SARS-CoV-2 infection has been associated with increased morbidity and mortality in organ transplant recipients, and data are limited on 2-dose or 3-dose vaccine immunogenicity against SARS-CoV-2 variants in this population. In this secondary analysis of a randomized trial of a third dose of mRNA-1273 vaccine versus placebo, the authors assessed neutralizing antibody responses against SARS-CoV-2 variants in transplant recipients after 2 and 3 vaccine doses.
Visual Abstract. SARS-CoV-2 Variant Neutralization After Vaccine in Transplant Recipients.

SARS-CoV-2 infection has been associated with increased morbidity and mortality in organ transplant recipients, and data are limited on 2-dose or 3-dose vaccine immunogenicity against SARS-CoV-2 variants in this population. In this secondary analysis of a randomized trial of a third dose of mRNA-1273 vaccine versus placebo, the authors assessed neutralizing antibody responses against SARS-CoV-2 variants in transplant recipients after 2 and 3 vaccine doses.
Abstract
Background:
COVID-19 is more severe in transplant recipients. Variants of concern have supplanted wild-type virus. In transplant recipients, data are limited on 2-dose or 3-dose vaccine immunogenicity against variant viruses.
Objective:
To assess neutralizing antibody responses against SARS-CoV-2 variants in transplant recipients after 2 and 3 vaccine doses.
Design:
Secondary analysis of a randomized, double-blind, controlled trial of a third dose of mRNA-1273 vaccine versus placebo. (ClinicalTrials.gov: NCT04885907)
Setting:
Single-center transplant program.
Patients:
Organ transplant recipients.
Intervention:
Third dose of mRNA-1273 vaccine versus placebo.
Measurements:
Sera were analyzed for neutralization against wild-type virus and the Alpha, Beta, and Delta variants using a surrogate virus neutralization assay and a spike-pseudotyped lentivirus assay.
Results:
A total of 117 transplant recipients were analyzed (60 in the mRNA-1273 group and 57 in the placebo group). Sera were obtained before and 4 to 6 weeks after the third dose. After 2 doses, the proportion of patients with positive neutralization for all 3 variants was small compared with wild-type virus. After the third dose of mRNA-1273 vaccine, the proportion with a positive neutralization response versus placebo was improved for all 3 variants as measured by both assays. Based on the pseudovirus neutralization assay against the Delta variant, 33 of 60 (55%) patients were positive in the mRNA-1273 group versus 10 of 57 (18%) in the placebo group (difference, 37 [95% CI, 19 to 53] percentage points). The differences were 36 (CI, 17 to 51) percentage points for the Alpha variant and 31 (CI, 15 to 46) percentage points for the Beta variant. In the mRNA-1273 group, lower neutralization values were observed for variants compared with wild-type virus, especially the Beta variant.
Limitations:
There is no clear correlate of protection for neutralizing antibody. This was a secondary analysis.
Conclusion:
In organ transplant recipients, a third dose of mRNA vaccine increases neutralizing antibody response against SARS-CoV-2 variants compared with placebo.
Primary Funding Source:
Ajmera Transplant Centre.
SARS-CoV-2 infection has been associated with increased morbidity and mortality in patients who are immunocompromised. Organ transplant recipients usually receive lifelong immunosuppression and have been shown to have a high rate of hospitalization and an approximate 2- to 5-fold increased mortality after SARS-CoV-2 infection (1). Preventive strategies are therefore critically important in this population. Two doses of mRNA vaccine have shown excellent immunogenicity in the general population (2); however, transplant recipients have very poor immunogenicity to 2 doses of mRNA vaccine, as demonstrated in multiple studies (3–5). In addition, breakthrough infections after 2 doses of mRNA vaccine have been commonly reported in transplant recipients, with significant resultant morbidity and mortality compared with breakthrough infections in the general population, which tend to be mild (6).
More recently, studies have shown that a third dose of mRNA vaccine improves immunogenicity in transplant recipients (7–9). We previously reported a randomized trial comparing a third dose of the mRNA-1273 vaccine (Moderna) versus placebo in organ transplant recipients who had completed the standard 2-dose vaccine series (7). Compared with placebo, the third dose resulted in significantly increased antibody titers as measured by anti–SARS-CoV-2 receptor-binding domain (RBD) antibodies. In addition, neutralization against wild-type virus and SARS-CoV-2–specific CD4+ T-cell responses were improved in the mRNA-1273 group. However, SARS-CoV-2 variants of concern (VOCs), especially the Delta variant, have rapidly supplanted wild-type virus as the dominant circulating strains in the community (10). In immunocompetent persons, studies have shown lower vaccine-induced virus neutralization ability against VOCs (11, 12). In addition, studies in the general population have shown decreased real-world efficacy of mRNA vaccines for prevention of SARS-CoV-2 VOC infection, although they seem to still be highly protective against severe disease requiring hospitalization (13, 14). However, in transplant recipients, there are limited data on either 2-dose or 3-dose vaccine immunogenicity as it relates to variant viruses.
Using sera obtained from the randomized placebo-controlled trial of a third dose of mRNA-1273 vaccine in organ transplant recipients, we performed neutralization assays against wild-type virus and the Alpha, Beta, and Delta SARS-CoV-2 variants after 2 and 3 doses of mRNA-1273 vaccine compared with third-dose placebo.
Methods
Design Overview, Setting, and Participants
In the primary trial (ClinicalTrials.gov: NCT04885907), 120 organ transplant recipients from a single center received 2 doses of mRNA-1273 vaccine (Spikevax [Moderna]) at the standard dosing interval (0 and 1 month). They were subsequently randomly assigned to either saline placebo or a third vaccine dose 2 months later. The primary hypothesis was that a third-dose booster would result in increased vaccine immunogenicity compared with placebo. The primary outcome of the original trial was the antibody response to the receptor-binding domain (anti-RBD). Primary trial details have been published previously (7). Inclusion criteria were adult patients (aged ≥18 years) who had received an organ transplant and had a functioning allograft. Exclusion criteria included previous SARS-CoV-2 infection, active cytomegalovirus infection, receipt of rituximab in the previous 6 months, treatment for acute rejection in the previous 30 days, and allergic reaction to a previous mRNA-1273 vaccination. Absence of previous diagnosis of COVID-19 was confirmed by testing for antinucleocapsid protein antibody (Abbott Laboratories) as per the manufacturer's instructions. The study was approved by the local institutional review board (CAPCR #21-5324), and all patients provided informed consent.
For the current study, sera were analyzed after the second vaccine dose and 4 to 6 weeks after the third dose (vaccine or placebo) for neutralization against the Alpha, Beta, and Delta variants compared with vaccine-homologous wild-type virus using the methods described later in this article. Laboratory personnel were blinded to treatment assignment.
Outcomes
Assessment of neutralizing antibody to wild-type virus and variants in the placebo and third-dose mRNA-1273 groups was a prespecified secondary outcome in the statistical analysis plan (version 2.0, June 2021; see the Supplement). Of note, the statistical analysis plan did not specifically mention variants. Comparison of neutralization titers between wild-type virus and variants after the third dose in the mRNA-1273 group was considered a post hoc analysis.
Neutralization Antibody Assessment
Surrogate Virus Neutralization Test
Neutralization assays were performed using 2 methods. The first was a commercially available surrogate virus neutralization test (cPass SARS-CoV-2 Neutralization Antibody Detection Kit [GenScript]) that was granted emergency use authorization by the U.S. Food and Drug Administration (FDA). The surrogate virus neutralization assay has been used in several previous studies and has been shown to have high sensitivity and specificity (with a recommended positive threshold of 30%), with excellent correlation with plaque reduction neutralization testing (15–17). Commercially available recombinant proteins for the Alpha, Beta, and Delta variants (GenScript) were used for the current study. The assay detects functional antibodies that neutralize the interaction between the spike protein receptor-binding domain (spike-RBD) and human angiotensin-converting enzyme 2 (ACE2). The assay was performed according to the manufacturer's specifications. Briefly, microplate wells were precoated with recombinant ACE2 and subsequently incubated with a mixture containing patient serum and horseradish peroxidase (HRP)–conjugated recombinant spike-RBD protein. The presence of neutralizing antibodies in sera prevents the binding of RBD to ACE2, which is resolved using a colorimetric enzyme-linked immunosorbent assay (ELISA) reaction. Percentage neutralization was calculated as (1 − optical density sample / optical density background) × 100. A cutoff of 30% or greater was used to define a positive test result per the manufacturer's instructions. This cutoff is based on manufacturer validation using a panel of confirmed COVID-19 patient sera, healthy control sera, and data from plaque reduction neutralization assays.
The conventional surrogate virus neutralization assay was modified to assess percentage neutralization against VOCs. The modified assay is not covered under the FDA emergency use authorization. No deviations were made from the original protocol (Supplement), except for use of alternate HRP-conjugated spike-RBD proteins corresponding to currently circulating VOCs; all reagents were purchased through GenScript. HRP-conjugated spike-RBDs corresponding to VOCs were diluted at 1:1000 in HRP dilution buffer, the same dilution used for the wild-type spike-RBD packaged with the kit. Recombinant spike-RBD proteins were produced to express key mutations corresponding to the Alpha (B.1.1.7), Beta (B.1.351), and Delta (B.1.617.2) variants. Alpha HRP-RBD expressed the N501Y mutation; Beta expressed N501Y, along with the K417N and E484K mutations; and Delta expressed the L452R and T478K mutations. All recombinant RBD proteins were validated for binding with human ACE2 protein in functional ELISA experiments. All recombinant proteins were expressed in mammalian cell lines. The variant constructs are commercially available but have not received FDA approval.
Spike-Pseudotyped Lentivirus Neutralization Assay
The pseudovirus neutralization assay was performed as described previously, with minor modifications (18). Pseudotyped lentivirus particles were generated from cotransfection of the viral packaging (psPAX2 [Addgene]), the ZsGreen and luciferase reporters (pHAGE-CMV-Luc2-IRES-ZsGreen-W), and the spike protein constructs (wild-type SARS-CoV-2 and the Alpha, Beta, and Delta variants) into HEK293TN cells (System Biosciences [LV900A-1]). Viral supernatants were harvested, clarified, and filtered through 0.45-μm filters before storage at −80 °C. A viral titer assay was performed using HEK293T-ACE2/TMPRSS2 cells, and a virus dilution resulting in more than 1000 relative luciferase units over the control was used in the neutralization assay (1:20 to 1:250 dilution of virus stock, depending on the virus titers of each variant). For the neutralization assay, diluted patient sera samples (1:22.5) were first prepared and serially diluted 3-fold over 7 dilutions, followed by incubation with diluted virus at a 1:1 ratio for 1 hour at 37 °C before addition to HEK293T-ACE2/TMPRSS2 cells. The infected cells were lysed after 48 hours using the Bright-Glo Luciferase Assay System (Promega), and the luminescence signals were detected using a PerkinElmer EnVision instrument. The 50% neutralization titers (ID50s) were calculated in GraphPad Prism 9 using a nonlinear regression algorithm (log[inhibitor] versus normalized response − variable slope). Both the HEK293TN and HEK293T-ACE2/TMPRSS2 cells were maintained at 85% confluency for no more than 25 passages.
Statistical Analysis
The analysis was performed on the per protocol sample, defined as patients who had provided blood samples at 4 to 6 weeks after the intervention (third dose of mRNA-1273 vaccine or placebo) (Figure 1). Descriptive statistics were used to outline the baseline characteristics of the cohort. For the surrogate virus neutralization titer, a neutralization cutoff of 30% or higher was prespecified for defining a positive test result as per the manufacturer's instructions. For the pseudovirus neutralization assay, the ID50 (inhibitory dilution with 50% virus neutralization) was converted to a log10 scale. In patients with an absence of 50% neutralization with undiluted serum, this equated to a log10 ID50 of zero (100 = a dilution of 1:1) and was considered the threshold for positive versus negative. After 2 and 3 doses, the proportion of patients meeting the positive threshold was determined for wild-type virus and each variant. For each variant, comparisons between mRNA-1273 vaccine and placebo after the third dose used the Yates-corrected χ2 test for the proportion exceeding the positive threshold for surrogate virus neutralization and pseudovirus neutralization. Confidence intervals for the difference between the mRNA-1273 and placebo groups in the proportion of patients exceeding the positive neutralization threshold were calculated using the normal-based approximation. This was considered a secondary end point analysis. The threshold for significance was set at P = 0.01. In the mRNA-1273 group, the median percentage neutralization after the third dose (surrogate virus neutralization) and the log10 ID50 (pseudovirus neutralization) were determined. Because this latter assessment was considered a post hoc analysis, no formal statistical comparisons of neutralization between variants were performed. All analyses were done using R, version 4.03 (R Foundation for Statistical Computing), and GraphPad Prism 9.
Figure 1. CONSORT (Consolidated Standards of Reporting Trials) diagram.
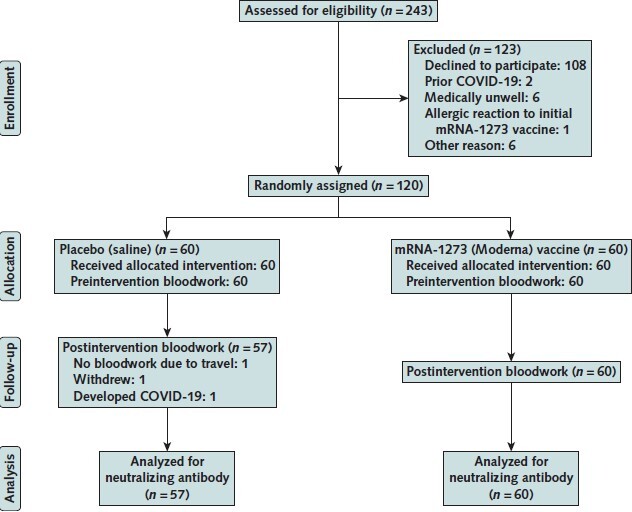
The figure shows the flow of the primary trial, indicating samples collected for determination of neutralizing antibody in the current study (n = 117).
Role of the Funding Source
The study was funded by the Ajmera Transplant Centre, University Health Network, the University of Toronto, and the University Health Network Foundation. The funding sources had no role in the design, conduct, analysis, or other aspects of the study. Vaccine was provided by the University Health Network pharmacy. Moderna had no role in any aspect of the study.
Results
Patient Characteristics
We randomly assigned 120 solid organ transplant recipients to receive either a third dose of mRNA-1273 vaccine or placebo. This analysis was performed in the 117 patients in whom serum samples were available 4 to 6 weeks after the third dose (Figure 1). All patients received 2 doses of mRNA-1273 vaccine at the standard interval of 0 and 1 month. Two months later, patients received either a third dose of mRNA-1273 vaccine (n = 60) or saline placebo (n = 57) in a randomized and double-blinded manner. Baseline demographic characteristics are shown in Table 1. Median age was 66.7 years (interquartile range, 63.6 to 71.4 years), and the type of transplants included kidney (n = 29), liver (n = 19), lung (n = 27), heart (n = 18), and pancreas (including kidney-pancreas) (n = 24). Median time from transplant was 3.2 years (interquartile range, 1.7 to 6.2 years). Although, by chance, minor differences in transplant types were present between the groups, they were well balanced in terms of type, dosages, and blood levels of immunosuppressive medications and baseline lymphocyte count. No adjustments to immunosuppression were made at the time of the third dose. No patient had a previous diagnosis of COVID-19; this was confirmed with SARS-CoV-2 antinucleocapsid protein antibody testing after the third dose for the whole cohort, which showed only a single low-level positive result in 1 patient in the placebo group. This patient was included among the 57 patients who received placebo and are analyzed here.
Table 1.
Patient Characteristics in the Per Protocol Sample (n = 117)

Neutralization Response Using Surrogate Virus Neutralization Test
After 2 vaccine doses, the proportions of patients with positive neutralization for wild-type virus and the Alpha, Beta, and Delta variants were 40 of 117 (34%), 17 of 117 (15%), 15 of 117 (13%), and 21 of 117 (18%), respectively (Figure 2, top). The proportion of patients with a positive neutralization result was lower for all 3 variants versus wild-type virus (differences in proportions above the threshold compared with wild-type virus: Alpha, −19 [95% CI, −8 to −31] percentage points; Beta, −21 [CI, −10 to −32] percentage points; Delta, −16 [CI, −4 to −28] percentage points). The smallest proportion of positivity was observed for the Beta variant. After the third dose of mRNA-1273 vaccine, the proportions above the positive threshold for wild-type virus and the Alpha, Beta, and Delta variants were 36 of 60 (60%), 34 of 60 (57%), 30 of 60 (50%), and 33 of 60 (55%), respectively (Figure 2, bottom). Compared with placebo, the response to mRNA-1273 vaccine was greater for each variant: The differences in the proportion above the threshold were 45 (CI, 28 to 61) percentage points for the Alpha variant, 38 (CI, 21 to 55) percentage points for the Beta variant, and 37 (CI, 20 to 55) percentage points for the Delta variant (P < 0.001 for all comparisons) (Figure 2, bottom). In the third-dose mRNA-1273 group (n = 60), the median percentage neutralizations after vaccination for wild-type virus and the Alpha, Beta, and Delta variants were 71%, 55%, 34%, and 43%, respectively (Figure 2, bottom).
Figure 2. Dot plots showing surrogate virus neutralization assay for wild-type and variant viruses.
![Figure 2. Dot plots showing surrogate virus neutralization assay for wild-type and variant viruses. Each circle represents an individual patient. The dotted lines represent the cutoff of 30% for assay positivity, and the dotted areas represent negative neutralization results. The numbers above each column indicate the proportion of patients with a positive assay result (≥30% neutralization). Top. Percentage neutralization against wild-type virus and the Alpha, Beta, and Delta variants after 2 doses of mRNA-1273 vaccine in organ transplant recipients (n = 117). The proportion of patients with a positive neutralization result was lower for all 3 variants versus wild-type virus (differences in the proportion above the threshold compared with wild-type virus: Alpha, −19 [95% CI, −8 to −31] percentage points; Beta, −21 [CI, −10 to −32] percentage points; Delta, −16 [CI, −4 to −28] percentage points). Bottom. Percentage neutralization after placebo (n = 57) or the third dose of mRNA-1273 vaccine (n = 60) for wild-type virus and the Alpha, Beta, and Delta variants. The horizontal lines represent median values. The differences in the proportion above the threshold for the mRNA-1273 vaccine versus placebo were 45 (CI, 28 to 61) percentage points for the Alpha variant, 38 (CI, 21 to 55) percentage points for the Beta variant, and 37 (CI, 20 to 55) percentage points for the Delta variant (P < 0.001 for all comparisons using the Yates-corrected χ2 test).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c8b7/8628360/91a08756f10e/aim-olf-M213480-M213480ff2.jpg)
Each circle represents an individual patient. The dotted lines represent the cutoff of 30% for assay positivity, and the dotted areas represent negative neutralization results. The numbers above each column indicate the proportion of patients with a positive assay result (≥30% neutralization). Top. Percentage neutralization against wild-type virus and the Alpha, Beta, and Delta variants after 2 doses of mRNA-1273 vaccine in organ transplant recipients (n = 117). The proportion of patients with a positive neutralization result was lower for all 3 variants versus wild-type virus (differences in the proportion above the threshold compared with wild-type virus: Alpha, −19 [95% CI, −8 to −31] percentage points; Beta, −21 [CI, −10 to −32] percentage points; Delta, −16 [CI, −4 to −28] percentage points). Bottom. Percentage neutralization after placebo (n = 57) or the third dose of mRNA-1273 vaccine (n = 60) for wild-type virus and the Alpha, Beta, and Delta variants. The horizontal lines represent median values. The differences in the proportion above the threshold for the mRNA-1273 vaccine versus placebo were 45 (CI, 28 to 61) percentage points for the Alpha variant, 38 (CI, 21 to 55) percentage points for the Beta variant, and 37 (CI, 20 to 55) percentage points for the Delta variant (P < 0.001 for all comparisons using the Yates-corrected χ2 test).
Neutralization Response Using Pseudovirus Neutralization Assay
Neutralization responses were further assessed using a pseudotyped lentivirus neutralization assay for wild-type virus and the Alpha, Beta, and Delta variants. Results are expressed as the log10 ID50 and are shown in Figure 3. After the third dose of mRNA-1273 vaccine, detectable neutralizing antibody response (sufficient for 50% neutralization) against wild-type virus and the Alpha, Beta, and Delta variants was present in 35 of 60 (58%), 34 of 60 (57%), 23 of 60 (38%), and 33 of 60 (55%) patients, respectively. The proportion with detectable neutralizing antibodies was larger in the mRNA-1273 group than the placebo group for each variant. Differences in the proportions for mRNA-1273 vaccine versus placebo were 35 (CI, 17 to 51) percentage points for wild-type virus, 36 (CI, 17 to 51) percentage points for the Alpha variant, 31 (CI, 15 to 46) percentage points for the Beta variant, and 37 (CI, 19 to 53) percentage points for the Delta variant (P < 0.001 for all comparisons). In the mRNA-1273 group (n = 60), the median neutralization titer (log10 ID50 value) was 2.13 log10 for wild-type virus, 1.98 log10 for the Alpha variant, undetectable for the Beta variant, and 1.72 log10 for the Delta variant (Figure 3).
Figure 3. Dot plot showing spike-pseudotyped lentiviral neutralization assay for wild-type and variant viruses.
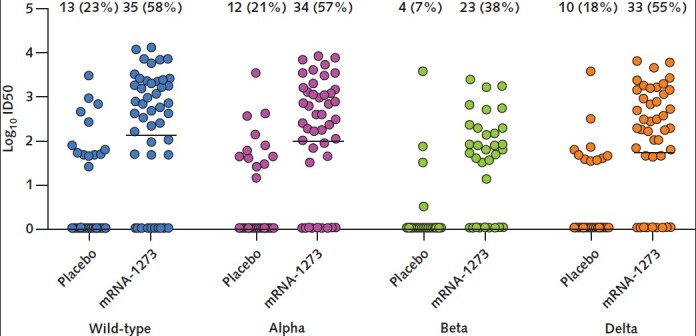
Results include patients after receipt of placebo (n = 57) or the third dose of mRNA-1273 vaccine (n = 60) for wild-type virus and the Alpha, Beta, and Delta variants. Results are expressed as log10-transformed ID50 values, which represent the serum dilution required to neutralize ≥50% of virus. Each circle represents an individual patient. The horizontal lines represent median values; the median for the Beta variant was undetectable in both the placebo group and the mRNA-1273 group. The numbers above each column indicate the proportion of patients with a detectable neutralization (ID50 >0). The differences in the proportion with detectable neutralization for mRNA-1273 vaccine versus placebo were 35 (95% CI, 17 to 51) percentage points for wild-type virus, 36 (CI, 17 to 51) percentage points for the Alpha variant, 31 (CI, 15 to 46) percentage points for the Beta variant, and 37 (CI, 19 to 53) percentage points for the Delta variant (P < 0.001 for all comparisons using the Yates-corrected χ2 test). ID50 = 50% neutralization titer.
Safety Analysis
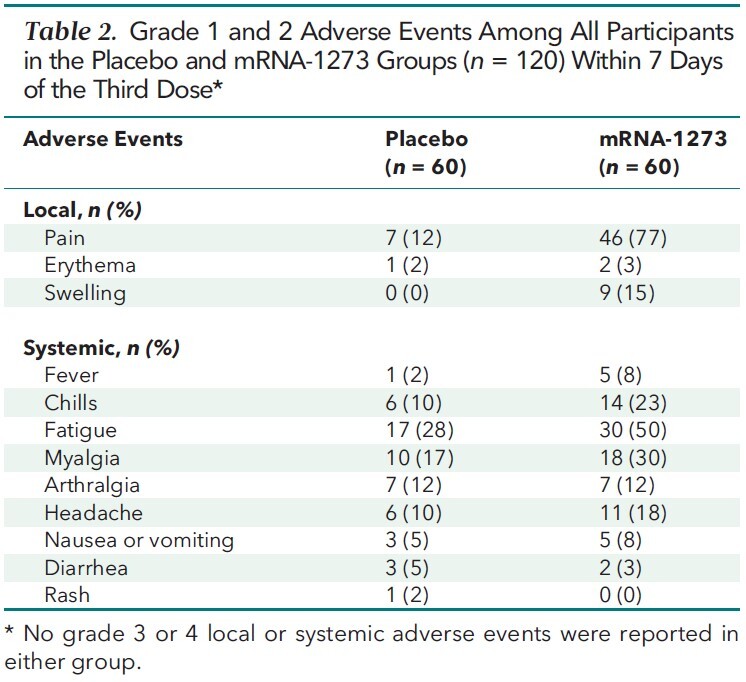
The third vaccine dose was well tolerated (Table 2). No grade 3 or 4 adverse events were observed. The most common adverse event was pain at the injection site, followed by fatigue and myalgia. No biopsy-proven or clinically treated acute rejection events were observed in either group at 1 and 3 months after the third-dose intervention. Graft function, as measured by routine organ-specific parameters (such as serum creatinine in kidney transplant recipients and pulmonary function in lung transplant recipients), remained stable in all patients at 1 and 3 months (data not shown).
Table 2.
Grade 1 and 2 Adverse Events Among All Participants in the Placebo and mRNA-1273 Groups (n = 120) Within 7 Days of the Third Dose*
Discussion
In this analysis, we first show that neutralizing antibody positivity after 2 doses of mRNA-1273 vaccine is low for all 3 SARS-CoV-2 VOCs (Alpha, Beta, and Delta). Subsequently, compared with placebo, a third dose resulted in a significant increase in the proportion of patients with a positive neutralization detection for all 3 variants, as determined by both the surrogate virus neutralization test and a pseudovirus neutralization assay. However, relative immunogenicity of a third dose seemed to be diminished against all 3 variants (especially the Beta variant) versus wild-type virus. The latter finding is consistent with data in healthy immunocompetent populations and likely relates to the fact that mRNA vaccines are manufactured based on the wild-type virus spike protein sequence (19). Taken together, the current data provide further support for a third vaccine dose in immunocompromised patients, even when current VOCs, such as the Delta variant, are the dominant circulating SARS-CoV-2 strains in the community.
A limitation of our study is that no clear protective correlate exists for current immunogenicity assays, including neutralization. However, Khoury and colleagues estimated that the 50% protective neutralization level in most studies was between 1:10 and 1:30 (1.0 log10 to 1.48 log10 ID50) (20). This is similar to the findings of Feng and colleagues, who suggested that the pseudovirus neutralization assay ID50 of 22 (1.34 log10 ID50) is associated with 60% vaccine efficacy (21). Although we acknowledge that this is a secondary analysis, we believe our findings, which are based on 2 separate measures of neutralization, are robust and important. The first measure is a surrogate virus neutralization assay that measures strength of the RBD-ACE2 receptor interaction. A limitation of the surrogate assay is that it does not account for neutralizing antibodies that bind to epitopes outside the RBD portion of the spike protein but are still encoded by the vaccine. To help address this, we subsequently performed an assay using a spike-pseudotyped lentivirus neutralization system (18). These assays have been commonly used in studies evaluating vaccine response in immunocompetent patients (22). In addition, the lentivirus-based assay we used here has been validated against plaque reduction neutralization testing (generally considered the gold standard but requiring special biocontainment facilities), both for wild-type virus and VOCs. This assay also allows a more accurate determination of the relative decrease in neutralization ability of wild-type virus versus variants. Based on this, neutralization in the third-dose mRNA-1273 group was lower for VOCs, especially the Beta variant, compared with wild-type virus. We previously showed improved SARS-CoV-2–specific CD4+ T-cell responses to a third dose of mRNA-1273 vaccine compared with placebo (7). It would be informative to assess variant-specific T-cell responses, but this is more technically challenging and difficult to interpret because stimulation with peptide pools derived from the variant virus spike sequences may result in overlapping T-cell reactivities, and also because clinical correlates for T-cell responses are poorly defined.
Several studies have now shown the potential benefit of a third vaccine dose in organ transplant recipients. However, we are currently unaware of any published studies that have evaluated virus neutralization against circulating VOCs with third-dose vaccinated samples. Kamar and colleagues evaluated 101 solid organ transplant recipients who were given 3 doses of BNT162b2 (Pfizer–BioNTech). They measured antispike antibody levels using an ELISA-based assay and found that detectable antibody levels increased from 40% to 68% (23). Benotmane and colleagues evaluated 3 doses of mRNA-1273 vaccine in persons who did not respond to 2 doses and found a 49% response as measured by anti-RBD antibodies (8). Neutralizing antibodies against wild-type virus were measured using a pseudovirus assay in a 3-dose study of 96 heart transplant recipients by Peled and colleagues (24). They observed a more than 9-fold increase in neutralization titer after the third dose. None of these studies evaluated the third vaccine dose in comparison with a placebo group but were instead single-group cohort studies.
In conclusion, our data suggest that in organ transplant recipients, 2 doses of mRNA-1273 vaccine provide minimal neutralizing protection against SARS-CoV-2 VOCs; however, a third dose increases immune response against variants compared with placebo. Overall, the third vaccine dose was safe and well tolerated. Importantly, there seemed to be no evidence that multiple doses precipitated acute rejection or graft dysfunction; however, the relatively small sample and short-term follow-up may preclude detection of a rare safety signal. Our findings may reasonably be applied to other groups of similarly immunocompromised patients, such as those receiving active cancer chemotherapy or biologics, but further studies would be beneficial in those populations.
Supplementary Material
Footnotes
This article was published at Annals.org on 23 November 2021.
References
- 1. Williamson EJ , Walker AJ , Bhaskaran K , et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-6. [PMID: ] doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steensels D , Pierlet N , Penders J , et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533-5. [PMID: ] doi: 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall VG , Ferreira VH , Ierullo M , et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021. [PMID: ] doi: 10.1111/ajt.16766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyarsky BJ , Werbel WA , Avery RK , et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204-6. [PMID: ] doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stumpf J , Siepmann T , Lindner T , et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [PMID: ] doi: 10.1016/j.lanepe.2021.100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qin CX , Moore LW , Anjan S , et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients [Letter]. Transplantation. 2021;105:e265-e266. [PMID: ] doi: 10.1097/TP.0000000000003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall VG , Ferreira VH , Ku T , et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients [Letter]. N Engl J Med. 2021;385:1244-6. [PMID: ] doi: 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benotmane I , Gautier G , Perrin P , et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021. [PMID: ] doi: 10.1001/jama.2021.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Bello A, Abravanel F, Marion O, et al.. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients [Letter]. Am J Transplant. 2021. [PMID: ] doi: 10.1111/ajt.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Callaway E . Delta coronavirus variant: scientists brace for impact. Nature. 2021;595:17-18. [PMID: ] doi: 10.1038/d41586-021-01696-3 [DOI] [PubMed] [Google Scholar]
- 11. Bates TA , Leier HC , Lyski ZL , et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun. 2021;12:5135. [PMID: ] doi: 10.1038/s41467-021-25479-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu K , Werner AP , Koch M , et al. Serum neutralizing activity elicited by mRNA-1273 vaccine [Letter]. N Engl J Med. 2021;384:1468-70. [PMID: ] doi: 10.1056/NEJMc2102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez Bernal J, Andrews N, Gower C, et al.. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585-94. [PMID: ] doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenforde MW , Self WH , Naioti EA , et al; IVY Network Investigators. Sustained effectiveness of Pfizer–BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1156-62. [PMID: ] doi: 10.15585/mmwr.mm7034e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan CW , Chia WN , Qin X , et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073-8. [PMID: ] doi: 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 16. Taylor SC , Hurst B , Charlton CL , et al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol. 2021;59. [PMID: ] doi: 10.1128/JCM.02438-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medeiros-Ribeiro AC , Aikawa NE , Saad CGS , et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744-51. [PMID: ] doi: 10.1038/s41591-021-01469-5 [DOI] [PubMed] [Google Scholar]
- 18. Abe KT , Li Z , Samson R , et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight. 2020;5. [PMID: ] doi: 10.1172/jci.insight.142362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinz FX , Stiasny K . Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. [PMID: ] doi: 10.1038/s41541-021-00369-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoury DS , Cromer D , Reynaldi A , et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205-11. [PMID: ] doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 21. Feng S , Phillips DJ , White T , et al; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021. [PMID: ] doi: 10.1038/s41591-021-01540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng C , Evans JP , Pearson R , et al. Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers, and convalescent plasma donors. JCI Insight. 2020;5. [PMID: ] doi: 10.1172/jci.insight.143213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamar N , Abravanel F , Marion O , et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients [Letter]. N Engl J Med. 2021;385:661-2. [PMID: ] doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peled Y , Ram E , Lavee J , et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. [PMID: ] doi: 10.1016/j.healun.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


