Abstract
A survey of Veterans Affairs Medical Centers on control of Carbapenem resistant Enterobacteriaceae and Carbapenem-producing CP-CRE demonstrated most facilities use VA guidelines but few screen for CRE/CP-CRE colonization regularly or regularly communicate CRE/CP-CRE status at patient transfer. Most respondents were knowledgeable about CRE guidelines, but cited lack of adequate resources.
Introduction:
Carbapenem-resistant Enterobacteriaceae (CRE) are multidrug-resistant gram-negative bacteria that colonize and infect many patients yearly. A subset of CRE, carbapenemase-producing CRE (CP-CRE), are worrisome as they may be responsible for increasing CRE spread in the US.1-3 The Department of Veterans Affairs (VA) disseminated guidelines in 2015 and updated them in 2017, with a focus on CP-CRE. The guidelines provide information on: 1) laboratory identification of CRE including PCR testing for carbapenemases; 2) CRE surveillance, including recommendations for active screening; and 3) infection prevention practices, such as contact precautions and communication within and across healthcare facilities. Findings related to VA guideline laboratory practices showed most VA laboratories reported using VA guidelines but variation existed on CP-CRE identification.4 We now report on an analogous survey identifying practices related to infection prevention and control of CRE and/or CP-CRE in VA facilities.
Methods:
A cross sectional study was conducted via an electronic survey of 161 Multidrug-resistant Organism (MDRO) Prevention Coordinators (MPCs) at 134 VA Medical Centers (VAMCs) between February 21- April 30, 2018. MPCs assist with data collection and reporting for MDRO surveillance at most facilities. The survey focused on experiences and practices related to CRE and/or CP-CRE (represented as “CRE/CP-CRE”). It was developed in collaboration with the VA MDRO Program Office and administered through Research Electronic Data Capture (REDCap). The Consolidated Framework for Implementation Research (CFIR) was used to develop and analyze implementation questions.5
Other characteristics collected about VA facilities included rural vs. urban location and facility complexity from the VA Office of Productivity, Efficiency, & Staffing (OPES) Facility Complexity Model.6 OPES classifies complexity based on patient characteristics, complex clinical research, and teaching programs (Levels 1a-c, 2, and 3). Levels 1a-c were considered high complexity and levels 2 and 3 were considered low complexity for this analysis.6
All survey responses were analyzed using descriptive statistics. Comparisons of facility complexity, rurality, reported CRE/CP-CRE experience, and nonresponse were assessed using Fisher’s exact test. Facility history of CRE/CP-CRE experience was defined as facilities reporting any CRE/CP-CRE. If no cases of CRE/CP-CRE were reported, facilities were defined as not having CRE/CP-CRE experience. Questions were categorized ‘agree’ if respondents ‘agreed’ or ‘strongly agreed’ with the statement and ‘did not agree’ if they responded ‘neutral’, ‘disagree’, or ‘strongly disagree’. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results:
Eighty-one MPCs (50.3% of 161 MPCs) from 79 unique facilities (58.9% of 134) responded, including two facilities with unique MPCs for acute care and long term-care unit(s) (n=4). Responding facilities did not differ significantly (p>0.05) from non-responding facilities by facility complexity (high complexity 69.1% vs. 63.8%), geographic location (urban: 79.0% vs. 83.8%), or presence of a long-term care center (85.2% vs. 92.5%) or spinal cord injury center (21% vs. 17.5%). 70.4% of respondents reported some CRE/CP-CRE experience.
Most MPCs reported using VA Guidelines (97.5%) (either 2017 or 2015). Seventeen MPCs (20.9%) reported routinely screening for CRE/CP-CRE colonization (Figure 1 shows the types of patients screened). The major reason reported for not screening was not having a CRE/CP-CRE problem (70.4%). Beyond implementing contact precautions, other infection control processes for CRE/CP-CRE positive patients included patient (24.7%) and/or nurse cohorting (7.4%).
Figure 1. Proportion of types of patients routinely screened (among those that do screening/active surveillance, n=17).
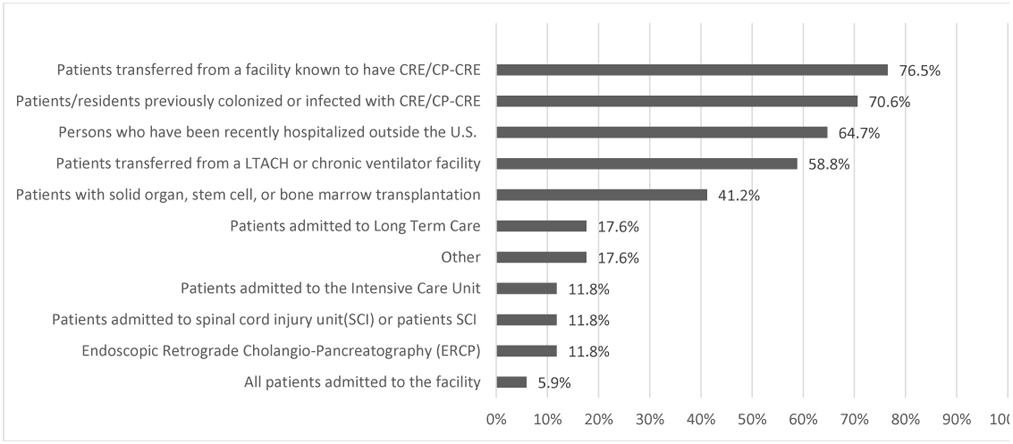
*Other includes: patients with multiple admissions to acute care, transfers from outside ICU, CLC transfers, or when physician suspects it
Nearly all MPCs (90.1%) used an electronic health record (EHR) flag available to notify providers about patients with CRE/CP-CRE, with half indicating the flag is ‘never’ discontinued (Table 1). Other flag removal responses included ‘after 1 year’ (15.1%) and ‘other’ criteria including but not limited to “two negative rectal swabs or “on a case-by-case basis”.
Table 1.
Survey responses by facility complexity, location, and CRE experience
| Survey response (# of responses for that question) |
Facility complexity | Facility location | CRE/CP-CRE Experience | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall responses (%) |
High (n=56) |
Low (n=25) |
p-value | Rural (n=17) |
Urban (n=64) |
p-value | Yes (n=57) |
No (n=24) |
p-value | |
| Guideline practices | ||||||||||
| No CRE/CP-CRE cases seen at facility (n=81) | 24 (29.6%) | 10 (18%) | 14 (56%) | <0.01 | 9 (53%) | 15 (24%) | 0.03 | NA | NA | NA |
| Conduct screening (n=81) | 17 (20.9%) | 13 (23%) | 4 (16%) | 0.56 | 4 (24%) | 13 (21%) | 0.75 | 14 (25%) | 3 (13%) | 0.43 |
| Electronic health record flag (n=81) | 73 (51.3%) | 51 (91%) | 22 (88%) | 0.70 | 15 (88%) | 58 (91%) | 0.67 | 51 (90%) | 22 (92%) | 0.99 |
| Electronic health record flag never discontinued (n=73) | 39 (53.4%) | 28 (55%) | 11 (50%) | 0.80 | 7 (47%) | 32 (55%) | 0.58 | 32 (63%) | 7 (32%) | 0.02 |
| Communication (usually or always) | ||||||||||
| Non-VA Skilled Nursing Facility (n=81) | 18 (22.2%) | 12 (21%) | 6 (24%) | 0.78 | 4 (24%) | 14 (22%) | 0.99 | 14 (25%) | 4 (17%) | 0.56 |
| Non-VA Acute Care Facility (n=81) | 25 (15.1%) | 15 (27%) | 10 (40%) | 0.30 | 6 (35%) | 19 (30%) | 0.77 | 21 (37%) | 4 (17%) | 0.11 |
| VA Skilled Nursing Facility (n=81) | 31 (38.3%) | 25 (45%) | 6 (24%) | 0.09 | 5 (29%) | 26 (41%) | 0.58 | 27 (47%) | 4 (17%) | 0.01 |
| VA Acute Care Facility (n=81) | 34 (42.0%) | 27 (48%) | 7 (28%) | 0.14 | 5 (29%) | 29 (45%) | 0.28 | 30 (53%) | 4 (17%) | 0.003 |
| Use of Verbal, Written or Electronic communication (n=81) | 67 (82.7%) | 48 (86%) | 19 (76%) | 0.34 | 12 (71%) | 55 (86%) | 0.16 | 53 (93%) | 14 (58%) | 0.0005 |
| Guidelines Fully Implemented (n=81) | 46 (56.7%) | 34 (61%) | 12 (48%) | 0.34 | 7 (41%) | 39 (61%) | 0.17 | 36 (63%) | 10 (42%) | 0.09 |
Table 1 illustrates differences in responses by facility complexity level, location, and CRE/CP-CRE experience. Lower complexity and rural facilities were more likely to report no CRE/CP-CRE at their VAMC in the last year. Respondents from high complexity and urban sites were significantly more likely to report actively screening patients at admission. Respondents from facilities with CRE/CP-CRE experience were more likely to never discontinue the EHR flag.
Respondents indicated they typically received little to no information on patients’ CRE/CP-CRE status when transferred between facilities (Table 1). MPCs reported more often receiving communication on CRE status when their patient was transferred from a VA skilled nursing facility or acute-care hospital versus non-VA skilled nursing facility or acute-care hospital. Similarly, in acute care, information on patient CRE status was received most frequently from VA facilities vs. non-VA facilities. Respondents from facilities with CRE/CP-CRE experience more frequently reported ‘usually’ or ‘always’ obtaining CRE/CP-CRE status when transferring from a VA facility. Figure 2 shows patients’ CRE/CP-CRE status was most frequently communicated verbally or via inter-facility transfer forms.
Figure 2.
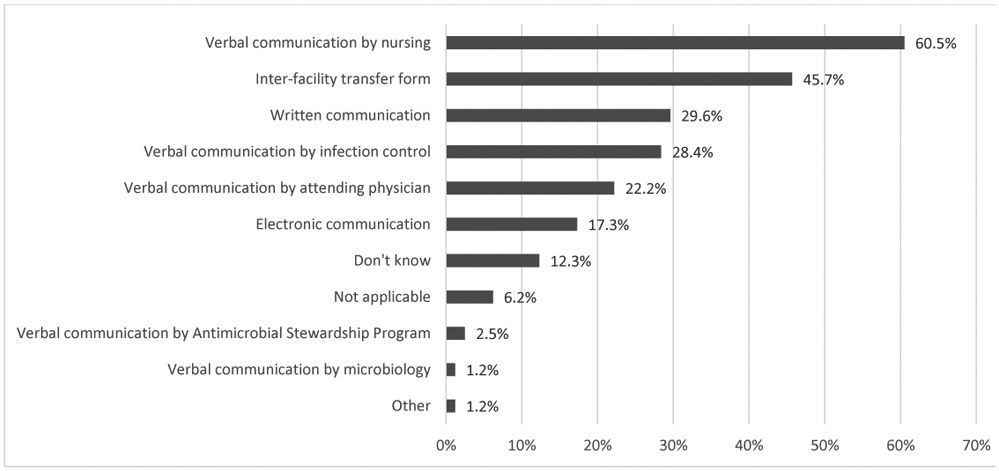
Mode of communication when transferring CRE/CP-CRE patients from their facility to another facility (n=81)
Most respondents ‘agreed/strongly agreed’ (85.0%) they are knowledgeable about the CRE/CP-CRE guidelines. However, only half ‘agreed’ they had the physical (56.8%), staffing (55.6%), and laboratory resources (65.1%) to accomplish guideline activities. More facilities experiencing CRE/CP-CRE described having adequate physical resources necessary to accomplish guidelines activities (75.0% vs. 49.0%, p=0.05). 56.8% reported the CRE/CP-CRE guidelines are ‘fully’ implemented at their facility. The main reason reported for guidelines not being fully implemented was lack of active surveillance (59.3%).
Discussion:
This survey found 29.6% of VA acute-care facilities report no experience with CRE/CP-CRE, and only a small percentage perform active surveillance for CRE/CP-CRE. As of 2017, CRE/CP-CRE has been reported in every state2 therefore it is critical all VA facilities are prepared. Implementing CRE/CP-CRE screening is not straightforward, requiring a multidisciplinary plan with allocated resources and time to establish a new process. At sites that are screening, we found variability in the types of patients screened and a reported lack of resources.
An interesting survey finding relates to CRE/CP-CRE EHR alerts.7 Currently there are no guidelines or conclusive evidence for discontinuing alerts and contact isolation for patients with CRE/CP-CRE.4 Hospitals determine their own protocol, based on expert opinion, local MDRO epidemiology, and previous experience with other MDROs. Though most VAMCs reported they never discontinue the alert, respondents also described other methods which could assist other facilities in establishing protocols for CRE/CP-CRE alerts and contact isolation.
Communication is a key strategy to improve infection control and interrupt the spread of CRE.8 Unfortunately, our survey showed positive CRE/CP-CRE status is communicated less than half the time upon patient, a finding previously demonstrated in other studies including those in non-VA settings.9,10 The greater reported transfer communication between VA facilities in our survey is encouraging and may be due to their common EHR. However, implementation of standardized inter-facility transfer notification and development of regional CRE/CP-CRE registries are important steps towards improving communication. To this end, VA is actively working on implementing a system-wide automated CP-CRE notification system.
Our study was limited by being conducted in the VA, where responses and practices may differ compared with non-VA hospitals. Furthermore, selection bias is possible with only 59% of VAMCs responding, however, there were no differences in key characteristics between responding and non-responding facilities. Finally, recall bias may have affected the accuracy of responses, given that this was a cross-sectional survey of self-reported practices related to CRE.
In conclusion, almost all MPCs reported using the VA CRE/CP-CRE guidelines. However, gaps in implementation include the lack of CRE/CPE CRE screening and communication about CRE/CP-CRE status during transfer. Additional work is needed to overcome barriers to guideline implementation and provide stronger education and resources to help support guideline activities for the prevention of MDROs.
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Financial support
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Quality Enhancement Research Initiative (grant no. QUE 15-269).
Footnotes
Conflicts of interest
All authors report no conflicts of interest or financial disclosures relevant to this article.
References:
- 1.Centers for Disease Control and Prevention. Antibiotic Resistant Threats in the United States, 2019. Atlanta; 2019. (5) [Google Scholar]
- 2.Centers for Disease Control and Prevention. FAQs about choosing and implementing a CRE definition. URL: https://www.cdc.gov/hai/organisms/cre/definition.html. Last accessed January 22, 2020. (3) [Google Scholar]
- 3.Fitzpatrick MA, Suda KJ, Ramanathan S, et al. Laboratory practices for identification and reporting of carbapenem-resistant Enterobacteriaceae in Department of Veterans Affairs facilities. Infect Control Hosp Epidemiol 2019; 40(4): 463–66. (7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bart Y, Paul M, Eluk O, Geffen Y, Rabino G, Hussein K. Risk factors for recurrence of carbapenem-resistant Enterobacteriaceae carriage: case-control study. Infect Control Hosp Epidemiol 2015;36: 936–941. [DOI] [PubMed] [Google Scholar]
- 5.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. (8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VHA Office of Productivity Efficiency & Staffing. Facility Complexity Model. Last accessed December 17, 2019.
- 7.Martischang R, Buetti N, Balmelli C, Saam M, Widmer A, Harbarth S. Nation-wide survey of screening practices to detect carriers of multi-drug resistant organisms upon admission to Swiss healthcare institutions. Antimicrob Resist Infect Control 2019; 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BY, Bartsch SM, Wong KF, et al. Simulation show hospitals that cooperate on infection control obtain better results than hospitals acting alone. Health Affairs 2012; 31(10): 2295–303. (26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trepanier P, Mallard K, Muenier D, Pike R, et al. Carbapenem-producing Enterobacteriaceae in the UK: a national study (EuSCAPE-UK) on prevalence, incidence, laboratory detection methods and infection control measures. J Antimicrob Chemother 2017; 72(2): 596–603. (18) [DOI] [PubMed] [Google Scholar]
- 10.Shimasaki T, Segreti J, Tomich A, Kim J, Hayden MK, Ling MY. Active screening and interfacility communication of carbapenem-resistant Enterobacteriaceae (CRE) in a tertiary-care hospital. Infect Control Hosp Epidemiol 2018; 39(9): 1058–62. (23) [DOI] [PMC free article] [PubMed] [Google Scholar]


