Abstract
Background:
We conducted a secondary analysis of changes in the Kansas City Cardiomyopathy Questionnaire (KCCQ)-12 over 30 days in a randomized trial of self-care coaching versus structured usual care in patients with AHF who were discharged from the ED.
Methods:
Patients in 15 EDs completed the KCCQ-12 at ED discharge and at 30 days. We compared change in KCCQ-12 scores between the intervention and usual care arms, adjusted for enrollment KCCQ-12 and demographic characteristics. We used linear regression to describe changes in KCCQ-12 summary scores and logistic regression to characterize clinically meaningful KCCQ-12 subdomain changes at 30 days.
Results:
There were 350 patients with both enrollment and 30-day KCCQ summary scores available; 166 allocated to usual care and 184 to the intervention arm. Median age was 64 years (IQR 55 to 70), 37% were female, 63% were African American, median KCCQ-12 summary score at enrollment was 47 (IQR 33 to 64). Self-care coaching resulted in significantly greater improvement in health status compared with structured usual care (5.4-point greater improvement, 95% CI, 1.12 to 9.68; p = 0.01). Improvements in health status in the intervention arm were driven by improvements within the symptom frequency [aOR 1.62, 95% CI, 1.01 to 2.59] and quality of life [aOR 2.39, 95% CI, 1.46 to 3.90] subdomains.
Conclusions:
In this secondary analysis, patients with AHF who received a tailored, self-care intervention after ED discharge had clinically significant improvements in health status at 30 days compared with structured usual care largely due to improvements within the symptom frequency and quality of life subdomains of the KCCQ-12.
Registration:
ClinicalTrials.gov Identifier: NCT02519283
Keywords: Kansas City Cardiomyopathy Questionnaire, KCCQ, KCCQ-12, GUIDED-HF, heart failure
Introduction
Heart failure (HF) is common with an estimated prevalence of 6 million Americans ≥20 years of age according to 2015 to 2018 data and is projected to increase by 46% from 2012 to 2030, affecting >8 million people ≥18 years of age.1 As many as 875,000 patients with HF visit the emergency department (ED) annually, with 54% being admitted to the hospital three or more times following diagnosis.2 Of the patients who present to the ED and are diagnosed with acute heart failure (AHF) as the cause of their symptoms, 84% are hospitalized. The 16% of patients with AHF who are discharged home3 rely on the ED to assist with the transition to outpatient care, including medication adjustments, education about worsening symptoms, and obtaining provider follow-up.4 However, ED-based transition initiatives are lacking, and ensuring optimal transitions of care for patients with AHF discharged from the ED is a critical, unmet need.
Patients with HF suffer from functional limitations and impaired quality of life,5, 6 thus it is important to understand the impact of HF interventions on these outcomes.7, 8 We conducted a randomized trial (GUIDED-HF) to evaluate if a tailored self-care intervention improves HF outcomes in patients with AHF discharged from the ED compared to structured usual care.9 The self-care intervention included a home visit within 7 days of discharge and twice monthly telephone-based coaching calls for 3 months performed by the study team, including study coordinators, nurses or paramedics who underwent protocol training. A key component of our outcome assessment was to determine the impact of our intervention on patient perceived health status. Our primary results suggested that a self-care intervention did not result in sustained improvement in the 90-day composite outcome of death, hospital admission, ED visit and Kansas City Cardiomyopathy Questionnaire (KCCQ-12) scores. However, we observed clinically important differences at 30 days between treatment arms in our primary outcome. A key component of this difference in efficacy was differences in changes in KCCQ scores between the intervention and usual care arms. The goal of this manuscript was to analyze changes in KCCQ-12 scores between enrollment and 30-day follow-up between trial arms.
Methods
KCCQ
The KCCQ-12 is a validated health status measure for patients with HF.10–12 It contains four subdomains: Physical Limitation, Symptom Frequency, Quality of Life, and Social Limitations. Each subdomain provides an individual score from 0 to 100, with 0 denoting the worst and 100 the best possible health status. The mean of the four subdomain scores are presented as a summary score, with differences of 5 points or greater considered to be clinically important.13–15
Design
Detailed study methodology for the GUIDED-HF Trial has been previously reported.16 In brief, patients ≥ 21 years old with a history of HF deemed by the treating emergency provider to have AHF, and who they planned to discharge after ED-based management (less than 23 hours of AHF care), were eligible for inclusion. Patients were excluded if they were unable to comply with the protocol due to psychiatric disease or distance from the hospital, if they had a systolic blood pressure less than 100 mmHg, evidence of acute coronary syndrome, or were undergoing outpatient inotrope infusion. Patients were enrolled in 15 geographically diverse EDs and randomized at the time of ED discharge to structured, usual care versus a self-care intervention. In keeping with the pragmatic nature of the trial, we designed the structured usual care arm discharge procedures to largely reflect usual practice, although the study team also performed HF medication reconciliation and arranged 7-day outpatient HF provider follow-up in the usual care arm. Patients randomized to the self-care intervention received these two structured usual care procedures, as well as a home visit within 7-days of ED discharge and twice-monthly self-care coaching calls for 3 months. Self-care coaching focused on daily weights, signs of worsening HF, low-salt diet, monitoring fluid intake, and exercise. KCCQ scores were collected shortly prior to ED discharge (enrollment) and again 30 days after ED discharge. Protocols were approved by each site’s Institutional Review Board.
Data Collection
At enrollment, patient demographics, medical history, prior ejection fraction (EF), HF hospitalizations and ED visits in the previous 6 months, ED tests and treatments, and KCCQ-12 scores were prospectively collected and entered into a research electronic data capture (REDCap) platform.17, 18 Outcome assessors collecting 30-day KCCQ-12 scores by phone were blinded to the treatment arm. Per study protocol, KCCQ-12 scoring at 30 days had to be completed within a 5 day window. If patients were reached after this window, HF readmission and cardiovascular mortality events were still recorded in the primary analysis,9 however KCCQ-12 scores were not recorded. This timeframe was implemented to ensure comprehensive follow-up for readmission and mortality outcomes, and KCCQ-12 collection was reflective of a 30-day follow-up period. This narrow window led to increased missingness for 30-day KCCQ-12 scoring relative to HF readmission and mortality outcomes.
Statistical Analysis
Enrollment demographic and clinical characteristics were summarized using median (Interquartile Range [IQR]) or count (percentage), as appropriate. Comparisons between trial arms were conducted using Wilcoxon rank-sum tests for continuous variables and Pearson’s Chi-squared test for categorical variables. Changes in KCCQ-12 scores from enrollment to 30 days were summarized and analyzed both by summary score and, in each subdomain, as continuous variables. To help facilitate clinical interpretability of the mean differences in scores between groups, we also conducted responder analyses of the KCCQ-12 scores, dichotomizing the change in KCCQ-12 scores using previously established thresholds of clinically important changes consistent with prior investigations.19, 20 Specifically, we evaluated five separate outcomes using thresholds of clinically important differences, defined as deterioration (≤ −5 point loss), small improvement (>5 points), moderate improvement (>10 points), large improvement (>15 points), and very large improvement (>20 points). All analyses were conducted following the intention to treat principle.
Our primary question of interest was whether the tailored self-care intervention resulted in greater improvement in KCCQ-12 scores compared with structured usual care after adjusting for a priori determined covariates. To answer this question, we used multivariable linear regression for continuous 30-day changes in KCCQ-12 scores adjusting for enrollment KCCQ-12 scores, age, sex, race, systolic blood pressure, and estimated glomerular filtration rate, and prior EF. In order to further investigate the clinically meaningful thresholds of change in KCCQ-12 scores as separate outcomes, we also fit multivariable logistic regression models for dichotomized outcomes using the 5 thresholds above. These models adjusted for the same covariates and evaluated the summary score and each of the 4 sub-domains as outcomes. Missing data was handled by multiple imputation. Ten copies of the dataset were created. For each dataset, we replaced missing data with imputed values generated using the predictive mean matching approach. Then we used Rubin’s rule to summarize results from regression analysis across the 10 imputed datasets. All statistical analyses were performed using R Statistical Software, Version 3.5.2 (www.R-project.org).21
Results
A total of 491 patients were randomized. Twelve patients withdrew consent after randomization leaving 479 patients in the overall cohort. There were 350 with both KCCQ summary scores available at enrollment and at 30 (+4) days after enrollment; 166 allocated to usual care and 184 allocated to the intervention arm. Patients were excluded due to incomplete data including two deaths within 30 days, one in each study arm, three withdrawals after ED discharge but before the 30-day follow up, 10 with missing baseline KCCQ summary scores, and 114 had missing 30-day KCCQ scores because follow-up occurred after day 34 (sFig 1). There was no detectable difference in enrollment data between the two arms. Further, there were no differences in baseline KCCQ-12 scores between: 1) the 350 patients with complete enrollment and follow-up KCCQ available and 2) the 129 patients who had either died, withdrew or had missing enrollment and/or follow-up KCCQ scores (n=129) (sTable 1). In the 350 patients that formed the study cohort, the median age was 64 years (IQR 55 to 70), 37% were female, 63% were African American, and 42% had a left ventricular EF >50%. The median KCCQ-12 summary score at enrollment was 47 (IQR 33 to 64). Patients in the intervention arm were more likely to be taking beta-blockers and less likely to be taking ACE inhibitors, otherwise there were no significant difference between study arms in enrollment characteristics or enrollment KCCQ-12 overall summary scores (Table 1).
Table 1.
Enrollment Characteristics by Study Arms
| Characteristic | Intervention (n=166) | Usual Care (n=184) | Combined (n=350) | p-value | |
|---|---|---|---|---|---|
| Median Age (interquartile range), years | 64 (54, 70) | 63 (56, 70) | 64 (55, 70) | 0.94 | |
| Female, n (%) | 65 (35) | 66 (40) | 131 (37) | 0.39 | |
| Race, n (%) | 0.66 | ||||
| American Indian or Alaskan Native | 0 | 1 (1) | 1 (0) | ||
| Black/African American | 121 (66) | 100 (60) | 221 (63) | ||
| Native Hawaiian or Pacific Islander | 0 | 1 (1) | 1 (0) | ||
| White non-Hispanic | 58 (32) | 60 (36) | 118 (34) | ||
| White Hispanic | 4 (2) | 3 (2) | 7 (2) | ||
| Declined to Disclose | 1 (1) | 1 (1) | 2 (1) | ||
| Vulnerable Population, n (%) | 143 (78) | 120 (72) | 263 (75) | 0.24 | |
| Brief Health Literacy Score < 9 | 22 (12) | 17 (10) | 39 (11) | 0.60 | |
| Median National ADI Rank (interquartile range) | 83 (58, 96) | 83 (58, 95) | 83 (57, 96) | 0.63 | |
| Low SES, n (%) | 85 (49) | 72 (44) | 157 (46) | 0.42 | |
| Mean Body Mass Index (kg/m2) | 33 (28, 41) | 36 (30, 43) | 34 (29, 42) | 0.05 | |
| Chronic Medical Conditions, n (%) | |||||
| Myocardial Infarction | 54 (29) | 52 (31) | 106 (30) | 0.52 | |
| Hypertension | 173 (94) | 154 (93) | 327 (93) | 0.64 | |
| Diabetes Mellitus | 105 (57) | 89 (54) | 194 (55) | 0.52 | |
| CKD | 54 (29) | 41 (25) | 95 (27) | 0.37 | |
| COPD | 70 (38) | 53 (32) | 123 (35) | 0.41 | |
| Prior EF, n (%) | 0.36 | ||||
| Normal | 63 (37) | 74 (47) | 137 (42) | ||
| Moderately reduced | 50 (29) | 38 (24) | 88 (27) | ||
| Severely reduced | 53 (31) | 43 (27) | 96 (29) | ||
| Not reported | 4 (2) | 3 (2) | 7 (2) | ||
| NYHA Class, n (%) | 0.42 | ||||
| I | 26 (14) | 29 (19) | 55 (16) | ||
| II | 89 (49) | 63 (41) | 152 (45) | ||
| III | 54 (30) | 50 (32) | 104 (31) | ||
| IV | 12 (7) | 13 (8) | 25 (7) | ||
| Mean number of visits for HF in last 6 months | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.46 | |
| Mean number of hospital admissions for HF in last 6 months | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.72 | |
| Initial ED testing (interquartile range) | |||||
| Median SBP | 143 (126, 164) | 142 (125, 164) | 143 (126, 164) | 0.80 | |
| Calculated eGFR | 65 (50, 80) | 61 (50, 85) | 63 (50, 82) | 0.70 | |
| BUN | 19 (14, 27) | 19 (14, 26) | 19 (14, 26) | 0.56 | |
| BNP | 603 (263, 1336) | 438 (148, 1152) | 520 (182, 1256) | 0.13 | |
| Troponin I | 0.026 (0.012, 0.040) | 0.021 (0.012, 0.040) | 0.024 (0.012, 0.040) | 0.87 | |
| Troponin T | 0.010 (0.007, 0.033) | 0.030 (0.020, 0.045) | 0.030 (0.010, 0.035) | 0.27 | |
| Guideline Directed Medical Therapy at ED Discharge, n (%) | |||||
| Diuretic* | 163 (89) | 148 (89) | 311 (89) | 0.87 | |
| BB | 143 (78) | 112 (68) | 255 (73) | 0.04 | |
| ACEi | 58 (36) | 85 (47) | 143 (41) | 0.04 | |
| ARB | 31 (17) | 40 (25) | 71(21) | 0.09 | |
| Aldosterone Antagonist† | 38 (21) | 36 (22) | 74 (21) | 0.74 | |
| KCCQ-12 scores at enrollment (interquartile range) | |||||
| Summary | 49 (36, 65) | 44 (31, 63) | 47 (33, 64) | 0.10 | |
| Physical limitations | 67 (50, 75) | 67 (50, 83) | 67 (50, 75) | 0.60 | |
| Symptom frequency | 44 (27, 62) | 39 (21, 58) | 42 (25, 60) | 0.06 | |
| Quality of life | 38 (12, 62) | 38 (12, 50) | 38 (12, 62) | 0.23 | |
| Social limitations | 50 (25, 75) | 42 (25, 75) | 50 (25, 75) | 0.14 | |
ACEi, Angiotensin-converting enzyme inhibitors; ADI, area deprivation index; ARB, Angiotensin II receptor blocker; BB, beta blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; NYHA, New York Heart Association Functional Classification for Heart Failure; SBP, systolic blood pressure; SES, socioeconomic status.
diuretics included furosemide (Lasix) and bumetanide (Bumex)
Aldosterone antagonists included: Spironolactone (Aldactone)
Patients in the intervention arm experienced an adjusted 5.4-point improvement (95% CI, 1.12–9.68; P=0.01) in KCCQ-12 summary scores at 30 days compared with the usual care arm. The symptom frequency and quality of life sub-domains demonstrated similar improvements in the intervention arm (symptom frequency Beta = 5.62, 95% CI, 0.21–11.03, p = 0.04; quality of life Beta = 7.42, 95% CI, 1.71– 13.13, p = 0.01) (Fig 1).
Fig 1. Effect size between intervention and usual care from enrollment to 30 days.
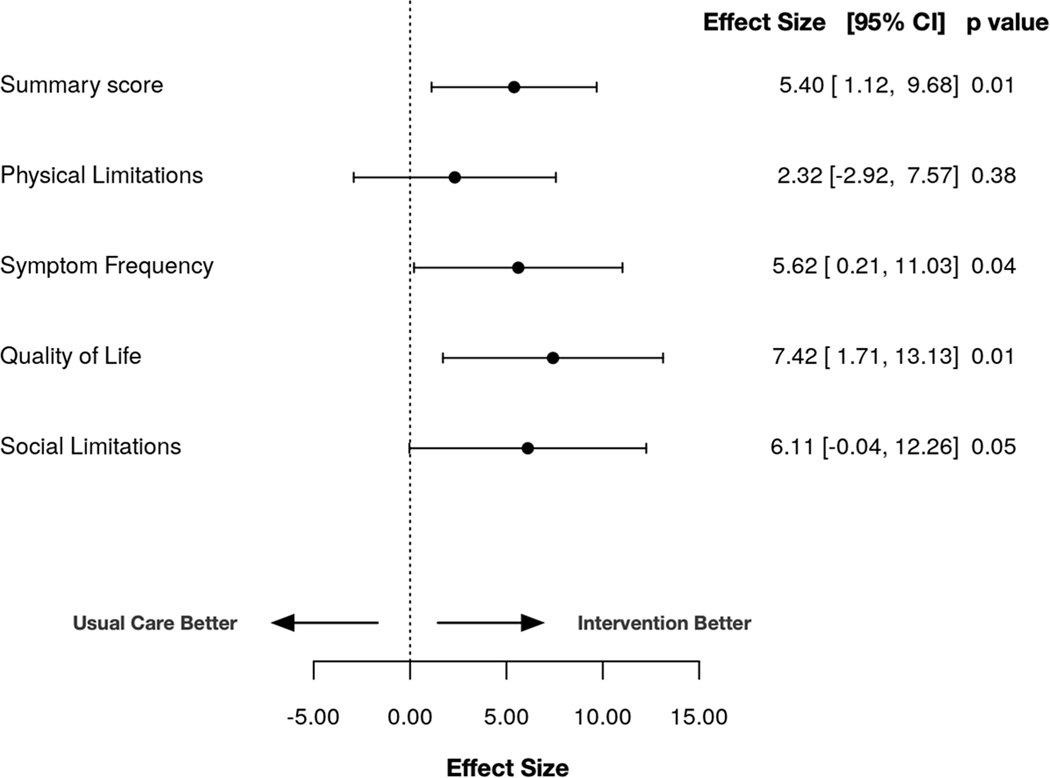
Linear regression analyses show 5.4-point greater improvements in KCCQ-12 summary scores at 30 days and similar associations in the symptom frequency and quality of life sub-domains.
In unadjusted analyses, relative to the usual care arm, we observed a greater proportion of patients in the intervention arm with improvements in KCCQ-12 summary scores [small (>5 point) 61% vs 53% p=0.11, moderate (>10 point) 49% vs 39% p =0.05, large (>15 point) 39% vs 29% p = 0.04, very large (>20 point) 30% vs 23% p = 0.11]. Additionally, relative to structured usual care, fewer patients in the intervention arm (20% vs 30%, p = 0.04) experienced a deterioration in KCCQ-12 overall summary scores (≤−5 points) at 30 days (Fig 2A). This yielded numbers needed to treat (NNT) of 12, 10, 10, and 14 respectively for small, moderate, large, and very large improvements in KCCQ-12 overall summary scores. Adjusted regression models suggested patients in the intervention arm had increased odds of improvement in KCCQ-12 overall summary scores compared to the usual care arm at 30 days for small (>5 points, aOR 1.48, 95% CI, 0.95–2.33; p = 0.09), moderate (>10 points, aOR 1.66, 95% CI, 1.05–2.26; p = 0.03;), large (> 15 points, aOR 1.66, 95% CI, 1.05–2.62; p = 0.03), and very large (>20 points, aOR 1.64, 95% CI, 0.98–2.74; p = 0.06) improvements, and a decreased odds of deterioration (≤ −5 points, aOR 0.56, 95% CI, 0.34–0.95; p = 0.03) (Fig 2B). In our adjusted regression models, none of the included covariates were significant predictors of change in KCCQ-12 summary scores at 30 days with the exception of baseline KCCQ-12 summary scores for deterioration, small, moderate, large, and very large improvements; prior EF for large improvement; and ED systolic blood pressure for large and very large improvements (sTable II). Patients within the intervention arm had increased odds of improvement in the symptom frequency and quality of life subdomains at 30 days for all improvement threshold levels (Fig 3, D and F). There was no observed association for improvement in the physical limitations and social limitations subdomains (Fig 3, B and H). In an exploratory analysis, we removed the quality of life subdomain to yield the KCCQ clinical summary score.22 The differences between study arms remained significant even after the removal of quality of life with a 4.96-point (0.58–9.33, p=0.03) difference between intervention and usual care arms at 30 days.
Fig 2. Responder analysis of change in KCCQ-12 summary score at 30 days with self-care intervention versus usual care.
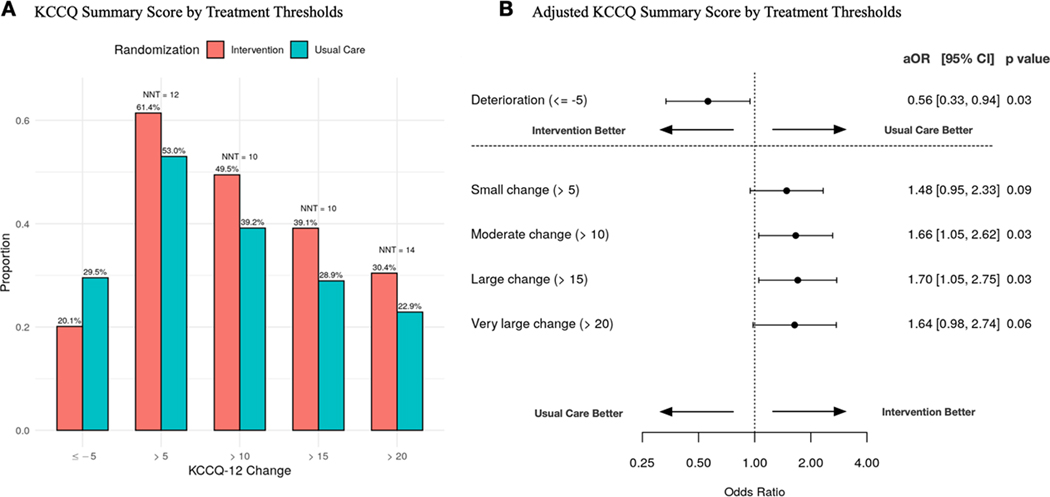
Unadjusted small, moderate, and large changes in KCCQ-12 Summary Scores in self-care intervention versus usual care at 30 days (A). Associated small, moderate, and large changes in KCCQ-12 summary scores using adjusted regression analyses (B). Abbreviations: KCCQ-12, 12 item Kansas City Cardiomyopathy Questionnaire; OR, odds ratio; CI, confidence interval.
Fig 3. Responder analysis of change in KCCQ-12 subdomain scores at 30 days with self-care intervention versus usual care.
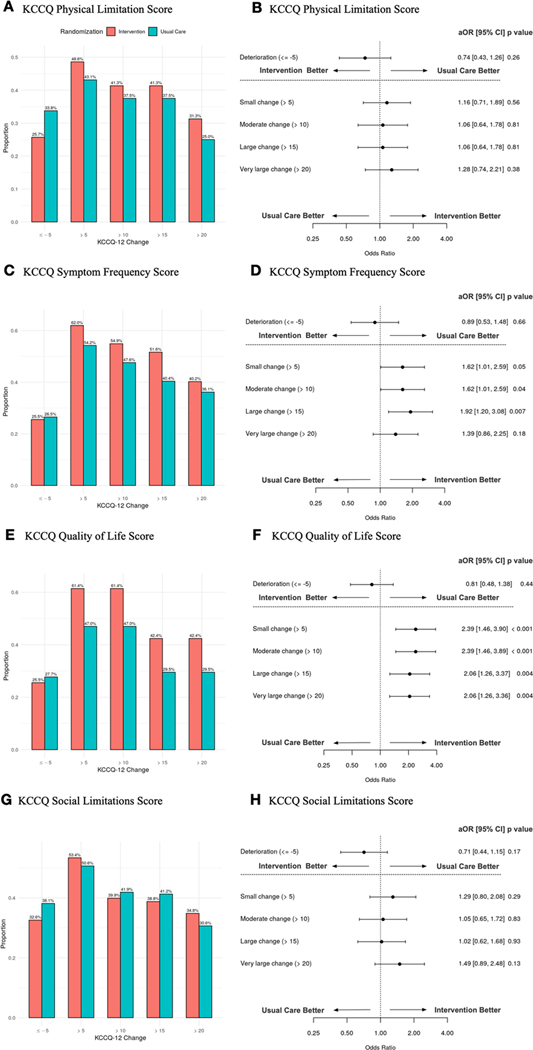
Unadjusted small, moderate, and large changes in KCCQ-12 Physical Limitation Score (A), Symptom Frequency Score (C), Quality of Life Score (E), and Social Limitation Score (G) with self-care intervention versus usual care at 30 days. Associated small, moderate, and large changes in KCCQ-12 Physical Limitation Score (B), Symptom Frequency Score (D), Quality of Life Score (F), and Social Limitation Score (H) using adjusted regression analyses. Abbreviations: KCCQ-12, 12 item Kansas City Cardiomyopathy Questionnaire; OR, odds ratio; CI, confidence interval.
Discussion
Patients with chronic HF frequently present to the ED with AHF and, when not hospitalized, their successful transition to the outpatient setting relies largely on the emergency care system and its providers. Our analysis of 350 patients included in our randomized trial of transitional care suggests patients in the intervention arm had significantly greater improvements in their health status at 30 days, as measured by the KCCQ-12 overall summary score. An estimated 12 patients were needed to treat to achieve a clinically important improvement (>5 points) in health status, primarily driven by improvements in their HF symptoms and quality of life. Importantly, no individual covariate (age, sex, race, systolic blood pressure, and estimated glomerular filtration rate, and prior EF) with the exception of enrollment KCCQ-12 score remained consistently significant in the adjusted logistic regression models used to predict meaningful clinical changes in KCCQ-12 summary score in the intervention arm. Our findings add to the current literature describing both the impact of self-care interventions on health status, and the changes in KCCQ-12 in patients 30 days after discharge from an ED visit for AHF without hospitalization.
Self-care interventions in patients with HF has been shown to prevent re-admission and improve quality of life in several meta analyses.23–26 Although we did not conduct a cost-effectiveness analysis, prior work has reported significant savings in self-management interventions due to reduced resource utilization.27, 28 The reduced resource utilization and cost savings in safely avoiding return ED visits and subsequent hospital admissions may offset the small incremental cost of time spent conducting a home visit and self-care coaching. Further, our tailored, self-care intervention was implemented by a variety of healthcare providers (paramedics, nurses, coordinators), and could be delivered by staff already present in the ED, without requiring new training or hiring new types of staff to complete this initiative.
Seventy five percent of our cohort had vulnerable characteristics defined as (1) non-White race/ethnicity, (2) brief health literacy score less than 9,29 or (3) a national area deprivation index (ADI) score greater than 85.30 This is of particular significance given that vulnerable patients often use the ED as their main source of health care. As noted in the original trial, vulnerable patients in the intervention arm experienced similar early benefit consistent with the overall population effect.
Our results are similar to those reported in patients with AHF who are hospitalized. A prior AHF study conducted by Sauser, et al. followed patients from the ED through hospital admission to 30 days post discharge. They noted KCCQ summary scores were lowest at presentation to the ED, improved during hospitalization (+11.9 points) and were highest at 30 days (+17.8 points).12 Our study demonstrates improvements in KCCQ summary scores in the intervention arm at 30 days, but is unique in characterizing patients who are discharged after only ED-based management. These improvements may be even more impactful considering patients discharged from the ED are less ill than those discharged from the hospital. Considering the cost and resource utilization resulting from a hospitalization, we believe these findings represent important incremental improvements in strategies of care in the subset of patients with HF who are discharged.
Our study suggests significant differences in 30-day health status between patients randomized to the intervention when compared with structured usual care, but these findings should be interpreted in the context of the following potential limitations. First, while we identified improvement in health status at 30 days, our primary analysis suggested this improvement was not sustained at 90 days. The temporal relationship of ED discharge with 30-day outcomes is impactful in the emergency department setting and several AHF tools have been developed predicting 30-day outcomes.31, 32 This does suggest however, a repeat home visit or additional HF provider follow-up may be needed between 30 and 90 days to sustain the observed early health status benefit. This added cost may also need to be considered in a cost-effectiveness analysis. Second, a slow accrual rate was observed in the primary study. A lower-than-expected ED discharge rate suggests our intervention may be of greatest utility at ED sites where a high proportion of patients are discharged home after ED-based management. Quality improvement efforts to increase the discharge to home rate of HF exacerbations presenting to the ED are ongoing.33 Third, not all patients approached for the study consented to participate, and patients were excluded if they lived too far away from the enrolling institution for a home visit to be conducted. We did not describe a distance but left it up to the study team to determine. Ultimately, we amended this to include telehealth visits and so distance did not exclude anybody from participation. Telehealth visits were conducted in lieu of home visits for 11% of the intervention arm in the original trial. This adjuvant may serve an unmet need for those patients with geographic limitations. Receptivity to telehealth is likely to have improved as a result of the coronavirus pandemic. Fourth, complete KCCQ-12 overall summary scores at enrollment and 30-day follow-up were available for 350 of the 479 patients (73%) in the GUIDED-HF Trial. The majority of patients were excluded due to our strict data collection time window for 30-day KCCQ scores (26–34 days). Patient death or withdrawal resulted in five patients being excluded, no baseline KCCQ-12 score excluded ten, and inability to contact patient within the strict KCCQ-12 follow-up time window excluded 114 patients (sFig I). While patients could recall and report HF events such as ED revisits and hospital admission at later time points, to minimize missingness on these events, the study team was not able to capture KCCQ-12 scores outside the +/− 4-day window. This may have biased the results and favored those who responded in a timely manner. However, there were no differences in baseline KCCQ-12 scores or enrollment characteristics between those included in the analysis and those unavailable for follow-up at 30 days (sTable I). Finally, these results may not be generalizable to more acutely-ill patients with HF, as these patients were specifically excluded. Thus, patients within our cohort had 0 to 1 hospital admissions for HF in the last 6 months, median systolic blood pressures of 143, and were deemed by emergency physician to be eligible for discharge.
Conclusion
Our findings in this secondary analysis suggest patients with AHF who received a tailored, self-care intervention after ED discharge had clinically significant improvements in health status at 30 days compared with structured usual care, largely due to improvements within the symptom frequency and quality of life subdomains of the KCCQ-12.
Supplementary Material
“What is Known”
The majority of patients who present to the emergency department (ED) with acute heart failure (AHF) are hospitalized.
ED-based transition initiatives to assist with the transition to outpatient care, including medication adjustments, education about worsening symptoms, and obtaining provider follow-up is a critical, unmet need.
The GUIDED-HF trial observed improvement in its composite primary outcome at 30 days as a result of its tailored self-care program
“What the Study Adds”
This secondary analysis of the GUIDED-HF trial evaluated changes in the Kansas City Cardiomyopathy Questionnaire (KCCQ)-12 over 30 days in patients with AHF who were discharged from the ED.
Patients with AHF who received the tailored, self-care intervention after ED discharge had clinically significant improvements in health status at 30 days compared with structured usual care, largely due to improvements within the symptom frequency and quality of life subdomains of the KCCQ-12.
Acknowledgments
Funding: This work was funded by Patient-Centered Outcomes Research Institute (PCORI) Award AD-1409–21656. REDCap was funded by UL1TR000445 from NCATS/NIH.
Abbreviations List:
- AHF
Acute Heart failure
- HF
Heart Failure
- ED
Emergency Department
- EF
Ejection Fraction
- GUIDED-HF
Get with the Guidelines in Emergency Department Patients with Heart Failure
- KCCQ
Kansas City Cardiomyopathy Questionnaire
Footnotes
Potential Conflicts of interest:
Dr. Stubblefield reports no COI.
Dr. Storrow reports no COI.
Dr. Spertus reports that, relevant to this work, he owns the copyright to the Kansas City Cardiomyopathy Questionnaire. He also serves as a consultant for Bayer, AstraZeneca, Myokardia, Merck, Amgen, Novartis, United Healthcare and Janssen; has equity in Health Outcomes Sciences and serves on the Board of Directors for Blue Cross-Blue Shield of Kansas City.
Dr. Pang is a consultant for Baxter, BMS, and Merck. He has received research or other support from: BMS, Roche, Novartis, PCORI, AHA, NHLBI, AHRQ, OrthoDiagnostics, Abbott, and Beckman Coulter.
Dr. Levy received grant/research support from NIH/NHLBI, NIH/NIMHD, PCORI, AHRQ, EMF, BCBSMF, MDHHS, and MHEF and consultant support/other from Apex Innovations, AstraZeneca, BMS, Mespere, Novartis, Cardionomics, Baim Institute, Ortho Clinical Diagnostics, Roche Diagnostics, Siemens, and the Hospital Quality Foundation. Dr. Levy is also Chair of the Accreditation Oversight Committee for the American College of Cardiology (ACC) and a member of the ACC’s National Cardiovascular Data Registry Oversight Committee.
Dr. Butler is a consultant for Abbott, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Relypsa, Vifor.
Dr. Chang reports no COI.
Dr. Char reports no COI.
Dr. Diercks reports research funding from Abbott, Ortho Clinical Diagnostics, Roche, and Siemens. Her stock/ownership interests are Emergencies in Medicine, LLC.
Dr. Fermann reports research funding from Siemens, Ortho Diagnostics, NIH (NHLBI/NINDS/NIMH). Speaker’s Bureau for Portola and Janssen. Advisory Board for Portola and Janssen.
Dr. Han is supported by the National Institutes of Health (NIH) under award number
R21AG06312, R56HL141567 and R01AG065249. Dr. Han also receives research funding from Bristol Myers Squibb, Boehringer Ingelheim, and Merck.
Dr. Hiestand reports research funding from Siemens.
Dr. Hogan reports no COI.
Dr. Kahn reports no COI.
Dr. Lindenfeld is a consultant for Abbott, AstraZeneca, CVRx, Boehringer Ingelheim, Edwards LifeSciences, Impulse Dynamics, VWave. She receives funding from Astra Zeneca, Volumetric, Sensible Medical.
Dr. McNaughton reports no COI relevant to this work. She receives research support from the NHLBI, the VA, and Pfizer.
Ms. Miller reports no COI.
Dr. Peacock reports grants from Abbott, Brainbox, Calcimedica, CSL Behring, Ortho Clinical Diagnostics, Relypsa, Roche, Salix, Siemens. He is a consultant for Abbott, Astra-Zeneca, Beckman, Bosch, Fast Biomedical, Forrest Devices, Ischemia Care, Dx, Instrument Labs, Janssen, Nabriva, Ortho Clinical Diagnostics, Osler, Relypsa, Roche, Quidel, Salix, Siemens. His Stock/Ownership Interests are AseptiScope Inc, Brainbox Inc, Comprehensive Research Associates LLC, Emergencies in Medicine LLC, Forrest Devices, Ischemia DX LLC.
Dr. Schrock reports no COI.
Dr. Self reports no COI.
Dr. Singer reports research funding or consulting fees from Alexion Pharmaceutical, Janssen, Pfizer, BMS, Mallinckrodt.
Dr. Sterling is partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 1U54GM115428.
Dr. Collins received grant research support from NIH/NHLBI, PCORI, AHRQ, Beckman Colter and consultant support/other from Ortho Clinical, Boehringer Ingelheim, Vixiar, BMS.
Disclosures:
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI) or its Board of Governors. Findings and conclusions similarly do not represent the Get with The Guidelines (GWTG) program or the American Heart Association (AHA).
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND and Roger VL. Hospitalizations after heart failure diagnosis a community perspective. Journal of the American College of Cardiology. 2009;54:1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storrow AB, Jenkins CA, Self WH, Alexander PT, Barrett TW, Han JH, McNaughton CD, Heavrin BS, Gheorghiade M and Collins SP. The burden of acute heart failure on U.S. emergency departments. JACC Heart Fail. 2014;2:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins SP and Storrow AB. Moving toward comprehensive acute heart failure risk assessment in the emergency department: the importance of self-care and shared decision making. JACC Heart Fail. 2013;1:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvert MJ, Freemantle N and Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–51. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R and Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. European heart journal. 2002;23:1867–76. [DOI] [PubMed] [Google Scholar]
- 7.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–10. [DOI] [PubMed] [Google Scholar]
- 8.Rumsfeld JS, Alexander KP, Goff DC Jr., Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ, American Heart Association Council on Quality of C, Outcomes Research CoC, Stroke Nursing CoE, Prevention CoPVD and Stroke C. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–49. [DOI] [PubMed] [Google Scholar]
- 9.Collins SP, Liu D, Jenkins CA, Storrow AB, Levy PD, Pang PS, Chang AM, Char D, Diercks DJ, Fermann GJ, Han JH, Hiestand B, Hogan C, Kampe CJ, Khan Y, Lee S, Lindenfeld J, Martindale J, McNaughton CD, Miller KF, Miller-Reilly C, Moser K, Peacock WF, Robichaux C, Rothman R, Schrock J, Self WH, Singer AJ, Sterling SA, Ward MJ, Walsh C and Butler J. Effect of a Self-care Intervention on 90-Day Outcomes in Patients With Acute Heart Failure Discharged From the Emergency Department: A Randomized Clinical Trial. JAMA Cardiol. 2021;6:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus JA and Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR and Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. Journal of the American College of Cardiology. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 12.Sauser K, Spertus JA, Pierchala L, Davis E and Pang PS. Quality of life assessment for acute heart failure patients from emergency department presentation through 30 days after discharge: a pilot study with the Kansas City Cardiomyopathy Questionnaire. J Card Fail. 2014;20:18–22. [DOI] [PubMed] [Google Scholar]
- 13.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS and Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 14.Flynn KE, Lin L, Ellis SJ, Russell SD, Spertus JA, Whellan DJ, Pina IL, Fine LJ, Schulman KA, Weinfurt KP and Investigators H-A. Outcomes, health policy, and managed care: relationships between patient-reported outcome measures and clinical measures in outpatients with heart failure. Am Heart J. 2009;158:S64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL and Weinfurt KP. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;163:88–94 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fermann GJ, Levy PD, Pang P, Butler J, Ayaz SI, Char D, Dunn P, Jenkins CA, Kampe C, Khan Y, Kumar VA, Lindenfeld J, Liu D, Miller K, Peacock WF, Rizk S, Robichaux C, Rothman RL, Schrock J, Singer A, Sterling SA, Storrow AB, Walsh C, Wilburn J and Collins SP. Design and Rationale of a Randomized Trial of a Care Transition Strategy in Patients With Acute Heart Failure Discharged From the Emergency Department: GUIDED-HF (Get With the Guidelines in Emergency Department Patients With Heart Failure). Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN and Consortium RE. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, Drazner MH, Fong MW, Givertz MM, Gordon RA, Jermyn R, Katz SD, Lamba S, Lanfear DE, LaRue SJ, Lindenfeld J, Malone M, Margulies K, Mentz RJ, Mutharasan RK, Pursley M, Umpierrez G and Kosiborod M. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463–1476. [DOI] [PubMed] [Google Scholar]
- 20.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Kober L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjostrand M, Langkilde AM and McMurray JJV. Effects of Dapagliflozin on Symptoms, Function, and Quality of Life in Patients With Heart Failure and Reduced Ejection Fraction: Results From the DAPA-HF Trial. Circulation. 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R: A language and environment for statistical computing [computer program]. Version 3.5.2. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 22.Spertus JA, Jones PG, Sandhu AT and Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2020;76:2379–2390. [DOI] [PubMed] [Google Scholar]
- 23.Jovicic A, Holroyd-Leduc JM and Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ditewig JB, Blok H, Havers J and van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78:297–315. [DOI] [PubMed] [Google Scholar]
- 25.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D and Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015:CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonkman NH, Westland H, Groenwold RH, Agren S, Atienza F, Blue L, Bruggink-Andre de la Porte PW, DeWalt DA, Hebert PL, Heisler M, Jaarsma T, Kempen GI, Leventhal ME, Lok DJ, Martensson J, Muniz J, Otsu H, Peters-Klimm F, Rich MW, Riegel B, Stromberg A, Tsuyuki RT, van Veldhuisen DJ, Trappenburg JC, Schuurmans MJ and Hoes AW. Do Self-Management Interventions Work in Patients With Heart Failure? An Individual Patient Data Meta-Analysis. Circulation. 2016;133:1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koelling TM, Johnson ML, Cody RJ and Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–85. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, Crombie P and Vaccarino V. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. Journal of the American College of Cardiology. 2002;39:83–9. [DOI] [PubMed] [Google Scholar]
- 29.McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB and Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C and Smith M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossello X, Bueno H, Gil V, Jacob J, Javier Martin-Sanchez F, Llorens P, Herrero Puente P, Alquezar-Arbe A, Raposeiras-Roubin S, Lopez-Diez MP, Pocock S and Miro O. MEESSI-AHF risk score performance to predict multiple post-index event and post-discharge short-term outcomes. Eur Heart J Acute Cardiovasc Care. 2020;10:142–152. [DOI] [PubMed] [Google Scholar]
- 32.Miro O, Rossello X, Platz E, Masip J, Gualandro DM, Peacock WF, Price S, Cullen L, DiSomma S, de Oliveira MT Jr., McMurray JJ, Martin-Sanchez FJ, Maisel AS, Vrints C, Cowie MR, Bueno H, Mebazaa A, Mueller C and Study Group on Acute Heart Failure of the Acute Cardiovascular Care Association of the European Society of C. Risk stratification scores for patients with acute heart failure in the Emergency Department: A systematic review. Eur Heart J Acute Cardiovasc Care. 2020;9:375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hejjaji V, Scholes A, Kennedy K, Sperry B, Khariton Y, Dean E, Lee DS and Spertus JA. Systemizing the Evaluation of Acute Heart Failure in the Emergency Department: A Quality Improvement Initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


