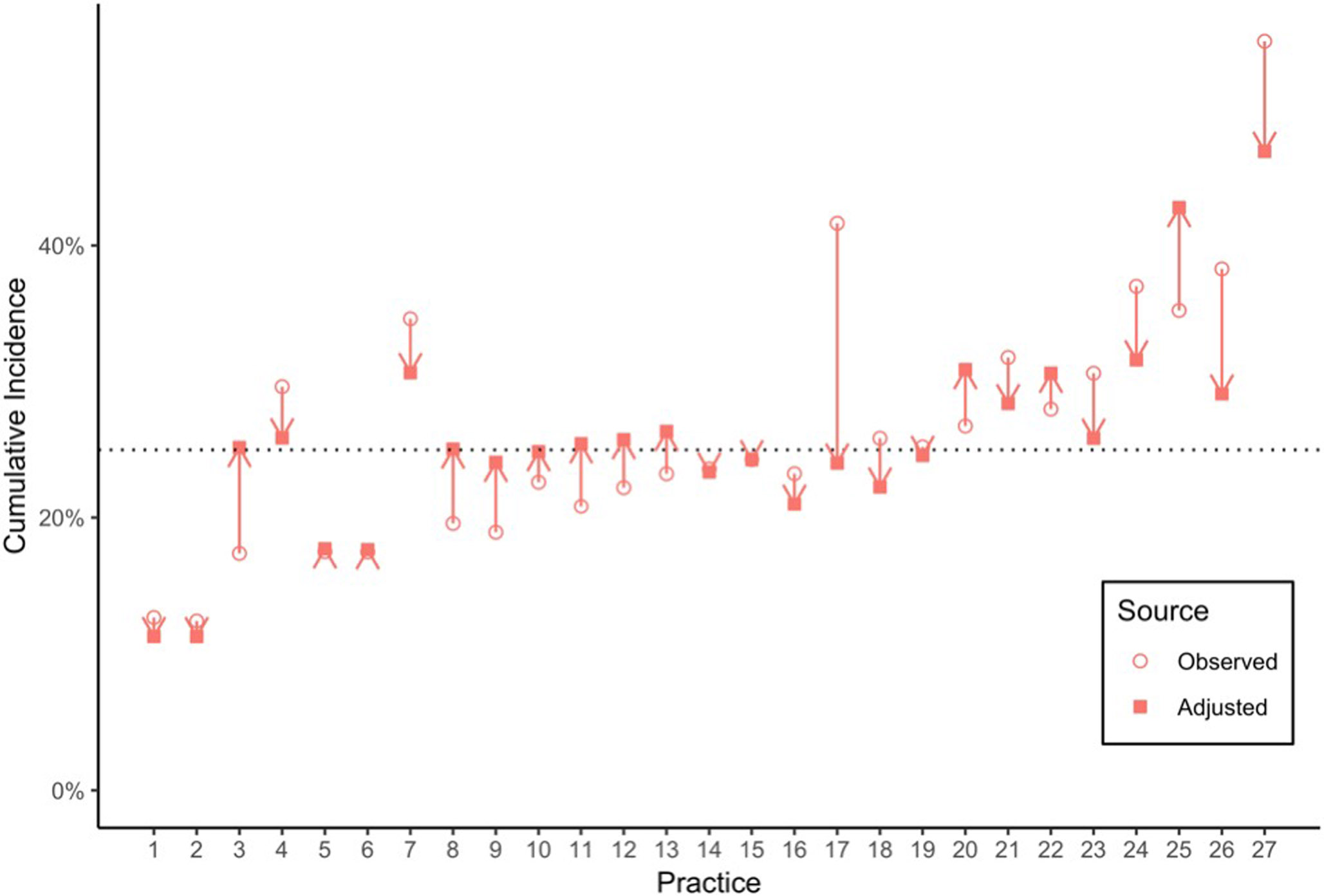
Variation in cumulative incidence of first athma diagnosis by primary care practice at age 6, comparing observed incidence to adjusted* incidence
*Adjusted for age, number of prior wheezing episodes, gender, race, ethnicity, and preterm birth, as well as prior or concomitant diagnosis of allergic rhinitis and food allergy
Sensitivity analyses
Seventeen percent of the cohort had ≥2 asthma diagnoses and the odds of asthma diagnosis given a wheezing episode was higher than in the main analysis (OR 48.92, 95% CI 45.69–52.38). Each additional wheezing episode was also associated with a higher odds of asthma diagnosis (OR 1.47, 95% CI 1.46-1.48). Other associations were qualitatively similar. When restricting the sample to the “proximity cohort” of the 4 clinics and including ED and hospital utilization data, associations were similar to the main model. While the ICC for second asthma diagnosis model was similar to the main model (0.047), the ICC for the proximity cohort model was 0.0014, indicating that less variability was explained by practice site. (Table 3)
Discussion
In this longitudinal study of children ages 6 months up to 8 years followed in primary care, 40% had at least one diagnosed wheezing episode and 21% received an asthma diagnosis. Cumulative incidence of asthma diagnosis varied substantially by practice, ranging from 11% to 47% by age 6 years after accounting for demographic and clinical factors commonly associated with asthma.
First, with regard to incidence of wheezing and asthma diagnosis, the percentage of children in our cohort with wheezing was similar to another often-cited longitudinal cohort5, while the rates of asthma diagnosis are slightly higher than National Health Information Survey (NHIS) reports. In 2017, 15% of parents in the Northeast US reported that their children have ever been diagnosed with asthma.17 We found that 21% of the children in our cohort had one asthma diagnosis and 17% had two or more diagnoses. While those diagnosed with asthma had longer followup duration (87 months vs 61 months) in our dataset, this would not have biased incidence estimates given our use of survival analysis. Further, these percentages are consistent with both door-to-door screening performed in neighborhoods where four of the current study’s practices are located18 and an earlier analysis performed using a smaller date range of the same health system’s data.19
The main finding from this study is the degree of variability in cumulative asthma incidence across pediatric practices. It is important to note that after adjusting for patient characteristics and clinical factors, variability between practices decreased and cumulative incidence by age 6 within the IQR was 25 to 29%. A post-hoc comparison of one outlier practice grouping with high proportion of asthma diagnosis to the overall cohort revealed differences in observed characteristics, such as geographic location, patient characteristics, prescribing practices, and diagnostic patterns. While practices outside the IQR may also have differed on unobserved characteristics, the explanation of this variability most relevant to our initial hypothesis is that the differential rates of asthma diagnosis may reflect differential thresholds for asthma diagnosis at the practice or provider level. Though there are a number of potential alternative explanations to consider, this explanation is both plausible and supported by a wide breadth of existing literature on variation in diagnosis and treatment in health care.20,21
While provider variation in the diagnosis of asthma in young children has not been extensively studied, one prior study examined the variability in symptom description and diagnostic labeling of asthma among 113 pediatricians who were presented with a series of 5 audiovisual cases of differing asthma presentations developed by the International Study of Asthma and Allergies in Childhood. The proportion of participants who identified asthma as one of the potential diagnoses ranged from 70% to 93% depending on the case and <50% of participants identified asthma as a potential diagnosis in all cases.22 To better understand variability in diagnosis, further research should investigate additional factors that may contribute to asthma diagnosis thresholds, such as provider type (physician versus advanced practice provider), physician training23 (location of residency training, years from residency, etc.), or practice environment (small vs large, academic vs private, etc.).
A second explanation is that this variability may be appropriate based on differential population-level asthma risk factors that were not accounted for in our analysis. For example, 25% of the cohort resided in Philadelphia, which has the highest rate of childhood poverty of the 10 largest U.S. cities, in addition to poor housing stock. Both poverty and poor housing stock are associated with higher asthma rates in the US and a sizeable literature supports the characterization of “inner-city asthma”, which refers to both a higher baseline asthma incidence and higher asthma morbidity in inner-city settings.24-26 In our sensitivity analysis of the cohort restricted to four inner-city practices, asthma diagnosis incidence remained similar to that for the full cohort. If asthma rates are truly higher in the inner-city, then this may indicate over diagnosis in non-inner-city practices. Recent studies using NHIS data, however, have challenged the “inner-city asthma” concept, finding only slightly higher prevalence of asthma in inner-city areas that was largely explained by demographic factors of gender, age, race/ethnicity, and region.27,28 Further, differential genetic propensity29 and indoor and outdoor environmental exposures30,31 contribute to asthma risk, both in inner city settings and more generally. Subsequent studies should assess how differing population-level characteristics, such as geographic location, poverty rates, genetic propensity, and environmental exposures, influence asthma incidence and, similarly, clinicians’ propensity to diagnose asthma.
Some degree of variability in asthma diagnosis may be appropriate given the heterogeneous nature of asthma and wheezing illnesses, particularly in young children. In the last decade or so, several experts have recommended moving away from the label asthma in young children, instead focusing on categories of recurrent wheezing.32 Recurrent wheezing, in conjunction with several other discrete risk factors, can be used to approximate likelihood of future asthma diagnosis using the Asthma Predictive Index (API) or similar predictive models.9,33 Specificity of asthma diagnosis may be enhanced by incorporating number of wheezing episodes and other features of these predictive indicies, such as diagnosis of allergic rhinitis, family history of a first degree relative with asthma, and eosinophilia, into EHR-based clinical decision support. Additionally, children with persistent wheezing patterns during preschool and school age are likely to have persistent wheezing into adulthood34 and those with lower pulmonary function at age 7 have been shown to have higher diagnosis of Asthma-COPD Overlap Syndrome at older ages.35
Similarly, a number of recent studies have characterized phenotypes of wheezing illnesses using unsupervised analytic approaches. 36-40 While no clear consensus has emerged on how wheezing phenotypes should be characterized or differentially treated41,42 , this approach may provide more useful clinical information on the nature of a patient’s illness (i.e. frequency and pattern of prior wheezing illnesses, associated conditions, etc.) than a simple label of asthma or likelihood of future asthma diagnosis. To further explore this hypothesis, future studies should investigate how wheezing phenotypes vary by practice location, as well as interactions with area-level factors such as poverty and pollution.
This study has several limitations. Because we were only able to characterize a number of discrete characteristics that are reliably recorded in the medical record, several characteristics that may be of use to establish a diagnosis of asthma – such as family history, breathing difficulties not characterized as wheezing or coughing, wheezing identified either during history taking or on physical exam, second hand smoke exposure, socioeconomic status, etc. – were not included in our analysis. These factors may contribute to unexplained variability in asthma diagnosis rates and should be included in future research on this subject.
Second, we captured only clinical events for which patients presented to the primary care clinic. Thus, if there is variability by clinic site as to what threshold families use to seek primary care (versus treatment at home, ED, or hospital), clinic-level percentage of asthma diagnosis may be differentially underestimated or overestimated. While we could not ascertain treatment at home, we were able to assess visits to the ED and hospital for four inner-city clinics whose patients reliably seek emergency and hospital care at our institution. The proportion of variability in asthma diagnosis explained by practice site (ICC) was substantially smaller in this sensitivity analysis, suggesting that including ED and hospital exacerbations may explain some of the practice-level variability. However, this analysis contained only four relatively homogeneous practices and a post-hoc analysis excluding ED and hospital visits for these 4 practices yielded a comparably low ICC of 0.003, suggesting that the small number of practices in the model led to the smaller ICC, not the inclusion of ED and hospital visits.
Lastly, our analysis relied on physician diagnosis and there may be practice or provider-level variability in how these episodes are coded by providers. We attempted to account for this by including numerous SNOMED concept codes representing a comprehensive list of diagnoses for each condition, as well as including visits with prescribed beta agonists without wheezing diagnoses as wheezing episodes. If, however, there was coding of conditions not included in our SNOMED concept codes that was differential by practice site (e.g. all patients presenting with wheezing at one practice were coded as respiratory distress and not wheezing), this could distort our estimates.
In this real-world, longitudinal cohort of children followed in primary care from age 6 months up to as old as 8 years, we found four-fold variability in the proportion of children diagnosed with asthma by practice site that was not explained by the demographics and clinical characteristics of these children. Given the potential implications of this degree of variability in clinical care, quality improvement, and healthcare value and costs, more extensive investigation into its sources, such as differing provider thresholds for asthma diagnosis and area-level exposures that lead to differing prevalence of wheezing disorders labeled “asthma”, are warranted.
Supplementary Material
What’s New:
After controlling for associated risk factors, we found wide practice-level variability in the proportion of young children diagnosed with asthma across a large pediatric primary care network, raising further questions about the use of this diagnostic label in young children.
Acknowledgments:
The authors acknowledge Claudia Salzberg, Rahul Darwar, and Ritu Kare for their assistance in project management and dataset preparation.
Funding Source:
This work was supported by a grant from the Commonwealth Universal Research Enhancement (C.U.R.E) program funded by the Pennsylvania Department of Health (SAP #4100072543, PI Christopher B. Forrest, MD, PhD). Dr Kenyon’s time on this project was supported by a K-award from the NHLBI (1K23HL136842-01A1).
Abbreviations:
- EMR
Electronic medical record
- SNOMED CT
Systematized Nomenclature of Medicine -- Clinical Terms
- COAST
Childhood Origins of Asthma Study
- NHIS
National Health Information Survey
- ED
Emergency department
- ICC
Intra-class correlation coefficient
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043 [DOI] [PubMed] [Google Scholar]
- 3.2018 GINA Report: Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma - GINA. https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed January 26, 2019.
- 4.Bacharier LB. The recurrently wheezing preschool child-benign or asthma in the making? Ann Allergy Asthma Immunol. 2015;115(6):463–470. doi: 10.1016/j.anai.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301 [DOI] [PubMed] [Google Scholar]
- 6.Bush A, Fleming L. Is asthma overdiagnosed? Arch Dis Child. 2016;101(8):688–689. doi: 10.1136/archdischild-2015-309053 [DOI] [PubMed] [Google Scholar]
- 7.Kliegman R, Behrman RE, Nelson WE, eds. Nelson Textbook of Pediatrics. Edition 20. Phialdelphia, PA: Elsevier; 2016. https://www.clinicalkey.com/#!/browse/book/3-s2.0-C20120035867. [Google Scholar]
- 8.Brand PLP, Caudri D, Eber E, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur Respir J. 2014;43(4):1172–1177. doi: 10.1183/09031936.00199913 [DOI] [PubMed] [Google Scholar]
- 9.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111 [DOI] [PubMed] [Google Scholar]
- 10.Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent disease among children who wheeze during early life. European Respiratory Journal. 2003;22(5):767–771. doi: 10.1183/09031936.03.00005903 [DOI] [PubMed] [Google Scholar]
- 11.Caudri D, Wijga A, A Schipper CM, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol. 2009;124(5):903–910.e1-7. doi: 10.1016/j.jaci.2009.06.045 [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Martinez CE, Sossa-Brice?o MP, Castro-Rodriguez JA. Factors predicting persistence of early wheezing through childhood and adolescence: a systematic review of the literature. Journal of Asthma and Allergy. 2017;Volume 10:83–98. doi: 10.2147/JAA.S128319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing Rhinovirus Illnesses in Early Life Predict Asthma Development in High-Risk Children. American Journal of Respiratory and Critical Care Medicine. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemporary Clinical Trials. 2012;33(5):869–880. doi: 10.1016/j.cct.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozano P Use of health services by African-American children with asthma on Medicaid. JAMA: The Journal of the American Medical Association. 1995;274(6):469–473. doi: 10.1001/jama.274.6.469 [DOI] [PubMed] [Google Scholar]
- 16.Gerber JS, Prasad PA, Localio AR, et al. Racial Differences in Antibiotic Prescribing by Primary Care Pediatricians. Pediatrics. 2013;131(4):677–684. doi: 10.1542/peds.2012-2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHIS - Tables of Summary Health Statistics. https://www.cdc.gov/nchs/nhis/shs/tables.htm. Published November 30, 2018. Accessed January 22, 2019.
- 18.Bryant-Stephens T, West C, Dirl C, Banks T, Briggs V, Rosenthal M. Asthma prevalence in Philadelphia: description of two community-based methodologies to assess asthma prevalence in an inner-city population. J Asthma. 2012;49(6):581–585. doi: 10.3109/02770903.2012.690476 [DOI] [PubMed] [Google Scholar]
- 19.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMCPediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615 [DOI] [PubMed] [Google Scholar]
- 21.Mangione-Smith R, DeCristofaro AH, Setodji CM, et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357(15):1515–1523. doi: 10.1056/NEJMsa064637 [DOI] [PubMed] [Google Scholar]
- 22.Van Sickle D, Magzamen S, Truelove S, Morrison T. Remote Monitoring of Inhaled Bronchodilator Use and Weekly Feedback about Asthma Management: An Open-Group, Short-Term Pilot Study of the Impact on Asthma Control. Baradaran HR, ed. PLoS ONE. 2013;8(2):e55335. doi: 10.1371/journal.pone.0055335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn S, Wi C-I, Juhn YJ, Liu H. Analysis of Clinical Variations in Asthma Care Documented in Electronic Health Records Between Staff and Resident Physicians. Stud Health Technol Inform. 2017;245:1170–1174. [PMC free article] [PubMed] [Google Scholar]
- 24.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125(3):540–544. doi: 10.1016/j.jaci.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 25.Matsui EC. Environmental exposures and asthma morbidity in children living in urban neighborhoods. Allergy. 2014;69(5):553–558. doi: 10.1111/all.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gergen PJ, Togias A. Inner City Asthma. Immunology and Allergy Clinics of North America. 2015;35(1):101–114. doi: 10.1016/j.iac.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J Allergy Clin Immunol. 2015;135(3):655–662. doi: 10.1016/j.jaci.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol. 2017;140(3):822–827. doi: 10.1016/j.Jaci.2017.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers DA, Bleecker ER, Holloway JW, Holgate ST. Asthma genetics and personalised medicine. Lancet Respir Med. 2014;2(5):405–415. doi: 10.1016/S2213-2600(14)70012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor GT, Lynch SV, Bloomberg GR, et al. Early-life home environment and risk of asthma among inner-city children. Journal of Allergy and Clinical Immunology. 2018;141(4):1468–1475. doi: 10.1016/j.jaci.2017.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollock J, Shi L, Gimbel RW. Outdoor Environment and Pediatric Asthma: An Update on the Evidence from North America. Can Respir J. 2017;2017:8921917. doi: 10.1155/2017/8921917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigelman A, Bacharier LB. Management of preschool recurrent wheezing and asthma: a phenotype-based approach. Curr Opin Allergy Clin Immunol. 2017;17(2):131–138. doi: 10.1097/ACI.0000000000000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol. 2010;126(2):212–216. doi: 10.1016/j.jaci.2010.06.032 [DOI] [PubMed] [Google Scholar]
- 34.Grad R, Morgan WJ. Long-term outcomes of early-onset wheeze and asthma. J Allergy Clin Immunol. 2012;130(2):299–307. doi: 10.1016/j.jaci.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bui DS, Burgess JA, Lowe AJ, et al. Childhood Lung Function Predicts Adult Chronic Obstructive Pulmonary Disease and Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome. Am J Respir Crit Care Med. 2017;196(1):39–46. doi: 10.1164/rccm.201606-1272OC [DOI] [PubMed] [Google Scholar]
- 36.Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974–980. doi: 10.1136/thx.2007.093187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granell R, Henderson AJ, Sterne JA. Associations of wheezing phenotypes with late asthma outcomes in the Avon Longitudinal Study of Parents and Children: A population-based birth cohort. Journal of Allergy and Clinical Immunology. 2016;138(4):1060–1070.e11. doi: 10.1016/j.jaci.2016.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belgrave DCM, Simpson A, Semic-Jusufagic A, et al. Joint modeling of parentally reported and physician-confirmed wheeze identifies children with persistent troublesome wheezing. J Allergy Clin Immunol. 2013;132(3):575–583.e12. doi: 10.1016/j.jaci.2013.05.041 [DOI] [PubMed] [Google Scholar]
- 39.Collins SA, Pike KC, Inskip HM, et al. Validation of novel wheeze phenotypes using longitudinal airway function and atopic sensitization data in the first 6 years of life: evidence from the Southampton Women’s survey. Pediatr Pulmonol. 2013;48(7):683–692. doi: 10.1002/ppul.22766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacharier LB, Beigelman A, Calatroni A, et al. Longitudinal Phenotypes of Respiratory Health in a High-Risk Urban Birth Cohort. Am J Respir Crit Care Med. 2019;199(1):71–82. doi: 10.1164/rccm.201801-0190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Depner M, Fuchs O, Genuneit J, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189(2):129–138. doi: 10.1164/rccm.201307-1198OC [DOI] [PubMed] [Google Scholar]
- 42.Belgrave D, Simpson A, Custovic A. Challenges in interpreting wheeze phenotypes: the clinical implications of statistical learning techniques. Am J Respir Crit Care Med. 2014;189(2):121–123. doi: 10.1164/rccm.201312-2206ED [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


